Abstract
Context
Kidney complications may be considerably higher in patients with chronic hypoparathyroidism (hypoPT) treated with activated vitamin D and calcium supplementation.
Objective
We aimed to investigate the risk of chronic kidney disease (CKD), urolithiasis, and hospitalization in patients with chronic hypoPT.
Methods
In this population-based cohort study in Sweden, national registries (Swedish National Patient Register, Swedish Prescribed Drug Register, and Total Population Register, 1997–2018) were used to identify patients with chronic hypoPT and controls matched by sex, age, and county of residence. We determined time to CKD and urolithiasis diagnosis, and incidence rates of hospitalization.
Results
A total of 1562 patients with chronic hypoPT without preexisting CKD and 15 620 controls were included. The risk of developing CKD was higher in patients with chronic hypoPT compared with controls (hazard ratio [HR] 4.45; 95% CI, 3.66-5.41). In people without prior urolithiasis (n = 1810 chronic hypoPT and n = 18 100 controls), the risk of developing urolithiasis was higher in patients with chronic hypoPT (HR 3.55; 95% CI, 2.84-4.44) compared with controls. Patients with chronic hypoPT had higher incidence rates for all-cause hospitalization (49.59; 95% CI, 48.50-50.70, per 100 person-years vs 28.43; 95% CI, 28.15-28.71, respectively) and for CKD (3.46; 95% CI, 3.18-3.76, per 100 person-years vs 0.72; 95% CI, 0.68–0.77, respectively), compared with controls. Men with hypoPT appear to have a higher risk of CKD than women.
Conclusion
Patients with chronic hypoPT had an increased risk of CKD, urolithiasis, and hospitalization compared with controls.
Keywords: hypoparathyroidism, chronic kidney disease, urolithiasis, epidemiology
Parathyroid hormone (PTH) plays a central role in mineral homeostasis by stimulating the reabsorption of calcium in the kidney and increasing renal phosphate excretion (1). It also stimulates renal conversion of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D, a hormone that enhances absorption of calcium and phosphate from the gastrointestinal tract (2). PTH increases bone turnover and, therefore, the release of calcium and phosphate into the circulation. Hypoparathyroidism (hypoPT) is a rare endocrine disease characterized by hypocalcemia and undetectable or inappropriately low levels of PTH (3). The estimated prevalence of chronic hypoPT varies between 5.3 and 37 per 100 000 in the United States and Europe (4-7). Anterior neck surgery is the most common cause of acquired chronic hypoPT and is responsible for approximately 75% of cases. Other causes are autoimmune, idiopathic, and genetic disorders and, even more rare, infiltrative disorders, such as metastatic disease or iron overload in the parathyroid glands (8). The primary biochemical abnormalities resulting from PTH deficiency are hypocalcemia, hyperphosphatemia, and increased urinary calcium loss (3). Conventional therapy for chronic hypoPT includes active vitamin D and calcium supplementation. Thiazide diuretics are used in patients with marked hypercalciuria together with magnesium and potassium supplementation, as needed. Conventional therapy can correct the hypocalcemia associated with hypoPT, but it does not replace other functions of PTH and can lead to or worsen hypercalciuria (9, 10). Conversely, conventional therapy can cause hypercalcemia, hypercalciuria, and increased calcium-phosphorus products, which might increase the risk of urolithiasis, nephrocalcinosis, and chronic kidney disease (CKD) (8, 10-12).
Current evidence on long-term risks of CKD and urolithiasis in patients with chronic hypoPT is still limited. Previous studies are mainly restricted to either postsurgical or nonsurgical cases and often based on small sample sizes. Therefore, there is a need for larger studies on kidney complications in this rare disease. By merging data from population-based registries in Sweden, we identified a large cohort of patients with chronic hypoPT on conventional treatment and assessed the risk of kidney complications and hospitalization compared to matched controls.
Methods
The Registries
Since 1947, all Swedish residents are given a unique national registration number, enabling linking of data between national population registries. The Swedish Total Population Register covers demographic information on the Swedish population (13). The Swedish National Patient Register includes inpatient records since 1964 and outpatient records since 2001. It contains information on personal identity number, age, sex, dates of hospital admission and discharge, as well as codes for all surgical procedures and discharge diagnoses. It is mandatory for all health-care providers to report to the register (14). The Swedish Prescribed Drug Register started in July 2005 and covers information on all drugs dispensed by prescription in Sweden, regardless of reimbursement status. It does not include drugs bought over the counter or treatment received when hospitalized. The Prescribed Drug Register uses the Anatomical Therapeutic Chemical (ATC) classification system (15). In general, only a 3-month supply of a drug treatment can be dispensed at a time and a prescription is valid for 12 months, after which patients need a renewal.
Identification of Patients With Chronic Hypoparathyroidism
From the National Patient Register, we identified all patients with a diagnosis of hypoparathyroidism (ICD-10, E20.0, E20.2–9), postsurgical hypoparathyroidism (ICD-10 E89.2), DiGeorge syndrome (ICD-10 D82.1), or autoimmune polyendocrine syndrome (ICD-10 E31.0) between 1997 and 2018. Some patients develop transient hypoPT after anterior neck surgery and most of these cases resolve within 6 to 12 months of surgery (16). To further increase the diagnostic accuracy of chronic hypoPT and make sure that we did not include patients with transient hypoPT or patients with DiGeorge syndrome without hypoPT, we linked our data to the Prescribed Drug Register. Patients with a diagnosis of hypoPT before 2005 had to have at least 2 dispensations of active vitamin D (dihydrotachysterol, ATC A11CC02; alfacalcidol, ATC A11CC03; calcitriol, ATC A11CC04), with or without calcium, the first year after the start of the Prescribed Drug Register, thus between 2005 and 2006. We included patients who were reported to the National Patient Register in 2005 or later only if they had at least 2 dispensations of active vitamin D, with or without calcium, 13 to 24 months after first entry in the National Patient Register with the hypoPT diagnosis. We excluded patients with hypoPT if they had less than 2 dispensations of active vitamin D the last year of follow-up. Thus, only patients with corresponding ICD codes that fulfilled the dispensation criteria were included in this study. To date, PTH analogs are rarely used in the treatment of chronic hypoPT in Sweden. The ATC code H05AA was used to assess the use of PTH analogues in patients with chronic hypoPT and controls.
The validation of similar diagnostic criteria to identify patients in Sweden with chronic hypoPT through medical chart review in a subset of patients has been described earlier (17). In the validation study, patients were included if they had a diagnosis correspondent of hypoPT reported to the National Patient Register and had received 3 or more dispensations of conventional treatment during the first year after diagnosis or had 2 dispensations per year in 2 consecutive years. The positive predictive value of correct diagnosis was 91% in 120 randomly selected cases.
For each patient with an ICD code corresponding to hypoPT and fulfilling the dispensation criteria, we matched 10 controls by sex, birth year, and county of residence, through the Total Population Register. The study entry for cases with chronic hypoPT was the date when the first ICD code of hypoPT was reported to the National Patient Register. The study entry for the matched controls started when the corresponding case was first reported to the National Patient Register with an ICD code of chronic hypoPT. End of follow-up for both cases and controls was December 31, 2018, or the date of last activity in the registries.
Exclusion of Patients With Prior Kidney Disease or Urolithiasis
In the analysis of CKD, we excluded the set of patient and matched controls if either the patient or one of the controls had a diagnosis in the National Patient Register indicating preexisting CKD, before or within 12 months after study entry, using the following ICD-10 codes of CKD: E10.2: Type 1 diabetes mellitus with kidney complications; E11.2: Type 2 diabetes mellitus with kidney complications; I12.0 and I12.9: Hypertensive chronic kidney disease; N00–08: Glomerular diseases; N10–16: Renal tubulo-interstitial diseases (except N10.9, Acute tubulo-interstitial nephritis); N18–19: Chronic Kidney Disease and Unspecified kidney failure; N25–29: Other disorders of kidney and ureter; and Q61: Cystic kidney disease. Likewise, in the analysis of urolithiasis, we excluded the set of patient and matched controls if either the patient or the control had the ICD-10 codes N20–23, Urolithiasis, at baseline or within 1 year from study entry (Figure S1 (18)).
Outcomes and Covariates of Interest
The primary outcomes were CKD of any cause and urolithiasis occurring at least 12 months after the date of inclusion. In the analysis of CKD, we used a composite measure, defined as having one of the ICD codes of CKD as defined above. We did not use Q61: Cystic kidney disease, as an outcome since it is an inherited disorder rather than a potential complication to chronic hypoPT. For urolithiasis, we used the ICD-10 codes N20–23. Analyses were performed for all cases and separately for postsurgical and nonsurgical cases and by sex. In a sensitivity analysis, the time from inclusion to the first event of either outcome was increased from 12 months to 3, 5, and 7 years. Comorbidities at the time of inclusion were identified using ICD codes (19), and included a history of myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, diabetes, and hypertension (definitions are listed in Table S1 (18)).
Statistical Analyses
Categorical variables were summarized using frequency and percentage. Continuous variables were summarized using mean and SD or median and interquartile range (IQR) as appropriate. Rates of hospitalization for all causes and due to chronic kidney disease or urolithiasis during follow-up were summarized as event counts per 100 person-years of follow-up and presented with the associated Poisson 95% confidence intervals. Analysis of time-to-event outcome predictors in the matched sample were analyzed using a marginal Cox model. Analysis of time to outcome predictors in the chronic hypoPT subgroup were analyzed using a Cox proportional hazards regression. Hazard proportionality was assessed via analysis of scaled Schoenfeld residuals. Comparative time-to-event was visualized using Kaplan-Meier plots. For all analyses, P < 0.05 was considered significant. All analyses were conducted using Stata version 16.1 (StataCorp, College Station, Texas) and R version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Characteristics
In total, we identified 1982 patients with chronic hypoPT and 19 820 matched controls during the period 1997 to 2018. After excluding the set of patients with chronic hypoPT and controls with preexisting CKD, the remaining 1562 patients with chronic hypoPT and 15 620 controls were included in the analyses of the development of CKD. Of these, mean age at inclusion was 53 (± 19) years, and among the patients, 73% were postsurgical (Table 1). Mean follow-up time was 9.8 (± 6.0) years among people with chronic hypoPT and 10.2 (± 6.0) years among controls; median number of outpatient visits per year was 3.5 (IQR, 2.0-6.2) for patients with chronic hypoPT, compared with 1.3 (IQR, 0.7-2.6) for controls. In all, 12 (0.7%) patients with chronic hypoPT and 13 (0.07%) controls had at least one recorded dispensation of PTH analogues during follow-up.
Table 1.
Baseline characteristics of patients with chronic hypoparathyroidism and matched controls in Sweden between 1997 and 2018, without preexisting chronic kidney disease or urolithiasis
| Characteristics | Without preexisting CKD | Without preexisting urolithiasis | ||
|---|---|---|---|---|
| Chronic hypoparathyroidism | Controls | Chronic hypoparathyroidism | Controls | |
| No. of patients | 1,562 | 15,620 | 1,810 | 18,100 |
| Age, years, mean (SD) | 53.3 (19.1) | 53.3 (19.1) | 53.5 (19.6) | 53.5 (19.6) |
| Sex | ||||
| Women | 1,211 (77.5) | 12,110 (77.5) | 1,400 (77.4) | 14,000 (77.4) |
| Men | 351 (22.5) | 3,510 (22.5) | 410 (22.6) | 4,100 (22.6) |
| Follow-up time, years (Mean, SD) | 9.8 (6.0) | 10.2 (6.0) | 9.3 (6.0) | 9.8 (6.0) |
| Follow-up time, years, median (IQR) | 9.1 (4.6–15.2) | 9.8 (5.0–15.9) | 8.6 (4.1–14.5) | 9.1 (4.5–15.3) |
| Outpatient visits per year | 3.5 [2.0–6.2] | 1.3 [0.7–2.6] | 3.5 [2.0–6.1] | 1.4 [0.7–2.7] |
| Etiology | ||||
| Postsurgical hypoparathyroidism | 1,133 (72.5) | N/A | 1,300 (71.8) | N/A |
| DiGeorge syndrome | 10 (0.6) | N/A | 21 (1.2) | N/A |
| Autoimmune polyendocrine syndrome | 7 (0.4) | N/A | 18 (1.0) | N/A |
| Idiopathic hypoparathyroidism | 190 (12.2) | N/A | 219 (12.1) | N/A |
| Other or unspecified hypoparathyroidism | 222 (14.2) | N/A | 252 (13.9) | N/A |
| Dispensations of active vitamin D per yeara | 6.2 (4.0–10.4) | N/A | 6.1 (4.1–10.3) | N/A |
| Dispensations of calcium supplementation per year | 3.3 (1.1–6.6) | N/A | 3.4 (1.1–6.4) | N/A |
| Comorbidities | ||||
| Diabetes | 120 (7.7) | 505 (3.2) | 139 (7.7) | 639 (3.5) |
| Hypertension | 305 (19.5) | 1,157 (7.4) | 373 (20.6) | 1,485 (8.2) |
| Myocardial infarction | 38 (2.4) | 242 (1.6) | 49 (2.7) | 311 (1.7) |
| Congestive heart failure | 65 (4.2) | 270 (1.7) | 87 (4.8) | 349 (1.9) |
| Peripheral vascular disease | 25 (1.6) | 132 (0.9) | 33 (1.8) | 177 (1.0) |
| Cerebrovascular disease | 69 (4.4) | 401 (2.6) | 90 (5.0) | 540 (3.0) |
| Chronic pulmonary disease | 105 (6.7) | 508 (3.3) | 136 (7.5) | 645 (3.6) |
Values for continuous data are mean ± SD or median [IQR] and count (%) for categorical data. ICD codes of etiology: Postsurgical hypoparathyroidism (E89.2), DiGeorge syndrome (D82.1), Autoimmune polyendocrine syndrome (E31.0), Idiopathic hypoparathyroidism (E20.0), Other or unspecified hypoparathyroidism (E20.2–9). Abbreviations: CKD, chronic kidney disease; IQR, interquartile range; N/A, not applicable.
a Dispensation of any of the ATC codes A11CC02, A11CC03, A11CC04.
Further, after excluding patients with chronic hypoPT and controls with preexisting urolithiasis from the overall cohort of 1982 patients and 19 820 controls, the remaining 1810 patients with chronic hypoPT and 18 100 controls were included in the analyses of the development of urolithiasis. Mean age at inclusion was 54 (± 20) years, and among the patients, 72% were postsurgical (Table 1). Mean follow-up time was 9.3 (± 6.0) years among people with chronic hypoPT and 9.8 (± 6.0) years among controls; median number of outpatient visits per year was 3.5 (IQR, 2.0-6.1) for patients with chronic hypoPT, compared with 1.4 (0.7-2.7) for controls. In all, 11 (0.7%) patients with chronic hypoPT and 11 (0.07%) controls had at least one recorded dispensation of PTH analogues during follow-up.
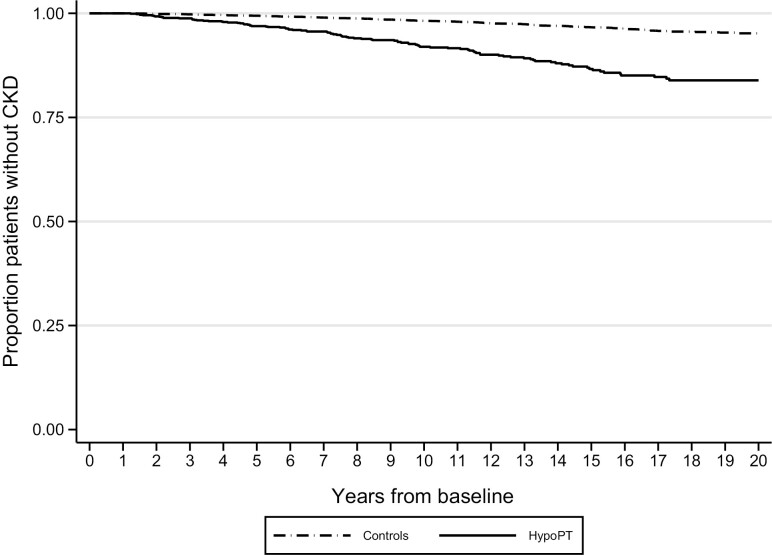
The Risk of Chronic Kidney Disease
During follow-up, a total of 136 (8.7%) patients with hypoPT developed CKD, compared with 309 (2.0%) among the controls (Table S2 (18)). The risk of developing de novo CKD was higher in patients with chronic hypoPT compared with controls (unadjusted hazard ratio [HR] 4.45; 95% CI, 3.66-5.41, Table 2). The unadjusted HR was 4.11 (95% CI, 3.29-5.17) for postsurgical patients (n = 1133, Fig. 1) and 5.74 (95% CI, 3.99-8.27) for nonsurgical patients (n = 429, Figure S2 (18)) with chronic hypoPT compared to controls. There was no difference in the risk of CKD between postsurgical and nonsurgical chronic hypoPT patients (P = 0.98). In a sensitivity analysis, we increased the time from inclusion to first event of CKD from 12 months to 3, 5, or 7 years. Patients with chronic hypoPT remained at similar increased risk compared with controls even if the time from inclusion increased (Table S3 (18)). When restricting to events 5 years after inclusion, the unadjusted HR for CKD was 4.09 (95% CI, 3.23-5.19) comparing patients with chronic hypoPT to controls. After adjustment for comorbidities, the results were attenuated but significant; the adjusted HR for CKD was 3.55 (95% CI, 2.77-4.54) comparing patients with chronic hypoPT to controls and when restricting to events 5 years after inclusion (Table S3 (18)).
Table 2.
The risk of chronic kidney disease and hospitalization in patients with chronic hypoparathyroidism, compared with matched controls
| Chronic kidney disease | Hospitalization for chronic kidney disease | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| Chronic hypoparathyroidism | 4.45 (3.66–5.41) | <0.001 | 3.49 (2.84–4.30) | <0.001 |
| Postsurgical | 4.11 (3.29–5.17) | <0.001 | 3.34 (2.64–4.23) | <0.001 |
| Nonsurgical | 5.74 (3.99–8.27) | <0.001 | 3.96 (2.76–5.68) | <0.001 |
| Adjusted hazard ratio (95% CI) | P value | Adjusted hazard ratio (95% CI) | P value | |
| Chronic hypoparathyroidism | 3.64 (2.96–4.49) | <0.001 | 2.68 (2.16–3.31) | <0.001 |
| Postsurgical | 3.40 (2.68–4.32) | <0.001 | 2.65 (2.06–3.39) | <0.001 |
| Nonsurgical | 4.96 (3.33–7.39) | <0.001 | 2.96 (1.98–4.44) | <0.001 |
Time-to-event analysis with Cox proportional hazards regression, using the first record of an ICD code corresponding of chronic kidney disease or hospitalization with chronic kidney disease as primary diagnosis. Adjusted for myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, diabetes, and hypertension.
Figure 1.
Proportion of individuals without chronic kidney disease in patients with postsurgical chronic hypoparathyroidism (n = 1133) compared with matched controls (n = 11 330).
The Risk of Urolithiasis
During follow-up, 108 (6.0%) of all patients developed urolithiasis, compared with 229 (1.3%) among the controls. A minority (12 cases among patients with hypoPT and 42 among controls) were lower tract calculi. Thus, most patients developed nephrolithiasis. The risk of developing de novo urolithiasis was higher in patients with chronic hypoPT, compared with controls (unadjusted HR 3.55; 95% CI, 2.84-4.44, Table 3). The differences of proportion of patients without urolithiasis over the follow-up period is shown in Figure S3 (18). Compared with controls, the risk of urolithiasis was higher in both the postsurgical (unadjusted HR 3.61; 95% CI, 2.79-4.67) and the nonsurgical subpopulation (unadjusted HR 3.39; 95% CI, 2.17-5.28). There was no difference in the risk of urolithiasis between postsurgical and nonsurgical patients (P = 0.69). Patients with chronic hypoPT remained at increased risk for urolithiasis compared with controls even when we increased the time to first event (Table S3 (18)). When restricting to events 5 years after inclusion, the adjusted HR for urolithiasis was 3.23 (95% CI, 2.51-4.16) in patients with chronic hypoPT compared with controls. The results were similar after adjustment for comorbidities (Table S3 (18)).
Table 3.
The risk of urolithiasis and hospitalization in patients with chronic hypoparathyroidism, compared to matched controls
| Urolithiasis | Hospitalization for urolithiasis | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| Chronic hypoparathyroidism | 3.55 (2.84–4.44) | <0.001 | 3.64 (2.95–4.48) | <0.001 |
| Postsurgical | 3.61 (2.79–4.67) | <0.001 | 3.51 (2.78–4.44) | <0.001 |
| Nonsurgical | 3.39 (2.17–5.28) | <0.001 | 4.06 (2.82–5.84) | <0.001 |
| Adjusted hazard ratio (95% CI) | P value | Adjusted hazard ratio (95% CI) | P value | |
| Chronic hypoparathyroidism | 3.27 (2.54–4.20) | <0.001 | 3.94 (2.72–5.71) | <0.001 |
| Postsurgical | 3.27 (2.44–4.40) | <0.001 | 3.45 (2.21–5.39) | <0.001 |
| Nonsurgical | 3.21 (1.97–5.22) | <0.001 | 5.93 (3.03–11.61) | <0.001 |
Time-to-event analysis with Cox proportional hazards regression, using the first record of an ICD code corresponding of urolithiasis or hospitalization with urolithiasis as primary diagnosis. Adjusted for myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, diabetes, and hypertension.
Hospitalization for Chronic Kidney Disease or Urolithiasis
The risk of hospitalization due to CKD was higher (unadjusted HR 3.49; 95% CI, 2.84-4.30) in patients with chronic hypoPT (n = 1562), compared with controls (Table 2). The risk was similar in the postsurgical (unadjusted HR 3.34; 95% CI, 2.64-4.23) and the nonsurgical subpopulation (unadjusted HR 3.96; 95% CI, 2.76-5.68). The risk of hospitalization due to urolithiasis for patients with chronic hypoPT was increased (unadjusted HR 3.64; 95% CI, 2.95-4.48), compared with controls (Table 3). Moreover, the risk was similar in the postsurgical (unadjusted HR 3.51; 95% CI, 2.78-4.44) and nonsurgical subpopulation (unadjusted HR 4.06; 95% CI, 2.82-5.84). There were no differences in the risk of hospitalization due to CKD or urolithiasis between patients with postsurgical and nonsurgical hypoPT (unadjusted HR 0.97; 95% CI, 0.66-1.45 and unadjusted HR 1.08; 95% CI, 0.73-1.62, respectively).
Incidence rates for all-cause hospitalization among patients without preexisting CKD was higher in patients with chronic hypoPT compared with controls (49.6; 95% CI, 48.5-50.7, per 100 person-years vs 28.4; 95% CI, 28.2-28.7, per 100 person-years, respectively) (Table 4). Additionally, incidence rates for hospitalization due to CKD was higher in patients with chronic hypoPT compared with controls (3.5; 95% CI, 3.2-3.8, per 100 person-years vs 0.7; 95% CI, 0.7-0.8, per 100 person-years, respectively). Among patients without preexisting urolithiasis, patients with chronic hypoPT had a higher incidence rate for all-cause hospitalization (47.8; 95% CI, 46.6-48.9, per 100 person-years) compared with controls (27.3; 95% CI, 27.0-27.6, per 100 person-years). Moreover, patients with chronic hypoPT had higher incidence rates for hospitalization due to urolithiasis, compared with controls.
Table 4.
Rates of hospitalization for all causes and due to chronic kidney disease or urolithiasis during follow-up
| Incidence rates for hospitalization per 100 person-years (95% CI) | |||
|---|---|---|---|
| Hypoparathyroidism | Controls | P value | |
| Cohort on chronic kidney disease | |||
| All causes | 49.59 (48.50–50.70) | 28.43 (28.15–28.71) | <0.001 |
| Chronic kidney disease | 3.46 (3.18–3.76) | 0.72 (0.68–0.77) | <0.001 |
| Cohort on urolithiasis | |||
| All causes | 47.77 (46.64–48.92) | 27.33 (27.04–27.62) | <0.001 |
| Urolithiasis | 0.76 (0.62–0.91) | 0.14 (0.12–0.16) | <0.001 |
Risk Factors for Chronic Kidney Disease and Urolithiasis
Risk factors for CKD and urolithiasis in patients with chronic hypoPT are presented in Table 5. Compared to women, men with chronic hypoPT had a higher risk for CKD (HR 2.12; 95% CI, 1.51-3.02) and urolithiasis (HR 1.90; 95% CI, 1.23-2.93). Other risk factors for CKD included congestive heart failure (HR 10.04; 95% CI, 6.00-16.81), diabetes (HR 4.08; 95% CI, 2.63-6.32) and hypertension (HR 2.59; 95% CI, 1.78-3.78). None of the evaluated comorbidities were associated with an increased risk for urolithiasis.
Table 5.
Risk factors for chronic kidney disease and urolithiasis in patients with chronic hypoparathyroidism
| Characteristics | Without preexisting CKD | Without preexisting urolithiasis | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| Age, years | 1.04 (1.03–1.05) | <0.001 | 1.00 (0.99–1.01) | 0.871 |
| Sex | ||||
| Women | Reference | Reference | ||
| Men | 2.14 (1.51–3.02) | <0.001 | 1.90 (1.23–2.93) | 0.004 |
| Comorbidities | ||||
| Diabetes | 4.08 (2.63–6.32) | <0.001 | 1.07 (0.47–2.46) | 0.870 |
| Hypertension | 2.59 (1.78–3.78) | <0.001 | 1.12 (0.64–1.95) | 0.702 |
| Myocardial infarction | 5.27 (2.67–10.41) | <0.001 | 1.32 (0.32–5.38) | 0.698 |
| Congestive heart failure | 10.04 (6.00–16.81) | <0.001 | 1.41 (0.44–4.50) | 0.557 |
| Peripheral vascular disease | 6.03 (2.45–14.84) | <0.001 | 2.49 (0.61–10.16) | 0.203 |
| Cerebrovascular disease | 3.80 (2.04–7.08) | <0.001 | 1.60 (0.58–4.38) | 0.360 |
| Chronic pulmonary disease | 1.44 (0.70–2.96) | 0.319 | 2.01 (0.97–4.17) | 0.062 |
Abbreviation: CKD, chronic kidney disease.
Comparing Men and Women With Hypoparathyroidism
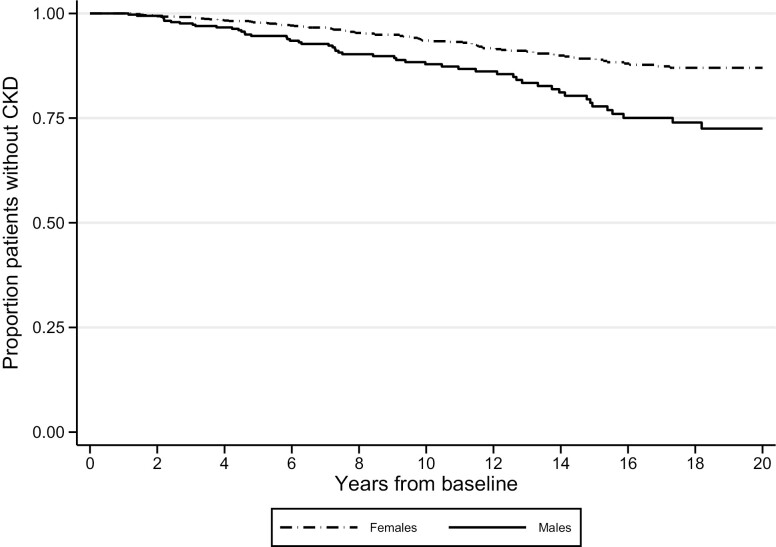
The majority of included patients were women (77.5% in the CKD cohort and 77.4% in the urolithiasis cohort, Table 1). More men than women with chronic hypoPT developed CKD during follow-up (Fig. 2). Men with postsurgical chronic hypoPT had an increased risk of CKD (unadjusted HR 1.75; 95% CI, 1.37-2.22), compared with women. The same was found in the nonsurgical subpopulation, where men also had an increased risk of CKD (unadjusted HR 2.63; 95% CI, 1.67-4.17), compared to women. Further, the risk of urolithiasis was higher in men in both postsurgical (unadjusted HR 2.65; 95% CI, 2.04-3.23) and nonsurgical patients (unadjusted HR 2.27; 95% CI, 1.49-3.45), compared with women. Moreover, men had a higher risk of hospitalization due to urolithiasis, both in patients with postsurgical hypoPT (unadjusted HR 2.17; 95% CI, 1.61-2.94) and nonsurgical hypoPT (unadjusted HR 2.04; 95% CI, 1.30-3.23). Men also had a higher risk of hospitalization due to CKD compared with women, both in patients with postsurgical (unadjusted HR 2.04; 95% CI, 1.52-2.78) and nonsurgical chronic hypoPT (unadjusted HR 2.04; 95% CI, 1.32-3.23).
Figure 2.
Proportion of individuals without chronic kidney disease among men and women with chronic hypoparathyroidism.
Discussion
Using high-quality population registries in Sweden and strict inclusion criteria by combining diagnostic codes and drug dispensation, we identified almost 2000 patients with chronic hypoPT between 1997 and 2018. Patients with chronic hypoPT are at more than 4 times higher risk of developing de novo CKD and almost 4 times higher of developing urolithiasis, compared with controls. Additionally, we found that patients with chronic hypoPT had a higher incidence rate of hospitalization due to any cause, CKD, and urolithiasis. Finally, our results indicate that men compared to women with chronic hypoPT are at even higher risk of CKD, urolithiasis, and hospitalization due to these conditions.
Conventional treatment for chronic hypoPT corrects hypocalcemia by increasing the intestinal calcium absorption but does not affect the renal calcium reabsorption and urinary phosphate excretion (20). Thus, it is hypothesized that both the use of conventional treatment with active vitamin D and calcium supplements and the absence of stimulatory effect of PTH on the reabsorption of calcium in the renal tubules in patients with chronic hypoPT increase the risk of developing kidney complications (21). In our study, a small number of individuals in the control group received PTH analogues, which probably corresponds to osteoporosis treatment.
Several studies have shown an increased risk of CKD in patients with chronic hypoPT (21). A Danish population-based study by Underbjerg et al, one of the largest studies, including 688 postsurgical patients with chronic hypoPT, showed an almost 5-fold increased risk of urolithiasis and CKD (22). The same research group also assessed the risk of urolithiasis and CKD in individuals with nonsurgical hypoPT. Again, the risk of CKD compared to matched controls was increased, although they did not find a significantly increased risk of urolithiasis (23). This can be compared to the results of our study, showing a 3- to 4-fold increased risk of CKD and urolithiasis in both postsurgical and nonsurgical chronic hypoPT. More recently, Gosmanova et al, identified more than 8000 patients with chronic hypoPT on conventional treatment and showed an increased risk of incident CKD (HR 2.91; 95% CI, 2.61-3.25), progression of CKD stage and progression to end-stage kidney disease compared to persons without hypoPT during a 5-year follow-up (24). Bergenfelz et al showed an almost 5-fold increased risk of CKD in patients with postsurgical hypoPT in Sweden who underwent total thyroidectomy, compared with patients who underwent total thyroidectomy but did not develop postsurgical hypoPT (25). Our study shows an increased risk of CKD in hypoPT that is similar to other large studies. Our study distinguishes itself from several others by including both postsurgical and nonsurgical cases, and also by having a longer follow-up time. In contrast to Underbjerg et al, our data also indicate a significantly increased risk of urolithiasis in patients with nonsurgical hypoPT (23). This difference could be explained by our better statistical power, since our cohort is almost 3 times as large. Indeed, patients with nonsurgical chronic hypoPT have previously been reported to have an increased risk of nephrolithiasis and CKD (26).
What causes CKD in patients with hypoPT? Previous studies in patients with chronic hypoPT have linked elevated serum calcium concentration and increased calcium-phosphate product level with increased risk of CKD (11, 27, 28). A recent study showed that hypercalciuria and renal calcification did not fully explain the increased risk of renal insufficiency in patient with chronic hypoPT (29). The role of serum phosphate in the risk for urolithiasis or CKD is not well understood. Studies in the general population have reported that high serum phosphate was associated with harmful effects on renal function (30) and impairment of microvascular function in individuals with normal renal function (31). Likewise, it is unclear whether the lack of PTH per se has any role in CKD development. In rodents, the renal vasculature is sensitive to vasodilation by PTH (32), and PTH has been shown to exert a relaxant effect on vascular smooth muscles cells that could be a potent modulator of renal hemodynamics and glomerular filtration rate (33).
Little is known about the rate of hospitalization due to CKD or urolithiasis in patients with chronic hypoPT. Underbjerg et al (22) reported a more than 3-fold higher risk of hospitalization for renal insufficiency or nephrolithiasis in patients with postsurgical hypoPT, compared with matched controls. Our results are similar, suggesting approximately 3 to 4 times higher risk of hospitalization with CKD or urolithiasis in both postsurgical and nonsurgical patients. Furthermore, we found that patients with chronic hypoPT had a higher incidence rate of hospitalization due to any cause, CKD, and urolithiasis. In a risk factor analysis in patients with chronic hypoPT, we found that comorbidities such as cardiovascular disease, diabetes, and hypertension were associated with an increased risk of CKD. This is in agreement with previous findings (34, 35).
We found that men compared to women with chronic hypoPT had a higher risk of urolithiasis, CKD, and hospitalization. Although women are more prevalent in the general CKD population (36), men are more likely to develop to more advanced CKD stages (37, 38). Also, CKD awareness is lower in women (39) and some findings indicate that the use of diagnostic codes for CKD is higher in men (40). These factors might contribute to the higher risk of CKD found in men in our study. Furthermore, urolithiasis is more common among men in the general population (41), which could also translate into a higher risk for men with chronic hypoPT.
The strengths of this study include the use of high-quality nationally representative registries and data linkage to combine ICD codes with drugs dispensed on prescription to identify patients with chronic hypoPT. Although misclassification of disease is a common limitation in observational studies, we previously validated the concordance between ICD-10 codes and clinical diagnosis of hypoPT. In the validation study, we found a positive predictive value of 91% using a similar method to find cases from the registries as described above (17). Another strength of this study is the large number of patients with chronic hypoPT included, given the rarity of the disease. The classification of CKD based on estimated glomerular filtration rate (eGFR) was first presented in global guidelines in 2005. Before that, clinical praxis was to use renal-specific diagnoses. Since both stage-specific and renal-specific codes are used clinically, we defined the ICD-10 codes of both stage-specific and renal-specific diagnoses to measure CKD.
Our study has limitations. We did not include biochemical data on kidney function, such as eGFR. Our study might have missed patients with chronic hypoPT if they did not have the diagnostic codes. We did not exclude incident patients with chronic hypoPT who might have develop CKD or urolithiasis before they were diagnosed with hypoPT but lacked a corresponding diagnostic code. However, this is unlikely to change our findings; when we increased the time from inclusion to the first outcome event to 3, 5, and 7 years in a sensitivity analysis, we found that the risk of CKD and urolithiasis remained increased compared to the controls. Further, we do not have data on vitamin D dosages or calcium load, thus are not able to assess whether prescriptions follow regional guidelines or if a dose-response relationship exists.
In summary, our findings suggest that patients with chronic hypoPT on conventional treatment in Sweden have an increased risk of CKD, urolithiasis, and hospitalization, compared with controls. The risk for men appears to be even higher than for women. This should raise awareness among treating physicians to monitor for modifiable risk factors for CKD and kidney stones.
Glossary
Abbreviations
- CKD
chronic kidney disease
- HR
hazard ratio
- hypoPT
hypoparathyroidism
- IQR
interquartile range
- PTH
parathyroid hormone
Contributor Information
Oskar Swartling, Department of Medicine, Clinical Epidemiology Division, Karolinska Institutet, Stockholm 171 77, Sweden.
Marie Evans, Renal unit, Department of Clinical Sciences, Interventions and Technology (CLINTEC), Karolinska Institutet and Karolinska University Hospital, Stockholm 141 52, Sweden; Swedish Renal Registry, Department of Internal Medicine, Ryhov Regional Hospital, Jönköping 551 11, Sweden.
Tim Spelman, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm 171 77, Sweden.
Wafa Kamal, Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm 171 76, Sweden; Department of Endocrinology, Metabolism and Diabetes, Karolinska University Hospital, Stockholm 171 76, Sweden.
Olle Kämpe, Department of Endocrinology, Metabolism and Diabetes, Karolinska University Hospital, Stockholm 171 76, Sweden; Department of Medicine, Solna, Karolinska Institutet, Stockholm 171 76, Sweden; K.G. Jebsen Center for Autoimmune Diseases, University of Bergen, Bergen 5021, Norway.
Michael Mannstadt, Endocrine Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA.
Ylva Trolle Lagerros, Department of Medicine, Clinical Epidemiology Division, Karolinska Institutet, Stockholm 171 77, Sweden; Center for Obesity, Academic Specialist Center, Stockholm Health Services, Stockholm 113 65, Sweden.
Sigridur Björnsdottir, Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm 171 76, Sweden; Department of Endocrinology, Metabolism and Diabetes, Karolinska University Hospital, Stockholm 171 76, Sweden.
Funding Support
This study was supported by a research grant from Shire and grants from the Swedish Research Council, Knut and Alice Wallenberg Foundation, Novo Nordisk Foundation, Torsten and Ragnar Söderberg’s Foundations, and Kristian Gerhard Jebsen Foundation. O.S. has received support from the Clinical Scientist Training Program (CSTP) and research internship at Karolinska Institutet. Y.T.L. was supported by Region Stockholm (clinical research appointment).
Author Contributions
O.S. and S.B. initiated the study and take responsibility for the integrity of the data and its accuracy. T.S. performed statistical analyses. O.S. and S.B. drafted the manuscript, which was developed to its final form by all coauthors. All authors contributed to the design of the study; interpretation of data; and to the critical revision of the manuscript for important intellectual content. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Disclosures
S.B. has received institutional research grant from Shire. O.K. is a board member of Navinci Diagnostics AB, Uppsala, Sweden. M.M. has served as an advisory board member, consultant, and research investigator for Shire, a Takeda company; as a consultant and research investigator for Chugai Pharmaceutical Co., Ltd; and as a consultant for Amolyt Pharma. The remaining authors have nothing to declare.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Shoback D. Clinical practice. Hypoparathyroidism. N Engl J Med. 2008;359(4):391-403. [DOI] [PubMed] [Google Scholar]
- 2. Khan MI, Waguespack SG, Hu MI. Medical management of postsurgical hypoparathyroidism. Endocr Pract. 2011;17(Suppl 1):18-25. [DOI] [PubMed] [Google Scholar]
- 3. Mannstadt M, Bilezikian JP, Thakker RV, et al. Hypoparathyroidism. Nat Rev Dis Primers. 2017;3:17055. [DOI] [PubMed] [Google Scholar]
- 4. Powers J, Joy K, Ruscio A, Lagast H. Prevalence and incidence of hypoparathyroidism in the United States using a large claims database. J Bone Miner Res. 2013;28(12):2570-2576. [DOI] [PubMed] [Google Scholar]
- 5. Clarke BL, Brown EM, Collins MT, et al. Epidemiology and diagnosis of hypoparathyroidism. J Clin Endocrinol Metab. 2016;101(6):2284-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Astor MC, Løvås K, Debowska A, et al. Epidemiology and health-related quality of life in hypoparathyroidism in Norway. J Clin Endocrinol Metab. 2016;101(8):3045-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cipriani C, Pepe J, Biamonte F, et al. The epidemiology of hypoparathyroidism in Italy: an 8-year register-based study. Calcif Tissue Int. 2017;100(3):278-285. [DOI] [PubMed] [Google Scholar]
- 8. Bilezikian JP, Khan A, Potts JT, et al. Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res. 2011;26(10):2317-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brandi ML, Bilezikian JP, Shoback D, et al. Management of hypoparathyroidism: summary statement and guidelines. J Clin Endocrinol Metab. 2016;101(6):2273-2283. [DOI] [PubMed] [Google Scholar]
- 10. Bilezikian JP, Brandi ML, Cusano NE, et al. Management of hypoparathyroidism: present and future. J Clin Endocrinol Metab. 2016;101(6):2313-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mitchell DM, Regan S, Cooley MR, et al. Long-term follow-up of patients with hypoparathyroidism. J Clin Endocrinol Metab. 2012;97(12):4507-4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rejnmark L, Underbjerg L, Sikjaer T. Hypoparathyroidism: replacement therapy with parathyroid hormone. Endocrinol Metab (Seoul). 2015;30(4):436-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ludvigsson JF, Almqvist C, Bonamy AK, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31(2):125-136. [DOI] [PubMed] [Google Scholar]
- 14. Laugesen K, Ludvigsson JF, Schmidt M, et al. Nordic health registry-based research: a review of health care systems and key registries. Clin Epidemiol. 2021;13:533-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register--opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16(7):726-735. [DOI] [PubMed] [Google Scholar]
- 16. Mihai R, Thakker RV. MANAGEMENT OF ENDOCRINE DISEASE: Postsurgical hypoparathyroidism: current treatments and future prospects for parathyroid allotransplantation. Eur J Endocrinol. 2021;184(5):R165-RR75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kamal W, Björnsdottir S, Kämpe O, Trolle Lagerros Y. Concordance between ICD-10 codes and clinical diagnosis of hypoparathyroidism in Sweden. Clin Epidemiol. 2020;12:327-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Swartling O, Evans M, Spelman T, et al. Supplemental data. Mendeley Data. Deposited July 6, 2022. 10.17632/pdmgg3h58g.1 [DOI]
- 19. Ludvigsson JF, Appelros P, Askling J, et al. Adaptation of the Charlson comorbidity index for register-based research in Sweden. Clin Epidemiol. 2021;13:21-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bilezikian JP. Hypoparathyroidism. J Clin Endocrinol Metab. 2020;105(6):1722-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gosmanova EO, Houillier P, Rejnmark L, Marelli C, Bilezikian JP. Renal complications in patients with chronic hypoparathyroidism on conventional therapy: a systematic literature review: renal disease in chronic hypoparathyroidism. Rev Endocr Metab Disord. 2021;22(2):297-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Underbjerg L, Sikjaer T, Mosekilde L, Rejnmark L. Cardiovascular and renal complications to postsurgical hypoparathyroidism: a Danish nationwide controlled historic follow-up study. J Bone Miner Res. 2013;28(11):2277-2285. [DOI] [PubMed] [Google Scholar]
- 23. Underbjerg L, Sikjaer T, Mosekilde L, Rejnmark L. The epidemiology of nonsurgical hypoparathyroidism in Denmark: a nationwide case finding study. J Bone Miner Res. 2015;30(9):1738-1744. [DOI] [PubMed] [Google Scholar]
- 24. Gosmanova EO, Chen K, Rejnmark L, et al. Risk of chronic kidney disease and estimated glomerular filtration rate decline in patients with chronic hypoparathyroidism: a retrospective cohort study. Adv Ther. 2021;38(4):1876-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bergenfelz A, Nordenstrom E, Almquist M. Morbidity in patients with permanent hypoparathyroidism after total thyroidectomy. Surgery. 2020;167(1):124-128. [DOI] [PubMed] [Google Scholar]
- 26. Kim SH, Rhee Y, Kim YM, et al. Prevalence and complications of nonsurgical hypoparathyroidism in Korea: a nationwide cohort study. PLoS One. 2020;15(5):e0232842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vadiveloo T, Donnan PT, Leese CJ, Abraham KJ, Leese GP. Increased mortality and morbidity in patients with chronic hypoparathyroidism: a population-based study. Clin Endocrinol (Oxf). 2019;90(2):285-292. [DOI] [PubMed] [Google Scholar]
- 28. Underbjerg L, Sikjaer T, Rejnmark L. Long-term complications in patients with hypoparathyroidism evaluated by biochemical findings: a case-control study. J Bone Miner Res. 2018;33(5):822-831. [DOI] [PubMed] [Google Scholar]
- 29. Ridder LO, Harslof T, Sikjaer T, Underbjerg L, Rejnmark L. Determinants of hypercalciuria and renal calcifications in chronic hypoparathyroidism: a cross-sectional study. Clin Endocrinol (Oxf). 2021;95(2):286-294. [DOI] [PubMed] [Google Scholar]
- 30. Moon H, Chin HJ, Na KY, et al. Hyperphosphatemia and risks of acute kidney injury, end-stage renal disease, and mortality in hospitalized patients. BMC Nephrol. 2019;20(1):362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ginsberg C, Houben A, Malhotra R, et al. Serum phosphate and microvascular function in a population-based cohort. Clin J Am Soc Nephrol. 2019;14(11):1626-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Endlich K, Massfelder T, Helwig JJ, Steinhausen M. Vascular effects of parathyroid hormone and parathyroid hormone-related protein in the split hydronephrotic rat kidney. J Physiol. 1995;483(Pt 2):481-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Philbrick WM, Wysolmerski JJ, Galbraith S, et al. Defining the roles of parathyroid hormone-related protein in normal physiology. Physiol Rev. 1996;76(1):127-173. [DOI] [PubMed] [Google Scholar]
- 34. Jager KJ, Fraser SDS. The ascending rank of chronic kidney disease in the global burden of disease study. Nephrol Dial Transplant. 2017;32(suppl_2):ii121-ii8. [DOI] [PubMed] [Google Scholar]
- 35. Denker M, Boyle S, Anderson AH, et al. Chronic Renal Insufficiency Cohort Study (CRIC): overview and summary of selected findings. Clin J Am Soc Nephrol. 2015;10(11):2073-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carrero JJ, Hecking M, Chesnaye NC, Jager KJ. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol. 2018;14(3):151-164. [DOI] [PubMed] [Google Scholar]
- 37. Swartling O, Rydell H, Stendahl M, Segelmark M, Trolle Lagerros Y, Evans M. CKD progression and mortality among men and women: a nationwide study in Sweden. Am J Kidney Dis. 2021;78(2):190-9 e1. [DOI] [PubMed] [Google Scholar]
- 38. Minutolo R, Gabbai FB, Chiodini P, et al. Sex differences in the progression of CKD among older patients: pooled analysis of 4 cohort studies. Am J Kidney Dis. 2020;75(1):30-38. [DOI] [PubMed] [Google Scholar]
- 39. Hodlmoser S, Winkelmayer WC, Zee J, et al. Sex differences in chronic kidney disease awareness among US adults, 1999 to 2018. PLoS One. 2020;15(12):e0243431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bello AK, Ronksley PE, Tangri N, et al. Quality of chronic kidney disease management in Canadian primary care. JAMA Netw Open. 2019;2(9):e1910704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sorokin I, Mamoulakis C, Miyazawa K, Rodgers A, Talati J, Lotan Y. Epidemiology of stone disease across the world. World J Urol. 2017;35(9):1301-1320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.