Abstract
Objectives
To measure the unit-level variation in Acute Kidney Injury (AKI) incidence post-thoracic surgery over a contemporary 1-year period. Secondary aims include examining the associations with sex, age group, operation type, length of stay and mortality.
Design
A multicentre, observational, retrospective study in thoracic surgery.
Setting
17 of 35 Society for Cardiothoracic Surgery of Great Britain and Ireland (SCTS) units participated. The student wing, known as SCTS STUDENTS, supported data collection.
Participants
Overall, 15 229 patients were collected of which 15 154 were included for analysis after exclusions. All patients (age≥18 years) undergoing any thoracic surgery from 1 April 2016 to 31 March 2017 were included. For analysis, we excluded patients with preoperative end-stage renal failure and those with incomplete data.
Main outcome measures
The primary outcome is the incidence of AKI within 7 days of the procedure or discharge date if earlier. Secondary outcomes include assessing associations with patient demographics (age, sex), type of procedure (open and minimally invasive), length of stay and mortality.
Results
Out of 15 154 patients AKI was diagnosed in 1090 patients (7.2%) within 7 days of surgery with AKI stage 1 (4.8%), stage 2 (1.7%) and stage 3 (0.7%). There was a statistically significant variation in AKI incidence between units from 3.1 to 16.1% (p<0.05). Significant differences between AKI and non-AKI were found in post-operative length of stay (7 vs 3 days, p<0.001), 30-day mortality (9 vs 1.6%, p<0.001), 90-day mortality (14.7 vs 4.4%, p<0.001) and 1-year mortality (23.1 vs 12.2 %, p<0.001).
Conclusions
Following thoracic surgery, AKI incidence ranged from 3.1% to 16.1% between units (p<0.05) with associations between AKI and both length of stay and mortality. We propose AKI as a suitable comparative and absolute quality measure in thoracic surgery. Reducing rates of AKI may improve patient outcomes, length of stay and reduce costs.
Keywords: Thoracic surgery, AUDIT, Acute renal failure
Strengths and limitations of this study.
Multi-centre Evaluation of Renal Impairment in Thoracic Surgery is one of the largest studies in acute kidney injury (AKI) and thoracic surgery worldwide.
We collected simple, robust and pragmatic data variables that were previously identified in the pilot study.
The observational design of this multicentre study does not allow conclusions regarding causal links between AKI and the outcomes.
The study did not collect comorbidities that have been previously associated with AKI as this was not the intent of the study objectives and design.
Introduction
To achieve the best patient outcomes after surgery and drive quality improvement, suitable outcome measures are needed. Traditionally, mortality has been used, but because of improved care, mortality is now very low in thoracic surgery. The 2019 lung cancer clinical outcomes project (LCCOP) report (for operations in 2017) gave survival rates of 98.1% at 30 days and 88.7% at 1-year post surgery for primary lung cancer in the National Health Service (NHS) in England.1 There were no negative outliers and one positive outlier at 30 days. At 1 year, there were no outliers. In the Society for Cardiothoracic Surgery of Great Britain and Ireland (SCTS) thoracic surgery audit2 from 1 April 2016 to 31 March 2017, 28 740 cases in total were reported to SCTS from units in Great Britain and Ireland. The overall in-hospital unadjusted mortality rate for this period was 1.16% (334 deaths/28 740 cases). This is reassuring for patients and clinicians. However, when an outcome has little variation, it means that there are limitations in using it to compare performance.
As a result, there is a need to identify and validate additional outcome measures. Such a metric should be (1) easy to reliably measure, (2) be associated with meaningful health and system outcomes and (3) show sufficient variation. This study aims to assess acute kidney injury (AKI)3 as such a performance measure.
AKI is not well documented in thoracic surgery. Only three relevant publications report an incidence of AKI post-thoracic surgery: 5.9% after all lung resections,4 and 6.8% and 10% after lung cancer resections.5 6 AKI is well recognised after cardiac surgery and is associated with worse morbidity, mortality and more costs.7–10 AKI has been studied in other surgical fields with rates from 7.4% to 12.2% in gastrointestinal surgery and 23.2% to 25.1% in vascular surgery.11
Our previous single-centre pilot study found an incidence of AKI post-thoracic surgery of 15.1% (86/568).12 AKI was also associated with a longer hospital stay. However, in order to explore variation, a single-centre study is not sufficient. Having multicentre estimates of incidence and baseline characteristics of AKI after thoracic surgery would allow benchmarking and quality improvement and standards to guide practice. In order to better understand AKI in thoracic surgery, we developed this project: ‘Multicentre Evaluation of Renal Impairment in Thoracic Surgery’ (MERITS).
The primary aim was to determine the unit-level variation in the incidence of AKI post thoracic surgery over a contemporary 1-year period. Secondary aims were to report associations with sex, age, operation type, length of stay and mortality. This study is not designed to show causation.
We now report significant variation of AKI incidence post thoracic surgery across the participating centres and found that AKI was associated with increased length of stay and mortality.
Methods
Study design
MERITS is a multicentre, observational, retrospective study in thoracic surgery, composed of a collaboration of 17 thoracic surgery centres participating in the already established SCTS thoracic surgery rolling audit. SCTS includes the thoracic surgery units from five different national healthcare systems (Eire (Ireland), England, Scotland, Northern Ireland and Wales)
All 35 hospitals in Great Britain and Ireland that offer adult thoracic surgery and report to the SCTS thoracic surgery audit were invited. Seventeen units participated. Each participating thoracic surgery unit team comprised a consultant thoracic surgeon lead, a day-to-day coordinator (usually a middle-grade doctor or a research nurse), and a group of medical students recruited by SCTS STUDENTS.
Inclusion and exclusion criteria
All patients (age≥18 years) undergoing any thoracic surgery from 1 April to 2016 to 31 March 2017 (date of first surgery within these dates) were included. For analysis, we excluded patients with preoperative end-stage renal failure and those with incomplete data.
Variables
Our previous pilot study12 had identified variables which were both pragmatic to collect, robust and clinically meaningful. These were: the submitted SCTS thoracic surgery operation code (refer to online supplemental file 1 and table 1), date of birth, operation, discharge, death (if applicable); sex; AKI stage (1, 2 or 3); peak creatinine; preoperative and postoperative renal replacement therapy. Thoracic surgery operations were recorded using the accepted SCTS code for 2016/2017. Survival was collected for 1-year post surgery.
Table 1.
Age, sex, operation mode and Society for Cardiothoracic Surgery of Great Britain and Ireland (SCTS) code and proportion with acute kidney injury (AKI)
| N | Level | Overall | AKI negative | AKI positive |
| 15 154 | 14 064 | 1090 | ||
| Gender n (%) | F | 6345 | 5967 (94.0) | 378 (6.0) |
| M | 8809 | 8097 (91.9) | 712 (8.1) | |
| Age group n (%) | Young | 5958 | 5686 (95.4) | 272 (4.6) |
| Old | 8197 | 7500 (91.5) | 697 (8.5) | |
| Oldest | 998 | 877 (87.9) | 121 (12.1) | |
| Operation access mode n (%) | OPEN | 5835 | 5260 (90.1) | 575 (9.9) |
| VATS | 7635 | 7180 (94.0) | 455 (6.0) | |
| ENDO | 1684 | 1624 (96.4) | 60 (3.6) | |
| SCTS operation code category n (%) | A—lung resections (primary malignant) | 4502 | 4052 (90.0) | 450 (10.0) |
| B—lung resections (all other pathologies) | 1930 | 1812 (93.9) | 118 (6.1) | |
| C—mesothelioma surgery (therapeutic) | 452 | 416 (92.0) | 36 (8.0) | |
| D—pleural procedures (other) | 3311 | 3084 (93.1) | 227 (6.9) | |
| E—chest wall/diaphragmatic procedures | 734 | 693 (94.4) | 41 (5.6) | |
| F—mediastinal procedures | 1484 | 1433 (96.6) | 51 (3.4) | |
| G—oesophageal/gastric procedures | 50 | 41 (82.0) | 9 (18) | |
| H—tracheal surgery | 13 | 12 (92.3) | 1 (7.7) | |
| I—other procedures | 939 | 847 (90.2) | 92 (9.8) | |
| Z—endoscopic procedures | 1684 | 1624 (96.4) | 60 (3.6) |
VATS, video-assisted thoracic surgery.
bmjopen-2021-058542supp001.pdf (33.8KB, pdf)
To accurately collect renal function data, each thoracic unit contacted their respective biochemistry department and extracted the AKI stage and peak creatinine up to 7 days from the operation or discharge date if earlier. AKI stage was calculated using the algorithm introduced by the NHS England Patient Safety Alert to standardise AKI identification.13 In 3 of 17 units, creatinine was collected manually, and the AKI staging was calculated following the same algorithm.
Our pilot study12 had previously found that urine volumes were not collected or recorded reliably; therefore, we did not collect this in MERITS. In modern thoracic surgery practice within our nations, urinary catheterisation and strict urine volume recording is not commonly performed, and so urine output is not a robust measure.
Outcome measures
The primary outcome is the incidence of AKI occurring within 7 days of the procedure or discharge date if earlier. Secondary outcomes include assessing associations with patient demographics (age group, sex), type of procedure (open and minimally invasive), length of stay and mortality.
Data quality, security and validation
The majority of data collectors were medical students who were recruited by SCTS STUDENTS and junior doctors. All participants were provided with an online training package as part of the local site set-up. They were supervised by a day-to-day coordinator (usually a middle-grade cardiothoracic surgeon or a research nurse) and a consultant surgeon. Data were entered locally onto a spreadsheet with each team securely retaining a non-anonymised version. A secure anonymised version was sent to the MERITS study centre. Validation with each centre was performed before analysis. Digital security followed General Data Protection Regulation (GDPR) guidelines.
Data were validated by two observers who were not involved in the original data collection. Individual unit analysis was shared with each unit lead for checking and approval.
Data collection period
The launch for MERITS was in March 2018 at the SCTS Annual Meeting in Glasgow. This was followed by local regulatory approvals. Site opening and the recruitment of students and other data collectors took place during Summer 2018. All participants were provided with site packs with access to key documents for the study design, including on-line training videos.14
Statistical analysis
Continuous variables were summarised with the following descriptive statistics, non-missing sample size, mean and 95% and 99.8% CIs or medians with IQR where appropriate. Categorical data such as AKI incidence was summarised using frequencies and percentages calculated using the non-missing sample size. Univariate hypothesis testing was undertaken by Mann Whitney U tests for continuous data and χ2 for categorical data.
Multivariate analysis was also undertaken using generalised linear mixed modelling (GLMM) to assess the associations between AKI incidence and the fixed effects of our covariates plus random variation in intercept among centres. Our fixed effects include age group (Young<60 years/Old 60–79 years/Oldest Old≥ 80 years),15 16 sex (M/F) and operation type (Open/VATs/Endoscopic). All centres were included as random effect intercepts with a fixed gradient. Model fit was assessed by the Hosmer-Lemeshow goodness of fit test, by computing receiver operating characteristics and Nakagawa’s pseudo r2 for mixed effect models. The associations of the fixed effects were estimated and reported as ORs with 95% CIs. The conditional modes of the random effect intercepts and their 95% CIs were also derived to assess centre specific variation in isolation from fixed effects.
Patient and public involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. No patients were asked to advise on interpretation or writing up of results. No plans have been made to disseminate the results of the research to study participants.
Results
Subjects
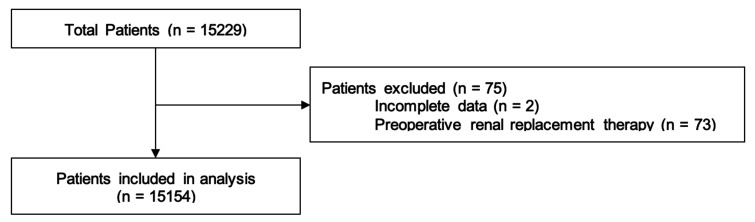
Overall, 15 229 patients were collected of which 15 154 were included for analysis after exclusions (figure 1). These were from 17 out of 35 thoracic surgical units in Great Britain and Ireland. Unit operative volumes ranged from 304 to 2416 patients per year. The total number of thoracic surgery operations submitted to SCTS in 2016–2017 was 28 740. This study represented 52.7% of all operations reported.
Figure 1.
Flow chart of inclusion and exclusion of patients.
Table 1 shows the sex, age groups, whether open, VATS or endoscopic and SCTS operation code category are shown along with the proportion with and without AKI.
Demographics
8809 (58.1%) patients were male and 6345 (41.9%) were female.
Average age at operation was 60.7±16.8 years. Age was divided into three categories; 5958 (39.3%) were<60 years, 8197 (54.1%) was 60–79 years and 998 (6.6%) were≥80 years. One patient’s age was not reliably confirmed.
Minimally invasive versus open surgery
The breakdown of operations as completed was as follows: 5835 (38.5%) operations were open, 7635 (50.4%) were minimally invasive video-assisted thoracic surgery (VATS), 1684 (11.1%) were endoscopic (such as bronchoscopy). Twenty cases were reported as robotic and were included with the minimally invasive VATS group.
SCTS operation code category
The breakdown of operations is also shown in table 1. The largest categories were lung resections for primary lung cancer (category A, 4502 cases, 29.8%), pleural diseases (category D, 3311 cases, 21.8%) and lung resections for reasons other than lung cancer (category B, 1930 cases, 12.8%). All lung resections (categories A and B) accounted for 42.6% of the workload.
Characteristics of AKI
Incidence of AKI
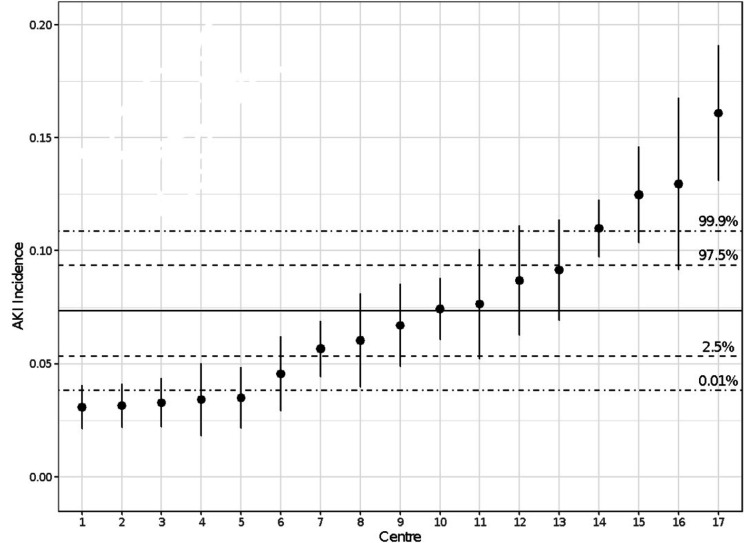
Of 15 154 patients, 1090 (7.2%) were found to have developed AKI within 7 days post-thoracic surgery: stage 1 (n=731; 4.8%); stage 2 (n=255; 1.7%); and stage 3 (n=104; 0.7%). AKI incidence ranged between 3.1% to 16.1%. The units have been listed in rank order from 1 to 17 (with 1 being the lowest rate of AKI and 17 the highest). This is shown numerically in table 2 and Forest plot figure 2.
Table 2.
AKI incidence (%) by unit in rank order
| Anonymised centre ID | Centre size | AKI negative | AKI positive |
| 1 | 1233 | 1195 (96.9) | 38 (3.1) |
| 2 | 1267 | 1227 (96.8) | 40 (3.2) |
| 3 | 1037 | 1003 (96.7) | 34 (3.3) |
| 4 | 497 | 480 (96.6) | 17 (3.4) |
| 5 | 716 | 691 (96.5) | 25 (3.5) |
| 6 | 615 | 587 (95.4) | 28 (4.6) |
| 7 | 1341 | 1265 (94.3) | 76 (5.7) |
| 8 | 513 | 482 (94.0) | 31 (6.0) |
| 9 | 716 | 668 (93.3) | 48 (6.7) |
| 10 | 1413 | 1308 (92.6) | 105 (7.4) |
| 11 | 458 | 423 (92.4) | 35 (7.6) |
| 12 | 518 | 473 (91.3) | 45 (8.7) |
| 13 | 645 | 586 (90.9) | 59 (9.1) |
| 14 | 2384 | 2122 (89.0) | 262 (11.0) |
| 15 | 922 | 807 (87.5) | 115 (12.5) |
| 16 | 301 | 262 (87.0) | 39 (13.0) |
| 17 | 578 | 485 (83.9) | 93 (16.1) |
AKI, acute kidney injury.
Figure 2.
Unadjusted Forest Plot for acute kidney injury (AKI) incidence among different units. Point ranges report the AKI proportion of that centre and the associated 95% CI. The solid horizontal line is the mean AKI incidence across all centres and the dashed lines represent the associated 95% and 99.8% CIs.
AKI rate in open and minimally invasive surgery
9.9% of patients undergoing open surgery developed AKI versus 6.0% undergoing VATS and 3.6% undergoing endoscopic procedures (table 1).
Adjusted AKI variation across units
To assess centre variation and associations more accurately between our covariates and AKI incidence, we undertook a multivariate analysis. Using the GLMM framework, we adjusted our observed clinically relevant variables by defining our fixed effects terms as age group, sex and operation type with each centre represented by a random effect intercept with a fixed gradient.
All fixed effects showed a significant relationship with developing AKI post operatively. Male patients had a 1.37× (95% CI 1.21 to 1.57; p<0.001) increased odds of developing AKI. Patients between the age of 60–79 had a 1.99× (95% CI 1.72 to 2.30; p<0.001) increased odds of developing AKI; ≥80 had a 3.01× (95% CI 2.4 to 3.8; p<0.001) increased odds of developing AKI. There was a 1.7× (95% CI 1.48 to 1.94; p<0.001) increased odds of developing AKI with open procedures compared with VATS (table 3).
Table 3.
AKI modelling for gender, age and operation type
| 95% CIs | |||||
| OR | Lower bound | Upper bound | P value | ||
| (Intercept) | 0.03 | 0.02 | 0.04 | <0.001 | |
| Gender | Female | 1.00 | Reference | ||
| Male | 1.37 | 1.20 | 1.57 | <0.001 | |
| Age | Youngest (<60) | 1.00 | Reference | ||
| Old (60–79) | 1.99 | 1.72 | 2.30 | <0.001 | |
| Oldest (≥ 80) | 3.02 | 2.40 | 3.80 | <0.001 | |
| Operation type | VATS | 1.00 | Reference | ||
| OPEN | 1.70 | 1.48 | 1.94 | <0.001 | |
| Endoscopy | 0.54 | 0.41 | 0.71 | <0.001 | |
AKI, acute kidney injury.
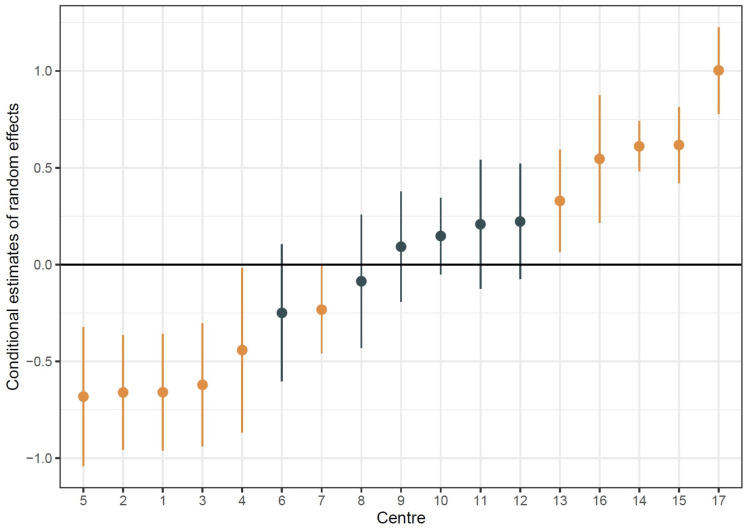
We then derived the conditional mode of the random intercepts for each centre to assess the adjusted centre-to-centre variation (figure 3). We found that there was significant variation in 11/17 (64.7%) of the sampled centres after adjusting for our observed covariates. This suggests that there was significant variation across the centres.
Figure 3.
Adjusted Forest Plot for acute kidney injury (AKI) incidence among different units. Point ranges represent the estimated conditional mode of the random intercept associated with each centre with the associated 95% CIs. Brown points represent centres that deviate significantly from average and black points represent non-significant centres.
Model diagnostics showed no evidence of lack of fit (HL test, p=0.32), and a reasonable level of discrimination with a c-statistic of 0.71. However, our model did not explain much of the variability in the data (Conditional pseudo r2=0.15), meaning that there are likely to be unobserved explanatory covariates.
Length of stay
Patients with AKIhad an increased median postoperative length of stay of 4 days as compared with non-AKI (7 versus 3 days; p<0.001) (table 4).
Table 4.
Associations between AKI and mortality and length of stay
| Level | AKI negative | AKI positive | P value | |
| N | 14 064 | 1090 | ||
| 30-day mortality (%) | Survived | 13 846 (98.4) | 992 (91.0) | <0.001 |
| Died | 218 (1.6) | 98 (9.0) | ||
| 90-day mortality (%) | Survived | 13 451 (95.6) | 930 (85.3) | <0.001 |
| Died | 613 (4.4) | 160 (14.7) | ||
| 365-day mortality (%) | Survived | 12 354 (87.8) | 838 (76.9) | <0.001 |
| Died | 1710 (12.2) | 252 (23.1) | ||
| Length of stay (median (IQR)) | 3.00 (2.00–6.00) | 7.00 (4.00–13.00) | <0.001 |
AKI, acute kidney injury.
The total increase in length of stay accounts for 4360 days across the 1090 AKI-positive patients or 5.1% (4360/86054) of the total number of days spent in the hospital after thoracic surgery in our study population.
Mortality
Patients with AKI (as compared with those without) had a significantly increased mortality at 30 days (AKI 9% vs no AKI 1.6%; p<0.001); 90 days (14.7% v 4.4%) and 1 year (23.1 vs 12.2%; p<0.001) (table 4).
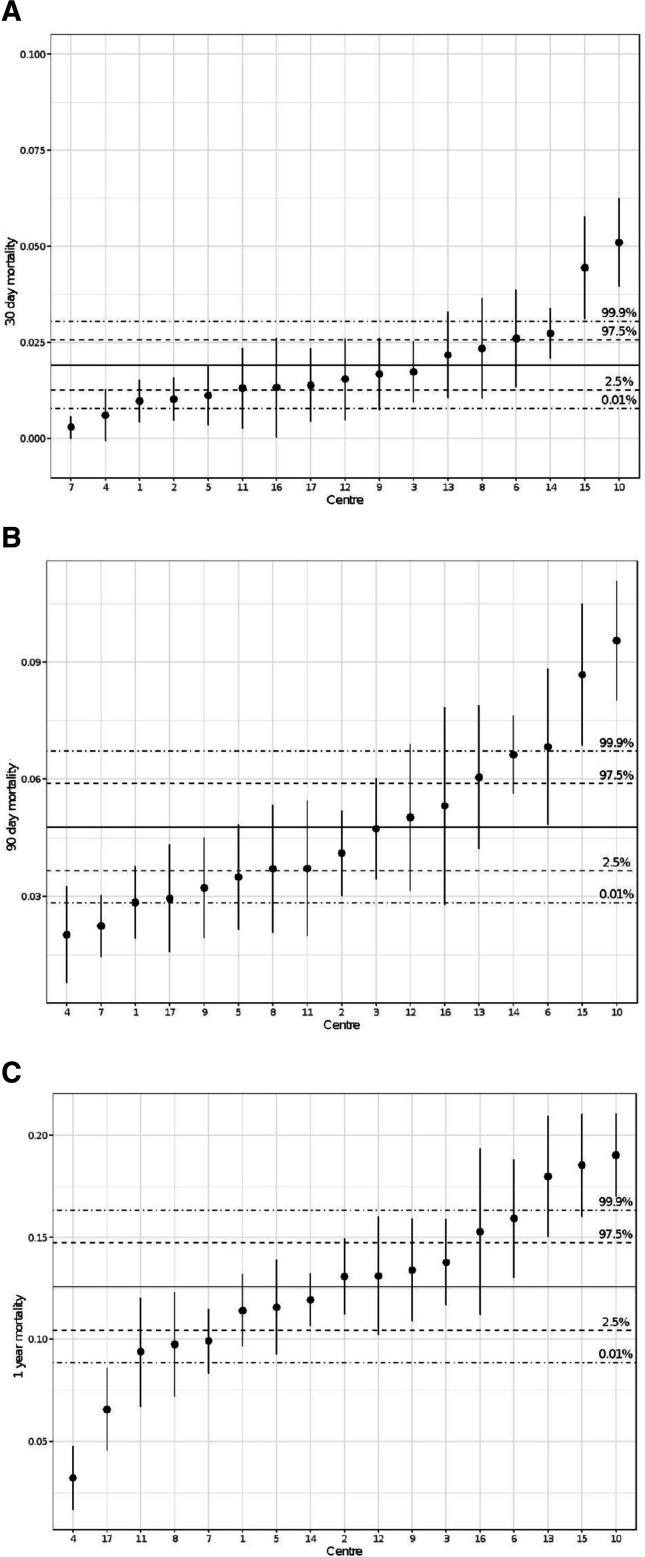
Across centres, we found that mortality varied between 0.3% and 5.1% at 30 days, 2.0% and 9.6% at 90 days and 3.2% and 19.0% at 1 year (figure 4a–c). We observed that the ranking of AKI differed from the ranking of mortality. For instance, the unit with the highest rate of AKI did not have the highest level of mortality. We also observed that the ranking of mortality changed over the three time points.
Figure 4.
Unadjusted forest plot for 30-day, 90-day and 1-year mortality among different units. Point ranges report the proportion of mortality of that centre and the associated 95% CI. The solid horizontal line is the mean mortality across all centres and the dashed lines represent the associated 95% and 99.8% confidence intervals.
Discussion
MERITS is the largest study to examine AKI after thoracic surgery and one of the largest such studies in a surgical population.4–6 Previous single-centre studies showed AKI rates that varied from 5.9% after all lung resections4 to 6.8% and 10% after lung cancer resections.5 6 Our earlier single-centre pilot study incorporating all procedures found a rate of 15.1%.12
The primary aim was to examine the unit variation in AKI incidence after thoracic surgery. This study of 17 units found an overall AKI rate of 7.2% with a range from 3.1% to 16.1%. The spread was statistically significant.
We have also shown that the post-thoracic surgery AKI variation was greater than the postoperative death rate reported in a similar period. In the 2019 LCCOP report, the overall in-hospital mortality was 1.26% (334 of 26 460 patients) with 1 positive unit outlier at 30 days and no unit outliers at 1 year.
Thus, we have shown that AKI has a greater variation in incidence than the death rate. In this study after adjustment, there are five positive and six negative statistical unit outliers (figure 3), which would support the use of AKI as a performance metric.
This study showed that the variation in AKI between units is greater than the variation in mortality. However, there was not a consistent relationship between AKI and mortality. For example, the unit with the highest rate of AKI (unit 17 in table 1 and figures 2 and 3) had a much lower mortality rate. The explanation for this is not obvious, and it is likely to be multifactorial. One explanation is that in that unit postoperative steps effectively treat AKI though do not prevent its occurrence as compared with other units. Examining the case-mix and different practices between units will be the start of exploring the reasons for this difference and this can drive quality improvement.
We went on to demonstrate a statistically significant association between AKI and length of stay and mortality. There are many studies in different clinical situations which observe similar findings. It is recognised that AKI is an independent predictor of death17 even with mild transient AKI post surgery.18 Patients who develop AKI are at increased risk of chronic kidney disease and end-stage renal failure.19
Because AKI is sometimes preventable and reducing its rate is associated with better outcomes, there are important potential health and economic benefits of monitoring and reducing AKI rates.20 There is a national programme in the UK to increase AKI awareness and to prevent and treat it.
The relationship between AKI and longer stay is also intuitively clear. In this study, the associated unadjusted median increase in bed occupancy is 5.1%, corresponding to 4360 days. While there will be various contributory factors, it follows that reducing postoperative AKI is also likely to reduce the length of stay and hospital costs.
We found that increased age and male sex were also associated with an increased risk of AKI. Various reasons can be speculated. Renal function declines with age and the nephrotoxic impact of surgery and anaesthesia may be greater. Perioperative hypotension, for example, may be less well tolerated.
Importantly, we found that open surgery is associated with a significantly greater risk of AKI than minimally invasive surgery. The reasons for this may be related to the greater tissue injury associated with an open operation, but there could also be other factors such as complexity and length of the surgery. We speculate that the latter is more likely and this is another area to be explored.
MERITS is one of the largest studies ever conducted in AKI and thoracic surgery worldwide. Furthermore, it is one of the largest collaborations of SCTS thoracic surgical units in and was achieved without any extra funding. This was only possible because of a strong collaborative professional culture including students recruited from SCTS STUDENTS. The success of the project also relied on collecting simple, robust and pragmatic data variables that were previously identified in the pilot study.
This study has some limitations. The observational design of this multicentre study precludes conclusions regarding causal links between AKI and the outcomes. AKI was diagnosed based on renal function only as urine output data could not be collected reliably. We were reliant on the coding of cases according to the SCTS database. The categorisation is high level and no intraoperative details are collected. The study also did not collect comorbidities that have been previously associated with AKI as this was not the intent of the study objectives and design. This could be addressed in a future study.
In summary, we have identified a significant variation in AKI rates between units post thoracic surgery. This will be due to multiple factors and reflect different surgical and anaesthetic strategies as well as patient heterogeneity. This is likely to include different approaches to perioperative cardiac output control, fluid management and use of nephrotoxic agents. Historically patients undergoing thoracic surgery were often relatively dehydrated on the basis that this may reduce the rate of acute lung injury associated with positive-pressure ventilation and surgical trauma. This is different to some of the concepts of enhanced recovery which encourage hydration and euvolaemia.21 It would be useful to consider the approach of the better performing units to determine what practices could be disseminated in line with the quality improvement strategy of the NHS and other health care systems.22
Supplementary Material
Acknowledgments
This work was supported by the Society for Cadiothoracic Surgery of Great Britain & Ireland (SCTS) and was made possible by the efforts of their student wing SCTS STUDENTS
Footnotes
Collaborators: Chief investigator: A.S. Coonar
Writing Committee: V. Naruka, M.A. Mckie, N. Ahmadi, C. Pama, A.S. Coonar
Steering committee: G. Aresu, A. Fry, S. Kendall, R. Page, C. Patvardhan, A. Peryt, R. Shah, D. West S. Wooley, S. Yeung
Thoracic Centre Principal Investigators: S. Stamenkovic (Barts Health NHS Trust, London), S. Shah (Basildon University Hospital, Basildon), A. Kirk (Golden Jubilee National Hospital, Glasgow), M. Loubani (Hull University Teaching Hospitals NHS Trust, Hull), J. Dunning (James Cook University Hospital, Middlesbrough), N. Chaudhuri and P. Tcherveniakov (Leeds Teaching Hospitals NHS Trust, Leeds), K. Rammohan (Manchester University NHS Foundation Trust, Manchester), J. Kadlec (Norfolk and Norwich University Hospitals NHS Foundation Trust, Norfolk and Norwich), A. Marchbank (Plymouth Hospitals NHS Trust, Plymouth), E.K.S Lim (Royal Brompton and Harefield NHS Foundation Trust, London), V. Zamvar (Royal Infirmary of Edinburgh, Edinburgh), A.S. Coonar (Royal Papworth Hospital, Cambridge), C. Tan (St George's University Hospitals NHS Foundation Trust, London), M. Hayward (University College London Hospitals NHS Foundation Trust, London), M. Kalkat (University Hospitals Birmingham NHS Foundation Trust, Birmingham), E. Woo (University Hospital Southampton NHS Foundation Trust, Southampton), V. Valtzoglou (University Hospital of Wales, Cardiff)
Local Coordinators (*) and Collaborators: Barts Health NHS Trust (London): O. Asemota, C. Evans, M. Lee, E. F. Tan, N. J. Yong; Basildon University Hospital (Basildon): V. Caruso, R. Leatherby; Golden Jubilee National Hospital (Glasgow): E. Allen, H. Ismahel, A. Patra; Hull University Teaching Hospitals NHS Trust (Hull): V. Crispi, A. Fort-Schaale, J. Green, E. Isaac*, J. Walshaw; James Cook University Hospital (Middlesbrough): C. P. Vidanapthirana*, J. Trevis; Leeds Teaching Hospitals NHS Trust (Leeds): M. Ashraf, M. Jabeen, Z. Syla*, S. Vijayapuri*; Manchester University NHS Foundation Trust (Manchester): T. Eadington*, B. Hama, O Karadakhy, M. Salehi*, M. Taylor*, Norfolk and Norwich University Hospitals NHS Foundation Trust (Norfolk and Norwich): M. Dixon, P. Njoku; Plymouth Hospitals NHS Trust (Plymouth): M. Halasa, N. Marshall, V. Palaniappan, L. Rogers*; Royal Brompton and Harefield NHS Foundation Trust (London): S. K. Bains, Y. N. Bashir, A. K. Bolina, H. Chavan, L. H. Cheng, J. Donovan*, A. G. Knighton, S. K. Longani, M. Nizami, P. D. Sousa*, B. A. Taylor, J. J. Teh, S. Zaman; Royal Infirmary of Edinburgh (Edinburgh): L. Clark; Royal Papworth Hospital (Cambridge): S. Cernic, H. Garland, A. GuéRoult, V. Naruka*, B. Ripoll, S. Saj, M. Tennyson; St George's University Hospitals NHS Foundation Trust (London): J. Abreu*, V. Beynon, M. Kaur, A. Nogueiro, A. Patel, V. Rohilla, N. Sahdev*, M. Sing, A. Sinha, J. Smelt*, M. Witcomb; University College London Hospitals NHS Foundation Trust (London): A. Antonopoulos*, A. Valnarov-Boulter; University Hospitals Birmingham NHS Foundation Trust (Birmingham): J. Cahill*, S. Khan*; University Hospital Southampton NHS Foundation Trust (Southampton): X. Liu, A. Tamburrini*, A. Visan; University Hospital of Wales (Cardiff): S. Algendy, T. Combellack*, R. Karsan, M. Musab*, A. Sayes, J. Williams*, S. Zouwail*
Contributors: Guarantor: ASC accepts responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish. Writing committee: VK has been involved in conceptualisation, design, methodology, data curation, project administration, validation, writing and reviewing the manuscript; MAM has been involved in data curation, formal analysis, methodology, validation, writing and reviewing the manuscript; NA has been involved in project administration, writing and reviewing the manuscript; EACP has been involved in the response to reviewers and data administration; ASC has been involved in conceptualisation, design, methodology, data curation, project administration, validation, supervision, writing and reviewing the manuscript. Steering committee: involved in validation and reviewing the manuscript. Thoracic Centre Principal Investigators from each centre held responsibility over data collection and validation. Local coordinators and collaborators were involved in data collection.
Funding: Statistical support was provided from the MRC Biostatistics Unit Cambridge through the Papworth Trials Unit Collaboration. No additional funds were used, and the work was done on a voluntary basis by all the collaborators. Royal Papworth Hospital Research & Development paid the article processing charge.
Competing interests: None
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The project was approved by the Clinical Audit and Effectiveness Department at the study centre which was Royal Papworth Hospital, Cambridge (Registration Number: 1702) with a waiver for the need for patient consent and was approved as a multicentre audit. This study was then registered as a clinical audit at each of the collaborating hospitals. The protocol and invitation to participate were disseminated widely through student networks and societies in Great Britain and Ireland.
References
- 1.LCCOP . Report | Society for cardiothoracic surgery, 2019. Available: https://scts.org/lccop-2019-report/ [Accessed 11 Mar 2021].
- 2.West D. Personal communication. SCTS Thoracic Surgery Audit Lead. 2021. [Google Scholar]
- 3.Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:1–138 http://www.kisupplements.org/article/S2157-1716(15)31044-3/fulltext [Google Scholar]
- 4.Ishikawa S, Griesdale DEG, Lohser J. Acute kidney injury after lung resection surgery: incidence and perioperative risk factors. Anesth Analg 2012;114:1256–62. 10.1213/ANE.0b013e31824e2d20 [DOI] [PubMed] [Google Scholar]
- 5.Licker M, Cartier V, Robert J, et al. Risk factors of acute kidney injury according to RIFLE criteria after lung cancer surgery. Ann Thorac Surg 2011;91:844–50. 10.1016/j.athoracsur.2010.10.037 [DOI] [PubMed] [Google Scholar]
- 6.Cardinale D, Cosentino N, Moltrasio M, et al. Acute kidney injury after lung cancer surgery: incidence and clinical relevance, predictors, and role of N-terminal pro B-type natriuretic peptide. Lung Cancer 2018;123:155–9. 10.1016/j.lungcan.2018.07.009 [DOI] [PubMed] [Google Scholar]
- 7.Loef BG, Epema AH, Smilde TD, et al. Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol 2005;16:195–200. 10.1681/ASN.2003100875 [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Delgado JC, Esteve F, Torrado H, et al. Influence of acute kidney injury on short- and long-term outcomes in patients undergoing cardiac surgery: risk factors and prognostic value of a modified RIFLE classification. Crit Care 2013;17:R293. 10.1186/cc13159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vives M, Wijeysundera D, Marczin N, et al. Cardiac surgery-associated acute kidney injury. Interact Cardiovasc Thorac Surg 2014;18:637–45. 10.1093/icvts/ivu014 [DOI] [PubMed] [Google Scholar]
- 10.Acute Kidney Injury and Prognosis After Cardiopulmonary Bypass: A Meta-analysis of Cohort Studies - American Journal of Kidney Diseases. Available: https://www.ajkd.org/article/S0272-6386(14)01252-9/fulltext [Accessed 25 Jun 2019]. [DOI] [PubMed]
- 11.Bell S, Davey P, Nathwani D, et al. Risk of AKI with gentamicin as surgical prophylaxis. J Am Soc Nephrol 2014;25:2625–32. 10.1681/ASN.2014010035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naruka V, Mckie MA, Khushiwal R, et al. Acute kidney injury after thoracic surgery: a proposal for a multicentre evaluation (MERITS). Interact Cardiovasc Thorac Surg 2019;29:861–6. 10.1093/icvts/ivz184 [DOI] [PubMed] [Google Scholar]
- 13.Algorithm for detecting acute repeat kidney injury (AKI) based on serum creatinine changes with time this algorithm relates to the NHS England patient safety alert: NHS/PSA/D/2014/010. Available: zotero://attachment/611/ [Accessed 25 Jun 2019].
- 14.MERITS Step by step video for data collection - YouTube.. Available: https://www.youtube.com/watch?v=a_PVuXU3bP8&feature=youtu.be [Accessed 25 Jun 2019].
- 15.World Health Organization . Integrated care for older people: guidelines on community-level interventions to manage declines in intrinsic capacity. Geneva: World Health organization, 2017. https://www.who.int/publications/i/item/9789241550109 [PubMed] [Google Scholar]
- 16.Oldest old | Encyclopedia.com. Available: https://www.encyclopedia.com/social-sciences/encyclopedias-almanacs-transcripts-and-maps/oldest-old [Accessed 11 May 2022].
- 17.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA 1996;275:1489–94. [PubMed] [Google Scholar]
- 18.O'Connor ME, Hewson RW, Kirwan CJ, et al. Acute kidney injury and mortality 1 year after major non-cardiac surgery. Br J Surg 2017;104:868–76. 10.1002/bjs.10498 [DOI] [PubMed] [Google Scholar]
- 19.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 2012;81:442–8. 10.1038/ki.2011.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doyle JF, Forni LG. Acute kidney injury: short-term and long-term effects. Critical Care 2016;20:188. 10.1186/s13054-016-1353-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the enhanced recovery after surgery (ERAS®) Society and the European Society of thoracic surgeons (ESTs). European Journal of Cardio-Thoracic Surgery 2019;55:91–115. 10.1093/ejcts/ezy301 [DOI] [PubMed] [Google Scholar]
- 22.Getting It Right First Time - GIRFT. Get. It Right First Time - GIRFT. Available: http://www.gettingitrightfirsttime.co.uk/ [Accessed 11 Mar 2021].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-058542supp001.pdf (33.8KB, pdf)
Data Availability Statement
Data are available upon reasonable request.