Abstract
Rationale: This study aimed to assess the safety, efficacy, and survival of 177Lu-DOTA-EB-TATE in patients with metastatic neuroendocrine tumors (NETs).
Methods: Thirty patients with metastatic NETs were prospectively enrolled and treated with 177Lu-DOTA-EB-TATE (3 intended cycles at 8 to 12-week intervals, 3.7 GBq/cycle). Treatment-related adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. The treatment response was graded according to RECIST 1.1 and PERCIST 1.0 criteria. Kaplan-Meier analysis was performed to calculate progression-free survival (PFS) and overall survival (OS).
Results: Patients tolerated therapy well without acute adverse effects. During peptide receptor radionuclide therapy (PRRT), no grade 4 toxicity was observed in any of the patients; grade 3 hematotoxicity was recorded in 4 patients, including grade 3 thrombocytopenia in 4 patients (13.3%) and grade-3 anemia in 1 patient (3.3%); grade 3 hepatotoxicity was recorded in 1 (3.3%) patient, and no grade 2/3/4 nephrotoxicity was observed. On long-term follow-up, none of the patients developed grade 4 hematotoxicity or nephrotoxicity of any grade, reversible grade 3 hematotoxicity (thrombocytopenia) occurred in 1 patient. There was no incidence of leukemia or myelodysplastic syndrome for the duration of follow-up. Of 27 patients with RECIST-measurable disease, partial response and stable disease were seen in 9 and 14 patients, respectively, resulting in a response rate of 33.3% and disease control rate of 85.2%. Of 29 patients evaluable for response on 68Ga-DOTATATE PET/CT, 14 had partial response and 11 had stable disease, with a response rate of 48.3% and disease control rate of 86.2%. The follow-up period ranged from 5 to 57 months after the first 177Lu-DOTA-EB-TATE PRRT with a median follow-up of 46 months. The median PFS was 36 months, and the median OS was not reached. Ki-67 index of greater than 10% was associated with poorer PFS (P = 0.012).
Conclusions: Our results suggest that PRRT with approximately 3.7 GBq 177Lu-DOTA-EB-TATE has acceptable toxicity profile and is effective in treating metastatic NET with high disease control rate. In addition, 177Lu-DOTA-EB-TATE achieved a favorable survival outcome with encouraging PFS.
Keywords: peptide receptor radionuclide therapy (PRRT), 177Lu-DOTA-EB-TATE, 177Lu-DOTA-TATE, neuroendocrine tumor
Introduction
Neuroendocrine tumors (NETs) represent a heterogenous group of neoplasms that arise from neuroendocrine cells throughout the body. The incidence rate of these tumors has increased substantially in recent years 1, 2. The choice of appropriate treatment in patients with unresectable metastatic NET is limited, and over 50% of NET patients are at an advanced stage at the time of diagnosis 3.
The majority of NETs overexpress somatostatin receptors (SSTRs), predominantly subtype 2 (SSTR2), which provides the basis for the use of SSTR2-targeted peptide receptor radionuclide therapy (PRRT). PRRT is a form of radiolabeled somatostatin analogue therapy, delivering radionuclides directly to NET tumor cells with high expression of SSTRs. At present, 177Lu-DOTATATE is the most frequently used radiopharmaceutical in patients with advanced NETs. Increasing evidence has confirmed the safety and efficacy of PRRT with 177Lu-DOTATATE for patients with advanced, progressive, and SSTR-positive NETs 4-6. In the prospective Phase 3 NETTER-1 trial, 177Lu-DOTATATE plus 30 mg long-acting octreotide demonstrated remarkedly longer progression-free survival and overall survival in midgut NET patients compared to 60 mg long-acting octreotide, which led to the approval of 177Lu-DOTATATE (Lutathera) by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) 7.
A potential disadvantage of 177Lu-DOTATATE is its rapid elimination from the blood stream, leading to insufficient tumor uptake and retention 8. To improve the efficacy of PRRT, Evans blue modified octreotate was synthesized and conjugated to DOTA chelator (DOTA-EB-TATE). Evans blue reversibly binds to serum albumin 9, 10, thus extending the effective plasma half-life of 177Lu-DOTA-TATE. In preclinical and first-in-human dosimetry studies, 177Lu-DOTA-EB-TATE demonstrated much higher tumor dose compared with 177Lu-DOTATATE 11, 12. Our prior dose escalation study showed that 177Lu-DOTA-EB-TATE with doses of 3.7 GBq/cycle achieved acceptable safety and satisfactory therapeutic efficacy after one-cycle treatment, and the more recent study of 177Lu-DOTA-EB-TATE with escalating doses in multiple cycles further supported this result 13, 14. In the present study, we aimed to further investigate the safety, efficacy, and long-term outcome of 177Lu-DOTA-EB-TATE with planned dose of 3.7 GBq/cycle in patients with metastatic NETs.
Materials and Methods
Patients
This clinical trial was approved by the Institutional Review Board of the Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College; written informed consent was obtained from all patients. The study was registered at ClinicalTrials.gov (NCT03478358). From August 2017 to November 2021, 30 patients (17 men and 13 women, age range, 15‒69) with metastatic NETs were prospectively enrolled in this trial to evaluate the safety and efficacy of repeated cycles of 177Lu-DOTA-EB-TATE. The inclusion criteria for PRRT were as follows: (1) histologically confirmed NETs with high SSTR2 expression determined by 68Ga-DOTATATE PET/CT imaging (modified Krenning score 3 = lesion uptake > liver; or 4 = lesion uptake ≥ spleen15), performed within 2 weeks before therapy; (2) white blood cell (WBC) ≥ 3 × 109/L; (3) platelets (PLT) ≥ 60 × 109/L; (4) hemoglobin (Hb) ≥ 90 g/L; (5) serum creatinine level < 150µmol/L; (6) patient's readiness to provide clinical information and follow-up. Exclusion criteria were as follows: (1) patients with no or low SSTR-expression on 68Ga-DOTATATE PET/CT (Krenning score less than 3); (2) pregnancy; (3) breast-feeding patients; (4) patients with known previous malignancies.
Treatment regimen
DOTA-EB-TATE was labeled with 177Lu using a previously published method 16. The radioisotope 177Lu, in the form of 177LuCl3 was purchased from LuMark®, IDB, Holland. Briefly, DOTA-EB-TATE (100 µg dissolved in 20 µL of absolute ethanol) was added to 200 µL 0.5 M NaOAc (pH 5.6), and then, the required amount of radioactivity of 177LuCl3 was added. The mixture was heated at 100 °C for 30 min and then purified by a preconditioned C18 light SEP-PAK cartridge and passed through a 0.22 µm aseptic filtration membrane directly into a sterile vial. The quality control was performed with analytical thin-layer chromatography (Bioscan, USA). CH3OH:NH4OAc (v/v 1:1) was used as the developing solution. The radiolabeling yield was greater than 90% and the radiochemical purity of 177Lu-DOTA-EB-TATE was more than 95%. The administration of radiopharmaceutical was performed in the ward. Pre-set dose of radiopharmaceutical was slowly administered intravenously over 30 min. In order to protect the kidneys, the mixture of arginine and 5% glucose solution (25 g/L, 1000 mL) was administered at least 30 min before radiopharmaceutical administration and lasted for 4 h. The planned administered dose per cycle was 3.7 GBq. The treatment was planned for up to 3 cycles, and cycles were repeated at intervals of 8 to 12 weeks.
Safety evaluation
All patients were clinically monitored during the administration of radiopharmaceutical and for 3 days thereafter as inpatients. Laboratory examinations including hematological parameters, renal function tests, and liver function tests were performed before, 2 weeks, and 4 weeks after each cycle during the course of PRRT, as well as during follow-up (at 4‒6 months intervals after completion of PRRT). Short- and long-term adverse events were graded in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0 (CTCAE 5.0). The rates of adverse events at baseline, 2 weeks, and 4 weeks after each cycle of therapy, and during follow-up were recorded.
Response evaluation
The treatment response assessment was performed 2-3 months after each cycle of PRRT. RECIST 1.1 criteria was applied to evaluate morphological response following PRRT with contrast-enhanced CT or MR. The molecular response was evaluated according to PERCIST 1.0 criteria 17 with 68Ga-DOTATATE PET/CT. Partial response was defined as ≥ 30% reduction in tracer uptake, progressive disease was defined as ≥ 30% increase in tracer uptake or appearance of new lesions; and stable disease as neither partial response nor progressive disease on 68Ga-DOTATATE PET/CT.
Progression-free survival and overall survival
Progression-free survival (PFS) was defined as the start of the first 177Lu-DOTA-EB-TATE PRRT cycle to date of radiographically confirmed progression by RECIST 1.1 or PERCIST 1.0 criteria. Overall survival (OS) was defined as the start of the first PRRT to death or last follow-up.
Statistical analysis
All statistical analyses were carried out using SPSS Statistics for Windows version 26.0 (IBM Corp, Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviations; non-continuous variables were expressed as counts and proportions. Differences between paired samples before and after treatment were compared using nonparametric testing or Student's test. Univariate Cox proportional hazards regression model was used to perform the survival analysis. Kaplan-Meier curves were used to depict the survival. All statistical tests were 2-tailed, and a P value less than 0.05 was considered statistically significant.
Result
Patients
The details of patients' baseline clinical characteristics are listed in Table 1 (Supplemental Table 1). In total, 30 patients who met the eligibility criteria were enrolled (17 men, 13 women). Of 30 patients, 18 patients (60%) had pancreatic NET, and the remaining 12 patients included rectal NET (n = 5), small intestinal NET (n = 4), paraganglioma (n = 2), and unknown primary site with metastases (n = 1). World Health Organization grades of tumor differentiation included grade 1 in 4 patients, grade 2 in 24 patients, and grade 3 in 2 patients. Approximately 3.7 GBq per cycle (average: 3.84 ± 0.70) of 177Lu-DOTA-EB-TATE was administered. Of 30 patients, 17 did not complete 3 cycles of 177Lu-DOTA-EB-TATE. Among those patients, 3 patients discontinued treatment because of hematotoxicity (grade 3 thrombocytopenia). Two patients withdrew voluntarily. Eight patients were delayed due to the COVID-19 lockdown. One patient discontinued treatment because of late enrollment time. Two patients discontinued treatment due to disease progression. One patient died due to pulmonary infection. The median cumulative administered activity was 8.97 GBq (range, 3.19‒14.32 GBq) over a median of 2 cycles (range, 1‒3). Treatment cycles and cumulative administered activity are summarized in Table 2.
Table 1.
Demographic and baseline clinical characteristics of patients
| Characteristic | Number | Percentage (%) |
|---|---|---|
| Male | 17 | 56.7 |
| Female | 13 | 43.3 |
| Age (years) | 50.1 ± 11.8 | |
| Primary tumor site | ||
| Pancreas | 18 | 60 |
| Rectum | 5 | 16.7 |
| Small intestine | 4 | 13.3 |
| Paraganglioma | 2 | 6.7 |
| Cancer of unknown primary | 1 | 3.3 |
| Tumor grade | ||
| G1 | 4 | 13.3 |
| G2 | 24 | 80 |
| G3 | 2 | 6.7 |
| Treatment before PRRT | ||
| Surgery | 17 | 56.7 |
| Somatostatin analogue | 20 | 66.7 |
| Chemotherapy | 12 | 40 |
| Everolimus | 4 | 13.3 |
| Radiotherapy | 1 | 3.3 |
| Tyrosine kinase inhibitor | 9 | 30 |
| Previously treated with PRRT | 4 | 13.3 |
| Transarterial chemoembolization |
4 | 13.3 |
| Metastases | ||
| Liver | 28 | 93.3 |
| Bone | 9 | 30 |
| Lymph nodes | 11 | 36.7 |
| Lung | 4 | 13.3 |
Table 2.
Treatment cycles and cumulative administered activity for 177Lu-DOTA-EB-TATE
| Number of PRRT cycles | Number of patients (n = 30) |
Percentage (%) | Cumulative activity (GBq) |
|---|---|---|---|
| 1 | 4 | 13.3% | 3.97 ± 0.24 |
| 2 | 13 | 43.3% | 7.78 ± 1.07 |
| 3 | 13 | 43.3% | 11.36 ± 2.01 |
Safety evaluation
Radiopharmaceutical administration was well tolerated without any serious acute adverse events. During the course of PRRT, no life-threatening grade 4 toxicity was observed in any of the patients. Grade 3 hematotoxicity was recorded in 4 patients (4/30, 13.3%), including grade 3 thrombocytopenia in 4 patients (13.3%), and grade 3 anemia in 1 patient (3.3%), who presented with grade 3 anemia already at baseline. There was a significant reduction in WBC counts (before therapy: 5.7 ± 2.0; after therapy: 4.3 ± 1.4 × 109/L, P = 0.034), platelet counts (before therapy: 245.4 ± 99; after therapy: 150.6 ± 97.8 × 109/L, P < 0.001), and hemoglobin (before therapy: 136.4 ± 13.7; after therapy: 120.2 ± 17.4 g/L, P = 0.017). Transient grade 3 hepatotoxicity (elevation of alanine aminotransferase, aspartate aminotransferase, and bilirubin) after the first cycle occurred in 1 patient, which spontaneously recovered to normal before the second cycle. No CTC-2/3/4 renal toxicity was observed in any of the patients. There was no statistically significant change observed in serum alanine aminotransferase (30.7 ± 17.4 vs. 36.1 ± 24.5, P = 0.094), aspartate aminotransferase (34.1 ± 13.6 vs. 34.4 ± 17.1, P = 0.178), alkaline phosphatase (136.1 ± 105.3 vs. 170.7 ± 165.5, P = 0.098), and creatinine level (70.6 ± 19.5 vs. 63.5 ± 9.0, P = 0.087) before and after treatment. The short-term toxicities were summarized in Table 3.
Table 3.
Hematotoxicity, hepatotoxicity, and nephrotoxicity before and after therapy according to CTCAE 5.0
| Toxicity | CTC-grade | Baseline | 1st cycle | 2nd cycle | 3rd cycle | |||
|---|---|---|---|---|---|---|---|---|
| 2 weeks | 4 weeks | 2 weeks | 4 weeks | 2 weeks | 4 weeks | |||
| Leukopenia | Grade-1 | 4 | 3 | 2 | 3 | 6 | 3 | 2 |
| Grade-2 | 0 | 3 | 3 | 3 | 4 | 3 | 2 | |
| Grade-3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Grade-4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Thrombocytopenia | Grade-1 | 0 | 3 | 3 | 1 | 3 | 1 | 3 |
| Grade-2 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | |
| Grade-3 | 0 | 0 | 2 | 1 | 1 | 1 | 0 | |
| Grade-4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Anemia | Grade-1 | 1 | 6 | 2 | 4 | 4 | 4 | 3 |
| Grade-2 | 2 | 0 | 2 | 1 | 1 | 0 | 1 | |
| Grade-3 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Grade-4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Nephrotoxicity | Grade-1 | 6 | 1 | 2 | 1 | 1 | 1 | 0 |
| Grade-2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Grade-3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Grade-4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Hepatoxicity | Grade-1 | 3 | 1 | 3 | 2 | 1 | 1 | 0 |
| Grade-2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Grade-3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Grade-4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
Long-term toxicity data were available for 29 patients. Long-term hematological event (thrombocytopenia, leukopenia, or anemia), occurring 7, 12, 7, 13, 23, and 21 months after the termination of PRRT, was documented in 6 patients. None of the patients developed grade 4 hematotoxicity. One patient (3.4%) had grade 3 hematotoxicity (thrombocytopenia). Five patients had grade 2 hematotoxicity, including leukopenia in 3 (10.3%), and anemia in 2 (6.9%). Three patients had grade 1 hematotoxicity, including leukopenia in 1 (3.4%), thrombocytopenia in 1 (3.4%), and anemia in 1 (3.4%).
There was no incidence of myelodysplastic syndrome or leukemia during the follow-up period. There was also no nephrotoxicity of any grade observed on long-term follow-up. Regarding hepatoxicity, only one patient had transient grade 1 toxicity (elevation of alanine aminotransferase). The long-term toxicities were summarized in Table 4.
Table 4.
Long-term hematotoxicity, nephrotoxicity, and hepatotoxicity according to CTCAE 5.0
| Toxicity | Grade 1 (no. of patients) | Grade 2 (no. of patients) | Grade 3 (no. of patients) | Grade 4 (no. of patients) |
|---|---|---|---|---|
| Leukopenia | 1 | 3 | 0 | 0 |
| Thrombocytopenia | 1 | 0 | 1 | 0 |
| Anemia | 1 | 2 | 0 | 0 |
| Nephrotoxicity | 0 | 0 | 0 | 0 |
| Hepatoxicity | 1 | 0 | 0 | 0 |
Efficacy of 177Lu-DOTA-EB-TATE
Of the 30 patients enrolled, tumor response assessment based on RECIST criteria was available for 27 patients after 177Lu-DOTA-EB-TATE PRRT. Nine patients had partial response and 14 patients had stable disease, resulting in a response rate of 33.3% and a disease control rate of 85.2%. Four of those 27 patients had progressive disease. Tumor response assessment based on 68Ga-DOTATATE PET/CT was available for 29 patients after 177Lu-DOTA-EB-TATE PRRT. The partial response was seen in 14 patients, stable disease in 11 patients, and progressive disease in 4 patients, with a response rate of 48.3% and disease control rate of 86.2%. According to the primary site, pancreatic NET and non-pancreatic NET showed no statistically significant difference in treatment response referring to RECIST (response rate: 33.3% vs. 33.3%, P = 1.000; disease control rate: 88.9% vs. 77.8%, P = 0.582) and PERCIST criteria (response rate: 58.8% vs. 33.3%, P = 0.264; disease control rate: 94.1% vs. 75%, P = 0.279). Representative cases of 177Lu-DOTA-EB-TATE PRRT efficacy on 68Ga-DOTATATE PET/CT are shown in Figure 1 and Supplemental Figure 1.
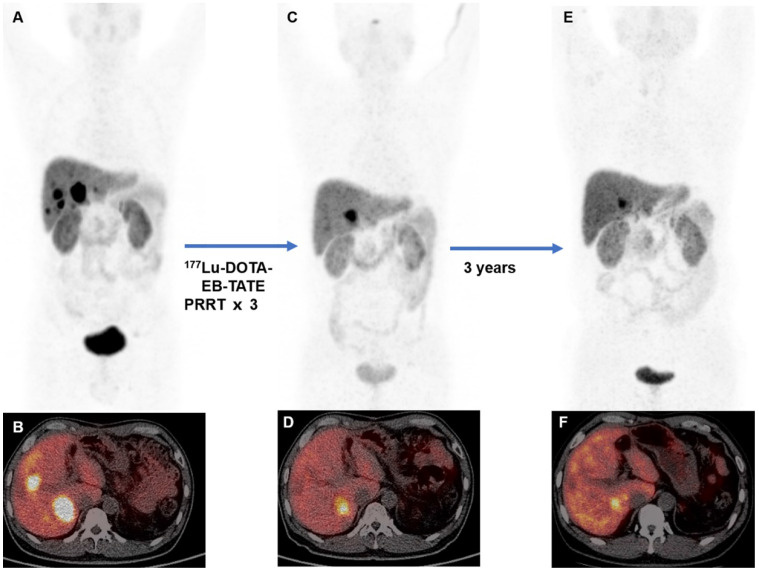
Figure 1.
A 45-year-old man with metastatic pancreatic neuroendocrine tumor (G1; Ki-67, 1%). The baseline 68Ga-DOTATATE PET/CT (A, MIP image; B, fused PET/CT) showed somatostatin receptor expression in liver metastases. The patient was treated with 3 cycles of 177Lu-DOTA-EB-TATE with cumulative administered activity of 12.4 GBq. Two months post-therapy 68Ga-DOTATATE PET/CT (C, MIP image; D, fused PET/CT) showed partial response. 68Ga-DOTATATE PET/CT at 3-year follow-up showed stable disease (E, MIP image; F, fused PET/CT) with progression-free survival of 43 months from the first cycle of 177Lu-DOTA-EB-TATE PRRT.
Four patients received PRRT before (PRRT re-treatment). Three patients were previously treated with 177Lu-DOTATATE PRRT (7.4 GBq/cycle), to which one showed partial response and two showed stable disease. One patient was previously treated with 177Lu-DOTA-EB-TATE (1.11 GBq/cycle) and demonstrated progressive disease. After repeated PRRT with 3.7 GBq/cycle of 177Lu-DOTA-EB-TATE, 3 patients (3/4) showed partial response and the remaining one patient (1/4) showed stable disease on 68Ga-DOTATATE PET/CT. An example is shown in Figure 2.
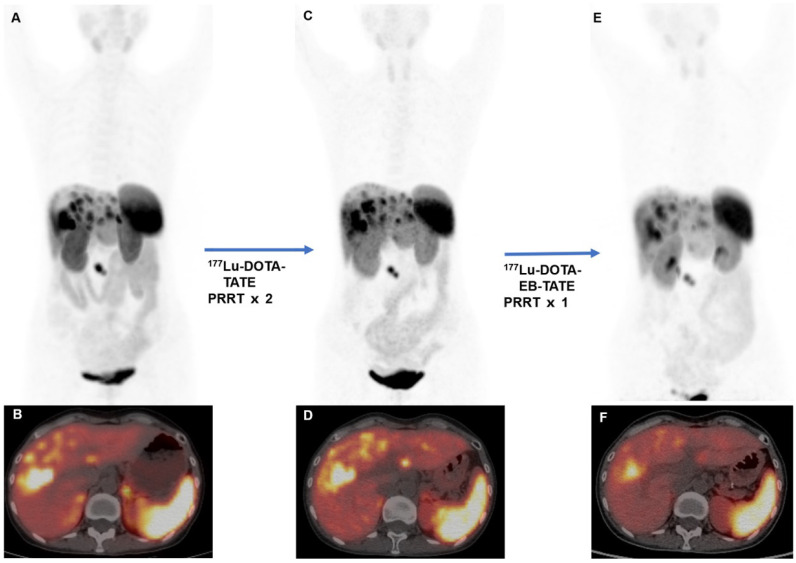
Figure 2.
A 51-year-old woman with metastatic pancreatic neuroendocrine tumor (G2; Ki-67, 5%). The patient was previously treated with 2 cycles of 177Lu-DOTATATE PRRT (cumulative activity, 14.8 GBq). The baseline 68Ga-DOTATATE PET/CT (A, MIP image; B, fused PET/CT) before 177Lu-DOTATATE demonstrated somatostatin receptor avid multiple metastases. Follow-up 68Ga-DOTATATE PET/CT (C, MIP image; D, fused PET/CT) performed 2 months after 2 cycles of 177Lu-DOTATATE showed stable disease. The patient was then admitted for 177Lu-DOTA-EB-TATE. Follow-up 68Ga-DOTATATE PET/CT (E, MIP image; F fused PET/CT) performed 2 months after 177Lu-DOTA-EB-TATE showed partial response (administered activity, 3.85 GBq). On follow-up, the disease remained stable with progression-free survival of 15 months.
Progression-free survival and overall survival
Follow-up data was available and ranges from 5 to 57 months after the start of the first PRRT in this study. The median follow-up period was 46 months (interquartile range, 13-50 months). The median PFS was 36 months, while the median OS was not reached. The observed PFS rates at 12 months, 24 months, and 36 months were 79.5%, 60.7%, and 47.2%, respectively; the observed OS rates at 12 months, 24 months, and 36 months were 92.9%, 79.7%, and 59.8%, respectively. In the univariate analysis, Ki-67 index was the only variable found to be significantly associated with PFS (Table 5). Therefore, a multivariate analysis was not performed. Of 14 patients with stable disease by RECIST criteria, those with partial response by PERCIST had longer PFS (median: not reached vs. 33 months; P = 0.817) and OS (median: not reached vs. 35 months; P = 0.109) than those with no response, whereas the difference was not statistically significant, probably due to the limited number of patients. Kaplan-Meier curves for PFS and OS after PRRT in this study are shown in Supplemental Figures 2 and 3.
Table 5.
Univariate Cox proportional hazards regression analysis of progression-free survival (PFS) and overall survival (OS)
| Factor | PFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Gender | ||||||
| Female | 1 | 1 | ||||
| Male | 1.083 | 0.330-3.554 | 0.896 | 2.612 | 0.504-13.543 | 0.253 |
| Age | ||||||
| ≤ 55 y | 1 | 1 | ||||
| > 55 y | 1.318 | 0.401-4.330 | 0.649 | 2.188 | 0.488-9.806 | 0.306 |
| Primary tumor type | ||||||
| Non-pancreatic | 1 | 1 | ||||
| Pancreatic | 0.523 | 0.159-1.721 | 0.286 | 0.308 | 0.060-1.594 | 0.160 |
| Ki-67 index | ||||||
| ≤ 10% | 1 | |||||
| > 10% | 4.973 | 1.430-17.293 | 0.012 | 1.527 | 0.341-6.838 | 0.580 |
| Number of organs involved | ||||||
| ≤ 1 | 1 | 1 | ||||
| > 1 | 0.309 | 0.082-1.169 | 0.084 | 0.898 | 0.200-4.035 | 0.888 |
| Bone metastases | ||||||
| No | 1 | 1 | ||||
| Yes | 2.135 | 0.623-7.313 | 0.227 | 1.260 | 0.242-6.552 | 0.783 |
| Hepatic tumor burden | ||||||
| ≤ 50% | 1 | 1 | ||||
| > 50% | 1.183 | 0.360-3.892 | 0.782 | 1.680 | 0.375-7.524 | 0.498 |
| Surgery of primary tumor | ||||||
| No | 1 | 1 | ||||
| Yes | 1.773 | 0.513-6.120 | 0.365 | 2.335 | 0.447-12.199 | 0.315 |
| Chemotherapy | ||||||
| No | 1 | 1 | ||||
| Yes | 2.849 | 0.826-9.817 | 0.097 | 1.140 | 0.255-5.100 | 0.864 |
Discussion
Hematological and renal toxicity are considered as the main side effects and dose-limiting factors for PRRT. In this study, the rate of severe hematotoxicity rate was rather low with 13.3% of the patients experiencing grade 3 thrombocytopenia, and 3.3% experiencing grade 3 anemia, who presented with grade 3 anemia already at baseline. This rate was similar to the previously reported 177Lu-DOTATATE PRRT studies that described grade 3/4 hematotoxicity rate of 3.1%-15% 7, 18-24. Recently, Garske-Román et al. reported data on 200 NET patients receiving 177Lu-DOTATATE, and grade 3/4 hematotoxicity was found in 30 patients (15%) 25. In the study of Bergsma et al., grade 3/4 hematotoxicity was observed in 11% of patients treated with 177Lu-DOTATATE PRRT 19. For the 4 patients presented with grade 3 thrombocytopenia in this study, all were previously treated with multiple courses of chemotherapy or sulfatinib/everolimus, which are risk factors associated with hematotoxicity 26. In addition, the platelet counts at baseline in 2 of them were only slightly higher than the lower limits (109 and 118 × 109/L; normal 100-300 × 109/L). These data confirmed the importance of baseline myeloid function and careful evaluation of risk factors for relevant toxicity. Regarding nephrotoxicity, no grade-2/3/4 toxicity was observed in our cohort, and renal function demonstrated by creatinine remained stable during the therapy.
Long-term hematotoxicity and nephrotoxicity are of particular concern in this trial. On follow-up, we observed low incidence of grade 3 hematotoxicity (3.4%) and no nephrotoxicity of any grade. Acute leukemia and MDS are rare delayed side effects and severe complications related to PRRT, which have been reported previously to occur in approximately 2% of patients 22. In our study, none of the patients developed MDS or leukemia during the follow-up period. Our data confirm that PRRT with 177Lu-DOTA-EB-TATE is a relatively safe therapeutic procedure in view of the low occurrences of short- and long-term toxicity.
In terms of efficacy, we observed a response rate of 33.3% and a disease control rate of 85.2% on RECIST criteria, and a response rate of 48.3% and a disease control rate of 86.2% on PERCIST criteria. These results are not inferior to that reported for 177Lu-DOTATATE PRRT. Recently, Sitani et al. retrospectively analyzed 468 NET patients who underwent PRRT with 177Lu-DOTATATE (dose: 5.55 to 7.4 GBq/cycle). They found a response rate of 30% and 31%, and a disease control rate of 90% and 88% on RECIST and PERCIST criteria, respectively 27. Another study performed by Parghane et al. analyzed 57 GEP-NET patients receiving PRRT with 177Lu-DOTATATE (dose: 7.4 GBq/cycle). They reported a response rate of 40% and 31%, and a disease control rate of 92.9% and 94.7% referring to RECIST and PERCIST criteria, respectively 28. In a recent meta-analysis on the efficacy of 177Lu-DOTATATE in NET patients, the pooled response rate and disease control rate were 27.58% and 79.14%, respectively, based on the RECIST criteria 29. These findings suggested that 3.7 GBq/cycle of 177Lu-DOTA-EB-TATE seems to be as effective as 5.55-7.4 GBq of 177Lu-DOTATATE for tumor control.
Treatment options are limited for patients who experience recurrence after PRRT, which led to the investigation of potential practice of PRRT re-treatment. Several studies have reported a safety profile for PRRT re-treatment that is similar to initial PRRT 30-32. However, the performance of PRRT re-treatment was reported to be poorer than initial PRRT 32, 33. The relatively poor performance of PRRT re-treatment was probably because patients have acquired a level of radio-resistance after previous PRRT. In the present study, 4 patients in our cohort were previously treated with PRRT, and repeated PRRT with 177Lu-DOTA-EB-TATE performed well for tumor control. Our preliminary findings indicate the potential benefit of 177Lu-DOTA-EB-TATE in PRRT re-treatment setting. In the future, it would make sense to apply 177Lu-DOTA-EB-TATE to PRRT re-treatment patients to investigate its potential value in this special setting.
In the present study, we observed a median PFS of 36 months after a median follow-up of 46 months, while the median OS was not reached. Our result compares favorably with those of previous 177Lu-DOTATATE studies, which reported a median PFS ranging from 26 to 37 months 4, 18, 23, 34. Ezzidin et al. in a retrospective study of 74 patients with metastatic NETs treated with 177Lu-DOTATATE, reported a median PFS of 26 months 35. In a large group of 443 NET patients treated with 177Lu-DOTATATE, Brabander et al. reported a median PFS of 29 months 5. In a recent study, Kennedy et al. retrospectively reviewed the long-term survival of 104 patients with advanced NETs treated with 177Lu-DOTATATE and reported a median PFS of 37 months 36. In the absence of a randomized trial comparing 177Lu-DOTA-EB-TATE with 177Lu-DOTATATE, these data seem to indicate that the survival benefit obtained with 177Lu-DOTA-EB-TATE at lower dose is not inferior to 177Lu-DOTATATE, at least in terms of PFS. We also found that Ki-67 index of greater than 10% was significantly associated with poorer PFS. A previous study has also reported that higher Ki-67 index was associated with poorer prognosis in NET patients treated with 177Lu-DOTATATE PRRT 35, and this finding warrants further investigation.
This study has several limitations. First, the main limitation of this study is the small population size, which might lead to some bias. The number of PRRT re-treatment patients was also limited. Still, our preliminary data were the first to report the potential benefit of 177Lu-DOTA-EB-TATE in this setting, encouraging study with larger sample size to provide stronger evidence for the effect of 177Lu-DOTA-EB-TATE in PRRT re-treatment patients. The second limitation is the lack of control group of standard PRRT with 177Lu-DOTATATE. Thus, the performance of 177Lu-DOTA-EB-TATE in this study was compared with the published literature. In the future, we will design a randomized controlled trial to compare 177Lu-DOTA-EB-TATE and standard 177Lu-DOTATATE to further determine the value of 177Lu-DOTA-EB-TATE. The third limitation is the limited length of follow-up period. Despite this shortcoming, we were able to report the long-term toxicity and survival outcome of 177Lu-DOTA-EB-TATE for the first time. Furthermore, the fact that most patients did not complete the planned 3 cycles due to various reasons may have affected the reported toxicity profile of 177Lu-DOTA-EB-TATE in this study.
Conclusion
Our results suggest that PRRT with approximately 3.7 GBq 177Lu-DOTA-EB-TATE has acceptable toxicity profile and is effective in treating metastatic NET with high disease control rate. In addition, 177Lu-DOTA-EB-TATE achieved a favorable survival outcome with encouraging PFS. Future prospective randomized studies in larger number of NET patients comparing 177Lu-DOTA-EB-TATE with standard 177Lu-DOTATATE are warranted.
Supplementary Material
Supplementary figures and table.
Acknowledgments
This study was supported by the Chinese Academy of Medical Science Innovation Fund for Medical Sciences (2021-I2M-1-016), the Capital Health Development Scientific Research Project (2018-1-4011), the National Natural Science Foundation of China (81871392), the National University of Singapore Start-up Grant (NUHSRO/2020/133/Startup/08), and National Medical Research Council (NMRC) Centre Grant Programme (CG21APR1005).
Author contributions
X.Y.C., J.J.Z. and Z.Z.H. initiated and designed the project; Y.Y.J., Q.X.L., G.C.W., H.M.C., R.X.W. and J.R.W. performed the experiments; Y.Y.J., Q.X.L. and G.C.W. assisted with data processing; Y.Y.J. analyzed the data and wrote the paper. Z.Z.H. supervised the study.
Abbreviations
- PRRT
peptide receptor radionuclide therapy
- NETs
neuroendocrine tumors
- CTCAE
Common Terminology Criteria for Adverse Events
- PFS
progression-free survival
- OS
overall survival
- SSTRs
somatostatin receptors
- EMA
European Medicines Agency
- FDA
Food and Drug Administration
- EB
Evans Blue
References
- 1.Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y. et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335–42. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE. et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 3.Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121:589–97. doi: 10.1002/cncr.29099. [DOI] [PubMed] [Google Scholar]
- 4.Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP. et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–30. doi: 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]
- 5.Brabander T, van der Zwan WA, Teunissen JJM, Kam BLR, Feelders RA, de Herder WW. et al. Long-Term Efficacy, Survival, and Safety of [(177)Lu-DOTA(0),Tyr(3)]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin Cancer Res. 2017;23:4617–24. doi: 10.1158/1078-0432.CCR-16-2743. [DOI] [PubMed] [Google Scholar]
- 6.Baum RP, Kulkarni HR, Singh A, Kaemmerer D, Mueller D, Prasad V. et al. Results and adverse events of personalized peptide receptor radionuclide therapy with (90)Yttrium and (177)Lutetium in 1048 patients with neuroendocrine neoplasms. Oncotarget. 2018;9:16932–50. doi: 10.18632/oncotarget.24524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B. et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125–35. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandström M, Garske-Román U, Granberg D, Johansson S, Widström C, Eriksson B. et al. Individualized dosimetry of kidney and bone marrow in patients undergoing 177Lu-DOTA-octreotate treatment. J Nucl Med. 2013;54:33–41. doi: 10.2967/jnumed.112.107524. [DOI] [PubMed] [Google Scholar]
- 9.Gibson JG, Evans WA. CLINICAL STUDIES OF THE BLOOD VOLUME. I. CLINICAL APPLICATION OF A METHOD EMPLOYING THE AZO DYE "EVANS BLUE" AND THE SPECTROPHOTOMETER. J Clin Invest. 1937;16:301–16. doi: 10.1172/JCI100859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spahr PF, Edsall JT. AMINO ACID COMPOSITION OF HUMAN AND BOVINE SERUM MERCAPTALBUMINS. J Biol Chem. 1964;239:850–4. [PubMed] [Google Scholar]
- 11.Tian R, Jacobson O, Niu G, Kiesewetter DO, Wang Z, Zhu G. et al. Evans Blue Attachment Enhances Somatostatin Receptor Subtype-2 Imaging and Radiotherapy. Theranostics. 2018;8:735–45. doi: 10.7150/thno.23491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Wang H, Jacobson O, Cheng Y, Niu G, Li F. et al. Safety, Pharmacokinetics, and Dosimetry of a Long-Acting Radiolabeled Somatostatin Analog (177)Lu-DOTA-EB-TATE in Patients with Advanced Metastatic Neuroendocrine Tumors. J Nucl Med. 2018;59:1699–705. doi: 10.2967/jnumed.118.209841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Q, Cheng Y, Zang J, Sui H, Wang H, Jacobson O. et al. Dose escalation of an Evans blue-modified radiolabeled somatostatin analog (177)Lu-DOTA-EB-TATE in the treatment of metastatic neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2020;47:947–57. doi: 10.1007/s00259-019-04530-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Q, Zang J, Sui H, Ren J, Guo H, Wang H. et al. Peptide Receptor Radionuclide Therapy of Late-Stage Neuroendocrine Tumor Patients with Multiple Cycles of (177)Lu-DOTA-EB-TATE. J Nucl Med. 2021;62:386–92. doi: 10.2967/jnumed.120.248658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krenning EP, Bakker WH, Breeman WA, Koper JW, Kooij PP, Ausema L. et al. Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin. Lancet. 1989;1:242–4. doi: 10.1016/s0140-6736(89)91258-0. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Cheng Y, Zhang J, Zang J, Li H, Liu Q. et al. Response to Single Low-dose (177)Lu-DOTA-EB-TATE Treatment in Patients with Advanced Neuroendocrine Neoplasm: A Prospective Pilot Study. Theranostics. 2018;8:3308–16. doi: 10.7150/thno.25919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122s–50s. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabet A, Dautzenberg K, Haslerud T, Aouf A, Sabet A, Simon B. et al. Specific efficacy of peptide receptor radionuclide therapy with (177)Lu-octreotate in advanced neuroendocrine tumours of the small intestine. Eur J Nucl Med Mol Imaging. 2015;42:1238–46. doi: 10.1007/s00259-015-3041-6. [DOI] [PubMed] [Google Scholar]
- 19.Bergsma H, Konijnenberg MW, Kam BL, Teunissen JJ, Kooij PP, de Herder WW. et al. Subacute haematotoxicity after PRRT with (177)Lu-DOTA-octreotate: prognostic factors, incidence and course. Eur J Nucl Med Mol Imaging. 2016;43:453–63. doi: 10.1007/s00259-015-3193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabet A, Haslerud T, Pape UF, Sabet A, Ahmadzadehfar H, Grünwald F. et al. Outcome and toxicity of salvage therapy with 177Lu-octreotate in patients with metastatic gastroenteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2014;41:205–10. doi: 10.1007/s00259-013-2547-z. [DOI] [PubMed] [Google Scholar]
- 21.Delpassand ES, Samarghandi A, Zamanian S, Wolin EM, Hamiditabar M, Espenan GD. et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE for patients with somatostatin receptor-expressing neuroendocrine tumors: the first US phase 2 experience. Pancreas. 2014;43:518–25. doi: 10.1097/MPA.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 22.Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M. et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015;42:5–19. doi: 10.1007/s00259-014-2893-5. [DOI] [PubMed] [Google Scholar]
- 23.Paganelli G, Sansovini M, Ambrosetti A, Severi S, Monti M, Scarpi E. et al. 177 Lu-Dota-octreotate radionuclide therapy of advanced gastrointestinal neuroendocrine tumors: results from a phase II study. Eur J Nucl Med Mol Imaging. 2014;41:1845–51. doi: 10.1007/s00259-014-2735-5. [DOI] [PubMed] [Google Scholar]
- 24.Sabet A, Ezziddin K, Pape UF, Ahmadzadehfar H, Mayer K, Pöppel T. et al. Long-term hematotoxicity after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med. 2013;54:1857–61. doi: 10.2967/jnumed.112.119347. [DOI] [PubMed] [Google Scholar]
- 25.Garske-Román U, Sandström M, Fröss Baron K, Lundin L, Hellman P, Welin S. et al. Prospective observational study of (177)Lu-DOTA-octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (NETs): feasibility and impact of a dosimetry-guided study protocol on outcome and toxicity. Eur J Nucl Med Mol Imaging. 2018;45:970–88. doi: 10.1007/s00259-018-3945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kesavan M, Turner JH. Myelotoxicity of Peptide Receptor Radionuclide Therapy of Neuroendocrine Tumors: A Decade of Experience. Cancer Biother Radiopharm. 2016;31:189–98. doi: 10.1089/cbr.2016.2035. [DOI] [PubMed] [Google Scholar]
- 27.Sitani K, Parghane RV, Talole S, Basu S. Long-term outcome of indigenous (177)Lu-DOTATATE PRRT in patients with Metastatic Advanced Neuroendocrine Tumours: a single institutional observation in a large tertiary care setting. Br J Radiol. 2021;94:20201041. doi: 10.1259/bjr.20201041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parghane RV, Bhandare M, Chaudhari V, Ostwal V, Ramaswamy A, Talole S. et al. Surgical Feasibility, Determinants, and Overall Efficacy of Neoadjuvant (177)Lu-DOTATATE PRRT for Locally Advanced Unresectable Gastroenteropancreatic Neuroendocrine Tumors. J Nucl Med. 2021;62:1558–63. doi: 10.2967/jnumed.120.258772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Song Q, Cai L, Xie Y, Chen Y. The efficacy of (177)Lu-DOTATATE peptide receptor radionuclide therapy (PRRT) in patients with metastatic neuroendocrine tumours: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2020;146:1533–43. doi: 10.1007/s00432-020-03181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strosberg J, Leeuwenkamp O, Siddiqui MK. Peptide receptor radiotherapy re-treatment in patients with progressive neuroendocrine tumors: A systematic review and meta-analysis. Cancer Treat Rev. 2021;93:102141. doi: 10.1016/j.ctrv.2020.102141. [DOI] [PubMed] [Google Scholar]
- 31.Vaughan E, Machta J, Walker M, Toumpanakis C, Caplin M, Navalkissoor S. Retreatment with peptide receptor radionuclide therapy in patients with progressing neuroendocrine tumours: efficacy and prognostic factors for response. Br J Radiol. 2018;91:20180041. doi: 10.1259/bjr.20180041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Zwan WA, Brabander T, Kam BLR, Teunissen JJM, Feelders RA, Hofland J. et al. Salvage peptide receptor radionuclide therapy with [(177)Lu-DOTA,Tyr(3)]octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2019;46:704–17. doi: 10.1007/s00259-018-4158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudisile S, Gosewisch A, Wenter V, Unterrainer M, Böning G, Gildehaus FJ. et al. Salvage PRRT with (177)Lu-DOTA-octreotate in extensively pretreated patients with metastatic neuroendocrine tumor (NET): dosimetry, toxicity, efficacy, and survival. BMC Cancer. 2019;19:788. doi: 10.1186/s12885-019-6000-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ezziddin S, Khalaf F, Vanezi M, Haslerud T, Mayer K, Al Zreiqat A. et al. Outcome of peptide receptor radionuclide therapy with 177Lu-octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2014;41:925–33. doi: 10.1007/s00259-013-2677-3. [DOI] [PubMed] [Google Scholar]
- 35.Ezziddin S, Attassi M, Yong-Hing CJ, Ahmadzadehfar H, Willinek W, Grünwald F. et al. Predictors of long-term outcome in patients with well-differentiated gastroenteropancreatic neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med. 2014;55:183–90. doi: 10.2967/jnumed.113.125336. [DOI] [PubMed] [Google Scholar]
- 36.Kennedy KR, Turner JH, MacDonald WBG, Claringbold PG, Boardman G, Ransom DT. Long-term survival and toxicity in patients with neuroendocrine tumors treated with (177) Lu-octreotate peptide radionuclide therapy. Cancer. 2022;128:2182–92. doi: 10.1002/cncr.34191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures and table.