Abstract
Objective
It is still unclear how glucocorticoids (GCs) affect the long-term clinical course of patients with SLE. The objective of this study is to explore the factors associated with GC-free treatment status.
Methods
Using data from the lupus registry of nationwide institutions, GC dose at registration was compared between short, middle and long disease durations of <5, 5–20 and ≥20 years, respectively. After excluding patients who never used GC, we evaluated the relationship between GC-free status and chronic damage using Systemic Lupus International Collaborating Clinics Damage Index.
Results
GC doses at enrolment of the 1019 patients were as follows: GC-free in 101 (10%); 0<prednisolone (PSL) ≤5 mg/day in 411 (40%); 5<PSL ≤7.5 in 169 (17%); 7.5<PSL ≤10 in 194 (19%) and PSL≥10 in 144 (14%) patients. Of the patients who were not currently using GCs, patients who never used GC more frequently had short disease duration (66% with short, 23% with middle and 17% with long disease duration, p=0.00029). Univariate analysis of patients who underwent GC treatment showed that patients without GCs exhibited older age, lower disease activity, less immunosuppressant and hydroxychloroquine use and higher C3 levels. Among patients with a disease duration of ≥20 years, GC-free status was more frequent in patients without chronic damage (11% vs 4%, p=0.023). After adjusting for age, sex and disease activity, no chronic damage accrual was associated with GC-free status (OR 3.6, 95% CI 1.1 to 11.3).
Conclusion
Even in the patients with long disease duration, one-point GC-free treatment status might be related to no chronic damage accrual.
Keywords: systemic lupus erythematosus; glucocorticoids; outcome assessment, health care; epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
The accumulation of chronic damage is associated with increased mortality and reduced quality of life in patients with SLE.
Glucocorticoid (GC) use is a well-known risk factor for chronic damage accrual.
Disease duration is reportedly associated with chronic damage.
WHAT THIS STUDY ADDS
GC use is avoidable in the long-term care of patients with SLE.
Even in patients with long disease duration, one-point GC-free treatment status might be related to no chronic damage accrual.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
It might be important to achieve the GC-free treatment status even in patients with long disease duration.
Introduction
Glucocorticoids (GCs) are still the mainstay of treatment for SLE. Owing to the contribution of several novel therapeutic agents, the treat-to-target principle has been established to care for patients with SLE. This principle stipulates that patients with SLE should be in remission without the administration of GCs as much as possible.1 Although a recent randomised controlled trial showed that GC withdrawal increases relapse risk in patients with SLE, 73% of patients without GC use could maintain remission.2 Another observational study showed that GC withdrawal was attempted in 91 patients, with 85% successfully withdrawing from GCs and maintaining remission,3 whereas recent cohort studies showed that only 20%–30% of patients with SLE had not taken any GCs.4 5 Previous reports have shown that younger age, disease activity and short disease duration are risk factors for relapse in GC-free patients.6–8 However, it is still unclear which patients could withdraw from GCs.
The prevention of chronic damage accrual has become one of the topmost current treatment targets, next to decreasing mortality. The accumulation of chronic damage is associated with increased mortality, and with reduced quality of life.9–11 Previous studies showed that disease activity and GC use are related to chronic damage accrual.12–17 However, it is still unclear how GCs affect the long-term clinical course of patients with SLE because it is difficult to estimate the cumulative GC dose in the patients with SLE with long disease duration.
This study explored the factors associated with GC-free treatment status using data from one of the world’s largest SLE registries.
Methods
Study design and setting
This cross-sectional study used data acquired through a multidisciplinary cohort study (the lupus registry of nationwide institutions (LUNA)), which was conducted in 2016 to investigate the association between clinical manifestations, socioeconomic backgrounds and outcomes in patients with SLE reported from 10 Japanese institutions. LUNA contains data on patients aged 20 years or older, diagnosed according to the revised 1997 American College of Rheumatology criteria for SLE classification18; approximately 1.7% of patients with SLE in Japan have been registered in LUNA, accounting for around 1000 cases.
Patient selection and outcome measure
This study was performed using electronic medical records or self-administered questionnaires completed by registered patients between April 2016 and June 2020. All data were collected at the time of registration. Patients with GC dose data at registration were enrolled in the study. The primary outcome measure was the GC-free treatment status at registration.
The collected data were as follows: age, sex, disease duration, prednisolone dose (current and past maximum), hydroxychloroquine use, immunosuppressant use, SLE Disease Activity Index 2000 (SLEDAI-2K),19 Systemic Lupus International Collaborating Clinics Damage Index (SLICC-DI),20 antidouble stranded DNA antibody level, complement (C3, C4, CH50) level and serum creatinine level.
Statistical analysis
First, the descriptive statistics of the enrolled patients were expressed as the median and IQR for continuous variables, and as n (%) for categorical variables. Subsequently, the GC dose at registration was described and compared among different disease durations divided by quartile points: short, middle and long disease durations being <5, 5–20 and ≥20 years, respectively. After excluding patients who had never used GCs and those without previous GC treatment information, we compared the characteristics of patients treated with and without GCs at registration.
Finally, in each disease duration group, we evaluated the relationship between GC-free treatment status and no chronic damage accrual (SLICC-DI=0) by univariate analysis. In patients with long disease duration, possible confounders, which were selected based on the findings of previous reports, were adjusted by logistic regression analysis. We used multiple imputation to handle the uncertainty caused by missing values of potential confounders, on the assumption of them missing at random.
Continuous variables were compared using the Mann-Whitney U test or Student’s t-test, depending on data distribution, and categorical variables were compared using the χ2 test or Fisher’s direct probability test, as appropriate. Statistical significance was set at p<0.05. All statistical analyses were performed using the JMP V.11.2.0 software package (SAS Institute, Cary, North Carolina, USA), and the Statistical Package of Stata, V.17.0 (StataCorp, College Station, Texas, USA).
Results
Enrolled patient characteristics
All patients registered in the LUNA had information on the current GC dose. The median (IQR) age of the enrolled 1019 patients was 45 (35–57) years, and 895 (88%) patients were female. The median (IQR) disease duration was 12 (5–20) years. The median (IQR) SLEDAI and total SLICC-DI scores at registration were 4 (2–8) and 1 (0–2), respectively. Immunosuppressants and hydroxychloroquine were used in 623 (61%) and 274 (27%) patients, respectively.
Glucocorticoid treatment status
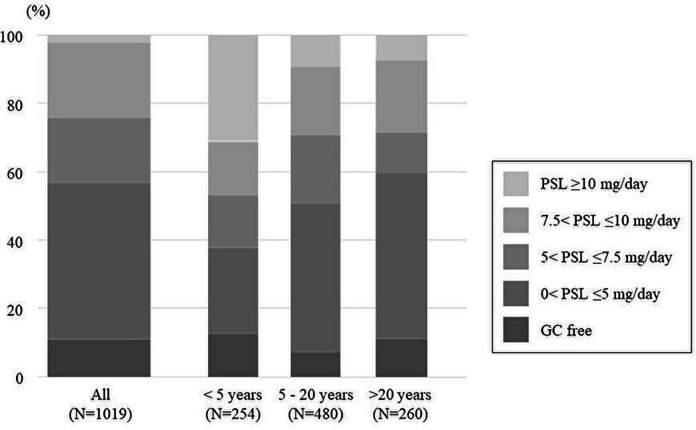
The GC doses at registration were as follows: GC-free in 101 (10%) patients; 0<prednisolone (PSL) ≤5 mg/day in 411 (40%) patients; 5<PSL ≤7.5 mg/day in 169 (17%) patients; 7.5<PSL ≤10 mg/day in 194 (19%) patients and PSL ≥10 mg/day in 144 (14%) patients (figure 1). The GC doses at registration were statistically different among the different disease durations (p<0.0001, figure 1). The proportion of GC-free treatment statuses also differed statistically among different disease durations (32 of 254 (13%) patients with short disease duration, 35 of 480 (7%) patients with middle disease duration and 29 of 260 (11%) patients with long disease duration, p=0.043). Patients with long disease duration were treated with immunosuppressants and/or hydroxychloroquine less frequently than those with other disease durations (immunosuppressant use, 134 of 260 (52%) patients with long disease duration vs 476 of 734 (65%) patients with the other disease durations, p=0.0002; hydroxychloroquine use, 55 of 260 (21%) patients vs 212 of 733 (29%) patients, p=0.015).
Figure 1.
Glucocorticoid dose among different disease durations. GC, glucocorticoid; PSL, prednisolone.
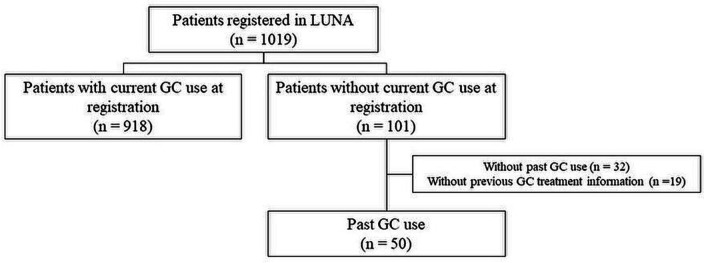
Of the patients who were not currently using GCs, short disease duration was more frequent in patients who never used GCs (21 of 32 (66%) patients in short disease duration, 7 of 30 (23%) patients with middle disease duration and 3 of 18 (17%) patients with long disease durations, p=0.00029). After excluding patients with never used GC and those without previous GC treatment information, the proportion of patients without GC use did not differ statistically among different disease durations (11 of 233 (5%) patients with short disease duration, 23 of 468 (5%) patients with middle disease duration and 15 of 246 (6%) patients with long disease duration, p=0.74) (figure 2, online supplemental figure 1). The characteristics of the patients with and without GCs among the patients with previous GC treatment history are shown in table 1. The patients without GCs were older, had lower SLEDAI, used less immunosuppressant and hydroxychloroquine and had higher C3 levels than those with GCs.
Figure 2.
Patient selection flow chart. GC, glucocorticoid; LUNA, the lupus registry of nationwide institutions.
Table 1.
Comparison of characteristics between patients with and without GCs
| Characteristics | Without GCs (n=50) |
Number of missing data | With GCs (n=918) |
Number of missing data | P value |
| Age, median IQR, years | 50.5 (40.8–60.5) | 0 | 44.5 (34–56) | 0 | 0.012 |
| Female patients, n (%) | 42 (84) | 0 | 807 (88) | 0 | 0.41 |
| Disease duration, median IQR, years* | 13 (6–22.5) | 1 | 12 (6–20) | 20 | 0.41 |
| <5 years, n (%) | 11 (22) | 222 (25) | |||
| 5–20 years, n (%) | 23 (47) | 445 (50) | |||
| ≥20 years, n (%) | 15 (31) | 231 (26) | |||
| SLEDAI-2K, median IQR* | 2 (0–6) | 9 | 4 | 223 | 0.027 |
| SLICC-DI, median IQR* | 0 (0–2) | 0 | 1 (0–2) | 0 | 0.61 |
| Maximum PSL dose after diagnosis, median IQR, mg/day | 50 (30–60) | 0 | 40 (30–50) | 70 | 0.48 |
| Current immunosuppressant use, n (%) | 21 (42) | 0 | 596 (65) | 0 | 0.0010 |
| Current hydroxychloroquine use, n (%) | 2 (4) | 0 | 262 (29) | 0 | <0.0001 |
| C3, median IQR, mg/dL | 88 (75.5–103.0) | 4 | 80 (68–93) | 80 | 0.033 |
| C4, median IQR, mg/dL | 17.65 (14.4–22.3) | 4 | 16 (11–21.9) | 144 | 0.12 |
| CH50, median IQR, U/mL | 37.6 (32.8–46.6) | 4 | 37 (29.8–44.6) | 126 | 0.20 |
| Anti-ds-DNA antibody, median IQR, EU/mL | 7.6 (1.7–10.1) | 3 | 9.15 (2.4–20.8) | 24 | 0.23 |
| Serum creatinine, median IQR, mg/dL | 0.66 (0.60–0.74) | 1 | 0.69 (0.59–0.84) | 2 | 0.31 |
*Mann-Whitney U test.
ds-DNA, double stranded DNA; GC, glucocorticoid; PSL, prednisolone; SLEDAI-2K, SLE Disease Activity Index 2000; SLICC-DI, Systemic Lupus International Collaborating Clinics Damage Index.
lupus-2022-000772supp001.pdf (29.7KB, pdf)
Glucocorticoid treatment status and chronic damage accrual in each disease duration
The proportions of each item of SLICC-DI among different disease durations are shown in online supplemental table 1. Ocular, renal, cardiovascular, peripheral vascular, musculoskeletal and diabetic impairments increased with the duration of disease.
lupus-2022-000772supp002.pdf (60KB, pdf)
GC-free treatment status was more frequent in patients without chronic damage accrual (SLICC-DI=0), than in those with chronic damage and long disease duration (9 of 82 (11%) patients without chronic damage vs 6 of 164 (4%) patients with chronic damage, p=0.023). In comparison, there was no significant difference in the frequency of GC-free treatment status between the patients with and without chronic damage in short disease duration (5 of 100 (5%) patients with chronic damage vs 6 of 133 (5%) patients without chronic damage, p=0.86) and middle disease duration (12 of 239 (5%) patients with chronic damage vs 11 of 229 (5%) patients without chronic damage, p=0.91). Moreover, even after adjusting for age, sex and SLEDAI-2K as confounding factors using logistic regression analysis, no chronic damage accrual was found to be associated with GC-free treatment status (OR 3.6, 95% CI 1.1 to 11.3) (table 2).
Table 2.
Association of GC-free treatment status and SLICC-DI in the patients with long disease duration by logistic regression analysis, after multiple imputation of missing values
| Crude | Adjusted | |||
| OR (95% CI) |
P value | OR (95% CI) |
P value | |
| SLICC-DI (=0) | 3.3 (1.1 to 9.46) | 0.03 | 3.6 (1.1 to 11.3) | 0.03 |
| Age (per 1 year older) | 1.1 (1.0 to 1.1) | 0.03 | ||
| Female | 1.2 (0.1 to 10.2) | 0.90 | ||
| SLEDAI-2K | 0.9 (0.7 to 1.0) | 0.11 | ||
GC, glucocorticoid; SLEDAI-2K, SLE Disease Activity Index 2000; SLICC-DI, Systemic Lupus International Collaborating Clinics Damage Index.
Discussion
In the present study, we focused on the GC-free treatment status related to disease duration and chronic damage accrual. Of the enrolled patients, only 10% did not receive GCs at registration. The patients without GCs were older, had a lower SLEDAI-2K score, used less immunosuppressant and hydroxychloroquine and had higher C3 levels than those with GCs. In patients with ≥20 years of disease duration, no chronic damage accrual was independently related to GC-free treatment status.
Recent cohort studies have shown that 20%–30% of patients with SLE do not receive GCs.4 5 Hydroxychloroquine use was less frequent in this study than in recent cohort studies (27% vs 60%–74%) because hydroxychloroquine use for SLE was approved just 6 years ago in Japan. Because it is well known that immunosuppressant and hydroxychloroquine use could reduce GC dose without relapse, the concomitant use of immunosuppressants and hydroxychloroquine may help increase the proportion of patients without GCs. Because recent patients were treated with immunosuppressants and hydroxychloroquine without GCs, more patients could withdraw from GCs with extended observation.
No damage accrual was related to one-point GC-free treatment status even in the patients with a disease duration of ≥20 years. Disease duration was reportedly associated with chronic damage,13–15 21 and it is a risk factor for cardiovascular events independent of chronic damage.22 GC use is also a well-known risk factor for chronic damage accrual.13–15 In the present study, the patients who never used GC decreased time-dependent manner. These results indicate that GC use is avoidable in the long-term care of the patients with SLE. We could not evaluate the cumulative GC dose in the present study. Since the patients without GC might include the patients with high cumulative GC dose, the relationship between achievement of GC and chronic damage might be underestimated. Nevertheless, at least one-point GC-free treatment status was statistically related to no damage accrual in the patients with a longer disease duration. Therefore, it might be important to achieve the GC-free treatment status even in the patients with long disease duration. Since the patients without GCs used less immunosuppressant and hydroxychloroquine, the patients with mild disease activity might be easy to withdraw from GC treatment.
This study had some limitations. First, this was a cross-sectional study; thus, we could not conclude by including the GC-treatment and free period and their cumulative dose. Second, the sample size of the patients with a long disease duration may not be sufficient for multivariate analysis.
Conclusion
One-point GC-free treatment status might be related to no chronic damage accrual even in the patients with long disease duration.
Acknowledgments
We express our gratitude to Tomomi Maruyama and Tomoko Takamae for their assistance in data management. We are also grateful to all collaborators who are working on LUNA.
Footnotes
Contributors: K-eS contributed to the conception and the design of the work, analysed and interpreted the patient data, and was a major contributor in writing the manuscript. K-eS accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish. YMi analysed the patient data. All authors contributed to the design of the work, read and approved the final manuscript.
Funding: This work was supported by JSPS KAKENHI Grant Number JP19K1053.
Competing interests: K-eS received a speaker’s fee from GlaxoSmithKline and a research grant from Pfizer.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. The datasets used and analysed in the present study will be shared on reasonable request to the ethics committee of Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences (mae6605@adm.okayama-u.ac.jp).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study protocol was approved by the ethics committee of Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences (authorisation number: Ken1909-025). This study was conducted according to the Declaration of Helsinki and the ethical guidelines for epidemiological research in Japan. All patients provided written informed consent to participate in the registry and gave permission to have their data published.
References
- 1.van Vollenhoven RF, Mosca M, Bertsias G, et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international Task force. Ann Rheum Dis 2014;73:958–67. 10.1136/annrheumdis-2013-205139 [DOI] [PubMed] [Google Scholar]
- 2.Mathian A, Pha M, Haroche J, et al. Withdrawal of low-dose prednisone in SLE patients with a clinically quiescent disease for more than 1 year: a randomised clinical trial. Ann Rheum Dis 2020;79:339–46. 10.1136/annrheumdis-2019-216303 [DOI] [PubMed] [Google Scholar]
- 3.Tani C, Elefante E, Signorini V, et al. Glucocorticoid withdrawal in systemic lupus erythematosus: are remission and low disease activity reliable starting points for stopping treatment? A real-life experience. RMD Open 2019;5:e000916. 10.1136/rmdopen-2019-000916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Touma Z, Hoskin B, Atkinson C, et al. Systemic lupus erythematosus symptom clusters and their association with Patient‐Reported outcomes and treatment: analysis of Real‐World data. Arthritis Care Res 2022;74:1079–88. 10.1002/acr.24546 [DOI] [PubMed] [Google Scholar]
- 5.Kandane-Rathnayake R, Louthrenoo W, Golder V, et al. Independent associations of lymphopenia and neutropenia in patients with systemic lupus erythematosus: a longitudinal, multinational study. Rheumatology 2021;60:5185–93. 10.1093/rheumatology/keab217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steiman AJ, Urowitz MB, Ibañez D, et al. Prolonged clinical remission in patients with systemic lupus erythematosus. J Rheumatol 2014;41:1808–16. 10.3899/jrheum.131137 [DOI] [PubMed] [Google Scholar]
- 7.Moroni G, Longhi S, Giglio E, et al. What happens after complete withdrawal of therapy in patients with lupus nephritis. Clin Exp Rheumatol 2013;31:S75–81. [PubMed] [Google Scholar]
- 8.Moroni G, Gallelli B, Quaglini S, et al. Withdrawal of therapy in patients with proliferative lupus nephritis: long-term follow-up. Nephrol Dial Transplant 2006;21:1541–8. 10.1093/ndt/gfk073 [DOI] [PubMed] [Google Scholar]
- 9.Bruce IN, O'Keeffe AG, Farewell V, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the systemic lupus international collaborating clinics (SLICC) inception cohort. Ann Rheum Dis 2015;74:1706–13. 10.1136/annrheumdis-2013-205171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabbani MA, Habib HB, Islam M, et al. Early renal damage assessed by the SLICC/ACR damage index is predictor of severe outcome in lupus patients in Pakistan. Lupus 2010;19:1573–8. 10.1177/0961203310375704 [DOI] [PubMed] [Google Scholar]
- 11.Conti F, Perricone C, Reboldi G, et al. Validation of a disease-specific health-related quality of life measure in adult Italian patients with systemic lupus erythematosus: LupusQoL-IT. Lupus 2014;23:743–51. 10.1177/0961203314524466 [DOI] [PubMed] [Google Scholar]
- 12.Ugarte-Gil MF, Acevedo-Vásquez E, Alarcón GS, et al. The number of flares patients experience impacts on damage accrual in systemic lupus erythematosus: data from a multiethnic Latin American cohort. Ann Rheum Dis 2015;74:1019–23. 10.1136/annrheumdis-2013-204620 [DOI] [PubMed] [Google Scholar]
- 13.Gonçalves MJ, Sousa S, Inês LS, et al. Characterization of damage in Portuguese lupus patients: analysis of a national lupus registry. Lupus 2015;24:256–62. 10.1177/0961203314555172 [DOI] [PubMed] [Google Scholar]
- 14.Conti F, Ceccarelli F, Perricone C, et al. The chronic damage in systemic lupus erythematosus is driven by flares, glucocorticoids and antiphospholipid antibodies: results from a monocentric cohort. Lupus 2016;25:719–26. 10.1177/0961203315627199 [DOI] [PubMed] [Google Scholar]
- 15.Tarr T, Papp G, Nagy N, et al. Chronic high-dose glucocorticoid therapy triggers the development of chronic organ damage and worsens disease outcome in systemic lupus erythematosus. Clin Rheumatol 2017;36:327–33. 10.1007/s10067-016-3492-6 [DOI] [PubMed] [Google Scholar]
- 16.Tselios K, Gladman DD, Touma Z, et al. Disease course patterns in systemic lupus erythematosus. Lupus 2019;28:114–22. 10.1177/0961203318817132 [DOI] [PubMed] [Google Scholar]
- 17.Jolly M, Pickard AS, Wilke C, et al. Lupus-specific health outcome measure for us patients: the LupusQoL-US version. Ann Rheum Dis 2010;69:29–33. 10.1136/ard.2008.094763 [DOI] [PubMed] [Google Scholar]
- 18.Hochberg MC. Updating the American College of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. 10.1002/art.1780400928 [DOI] [PubMed] [Google Scholar]
- 19.Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002;29:288–91. [PubMed] [Google Scholar]
- 20.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the systemic lupus international collaborating Clinics/American College of rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996;39:363–9. 10.1002/art.1780390303 [DOI] [PubMed] [Google Scholar]
- 21.Ceccarelli F, Olivieri G, Pirone C, et al. The impacts of the clinical and genetic factors on chronic damage in Caucasian systemic lupus erythematosus patients. J Clin Med 2022;11:3368. 10.3390/jcm11123368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nived O, Ingvarsson RF, Jöud A, et al. Disease duration, age at diagnosis and organ damage are important factors for cardiovascular disease in SLE. Lupus Sci Med 2020;7:e000398. 10.1136/lupus-2020-000398 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
lupus-2022-000772supp001.pdf (29.7KB, pdf)
lupus-2022-000772supp002.pdf (60KB, pdf)
Data Availability Statement
Data are available on reasonable request. The datasets used and analysed in the present study will be shared on reasonable request to the ethics committee of Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences (mae6605@adm.okayama-u.ac.jp).