Abstract
Objective
To quantify the effects of a series of text messages (safetxt) delivered in the community on incidence of chlamydia and gonorrhoea reinfection at one year in people aged 16-24 years.
Design
Parallel group randomised controlled trial.
Setting
92 sexual health clinics in the United Kingdom.
Participants
People aged 16-24 years with a diagnosis of, or treatment for, chlamydia, gonorrhoea, or non-specific urethritis in the past two weeks who owned a mobile phone.
Interventions
3123 participants assigned to the safetxt intervention received a series of text messages to improve sex behaviours: four texts daily for days 1-3, one or two daily for days 4-28, two or three weekly for month 2, and 2-5 monthly for months 3-12. 3125 control participants received a monthly text message for one year asking for any change to postal or email address. It was hypothesised that safetxt would reduce the risk of chlamydia and gonorrhoea reinfection at one year by improving three key safer sex behaviours: partner notification at one month, condom use, and sexually transmitted infection testing before unprotected sex with a new partner. Care providers and outcome assessors were blind to allocation.
Main outcome measures
The primary outcome was the cumulative incidence of chlamydia or gonorrhoea reinfection at one year, assessed by nucleic acid amplification tests. Safety outcomes were self-reported road traffic incidents and partner violence. All analyses were by intention to treat.
Results
6248 of 20 476 people assessed for eligibility between 1 April 2016 and 23 November 2018 were randomised. Primary outcome data were available for 4675/6248 (74.8%). At one year, the cumulative incidence of chlamydia or gonorrhoea reinfection was 22.2% (693/3123) in the safetxt arm versus 20.3% (633/3125) in the control arm (odds ratio 1.13, 95% confidence interval 0.98 to 1.31). The number needed to harm was 64 (95% confidence interval number needed to benefit 334 to ∞ to number needed to harm 24) The risk of road traffic incidents and partner violence was similar between the groups.
Conclusions
The safetxt intervention did not reduce chlamydia and gonorrhoea reinfections at one year in people aged 16-24 years. More reinfections occurred in the safetxt group. The results highlight the need for rigorous evaluation of health communication interventions.
Trial registration
ISRCTN registry ISRCTN64390461.
Introduction
The burden of sexually transmitted infections (STIs) such as chlamydia and gonorrhoea is highest in people aged 16-24 years.1 2 Limited knowledge of risk reduction strategies and poor sexual communication skills might contribute to this increased risk.3 4
Health communications delivered by text message are effective, cheap, and highly cost effective for some behaviours, such as smoking cessation.5 6 The World Health Organization currently recommends the use of digital health communication for strengthening health systems, including for sexual and reproductive health, provided that privacy and sensitivity concerns can be taken into consideration.7 The covid-19 pandemic has led to an expansion in the use of digital technologies, including text messages supporting healthcare systems. Current guidance and practice reflect an assumption that provided privacy and sensitivity concerns are considered, digital health communication using text messages poses no risk of harm.7
We developed an intervention, safetxt, delivered by text messages to improve safer sex behaviours in people aged 16-24 with chlamydia or gonorrhoea. In qualitative interviews and a pilot trial with 200 participants, we found that our interactive support via text message was acceptable, and reportedly altered the behaviours targeted. The trial methods were feasible. A main trial to establish the effect on STIs was warranted.8 9
Methods
We quantified the effects of the safetxt intervention on chlamydia and gonorrhoea reinfection at one year and hypothesised that safetxt would reduce the risk of both at one year and improve three key safer sex behaviours: partner notification at one month, condom use, and STI testing before unprotected sex with a new partner.
In this parallel group individual level randomised superiority trial, care providers and outcome assessors were blind to allocation. Participants were recruited from 92 sexual health clinics in the United Kingdom, and the intervention was delivered in the community by mobile phone. Our methods were prespecified and are published in the trial protocol.10
Eligible participants were aged 16-24 years, owned a mobile phone, were able to provide informed consent, and either had a diagnosis of or had started treatment for chlamydia or gonorrhoea or non-specific urethritis in the past two weeks. We excluded those known to be a sexual partner of someone already recruited to the trial. Participants provided written informed consent in person or via the trial enrolment website.
Randomisation and masking
An automated, independent, computer based system remote from the recruiting sites generated the randomisation sequence. Automated links between the web based enrolment randomisation system and system sending intervention and control group messages ensured allocation concealment. An information technologist with no role in research aspects of the trial monitored all systems. Laboratory staff were masked to treatment. The statisticians were masked to treatment allocation until the code was broken after the main analysis. Owing to the nature of the intervention, participants could surmise their allocated treatment.
Procedures
Safetxt aimed to reduce chlamydia and gonorrhoea reinfection by encouraging participants to correctly follow instructions for STI treatment, including informing partners about their own infection, promoting condom use, and encouraging participants to seek STI testing before unprotected sex with a new partner.8 Safetxt was developed based on the COM-B (capability, opportunity, motivation, and behaviour) model and evidence on factors that influence behaviours. To ensure that the intervention was acceptable and accessible, we shaped the content based on the views of 64 users who varied by gender, sexual orientation, sociodemographic background, ethnicity, and area of residence. The content that promoted condom use and STI testing was informed by existing face-to-face interventions shown to increase condom use and reduce STIs. Safetxt uses a novel approach to support partner notification by providing non-blaming and non-stigmatising information and examples of how others, in a range of relationships, told their partners about an infection. Safetxt comprises educational, enabling, and incentivising behaviour change strategies and 12 evidence based behaviour change techniques: information about the health consequences of behaviour, instruction on how to carry out the behaviour, demonstrations of risk reduction behaviour, social support, emotional support, social rewards, non-specific incentives, encouragement to add objects to the environment to trigger behaviours, anticipated regret, problem solving, action planning techniques, and reframing.11 The information on safer sexual practices was in accordance with existing guidelines.12 The messages were tailored according to sex or gender and sexual orientation. All participants who have sex with men received messages about how others had negotiated condom use. Women and those who have sex with women were sent messages about emergency contraception. Men who have sex with men were sent messages about HIV post-exposure prophylaxis. Women who only have sex with women were not sent messages about condom use. The information provided was specific to the STI diagnosed. This tailoring resulted in different numbers of messages being sent to those of different sex or gender and sexual orientation.
The core message sets included 42 messages for women who have sex with women, 74 for women who have sex with men or with men and women, 69 for men who have sex with women, 76 for men who have sex with men, and 79 for men who have sex with men and women. Recipients could request additional messages on specific topics. Participants were sent text messages starting on the day of randomisation: four texts daily for days 1-3, one or two daily for days 4-28, two or three weekly for month 2, and 2-5 monthly for months 3-12 (see examples of messages in appendix 1).
Participants in the control group received a monthly text message asking for any changes to their postal or email address. All participants received usual care and were free to seek any other existing services or support.
The text messages were sent automatically. Participants were able to stop the messages or set times when they did not want to receive them. Self-reported outcomes were assessed at one and 12 months by postal paper based questionnaire or the trial web based data entry form. At 12 months, chlamydia and gonorrhoea infections were assessed by nucleic acid amplification test using self-sampling postal kits. One accredited laboratory assessed the postal tests at 12 months. Chlamydia and gonorrhoea reinfections during 12 months’ follow-up were assessed by checking the records of clinics where participants reported they had completed tests.
Outcomes
The primary outcome was the incidence of chlamydia or gonorrhoea reinfection at one year. Secondary outcomes at four weeks were informing the last sexual partner before the test to seek treatment, clinic attendance by partner for treatment, taking prescribed antibiotics and avoiding sex for seven days after treatment, and condom use at last sexual encounter. Intermediate outcomes at four weeks were knowledge related to STIs (the consequences of behaviour and how to avoid infection), attitude towards notification of partners, and self-efficacy about correct condom use, negotiating condom use, and telling a partner about an infection. Secondary outcomes at one year were STI diagnosis after joining the trial (self-report confirmed by postal test results and clinic records), condom use at last sexual encounter, STI self-testing before sex with most recent new partner (self-reported and confirmed by clinic record of a test), sex with someone new since joining the trial, condom use at first sexual encounter with someone new, participants’ report that the last new partner was tested for STI before having sex with them, and number of sexual partners since joining the trial. Process outcomes at one year were reading and sharing of intervention content, number of text messages read, whether anyone else read the messages, and, if yes, how the participant felt about the messages being read, and reading someone else’s messages in the trial (control group) and someone else in the trial reading the participant’s messages (intervention group). Data on adverse events were collected on experience of partner violence and road traffic incidents when the participant was the driver in the past year (as road traffic incidents are a known harm of mobile phone use if used whilst driving).
Statistical analyses
Assuming an event rate for the cumulative incidence of chlamydia or gonorrhoea of 20%,8 13 the trial was designed to detect a reduction in chlamydia or gonorrhoea reinfection from 20% to 16% (relative risk 0.8). To detect this difference a trial with 5000 participants would have 90% power using an α level of 0.05. The sample size calculation allows for 2% of participants in the control arm viewing the intervention messages (as seen in the pilot study) and up to 20% losses to follow-up. The trial steering committee reviewed the (masked) event rate after 546 patients had completed 12 months’ follow-up, and it recommended an increase in the sample size to 6250 because of a lower than expected event rate of 15.6%.
The primary analysis was by intention to treat. A detailed statistical analysis plan was published on the trial website before analysis and unblinding.14 For the primary outcome we compared the cumulative incidence of chlamydia or gonorrhoea reinfection at one year in each group using logistic regression. We used multiple imputation by chained equations (MICE) using the predictors of the outcome identified in the baseline data and in four week data to impute one year outcome data.15 We adjusted the primary analysis regression for the prespecified baseline covariates (age, type of STI at baseline, sex or gender, sexual orientation, and ethnicity).16 We report the adjusted odds ratios with 95% confidence intervals. The analysis of the secondary outcomes was similar to the analysis of the primary outcome. A complete case analysis was conducted as a supplementary analysis. We conducted prespecified subgroup analysis on the imputed dataset for age (16-19 years; 20-24 years), sex or gender (female-woman; male-man), sex or gender sexual orientation (men who have sex with men, and men who have sex with men and women; men who have sex with women; women who have sex with men, and women who have sex with men and women), ethnic group (white British/other white ethnicity; black/black British; all other groups), and adjusted indices of multiple deprivation17 (first and second fifths (least deprived), third fifth, and fourth and fifth fifths (most deprived)). Across the subgroups, we assessed heterogeneity of treatment effect and estimated odds ratios with 99% confidence intervals.14 18 For the intermediate outcomes, we carried out a complete case analysis and compared the summed scores using a linear regression. We conducted structural equation modelling using the latent variable intermediate outcomes derived from confirmatory factor analysis for the intermediate outcomes (see appendix 2 for full details). All analyses were done using Stata v 15.1. This study had no data monitoring committee.
Patient and public involvement
Patients and members of the public were involved in all phases of this study. Before developing the intervention, we discussed possible safer sex interventions in five discussion groups with people aged 16-24 years based in Southwark Further Education College (total 25 participants). The students were enthusiastic about receiving information and support via mobile phone. We worked with patients, who were recruited as research participants in focus groups, to design the content of the intervention.8 We met with 14 patient representatives from King’s College Hospital Sexual and Reproductive Health user group who helped design the patient information, questionnaires, and consent and follow-up procedures. A patient representative was included in the trial steering committee. A group of patient representatives are actively involved in disseminating the trial results.
Results
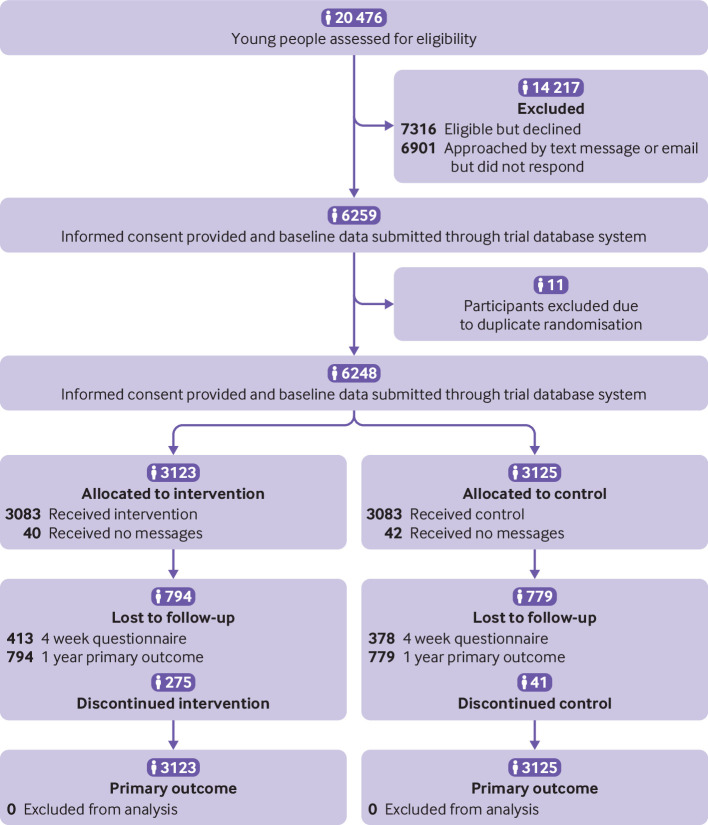
Between 1 April 2016 and 23 November 2018, we assessed 20 476 young people for eligibility. Of these, we excluded 14 217 before randomisation (7316 were not eligible and 6901 were eligible and approached by text message or email but did not respond) (fig 1). Informed consent was provided, and baseline data for 6259 participants was submitted through the trial database system. Eleven participants were excluded owing to duplicate randomisations. Of the remaining 6248 participants, 3123 were randomised to the intervention arm and 3125 to the control arm. Overall, 281/6248 (4.5%) participants withdrew from the trial before follow-up: 134/3123 (4.3%) in the intervention group and 147/3125 (4.7%) in the control group. A total of 4675 (74.8%) participants provided data for the primary outcome (safetxt: 2329/3123 (74.6%); control: 2346/3125 (75.0%)). All participants were included in the intention-to-treat analysis, with missing data imputed using MICE. A total of 2167/2412 (89.8% of the intervention group) respondents read all or most of the messages (2167/3125 (69.3%) of the intervention group).
Fig 1.
Consolidated standards of reporting trials (CONSORT) diagram
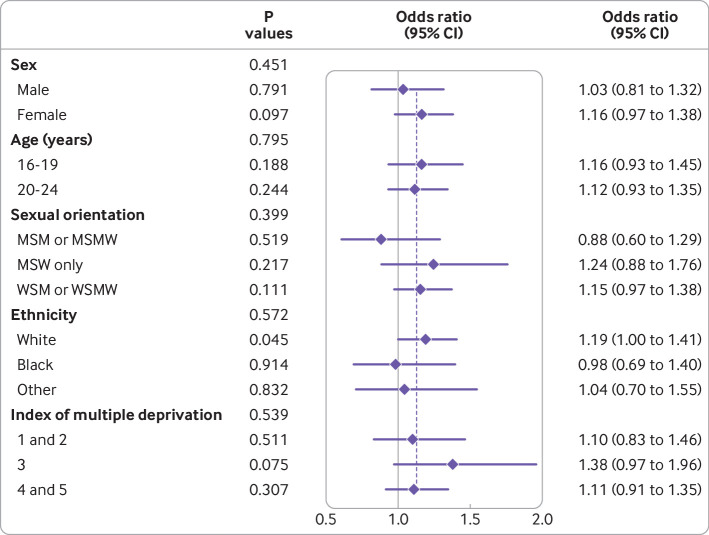
Baseline sociodemographic characteristics were similar between the groups (table 1). Table 2 shows the results for the primary and secondary outcomes. The cumulative incidence of chlamydia or gonorrhoea reinfection was 22.2% (693/3123) in the intervention arm versus 20.3% (633/3125) in the control arm (odds ratio 1.13, 95% confidence interval 0.98 to1.31, P=0.08). When only those participants with complete primary outcome data (4675/6248) were included in the primary analysis model, the odds ratio was 1.14 (0.98 to 1.31, P=0.08). In a per protocol analysis that was not prespecified, the corresponding intervention effect was 1.17 (0.99 to 1.38, P=0.06, see appendix 2, table S3). We found no evidence to suggest that the effect of the intervention was different among participants in any of the prespecified subgroups (fig 2).
Table 1.
Baseline characteristics of participants with a history of chlamydia, gonorrhoea, or non-specific urethritis assigned to a series of text messages to improve sexual health (safetxt intervention) or to text messages querying change of address (control group). Values are numbers (percentages) unless stated otherwise
| Characteristics | Safetxt group (n=3123) | Control group (n=3125) |
|---|---|---|
| Age group (years) | ||
| 16-19 | 1189 (38.1) | 1117 (35.7) |
| 20-24 | 1934 (61.9) | 2008 (64.3) |
| Mean (SD) age (years) (based on integer) | 20.3 (2.1) | 20.4 (2.1) |
| Gender | ||
| Female | 2047 (65.5) | 2020 (64.6) |
| Male | 1065 (34.1) | 1097 (35.1) |
| Non-binary | 11 (0.4) | 8 (0.3) |
| Ethnicity | ||
| White British/other white | 2428 (77.7) | 2436 (78.0) |
| Black/black British-Caribbean, African, other | 380 (12.2) | 347 (11.1) |
| Asian/Asian British-Bangladeshi, Chinese, Indian, Pakistani, other | 89 (2.8) | 91 (2.9) |
| Mixed | 174 (5.6) | 205 (6.6) |
| Other | 52 (1.7) | 46 (1.5) |
| Index of multiple deprivation fifth* | n=3099 | n=3096 |
| 1st (least deprived) | 439 (14.2) | 424 (13.7) |
| 2nd | 516 (16.7) | 527 (17.0) |
| 3rd | 608 (19.6) | 590 (19.1) |
| 4th | 768 (24.8) | 761 (24.6) |
| 5th (most deprived) | 768 (24.8) | 794 (25.6) |
| Educational level† | n=2996 | n=2990 |
| Primary and secondary (age ≤16 years) | 436 (14.6) | 450 (15.1) |
| Secondary onwards (age ≥17 years) | 1352 (45.1) | 1348 (45.1) |
| Still in full time education | 1208 (40.3) | 1192 (39.9) |
| Gender and sexual orientation | ||
| Women who have sex with men only | 1901 (60.9) | 1855 (59.4) |
| Men who have sex with women only | 790 (25.3) | 778 (24.9) |
| Women who have sex with women only | 20 (0.6) | 17 (0.5) |
| Men who have sex with men only | 226 (7.2) | 258 (8.3) |
| Women who have sex with women and men | 125 (4.0) | 147 (4.7) |
| Men who have sex with women and men | 49 (1.6) | 60 (1.9) |
| Those with non-binary gender who have sex with men | 7 (0.2) | 3 (0.1) |
| Those with non-binary gender who have sex with women | 1 (0) | 2 (0.1) |
| Those with non-binary gender who have sex with women and men | 3 (0.1) | 3 (0.1) |
| Not stated | 1 (0) | 2 (0.1) |
| Baseline diagnosis | ||
| Chlamydia | 2449 (78.4) | 2433 (77.9) |
| Gonorrhoea | 283 (9.1) | 303 (9.7) |
| Gonorrhoea and chlamydia | 159 (5.1) | 155 (5.0) |
| Gonorrhoea or non-specific urethritis | 27 (0.9) | 32 (1.0) |
| Non-specific urethritis | 125 (4.0) | 123 (3.9) |
| Unknown | 80 (2.6) | 79 (2.5) |
| Condom used during last sexual encounter | ||
| Yes | 747 (23.9) | 806 (25.8) |
| No | 2314 (74.1) | 2273 (72.7) |
| Unsure | 62 (2.0) | 46 (1.5) |
| Condom used during first sexual encounter with last new partner | ||
| Yes | 981 (31.4) | 1035 (33.1) |
| No | 2065 (66.1) | 2010 (64.3) |
| Unsure | 77 (2.5) | 80 (2.6) |
| Tested before sex with last new partner | ||
| Yes | 1242 (39.8) | 1243 (39.8) |
| No | 1798 (57.6) | 1787 (57.2) |
| Unsure | 83 (2.7) | 95 (3) |
| Partner tested before sex with last new partner | n=3120 | n=3125 |
| Yes | 437 (14) | 457 (14.6) |
| No | 1189 (38.1) | 1181 (37.8) |
| Unsure | 1494 (47.9) | 1487 (47.6) |
| No of partners in past 12 months | n=3120 | n=3122 |
| 0 | 5 (0.2) | 2 (0.1) |
| 1 | 496 (15.9) | 538 (17.2) |
| ≥2 | 2619 (83.9) | 2582 (82.7) |
Reduced denominator—index of multiple deprivation fifth was missing for some participants who provided an invalid postcode.
Reduced denominator—education information was missing for some participants due to non-response.
Table 2.
Primary and secondary outcomes in participants with a history of chlamydia, gonorrhoea, or non-specific urethritis assigned to a series of text messages to improve sexual health (safetxt intervention) or to text messages querying change of address (control group). Values are numbers (percentages) estimated from imputed data
| Outcomes | Safetxt group (n=3123) | Control group (n=3125) | Odds ratio (95% CI) | P value |
|---|---|---|---|---|
| Primary outcome (1 year) | ||||
| Cumulative incidence of chlamydia or gonorrhoea reinfection | 693 (22.2) | 633 (20.3) | 1.13 (0.98 to 1.31) | 0.09 |
| Secondary outcomes (4 weeks) | ||||
| Correctly treated for STI (took prescribed antibiotics and avoided sex for 7 days after treatment) | 2798 (89.6) | 2769 (88.6) | 1.11 (0.94 to 1.32) | 0.22 |
| Participant told last partner they had sex with before testing positive to get treatment | 2673 (85.6) | 2625 (84.0) | 1.14 (0.99 to 1.33) | 0.08 |
| Partner attended clinic for treatment (identified from clinic records) | 365 (11.7) | 406 (13.0) | 0.88 (0.75 to 1.02) | 0.10 |
| Condom use at last sexual encounter | 1312 (42.0) | 1238 (39.6) | 1.12 (1.00 to 1.25) | 0.05 |
| Secondary outcomes (1 year) | ||||
| Condom use at last sexual encounter | 1056 (33.8) | 975 (31.2) | 1.14 (1.01 to 1.28) | 0.04 |
| ≥2 sexual partners since joining the trial | 1777 (56.9) | 1713 (54.8) | 1.11 (1.00 to 1.24) | 0.06 |
| Sex with someone new since joining the trial | 2177 (69.7) | 2106 (67.4) | 1.13 (1.00 to 1.28) | 0.06 |
| Condom use at first sex with most recent new partner | 1699 (54.4) | 1522 (48.7) | 1.27 (1.11 to 1.45) | 0.001 |
| STI testing for self, before first sexual encounter with most recent new partner (self-reported) | 2067 (66.2) | 2128 (68.1) | 0.92 (0.79 to 1.06) | 0.24 |
| STI testing for self, before first sexual encounter with most recent new partner (testing confirmed by clinic record) | 1234 (39.5) | 1278 (40.9) | 0.95 (0.82 to 1.10) | 0.48 |
| Most recent new partner was tested for STI before sex with participant | 977 (31.3) | 881 (28.2) | 1.15 (0.88 to 1.51) | 0.28 |
| Road traffic incident in past year when participant was driver | 106 (3.4) | 100 (3.2) | 1.05 (0.76 to 1.47) | |
| Experience of partner violence in past year | 103 (3.3) | 103 (3.3) | 1.01 (0.75 to 1.38) | |
| Diagnosis of “any” STI after joining trial according to postal test results and clinic records | 693 (22.2) | 647 (20.7) | 1.10 (0.95 to 1.29) | 0.21 |
STI=sexually transmitted infection.
Analyses based on intention-to-treat principle; logistic regression analysis adjusted for prespecified baseline covariates (age, type of STI at baseline, sexual orientation, and ethnicity).
Fig 2.
Primary outcome by prespecified subgroups. MSM=men who have sex with men; MSMW=men who have sex with men and women; MSW=men who have sex with women; WSM=women who have sex with men; WSMW=women who have sex with men and women
At four weeks, 85.6% (2673/3123) of participants in the intervention arm versus 84.0% (2625/3125) in the control arm had notified the last partner they had sex with before testing positive to get treatment (odds ratio 1.14, 95% confidence interval 0.99 to 1.33, P=0.08), 89.6% (2798/3123) in the intervention arm versus 88.6% (2769/3125) in the control arm followed the correct treatment for STIs (1.11, 0.94 to 1.32, P=0.22), and, according to data from clinics, the partners of 11.7% (365/3123) of participants in the intervention arm versus 13.0% (406/3125) in the control arm attended for treatment (0.88, 0.75 to 1.02, P=0.10) (table 2). At four weeks, 42.0% (1312/3123) of participants in the intervention arm versus 39.6% (1238/3125) in the control arm (1.12, 1.00 to 1.25, P=0.05) reported using a condom at last sexual encounter (table 2). This difference was sustained at 12 months (33.8% (1056/3123) intervention v 31.2% (975/3125) control, 1.14, 1.01 to 1.28, P=0.04). At one year, 54.4% (1699/3123) of participants in the intervention arm versus 48.7% (1522/3125) in the control arm reported using a condom at first sexual encounter with their most recent new partner (1.27, 1.11 to 1.45, P=0.001) (table 2). No difference was found in participants testing before sex with a new partner according to self-report (66.2% (2067/3123) intervention v 68.1% (2128/3125) control, 0.92, 0.79 to 1.06, P=0.24) or self-report confirmed by clinic test (39.5% (1234/3123) intervention v 40.9% (1278/3125) control, 0.95, 0.82 to 1.10, P=0.48). The most recent new partner of 31.3% (977/3123) of participants in the intervention arm versus 28.2% (881/3125) in the control arm was tested for an STI before sex with the participant (1.15, 0.88 to 1.51, P=0.28) (table 2). Since joining the trial, 56.9% (1777/3123) of participants in the intervention arm versus 54.8% (1713/3125) in the control arm reported having two or more partners (1.11, 1.00 to 1.24, P=0.06), and 69.7% (2177/3123) in the intervention arm versus 67.4% (2106/3125) in the control arm reported having sex with someone new (1.13, 1.00 to 1.28, P=0.06) (table 2). The diagnosis of any STI was reported in 22.2% (693/3123) of participants the intervention arm versus 20.7% (647/3125) in the control arm (1.10, 0.95 to 1.29, P=0.21) (table 2). Self-reported partner violence or road traffic incidents were similar between the groups. Table 3 shows intermediate and process outcomes (also see tables S1 and S2 in appendix 2). The intervention was associated with small increases in the intermediate outcomes: knowledge related to STIs (coefficient 0.10, P=0.04) and correct condom use self-efficacy (0.32, P<0.001).
Table 3.
Intermediate and process outcomes in participants with a history of chlamydia, gonorrhoea, or non-specific urethritis assigned to a series of text messages to improve sexual health (safetxt intervention) or to text messages querying change of address (control group). Values are numbers (percentages) unless stated otherwise
| Outcomes | Safetxt group | Control group | Coefficient* (95% CI) | P value |
|---|---|---|---|---|
| Mean (SD) intermediate outcomes (summed items) | n=2656 | n=2705 | ||
| Knowledge related to STIs | 12.38 (1.84) | 12.29 (1.84) | 0.10 (0.01 to 0.20) | 0.04 |
| Attitude towards partner notification | 11.59 (1.74) | 11.63 (1.74) | −0.04 (−0.14 to 0.05) | 0.37 |
| Self-efficacy in telling partner about an infection | 11.55 (3.80) | 11.53 (3.90) | 0.04 (−0.17 to 0.24) | 0.72 |
| Correct condom use self-efficacy | 14.57 (2.90) | 14.27 (2.97) | 0.32 (0.16 to 0.47) | <0.001 |
| Self-efficacy in negotiating condom use | 11.35 (2.50) | 11.32 (2.60) | 0.03 (−0.10 to 0.17) | 0.64 |
| Intermediate outcomes (structural equation model)† | ||||
| Knowledge related to STIs | - | - | 0.081 | 0.02 |
| Attitude towards partner notification | - | - | 0.031 | 0.39 |
| Self-efficacy in telling partner about an infection | - | - | 0.020 | 0.55 |
| Correct condom use self-efficacy | - | - | 0.118 | <0.001 |
| Sharing of intervention content | n=2414 | n=2453 | ||
| Participant knew someone taking part in the study: | 137 (5.7) | 141 (5.8) | ||
| They read participant’s messages | 37 (1.5) | 32 (1.3) | ||
| Participant read their messages | 38 (1.6) | 34 (1.4) | - | - |
| Reading intervention content | n=2416 | |||
| Did anyone read the messages sent to you?: | 342 (14.2) | NA | ||
| Yes | 1971 (81.6) | NA | ||
| No or unsure | 103 (4.3) | NA | - | - |
| How did you feel about them reading the messages?: | n=342 | |||
| Happy | 224 (65.5) | NA | ||
| Unhappy | 35 (10.2) | NA | ||
| Unsure | 83 (24.3) | NA | ||
| How many of the messages did you read?: | n=2412 | |||
| All | 1506 (62.4) | |||
| Most | 661 (27.4) | |||
| Few | 229 (9.5) |
STI=sexually transmitted infection; NA=not applicable.
Regression of summed items, adjusted for same baseline characteristics as primary analysis: age, ethnicity, type of infection at baseline, sexual orientation group. Ranges of possible scores: Knowledge 3-15; attitude towards partner notification 3-15; self-efficacy in telling a partner about an infection 4-20; correct condom use self-efficacy 4-20; self-efficacy in negotiating condom use 3-15.
Results from structural equation model (using latent variable process outcomes). Coefficients are standardised so that the interpretation is that compared with the control group, the intervention group has 0.081 standard deviations greater knowledge related to STIs. Adjusted for same baseline characteristics as primary analysis: age, ethnicity, type of infection at baseline, sexual orientation group.
Similar findings to those of the main analysis were obtained from additional sensitivity analyses that were not prespecified (see appendix 2). Analyses were undertaken under different assumptions from those of the primary analysis missing-at-random assumption and included a post hoc analysis adding baseline number of partners (<2 or ≥2 partners) to the imputation model as an additional covariate for the primary outcome and the outcome number of partners. An additional analysis found the number need to harm was 64 (95% confidence interval number needed to benefit 334 to ∞ to number need to harm 24).
Discussion
Our text messaging intervention (safetxt) targeting partner notification, condom use, and STI testing did not reduce the risk of chlamydia or gonorrhoea reinfection at one year. More infections occurred in the safetxt intervention group compared with control group that only received text messages to query any changes to postal or email address. Some increase was found in self-reported precautionary behaviours such as condom use, but the number of STIs was not reduced. Although our intervention did not target sexual partnerships, the proportion of people with a new partner and with two or more partners at one year was higher in the intervention group.
Strengths and limitations of this study
We ensured allocation concealment by using a web based randomisation system. Baseline prognostic factors were well balanced between the two groups. Data collectors, laboratory analysts, and statistical analysts were masked to treatment allocation. Chlamydia and gonorrhoea were diagnosed using nucleic acid amplification polymerase chain reaction tests with high sensitivity and specificity. The primary analyses were on an intention-to-treat basis. Our recruitment across sociodemographic groups and sexualities, with no evidence of heterogeneity of effects in subgroups, suggests the results are generalisable.
Our trial has some limitations. A high proportion of eligible people declined to participate in the study. Many were only approached by text message or email and did not respond. Compared with the general UK population, those living in areas with a high index of multiple deprivation (fourth and fifth fifths) and ethnic minorities were well represented in our trial. Although 2162 men took part, men were under-represented and women over-represented in the trial. The primary outcome was only available for 75% of participants in each group. Although we used evidence based methods to achieve higher follow-up for laboratory assessed chlamydia and gonorrhoea than previous similar trials,19 some potential for bias remains. In our primary analysis we used multiple imputation methods to reduce bias and increase precision of the effect estimates.5 15 The similarity of findings in our primary analysis and all sensitivity analyses is reassuring. Many of our secondary outcomes were self-reported and could be influenced by social desirability bias.
Comparison with other studies
Our trial explored the effects of safer sex text message support on objectively measured STI outcomes.20 21 In previous trials the effects of interventions delivered by text message on condom use in the long term (12 months) were uncertain (pooled effect estimate relative risk 1.10, 95% confidence interval 0.77 to 1.56, I2=7%; three trials, n=667) and at risk of bias from incomplete follow-up, selection bias in a cluster randomised controlled trial, and lack of blinding of those collecting follow-up data.8 20 21 22 23 The effects of our text message intervention on condom use at one year are modest (odds ratio 1.14, 95% confidence interval 1.01 to 1.28, P=0.038) but larger than those reported for single sessions of face-to-face counselling or other forms of remote support such as telephone counselling, videos, or websites at 12 months.24 25
In a recent systematic review, the pooled odds ratio for text messages on STI testing within 12 months was 1.83 (95% confidence interval 1.41 to 2.36; seven trials, n=2151) (moderate certainty evidence).20 Our trial found no benefit on STI testing before sex with new partners. Before the main and pilot trial of safetxt, a Ugandan trial had reported an odds ratio of 1.54 (95% confidence interval 0.85 to 2.79) for the effect of text messages on partner attendance for syphilis testing and treatment at next antenatal care visit.20 21 26
The effects of text messages in promoting sexual health might be context and content specific. The effect on STIs of digital messages targeting condom use and STI testing only among those reporting risky behaviour but with no STI diagnosed remains uncertain.
Our intervention was not associated with a reduction in risk of chlamydia or gonorrhoea at one year and could have increased risk. We explored methodological reasons for the unexpected results. Firstly, we conducted analyses using a range of different assumptions about STI rates in those lost to follow-up in both groups. An active care seeking effect resulting in more people with STI in the intervention group being identified is unlikely as follow-up for the primary outcome was similar in both groups (slightly higher in the control group), and the results of sensitivity analysis testing assumptions that the outcome data are not missing at random provided results that were similar to the findings of our main trial analysis (see appendix 2). Secondly, we explored if these findings could have occurred as a result of small chance imbalances in sexual behaviour (number of partners in preceding 12 months) between the intervention group and control group at baseline. The consistency of the results of all these analyses combined with the slightly increased effect size in the per protocol analysis adds to the weight of evidence suggesting our intervention increased the risk of STIs. When we pooled the results of the trial with data from pilot trial participants receiving the same intervention targeting partner notification, condom use, and STI testing, risk of reinfection remained similar (pooled odds ratio 1.12, 95% confidence interval 0.99 to 1.26, P=0.08, I2=0%, see supplementary figure S1).
Sexual behaviour is complex, and a review of our qualitative research findings and open feedback comments provided by participants raises a potential mechanism for the slightly higher number of infections in the intervention group.9 This concerned a reduction in felt stigma (internal stigma or self-stigmatisation) in having an STI, leading to the intervention group having more partners than the control group and hence more STIs. To encourage partner notification, the intervention adopted a non-stigmatising approach to providing information and support. Recipients reported a reduction in felt stigma about having an STI as a benefit of the intervention.9 Lower levels of stigma are associated with higher precautionary and treatment behaviours (eg, condom use, STI testing, and STI treatment), improved emotional and mental wellbeing, lower pregnancy rates in adolescents, and also higher numbers of sexual partners.27 28 29 Lower stigma measured at a societal level is associated with higher country level STIs.30 If our intervention reduced felt stigma about having an STI, this could have resulted in recipients having more partners than the control group leading to a higher risk of STIs. Some group based behavioural interventions have been associated with increased numbers of STIs, thought to be due to the aggregation of at risk individuals.31 Although in this trial we did not introduce participants to each other, they reported a reduced sense of isolation in having an STI—realising they were “not the only one.”
Data from our trial show that between baseline and 12 month follow-up self-reported condom use at last sex increased in both groups (23.9% intervention and 25.8% control; 33.8% intervention and 31.2% control, respectively), and the number of participants who reported having two or more partners was noticeably reduced (83.9% intervention and 82.7% control; 56.9% intervention and 54.8% control, respectively). Large behavioural changes occurred among the participants in our trial who had a diagnosis of an STI, received current care pathways in community sexual and reproductive health clinics or genitourinary clinics, and may have accessed information currently available in the public domain. Compared with this finding, the effect of text messages was small. Safer sex behaviours are influenced by a wide range of individual, interpersonal, societal, and structural factors, which could also explain why the intervention effects were small.
Policy implications
Our qualitative research suggests that from the perspective of young people, the safetxt intervention positively impacts on broader aspects of sexual and reproductive wellbeing, such as confidence, agency, and communication about sexual health with siblings, friends, and partners.9 In this trial our intervention showed benefits on some measures of sexual health, such as self-efficacy in condom use and condom use in itself, whereas the intervention’s public health effects on STIs were in the direction of harm. This illustrates the importance of rigorously evaluating the impact of novel health communication interventions on objective public health outcomes.
It is not likely that the approaches to promoting condom use resulted in increased numbers of STIs, as these methods were adapted from, and similar to, the content of face-to-face interventions that have been shown to increase condom use and reduce the number of STIs.32 33 34
Our text message intervention was grounded in psychological theory, incorporating the best evidence on health behaviour change, but it did not have the effects we anticipated. In light of our results, WHO should revise its endorsement of digital behaviour change communication for strengthening health systems, to specify which topics and content WHO endorses.
Conclusions
Safetxt did not reduce STIs. More reinfections occurred in the intervention group. Our results highlight the need for rigorous evaluation of health communication interventions. Future work could evaluate the effect of interventions promoting condom use and STI testing in those at risk but with a diagnosis of an STI. Further research should focus on how to reduce the stigma associated with STIs to benefit wellbeing, treatment, and precautionary behaviours for those with a diagnosis of an STI, without increasing the risk of infection.
What is already known on this topic
Behaviour change interventions delivered by automated text message (such as for smoking cessation) can be highly cost effective
A review on the effects of sexual health interventions delivered by text message found little high quality evidence
The effects on key behaviours such as condom use, partner notification, and outcomes of sexually transmitted infections (STIs) were uncertain
What this study adds
The safetxt intervention using a mobile phone and targeting safer sex behaviours was not associated with a reduction in incidence of chlamydia or gonorrhoea at one year; more infections occurred in the intervention group
Saftext was associated with an increase in some self-reported measures of sexual health, such as self-efficacy in condom use and condom use in itself
WHO should revise its endorsement of digital behaviour change communication for strengthening health systems, to specify which topics and content WHO endorses
Acknowledgments
Contributing Trusts
Berkshire Healthcare NHS Foundation Trust—Nisha Pal, Matthew Hamill, Kate Rabjohn, Sunita Baniya, Rhona Merricks; Bridgewater Community Healthcare—Debashis Mandal, Dona McManus, Gail Hampal, Sandra Mason; Brighton and Sussex University Hospitals—Afra Barrett, Amanda Clarke, Celia Richardson, Emma Collins, Justine Orme, Lisa Barbour, Louise Kerr, Michelle Hawkins, Sarah Smith, Tamara Woodroffe, Victoria Cook; Calderdale and Huddersfield NHS Foundation Trust—Alison Wilson, Asifa Ali, Emma Street, Marie Home, Susan Kilroy, Tonicha Nortcliffe, Wendy Cook, Annalisa Dance, Catherine Hawksworth, Susan Kilroy, Amanda Clarkson-Kearsley, Mohammad Irfan Alam, Rebecca Jenkins, Maneh Farazmand, Kathryn Hanson, Joseph Deering; Cambridgeshire Community Services NHS Trust—Carl Turner, Samantha Nunn, Sam Davies, Claire Abbs, Bethany Burgess, Muhammad Patel, Gemma-Lea Green, Evie Wooltorton, Katie Kankanamge, Beverley Walmsley, Sonya Issitt, Heather Bennett, Steve Macfarlane, Nina Frusher, Gemma Owen, Maxine Emerson, Clare Lee, Sarah Montagu, Laura Nicol, Carie Coleman, Laura Chase, Elizabeth Hodges, Dawn Cooper, James Pamment, Katrina Nethercott, Eleni Kouffi, Jenny Onyon, Carina Taylor, Amy Spikes, Clare Allen, Alison Shaw, Lorraine Newstead, Jeanette Gowing, Julie Turner, Val Seaman, Julia Ball; Central and North West London NHS Foundation Trust—Abigail Severn, Andrea Cartier, Jack Brophy, Anna-Lena Salz, Rebecca Matthews, Alexandra Rolland, John Saunders; Central London Community Healthcare NHS Trust—Sarah Edwards, Jackie Austin, Liz Craggs, Julie Carter, Jennifer Kent, Holly Le Blond, Aicha Kallo, Tracey Callan, Ann Gardner, Aliki Rizou; Central Manchester University Hospitals—Lisa Southon, Denise Kadiu, Denise Donahue; Chelsea and Westminster Hospital NHS Foundation Trust—Roberta Brum, Sara Day, Neil Turner, Grainne Cooney, Ann Sullivan, Clare Turvey, Sophie Hobday, Serah Duro, Jessica Whittock, Ceri Evans, Simon Paragreen, Rachel Jones, Michael Rayment; County Durham and Darlington NHS Foundation Trust—Sarah Duncan, Alison Wardropper, Melanie Kent, Nicola Hewitson; Coventry and Warwickshire Partnership Trust—Amine Abbs, Anthony Wood, Jane Drewery, Kay Wright, Kerry Flahive, Marie McCauley, Nyaradzo Nyamayaro, Rachel Califano, Elizabeth Vassell, Sris Allen, Teresa McIntyre; Cumbria Partnership NHS Foundation Trust—Charlotte Halliday, Helen Fairlamb, Leon Jonker, Matt Philiips, Sarah Thornthwaite, Jose Schutter, Danielle Smith, Emma Meekley, Tracy Armstrong, Sam Grimwood, Laura Fugler, Rachel Hardy; Dorset County Hospital NHS Foundation Trust—Sue Hyett, Simone Caddy, the GUM team; Dorset HealthCare—Ciarán Newell, Hazel Burt, Jo Goodman, Kim Meldrum; East Sussex Healthcare NHS Trust—Harish Patel, Anne Cowley, Kazeem Aderogba; NHS Lothian—Anne Johnstone, Jack Perry, Sharon Cameron; George Eliot Hospital NHS Trust—Loay David, Jessica Gunn, Karen Chambers, Karen Peacock; Homerton University Hospital—Katherine Coyne, Monica James; Kent Community Health Foundation Trust—Charlie Marlow, Karen Thorogood, Lee Tomlinson, Serena Mansfield, Leah Elliot, Tracy Hazelton, Brian Connolly, Nikki Crisp, Mun-Yee Tung; King's College Hospital NHS Foundation Trust—Hannah McCulloch, Alessandra Morelli; Kingston Hospital NHS Foundation Trust—Andrew Swain, Bavitha Nathan, India McKenley, Isabel Bradley, Jennifer Crooks, Judith Murray, Nikita Michael, Ross Anderson, Richard Sims, Hannah White; Lancashire Care NHS Foundation Trust—Lisa Wadeson, Tessa Malone, Gillian Welch, Rebecca Davies, Yu Wilson, the Lancashire Sexual Health Team, Lancashire and South Cumbria Research and Development Team; Lewisham and Greenwich NHS Trust—Praveen Jayadera, Hannah Kelly, Emily Mabonga, Christos Tsintikidis; Luton and Dunstable University Hospital—Mohanarathi Kawsar, Memory Kakowa; Maidstone and Tunbridge Wells NHS Trust—Lesley Navaratne, Rita Joseph, Laura Reid, Aimee Williams, Joan Miller, Lindsey Hamilton, Beth Jones, Paige Halliwell, Stephanie McKinley, Banhur Sandhu; Midlands Partnership NHS Foundation Trust—Andrea Ng, Anna Stegeman, Daniel Crawshaw, Andy Taylor, Karen Townshend, Jo Tomlinson, Lisa Goodall, Jo Meikle, Julie Farr; Milton Keynes University Hospital NHS Foundation Trust—Dushyant Mital, Patricia Williams, Annie Rose, Cheryl Padilla-Harris, Kerry Fendick, Anne Somerton, Brenda Kell, Miriam Leah, Christopher Ford, Carole Holder, Veronia Edgell; Newcastle upon Tyne NHS Foundation Trust—Mayur Chauhan, Victoria Murtha, Laura Shewan, Lorraine Neild, Diane Tregenning, Tara Stothard, Lesley Harvey, Jane Taylor, Hannat Akintomide; North Devon Healthcare NHS Trust—Sophia Davies, April Brooks, Clare Davidson, Fiona Stonham, Beverly Ripper, Alison Wesley, Abbey Eboigbe, Louise Pritchard, Laurance Druiff, Jan Freer, Maggie Rice, Tess Taylor, Joanne Hamilton, Jane Hunt, Geraldine Belcher, Sally Tettersell, Martin Howard, Helen Black; Northamptonshire Healthcare NHS Foundation Trust—Sophie Herbert, Kim Burke, Lizzie Biswell ; Nottingham University Hospital NHS Trust—Ashini Fox, Katie Smith, Sarah Flew, Laura Anderson; Plymouth Hospitals NHS Trust—Zoe Warwick, Hannah Gott, Hannah Jory, Suzanne Price; Rotherham NHS Foundation Trust—Nadi Gupta, Rachel Walker, Dawn Collier, Kathryn Dixon, Carol Weston, Victoria Murray, Alison Humphries; Royal Berkshire NHS Foundation Trust—Fabien Chen, Sue Hallett, Candy Brown, Sheila O’Connor, Julia Tassano-Smith, Chrissie Ambrose, Emma Craig, Shanies Mughal, Chelsea Gallagher, Makini Jones; Royal Bournemouth and Christchurch Hospital NHS Foundation Trust—Elbushra Herieka, Michaela Osborne, Sarah Andrews, Steven Williams, Annamaria Wilce, Emma Gunter, Luke Vamplew, Jessica Kelly, Debbie Dawson; Royal Cornwall Hospitals NHS Trust—Sara McNamara, Julie Crockford, Emma Gardner, Pam Gates, Olivia Dunlop; Royal Free NHS Foundation Trust—Dan Ivens, Monte Fields, George Matchiek, Tony Robinson, Mirelle Harris, Louie Pong, Dawn Whittaker, Barbara Danielski, Silvia Belmondo, Joanna Darim; Royal Liverpool and Broadgreen University Hospitals—Christine Bates, Mark Lawton, Martyn Wood, Damitha Edirisinghe, R Thomson-Glover, Graham Sweeney, Deborah Scanlon, Rachel Robinson, Deven Williams; Royal Wolverhampton NHS Trust—Anjum Tariq, Sarah Milgate; Sheffield Teaching Hospitals NHS Foundation Trust—Gill Bell, Aimee Card, Lauren Theaker, Jennifer Warvell, Jo Brown, Leisa Broadhurst, Miriam McChelka, Sally Howlett, Julie Barratt, Sarah Birch; Solent NHS Trust—Rajul Patel, Johanna Turpitt, Jane Whitehead, Tracy Callen; Somerset Partnership NHS Foundation Trust—Carinna Vickers, Paula Hill, Jane Scott, Kerry Lucas, Lucy Ayres, Olivia Archer; Torbay and South Devon NHS Foundation Trust—Louise Dawkins, Pauline Fitzell, Josie Garfield-Smith, Kathy Horan, Justine Wright; University Hospitals Birmingham—Jonathon Ross, Magdalena Nowacka, Monica Okriak, Louise Parish, Liza Nicholls, Francine Bridge, Sarah Brown, Christine Carter, Satwant Kaur, Sonia Graham, Alzcess Joe, Kelly Hollier, Jayne Rankin, Holly Whitehouse, Victoria Hardy, Sharon Hackett, Tessa Lawrence, David Tyrrell; University Hospitals Bristol NHS Foundation Trust—Megan Crofts, James Gabb, Michael Clark, Helen Wheeler; Warrington and Halton Hospitals NHS Foundation Trust—Debashis Mandal, Sandra Mason, Helen Whittle; West Middlesex University Hospital—Shamela De Silva, Marie-Louise Svensson, Ursula Kirwan, Metod Oblak, Arshia Tavender, Lynsey Barry; Wirral Community NHS Trust—Rachael Ellks, Martyn Wood, Elizabeth Anderson.
Trial steering committee
Pippa Oakeshott (chair), Caroline Free (chief investigator), Andrew Copas, Michael Brady, Michael Ussher, Colum McGrady (patient representative); observers: Rosemary Knight, Kimberley Potter; as risk of harm from the intervention was considered low, the analysis was conducted once at the end of the trial. No separate data monitoring and ethics committees existed. The trial steering committee took on the ethical responsibility for the trial and monitored adverse events.
Trial management group
Caroline Free, Ona McCarthy, Melissa Palmer, Irrfan Ahmed, Kimberley Potter, Lauren Jerome, Rosemary Knight, Megan Knight, Zahra Jamal, Faran Dhaliwal, Rebecca Matthews (December 2015 to August 2016), Rebecca Swinson (August 2016 to August 2017).
Trial statistician
James Carpenter, Tim Morris, Phil Edwards. We thank George K Ploubidis for senior oversight of the analysis of intermediate outcomes and measures.
Software and IT support
Irfan Ahmed.
Web extra.
Extra material supplied by authors
Supplementary material: Appendices 1 and 2, including supplementary tables S1-S3, figure S1, and additional information
Contributors: CF, MJP, OLM, IR, LJ, SB, and KP wrote the report with comments from KT, LM, KD, MK, JRC, TPM, ZJ, FD. RF, FCIH, AG, KW, PB, JB, TC, PE, and GH. SM, CF, and PB conceptualised the intervention. CF, OLM, RSF, FH, PB, KW, JVB, GH, SM, and KD designed the intervention. CF, OLM, TC, PE, and IR designed the trial. TPM, JRC, and PE were trial statisticians. TPM, JRC, and MJP conducted the trial analysis. MJP conducted the analysis of process measures. SB conducted the pooled analyses of data. KP, OLM, LJ, MJP, MK, and ZJ were responsible for project administration. OLM, LJ, MK, MJP, KP, FD, ZJ, and CF developed the trial recruitment and follow-up procedures. KP was responsible for the day-to-day running of the trial. LJ, MK, and MJP assisted recruitment. LJ, MK, ZJ, and FD undertook trial follow-up. MJP, TPM, and JRC prepared figures and tables. CF, OLM, RSF, FCIH, PB, KW, GH, SM, KD, PE, IR, and GP contributed to writing the grant application. CF is the guarantor and accepts full responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish. The corresponding author (CF) attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: The National Institute for Health and Care Research (NIHR) Public Health Research Board funded the trial, but had no role in the design, conduct, analysis, interpretation, or writing up of the results. The intervention development work was funded by the NIHR Health Technology Assessment Programme (project No 10/93/04). This trial was funded by the NIHR Public Health Research programme (project No 14/182/07). JRC and TPM are funded by the Medical Research Council (grant No MC_UU_00004/07). The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the National Institute for Health and Care Research (NIHR) Public Health Research and no support from any organisation for the submitted work JRC and TPM are funded by the Medical Research Council; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years except PB who is a medical director of a not-for-profit organisation (SH:24) providing online sexual health services; no other relationships or activities that could appear to have influenced the submitted work. TM receives personal income from consultancy for Kite Pharma and Alliance Pharmaceuticals. KMET received income from GlaxoSmithKline, statistical consultancy roles in Novartis, AstraZeneca and GlaxoSmithKline, and membership of a data safety monitoring board for Pfizer.
The lead author (CF) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: The results will be emailed to the participants who requested them and will be disseminated to all participating clinics. Patient representatives are developing a dissemination plan that they will be actively involved in, including dissemination via social media.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
This study was approved by the NHS Health Research Authority-London-Riverside research ethics committee (reference 15/LO/1665) and the London School of Hygiene and Tropical Medicine.
Data availability statement
After publication of the primary and secondary analyses detailed in the statistical analysis plan, individual deidentified patient data, including a data dictionary, will be made available via our data sharing portal FreeBIRD website indefinitely. The trial protocol, statistical analysis plan, and trial publications will be freely available online.
References
- 1. Cates W, Jr, Wasserheit JN. Genital chlamydial infections: epidemiology and reproductive sequelae. Am J Obstet Gynecol 1991;164:1771-81. 10.1016/0002-9378(91)90559-A [DOI] [PubMed] [Google Scholar]
- 2. Sonnenberg P, Clifton S, Beddows S, et al. Prevalence, risk factors, and uptake of interventions for sexually transmitted infections in Britain: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). Lancet 2013;382:1795-806. 10.1016/S0140-6736(13)61947-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization DoRHaR . Defining sexual health: Report of a technical consultation on sexual health. WHO, 2006. [Google Scholar]
- 4. Marston C, King E. Factors that shape young people’s sexual behaviour: a systematic review. Lancet 2006;368:1581-6. 10.1016/S0140-6736(06)69662-1 [DOI] [PubMed] [Google Scholar]
- 5. Free C, Knight R, Robertson S, et al. Smoking cessation support delivered via mobile phone text messaging (txt2stop): a single-blind, randomised trial. Lancet 2011;378:49-55. 10.1016/S0140-6736(11)60701-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guerriero C, Cairns J, Roberts I, Rodgers A, Whittaker R, Free C. The cost-effectiveness of smoking cessation support delivered by mobile phone text messaging: Txt2stop. Eur J Health Econ 2013;14:789-97. 10.1007/s10198-012-0424-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization . WHO guideline: recommendations on digital interventions for health system strengthening. WHO, 2019. [PubMed] [Google Scholar]
- 8. Free C, McCarthy O, French RS, et al. Can text messages increase safer sex behaviours in young people? Intervention development and pilot randomised controlled trial. Health Technol Assess 2016;20:1-82. 10.3310/hta20570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. French RS, McCarthy O, Baraitser P, Wellings K, Bailey JV, Free C. Young People’s Views and Experiences of a Mobile Phone Texting Intervention to Promote Safer Sex Behavior. JMIR Mhealth Uhealth 2016;4:e26. 10.2196/mhealth.4302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Free C, McCarthy OL, Palmer MJ, et al. Safetxt: a safer sex intervention delivered by mobile phone messaging on sexually transmitted infections (STI) among young people in the UK - protocol for a randomised controlled trial. BMJ Open 2020;10:e031635. 10.1136/bmjopen-2019-031635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Michie S, Wood CE, Johnston M, Abraham C, Francis JJ, Hardeman W. Behaviour change techniques: the development and evaluation of a taxonomic method for reporting and describing behaviour change interventions (a suite of five studies involving consensus methods, randomised controlled trials and analysis of qualitative data). Health Technol Assess 2015;19:1-188. 10.3310/hta19990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clutterbuck DJ, Flowers P, Barber T, et al. UK National Guidelines on safer sex advice - The Clinical Effectiveness Group of the British Association for Sexual Health and HIV (BASHH) and the British HIV Association (BHIVA), July 2012. British Association for Sexual Health and HIV, 2012. [Google Scholar]
- 13. Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with Chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis 2009;36:478-89. 10.1097/OLQ.0b013e3181a2a933 [DOI] [PubMed] [Google Scholar]
- 14.Free C, Carpenter JR, and trial team. safetxt: A randomised controlled trial of an intervention delivered by mobile phone messaging to reduce sexually transmitted infections (STI) by increasing sexual health precaution behaviours in young people - Statistical Analysis Plan, SAP: Version 6- 10/06/2020 London: London School of Hygiene and Tropical Medicine; 2020. https://safetxt.lshtm.ac.uk/files/2020/06/safetxt-SAP-v-6-final-10th-June.pdf.
- 15. Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. 10.1136/bmj.b2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. European Medicines Agency, Committee for Medicinal Products for Human Use . Guideline on adjustment for baseline covariates in clinical trials. 2015. [Google Scholar]
- 17. Abel G, Payne R, Barclay M. UK Deprivation Indices Bristol. University of Bristol, 2016, https://data.bris.ac.uk/data/dataset/1ef3q32gybk001v77c1ifmty7x. [Google Scholar]
- 18. Kasenda B, Schandelmaier S, Sun X, et al. DISCO Study Group . Subgroup analyses in randomised controlled trials: cohort study on trial protocols and journal publications. BMJ 2014;349:g4539. 10.1136/bmj.g4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bailey JV, Murray E, Rait G, et al. Interactive computer-based interventions for sexual health promotion. Cochrane Database Syst Rev 2010;(9):CD006483. 10.1002/14651858.CD006483.pub2 [DOI] [PubMed] [Google Scholar]
- 20. Berendes S, Gubijev A, McCarthy OL, Palmer MJ, Wilson E, Free C. Sexual health interventions delivered to participants by mobile technology: a systematic review and meta-analysis of randomised controlled trials. Sex Transm Infect 2021;97:190-200. 10.1136/sextrans-2020-054853 [DOI] [PubMed] [Google Scholar]
- 21. Palmer MJ, Henschke N, Villanueva G, et al. Targeted client communication via mobile devices for improving sexual and reproductive health. Cochrane Database Syst Rev 2020;8:CD013680. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lim MS, Hocking JS, Aitken CK, et al. Impact of text and email messaging on the sexual health of young people: a randomised controlled trial. J Epidemiol Community Health 2012;66:69-74. 10.1136/jech.2009.100396 [DOI] [PubMed] [Google Scholar]
- 23. Rokicki S, Cohen J, Salomon JA, Fink G. Impact of a Text-Messaging Program on Adolescent Reproductive Health: A Cluster-Randomized Trial in Ghana. Am J Public Health 2017;107:298-305. 10.2105/AJPH.2016.303562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bailey JV, Wayal S, Aicken CRH, et al. Interactive digital interventions for prevention of sexually transmitted HIV. AIDS 2021;35:643-53. 10.1097/QAD.0000000000002780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Henderson JT, Senger CA, Henninger M, Bean SI, Redmond N, O’Connor EA. Behavioral Counseling Interventions to Prevent Sexually Transmitted Infections: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2020;324:682-99. 10.1001/jama.2020.10371 [DOI] [PubMed] [Google Scholar]
- 26. Parkes-Ratanshi R, Mbazira Kimeze J, Nakku-Joloba E, et al. Low male partner attendance after syphilis screening in pregnant women leads to worse birth outcomes: the Syphilis Treatment of Partners (STOP) randomised control trial. Sex Health 2020;17:214-22. 10.1071/SH19092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hall KS, Morhe E, Manu A, et al. Factors associated with sexual and reproductive health stigma among adolescent girls in Ghana. PLoS One 2018;13:e0195163. 10.1371/journal.pone.0195163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hatzenbuehler ML, Phelan JC, Link BG. Stigma as a fundamental cause of population health inequalities. Am J Public Health 2013;103:813-21. 10.2105/AJPH.2012.301069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parker R, Aggleton P. HIV and AIDS-related stigma and discrimination: a conceptual framework and implications for action. Soc Sci Med 2003;57:13-24. 10.1016/S0277-9536(02)00304-0 [DOI] [PubMed] [Google Scholar]
- 30. Pachankis JE, Hatzenbuehler ML, Hickson F, et al. Hidden from health: structural stigma, sexual orientation concealment, and HIV across 38 countries in the European MSM Internet Survey. AIDS 2015;29:1239-46. 10.1097/QAD.0000000000000724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Imrie J, Stephenson JM, Cowan FM, et al. Behavioural Intervention in Gay Men Project Study Group . A cognitive behavioural intervention to reduce sexually transmitted infections among gay men: randomised trial. BMJ 2001;322:1451-6. 10.1136/bmj.322.7300.1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Free C, Roberts IG, Abramsky T, Fitzgerald M, Wensley F. A systematic review of randomised controlled trials of interventions promoting effective condom use. J Epidemiol Community Health 2011;65:100-10. 10.1136/jech.2008.085456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jemmott JB, 3rd, Jemmott LS, Braverman PK, Fong GT. HIV/STD risk reduction interventions for African American and Latino adolescent girls at an adolescent medicine clinic: a randomized controlled trial. Arch Pediatr Adolesc Med 2005;159:440-9. 10.1001/archpedi.159.5.440 [DOI] [PubMed] [Google Scholar]
- 34. Shain RN, Piper JM, Newton ER, et al. A randomized, controlled trial of a behavioral intervention to prevent sexually transmitted disease among minority women. N Engl J Med 1999;340:93-100. 10.1056/NEJM199901143400203 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material: Appendices 1 and 2, including supplementary tables S1-S3, figure S1, and additional information
Data Availability Statement
After publication of the primary and secondary analyses detailed in the statistical analysis plan, individual deidentified patient data, including a data dictionary, will be made available via our data sharing portal FreeBIRD website indefinitely. The trial protocol, statistical analysis plan, and trial publications will be freely available online.