Abstract
Background:
Children born extremely preterm disproportionately experience sequelae of preterm birth compared to those born at later gestational ages, including higher prevalence of autism spectrum disorder (ASD) and associated behaviors.
Aim:
Explore effects of combined dietary docosahexaenoic acid, eicosapentaenoic acid, gamma-linolenic acid, and oleic acid (omega 3-6-9) on caregiver-reported behavior and sleep in toddlers born at ≤29 weeks' gestation who were exhibiting symptoms commonly seen with ASD.
Study design:
90-day randomized (1:1), double blinded, placebo-controlled trial.
Subjects:
Thirty-one children aged 18–38 months received omega 3-6-9 (n = 15) or canola oil placebo (n = 16).
Outcome Measures:
Mixed effects regression analyses followed intent to treat and explored treatment effects on measures of caregiver-reported behavior (Child Behavior Checklist 1.5–5, Toddler Behavior Assessment Questionnaire – Short Form, Vineland Adaptive Behavior Scales, 2nd Edition) and sleep (Children's Sleep Habits Questionnaire, Brief Infant Sleep Questionnaire).
Results:
Twenty-nine of 31 (94%; ntx = 13, nplacebo = 16) children randomized had data available for at least one outcome measure, 27 (87%; ntx = 12, nplacebo = 15) had complete outcome data. Children randomized to omega 3-6-9 experienced a medium magnitude benefit of supplementation on anxious and depressed behaviors (ΔDifference = −1.27, d = −0.58, p = 0.049) and internalizing behaviors (ΔDifference = −3.41, d = −0.68, p = 0.05); and a large magnitude benefit on interpersonal relationship adaptive behaviors (ΔDifference = 7.50, d = 0.83, p = 0.01), compared to placebo. No effects were observed on other aspects of behavior or sleep.
Conclusions:
Findings provide preliminary support for further exploration of omega 3-6-9 during toddlerhood to improve socioemotional outcomes among children born preterm, especially for those showing early symptoms commonly seen with ASD. Results need to be replicated in a larger sample.
Trial registration:
Registered with ClinicalTrials.gov: NCT01683565.
Keywords: Behavior, Docosahexaenoic acid (DHA), Gamma-linolenic acid (GLA), Premature birth, Randomized clinical trial, Sleep
1. Introduction
One of every 10 US-born babies is born preterm (<37 completed weeks' gestation) [1]. Children born preterm experience higher rates of cognitive impairments, behavioral problems, socioemotional difficulties, and sleep disturbances, versus children born at term [2-8]. Several studies report that children born preterm are at increased risk for autism spectrum disorder (ASD), with one meta-analysis reporting that children born at <37 weeks are more than 1.3 times as likely to have ASD, compared to term children [9-11]. Furthermore, children of the lowest gestational ages demonstrate the highest ASD prevalence and disproportionately experience long-term sequalae of preterm birth compared to those born at later gestational ages [2,10,12,13].
The omega-3 fatty acids eicosapentaenoic acid (EPA) and its downstream product, docosahexaenoic acid (DHA), play a role in structural and functional brain development. DHA is the major structural fatty acid in the developing brain, and DHA accretion is rapid through age 2 years [14-18]. These fatty acids, along with an omega-6 fatty acid called gamma-linolenic acid (GLA) have anti-inflammatory properties, and DHA is a key player in neurotransmitter function, synaptogenesis, gene expression, membrane fluidity, neurogenesis, neuroplasticity, and anti-inflammation [19-22]. Omega-9 fatty acids may promote metabolic health, including an improved lipid profile [22,23]. Children born preterm miss much of the rapid acquisition of DHA and other fatty acids that occurs in the third trimester of pregnancy [24].
A meta-analysis of 15 studies reported that young children with ASD have lower blood levels of the omega-3 fatty acids DHA, EPA, and the omega-6 fatty acid arachidonic acid (AA) compared to their typically developing peers, suggesting that interventions to increase these fatty acids may have benefit [25]. Findings from trials which supplemented the diets of children with ASD with omega-3 fatty acids in early childhood are mixed. Some report that, compared to placebo, supplementation results in reduction of externalizing behaviors, stereotypy, lethargy, and irritability, and improvement in social-awareness, while others report that supplementation is associated with an increased display of externalizing behaviors [26-30]. No effects of omega-3 supplementation on adaptive functioning, sleep, and several other aspects of behavior in young children with ASD were identified [27-31]. One study reported benefit of omega-3 supplementation treatment on broad aspects of ASD as measured by the Autism Treatment Evaluation Checklist, however this was not a placebo-controlled trial [32]. The supplementation period in these trials ranged from 6 weeks to 1 year, with three, 3-month trials reporting positive effects of supplementation on aspects of child behavior [27,31,32]. Previous trials in which eligible children were aged two to eight years old and had ASD did not include omega-6 fatty acids which share enzyme-driven metabolic pathways with omega-3 fatty acids and may help promote anti-inflammatory properties. These studies supplemented diets with omega-3 fatty acids, but supplementation of an omega-3 and omega-6 fatty acid combination in early childhood may help address deficits typically seen in children born preterm who display behaviors commonly seen with ASD in ways not enhanced by omega-3 supplementation alone.
The objective of this secondary analysis was to test the effect of EPA (omega-3), DHA (omega-3), GLA (omega-6), and oleic acid (omega-9) (omega 3-6-9) supplementation for 90-days on multiple aspects caregiver-reported behavioral and sleep outcomes in a sample of toddlers born at ≤29 weeks' gestation who were exhibiting symptoms commonly seen with ASD during toddlerhood.
2. Method
2.1. Study design and setting
The Preemie Tots trial was a single-site randomized, double blinded, placebo-controlled trial (clinicaltrials.gov #: NCT01683565). The study was prospectively reviewed and approved by the Institutional Review Board at Nationwide Children's Hospital (Columbus, OH, USA). Study enrollment took place between November 2012 and January 2015, and data collection for all participants was completed by March 2015. Each child's legal guardian/caregiver provided written informed consent for participation. Examination of behavior and sleep in this cohort was not a pre-specified outcome of the trial, but measures of these constructs were included in the study protocol from the beginning to enable exploration of treatment effects on these domains. No adverse events were found to be serious and related to the treatment. The full protocol, detailed adverse event data, analysis of fatty acid biomarkers, and the primary outcomes (ASD symptoms and other problem behaviors) which showed that children assigned to the treatment group exhibited a greater reduction in ASD symptoms, compared to those assigned to placebo are presented elsewhere [33].
2.2. Participants and sample size
Participants were children 18–38 months of calendar age who were 1) born at ≤29 completed weeks' gestation and 2) admitted to a Nationwide Children's Hospital NICU post birth and survived to discharge or 3) had a Neonatology Clinic follow up visit scheduled at Nationwide Children's Hospital.
Caregivers of potentially eligible children (n = 1121) were invited to complete a postal questionnaire (53% response proportion) that comprised the Pervasive Developmental Disorders Screening Test – II, Stage 2 (PDDST-II); the Brief Infant Toddler Social and Emotional Assessment (BITSEA); and one item from the Ages and Stages Questionnaire: Social Emotional (ASQ:SE) to assess joint attention (“Looks in the direction you are pointing, when you are pointing at something”) [34-36]. Children who exceeded pre-specified cutoffs (the Preemie Tots trial used more stringent criteria than recommended by instrument developers) on the PDDST-II (>5) or on the BITSEA competence scale (<15th percentile), or who did not respond to their name based on a single item from the BITSEA (caregivers responded not true/rarely to “Looks right at you when you say his/her name”), or did not participate in joint attention (caregiver responded not true/rarely to the ASQ:SE item) were considered to have significant ASD symptoms and were further assessed for trial eligibility (n = 201). All children exceeded the pre-specified cutoff on at least one measure, 32% exceeded the pre-specified cutoff on more than on measure.
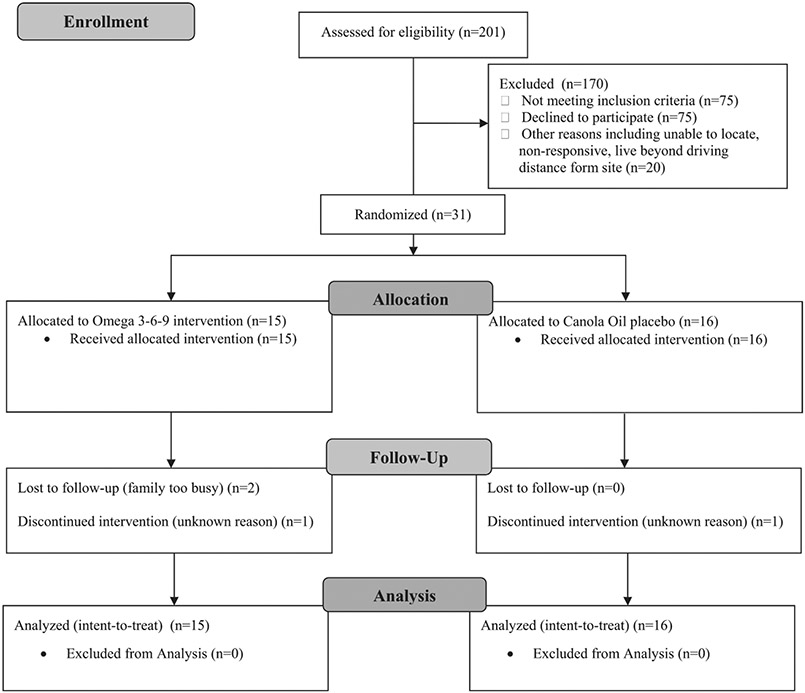
Additional eligibility criteria for the trial included: weight between the 5th and 95th percentiles for age and sex per the World Health Organization (WHO) growth standards (nexcluded = 20) and English language ability adequate to understand the study required of the caregiver (nexcluded = 13). Children were excluded if they consumed fatty acid supplements, fatty fish, or nutritional support beverages with DHA more than twice weekly (nexcluded = 2); were known to be unable to tolerate venipuncture (nexcluded = 2); or had any of the following conditions that would make the child unable to benefit from the intervention, difficult to assess, or put them at possible safety risk (nexcluded = 38): developmental syndromes (Fragile X, Rett, Angelman, Tuberous Sclerosis), deafness, blindness, a major medical condition or malformation that would preclude participation (e.g., feeding tube), a score of <70 on their most recent Bayley Scales of Infant and Toddler Development-3 (Bayley-3) cognitive clinical assessment during the previous year, quadriplegic cerebral palsy, fish or canola allergy, Type I diabetes, bleeding disorder, or a recent non-febrile seizure without a clear and resolved etiology. In addition, 20 children were unable to be located, were non-responsive to recruitment attempts, or lived beyond driving distance to the research site (Fig. 1). Children who met the eligibility criteria were invited to enroll in the clinical trial (n = 106). The goal was to enroll 40 children as this would be sufficient to confirm trial feasibility and estimate effect sizes for a full-scale trial; however, funding limitations capped enrollment at 31.
Fig. 1.
Participant CONSORT flow diagram, Preemie Tots trial (n = 31), 2012–2015.
2.3. Randomization, blinding and intervention
Children were allocated to one of four color-coded groups (two groups for treatment, two groups for placebo) using a randomization scheme with a varying block size of multiples of 4 and with 1:1 allocation to treatment and placebo. Four randomization groups were used to preserve blinding of treatment assignment as much as possible, should unblinding of a child have been necessary for emergency reasons. A statistician who had no contact with participants prepared the randomization scheme and assigned identification numbers. Sequentially numbered, opaque, sealed, tamper-resistant envelopes, each containing a unique identification number and treatment assignment were created before the study began. Immediately after enrollment, study staff opened the next sequentially numbered envelope to randomize children to treatment or placebo. All investigators, staff, and participants remained blinded throughout the trial. Because multiple births are common among preterm births, they are routinely included in clinical trials related to prematurity outcomes. This increases generalizability to the larger preterm population. As is commonly done, members of sets of twins were randomized together to the same group to reduce the likelihood of accidental treatment crossover and to increase the consent rate among families with multiples [37].
The treatment group was assigned to 90 days of daily oral omega 3-6-9 fatty acid supplementation in the form of a lemon-flavored fish and borage oil (706 mg total omega-3 fatty acids: 338 mg EPA, 225 mg DHA; 280 mg total omega-6 fatty acids: 83 mg GLA; and 306 mg total omega-9 fatty acids (oleic acid); Omega-3-6-9 JuniorTM, Nordic Naturals, Inc., Watsonville, CA). The placebo group was assigned to daily canola oil (124 mg palmitic acid, 39 mg stearic acid, 513 mg linoleic acid (LA), 225 mg α-linolenic acid (ALA); 1346 mg oleic acid). The placebo was prepared with lemon oil flavor by the Nationwide Children's Hospital Investigational Drug Service (IDS) to match the treatment product, and both products were packaged identically and distributed directly to the family by the hospital pharmacy. The 90-day timeframe was selected based on previous trials and to allow incorporation of DHA into neuronal cell membranes, while maximizing enrollment and retention of participants. Previous analysis of fatty acid biomarkers in this trial showed increases in concentrations of EPA, DHA, oleic acid, total n–3 FAs, and a trend toward an increase in dihomo-γ-linolenic acid (DGLA, downstream product of GLA) for the treatment group, confirming that the 90-day timeframe was sufficient [33].
2.4. Data collection
Data were collected in designated clinical research space at Nationwide Children's Hospital. The baseline study visit (day 0) included age-appropriate caregiver-completed questionnaires which collected demographics and caregiver ratings of the child's behavior and sleep. At the last study visit (day 90), behavior and sleep were assessed again and caregivers were asked to guess their child's treatment assignment. The child's electronic medical record was the source of gestational age and birthweight information.
2.5. Behavior
Caregivers indicated how true (not true (0), sometimes true (1), often true (2)) items on the Child Behavior Checklist 1.5–5 (CBCL) were of their child in the previous two months. Items were summed to derive scores on the CBCL Syndrome Scales (emotionally reactive, anxious/depressed, somatic complaints, withdrawn, sleep problems, attention problems, aggressive behavior), Problem Scales (internalizing, externalizing, total, stress), and Diagnostic and Statistical Manual of Mental Disorders (DSM)-oriented scales (affective problems, anxiety problems, pervasive developmental problems, attention deficit/hyperactivity problems, oppositional defiant problems) [38]. The CBCL has high test-retest reliability (>0.74) and discriminates between children referred and not referred for clinical follow-up [38]. Higher raw scores are indicative of more problems in the domain.
Caregivers reported how often their child engaged in various behaviors in the past month on the Toddler Behavior Assessment Questionnaire – Short Form (TBAQ-SF) [39]. The TBAQ-SF consists of 56 items that assess activity level, anger, inhibitory control, interest, pleasure, and social fearfulness. Caregivers rated each item on a scale from 1 (never) to 7 (always). The mean of all items from each domain represents the score on that domain. Higher scores represent more frequent behaviors associated with the domain.
Caregivers assessed the child's daily social functioning by completing the socialization domain of the Vineland Adaptive Behavior Scales, 2nd Edition (VABS-2), which comprises: play and leisure time, interpersonal relationships, and coping skills [40]. Each subdomain presents the caregiver with a series of behaviors of increasing developmental ability. Caregivers reported how often the child performed each behavior at the time of survey completion on a scale ranging from 0 (never) to 2 (usually). Three items from the coping skills subdomain were inadvertently omitted from the survey. Therefore, this subdomain was scored based on 27 items, rather than 30 items in the subdomain. Higher raw scores on each subdomain reflect a higher frequency of behavior in that subdomain.
2.6. Sleep
The caregiver-completed Children's Sleep Habits Questionnaire (CSHQ) assessed child sleep habits and difficulties during the previous week [41]. Caregivers reported the frequency of sleep behavior on a scale from 1 (rarely) to 3 (usually). Scores represent the mean of items in each domain: daytime sleepiness, night wakings, bedtime resistance, parasomnias, sleep anxiety, sleep onset delay, sleep disordered breathing, and sleep duration. Additionally, the mean of all items represents a total sleep score. Higher scores represent more sleep problems in the domain.
Caregivers reported the following sleep durations at the time of survey completion on the Brief Infant Sleep Questionnaire (BISQ): nocturnal sleep, daytime sleep, total sleep within a 24-h period, nocturnal sleep-onset, and wakefulness during night hours [42]. Caregiver reports on the BISQ were validated with actigraphy and sleep logs when the measure was developed [43].
2.7. Compliance and follow-up
Compliance with taking the supplement or placebo was assessed in two ways: caregiver-completed daily diaries (calculated as the proportion of days the supplement/placebo was consumed divided by the total days enrolled) and measurement of the amount of remaining oil in bottles returned to IDS (calculated as the amount of missing oil divided by the expected amount, similar to pill count method). Families were contacted regularly to encourage compliance and address participation barriers.
2.8. Statistical analysis
All analyses used SAS software (v9.4, SAS Institute) and were conducted according to intent-to-treat methods. No interim analyses were conducted. Analyses of treatment effects compared the change in behavior and sleep domains between groups, controlling for baseline characteristics, using a linear mixed model (analogous to ANCOVA), per the method of Winkens et al. which leverages maximum likelihood estimation to account for missing data for continuous variables [44]. Treatment-by-time interaction terms were included as fixed effects and served as estimates of treatment effect. Analyses included a random effect for the family to address statistical non-independence because of the inclusion of multiple-gestation births. Adjustments for multiple comparisons were not made, per published guidance [45,46]. Due to the potential for type I error due to multiple comparisons, results should be interpreted as exploratory. Group mean differences divided by the standard deviation were calculated as standardized effect sizes (Cohen's d), and interpreted as follows: 0.2 represents a small treatment effect, 0.5 a medium treatment effect, and 0.8 a large treatment effect [47]. No subgroup analyses were conducted.
3. Results
3.1. Participant characteristics
Baseline characteristics were similar between treatment groups (Table 1). The median age of children was 27 (IQR = 13) months at enrollment, the median gestational age was 27 (IQR = 4) completed weeks', the majority (68%) of children were male, and 68% scored below the pre-specified cutoff on one (verses 2 or more) screening tools. There were 4 sets of twins. All randomized children received their allocated intervention. Most respondents were mothers (97%) with a median age of 33 (IQR = 11) years at enrollment; 58% were married or living with a partner, 55% reported an annual household income of <$35,000 USD, and 35% had a high school/GED or lower level of education. Twenty-nine (94%) children randomized had data available for at least one outcome measure, 27 (87%) had outcome data for each measure.
Table 1.
Participant characteristics at baseline, Preemie Tots trial (n = 31), 2012–2015.
| Baseline characteristics | Omega 3-6-9 Group (n = 15) |
Placebo Group (n = 16) |
Full Sample (n = 31) |
Number of Missing Observations |
|---|---|---|---|---|
| Child characteristics | ||||
| Age at randomization, months, unadjusted for prematurity, median (interquartile range (IQR)) | 30 (11) | 25 (12) | 27 (13) | 0 |
| Gestational age at delivery, completed weeks, median (IQR) | 27 (3) | 28 (4) | 27 (4) | 0 |
| Sets of twins, n | 1 | 3 | 4 | 0 |
| Gender, male, n (%) | 8 (53) | 13 (81) | 21 (68) | 0 |
| Birthweight, grams, mean (standard deviation (SD)) | 940 (241) | 1021 (361) | 982 (306) | 2 |
| Race, n (%) – Caucasian/White | 8 (53) | 10 (62) | 18 (58) | 0 |
| African-American/Black | 4 (27) | 6 (38) | 10 (32) | – |
| Other or multiple races | 3 (20) | 0 (0) | 3 (10) | – |
| Ethnicity, n (%) – Hispanic or Latino | 3 (20) | 0 (0) | 3 (10) | 0 |
| Child diagnosed with ASD or other developmental disability prior to enrollment, n (%) | 4 (27) | 2 (13) | 6 (19) | 0 |
| Postal questionnaire screening results | ||||
| Responds to name (not true/rarely), n (%) | 1 (7) | 0 (0.00) | 1 (3) | 0 |
| Responds to joint attention (not true/rarely), n (%) | 1 (7) | 0 (0.00) | 1 (3) | 0 |
| Pervasive developmental disorders screening test – II, stage 2 (PDDST-II, >5), n (%) | 10 (67) | 10 (63) | 20 (65) | 0 |
| Brief Infant toddler social and emotional assessment competence (BITSEA, <15th percentile), n (%) | 9 (60) | 11 (69) | 20 (65) | 0 |
| Total number of screening measures failed (via postal questionnaire, max = 4), n (%) – 1 measure | 10 (67) | 11 (69) | 21 (68) | 0 |
| 2 measures | 4 (27) | 5 (31) | 9 (29) | – |
| 3 measures | 1 (7) | 0 (0) | 1 (3) | – |
| Caregiver and household characteristics | ||||
| Caregiver age at enrollment, median (IQR) | 34 (11) | 33 (9) | 33 (11) | 0 |
| Caregiver relationship to child, n (%) – Mother | 15 (100) | 15 (94) | 30 (97) | 0 |
| Caregiver marital status, n (%) – married or living with partner | 9 (60) | 9 (56) | 18 (58) | 0 |
| Separated | 1 (7) | 0 (0) | 1 (3) | – |
| Single never married or not living with partner | 5 (33) | 7 (44) | 12 (39) | – |
| Annual household income <$35,000 USD, n (%) | 10 (67) | 7 (44) | 17 (55) | 0 |
| Caregiver education, n (%) – high school/GED or less | 5 (33) | 6 (38) | 11 (35) | 0 |
| Some college/associate's degree | 5 (33) | 2 (13) | 7 (23) | – |
| Bachelor's degree or higher | 5 (33) | 8 (50) | 13 (42) | – |
3.2. Behavior
3.2.1. Baseline
At baseline, 71% (n = 22) of children scored in the borderline or clinical range (t-score > 65) on at least one CBCL scale. Children were reported as displaying inhibitory control (mean = 3.6, SD = 1.2) and interest (mean = 3.9, SD = 0.7) about half the time or less, while behaviors associated with the activity level (mean = 4.5, SD = 1.0), pleasure (mean = 4.3, SD = 1.1), anger (mean = 4.3, SD = 1.2), and social fearfulness (mean = 4.2, SD = 1.3) domains were reported as happening about half the time or more, per the TABQ-SF.
Baseline sleep characteristics as measured by the CSHQ reflected sleep behaviors in the rarely to sometimes range on all domains: bedtime resistance (median = 1.7, IQR = 1.3), night wakings (median = 1.3, IQR = 0.7), sleep anxiety (median = 1.5, IQR = 1.0), daytime sleepiness (median = 1.4, IQR = 0.4), parasomnias (median = 1.3, IQR = 0.3), sleep onset delay (median = 1.0, IQR = 2.0). sleep disordered breathing (median = 1.0, IQR = 0.3), and sleep duration (median = 1.0, IQR = 0.7). Children were reported to sleep 11.1 h (SD = 2.2) per 24 h, with approximately 8.9 h (SD = 1.7) comprising nocturnal sleep duration. Caregivers reported that it took 20 min (IQR = 45) to put the child to sleep, however, 45% reported that it took 30 min or more to put their child to sleep. Caregivers reported that children experienced about 20 min (IQR = 45) of night wakefulness, with 45% of caregivers reporting that their child spent one hour or more in night wakefulness.
3.2.2. Treatment effects
Changes in outcomes from baseline to the end of the trial are reported in Table 2. After 90 days of supplementation, children randomized to omega 3-6-9 were reported by their caregivers to experience a greater reduction in anxious and depressed behaviors (ΔDifference = −1.27, d = −0.58, p = 0.049), as well as internalizing problems (ΔDifference = −3.41, d = −0.68, p = 0.05) as measured by the CBCL, compared to placebo. The magnitude of these effects was medium. Children randomized to omega 3-6-9 were reported by their caregivers to experience a greater improvement in their interpersonal relationship skills (ΔDifference = 7.50, d = 0.83, p = 0.01), per the VABS-2. The magnitude of this effect was large. Several outcomes had non-statistically significant medium effects: changes in emotionally reactive behavior (ΔDifference = −1.46, d = −0.64, p = 0.06) on the CBCL; and pleasure (ΔDifference = 0.46, d = 0.68, p = 0.09), and social fearfulness (ΔDifference = −0.54, d = −0.63, p = 0.12) on the TBAQ-SF favored the omega 3-6-9 group; changes in night wakings (ΔDifference = 0.23, d = 0.59, p = 0.09) and sleep duration ((ΔDifference = 0.16, d = 0.53, p = 0.14) per the CSHQ favored placebo. Differences in change between the treatment and placebo groups on the CBCL DSM-oriented scales and the BISQ were not statistically significant, and effects were of small magnitude.
Table 2.
Change in outcomes from baseline to trial end, Preemie Tots trial (n = 31), 2012–2015.
| |
Baseline |
Trial end |
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Measure | N (Omega 3-6-9/ placebo) |
Omega 3-6- 9 |
Placebo | N (Omega 3-6-9/ placebo) |
Omega 3-6- 9 |
Placebo | Difference in change (95% CI)a |
Effect size (d) |
p- value |
| Child Behavior Checklist (CBCL) | |||||||||
| Higher scores = more behavior indicative of domain | |||||||||
| Syndrome scales | |||||||||
| Emotionally reactive | 15/16 | 3.73 (3.35) | 2.56 (2.19) | 12/16 | 2.42 (1.98) | 2.69 (3.22) | −1.46 (−3.01, 0.09) | −0.64 | 0.06 |
| Anxious/depressed | 15/16 | 3.40 (3.29) | 3.38 (2.68) | 12/16 | 2.42 (1.98) | 3.38 (2.39) | −1.27 (2.53, 0.00) | −0.58 | 0.049 |
| Somatic complaints | 15/16 | 2.73 (3.83) | 1.81 (2.32) | 12/16 | 1.58 (1.56) | 1.75 (2.54) | 0.53 (−1.49, 0.43) | −0.35 | 0.27 |
| Withdrawn | 15/16 | 2.80 (2.60) | 3.81 (3.12) | 12/16 | 2.42 (2.87) | 3.06 (2.77) | 0.36 (−0.88, 1.59) | 0.08 | 0.56 |
| Sleep problems | 15/16 | 3.53 (3.80) | 3.50 (3.50) | 12/16 | 3.17 (3.81) | 2.88 (2.53) | 0.57 (−1.02, 2.15) | 0.24 | 0.47 |
| Attention problems | 15/16 | 4.20 (2.68) | 4.38 (2.03) | 12/16 | 3.67 (3.08) | 4.06 (1.81) | −0.08 (−1.45, 1.29) | −0.15 | 0.91 |
| Aggressive behavior | 15/16 | 13.60 (9.79) | 13.75 (7.68) | 12/16 | 10.58 (8.67) | 11.38 (7.46) | −0.31 (−4.19, 3.56) | −0.05 | 0.87 |
| Problem scales | |||||||||
| Internalizing | 15/16 | 12.67 (10.24) | 11.56 (8.34) | 12/16 | 8.83 (7.04) | 10.69 (8.67) | −3.41 (−6.87, 0.06) | −0.68 | 0.05 |
| Externalizing | 15/16 | 17.80 (11.83) | 18.13 (8.66) | 12/16 | 14.25 (10.26) | 15.44 (8.29) | −0.35 (−4.58, 3.88) | −0.05 | 0.87 |
| Total problems | 15/16 | 48.67 (32.05) | 46.63 (21.61) | 12/16 | 37.92 (29.14) | 42.50 (22.64) | −5.42 (−16.44, 5.61) | −0.34 | 0.32 |
| Stress problems | 15/16 | 2.40 (2.20) | 1.94 (1.84) | 12/16 | 1.83 (1.85) | 2.06 (1.77) | −0.64 (1.63, 0.35) | −0.42 | 0.20 |
| DSM-oriented Scales | |||||||||
| Affective problems | 15/16 | 3.07 (3.24) | 2.75 (2.56) | 12/16 | 2.75 (2.96) | 2.56 (3.12) | −0.14 (−1.50, 1.22) | −0.05 | 0.83 |
| Anxiety problems | 15/16 | 4.40 (3.44) | 3.94 (2.72) | 12/16 | 3.67 (2.99) | 4.00 (2.13) | −0.74 (−1.96, 0.49) | −0.37 | 0.23 |
| Pervasive developmental problems | 15/16 | 5.87 (5.10) | 6.44 (3.81) | 12/16 | 4.58 (4.40) | 5.25 (4.11) | −0.39 (−2.68, 1.89) | −0.14 | 0.73 |
| Attention deficit/hyperactivity problems | 15/16 | 6.13 (3.74) | 6.94 (2.52) | 12/16 | 4.92 (4.19) | 5.94 (2.64) | −0.17 (2.09, 1.75) | −0.07 | 0.86 |
| Oppositional defiant problems | 15/16 | 4.00 (3.44) | 4.44 (3.18) | 12/16 | 3.50 (3.63) | 3.50 (2.99) | 0.35 (−1.2, 2.21) | 0.13 | 0.71 |
| Toddler Behavior Assessment Questionnaire – Short Form (TBAQ-SF) | |||||||||
| Higher scores = more behavior indicative of domain | |||||||||
| Activity level | 15/16 | 4.96 (0.94) | 4.08 (0.97) | 13/15 | 4.69 (1.93) | 4.08 (1.03) | −0.11 (−0.57, 0.36) | −0.24 | 0.64 |
| Anger | 15/16 | 4.42 (1.45) | 4.17 (0.86) | 13/15 | 4.18 (1.00) | 4.24 (1.04) | −0.13 (−0.78, 0.53) | −0.11 | 0.70 |
| Inhibitory control | 15/16 | 3.32 (1.25) | 3.84 (1.13) | 13/15 | 3.67 (0.90) | 3.80 (0.91) | 0.07 (−0.58, 0.7) | 0.04 | 0.83 |
| Interest | 15/16 | 3.86 (0.76) | 4.00 (0.74) | 13/15 | 3.96 (0.93) | 4.07 (0.72) | −0.02 (−0.44, 0.41) | −0.03 | 0.94 |
| Pleasure | 15/16 | 4.58 (1.16) | 4.05 (1.00) | 13/15 | 5.22 (0.78) | 4.20 (1.22) | 0.46 (−0.08, 1.01) | 0.68 | 0.09 |
| Social fearfulness | 15/16 | 3.95 (1.44) | 4.52 (1.01) | 13/15 | 3.90 (1.22) | 4.77 (1.29) | −0.54 (−1.22, 0.15) | −0.63 | 0.12 |
| Vineland Adaptive Behavior Scales, 2nd Edition (VABS-2) | |||||||||
| Higher scores = more behavior indicative of domain | |||||||||
| Play and leisure time | 15/13 | 17.13 (8.26) | 17.92 (11.67) | 12/14 | 21.23 (8.50) | 19.57 (6.58) | 1.39 (−4.51, 7.29) | 0.20 | 0.63 |
| Interpersonal relationships | 15/15 | 29.07 (11.34) | 26.87 (9.66) | 13/15 | 37.23 (8.76) | 28.60 (7.66) | 7.50 (1.63, 13.67) | 0.83 | 0.01 |
| Coping skills | 15/13 | 8.33 (6.69) | 6.23 (3.24) | 13/14 | 9.83 (6.97) | 8.14 (5.49) | 1.11 (−2.38, 4.60) | 0.16 | 0.52 |
| Children's Sleep Habits Questionnaire (CSHQ) | |||||||||
| Higher scores = more disturbed sleep behavior in domain | |||||||||
| Daytime sleepiness | 15/16 | 1.48 (0.37) | 1.47 (0.26) | 12/15 | 1.46 (0.37) | 1.36 (0.26) | 0.06 (−0.13, 0.25) | 0.22 | 0.55 |
| Night wakings | 15/16 | 1.56 (0.54) | 1.65 (0.46) | 12/15 | 1.53 (0.59) | 1.44 (0.41) | 0.23 (−0.04, 0.51) | 0.59 | 0.09 |
| Bedtime resistance | 15/16 | 1.61 (0.71) | 1.69 (0.57) | 12/15 | 1.46 (0.52) | 1.61 (0.58) | −0.02 (−0.31, 0.28) | −0.08 | 0.91 |
| Parasomnias | 15/16 | 1.32 (0.22) | 1.48 (0.32) | 12/15 | 1.33 (0.19) | 1.32 (0.17) | 0.07 (−0.05, 0.19) | 0.29 | 0.25 |
| Sleep anxiety | 15/16 | 1.52 (0.51) | 1.61 (0.44) | 12/15 | 1.52 (0.57) | 1.42 (0.40) | 0.18 (−0.11, 0.46) | 0.41 | 0.22 |
| Sleep onset delayb | 15/16 | 1.60 (0.21) | 1.81 (0.91) | 12/15 | 1.75 (0.97) | 1.73 (0.88) | 0.27 (−0.21, 0.75) | 0.43 | 0.26 |
| Sleep disordered breathing | 15/16 | 1.16 (0.21) | 1.29 (0.36) | 12/15 | 1.13 (0.24) | 1.18 (0.31) | −0.02 (−0.20, 0.17) | −0.08 | 0.87 |
| Sleep duration | 15/16 | 1.38 (0.64) | 1.40 (0.60) | 12/15 | 1.33 (0.57) | 1.13 (0.25) | 0.16 (−0.06, 0.38) | 0.53 | 0.14 |
| Total score | 15/16 | 1.47 (0.26) | 1.54 (0.28) | 12/15 | 1.43 (0.28) | 1.40 (0.16) | 0.07 (−0.05, 0.18) | 0.38 | 0.25 |
| Brief Infant Sleep Questionnaire (BISQ, minutes) | |||||||||
| Higher scores = more time spent in domain | |||||||||
| Nocturnal sleep duration | 15/16 | 518.07 (121.62) | 545.63 (88.47) | 12/15 | 541.25 (118.02) | 540.00 (57.82) | 16.51 (−26.83, 59.85) | 0.26 | 0.44 |
| Daytime sleep duration | 15/16 | 116.47 (100.92) | 148.13 (82.32) | 12/15 | 130.00 (59.08) | 149.07 (117.61) | −18.59 (−79.99, 42.80) | −0.10 | 0.54 |
| Total sleep duration | 15/16 | 634.53 (151.84) | 693.75 (102.69) | 12/15 | 671.25 (95.11) | 689.07 (147.44) | −9.92 (−93.14, 73.21) | −0.03 | 0.81 |
| Sleep onset duration | 15/16 | 29.47 (30.44) | 38.63 (25.08) | 12/15 | 27.50 (23.98) | 31.93 (33.09) | 6.53 (−15.98, 29.05) | 0.07 | 0.56 |
| Night wakefulness | 15/16 | 39.67 (74.89) | 64.06 (62.24) | 12/15 | 38.33 (53.24) | 27.47 (42.92) | 23.37 (−8.60, 55.34) | 0.39 | 0.15 |
Difference in change column is based on mixed effects model (analogous to ANCOVA) using maximum likelihood to account for missing data and thus may differ from the results of the raw summary statistics for the within-group mean change.
The variance of this model with the random effect for the family unit within the mixed model was estimated to be zero; therefore, effect for the family unit was removed from the model.
3.2.3. Compliance and blinding
Compliance was high: children consumed 83% of the prescribed dose based on caregiver-completed diaries and IDS volume calculations. At the end of the trial, 70% of caregivers in the treatment group and 57% of those in the placebo group did not accurately guess their child's treatment assignment, suggesting blinding remained intact (McNemar's χ2 = 1.00, p = 0.32).
4. Discussion
In this randomized, double blind, placebo-controlled trial of omega 3-6-9 fatty acids in an at-risk preterm sample showing symptoms commonly associated with ASD, we found that supplementation for 90 days resulted in greater reduction of anxious and depressed and internalizing behaviors, compared to placebo. Additionally, children randomized to omega 3-6-9 were reported to have significantly greater gains in interpersonal relationship skills, compared to placebo. Effects are of medium-to-large magnitude. No statistically significant effects of supplementation were found on other aspects of behavior or sleep. Combined with other results from the Preemie Tots trial, which report benefit of supplementation on behaviors commonly associated with ASD and gesture use without negative effects on behaviors that have been reported in omega-3 only trials, a combination of omega-3-6-9 fatty acids in toddlerhood and early childhood may provide benefit for children born preterm who are experiencing symptoms commonly seen with ASD, however, results need to be replicated in a larger trial [33,48].
Children randomized to omega 3-6-9 were reported to have nearly a 1-point decrease in the raw score of the anxious/depressed syndrome scale on the CBCL, compared to no change in the placebo group. Although both groups experienced improvements in internalizing behaviors (comprised emotionally reactive, anxious/depressed, somatic complaints, withdrawn behaviors) and interpersonal relationships (including responding to others, expressing and recognizing emotions, imitating, social communication, thoughtfulness, friendship), which may be explained by age-related development, benefits experienced by the treatment group were statistically significantly more pronounced: 3.84 vs 0.87 reduction in internalizing behaviors per the CBCL and 8.16 vs 1.73 improvement in interpersonal relationship skills per the VABS-2, suggesting supplementation may enhance age-related development in these domains. The interpersonal relationship score of children in the treatment group after 90 days of supplementation is characterized as representing an “adequate” adaptive level according to instrument developers, compared to a “moderately low” adaptive level that corresponds to the interpersonal relationship score of the placebo group after 90 days of supplementation [40]. Studies of children under 5 years with ASD report mixed effects of supplementation on externalizing behaviors. While one study showed benefit of DHA supplementation on externalizing behaviors, another reported that DHA + EPA supplementation had a negative effect on externalizing behaviors [27,28]. This study did not observe an effect of supplementation on externalizing behaviors per the CBCL, and previous work in this cohort reported no effect of supplementation on externalizing behaviors per the BITSEA [33].
Reviews of research involving children aged two to eight years old showed no benefit of omega-3 supplementation for improving behaviors such as internalizing, adaptive functioning, and hyperactivity in children with ASD [49-52]. A key difference between previous trials of young children (i.e., age 8 years and younger) and the Preemie Tots trial is the combination of long-chain polyunsaturated fatty acids used. However, three uncontrolled trials that included children with ASD up to age 18 years, included omega-3 and omega-6 fatty acids, similar to the Preemie Tots trial. A trial of children aged three to ten years old which supplemented their diets for 3 months with DHA (247 mg) and GLA (40 mg) reported improvements in a wide range of learning and language skills [53]. A second trial of children aged three to 11 years old with ASD that supplemented children for 3 months with DHA (240 mg), EPA (52 mg), GLA (48 mg), and AA (20 mg) reported that 67% of the children showed statistically significant reduction in autism severity [54]. A third trial examined the effect of DHA (840 mg), EPA (192 mg), GLA (144 mg), and AA (66 mg) supplementation in children aged seven to 18 years for three months reported statistically significant improvements across several domains of social responsiveness and attention [55]. The non-randomized design of these trials makes it difficult to attribute the effects to the treatment, and the wide age ranges of children treated limits an understanding of the possible effects during early childhood when intervention may be most impactful. The Preemie Tots trial builds on this information by testing a promising fatty acid combination within a narrower and younger age range using a randomized placebo-controlled design at a developmental period that is particularly amendable to intervention.
Results of the Preemie Tots trial may support early supplementation with a combination of omega 3-6-9 for children born preterm who are showing early behaviors commonly associated with ASD. Supplementing the diets at this young age may be advantageous because this developmental period is critical for and receptive to early intervention. Additionally, nearly 80% of caregivers of children with ASD report the use of at least one dietary supplement; with omega-3 supplementation being among the most common [56]. The omega 3-6-9 supplement used in the Preemie Tots trial resulted in medium magnitude reduction of anxious and depressed behaviors, as well as internalizing problems. Additionally, there was a large magnitude improvement in interpersonal relationship skills for children in the treatment group, a developmental area for which there is currently no pharmacologic intervention. The findings of this trial, along with the high use of nutritional supplements in this population and the low risk profile of long chain polyunsaturated fatty acids suggests that readily available, cost-effective nutritional interventions such omega 3-6-9 supplements for children may be an effective treatment for children at risk for developmental challenges in early childhood. However, given the small sample size and exploratory nature of this trial, results need to be replicated in a larger sample to make any definitive clinical recommendations.
Other important methodological differences exist between the Preemie Tots trial and others which focused on the early childhood developmental period in children with ASD. The Preemie Tots trial supplemented children ranging from age 18 to 38 months, whereas other trials initiated supplementation later, included larger age ranges of children, and/or provided supplementation for a different period of time. One trial which compared a DHA supplement to a low sugar diet began supplementation at 41.7 months, on average [27]. Four placebo-controlled trials of omega-3 supplementation enrolled children between the ages of 2–5 years, 2–8 years, 3–8 years, or 5–8 years [26,28,30,31]. The placebo-controlled trials supplemented children's' diets for 6 weeks, 3 months, 6 months, or 12 months; whereas the omega-3 and low sugar diet included 3 months of supplementation. Another notable difference is that although some children in the Preemie Tots trial had an ASD diagnosis at enrollment, it was not an inclusion criterion, therefore the range of symptom severity may have been larger than that in trials that included only children with an ASD diagnosis, and thus direct comparisons to studies that included only children with ASD cannot be made. Collectively, these key differences make comparisons across studies difficult and may help explain the benefits of omega 3-6-9 supplementation on aspects of behavior reported here that have not been previously reported in cohorts of young children with ASD.
This study has limitations. This study relied on caregiver-reports of behavior and sleep, therefore, caregiver bias cannot be ruled out. Although, caregivers were blinded and poor at guessing their child's treatment assignment, the study did not collect information as to why caregivers thought their child was in which group. The sample size for this pilot study was small and limits the generalizability of the findings to larger samples of children born preterm. The benefit for children randomized to omega 3-6-9 compared to placebo may be a chance effect due to multiple comparisons. The Preemie Tots trial focused on children born preterm who were demonstrating early symptoms commonly associated with ASD, rather than children who had an ASD diagnosis. This may limit the generalizability of the findings to all children with ASD. Each of the outcome measures had a different reporting timeframe, per standardized instructions, which may explain the lack of significant findings across all measures. Finally, three items from the coping skills subdomain of the VABS-2 were inadvertently omitted from the survey. Although this may present a challenge in comparing this outcome to the current literature in a descriptive nature, the focus of this paper was to examine change in outcomes from baseline to the end of the trial. Therefore, the results are interpretable in terms of treatment effects on coping skills.
The trial has several strengths. Participants were recruited from a roster of all children who required NICU care. As such, our source population was not subject to participation bias which may be seen in studies that rely purely on volunteers or patients of neonatal follow-up clinics and our findings may be more generalizable to the larger population of children born at ≤29 weeks' gestation, especially those demonstrating symptoms commonly associated with ASD. Treatment compliance in this cohort was greater than 80%, based on multiple reporting methods, illustrating that this is a feasible approach for young children.
Children born very preterm (<29 completed weeks' gestation) experience significant long-term difficulties in internalizing and competence behaviors compared to those born at term [4]. Although previous studies of omega-3 supplementation do not clearly support supplementation in children with ASD, the current trial which used a combination of omega 3-6-9 fatty acids found potential benefit of supplementation for children showing symptoms commonly associated with ASD at a young age, often before a diagnosis is feasible. The present study may provide preliminary support for further exploration of omega 3-6-9 during toddlerhood to improve a range of socioemotional outcomes among infants born preterm, especially those who are showing early symptoms associated with ASD. However, due to the exploratory nature of these outcomes and the small sample size, results should not be interpreted as definitive and need to be replicated in a larger sample.
Acknowledgements
We thank the participants; Nationwide Children's Hospital Division of Neonatology; Nationwide Children's Hospital Investigational Drug Service; Nordic Naturals; Welsh, Holme, & Clark Co., Inc. as well as the entire Preemie Tots Research Team: Yvette Bean, Anne Brown, Kendra Heck, Chenali Jayadeva, Julia Less, Sarah Landry, and Kanima Smith for data collection and administrative support. This work was supported by grants from The Marci and Bill Ingram Fund for Autism Spectrum Disorders Research, Cures Within Reach, Award Number Grant UL1TR001070 from the National Center For Advancing Translational Sciences/NIH, and internal support from the Abigail Wexner Research Institute at Nationwide Children’s Hospital. Nordic Naturals, Inc., provided the investigational product at no cost; and Welsh, Holme, & Clark Co., Inc., provided canola oil at no cost. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Advancing Translational Sciences or the National Institutes of Health. Neither the study sponsors nor product providers had a role in the study design; the collection, analysis, and interpretation of data; writing of this report; or the decision to submit the manuscript for publication.
Footnotes
CRediT authorship contribution statement
Kelly M. Boone: Validations, Formal Analysis, Investigation, Data Curation, Writing – Original Draft, Visualization, Project Administration.
Dr. Mark A. Klebanoff: Conceptualization, Methodology, Writing – Review & Editing, Funding Acquisition.
Dr. Lynette K. Rogers: Conceptualization, Methodology, Writing – Review & Editing, Funding Acquisition.
Dr. Joseph Rausch: Methodology, Formal Analysis, Writing – Review & Editing.
Dr. Daniel L. Coury: Writing – Review & Editing, Supervision.
Dr. Sarah A. Keim: Conceptualization, Methodology, Formal Analysis, Investigation, Data Curation, Writing – Review & Editing, Supervision, Project Administration, Funding Acquisition.
Declaration of competing interest
Daniel L. Coury, MD reports the following disclosures: research grant support from GW Biosciences, Stalicla and Stemina; and service on advisory boards for BioRosa, Cognoa, GW Biosciences, MaraBio, Quadrant, and Stalicla. Kelly M. Boone, Mark A. Klebanoff, Lynette K. Rogers, Joseph Rausch, and Sarah A. Keim have no conflicts of interest relevant to this work to disclose.
References
- [1].Centers for Disease Control and Prevention, Preterm birth. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm, 2020. (accessed 31 August 2021).
- [2].Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ, Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis, J. Am. Med. Assoc 288 (2002) 728–737, 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- [3].Luu TM, Rehman Mian MO, Nuyt AM, Long-term impact of preterm birth: neurodevelopmental and physical health outcomes, Clin. Perinatol 44 (2017) 305–314, 10.1016/j.clp.2017.01.003. [DOI] [PubMed] [Google Scholar]
- [4].Spittle AJ, Treyvaud K, Doyle LW, Roberts G, Lee KJ, Inder TE, Cheong JLY, Hunt RW, Newnham CA, Anderson PJ, Early emergence of behavior and social-emotional problems in very preterm infants, J. Am. Acad. Child Adolesc. Psychiatry 48 (2009) 909–918, 10.1097/CHI.0b013e3181af8235. [DOI] [PubMed] [Google Scholar]
- [5].Twilhaar ES, de Kieviet JF, Aarnoudse-Moens CS, van Elburg RM, Oosterlaan J, Academic performance of children born preterm: a meta-analysis and meta-regression, Arch. Dis. Child Fetal Neonatal Ed 103 (2017) F322–F330, 10.1136/archdischild-2017-312916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yiallourou SR, Arena BC, Wallace EM, Odoi A, Hollis S, Weichard A, Horne RSC, Being born too small and too early may alter sleep in childhood, Sleep 41 (2017), zsx193, 10.1093/sleep/zsx193. [DOI] [PubMed] [Google Scholar]
- [7].Linsell L, Johnson S, Wolke D, Morris J, Kurinczuk JJ, Marlow N, Trajectories of behavior, attention, social and emotional problems from childhood to early adulthood following extremely preterm birth: a prospective cohort study, Eur. Child Adolesc. Psychiatry 28 (2019) 531–542, 10.1007/s00787-018-1219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Loe IM, Heller NA, Chatav M, Behavior problems and executive function impairments in preterm compared to full term preschoolers, Early Hum. Dev 130 (2019) 87–95, 10.1016/j.earlhumdev.2019.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Agrawal S, Rao SC, Bulsara MK, Patole SK, Prevalence of autism spectrum disorder in preterm infants: a meta-analysis, Pediatrics 142 (2018), e20180134, 10.1542/peds.2018-0134. [DOI] [PubMed] [Google Scholar]
- [10].Kuzniewicz MW, Wi S, Qian Y, Walsh EM, Armstrong MA, Croen LA, Prevalence and neonatal factors associated with autism spectrum disorders in preterm infants, J. Pediatr 164 (2014) 20–25, 10.1016/j.jpeds.2013.09.021. [DOI] [PubMed] [Google Scholar]
- [11].Wang C, Geng H, Liu W, Zhang G, Prenatal, perinatal, and postnatal factors associated with autism: a meta-analysis, Medicine 96 (2017), e6696, 10.1097/md.0000000000006696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shah PE, Kaciroti N, Richards B, Lumeng JC, Gestational age and kindergarten school readiness in a national sample of preterm infants, J. Pediatr 178 (2016) 61–67, 10.1016/j.jpeds.2016.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Myrhaug HT, Brurberg KG, Hov L, Markestad T, Survival and impairment of extremely premature infants: a meta-analysis, Pediatrics 143 (2019), e20180933, 10.1542/peds.2018-0933. [DOI] [PubMed] [Google Scholar]
- [14].Bazan NG, Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors, Trends Neurosci. 29 (2006) 263–271, 10.1016/j.tins.2006.03.005. [DOI] [PubMed] [Google Scholar]
- [15].Coti Bertrand P., O'Kusky JR, Innis SM, Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain, J. Nutr 136 (2006) 1570–1575, 10.1093/jn/136.6.1570. [DOI] [PubMed] [Google Scholar]
- [16].Kitajka K, Puskas LG, Zvara A, Hackler L Jr., Barcelo-Coblijn G, Yeo YK, Farkas T, The role of n-3 polyunsaturated fatty acids in brain: modulation of rat brain gene expression by dietary n-3 fatty acids, Proc. Natl. Acad. Sci. U. S. A 99 (2002) 2619–2624, 10.1073/pnas.042698699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Salem N, Litman B, Kim HY, Gawrisch K, Mechanisms of action of docosahexaenoic acid in the nervous system, Lipids 36 (2001) 945–959, 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- [18].Simopoulos AP, Omega-3 fatty acids in inflammation and autoimmune diseases, J. Am. Coll. Nutr 21 (2002) 495–505, 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- [19].Innis SM, de la Presa Owens S, Dietary fatty acid composition in pregnancy alters neurite membrane fatty acids and dopamine in newborn rat brain, J. Nutr 131 (2001) 118–122, 10.1093/jn/131.1.118. [DOI] [PubMed] [Google Scholar]
- [20].Janssen CIF, Kiliaan AJ, Long-chain polyunsaturated fatty acids (lcpufa) from genesis to senescence: the influence of lcpufa on neural development, aging, and neurodegeneration, Prog. Lipid Res 53 (2014) 1–17, 10.1016/j.plipres.2013.10.002. [DOI] [PubMed] [Google Scholar]
- [21].Shaw DI, Hall WL, Jeffs NR, M WC, comparative effects of fatty acids on endothelial inflammatory gene expression, Eur. J. Nutr 46 (2007) 321–328, 10.1007/s00394-007-0669-4. [DOI] [PubMed] [Google Scholar]
- [22].Alagawany M, Elnesr SS, Farag MR, El-Sabrout K, Alqaisi O, Dawood MAO, Soomro H, Abdelnour SA, Nutritional significance and health benefits of omega-3, -6 and -9 fatty acids in animals, Anim. Biotechnol (2021) 1–13, 10.1080/10495398.2020.1869562. [DOI] [PubMed] [Google Scholar]
- [23].Johnson M, Bradford CN, Omega-3, omega-6 and omega-9 fatty acids: implications for cardiovascular and other diseases, J. Glycomics Lipidomics 4 (2014) 1–8. [Google Scholar]
- [24].Innis SM, Essential fatty acid transfer and fetal development. Placenta 26 (Suppl A) (2005) S70–S75, 10.1016/j.placenta.2005.01.005. [DOI] [PubMed] [Google Scholar]
- [25].Mazahery H, Stonehouse W, Delshad M, Kruger MC, Conlon CA, Beck KL, von Hurst PR, Relationship between long chain n-3 polyunsaturated fatty acids and autism spectrum disorder: systematic review and meta-analysis of case-control and randomised controlled trials, Nutrients 9 (2017), 10.3390/nu9020155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bent S, Hendren RL, Zandi T, Law K, Choi JE, Widjaja F, Kalb L, Nestle J, Law P, Internet-based, randomized, controlled trial of omega-3 fatty acids for hyperactivity in autism, J. Am. Acad. Child Adolesc. Psychiatry 53 (2014) 658–666, 10.1016/j.jaac.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Johnson CR, Handen BL, Zimmer M, Sacco K, Polyunsaturated fatty acid supplementation in young children with autism, J. Dev. Phys. Disabil 22 (2010) 1–10, 10.1007/s10882-009-9152-x. [DOI] [Google Scholar]
- [28].Mankad D, Dupuis A, Smile S, Roberts W, Brian J, Lui T, Genore L, Zaghloul D, Iaboni A, Marcon PM, Anagnostou E, A randomized, placebo controlled trial of omega-3 fatty acids in the treatment of young children with autism, Mol Autism. 6 (2015) 18, 10.1186/s13229-015-0010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mazahery H, Conlon CA, Beck KL, Mugridge O, Kruger MC, Stonehouse W, Camargo CA Jr., Meyer BJ, Jones B, von Hurst PR, A randomised controlled trial of vitamin d and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder, J. Steroid Biochem. Mol. Biol 187 (2019) 9–16, 10.1016/j.jsbmb.2018.10.017. [DOI] [PubMed] [Google Scholar]
- [30].Mazahery H, Conlon CA, Beck KL, Mugridge O, Kruger MC, Stonehouse W, Camargo CA Jr., Meyer BJ, Tsang B, Jones B, von Hurst PR, A randomised-controlled trial of vitamin d and omega-3 long chain polyunsaturated fatty acids in the treatment of core symptoms of autism spectrum disorder in children, J. Autism Dev. Disord 49 (2019) 1778–1794, 10.1007/s10803-018-3860-y. [DOI] [PubMed] [Google Scholar]
- [31].Bent S, Bertoglio K, Ashwood P, Bostrom A, Hendren RL, A pilot randomized controlled trial of omega-3 fatty acids for autism spectrum disorder, J. Autism Dev. Disord 41 (2011) 545–554, 10.1007/s10803-010-1078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Meiri G, Bichovsky Y, Belmaker RH, Omega 3 fatty acid treatment in autism, J. Child Adolesc. Psychopharmacol 19 (2009) 449–451, 10.1089/cap.2008.0123. [DOI] [PubMed] [Google Scholar]
- [33].Keim SA, Gracious B, Boone KM, Klebanoff MA, Rogers LK, Rausch J, Coury DL, Sheppard KW, Husk J, Rhoda DA, Ω-3 and ω-6 fatty acid supplementation may reduce autism symptoms based on parent report in preterm toddlers, J. Nutr 148 (2018) 227–235, 10.1093/jn/nxx047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Briggs-Gowan MJ, Carter AS, The Brief Infant-Toddler Social and Emotional Assessment (BITSEA) Examiner’s Manual, Harcourt Assessment, Inc., San Antonio, TX, 2006. [Google Scholar]
- [35].Siegel B, Pervasive Developmental Disorders Screening Test-II (PDSDST-II): Early Childhood Screener for Autistic Spectrum Disorders, Harcourt Assessment, Inc., San Antonio, TX, 2004. [Google Scholar]
- [36].Squires J, Bricker D, Twombly E, Ages & Stages Questionnaires®: Social-Emotional (ASQ:SE), Paul H. Brookes Publishing Co., Inc., Baltimore, MD, 2002. [Google Scholar]
- [37].Bernardo J, Nowacki A, Martin R, Fanaroff JM, Hibbs AM, Multiples and parents of multiples prefer same arm randomization of siblings in neonatal trials, J. Perinatol 35 (2015) 208–213, 10.1038/jp.2014.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Achenbach TM, Rescorla LA, Manual for the ASEBA Preschool Forms and Profiles, University of Vermont, Research Center for Children, Youth, & Families, Burlington, VT, 2000. [Google Scholar]
- [39].Goldsmith HH, Studying temperament via construction of the toddler behavior assessment questionnaire. Child Dev. 67 (1996) 218–235. [PubMed] [Google Scholar]
- [40].Sparrow SS, Cicchetti D, Balla DA, Vineland Adaptive Behavior Scales, 2nd ed., American Guidance Service, Circle Pines, MN, 2005. [Google Scholar]
- [41].Owens JA, Spirito A, McGuinn M, The Children's Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children, Sleep 23 (2000) 1043–1051. [PubMed] [Google Scholar]
- [42].Sadeh A, A brief screening questionnaire for infant sleep problems: validation and findings for an internet sample, Pediatrics 113 (2004) e570–e577, 10.1542/peds.113.6.e570. [DOI] [PubMed] [Google Scholar]
- [43].Huang YS, Paiva T, Hsu JF, Kuo MC, Guilleminault C, Sleep and breathing in premature infants at 6 months post-natal age, BMC Pediatr. 14 (2014) 303, 10.1186/s12887-014-0303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Winkens B, van Breukelen GJ, Schouten HJ, Berger MP, Randomized clinical trials with a pre- and a post-treatment measurement: repeated measures versus ancova models, Contemp. Clin. Trials 28 (2007) 713–719, 10.1016/j.cct.2007.04.002. [DOI] [PubMed] [Google Scholar]
- [45].Rothman KJ, Six persistent research misconceptions, J. Gen. Intern. Med 29 (2014) 1060–1064, 10.1007/s11606-013-2755-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Rothman KJ, No adjustments are needed for multiple comparisons, Epidemiology 1 (1990) 43–46. [PubMed] [Google Scholar]
- [47].Cohen J, Statistical Power Analysis for the Behavioral Sciences, 2nd ed., Erlbaum, Hillsdale, NJ, 1998. [Google Scholar]
- [48].Sheppard KW, Boone KM, Gracious B, Klebanoff MA, Rogers LK, Rausch J, Bartlett C, Coury DL, Keim SA, Effect of omega-3 and -6 supplementation on language in preterm toddlers exhibiting autism spectrum disorder symptoms, J. Autism Dev. Disord 47 (2017) 3358–3369, 10.1007/s10803-017-3249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].James S, Montgomery P, Williams K, Omega-3 fatty acids supplementation for autism spectrum disorders (ASD), Cochrane Database Syst. Rev (2011), Cd007992, 10.1002/14651858.CD007992.pub2. [DOI] [PubMed] [Google Scholar]
- [50].Karhu E, Zukerman R, Eshraghi RS, Mittal J, Deth RC, Castejon AM, Trivedi M, Mittal R, Eshraghi AA, Nutritional interventions for autism spectrum disorder, Nutr. Rev 78 (2020) 515–531, 10.1093/nutrit/nuz092. [DOI] [PubMed] [Google Scholar]
- [51].Sathe N, Andrews JC, McPheeters ML, Warren ZE, Nutritional and dietary interventions for autism spectrum disorder: a systematic review, Pediatrics 139 (2017), 10.1542/peds.2017-0346. [DOI] [PubMed] [Google Scholar]
- [52].Williamson E, Sathe NA, Andrews JC, Krishnaswami S, McPheeters ML, Fonnesbeck C, Sanders K, Weitlauf A, Warren Z, AHRQ comparative effectiveness reviews, in medical therapies for children with autism spectrum disorder—an update. 2017, in: Agency for Healthcare Research and Quality (US), Rockville (MD), 2018. [PubMed] [Google Scholar]
- [53].Patrick L, Salik R, The effect of essential fatty acid supplementation on language development and learning skills in autism and Asperger's syndrome, Autism Asperger's Digest. (2005) 36–37. Jan–Feb. [Google Scholar]
- [54].Meguid NA, Atta HM, Gouda AS, Khalil RO, Role of polyunsaturated fatty acids in the management of Egyptian children with autism, Clin. Biochem 41 (2008) 1044–1048, 10.1016/j.clinbiochem.2008.05.013. [DOI] [PubMed] [Google Scholar]
- [55].Ooi YP, Weng SJ, Jang LY, Low L, Seah J, Teo S, Ang RP, Lim CG, Liew A, Fung DS, Sung M, Omega-3 fatty acids in the management of autism spectrum disorders: Findings from an open-label pilot study in Singapore, Eur. J. Clin. Nutr 69 (2015) 969–971, 10.1038/ejcn.2015.28. [DOI] [PubMed] [Google Scholar]
- [56].Trudeau MS, Madden RF, Parnell JA, Gibbard WB, Shearer J, Dietary and supplement-based complementary and alternative medicine use in pediatric autism spectrum disorder, Nutrients 11 (2019) 1, 10.3390/nu11081783. [DOI] [PMC free article] [PubMed] [Google Scholar]