Abstract
Beginning with the outbreak of COVID-19 at the dawn of 2020, the continuing spread of the pandemic has challenged the healthcare market and the supply chain of Personal Protective Equipment (PPE) around the world. Moreover, the emergence of the variants of COVID-19 occurring in waves threatens the sufficient supply of PPE. Among the various types of PPE, N95 Respirators, surgical masks, and medical gowns are the most consumed and thus have a high potential for a serious shortage during such emergencies. Considering the unanticipated demand for PPE during a pandemic, re-processing of used PPE is one approach to continue to protect the health of first responders and healthcare personnel. This paper evaluates the viability and efficacy of using FDA-approved electron beam (eBeam) sterilization technology (ISO 11137) to re-process used PPE. PPEs including 3M N95 Respirators, Proxima Sirus gowns, and face shields were eBeam irradiated in different media (air, argon) over a dose range of 0–200 kGy. Several tests were then performed to examine surface properties, mechanical properties, functionality performance, discoloration phenomenon, and liquid barrier performance. The results show a reduction of filtration efficiency to about 63.6% in the N95 Respirator; however, charge regeneration may improve the re-processed efficiency. Additionally, mechanical degradation was observed in Proxima Sirus gown with increasing dose up to 100 kGy. However, no mechanical degradation was observed in the face shields after 10 times donning and doffing. Apart from the face shield, N95 Respirators and Proxima Sirus gown both show significant mechanical degradation with ebeam dose over sterilization doses (>25 kGy), indicating that eBeam technology is not appropriate for the re-processing these PPEs.
Keywords: N95 respirator, Medical gown, Face shield, Electron beam, Reprocessing, Personal protective equipment
1. Introduction
The outbreak of the COVID-19 pandemic caused by the coronavirus SARS-CoV-2 began at the dawn of 2020 and has deeply impacted the way we live. As reported by World Health Organization (WHO) as of March 30, 2022, there are 483.55 million confirmed cases, including 6.13 million deaths worldwide (World Health Organization, 2021). The corresponding severe shortage of Personal Protective Equipment (PPE) has caught the spotlight due to the lack of effective action to distribute and maintain inventories, as well as a dearth of required labor capabilities and raw materials (Emanuel et al., 2020; Shah et al., 2019). In March 2020, World Health Organization modeling warned that 89 million medical masks, 76 million examination gloves, and 1.6 million pairs of goggles would be required worldwide for the COVID-19 response each month, with a dire shortage putting healthcare workers at risk (Feinmann, 2020). PPEs shortages were predicted even during the earlier influenza and SARS pandemics (Murray et al., 2010; Beckman et al., 2013; Srinivasan et al., 2004; Hines et al., 2014). With more research aimed at relieving the pandemic situation, in tandem with an increasingly fully vaccinated (two doses delivered) percentage in the United States (65.51% on March 9, 2022) (Statistics and Research), the PPE shortage has gained some relief. However, a significant shortage of essential PPE may break out due to the surge of Omicron and other new variants.
The PPEs that were in short supply included disposable surgical masks, respirators, hair coverings, aprons, and gowns. Among these PPEs, N95 respirators and surgical masks were in the greatest shortage. However, there is no way to decrease the usage of PPE of frontline medical workers during such an urgent pandemic. It is thus necessary to ration the use of PPE or to assess the possibility of re-using sterilized PPE. Heating (<100 °C) under different humidity, ultraviolet (UV) sterilization, standard hospital sterilization technologies (autoclave treatment, ethylene oxide gassing, low-temperature hydrogen peroxide gas plasma treatment, vaporous hydrogen peroxide exposure (VHP), and peracetic acid dry fogging), standardized drying, and steam sterilization processes are some of the effective methods for re-using masks (Liao et al., 2020) (Kumar et al., 2020) (de Man et al., 2020). In addition, the FDA has recently granted a EUA for convalescent plasma treatment to treat the coronavirus (US Food and Drug, 2020).
Ionizing radiation such as gamma rays, electron beams, and X-rays are common modalities for sterilization in the medical device industry. There are established standards for these technologies, e.g., ANSI/AAMI/ISO11137-Sterilization of health care products-Radiation (Association for the Advancement of Medical Instrumentation, 2006), AAMI TRI17-2017: Compatibility of materials subject to sterilization (TIR17 AAM, 2017), and some ASTM Standards on Dosimetry for Radiation Processing, such as ISO/ASTM 51649 – Dosimetry for E-Beam facilities (ISO/ASTM-51649, 2018), ISO/ASTM 51631 – Calorimetric dosimetry system for E-Beam (ISO/ASTM-51631, 2020), etc. Considering the device performance after treatment, our previous work (Fifield et al., 2019, 2021a, 2021b) has demonstrated that no significant changes in material properties or functionality have been observed in Becton, Dickinson and Company's medical devices after eBeam and X-ray treatment, and electron beam and X-ray methods have been suggested as viable alternatives to gamma-ray irradiation. The parameter that is calibrated to achieve the desired killing of microbial pathogens is the minimum absorbed dose (measured in kilograys). Extensive published information exists related to the minimum absorbed dose that is required for achieving specific log reductions of the viral pathogens. Often a pre-selected dose of 25 kGy is appropriate for medical devices (Association for the Advancement of Medical Instrumentation (AAMI), 2001). Likewise, prior work has shown 3 log reduction at 50 kGy of Porcine Epidemic Diarrhea Virus (PEDV) which is a pleomorphic, enveloped RNA virus, classified as a coronavirus under the family Coronaviridae (Trudeau et al., 2016). Both non-enveloped and enveloped viruses, such as Human Immunodeficiency Virus (RNA, enveloped), Bovine Viral Diarrhea Virus (RNA, enveloped), Hepatitis A Virus (RNA, non-enveloped), Porcine Parvovirus (DNA, non-enveloped), etc., have been processed by low dose gamma radiation and have been assured to be effectively decontaminated (Moore, 2012). The typical dose range for ionizing radiation of 15–50 kGy is usually bioburden-based and determined according to standard methods (ANSI 11137-2, 2019) (ANSI/AAMI/ISO 11137-2: 2013 (R2019), 2019) (AAMI TIR17:2017, 2018) (AAMI TIR17 : 2017, 2017).
The mechanism of RNA virus inactivation by high energy particles (electron beam, gamma rays, and X-rays), such as enveloped coronavirus, has been intensively investigated. Two theories that explain microbial inactivation induced by ionizing radiation are the direct-action theory and indirect-action theory. The first theory suggests that the breakage of DNA/RNA caused by ionization inactivates microorganisms (Dempsey and Thirucote, 1988). Photons and electrons with high energy strike the genetic material, leading to extensive double-stranded and single-stranded breaks. The indirect effect of ionizing radiation on microorganisms has been reported to involve the generation of highly reactive oxygen species (ROS: H2O2, –OH, HO2·) arising from the presence of water molecules in the cell. These responses induced by high energy, in turn, also initiate DNA and RNA strand breakages. Ionizing radiation at sufficiently high doses can also affect structural and functional proteins (enzymes), lipids, etc (Dickson and RicaRdo, 2001). Considering the virus responsible for the COVID-19 pandemic is SARS-CoV-2 (an enveloped RNA virus), eBeam technology could theoretically be used to decontaminate personal protective equipment exposed to the virus.
There is extensive information focusing on ionizing irradiation-induced effects on polymer properties available in the literature (Hill and Whittaker, 2016; Giberson and Harrington, 1958; Atchison, 2003; Gheysari and Behjat, 2001; CHARLESBY, 2009; Mishra et al., 2001; Dawes et al., 2007). Theoretically, crosslinking increases the molecular weight via bond formation, leading to weakened elongation at break and improved tensile strength with less mobility of polymer chains. Chain scission and oxidation are referring to degradation. The decreased molecular weight caused by chain scission potentially impairs mechanical properties. Common polymers like polyethylene (PE), polycarbonate (PC), and polypropylene (PP) generally obey this rule, but their behavior may somewhat differ depending on the sample additives and sample geometry cut used for testing. Polyester (PET), polypropylene (PP), polyisoprene (IR), and polyurethane (PR) are used to make the outer layer, filter layer, strap, and nose foam of the 3M respirator, respectively. Two densely-packed meltblown layers sandwiched between two strong, spunbond outer layers are the main material structures used to make the Proxima Sirus gown; both are polypropylene nonwovens. The headgear of the face shield (SellStrom S39110) is made of nylon and the visor is made of Polycarbonate. Irradiated medical device components made with PP and the corresponding PP dog-bones have been evaluated in terms of mechanical testing and discoloration testing, which suggested negligible changes with increasing doses up to 90 kGy for PP (Fifield et al., 2021a, 2021b). However, ionizing irradiated PP membranes of the facepiece respirator demonstrated mechanical integrity degradation when exposed to 50 kGy (Pirker et al., 2021). Nonwoven polypropylene has also been shown to have degradation due to reduced molecular weight from chain scission. Meltblown polypropylene nonwovens showed faster deterioration due to the lower molecular weight (MEDLINE. Proxima Surgical Gowns). The radiation effect on PC has been investigated in detail; however, the conclusions in terms of irradiation-induced effects are not consistent among various research reports, likely due to various geometries and manufacturing processes of the researched PP samples.
In this paper, we assessed the compatibility of eBeam technology for the re-processing PPEs. To evaluate the efficacy of electron beam irradiation in sterilization and re-processing of various PPEs for re-use, detailed research was required in following the CDC and FDA recognized standards. This paper focuses on characterizing the properties of PPE (N95 Respirators, surgical masks, and medical gowns) under various doses and conditions of eBeam irradiation treatment. While re-processing disposable PPE is not a desirable method of protecting healthcare workers, it is important to document the effects of various possible methods of re-processing on PPE for potential evaluation as alternatives in the ongoing and potential future pandemics and shortages.
2. Materials and methods
2.1. Preparation of samples
N95 Respirators (3M™ Particulate Respirator 8200/07023(AAD)), Proxima Sirus gowns (Medline Industries), and face shields (SellStrom S39110) were utilized, as shown in Table 1 . The N95 Respirators have three layers in which the inner and outer layers are made of polyester and the middle layer is a polypropylene filter. Proxima Sirus gowns are standard AAMI level 3 meeting the ANSI/AAMI PB70 standard (ANSI/AAMI PB70:2012, 2012). These samples were stored in a laboratory at room temperature (72–75 °F) and humidity of 55%–60%. A rotary cutter was used to cut gowns and respirators into samples to prevent distortion in pattern lines and fraying, which is important for tensile testing and other mechanical properties testing.
Table 1.
Conditions used for eBeam irradiation of various PPE samples.
| Sample types | Sample modality (kGy) |
|---|---|
| Proxima Sirus Gown | Control, 25 kGy, 50 kGy, 75 kGy, 100 kGy, 200 kGy |
| 3M 8200 Respirators | Control, open-air-25 kGy, sealed air-25 kGy, sealed argon-25 kGy, open air-100 kGy |
| Face Shield (SellStrom S39110) | 25 kGy |
It should be noted that a relatively small sample size of PPE was used due to the shortage of the products during the COVID19 pandemic. Except for the face shield testing and water impact testing for Proxima Sirus gown (one replicate each), at least 3–6 replicates were used in testing the PPEs. Detailed information about the replicates for each testing are listed in S-Table 1 in supplementary materials. In addition, raw data can also be found in the S-Table 2-9 in supplementary materials. Bar plots with 2σ error bar were generated for each testing. This represents an 80%–95% confidence interval, with the lower confidence interval corresponding to the smaller sample sizes.
2.2. Electron beam dosing studies
Irradiation of the target samples involved a vertically mounted 10 MeV, 18 kW electron beam linear accelerator at the National Center for Electron Beam Research at Texas A&M University. This facility utilizes a single conveyance system to move the product in and out of the process chamber. Samples were placed on the conveyor and exposed to defined eBeam doses by controlling the speed of the conveyor. For certain high doses, the samples were subjected to incremental dosing. Internationally traceable, industry-standard alanine dosimeters were used to measure the absorbed doses. The doses absorbed by the dosimeters were measured using a Bruker E-scan spectrometer (Bruker, Billerica, MA). Preliminary dose-mapping studies were performed to determine the ideal placement of the masks relative to the eBeam scan horn to ensure dose uniformity across the top and bottom of the masks. Doses in the range of 0–200 kGy were used in this study. Different irradiation conditions (sealed air, open-air, sealed argon) were employed to understand the irradiation-induced effect on N95 Respirators. The eBeam doses 25 kGy, 50 kGy, 75 kGy, and 100 kGy were utilized for the gowns. Only 25 kGy was utilized for the face shield. The dose rate of the eBeam source was 3 kGy/s, and thus the total time that the PPEs were under the e-beam scan horn ranged between a minimum of 8.3 s (for 25 kGy) and 33.3 s (for 100 kGy). Fig. 1 shows the scan and conveyor system with samples in the eBeam facility.
Fig. 1.
Photo showing the N-95 respirators placed on the conveyor system by the eBeam scan horn.
2.3. Characterization of PPE
Control and treated samples (Table 1) were employed to investigate functionality performance, surface properties, liquid barrier performance, material integrity, and mechanical properties, including filtration efficiency testing, strap integrity testing, surface wettability testing, yellowness index (YI) testing, surface charge measurements, hydrostatic pressure testing, water impact penetration testing, and morphological characterization scanning. A detailed description of the above tests for the respirators and gown, except for morphological characterization scanning, can be found in our previous study (Huang et al., 2022). Morphological characterization scanning was used in the present work to examine the effects of eBeam irradiation on the material structure and integrity. A benchtop Phenom SL SEM with EDS was used, located in the Baker Hughes Materials Laboratory, Zachry Engineering Education Complex at Texas A&M University, College Station. The SEM was operated at 5 kV in low vacuum mode (1 Pa) using a secondary electron detector (SED). Samples were prepared according to the SEM protocol, cut into 6 mm6 mm pieces, and fixed with conductive carbon adhesive tape on the sample holder.
For the face shield the functional test for EUA fabricated or recycled faceshields involves four procedures: 1) Inspection of each component, 2) Don and doff the face shield 10 times following CDC guidelines, 3) Qualitative visibility assessment, 4) Compatability with commonly used hospital disinfectants (e.g 70% ethanol wipe). Since the design and original material selection was a preexisting FDA-approved design the main concern was mechanical integrity during the Donning and doffing test (Mostaghimi et al.,; DtM-v3.1 Face Shield PPE, 2020).
Since the experimental and un-treated samples tested for tensile testing in this work were different from our previous plasma-treated work, the tensile testing parameters used are specifically listed in Table 2 .
Table 2.
Experimental parameters used in tensile testing.
| Materials | Specimen length (mm) | Gage length (mm) | Distance between grips (mm) | Displacement rate (mm/min) |
|---|---|---|---|---|
| Outer Layer-Polyester | 57.5 | 20 | 32.5 | 100 |
| Middle Layer (Filter)-Polypropylene | 57.5 | 20 | 32.5 | 100 |
| Inner Layer-Polyester | 57.5 | 20 | 32.5 | 100 |
| Proxima Sirus Gown | 57.5 | 20 | 100 | 300 |
3. Results and discussion
3.1. Filtration efficiency testing and strap integrity testing
A 1 cycle (100 kGy) eBeam irradiated 3M 8200 N95 Respirator was sent to the National Personal Protective Technology Laboratory (NPPTL) for functionality testing, including filtration efficiency testing and strap integrity testing. An efficiency of 63.6% was measured for the eBeam irradiated sample, while 95% is the minimum requirement for a qualified N95 Respirator (Centers for Disease Control and Prevention, 2021). This result was consistent with recent research focusing on the effect of eBeam irradiation on filtration efficiency (Pirker et al., 2021; Smietanko et al., 2020), indicating reduced filtration efficiency may result from the elimination of the electric charge from the filter's surface. However, some work has been done, revealing that recharging a respirator post-decontamination has great potential in recovering filtration efficiency (Hossain et al., 2020; Wang et al., 2020).
NPPTL also tested the strap integrity of an eBeam irradiated respirator, showing 12.10% and 7.53% decrease in recorded force in the top strap and force in the bottom strap respectively. The strap is made of polyisoprene, which also showed degradation after plasma ROS treatment in our previous work (Huang et al., 2022). The strap functions to provide an effective seal between the filter body and the wearer's face and there are more alternatives to polyisoprene to support this functionality. For instance, a strap made from polyester/nylon spandex and a combination of Lycra 784 and polyester materials (BYD mask and Prestige Ameritech) are two good potential alternatives to polyisoprene-based straps according to our previous work (Huang et al., 2022).
3.2. Surface wettability testing
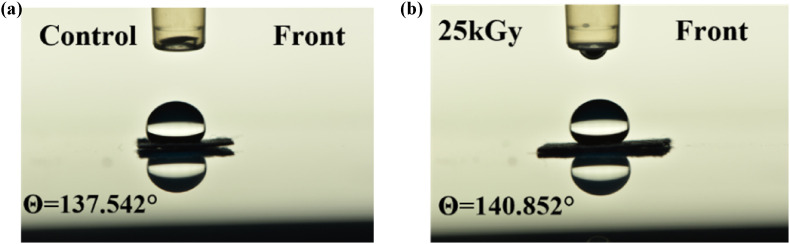
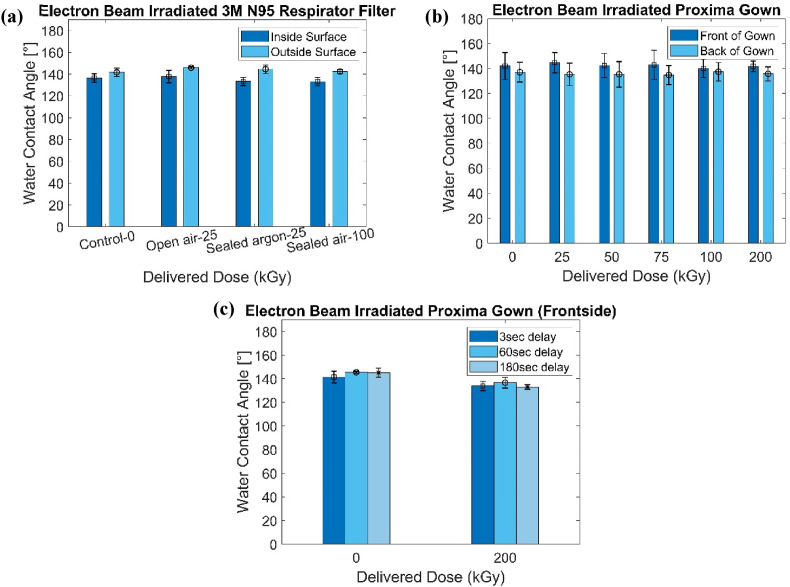
The wettability of the eBeam irradiated PPE was examined by contact angle measurements. Examples of control and 25 kGy irradiated 3M-8500 N95 Respirator filters are shown in Fig. 2 . 6 replicates of each of the different doses were prepared to be tested for each N95 respirator with different delivered doses. A careful analysis showed no obvious change occurring in the respirator's polypropylene-made filter with increased delivered dose, as shown in Fig. 3 (a). It was noticeable that the outside of the filter has a higher contact angle than the inside of the filter (note: the inside surface faces the mouth.). The difference between the outside and inside may be caused by the different treatment processes, resulting in different morphology of the surface. Also, the presence of impurities and surface roughness are other potential explanations for the larger contact angle of the outside surface of the filter. The outside surface has a high possibility of contacting impurities and rubbing with foreign bodies, resulting in more hydrophobicity (Gennes et al., 2013). Statistical analysis (Student t-test) for the 3M N95 respirator show there is a significant difference among open-air-25 kGy, Argon-25 kGy, and close-air-25 kGy for the outside surface, but the difference is less than 2%.
Fig. 2.
The contact angle of eBeam irradiated 3M-8500 N95 Respirator filters: (a) control sample, (b) 25 kGy sample.
Fig. 3.
The water contact angle of eBeam irradiated PPE: (a) N95 Respirator's filter, (b) Proxima Sirus Gown, (c) Proxima Sirus Gown (frontside) with 3 s, 60 s, and 180 s delayed measurements.
In addition, 6 specimens of Proxima Sirus gowns were prepared to be tested for each dose. Based on Fig. 3(b–c), it is evident there is no significant difference in contact angle with increasing delivered dose. Concerning the effect of the droplet's stability on the contact angle, we implemented delays between adding the water drop onto the sample surface and image capture for contact angle measurements of 3 s, 60 s, and 180 s. Results of the Proxima Sirus gown's frontside are shown in Fig. 3 (c), which reveals that the droplets lying on the surface of the gown are stable enough and the effect of delayed time for taking the photo is negligible. An 8-min video was also taken to track the dynamics of the droplet's contact angle, and the droplet had no obvious decrease in contact angle until ∼6 min. This observation further fortifies the results shown in Fig. 3(a–b). ANOVA test for Proxima Sirus gown were also performed and the high p-value leads to the conclusion that there is no significant difference for each comparison group.
3.3. Yellowness index (YI) testing
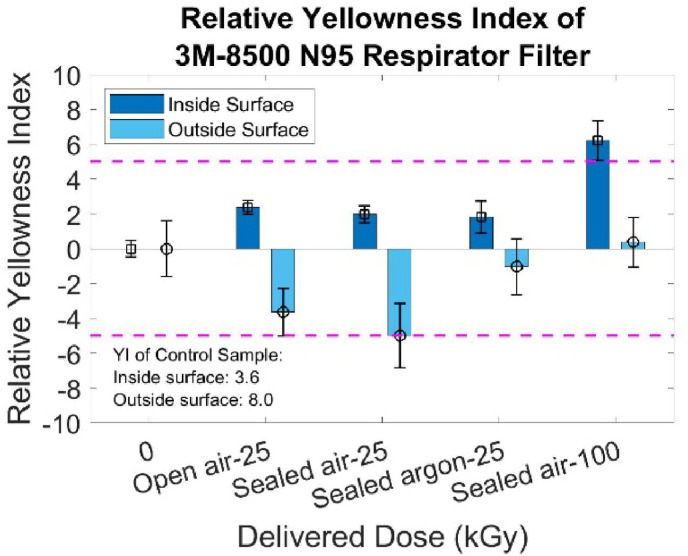
Five irradiation conditions of the N95 Respirator's filter were evaluated for possible discoloration with results shown in Fig. 4 . It shows the difference in YI (ΔYI) between the control sample and the treated sample. Notably, the ΔYI of the sealed air 100 kGy irradiated sample has a significant increase in its inside surface. However, all ΔYI <5 indicates that irradiated samples with doses below 100 kGy have negligible discoloration since the human eye cannot detect discoloration differences with ΔYI <5 (Holley and Agro, 1998). The same conclusion for all outside surfaces can also be drawn since the large error bar makes the YI difference acceptable. To summarize, a negligible difference in ΔYI exists in the N95 Respirator's filter (both inside and outside surface) irradiated with doses less than 25 kGy. Only the highest dose irradiated on the inside surface of the sample (sealed air-100 kGy) has ΔYI >5. Statistical analysis reveals a measurable difference between each group, but this result is not considered in detail since the YI change is barely observable by eyes and YI is not closely related to the performance degradation.
Fig. 4.
Relative Yellowness Index of 3M–8500 N95 Respirator changes with modality.
3.4. Hydrostatic pressure testing
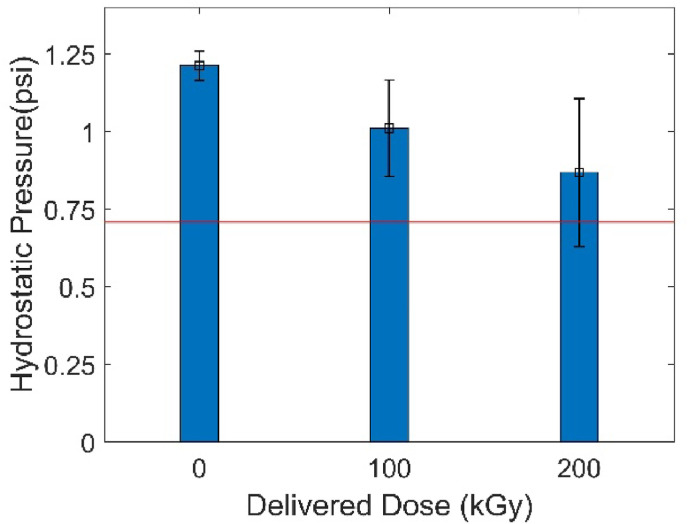
A self-assembled hydrostatic pressure tester was equipped to measure the hydrostatic pressure of the eBeam irradiated Proxima gowns, and the results are shown in Fig. 5 . Due to the limited number of PPE that were available for this study, only 3 replicates of each dose were utilized (control, 100 kGy, 200 kGy). According to the standard set by the CDC, to be a moderate water resistance AAMI level 3 gown, the gown should have hydrostatic pressure higher than 0.71 psi when the first three droplets penetrate the gown (Centers for Disease Control and Prevention, 2012). The average results in Fig. 5 indicate that all the control and 100 kGy irradiated Proxima Sirus AAMI gowns passed the testing with hydrostatic pressure higher than 0.71 psi. The success of the 100 kGy irradiated Proxima Sirus gown indicates the success of lower doses as well (25 kGy, 50 kGy, 75 kGy). Although the average value of the Proxima Sirus gown-200 kGy samples was larger than 0.71 psi, one of the tested samples (0.553 psi) was lower than 0.71 psi, making this dose level-200 kGy fail the test. ANOVA test was also performed and no significant difference among the treatment groups was observed.
Fig. 5.
Hydrostatic pressure of eBeam irradiated Proxima Sirus gowns.
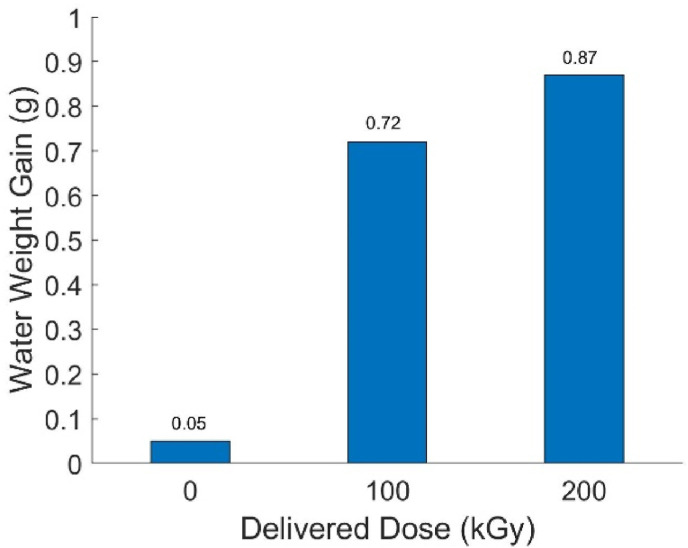
3.5. Impact penetration testing
Based on the AATCC test method 42–2017, a laboratory-fabricated device was made for impact penetration testing. The result of the gown testing is shown in Fig. 6 . According to the guidance of the CDC, to be a moderate water resistance gown that satisfies the requirement of the AAMI level 3, the weight gain of the blotting paper should be less than 1 g (Centers for Disease Control and Prevention, 2012). As such, we can conclude that the eBeam irradiated Proxima Sirus gown successfully pass the testing with lower than 1 g of water weight gain. Only one replicate of 100 kGy and 200 kGy samples were tested but their success again infers success at lower doses as well (25 kGy, 50 kGy, 75 kGy). However, it would appear that while technically still meeting the AAMI requirements there is a significant degradation in the gown's ability to prevent water penetration. Still, more replicates should be used to draw a strong conclusion.
Fig. 6.
Impact penetration testing results of eBeam irradiated Proxima Sirus gown.
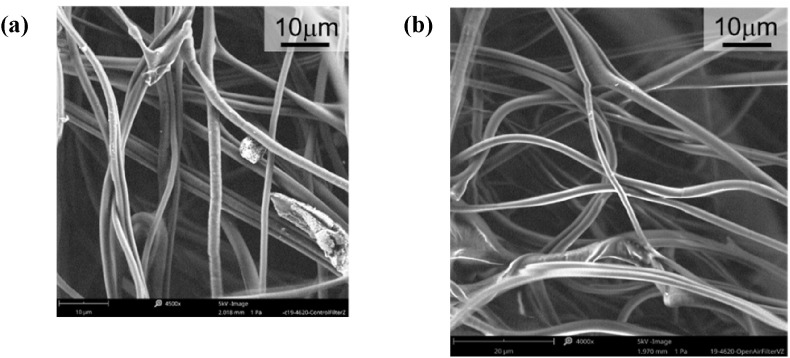
3.6. Surface morphology testing
The filtration efficiency of N95 Respirators has been widely researched, and there are five mechanisms normally activated for trapping the aerosol based on the sizes of particles. Among gravity sedimentation, inertial impaction, interception, diffusion, and electrostatic attraction, interception is closely related to the polypropylene fiber's integrity, which can be examined with scanning electron microscopes (SEM). In our present work, we characterized the mid-layer (filter) and shell of N95 Respirator and Proxima Sirus gowns after eBeam irradiation. The SEM images of the control and 25 kGy irradiated N95 Respirator's filter (polypropylene) are shown in Fig. 7 (a–b), which indicates no obvious damage or morphological change occurs in the N95 Respirator filter after irradiation. The same conclusion also has been drawn in the shell of N95 (Polyester) and Proxima Sirus gowns treated by eBeam. Our present SEM work is consistent with recent studies (Pirker et al., 2021; Smietanko et al., 2020).
Fig. 7.
SEM of N95 PP filter: (a) control specimen, (b) open-air treated.
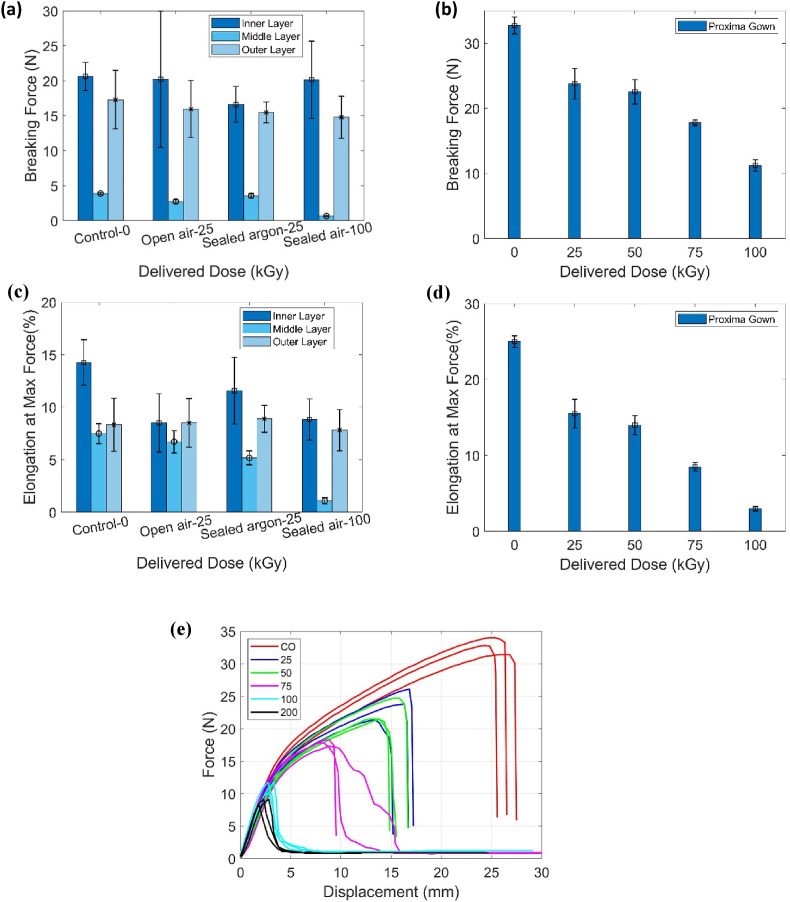
3.7. Mechanical properties testing
Samples of N95 Respirator layers (the mid-layer is meltblown polypropylene; the outer and inner layers are polyester) and Proxima Sirus gowns were cut for testing. Sirus gown is made of four layers of fabric to provide a high level of fluid repellency via spunbond meltblown spunbond (SMS) technology. Two densely-packed meltblown polypropylene layers are sandwiched between two strong, spunbond polypropylene outer layers (MEDLINE. Proxima Surgical Gowns). Fig. 8 shows the breaking force and the corresponding elongation at the max force of the filter and gown. Fig. 8 (a) and (c) indicate no significant differences among different gases at 25 kGy and the control samples. In brief, no degradation of mechanical properties occurs in the N95 filter upon <25 kGy irradiation for both polyesters made-layers and meltblown polypropylene made-filter. However, the 100 kGy irradiated filter (mid-layer) has a significant reduction in the breaking force and the corresponding elongation at max force, indicating the meltblown polypropylene has lower radiation resistance. For the Proxima Sirus gowns, Fig. 8 (b) and (d) indicate that with increasing dose from 0 to 100 kGy, both the breaking force and elongation at max force systematically decrease. Indeed, the force-displacement curves shown in Fig. 8 (e) show that the force to break decreases with increasing dose. This behavior of polypropylene (PP) is different than some conclusions generated in historical literature, where PP in the format of sheets has relatively high radiation resistance with dose up to 120 kGy (Fifield et al., 2021a) (Sabet et al., 2012). Spunbond polypropylene functions as a strong and durable layer, and meltblown polypropylene has excellent wicking and barrier properties. From that perspective, the decreased mechanical performance observed in filter and gown is attributed to the weak tensile properties of meltblown polypropylene layer since it has a high surface area which can be oxidized easier. The lower radiation resistance of the meltblown polypropylene layer compared to the polymer sheet may also be due to the big difference in the sample thickness and the morphology (Nishimoto et al., 1991). Meltblown polypropylene layers have macroscopic interconnected fibers with a rough diameter of 2–3 μm, while polymer sheets normally have nanoscale entanglement of polymer chains. Both N95 Respirators filters and Proxima Sirus gowns show a certain level of degradation after ebeam treatment, however, 10 times donning and doffing for face shield performed as mechanical testing indicates there is no significant degradation occurrence in face shield. In addition, inspection of each component, qualitative visibility assessment, and compatibility investigation with commonly used hospital disinfectants (e.g., 70% ethanol wipe) were performed without observation of any degradation.
Fig. 8.
(a–b) Are the measured breaking force of (a) the three treated layers of N95 Respirators and (b) Proxima Sirus Gowns. (c–d) Are the measured elongation at max force of (c) the three treated layers of N95 Respirators and (d) Proxima Sirus Gowns. (e) Presents force-displacement curves (to break) of Proxima Sirus gowns under tensile testing.
4. Conclusions
In this paper, we investigated the influence of high-energy electron beam doses on the properties of N95 Respirators, Proxima Sirus gowns, and face shields, including functionality performance, surface properties, liquid barrier performance, material integrity, and mechanical properties. Different PPE was irradiated under different doses and involved gases. Polyester (PEs), polypropylene (PP), nylon, and polycarbonate (PC) were involved in this work. PP was observed to degrade more substantially with dose than the other polymers, which differed from literature likely due to processing form and methods. As we mentioned above, the polypropylene used to make mask filters (N95 respirator) and gowns (Proxima Sirus) were processed via different technologies. Meltblown polypropylene fabric is used for the 3M respirator filter and the middle layer of the Proxima Sirus gown, and Spunbond polypropylene is used for the two outer layers of the gown considering the necessity of the strongness and durability of PPEs. The high surface area of the meltblown PP weaken the mechanical properties due to the tendency to oxidize readily. However, the general conclusions drawn from literature typically involve polypropylene plastic sheets. From this perspective, it is inappropriate to conclude PPEs made with the same type of radiation-resistant material are more likely to have consistent reprocessing results via ionizing radiation because there is a big difference existing in material manufacturing technologies even using the same polymer monomer. Overall, for disposable PPE there is important performance degradation that should be considered before any adoption of a re-processing program. Several specific conclusions can be drawn:
-
a.
A significant reduction of filtration efficiency (from 95% reduced to 63.6%) was observed in N95 Respirators, which indicates that electron beam cannot be used for re-use of 3M 8200/07023(AAD) Respirators. However, all the other modes of testing indicate no differences in properties between the control and irradiated samples at sterilization doses of 25 kGy. Degradation of the strength of the inner polypropylene filter layer is observable at doses of 100 kGy – and would indicate that it could break during use if re-processed multiple times. As such, with the conclusions drawn from other research regarding recharging respirators after decontamination to improve the efficiency, electron beam irradiated N95 Respirator may work with a post-irradiation recharging treatment – but only for a limited number of re-processing cycles.
-
b.
Technically the gown still met AAMI standards for liquid penetration up to 100 kGy. However, the water penetrating the gown was over 10 times the amount of an unirradiated gown, also significant mechanical degradation was detected in the Proxima Sirus gowns. Successful re-use of these gowns after eBeam decontamination is probably limited but could warrant further investigation since limited samples were utilized in the present work.
-
c.
The face shield was only tested for mechanical behavior, and no degradation was observed. Still, more replicates should be studied, as we have only one characterized sample herein.
These conclusions only apply to new PPE since only new PPE was utilized in this work for electron beam processing and post-irradiation characterization. Theoretically, PPE decontamination and re-use protocol would first require inspection of the used PPE to ensure that the field use did not damage the PPE. Only PPE which is intact and functional should be subject to a decontamination process. Testing and decontamination of used PPE were beyond the scope of this project. Cross-correlated effects or combined field use damage and decontamination damage are possible but unknown at this point.
Author statement
Min Huang: Investigation, Methodology, Validation, Formal Analysis, Writing - Original Draft. Md Kamrul Hasan: Investigation, Methodology, Validation, Formal Analysis, Writing - Original Draft. Suresh D. Pillai: Conceptualization, Resources, Writing - Review & Editing. Matt Pharr: Methodology, Validation, Formal Analysis, Writing - Review & Editing, Supervision. David Staack: Conceptualization, Investigation, Methodology, Validation, Formal Analysis, Resources, Writing - Review & Editing, Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was funded by internal resources from the Texas A&M Unviersity System.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.radphyschem.2022.110557.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- AAMI TIR17 : 2017 . 2017. COMPATIBILITY OF MATERIALS SUBJECT TO STERILIZATION. Arlington, vol. A. [Google Scholar]
- ANSI/AAMI PB70:2012 Liquid barrier performance and classification of protective apparel and drapes intended for use in health care facilities. American Nation Standard. 2012:1–38. [Google Scholar]
- ANSI/AAMI/ISO 11137-2: 2013 (R2019) 2019. NoSterilization of Health Care Products - Radiation - Part 2: Establishing the Sterilization Dose. Arlington, vol. A. [Google Scholar]
- Association for the Advancement of Medical Instrumentation (AAMI) 2001. Sterilization of Health Care Products — Radiation Sterilization — Substantiation of 25 kGy as a Sterilization Dose — Method VDmax. Arlington, vol. A. [Google Scholar]
- Association for the Advancement of Medical Instrumentation . 2006. Sterilization of Health Care Products--radiation--Part 2: Establishing the sterilization dose. [Google Scholar]
- Atchison G.J. Color and radical formation in irradiated polyvinyl chloride. J. Polym. Sci. 2003;49(152):385–395. [Google Scholar]
- Beckman S., Materna B., Goldmacher S., Zipprich J., D'Alessandro M., Novak D., Harrison R. Evaluation of respiratory protection programs and practices in California hospitals during the 2009-2010 H1N1 influenza pandemic. Am J Infect Control [Internet] 2013;41(11):1024–1031. doi: 10.1016/j.ajic.2013.05.006. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention NIOSH personal protective equipment information (PPE-Info) [internet] 2012. https://wwwn.cdc.gov/PPEInfo/Standards/Info/ANSI/AAMIPB70Class4 Available from:
- Centers for Disease Control and Prevention Decontaminated assessment results | NPPTL | NIOSH | CDC [internet] 2021. https://www.cdc.gov/niosh/npptl/respirators/testing/DeconResults.html [cited 2021 Apr 16]. Available from:
- Charlesby A. 2009. Radiation Mechanisms in Polymers; pp. 1–21. [Google Scholar]
- Dawes K., Glover L.C., Vroom D.A. Physical Properties of Polymers Handbook. 2007. The effects of electron beam and g-irradiation on polymeric materials; pp. 867–887. [Google Scholar]
- de Man P., van Straten B., van den Dobbelsteen J., van der Eijk A., Horeman T., Koeleman H. Sterilization of disposable face masks by means of standardized dry and steam sterilization processes; an alternative in the fight against mask shortages due to COVID-19. J Hosp Infect [Internet] 2020;105(2):356–357. doi: 10.1016/j.jhin.2020.04.001. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey D.J., Thirucote R.R. Sterilization of medical devices: a Review. J. Biomater. Appl. 1988;3(3):454–523. doi: 10.1177/088532828800300303. [DOI] [PubMed] [Google Scholar]
- Dickson J.S. In: Food Irradiation: Principles and Applications. RicaRdo M., editor. Wiley-Interscience; New York, New York, USA: 2001. Radiation inactivation of microorganisms; pp. 23–32. [Google Scholar]
- DtM-v3.1 face shield PPE, 3D printable headband NO LOGO [internet] https://3dprint.nih.gov/discover/3dpx-013359 NIH. 2020. Available from:
- Emanuel E.J., Persad G., Upshur R., Thome B., Parker M., Glickman A., Zhang C., Boyle C., Smith M., Phillips J.P. Fair allocation of scarce medical Resources in the time of Covid-19. N. Engl. J. Med. 2020;382:2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- Feinmann J. PPE: what now for the global supply chain? Bmj [Internet] 2020;1910(May):m1910. doi: 10.1136/bmj.m1910. Available from: [DOI] [PubMed] [Google Scholar]
- Fifield L.S., Pharr M., Staack D.A., Murphy M.K., Huang M., Hasan M.K. Transitioning from cobalt-60 to X-ray or e-beam for medical sterilization: filling data and education gaps. Trans. Am. Nucl. Soc. 2019;121:1089–1092. [Google Scholar]
- Fifield L.S., Pharr M., Staack D., Pillai S.D., Nichols L., McCoy J., Faucette T., Bisel T.T., Huang M., Hasan M.K., Perkins L., Cooley S.K., Murphy M. Direct comparison of gamma, electron beam and X-ray irradiation doses on characteristics of low-density polyethylene, polypropylene homopolymer, polyolefin elastomer and chlorobutyl rubber medical device polymers. Radiat Phys Chem [Internet. 2021;186(April) doi: 10.1016/j.radphyschem.2021.109505. Available from: [DOI] [Google Scholar]
- Fifield L.S., Pharr M., Staack D., Pillai S.D., Nichols L., McCoy J., Faucette T., Bisel T.T., Huang M., Hasan M.K., Perkins L., Cooley S.K., Murphy M.K. Direct comparison of gamma, electron beam and X-ray irradiation effects on single-use blood collection devices with plastic components. Radiat Phys Chem [Internet. 2021;180(November 2020) doi: 10.1016/j.radphyschem.2020.109282. Available from: [DOI] [Google Scholar]
- Gennes D., Pierre-Gilles, Brochard-Wyart F., Quere D. Springer Science & Business Media; 2013. Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves. [Google Scholar]
- Gheysari D., Behjat A. Radiation crosslinking of LDPE and HDPE with 5 and 10 MeV electron beams. Eur Polym J [Internet] 2001 https://www.sciencedirect.com/science/article/pii/S0014305701000842 Oct 1 [cited 2019 May 29];37(10):2011–6. Available from: [Google Scholar]
- Giberson R.C., Harrington R. Chemical and Physical Changes in Gamma Irradiation Plastics. 1958;8(509):372–7443. DOE Public Reading Room - Hanford Battelle P.O. Box 999 MS H2-53 Richland WA 99352. [Google Scholar]
- Hill D.J.T., Whittaker A.K. Encyclopedia of Polymer Science and Technology [Internet] John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2016. Radiation chemistry of polymers. [cited 2019 May 22]. pp. 1–58. Available from: [DOI] [Google Scholar]
- Hines L., Rees E., Pavelchak N. Respiratory protection policies and practices among the health care workforce exposed to influenza in New York State: evaluating emergency preparedness for the next pandemic. Am J Infect Control [Internet] 2014;42(3):240–245. doi: 10.1016/j.ajic.2013.09.013. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley W.W., Agro S.C. 1998. Advanced EVA-Based Encapsulants. NREL report. [Google Scholar]
- Hossain E., Bhadra S., Jain H., Das S., Bhattacharya A., Ghosh S., Levine D. Recharging and rejuvenation of decontaminated N95 masks. Phys Fluids [Internet] 2020;32(9) doi: 10.1063/5.0023940. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M., Hasan M.K., Rathore K., Baky M.A.H., Lassalle J., Kraus J., Burnette M., Campbell C., Wang K.P., Jemison H., Pillai S., Pharr M., Staack D. Plasma generated ozone and reactive oxygen species for point of use PPE decontamination system [internet] PLoS One. 2022 doi: 10.1371/journal.pone.0262818. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO/ASTM-51631 . ASTM Int West; Conshohocken, PA: 2020. Calorimetric dosimetry system for E-Beam. [Google Scholar]
- ISO/ASTM-51649 . ASTM Int West; Conshohocken, PA: 2018. Dosimetry for E-Beam facilities. [Google Scholar]
- Kumar A., Kasloff S., Leung A., Cutts T., Strong J., Hills K., Vazquez-Grande G., Rush B., Lother S., Zarychanski R., Krishnan J. 2020. N95 Mask Decontamination using Standard Hospital Sterilization Technologies. [Google Scholar]
- Liao L., Xiao W., Zhao M., Yu X., Wang H., Wang Q., Chu S., Cui Y. Can N95 respirators Be reused after disinfection? How many times? ACS Nano. 2020;14(5):6348–6356. doi: 10.1021/acsnano.0c03597. [DOI] [PubMed] [Google Scholar]
- MEDLINE. Proxima Surgical Gowns. MEDLINE Industry, LP. p. 10.
- Mishra R., Tripathy S.P., Dwivedi K.K., Khathing D.T., Ghosh S., Müller M., Fink D. Electron induced modification in polypropylene. Radiat Meas [Internet] 2001 https://www.sciencedirect.com/science/article/pii/S1350448701000385?via%3Dihub Dec 1 [cited 2019 Jun 12];33(6):845–50. Available from: [Google Scholar]
- Moore M.A. vol. 13. Cell and Tissue Banking; 2012. pp. 401–407. (Inactivation of Enveloped and Non-enveloped Viruses on Seeded Human Tissues by Gamma Irradiation). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostaghimi A, Antonini M-J, Plana D, Anderson PD, Beller B, Boyer EW, Fannin A, Freake J, Oakley R, Sinha MS, Sinha MS, Smith L, Van C, Yang H, Sorger PK, LeBoeuf NR, Yu SH. Regulatory and safety considerations in deploying a locally fabricated, reusable face shield in a hospital responding to the COVID-19 pandemic. Med (N Y). 1(1):139–151. [DOI] [PMC free article] [PubMed]
- Murray M., Grant J., Bryce E., Chilton P., Forrester L. Facial protective equipment, personnel, and pandemics: impact of the pandemic (H1N1) 2009 virus on personnel and use of facial protective equipment. Infect. Control Hosp. Epidemiol. 2010;31(10):1011–1016. doi: 10.1086/656564. [DOI] [PubMed] [Google Scholar]
- Nishimoto S.I., Kitamura K., Watanabe Y., Kagiya T. The correlation between the morphology and radiation resistance of polypropylene solid materials. Int J Radiat Appl Instrumentation Part. 1991;37(1):71–75. [Google Scholar]
- Pirker L., Krajnc A.P., Malec J., Radulović V., Gradišek A., Jelen A., Remskar M., Mekjavic I.B., Kovac J., Mozetic M., Snoj L. Sterilization of polypropylene membranes of facepiece respirators by ionizing radiation. J. Membr. Sci. 2021:619. doi: 10.1016/j.memsci.2020.118756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabet M., Hassan A., Ratnam C.T. Mechanical, electrical, and thermal properties of irradiated low-density polyethylene by electron beam. Polym. Bull. 2012;68(9):2323–2339. [Google Scholar]
- Shah R., Harding J., Brown J., Mckinlay C. Neonatal glycaemia and neurodevelopmental outcomes: a systematic Review and meta-analysis. Neonatology. 2019;115(2):116–126. doi: 10.1159/000492859. [DOI] [PubMed] [Google Scholar]
- Smietanko D.C., Gryczka U., Chemistry N., July T. Effect of electron beam irradiation on filtering facepiece respirators integrity and filtering efficiency. Author. 2020:1–14. [Google Scholar]
- Srinivasan A., Jernign D.B., Liedtke L., Strausbaugh L. Hospital preparedness for severe acute respiratory syndrome in the United States: views from a national survey of infectious diseases consultants. Clin. Infect. Dis. 2004;39(2):272–274. doi: 10.1086/421777. [DOI] [PubMed] [Google Scholar]
- Statistics and research: coronavirus (COVID-19) vaccinations [internet]. Our world in data. https://ourworldindata.org/covid-vaccinations Available from:
- TIR17 AAM . Association for the Advancement of Medical Instrumentation; Arlington, VA: 2017. Compatibility of materials subject to sterilization. [Google Scholar]
- Trudeau M.P., Verma H., Sampedro F., Urriola P.E., Shurson G.C., Mikelvey J., Pillai S.D., Goyal S.M. Comparison of thermal and non-thermal processing of swine feed and the use of selected feed additives on inactivation of porcine epidemic diarrhea virus (PEDV) PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0158128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Food & Drug . US Food & Drug; 2020. FDA Issues Emergency Use Authorization for Convalescent Plasma as Potential Promising COVID–19 Treatment, Another Achievement in Administration's Fight Against Pandemic | FDA [Internet]https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-convalescent-plasma-potential-promising-covid-19-treatment [cited 2020 Aug 31]. Available from: [Google Scholar]
- Wang D., Sun B.C., Wang J.X., Zhou Y.Y., Chen Z.W., Fang Y., Yue W.H., Liu S.M., Liu K.Y., Liu K.Y., Zeng X.F., Chu G.W., Chen J.F. Can masks Be reused after hot water decontamination during the COVID-19 pandemic? Engineering [Internet] 2020;6(10):1115–1121. doi: 10.1016/j.eng.2020.05.016. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization WHO coronavirus (COVID-19) dashboard [internet]. Word health organization. 2021. https://covid19.who.int/ Available from:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.