Abstract
Background
Many women with HIV (WWH) have suboptimal adherence to oral antiretroviral therapy (ART) due to multilevel barriers to HIV care access and retention. A long-acting injectable (LAI) version of ART was approved by the US Food and Drug Administration in January 2021 and has the potential to overcome many of these barriers by eliminating the need for daily pill taking. However, it may not be optimal for all WWH. It is critical to develop tools that facilitate patient-provider shared decision making about oral versus LAI ART modalities to promote women’s adherence and long-term HIV outcomes.
Objective
This study will develop and pilot test a web-based patient decision aid called i.ART+support (i.ARTs). This decision aid aims to support shared decision making between WWH and their providers, and help women choose between oral and LAI HIV treatment.
Methods
The study will occur in 3 phases. In phase 1, we will utilize a mixed methods approach to collect data from WWH and medical and social service providers to inform i.ARTs content. During phase 2, we will conduct focus groups with WWH and providers to refine i.ARTs content and develop the web-based decision aid. In phase 3, i.ARTs will be tested in a randomized controlled trial with 180 women in Miami, Florida, and assessed for feasibility, usability, and acceptability, as well as to evaluate the associations between receiving i.ARTs and viral suppression, ART pharmacy refills, and clinic attendance.
Results
This study was funded in March 2021. Columbia University’s IRB approved the study protocols (approval number IRB-AAAT5314). Protocols for phase 1 interviews have been developed and interviews with service providers started in September 2021. We will apply for Clinicaltrials.gov registration prior to phase 3, which is when our first participant will be enrolled in the randomized controlled trial. This is anticipated to occur in April 2023.
Conclusions
This study is the first to develop a web-based patient decision aid to support WWH choices between oral and LAI ART. Its strengths include the incorporation of both patient and provider perspectives, a mixed methods design, and implementation in a real-world clinical setting.
International Registered Report Identifier (IRRID)
DERR1-10.2196/35646
Keywords: patient decision aid, HIV treatment, oral ART, long-acting injectable ART, study protocol, women’s health
Introduction
Suboptimal antiretroviral therapy (ART) adherence among people with HIV has constrained efforts to curb the HIV epidemic in the United States [1]. Women face myriad barriers to HIV care and treatment and have historically been underrepresented in clinical trials for HIV treatment [2,3]. As a result, women with HIV (WWH) have lower care retention (58% retention versus 65% retention in the overall US population), which contributes to their increased mortality compared with men [1,4-7]. There is therefore an urgent need for strategies that optimize care engagement and viral suppression among WWH.
Long-acting injectable (LAI) ART may be a strategy to improve ART adherence and HIV outcomes for women [2,3,8]. The first LAI ART (cabotegravir/rilpivirine), which consists of monthly intramuscular injections rather than daily pills, was approved by the US Food and Drug Administration (FDA) in January 2021; a bimonthly version was approved in February 2022; and other LAI ART drugs are in advanced stages of clinical trials and expected to be available for HIV treatment in the near future [9]. Women comprised only 8%-33% of phase 2 and phase 3 LAI ART trial participants, and pregnant women were not included [10-13]. As such, gender-specific barriers, pregnancy-related interactions [13] and characteristics that promote ART adherence among WWH remain underexplored [14-16]. Furthermore, LAI ART research has occurred largely among clinical trial participants, whose optimal medication adherence and clinic attendance do not represent the majority of WWH [17].
While preliminary research suggests that most WWH would prefer LAI ART over their current daily oral medication [18], this research has also identified multilevel barriers to LAI ART uptake. At the individual level, these include women’s concerns about side effects, pregnancy-related interactions, and drug resistance if LAI ART is discontinued without oral ART initiation; at the clinic level, barriers include medical mistrust due to historic sterilization campaigns [19] and lack of provider knowledge and willingness to offer LAI ART; and at the structural level, barriers include gender-specific socioeconomic inequalities and dynamics such as low-wage employment with unstable scheduling, lack of transportation, and care-taking responsibilities. Furthermore, FDA indication still requires viral suppression via oral ART prior to initiating LAI. These multilevel barriers could all complicate the frequent visits that LAI ART administration will require [18,20-23]. WWH and providers may benefit from tools and strategies that help them to identify and address barriers that prevent uptake of, and adherence to, LAI ART.
Shared decision making in medical settings is often accomplished using patient decision aids. These evidence-based tools promote equity in medicine by increasing patients’ knowledge, decision-making power [24,25], and health outcomes [26-28]. This approach can also lower medical paternalism [24,29-31]. Patient decision aids can improve medication adherence directly [32], as well as through mediating factors (eg, patient satisfaction [33-35], efficacy [36-38], and communication) [33,39,40]. These tools are ideal for preference-sensitive decisions with multiple options [41,42]; however, no patient decision aids yet exist to facilitate women’s choice between HIV treatment modalities. New tools are urgently needed to ensure the successful and equitable integration of new technologies such as LAI ART into clinical settings [43].
This paper describes the protocol for a mixed methods study to develop, refine, and test a patient decision aid for WWH, called i.ART+support (i.ARTs). i.ARTs will facilitate shared decision making between WWH and providers to determine which HIV treatment (oral or LAI ART) best fits a woman’s preferences, addresses her unique barriers, and, in doing so, facilitates adherence. This study will be conducted within the MACS/WIHS Combined Cohort Study (MWCCS) [44] and will fill the aforementioned gaps in existing research regarding patient decision aids for WWH. MWCCS is the largest and oldest prospective epidemiological cohort study of HIV in the United States. The current cohort includes approximately 1800 women from geographically diverse sites across the United States. Each of these has associated clinical sites with medical and social service providers who work with WWH. The aim of this paper is to provide an overview of all procedures for our study focused on developing a patient decision aid to support WWH as they choose between LAI and oral formulations of ART.
Methods
Overview of the Study
Study methods are aligned with current systematic approaches to patient decision aid development [36,45,46], and will occur in 3 phases (Figure 1). Each phase is associated with 1 of the following study aims. Phase 1 will generate data to inform i.ARTs content using mixed methods research with WWH and providers. Phase 2 will iteratively develop i.ARTs as a web-based patient decision aid. Phase 3 will pilot test i.ARTs to assess feasibility, acceptability, and usability and to compare decisional outcomes and adherence data (including viral suppression, ART refills, and clinic attendance) between i.ARTs recipients and standard of care (control) WWH (n=180; 90 per group). Each phase includes distinct data collection activities, which are summarized in Table 1.
Figure 1.
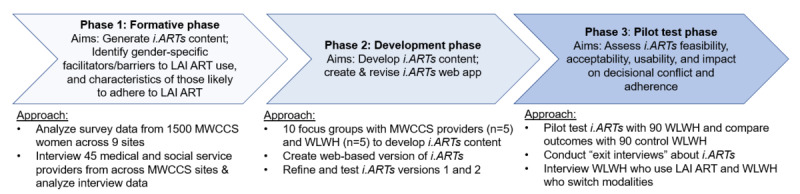
Study overview. i.ARTs: i.ART+support; LAI ART: long-acting injectable ART; MWCCS: MACS/WIHS Combined Cohort Study; WWH: women with HIV.
Table 1.
Overview of data sources and participants.
| Phase | Data source | Participants | Scope of data | Purpose |
| 1 | MWCCSa 2020 cohort survey | 1500 WWHb at 9 MWCCS sites | WWH perspectives on LAI ARTc and decision making; identify relevant barriers and facilitators to LAI ART uptake; characteristics of women who are most likely to adhere to LAI ART | Generate i.ARTsd content |
| 1 | Provider interviews | 45 medical/social service providers at MWCCS sites | Provider perspective on characteristics of women most likely to adhere to LAI ART; reasons providers would not offer LAI ART to a woman for whom it is clinically indicated; provider perceptions of multilevel barriers and facilitators to WWH’s ART use | Generate i.ARTs content |
| 2 | Focus groups | MWCCS providers, WWH in Miami, Florida | Feedback on successive iterations of i.ARTs | Develop and refine i.ARTs content |
| 3 | Baseline and postvisit survey | 180 WWH (90 from the intervention/i.ARTs arm and 90 from the control arm) | Information on decisional conflict (baseline and post), acceptability and satisfaction (post only), and sociodemographic and behavioral moderators (baseline only) | Assess i.ARTs acceptability and usability outcomes, assess impact of moderators |
| 3 | Exit interviews | 20 WWH who receive i.ARTs and 10 providers | WWH and provider perspectives on i.ARTs tool | Assess i.ARTs feasibility, usability, acceptability |
| 3 | Electronic medical record data | 180 WWH | i.ARTs adherence data for study participants, including clinic visits, medication refills, and viral load data | Preliminary impact of i.ARTs data on treatment-related outcomes |
aMWCCS: MACS/WIHS Combined Cohort Study.
bWWH: women with HIV.
cLAI ART: long-acting injectable antiretroviral therapy.
di.ARTs: i.ART+support
Phase 1: Formative Work to Inform i.ARTs Content
To ensure i.ARTs addresses a comprehensive array of factors that influence LAI ART uptake, phase 1 includes data collection that incorporates the perspectives of both WWH and providers at MWCCS sites.
Phase 1a will quantitatively assess WWH perspectives on LAI ART and decision making, identify relevant barriers and facilitators to LAI ART uptake, and identify the characteristics of women who are most likely to adhere to LAI ART. It utilizes survey data administered to the MWCCS study cohort starting in October 2020. Historically, this sample included 1661 WWH of whom 1610 have a history of taking oral ART [44]. None have participated in LAI ART clinical trials. Full descriptions of participant recruitment, selection, enrollment, and study procedures for MWCCS are available elsewhere [44]. Survey responses from the 2020 cohort were collected from fall 2020 through summer 2021, and include 15 items that assess LAI ART–related knowledge, interest, and potential barriers and facilitators to use. We will use multivariable logistic regression models to identify factors associated with WWH’s interest in, and barriers and facilitators to, LAI ART. While potential barriers and facilitators to LAI ART uptake can change over time (eg, transportation/housing), we will be looking at these associations cross sectionally and thus capturing these barriers at a given moment in time. The sample size provides the power to detect modest effect sizes in a multivariable logistic regression analysis (odds ratios ranging from 1.4 to 1.6), even with a low probability of LAI interest and a high squared multiple correlation (0.4-0.5) between predictor variables. This sample size will allow for subgroup analyses and still detect moderate effect sizes (eg, by age or MWCCS site).
Phase 1b assesses HIV provider perspectives on LAI ART for their female clients. It involves in-depth interviews with 30 medical (eg, infectious disease physicians, advanced practice registered nurses) and 15 social service providers (eg, case managers, HIV clinical social workers) who serve WWH across 9 MWCCS sites and affiliated clinical sites.
Provider participants will complete a 1-time, 45-60-minute interview, and receive a US $75 gift card as compensation. Interview domains will include (1) providers’ perceived characteristics of women most likely to adhere to LAI ART, particularly compared with oral ART (eg, housing, oral ART adherence, caregiving responsibilities, and clinic attendance); (2) reasons providers would not offer LAI ART to a woman for whom it is clinically indicated; (3) providers’ perspectives on decision making in the clinical setting and their experience in deciding which ART medications their WWH clients should take; and (4) multilevel barriers and facilitators to WWH’s ART use and solutions to overcome these adherence barriers. These domains are determined by formative work by the study team [18,20,22,23]. Interviews will be digitally recorded and professionally transcribed. Transcripts will be coded using a set of codes inductively identified from the data; these will be supplemented by codes derived from the existing literature. A thematic content analysis approach will be used to identify key findings within domains of interest [47]. Data will be summarized and applied to activities in phase 2, described in the following section.
Phase 2: i.ARTs Development
Phase 2 utilizes sequential focus groups with both WWH and medical and social service providers to determine the content of i.ARTs and create and revise the i.ARTs web-based app. Existing research indicates that web-based decision aid tools have advantages over paper-based ones, including interactive interfaces and visual filtering and sorting options [48]. This stage of i.ARTs development will follow International Patient Decision Aids Standards Collaboration (IPDAS) guidelines [46,49,50] and use the Ottawa Decision Support Framework [27,51-53]. This framework explains the relationship between participants’ decisional needs and decisional quality using both prescriptive [54] (based on rational actions, highest expected utility) and descriptive [55] (preferences-based, nonrational) decision theories.
An overview of the planned i.ARTs development process is included in Figure 2. We will conduct 2 sets of 5 focus groups: 1 set with 10 MWCCS providers recruited from the Phase 1 interview participants and the other set with 8-10 WWH recruited from the Miami MWCCS–affiliated clinical site. The Miami MWCCS site was selected due to the city’s high HIV incidence and low ART adherence, as well as a racially/ethnically diverse population [56-58]. WWH will be selected from Miami’s MWCCS community advisory board as well as by local MWCCS collaborators to ensure inclusion of women with a diverse range of perspectives, clinic attendance, and adherence. Each focus group will meet for approximately 60 minutes, and participants will be compensated US $40 per session for their time. We will record and transcribe the focus groups and review them to identify which items and topics should be included in the patient decision aid, as well as how to word specific items.
Figure 2.
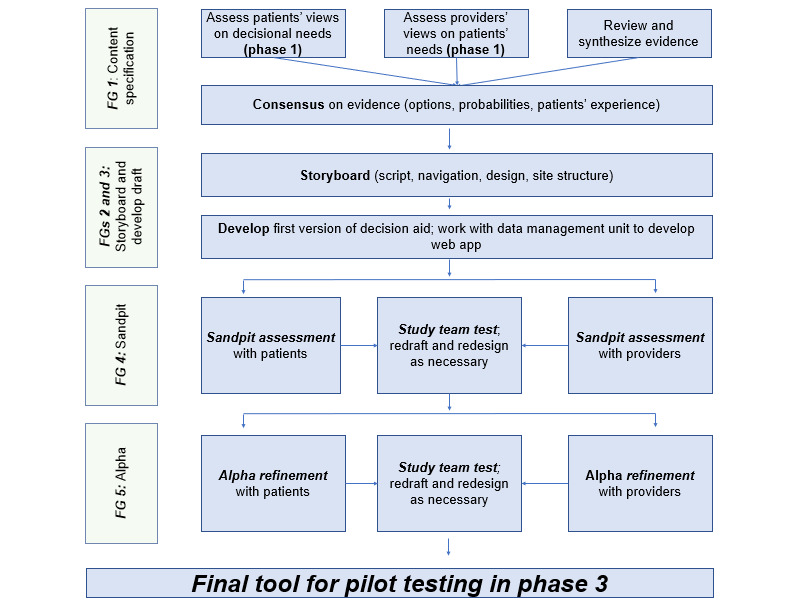
i.ARTs decision aid development process and focus groups (FGs). i.ARTs: i.ART+support.
Focus groups will use data from phase 1 to develop and finalize i.ARTs content and iteratively refine the web-based version of i.ARTs to prepare for the phase 3 pilot testing according to the schedule in Figure 3. Between each set of focus group meetings, the study team will integrate feedback from both the provider and WWH groups and either create the suggested content or make the suggested improvements to the i.ARTs program. This will ensure that women’s perspectives and realities will be captured to tailor the content within the patient decision aid.
Figure 3.
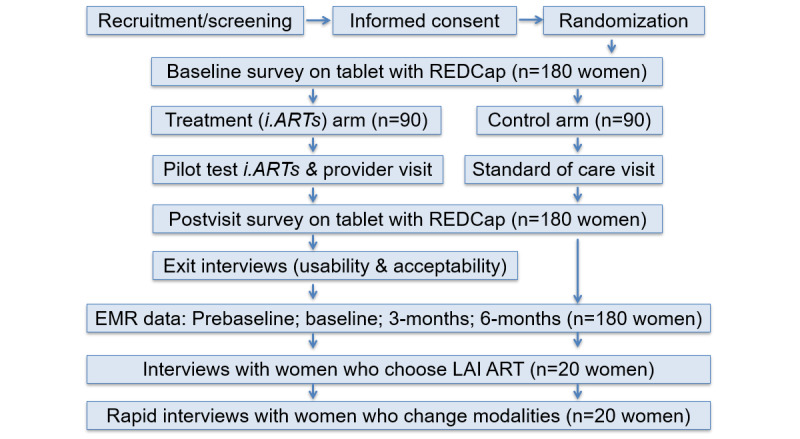
Pilot study flow and assessments. EMR: electronic medical record; iARTs: i.ART+support; LAI ART: long-acting injectable antiretroviral therapy.
Phase 3: Pilot Test i.ARTs
Study Design
Phase 3 will pilot test the final i.ARTs patient decision aid to assess feasibility, acceptability, and usability as well as to evaluate associations between receipt of i.ARTs and ART adherence, including viral suppression, ART refills, chosen ART modality, and clinic attendance. The pilot will be a 2-arm randomized controlled trial with 180 WWH. It will compare an intervention arm (n=90 WWH) that uses the i.ARTs decision aid tool developed in phases 1 and 2 with a standard-of-care control arm (n=90 WWH).
Setting, Participants, Recruitment, and Enrollment
Pilot testing will occur among WWH at the same Miami MWCCS clinical site where phase 2 focus groups occurred. The clinic is one of the largest hospital-based clinics in Miami, a city with one of the highest rates of incident and prevalent HIV infections in the United States. We will recruit 180 women from the existing client population over the course of 15 months (about 12 women per month). A full-time research associate will approach potential participants at their regular clinic visit, share information about the study, and invite them to take part in eligibility screening. Participants must meet the following eligibility criteria: (1) identify as female; (2) be receiving HIV-related care at the Miami site; (3) have a diagnosis of HIV infection; (4) aged 18 and older; (5) be interested in learning about LAI ART and open to discussing HIV treatment with their provider; and (6) be willing to provide informed consent.
Study Procedures
Randomization
Following informed consent and the baseline assessment (discussed later), we will use a web-based system to randomize women (1:1) to the i.ARTs intervention arm (n=90) or to the standard-of-care control arm (n=90). Neither participants nor researchers will be blind to study condition. Women in the control arm will receive standard of care as it exists at the time of LAI ART roll out: providers will offer women information about LAI ART and may strongly suggest which option she should choose. Women who participated in the focus groups will not be eligible for inclusion.
Women in the intervention arm will use i.ARTs as part of their regular participant visit. While the complete content of i.ARTs will be finalized in phase 2, the web-based i.ARTs app will guide WWH through the following activities: (1) provide education on different ART modalities, (2) utilize value clarification methods to guide women in ranking the prominence of relevant barriers and facilitators that may impact adherence to various ART modalities, (3) collect information on participant health history, (4) generate a suggested ART modality based on previous steps, (5) assist WWH in developing questions for their health care providers, and (6) generate an individual profile description as well as individualized suggestions to support adherence based on the modality selected (eg, a woman may be profiled as having transportation challenges; i.ARTs would suggest solutions—arranged transportation or transportation vouchers—that the clinic could employ to address these challenges). Participants will take a printout of this profile to their clinic visit with their provider. Intervention arm participants will receive US $15 for i.ARTs use.
Assessments
All women enrolled in the study will complete a baseline survey following informed consent and a follow-up survey immediately after their clinical visit. Surveys will be administered on tablets in a private room within the clinic and will use REDCap for data collection. Each survey will take approximately 25 minutes to complete, and women will receive US $25. Survey measures are described later and in Table 2.
Table 2.
i.ARTsa pilot study measures in phase 3.
| Outcomes | Description of measures | ||
| Primary | Assessed after i.ARTs use among women using i.ARTs (n=90) | ||
|
|
Feasibility | Adaptation of Thabane et al’s [59] framework [60,61] for assessing pilot studies: Process, Resources, Management, Science | |
|
|
Acceptability and usability | Acceptability [62] and CSQ-8b [63] to measure satisfaction with i.ARTs; exit interviews about i.ARTs features/protocol [62] | |
| Secondary | Assessed in baseline survey and postvisit survey for all women (n=180) | ||
|
|
Decisional conflict | Decisional Conflict Scale (16 items) [52]; satisfaction with the chosen method [64] | |
| Tertiary | Assessed 1-year prebaseline, at baseline, 3 months, and 6 months (all women; n=180) | ||
|
|
Viral suppression | Viral suppression, defined as viral load below limit of detection per assay used | |
|
|
Medication refills | Missed doses; report of medication refills/pharmacy pick up (WWHc on oral ARTd) | |
|
|
Clinic attendance | Missed or cancelled medical visits (<2 visits in a 6-month period for oral ART; missed monthly visit for LAIe ART) | |
|
|
ART modality | Adoption of LAI among patients who are virally suppressed | |
| Moderators | Assessed at baseline | ||
|
|
Sociodemographics | Race/ethnicity, age, housing stability, education, employment, income, distance from clinic, relationship status, children | |
|
|
Depression/anxiety | PHQ-8f (depression) [65]; GAD-7g [66] | |
|
|
Substance use | ASSISTh [67], an 8-item measure of problematic substance use | |
|
|
Stigma/discrimination | MDSi to measure racial discrimination [68]; HIV-related stigma | |
|
|
Self-efficacy | HIV-ASESj [38] | |
|
|
ART-related knowledge | Adaptation of Feldman’s [69] scale of oral ART knowledge; BMQk [70] | |
ai.ARTs: i.ART+support.
bCSQ-8: 8-item Client Satisfaction Questionnaire.
cWWH: women with HIV.
dART: antiretroviral therapy.
eLAI: long-acting injectable.
fPHQ-8: 8-item Patient Health Questionnaire.
gGAD-7: 7-item General Anxiety Disorder scale.
hASSIST: Alcohol, Smoking and Substance Involvement Screening Test.
iMDS: 10-item Multiple Discrimination Scale.
jHIV-ASES: HIV Treatment Adherence Self-Efficacy Scale.
kBMQ-18: 18-item Beliefs and Medicines Questionnaire.
A subsample of 20 intervention (i.ARTs) arm women and 10 providers will be randomly chosen to participate in an exit interview. This interview will occur either at the baseline visit or up to 1 month after i.ARTs use at the participant’s convenience. We will select every fourth woman in the intervention arm to complete the exit interview. Providers will be selected based on the number of i.ARTs participants they served in the first year of the pilot (we will randomly select 10 providers from all providers who serve above the median number of i.ARTs clients, which ensures that providers will have sufficient experience with the tool to provide feedback) and exit interviews with providers will be conducted in the final 3 months of data collection. These interviews will focus on patient and provider experiences with i.ARTs acceptability, feasibility, and usability, and the most useful ways to improve its integration into clinical practice. This will also include discussions of how women shared the results from the patient decision aid with their provider. Exit interviews will take approximately 30 minutes and participants will be provided US $30 in compensation.
The study will also use all 180 participants’ electronic medical records (EMRs) to assess the impact of the intervention on clinical outcomes (Table 2). The informed consent process will include the EMR abstraction release form and detailed information about the data abstraction process. Study staff will abstract data on viral suppression (all women), ART modality, clinic attendance (all women), and medication refill data (women on oral ART) from EMRs into a REDCap database. Abstraction will include data from 1-year prebaseline to 6 months postbaseline. This timeline is based on the frequency of HIV clinic visits (ie, approximately every 3 months) to ensure that women have at least one visit in the follow-up period.
Study Outcomes and Measures
All study outcomes are summarized in Table 2. Primary outcomes include i.ARTs feasibility, acceptability, and usability. To assess feasibility, we will adapt Thabane et al’s [59] framework [60,61]. For acceptability and usability, we will use the 8-item Client Satisfaction Questionnaire (CSQ-8) to assess participants’ attitudes, burden, ethics, coherence, opportunity cost, perceived effectiveness, and self-efficacy [63]. The secondary outcome is ART-related decisional conflict, a 16-item scale scored from 0 to 100 [52]. Scores of 0-25 are associated with “implementing decisions” and scores of 37.5-100 are associated with “decision delay/feeling unsure.” Tertiary outcomes are exploratory, and consist of information extracted from EMRs, including viral suppression at the most recent visit in the prior 6 months, ART modality, medication refills (oral ART), and clinic attendance in the prior 6 months.
In addition to the outcomes, the baseline survey will measure moderators associated with adherence, including sociodemographics, depression, anxiety, substance use, HIV-related stigma and discrimination, self-efficacy, and ART-related knowledge.
Analysis
The primary outcomes of feasibility, acceptability, and usability will be assessed descriptively. We will compare ART-related decisional conflict, clinic attendance, viral suppression, and medication refills between and within the 90 women in each arm (baseline vs postvisit surveys and EMR data at 3 and 6 months). For normally distributed data, we will first use t tests to compare the 2 arms and then use regression methods to develop a multivariable model of the outcome, with arm membership as the principal covariate, while controlling for the moderators listed in the previous sections and in Table 2. For nonnormally distributed data, we will employ chi-square tests and logistic regression; distributional properties of the outcome will determine the type of logistic regression (eg, binary, multinomial, or ordinal logistic regression models). We note that these are examples and the final approach will be determined based on the distribution of the data.
The primary analysis will use an intent-to-treat approach with the use of i.ARTs as a 1-time exposure, with α=.05, and 2-sided tests. The number of moderators included in the regression model will be used to make a Bonferroni adjustment to the α value. A secondary efficacy analysis will explore actual treatment taken. Our exploratory tertiary outcomes of viral suppression, clinic attendance, chosen ART modality, and medication refills will compare both between and within i.ARTs and control arm women (ie, baseline and postvisit surveys and EMR data at 3 and 6 months); we will also examine these outcomes for women who do not change regimens. We will use prebaseline EMR data to control for previous viral load. We will also compare how many women in each arm change modalities postbaseline to assess i.ARTs’ accuracy in identifying which HIV treatment modality is the best match. Sensitivity analyses will determine whether assumptions associated with each model are defensible.
Power Analysis and Sample Size
Our sample size is based on our secondary outcome of decisional conflict, as our primary outcomes of acceptability and feasibility are only assessed in the intervention group (as the control group will not experience i.ARTs). Scale developers use a moderate effect size (0.3-0.4) to determine sample size. Using the average weighted means and SDs from prior studies [52], we calculated sample size for α=.05, power=0.8 for an independent samples t test. Assuming a mean score of 21 (SD 16) in the intervention (i.ARTs) group and a mean score of 28 (SD 18) in the control group, the sample size of 90 in each group, for a total of 180, provides 80% power. Our tertiary outcomes are exploratory, but as 35% of US women are not adherent after 6 months, we are powered to detect basic differences between the intervention and control arms.
Ethics Approval
This study received approval from the Columbia University Institutional Review Board (IRB; approval number IRB-AAAT5314), and we will receive approval from University of Miami IRB once the clinical trial portion begins.
Results
This study was funded in March 2021. Protocols for phase 1 interviews have been developed and interviews with service providers started in September 2021. We will apply for Clinicaltrials.gov registration prior to phase 3, which is when our first participant will be enrolled in the randomized controlled trial. This is anticipated to occur in April 2023.
Discussion
Research Implications
This paper describes the protocol for developing and piloting the first decision aid to facilitate women’s decision making between LAI and oral ART modality for HIV treatment. This study will expand HIV treatment research in important ways. The field of HIV care has increasingly emphasized patient-centered care [42], as this approach improves quality of life, patient adherence, and health outcomes [28,42,71]. Patient decision aids are a key tool for patient-centered care, as they can enhance equity in medicine, activate patients to increase their knowledge and decision-making power, and lower medical paternalism [24,29-31]. They also enhance patient care by enabling shared discussions and outcomes that match patients’ needs [26,42,72].
As noted, patient decision aids have been successfully used in other areas of medicine, most similarly to this in facilitating contraception decision making among women [40,41]. However, as yet no patient decision aids exist to facilitate the decision-making process between oral and LAI HIV treatment. Thus, i.ARTs is urgently needed to promote equity in patient-provider decision making by helping WWH identify their preferences for oral or LAI ART. Further, developing i.ARTs specifically for women may help promote gender equity in the uptake of LAI ART. Examples from oral pre-exposure prophylaxis (PrEP) scale up show that a lack of decision aid tools can hinder uptake and lead to gender-related disparities [73-76]. Women not only have much lower rates of PrEP use than men but also have shorter periods of sustained PrEP use [75,77,78]. Our study to develop and pilot test the i.ARTs patient decision aid aims to prevent a similar gender gap for LAI ART uptake and thus a further exacerbation of disparities in care and treatment outcomes.
Furthermore, this study comes at a crucial moment in HIV treatment. As the menu of options for ART treatment expands, patients and providers will encounter different considerations and concerns regarding the various ART modalities. Despite LAI ART’s potential, we know little about the “real-world” facilitators and barriers WWH will face, or how providers will decide to whom to offer LAI ART. The FDA approved LAI ART in January 2021, and developing i.ARTs as LAI ART is rolled out has the potential to capture and address some of these real-world barriers and facilitators. In turn, it will provide data that can support the further dissemination of this new biomedical technology. In addition, i.ARTs can be updated to incorporate future ART modalities (eg, monthly oral medication [79,80], implants [81], subcutaneous injections [82]).
Study Strengths
Our study has multiple strengths that ensure the validity and applicability of its findings. As described in the previous sections, we incorporate patient and provider perspectives throughout i.ARTs development (phase 1, phase 2) and testing (exit interviews in phase 3). This ensures that i.ARTs content includes the full landscape of factors that influence LAI ART uptake, determine whether LAI or oral ART best fits a woman’s values and preferences, and will help us to identify potential differences in patient and providers’ beliefs regarding who should be offered LAI ART.
Next, our real-world clinic-based sample can provide insight into LAI ART uptake that samples of clinical trials participants cannot. As mentioned, women were underrepresented in LAI ART clinical trials [10-13]. Working with women from the MWCCS not only resolves this gender gap, but also provides data that trial- and clinic-based samples (eg, CFAR Network of Integrated Clinical Systems [CNICS] sites) cannot, as it includes out-of-care women who may face different and additional adherence barriers. Clinical trials include participants with high adherence to determine drug efficacy, but only 49% of MWCCS women would be eligible for AIDS Clinical Trials Group trials [44]. Furthermore, pilot testing i.ARTs in Miami, which leads the United States in HIV incidence, and whose HIV epidemic is marked by stark racial and ethnic disparities, will help us identify the full landscape of WWH’s barriers to ART use and potential solutions to address them. This will increase the relevance of i.ARTs for WWH across the United States.
Finally, our study benefits from its mixed methods approach [83], which increases validity by capitalizing on each method’s inherent strengths. The different methods answer complementary research questions: we will use the phase 1 survey and provider interviews to explore potential disconnects between who may be offered LAI ART and who wants it, which can affect patient equity; interviews with women who choose LAI ART will explore the mechanisms that drive viral suppression data abstracted from EMRs. We will integrate mixed method findings using “triangulation” (ie, use of 2 different methods to address the same research question) to improve reliability [84,85]. Consistency of findings across methodological approaches increases validity and reliability and suggests findings are not due to methodological artifacts [86]. This will allow us to see how themes vary across methods, leading to stronger findings than using each method independently.
Expected Challenges
As this study is set in real-world clinical settings, we expect challenges to incorporating i.ARTs delivery into existing clinical workflows. These include considerations such as available space for participants to complete i.ARTs, tech support for the computerized tool and printout, staffing resources, and limited clinician time with each patient to discuss the decision aid results. The study team will work with the clinic staff to determine how to ensure efficient delivery of i.ARTs in the clinical setting. Exit interviews will be used to collect data on these challenges to inform further scale-up or dissemination efforts of i.ARTs outside of the Miami-based clinic.
As LAI ART is being administered in a real-world setting, participating clinicians are subject to existing, and possibly changing, FDA approvals. While phase 3 trials are currently testing LAI ART for nonadherent patients, the FDA-approved LAI ART formulation is only for virally suppressed patients. To ensure i.ARTs incorporates all relevant drivers of ART use for all women, we aim to include nonadherent women in aim 3, pending FDA approval. If FDA approval only exists for virally suppressed WWH when aim 3 starts, then we will limit the pilot test to that population. Should FDA approval for nonadherent patients begin in the middle of the pilot, we may adjust eligibility criteria to ensure a standard participant population throughout the pilot period. The study team will carefully monitor developments in ART treatment technologies and adapt accordingly. In addition, LAI ART was initially approved as a monthly injection, and the US FDA has approved a bimonthly (ie, every 8 weeks) formulation of LAI ART. This may also affect women’s preferences as the study is rolled out.
New modalities of ART delivery may emerge prior to the pilot study period, including monthly oral medication [79,80], implants [81], and subcutaneous injections [82]. The addition of these new technologies could further complicate the decision-making process. As mentioned earlier, the i.ARTs tool is web based and thus easy to update as technology or treatment modalities change [87]. The web-based nature of this tool will allow us to adapt and expand i.ARTs to all genders in future studies. In addition, a similar approach could be used to develop a tool to facilitate women’s decision-making process between oral and LAI versions of PrEP, with a goal to limit HIV infections among women.
Acknowledgments
This project was funded by the National Institute of Mental Health (NIMH) to MMP (R34MH124552). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH. The study described in this protocol paper will utilize data collected by the MACS/WIHS Combined Cohort Study (MWCCS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). MWCCS (Principal Investigators): Atlanta Clinical Research Site (CRS; Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange and Elizabeth Golub), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Northern California CRS (Bradley Aouizerat, Jennifer Price, and Phyllis Tien), U01-HL146242; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; UAB-MS CRS (Mirjam-Colette Kempf, Jodie Dionne-Odom, and Deborah Konkle-Parker), U01-HL146192; UNC CRS (Adaora Adimora), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional cofunding from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), National Institute On Aging (NIA), National Institute of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute of Neurological Disorders And Stroke (NINDS), National Institute of Mental Health (NIMH), National Institute on Drug Abuse (NIDA), National Institute of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute on Minority Health and Health Disparities (NIMHD), and in coordination and alignment with the research priorities of the National Institutes of Health, Office of AIDS Research (OAR). MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), UL1-TR003098 (JHU ICTR), UL1-TR001881 (UCLA CTSI), P30-AI-050409 (Atlanta CFAR), P30-AI-073961 (Miami CFAR), P30-AI-050410 (UNC CFAR), P30-AI-027767 (UAB CFAR), and P30-MH-116867 (Miami CHARM). The authors gratefully acknowledge the contributions of the study participants and dedication of the staff at the MWCCS sites.
Abbreviations
- ART
antiretroviral therapy
- CNICS
CFAR Network of Integrated Clinical Systems
- EMR
electronic medical record
- FDA
Food and Drug Administration
- i.ARTs
i.ART+support
- IPDAS
International Patient Decision Aids Standards Collaboration
- IRB
institutional review board
- LAI ART
long-acting injectable ART
- MWCCS
MACS/WIHS Combined Cohort Study
- PrEP
pre-exposure prophylaxis
- WWH
women with HIV
Footnotes
Authors' Contributions: MMP, MA, MP, and VAS conceptualized the study and the methodology. MMP, MP, and VAS contributed to the statistical analysis plan. TM and MMP wrote the original manuscript. All authors reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest: AAA has received personal funds from Merck, Viiv, and Gilead for consulting; her institution has received funding from Merck and Gilead for her research.
References
- 1.Centers for Disease Control and Prevention (CDC) Women | Gender | HIV by Group | HIV/AIDS | CDC Internet. CDC. 2018. [2020-05-21]. https://www.cdc.gov/hiv/group/gender/women/index.html .
- 2.Rice Whitney S, Turan Bulent, Fletcher Faith E, Nápoles Tessa M, Walcott Melonie, Batchelder Abigail, Kempf Mirjam-Colette, Konkle-Parker Deborah J, Wilson Tracey E, Tien Phyllis C, Wingood Gina M, Neilands Torsten B, Johnson Mallory O, Weiser Sheri D, Turan Janet M. A Mixed Methods Study of Anticipated and Experienced Stigma in Health Care Settings Among Women Living with HIV in the United States. AIDS Patient Care STDS. 2019 Apr;33(4):184–195. doi: 10.1089/apc.2018.0282. https://europepmc.org/abstract/MED/30932700 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turan B, Rice WS, Crockett KB, Johnson M, Neilands TB, Ross SN, Kempf M, Konkle-Parker D, Wingood G, Tien PC, Cohen M, Wilson TE, Logie CH, Sosanya O, Plankey M, Golub E, Adimora AA, Parish C, Weiser SD, Turan JM. Longitudinal association between internalized HIV stigma and antiretroviral therapy adherence for women living with HIV: the mediating role of depression. AIDS. 2019 Mar 01;33(3):571–576. doi: 10.1097/QAD.0000000000002071. https://europepmc.org/abstract/MED/30702521 .00002030-201903010-00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puskas CM, Kaida A, Miller CL, Zhang W, Yip B, Pick N, Montaner JSG, Hogg RS. The adherence gap: a longitudinal examination of men's and women's antiretroviral therapy adherence in British Columbia, 2000-2014. AIDS. 2017 Mar 27;31(6):827–833. doi: 10.1097/QAD.0000000000001408.00002030-201703270-00011 [DOI] [PubMed] [Google Scholar]
- 5.Beer L, Mattson CL, Bradley H, Skarbinski J, Medical Monitoring Project Understanding Cross-Sectional Racial, Ethnic, and Gender Disparities in Antiretroviral Use and Viral Suppression Among HIV Patients in the United States. Medicine (Baltimore) 2016 Mar;95(13):e3171. doi: 10.1097/MD.0000000000003171. doi: 10.1097/MD.0000000000003171.00005792-201603290-00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mugavero MJ, Napravnik S, Cole SR, Eron JJ, Lau B, Crane HM, Kitahata MM, Willig JH, Moore RD, Deeks SG, Saag MS, Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) Cohort Study Viremia copy-years predicts mortality among treatment-naive HIV-infected patients initiating antiretroviral therapy. Clin Infect Dis. 2011 Nov;53(9):927–35. doi: 10.1093/cid/cir526. https://europepmc.org/abstract/MED/21890751 .cir526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pellowski JA, Price DM, Harrison AD, Tuthill EL, Myer L, Operario D, Lurie MN. A Systematic Review and Meta-analysis of Antiretroviral Therapy (ART) Adherence Interventions for Women Living with HIV. AIDS Behav. 2019 Aug;23(8):1998–2013. doi: 10.1007/s10461-018-2341-9. https://europepmc.org/abstract/MED/30443806 .10.1007/s10461-018-2341-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Selected National HIV Prevention and Care Outcomes (2015, 2016) CDC. 2017. [2019-05-08]. https://www.cdc.gov/hiv/pdf/library/slidesets/cdc-hiv-prevention-and-care-outcomes.pdf .
- 9.Food and Drug Administration (FDA) FDA Approves First Extended-Release, Injectable Drug Regimen for Adults Living with HIV. FDA. 2021. [2022-08-15]. https://www.fda.gov/news-events/press-announcements/fda-approves-first-extended-release -injectable-drug-regimen-adults-living-hiv .
- 10.Swindells S, Andrade-Villanueva J, Richmond GJ, Rizzardini G, Baumgarten A, Masiá Mar, Latiff G, Pokrovsky V, Bredeek F, Smith G, Cahn P, Kim Y, Ford SL, Talarico CL, Patel P, Chounta V, Crauwels H, Parys W, Vanveggel S, Mrus J, Huang J, Harrington CM, Hudson KJ, Margolis DA, Smith KY, Williams PE, Spreen WR. Long-Acting Cabotegravir and Rilpivirine for Maintenance of HIV-1 Suppression. N Engl J Med. 2020 Mar 19;382(12):1112–1123. doi: 10.1056/NEJMoa1904398. [DOI] [PubMed] [Google Scholar]
- 11.Overton ET, Richmond G, Rizzardini G, Jaeger H, Orrell C, Nagimova F, Bredeek F, García Deltoro Miguel, Swindells S, Andrade-Villanueva JF, Wong A, Khuong-Josses M, Van Solingen-Ristea R, van Eygen V, Crauwels H, Ford S, Talarico C, Benn P, Wang Y, Hudson KJ, Chounta V, Cutrell A, Patel P, Shaefer M, Margolis DA, Smith KY, Vanveggel S, Spreen W. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 48-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet. 2021 Dec 19;396(10267):1994–2005. doi: 10.1016/S0140-6736(20)32666-0.S0140-6736(20)32666-0 [DOI] [PubMed] [Google Scholar]
- 12.Margolis DA, Gonzalez-Garcia J, Stellbrink H, Eron JJ, Yazdanpanah Y, Podzamczer D, Lutz T, Angel JB, Richmond GJ, Clotet B, Gutierrez F, Sloan L, Clair MS, Murray M, Ford SL, Mrus J, Patel P, Crauwels H, Griffith SK, Sutton KC, Dorey D, Smith KY, Williams PE, Spreen WR. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017 Sep 23;390(10101):1499–1510. doi: 10.1016/S0140-6736(17)31917-7.S0140-6736(17)31917-7 [DOI] [PubMed] [Google Scholar]
- 13.Fernandez C, van Halsema CL. Evaluating cabotegravir/rilpivirine long-acting, injectable in the treatment of HIV infection: emerging data and therapeutic potential. HIV AIDS (Auckl) 2019;11:179–192. doi: 10.2147/HIV.S184642. doi: 10.2147/HIV.S184642.184642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margolis DA, Boffito M. Long-acting antiviral agents for HIV treatment. Curr Opin HIV AIDS. 2015 Jul;10(4):246–52. doi: 10.1097/COH.0000000000000169. https://europepmc.org/abstract/MED/26049949 .01222929-201507000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson JM, Flexner CW. Universal antiretroviral regimens: thinking beyond one-pill-once-a-day. Curr Opin HIV AIDS. 2017 Jul;12(4):343–350. doi: 10.1097/COH.0000000000000374. https://europepmc.org/abstract/MED/28368868 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nachman S, Townsend CL, Abrams EJ, Archary M, Capparelli E, Clayden P, Lockman S, Jean-Philippe P, Mayer K, Mirochnick M, McKenzie-White J, Struble K, Watts H, Flexner C. Long-acting or extended-release antiretroviral products for HIV treatment and prevention in infants, children, adolescents, and pregnant and breastfeeding women: knowledge gaps and research priorities. Lancet HIV. 2019 Aug;6(8):e552–e558. doi: 10.1016/S2352-3018(19)30147-X. https://linkinghub.elsevier.com/retrieve/pii/S2352-3018(19)30147-X .S2352-3018(19)30147-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandhi M, Ameli N, Bacchetti P, Sharp GB, French AL, Young M, Gange SJ, Anastos K, Holman S, Levine A, Greenblatt RM. Eligibility criteria for HIV clinical trials and generalizability of results: the gap between published reports and study protocols. AIDS. 2005 Nov 04;19(16):1885–96. doi: 10.1097/01.aids.0000189866.67182.f7.00002030-200511040-00018 [DOI] [PubMed] [Google Scholar]
- 18.Philbin MM, Parish CL, Kinnard EN, Reed SE, Kerrigan D, Alcaide ML, Cohen MH, Sosanya O, Sheth AN, Adimora AA, Cocohoba J, Goparaju L, Golub ET, Fischl M, Metsch LR. Multisite Study of Women Living With HIV's Perceived Barriers to, and Interest in, Long-Acting Injectable Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2020 Jul 01;84(3):263–270. doi: 10.1097/QAI.0000000000002337. https://europepmc.org/abstract/MED/32530905 .00126334-202007010-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark LR. Will the pill make me sterile? Addressing reproductive health concerns and strategies to improve adherence to hormonal contraceptive regimens in adolescent girls. J Pediatr Adolesc Gynecol. 2001 Nov;14(4):153–62. doi: 10.1016/s1083-3188(01)00123-1.S1083318801001231 [DOI] [PubMed] [Google Scholar]
- 20.Philbin MM, Parish C, Kinnard EN, Reed SE, Kerrigan D, Alcaide ML, Cohen MH, Sosanya O, Sheth AN, Adimora AA, Cocohoba J, Goparaju L, Golub ET, Fischl M, Metsch LR. Interest in Long-Acting Injectable Pre-exposure Prophylaxis (LAI PrEP) Among Women in the Women's Interagency HIV Study (WIHS): A Qualitative Study Across Six Cities in the United States. AIDS Behav. 2021 Mar;25(3):667–678. doi: 10.1007/s10461-020-03023-9. https://europepmc.org/abstract/MED/32910351 .10.1007/s10461-020-03023-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benning L, Mantsios A, Kerrigan D, Coleman JS, Golub E, Blackstock O, Konkle-Parker D, Philbin M, Sheth A, Adimora AA, Cohen MH, Seidman D, Milam J, Kassaye SG, Taylor T, Murray M. Examining adherence barriers among women with HIV to tailor outreach for long-acting injectable antiretroviral therapy. BMC Womens Health. 2020 Jul 25;20(1):152. doi: 10.1186/s12905-020-01011-8. https://bmcwomenshealth.biomedcentral.com/articles/10.1186/s12905-020-01011-8 .10.1186/s12905-020-01011-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerrigan D, Mantsios A, Grant R, Markowitz M, Defechereux P, La Mar M, Beckham SW, Hammond P, Margolis D, Murray M. Expanding the Menu of HIV Prevention Options: A Qualitative Study of Experiences with Long-Acting Injectable Cabotegravir as PrEP in the Context of a Phase II Trial in the United States. AIDS Behav. 2018 Nov;22(11):3540–3549. doi: 10.1007/s10461-017-2017-x. https://europepmc.org/abstract/MED/29290075 .10.1007/s10461-017-2017-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerrigan D, Mantsios A, Gorgolas M, Montes M, Pulido F, Brinson C, deVente J, Richmond GJ, Beckham SW, Hammond P, Margolis D, Murray M. Experiences with long acting injectable ART: A qualitative study among PLHIV participating in a Phase II study of cabotegravir + rilpivirine (LATTE-2) in the United States and Spain. PLoS One. 2018;13(1):e0190487. doi: 10.1371/journal.pone.0190487. https://dx.plos.org/10.1371/journal.pone.0190487 .PONE-D-17-20666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stacey D, Légaré France, Lewis K, Barry MJ, Bennett CL, Eden KB, Holmes-Rovner M, Llewellyn-Thomas H, Lyddiatt A, Thomson R, Trevena L. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017 Apr 12;4:CD001431. doi: 10.1002/14651858.CD001431.pub5. https://europepmc.org/abstract/MED/28402085 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stacey D, Kryworuchko J, Belkora J, Davison BJ, Durand M, Eden KB, Hoffman AS, Koerner M, Légaré France, Loiselle M, Street RL. Coaching and guidance with patient decision aids: A review of theoretical and empirical evidence. BMC Med Inform Decis Mak. 2013;13 Suppl 2:S11. doi: 10.1186/1472-6947-13-S2-S11. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/1472-6947-13-S2-S11 .1472-6947-13-S2-S11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango) Soc Sci Med. 1997 Mar;44(5):681–92. doi: 10.1016/s0277-9536(96)00221-3.S0277953696002213 [DOI] [PubMed] [Google Scholar]
- 27.O'Connor AM, Rostom A, Fiset V, Tetroe J, Entwistle V, Llewellyn-Thomas H, Holmes-Rovner M, Barry M, Jones J. Decision aids for patients facing health treatment or screening decisions: systematic review. BMJ. 1999 Sep 18;319(7212):731–4. doi: 10.1136/bmj.319.7212.731. https://europepmc.org/abstract/MED/10487995 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision making and patient outcomes. Med Decis Making. 2015 Jan;35(1):114–31. doi: 10.1177/0272989X14551638. https://europepmc.org/abstract/MED/25351843 .0272989X14551638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Légaré France, Ratté Stéphane, Gravel K, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals' perceptions. Patient Educ Couns. 2008 Dec;73(3):526–35. doi: 10.1016/j.pec.2008.07.018.S0738-3991(08)00350-9 [DOI] [PubMed] [Google Scholar]
- 30.Stacey D, Samant R, Bennett C. Decision making in oncology: a review of patient decision aids to support patient participation. CA Cancer J Clin. 2008;58(5):293–304. doi: 10.3322/CA.2008.0006. doi: 10.3322/CA.2008.0006.CA.2008.0006 [DOI] [PubMed] [Google Scholar]
- 31.Ankolekar A, Vanneste BGL, Bloemen-van Gurp E, van Roermund JG, van Limbergen EJ, van de Beek K, Marcelissen T, Zambon V, Oelke M, Dekker A, Roumen C, Lambin P, Berlanga A, Fijten R. Development and validation of a patient decision aid for prostate Cancer therapy: from paternalistic towards participative shared decision making. BMC Med Inform Decis Mak. 2019 Jul 11;19(1):130. doi: 10.1186/s12911-019-0862-4. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911-019-0862-4 .10.1186/s12911-019-0862-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trenaman L, Selva A, Desroches S, Singh K, Bissonnette J, Bansback N, Stacey D. A measurement framework for adherence in patient decision aid trials applied in a systematic review subanalysis. J Clin Epidemiol. 2016 Sep;77:15–23. doi: 10.1016/j.jclinepi.2016.03.032.S0895-4356(16)30129-9 [DOI] [PubMed] [Google Scholar]
- 33.Roberts KJ. Physician-patient relationships, patient satisfaction, and antiretroviral medication Adherence among HIV-infected adults attending a public health clinic. AIDS Patient Care STDS. 2002 Jan;16(1):43–50. doi: 10.1089/108729102753429398. [DOI] [PubMed] [Google Scholar]
- 34.Dang BN, Westbrook RA, Black WC, Rodriguez-Barradas MC, Giordano TP. Examining the link between patient satisfaction and adherence to HIV care: a structural equation model. PLoS One. 2013;8(1):e54729. doi: 10.1371/journal.pone.0054729. https://dx.plos.org/10.1371/journal.pone.0054729 .PONE-D-12-24455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jordan J, Cahn P, Goebel F, Matheron S, Bradley C, Woodcock A. Abacavir compared to protease inhibitors as part of HAART regimens for treatment of HIV infection: patient satisfaction and implications for adherence. AIDS Patient Care STDS. 2005 Jan;19(1):9–18. doi: 10.1089/apc.2005.19.9. [DOI] [PubMed] [Google Scholar]
- 36.Elwyn G, Kreuwel I, Durand MA, Sivell S, Joseph-Williams N, Evans R, Edwards A. How to develop web-based decision support interventions for patients: a process map. Patient Educ Couns. 2011 Feb;82(2):260–5. doi: 10.1016/j.pec.2010.04.034.S0738-3991(10)00281-8 [DOI] [PubMed] [Google Scholar]
- 37.Wolf MS, Davis TC, Osborn CY, Skripkauskas S, Bennett CL, Makoul G. Literacy, self-efficacy, and HIV medication adherence. Patient Educ Couns. 2007 Feb;65(2):253–60. doi: 10.1016/j.pec.2006.08.006.S0738-3991(06)00265-5 [DOI] [PubMed] [Google Scholar]
- 38.Johnson MO, Neilands TB, Dilworth SE, Morin SF, Remien RH, Chesney MA. The role of self-efficacy in HIV treatment adherence: validation of the HIV Treatment Adherence Self-Efficacy Scale (HIV-ASES) J Behav Med. 2007 Oct;30(5):359–70. doi: 10.1007/s10865-007-9118-3. https://europepmc.org/abstract/MED/17588200 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beach MC, Keruly J, Moore RD. Is the quality of the patient-provider relationship associated with better adherence and health outcomes for patients with HIV? J Gen Intern Med. 2006 Jun;21(6):661–5. doi: 10.1111/j.1525-1497.2006.00399.x. https://onlinelibrary.wiley.com/resolve/openurl?genre=article&sid=nlm:pubmed&issn=0884-8734&date=2006&volume=21&issue=6&spage=661 .JGI399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dehlendorf C, Reed R, Fitzpatrick J, Kuppermann M, Steinauer J, Kimport K. A mixed-methods study of provider perspectives on My Birth Control: a contraceptive decision support tool designed to facilitate shared decision making. Contraception. 2019 Nov;100(5):420–423. doi: 10.1016/j.contraception.2019.08.001.S0010-7824(19)30385-3 [DOI] [PubMed] [Google Scholar]
- 41.Dehlendorf C, Grumbach K, Schmittdiel JA, Steinauer J. Shared decision making in contraceptive counseling. Contraception. 2017 May;95(5):452–455. doi: 10.1016/j.contraception.2016.12.010. https://europepmc.org/abstract/MED/28069491 .S0010-7824(17)30002-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elwyn G, Dehlendorf C, Epstein RM, Marrin K, White J, Frosch DL. Shared decision making and motivational interviewing: achieving patient-centered care across the spectrum of health care problems. Ann Fam Med. 2014;12(3):270–5. doi: 10.1370/afm.1615. http://www.annfammed.org/cgi/pmidlookup?view=long&pmid=24821899 .12/3/270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ending the HIV Epidemic: A Plan for America. Health Resources and Services Administration (HRSA) 2019. [2022-08-15]. https://www.hrsa.gov/ending-hiv-epidemic .
- 44.Adimora AA, Ramirez C, Benning L, Greenblatt RM, Kempf M, Tien PC, Kassaye SG, Anastos K, Cohen M, Minkoff H, Wingood G, Ofotokun I, Fischl MA, Gange S. Cohort Profile: The Women's Interagency HIV Study (WIHS) Int J Epidemiol. 2018 Apr 01;47(2):393–394i. doi: 10.1093/ije/dyy021. https://europepmc.org/abstract/MED/29688497 .4904205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coulter A, Stilwell D, Kryworuchko J, Mullen PD, Ng CJ, van der Weijden Trudy. A systematic development process for patient decision aids. BMC Med Inform Decis Mak. 2013;13 Suppl 2:S2. doi: 10.1186/1472-6947-13-S2-S2. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/1472-6947-13-S2-S2 .1472-6947-13-S2-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vollmer Dahlke Deborah, Fair K, Hong YA, Beaudoin CE, Pulczinski J, Ory MG. Apps seeking theories: results of a study on the use of health behavior change theories in cancer survivorship mobile apps. JMIR Mhealth Uhealth. 2015 Mar 27;3(1):e31. doi: 10.2196/mhealth.3861. https://mhealth.jmir.org/2015/1/e31/ v3i1e31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Creswell J, Clark V. Designing and Conducting Mixed Methods Research. Thousand Oaks, CA: Sage Publications; 2017. Sep, pp. 1–520. [Google Scholar]
- 48.Wu JP, Damschroder LJ, Fetters MD, Zikmund-Fisher BJ, Crabtree BF, Hudson SV, Ruffin MT, Fucinari J, Kang M, Taichman LS, Creswell JW. A Web-Based Decision Tool to Improve Contraceptive Counseling for Women With Chronic Medical Conditions: Protocol For a Mixed Methods Implementation Study. JMIR Res Protoc. 2018 Apr 18;7(4):e107. doi: 10.2196/resprot.9249. https://www.researchprotocols.org/2018/4/e107/ v7i4e107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elwyn G, O'Connor A, Stacey D, Volk R, Edwards A, Coulter A, Thomson R, Barratt A, Barry M, Bernstein S, Butow P, Clarke A, Entwistle V, Feldman-Stewart D, Holmes-Rovner M, Llewellyn-Thomas H, Moumjid N, Mulley A, Ruland C, Sepucha K, Sykes A, Whelan T, International Patient Decision Aids Standards (IPDAS) Collaboration Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006 Aug 26;333(7565):417. doi: 10.1136/bmj.38926.629329.AE. https://europepmc.org/abstract/MED/16908462 .bmj.38926.629329.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.International Patient Decision Aid Standards (IPDAS) Collaboration 2012 Update of the IPDAS Collaboration Background Document. IPDAS. 2012. [2022-08-15]. http://ipdas.ohri.ca/resources.html .
- 51.Légaré France, O'Connor AM, Graham ID, Wells GA, Tremblay S. Impact of the Ottawa Decision Support Framework on the agreement and the difference between patients' and physicians' decisional conflict. Med Decis Making. 2006;26(4):373–90. doi: 10.1177/0272989X06290492.26/4/373 [DOI] [PubMed] [Google Scholar]
- 52.O'Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15(1):25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 53.O'Connor AM, Tugwell P, Wells GA, Elmslie T, Jolly E, Hollingworth G, McPherson R, Bunn H, Graham I, Drake E. A decision aid for women considering hormone therapy after menopause: decision support framework and evaluation. Patient Educ Couns. 1998 Mar;33(3):267–79. doi: 10.1016/s0738-3991(98)00026-3.S0738-3991(98)00026-3 [DOI] [PubMed] [Google Scholar]
- 54.Neumann JV, Morgenstern O, Kuhn H. Theory of Games and Economic Behavior (Commemorative Edition) Princeton, NJ: Princeton University Press; 2007. [Google Scholar]
- 55.Frisch D, Clemen RT. Beyond expected utility: rethinking behavioral decision research. Psychol Bull. 1994 Jul;116(1):46–54. doi: 10.1037/0033-2909.116.1.46. [DOI] [PubMed] [Google Scholar]
- 56.Florida Department of Health HIV among Women: Miami-Dade. Florida Department of Health. 2016. [2022-08-15]. https://tinyurl.com/2myxjzpr .
- 57.Florida Department of Health Department of Health: Epidemiology of HIV in Miami-Dade County. Florida Department of Health. 2018. [2022-08-15]. http://miamidade.floridahealth.gov/programs-and-services/infectious-disease-services/hiv-aids-services/_documents/2019/_documents/HIV-in-Miami-Dade-2018.pdf .
- 58.AIDSVu Local Data: Miami (Miami-Dade County) AIDSVu. [2022-08-15]. https://aidsvu.org/local-data/united-states/south/florida/miami/
- 59.Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, Robson R, Thabane M, Giangregorio L, Goldsmith CH. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010 Jan 06;10:1. doi: 10.1186/1471-2288-10-1. https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/1471-2288-10-1 .1471-2288-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tickle-Degnen L. Nuts and bolts of conducting feasibility studies. Am J Occup Ther. 2013;67(2):171–6. doi: 10.5014/ajot.2013.006270. https://europepmc.org/abstract/MED/23433271 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arain M, Campbell MJ, Cooper CL, Lancaster GA. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med Res Methodol. 2010 Jul 16;10:67. doi: 10.1186/1471-2288-10-67. https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/1471-2288-10-67 .1471-2288-10-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res. 2017 Jan 26;17(1):88. doi: 10.1186/s12913-017-2031-8. https://bmchealthservres.biomedcentral.com/articles/10.1186/s12913-017-2031-8 .10.1186/s12913-017-2031-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsubara C, Green J, Astorga LT, Daya EL, Jervoso HC, Gonzaga EM, Jimba M. Reliability tests and validation tests of the client satisfaction questionnaire (CSQ-8) as an index of satisfaction with childbirth-related care among Filipino women. BMC Pregnancy Childbirth. 2013 Dec 17;13:235. doi: 10.1186/1471-2393-13-235. https://bmcpregnancychildbirth.biomedcentral.com/articles/10.1186/1471-2393-13-235 .1471-2393-13-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dehlendorf C, Vittinghoff E, Fitzpatrick J, Fox E, Holt K, Reed R, Campora M, Sokoloff A, Kuppermann M. Patient-Centered Outcomes Research Institute (PCORI) Washington, DC: Patient-Centered Outcomes Research Institute; 2019. [2022-08-15]. A Decision Aid to Help Women Choose and Use a Method of Birth Control. https://www.pcori.org/document/decision-aid-help-women-choose-and-use- method-birth-control . [PubMed] [Google Scholar]
- 65.Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009 Apr;114(1-3):163–73. doi: 10.1016/j.jad.2008.06.026.S0165-0327(08)00282-6 [DOI] [PubMed] [Google Scholar]
- 66.Spitzer RL, Kroenke K, Williams JBW, Löwe Bernd. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006 May 22;166(10):1092–7. doi: 10.1001/archinte.166.10.1092.166/10/1092 [DOI] [PubMed] [Google Scholar]
- 67.WHO ASSIST Working Group The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction. 2002 Sep;97(9):1183–94. doi: 10.1046/j.1360-0443.2002.00185.x.185 [DOI] [PubMed] [Google Scholar]
- 68.Bogart LM, Wagner GJ, Green HD, Mutchler MG, Klein DJ, McDavitt B, Lawrence SJ, Hilliard CL. Medical mistrust among social network members may contribute to antiretroviral treatment nonadherence in African Americans living with HIV. Soc Sci Med. 2016 Sep;164:133–140. doi: 10.1016/j.socscimed.2016.03.028. https://europepmc.org/abstract/MED/27046475 .S0277-9536(16)30128-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feldman BJ, Fredericksen RJ, Crane PK, Safren SA, Mugavero MJ, Willig JH, Simoni JM, Wilson IB, Saag MS, Kitahata MM, Crane HM. Evaluation of the single-item self-rating adherence scale for use in routine clinical care of people living with HIV. AIDS Behav. 2013 Jan;17(1):307–18. doi: 10.1007/s10461-012-0326-7. https://europepmc.org/abstract/MED/23108721 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: The development and evaluation of a new method for assessing the cognitive representation of medication. Psychology & Health. 1999 Jan;14(1):1–24. doi: 10.1080/08870449908407311. [DOI] [Google Scholar]
- 71.Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. N Engl J Med. 2012 Mar 01;366(9):780–1. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- 72.Dehlendorf C, Fitzpatrick J, Steinauer J, Swiader L, Grumbach K, Hall C, Kuppermann M. Development and field testing of a decision support tool to facilitate shared decision making in contraceptive counseling. Patient Educ Couns. 2017 Jul;100(7):1374–1381. doi: 10.1016/j.pec.2017.02.009. https://europepmc.org/abstract/MED/28237522 .S0738-3991(17)30073-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith DK, Van Handel Michelle, Wolitski RJ, Stryker JE, Hall HI, Prejean J, Koenig LJ, Valleroy LA. Vital Signs: Estimated Percentages and Numbers of Adults with Indications for Preexposure Prophylaxis to Prevent HIV Acquisition--United States, 2015. MMWR Morb Mortal Wkly Rep. 2015 Nov 27;64(46):1291–5. doi: 10.15585/mmwr.mm6446a4. doi: 10.15585/mmwr.mm6446a4. [DOI] [PubMed] [Google Scholar]
- 74.Smith DK, Van Handel M, Grey J. Estimates of adults with indications for HIV pre-exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol. 2018 Dec;28(12):850–857.e9. doi: 10.1016/j.annepidem.2018.05.003.S1047-2797(17)31069-4 [DOI] [PubMed] [Google Scholar]
- 75.Smith DK, Chang M, Duffus WA, Okoye S, Weissman S. Missed Opportunities to Prescribe Preexposure Prophylaxis in South Carolina, 2013-2016. Clin Infect Dis. 2019 Jan 01;68(1):37–42. doi: 10.1093/cid/ciy441.5001363 [DOI] [PubMed] [Google Scholar]
- 76.Aaron E, Blum C, Seidman D, Hoyt MJ, Simone J, Sullivan M, Smith DK. Optimizing Delivery of HIV Preexposure Prophylaxis for Women in the United States. AIDS Patient Care STDS. 2018 Jan;32(1):16–23. doi: 10.1089/apc.2017.0201. https://europepmc.org/abstract/MED/29323558 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mayer KH, Chan PA, R Patel Rupa, Flash Charlene A, Krakower Douglas S. Evolving Models and Ongoing Challenges for HIV Preexposure Prophylaxis Implementation in the United States. J Acquir Immune Defic Syndr. 2018 Feb 01;77(2):119–127. doi: 10.1097/QAI.0000000000001579. https://europepmc.org/abstract/MED/29084044 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seidman D, Weber S. Integrating Preexposure Prophylaxis for Human Immunodeficiency Virus Prevention Into Women's Health Care in the United States. Obstet Gynecol. 2016 Jul;128(1):37–43. doi: 10.1097/AOG.0000000000001455.00006250-201607000-00007 [DOI] [PubMed] [Google Scholar]
- 79.Markowitz M, Sarafianos SG. 4'-Ethynyl-2-fluoro-2'-deoxyadenosine, MK-8591: a novel HIV-1 reverse transcriptase translocation inhibitor. Curr Opin HIV AIDS. 2018 Jul;13(4):294–299. doi: 10.1097/COH.0000000000000467. https://europepmc.org/abstract/MED/29697468 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eron J. Long Acting Therapy for the Treatment of HIV Infection. Looking to the Future; May 13-14, 2019; Rockville, MD. 2019. May 13, [Google Scholar]
- 81.Gatto GJ, Brand RM, Girouard N, Li LA, Johnson L, Marzinke MA, Krogstad E, Siegel A, Helms E, Demkovich Z, Luecke E, van der Straten A. Development of an end-user informed tenofovir alafenamide (TAF) implant for long-acting (LA)-HIV pre-exposure Prophylaxis (PrEP). HIV Research for Prevention (HIVR4P); October 21-25, 2018; Madrid, Spain. 2018. https://www.natap.org/2018/HIVR4P/HIVR4P_23.htm . [Google Scholar]
- 82.Su B, Yao C, Zhao Q, Cai W, Wang M, Lu H, Chen Y, Liu L, Wang H, He Y, Zheng Y, Li L, Chen J, Yu J, Zhu B, Zhao M, Sun Y, Lun W, Xia W, Sun L, Dai L, Jiang T, Wang M, Zheng Q, Peng H, Wang Y, Lu R, Hu J, Xing H, Shao Y, Xie D, Zhang T, Zhang F, Wu H, TALENT Study Team Efficacy and safety of the long-acting fusion inhibitor albuvirtide in antiretroviral-experienced adults with human immunodeficiency virus-1: interim analysis of the randomized, controlled, phase 3, non-inferiority TALENT study. Chin Med J (Engl) 2020 Nov 25;133(24):2919–2927. doi: 10.1097/CM9.0000000000001273. https://europepmc.org/abstract/MED/33252379 .00029330-202012200-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Greene JC, Caracelli VJ, Graham WF. Toward a Conceptual Framework for Mixed-Method Evaluation Designs. Educational Evaluation and Policy Analysis. 2016 Nov 23;11(3):255–274. doi: 10.3102/01623737011003255. [DOI] [Google Scholar]
- 84.Dellinger AB, Leech NL. Toward a Unified Validation Framework in Mixed Methods Research. Journal of Mixed Methods Research. 2016 Jun 29;1(4):309–332. doi: 10.1177/1558689807306147. [DOI] [Google Scholar]
- 85.Andrew S, Halcomb E. Mixed Methods Research for Nursing and the Health Sciences (1st edition) Hoboken, NJ: John Wiley & Sons, Ltd; 2009. [Google Scholar]
- 86.Clark VLP, Ivankova NV. Mixed Methods Research: A Guide to the Field. Thousand Oaks, CA: SAGE Publications; 2015. [Google Scholar]
- 87.Danaher BG, Brendryen H, Seeley JR, Tyler MS, Woolley T. From black box to toolbox: Outlining device functionality, engagement activities, and the pervasive information architecture of mHealth interventions. Internet Interv. 2015 Mar 01;2(1):91–101. doi: 10.1016/j.invent.2015.01.002. https://linkinghub.elsevier.com/retrieve/pii/S2214-7829(15)00003-2 .S2214-7829(15)00003-2 [DOI] [PMC free article] [PubMed] [Google Scholar]


