Abstract
Objective:
Medicinal plants having antioxidant potential possess numerous constituents which are responsible for different beneficial effects and are used as an alternative resource of medicine to lessen diseases linked with oxidative stress. Flavonoids are identified in the plants since ages and display wide spectrum of biological actions that might be able to stimulate the steps which are disturbed in different diseases. Flavonoids are significant natural compounds with various biologic properties, among which the most common is the anti-oxidant potential. Citrus flavonoids establish an important stream of flavonoids. Naringin, very common flavonoids present in the diet, belongs to the family of flavanone. It is the principal constituent of citrus family that contains flavonoids for example tomatoes, grapefruits and oranges.
Materials and Methods:
In this article, we reviewed naringin with respect to sources, chemical property, pharmacokinetics, pharmacological activity, and novel formulations. The literature survey has been done by searching different databases such as Psyc INFO, Science Direct, PubMed, EMBASE, Google, Google Scholar, Medline.
Results:
Naringin is known to behave as an antioxidant and possess anti-inflammatory, anti-apoptotic, anti-atherosclerotic, neuroprotective, anti-psychotic, anti-asthmatic, anti-diabetic, hepatoprotective, anti-tussive, cardioprotective, and anti-obesity activity. Further clinical studies using large sample sizes remain essential to obtain the appropriate dose and form of naringin for averting diseases. Furthermore, the therapeutic approach of these bioflavonoids is significantly inappropriate due to the lack of clinical evidence. Different plants must be explored further to find these bioflavonoids in them.
Conclusion:
The results of this exploration provides biological actions of bioflavonoid (naringin), predominantly on pharmacological and novel dosage forms of naringin.
Key Words: Naringin, Pharmacokinetics Pharmacological activity, Formulations, Flavonoids
Introduction
Flavonoids are natural phenolic compounds with a broad range of bioactivity. Almost 4000 flavonoids, primarily from fruits, herbs, and vegetables, have been detected so far (Cook and Samman, 1996 ▶). The basic structure of the flavonoids consists of fifteen carbon atoms and 3 rings, 2 of which are benzene rings linked to a 3-carbon chain (Croft, 1998 ▶). Plants contain many forms of flavonoids, such as flavanone, flavone, polymethoxylated flavone, flavonol, and anthocyanin (Bhagwat et al., 2011 ▶; Ma et al., 2020 ▶).
Flavanones are major flavonoids which are very common in citrus fruits. In glycoside and aglycone forms, citrus flavanones are found. The major flavanone aglycones found in citrus fruits are eriodictyol hesperetin, naringenin, and isosakuranetin. In most citrus fruits, flavonone glycosides are more abundant than the flavanone aglycones (Bhagwat et al., 2011 ▶; Ma et al., 2020 ▶). Flavones are a flavonoid subgroup which, through a C2-C3 double bond, differ from flavanones. Flavones in citrus fruits are also found in glycoside and aglycone forms. In some plants like lemon and lime, a very small number of flavonols are present in the citrus fruits (Bhagwat et al., 2011 ▶; Ma et al., 2020 ▶).
Anthocyanins are water soluble pigments that are synthesized by phenylpropanoid pathway (Bhagwat et al., 2011 ▶; Ma et al., 2020 ▶). Such flavonoids are strong free radical scavengers and in vivo, they avoid oxidative stress (Croft, 1998 ▶; Ross and Kasum, 2002 ▶). Flavonoids are versatile in characteristics and chemical structure as a group of polyphenolic compounds. In fruits, vegetables, seeds, flowers, nuts and bark, they occur naturally and serve as major part of our diet (Middleton, 1993 ▶; Ratty and Das, 1988 ▶; Hackett, 1996 ▶). A broad range of biological effects have been mentioned as antibacterial, anti-inflammatory, anti-allergic (Hanasaki et al., 1994 ▶; Cook and Samman, 1996 ▶) and vasodilatory (Duarte et al., 1993 ▶) activities.
De Vry first observed naringin in the flower of grapefruit plant that grown in Java in 1857, but his results were not published at that time (Rangaswami et al., 1993 ▶). The name naringin is most likely derived from the word "Narangi" in Sanskrit, meaning "Orange" (Sinclair, 1972 ▶). As a bitter flavonone glycoside, naringin is a major active ingredient in citrus fruits, such as Drynaria fortune, Citrus medica L and Citrus aurantium L (Chen et al., 2016 ▶). Naringin is extracted from grapefruit (Citrus plant) (Papasani et al., 2014 ▶). Naringin is a bioflavonoid (flavanone glycoside) that gives grape fruit juice a bitter taste. In grapefruit, pummelo, sour orange, trifoliate orange, and kumquat, naringin is the chief constituents (Horowitz and Gentili, 1969 ▶; Alam et al., 2020 ▶). Various therapeutic effects of naringenin (aglycone portion of naringin) such as cardioprotective (Arafa et al., 2005 ▶), cholesterol lowering, anti-Alzheimer's, nephroprotective, anti-aging, antihyperglycemic, anti-osteoporotic and gastroprotective (Jeon et al., 2004 ▶; Jagetia and Reddy, 2005 ▶), anti-inflammatory (Mohanty et al., 2020 ▶), antioxidant, anti-apoptotic, anti-carcinogenic, anti-osteoporotic and anti-ulcer activities have been studied (Wang et al., 2013 ▶; Rivoira et al., 2021 ▶).
For a long period of time, the plants containing naringin as a chemical constituent have been used as a natural source of medicine, and the use of plant compounds for pharmaceutical purposes has gradually increased in the world. The novelty of this manuscript justifies as, we collected the data related to characteristic, chemical constituents, pharmacological activities and novel formulations of naringin. Focus has been made on the pharmacological properties of naringin, so as to provide brief compiled information regarding its activities along with novel formulations as these formulations are hot topic for both clinicians and researchers. Nevertheless, the exact effects and mechanisms involved in pharmacological and toxicological effects of many of these chemicals remain to be cleared. This review aims to highlight the medicinal importance of bioflavonoid (naringin) and the journey of this folk ingredient to modern medicine.
Materials and Methods
The searches were limited to the English language. The search was done from 1965-2020 and data were included from year 1969-2020. The scientific name of the plants was identified from the standard herbal literature. For collecting data, books, online materials, thesis, and scientific journals were also considered. The authors have utilized broad Major Exploded Subject Headings (MesH) terms and these keywords [Naringin, Naringin fruit] with the following suffix and prefix [pharmacological activity, phytochemistry, clinical trial, history, pharmacokinetics, chemical structure, species, morphological], using these words, the search was done using following search engines such as Psyc INFO, Science Direct, PubMed, EMBASE, Google, Google Scholar, Medline. Initially, articles were downloaded which were available as open-access files, the articles which were subscription-based were not downloaded, only the abstract was copied from such papers. Later the guideline of Systematic Reviews and Meta‐Analyses (PRISMA) was followed, the manuscripts which were not pertinent to the title of this paper were discarded. Summarizing the search strategy we have downloaded 106 manuscripts and noted the abstract of 23 papers. From which a total of 39 manuscripts were deleted as they were non-relevant, the remaining manuscripts were used for preparing this manuscript. All collected publications were reviewed manually by the third person to remove the chance of bias and also checked regarding the conflict of interest.
Results
Source
Citrus fruits and Grapes contain a flavanone glycoside called naringin. It has a strong pungent taste for grapefruit juice (Jung et al., 2003 ▶; Kanaze et al., 2003 ▶). In grapefruit, pummelo, sour orange, trifoliate orange, and kumquat, naringin is also the primary bitter ingredient (Horowitz and Gentili, 1969 ▶). Naringin is a naringenin, an aglycone and neohesperidose flavanone glycoside bound to the -OH group at the carbon C-7 and it has a bitter taste (Braverman, 1949 ▶). When potassium hydroxide or another solid base is used, it is possible to make 1, 3-diphenylpropan-1-one from it, a compound that is 300–1800 folds sweeter as compared to sugar, with a refreshing sweet taste reminiscent of menthol (Jane, 2004 ▶). Naringin has a weak basic nature and is less soluble in water buffers (Tomasik, 2003 ▶). Rutinosc sugar molecule (L-rhamnose-D-glucose) is naringin and can be extracted with boiling mineral acid by hydrolysis. Naringenin is known as aglucose, which lacks bitter property of naringin. Although naringin is only moderately water-soluble (0.05% at 20°C), it might be crystallized when grapefruit is exposed to temperatures below freezing point (Hurst et al., 2018 ▶). Various Citrus species are listed in Table 1.
Table 1.
Total content of naringin in different citrus fruits
| S.N. | Citrus species | Naringin content (mg/ml) | Reference |
|---|---|---|---|
| 1 | Citrus (C.) sinensis | 21.3 | (Alam et al. 2014 ▶) |
| 2 | Citrus bergamia | 22.3 | |
| 3 | Citrus clementina | 8 | |
| 4 | Citrus reticulate | 3383.6 | |
| 5 | Citrus paradise | 230 | |
| 6 | Citrus aurantium | 19.7 | |
| 7 | Fruit juices/citrus juices (Naringin) | 15.6 | |
| 8 | Grapefruit, pure juice | 30.8 | |
| 9 | Pummelo, pure juice | 84.8 | |
| 10 | Orange (blond), pure juice | 7 | |
| 11 | Grapefruit (juice from concentrate) | 37.8 | |
| 12 | Pummelo hybrid/Grapefruit, pure juice | 45.1 | |
| 13 | Grapefruit, raw (color not specified)C. paradise (naringenin) | 53.0 | |
| 14 | Grapefruit, raw, white, all areas(C. paradisi) (naringenin) | 21.3 | |
| 15 | Grapefruit, raw, red and pink (C. paradisi) (naringenin) | 32.6 | |
| 16 | Grapefruit juice concentrate, frozen, white, unsweetened (naringenin) | 31.2 | |
| 17 | Grapefruit juice, canned, white, unsweetened (naringenin) | 18.0 | |
| 18 | Grapefruit juice, raw, white (naringenin) | 18.2 |
Chemical property of naringin
Naringin is a flavanone glycoside obtained from citrus and grapes fruits (Figure 1) (with molecular formula C27H32O14 and 580.4 g/mol molecular weight). At the 7-carbon position, two rhamnose units are attached to its aglycon portion, naringenin. Naringin and naringenin are potent antioxidants (Renugadevi and Prabu, 2009 ▶; Jung et al., 2003 ▶). Compared to naringenin, naringin is very less potent as the sugar moiety induces steric obstruction by the scavenging community in the former. Naringin is slightly water soluble. Naringin is broken down into its aglycon naringenin in the intestine by the gut microflora; it is then absorbed from the gut (Choudhury et al., 1999 ▶).
Figure 1.
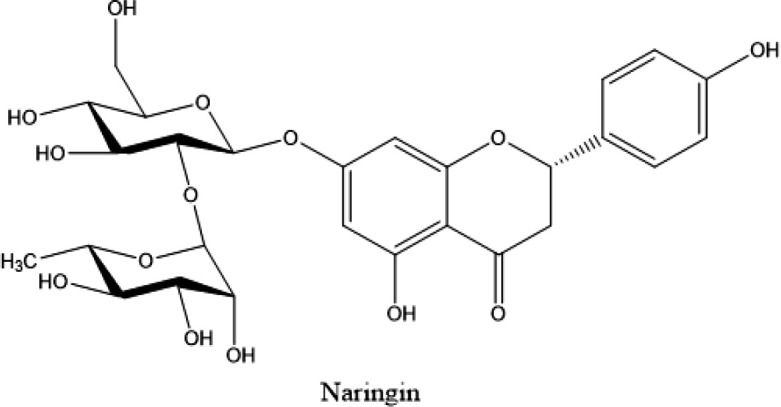
Chemical structure of naringin
Uses
Natural flavonoids have unique physicochemical and physiological features that allow them to perform a broad array of functions. Naringin has a variety of biological and pharmacological properties that serve to minimize the risk of many diseases (Yin et al., 2015 ▶). Naringin is distinguished from other bioflavonoids by its bioactivity in the regulation of heart rhythm, its cardioprotective effect (Rani et al., 2013 ▶), and its ways to enhance lipid profile (Chanet et al., 2012 ▶), owing to its ability to lower LDL cholesterol and triglyceride levels in the blood, thereby increasing HDL cholesterol levels. All of this helps to keep blood pressure under control and prevent atherosclerosis from developing. Furthermore, it has been proven to have antidiabetic characteristics (Shen et al., 2018 ▶) and enhance thermogenesis (Silver et al., 2011 ▶), making it an ideal element in dietary supplement formulations aimed at promoting health and weight loss.
Pharmacokinetics
The oral absorption of naringin, obtained from citrus fruits indicated that it was not well absorbed in the gastrointestinal tract (GIT) in its original form (Kanaze et al., 2004 ▶). Naringin free form was partially present in plasma in rats and humans, and the predominant metabolite was naringenin glucuronide (Felgines et al., 2000 ▶; Fang et al., 2006 ▶). Naringin was rapidly absorbed into the serum by entering the first concentration peak at 15 min and another at 3 hr oral dose of naringin monomer. It can not be observed after 480 min of dosing, which could be due to rapid metabolism (5.075 mg/kg). The area under the curve (AUCall) and mean residence time (MRT) were 274.8070 min, mg/L and 114.0243 min (Li et al., 2013 ▶).
Naringin can also influence the absorption, metabolism or removal process of candesartan (CDS), thereby affecting the absorption process in the intestine (Surampalli et al., 2015 ▶). It is suggested that the absorption of diterpenoid (oridonin) in rats is much greater than that of flavonoid glycoside (naringin) (Jin et al., 2015 ▶). Naringin was administered by duodenal cannula. The mean naringin Cmax occurred at 18.8±3.8 min in portal plasma (determined to achieve tmax in portal plasma) and was significantly greater than in mesenteric lymph fluid. The Cmax of naringin was approximately 1.7-fold higher in bile than in jugular plasma (Tsai and Tsai, 2012 ▶). The total absorption of naringin in rats and dogs was found to be 44.1% and 34.4%, respectively. It has also been reported that the pharmacokinetic evaluation of naringin were significantly altered by administration of high fat diet (Bai et al., 2020 ▶; Joshi et al., 2018 ▶).
Pharmacological activity
Naringin possesses potential pharmacological properties. The literature suggests that naringin has mainly antioxidant potential which provides a bridge between traditional medicine and western medicine due to its pharmacological potential. The literature available on these properties has been summarized below:
Anti-inflammatory activity
The anti-inflammatory effect of naringin in cisplatin-induced renal injury in rats has been studied. The administration of naringin on various doses (20, 50 or 100 mg/kg) has shown to protect against impaired renal function, eliminate the decrease in antioxidant enzymes and suppress increases in thiobarbituric acid reactive substances (TBARS), tumor necrosis factor (TNF-α) concentrations and nitrite (Chtourou et al., 2016 ▶). According to another study, naringin is an important anti-inflammatory agent in cisplatin-induced rats to attenuate chronic pulmonary neutrophilic inflammation (Nie et al., 2012 ▶). Anti-inflammatory effect of naringin in cigarette smoke-induced chronic bronchitis in guinea pig suggested that naringin may have novel therapeutic potential for chronic bronchitis treatment (Luo et al., 2012 ▶). Naringin inhibited the increased expression of TNF-α and high mobility group box protein 1 (HMGB-1). It was shown that oral naringin treatment may be beneficial in the treatment of patients with rheumatoid arthritis (Kawaguchi et al., 2011 ▶).
Anti-oxidant activity
Effects of hesperidin and naringin on antioxidant activity and plasma lipid profile in rats fed on diet containing cholesterol displayed that the bioactive citrus fruit compounds are effective reducers of plasma lipids and possess potential antioxidant activity (Gorinstein et al., 2007 ▶).
The assessment of antioxidant effects of naringin and probucol was reported by Jeon et al. In the antioxidant protection system, probucol was very potent, while naringin showed a comparable antioxidant potential based on increasing gene expression in antioxidant enzymes (Jeon et al., 2002 ▶). Results suggest that it was possible to defend against kidney disease at different doses of naringin, to eliminate the decrease in antioxidant enzyme activity and to suppress the rise in TBARS, nitrite, TNF alpha concentrations, and to enhance the histological changes caused by cisplatin (Chtourou et al., 2016 ▶). It also lowered the oxidative biomarkers malondialdehyde (MDA) and lactate dehydrogenase (LDH) and increased the levels of catalase (CAT) and superoxide dismutase (SOD) (Papasani et al., 2014 ▶).
Cancer
The in vivo action of naringin in Walker 256 carcinosarcoma bearing rats has been studied. Furthermore, complete tumor regression was present in 2 rats. Inhibition of tumor development, decreased IL-6 levels, TNF-alpha and augmented survival in Walker 256 carcinosarcoma-bearing rats treated with naringin strongly showed that it has anticarcinogenic potential (Camargo et al., 2012 ▶). Naringin treatment prevents lipid peroxidation and liver damage, proving the antioxidant protection mechanism in rats after diethylnitrosamine (DEN)-induced liver carcinogenesis (Thangavel et al., 2012 ▶).
Anti-tussive effect
The anti-tussive activity of naringin on electrical stimulation-induced cough in guinea pigs was recorded. Naringin is not a primary antitussive drug, either the sensory neuropeptide mechanism or the activation of ATP-sensitive K+ channels does not have a peripheral antitussive activity (Gao et al., 2011 ▶).
Anti-asthmatic effect
The anti-asthmatic activity of naringin was estimated. In this experiment the ovalbumin caused inflammation of the airways in the mouse. Flow cytometry experiments found that Th2 cells and enhanced Th1 cells were significantly inhibited by naringin. Naringin increased T-bet and inhibited GABA3 significantly (Guihua et al., 2016 ▶).
Cardiovascular disorders
Naringin inhibited lipopolysaccharide induced increased activity of TNF-alpha, IL-1β and IL-6 to relieve the inflammatory action in the heart. Additionally, supplementation of naringin significantly elevates SOD levels and prevents oxidative stress parameter compared to lipopolysaccharide-induced injury. Finally, treatment with naringin potentially reduced the expression of pro and anti-apoptotic (BAX and BCL-2) respectively in cardiac tissue (Xianchu et al., 2016 ▶). Naringin was shown to have anti-lipoperoxidative and antioxidant function in cardiac toxicity that was experimentally induced (Rajadurai and Prince, 2006 ▶).
Antiatherogenic effects
Naringin significantly decreased the development of fatty streaks and filtration of neointimal macrophages, it also inhibited the activity of intercellular adhesion molecule 1 (ICAM-1) in endothelial cells and has hepatoprotective role unlike lovastatin (Choe et al., 2001 ▶). Naringin decreased plasma non-HDL level and also endothelial dysfunction biomarkers, showing its protective impact (Chanet et al., 2012 ▶).
Anti-hypertensive effect
By downregulating the inflammatory markers including TNF-alpha and IL-β, naringin has prevented hypertension and ocular dysfunction. Naringin can be a useful medication to mitigate apoptosis, inflammatory markers and metabolic nucleotide disorders in hypertensive rats through the NOS/cGMP/PKG signaling pathways for identifying myocardial damage (Akintunde et al., 2020 ▶). Anti-hypertensive effect of naringin in renal artery occlusion induced hypertension in rats, showed altered left ventricular function at different time intervals after the clamp was removed. The study concluded that treatment with naringin has markedly altered SOD, MDA and GSH levels (Visnagri et al., 2015 ▶).
Anti-diabetic effect
When treated with naringin, the level of plasma cholesterol and triglyceride were reduced from 84.84±1.62 to 55.59±1.50 mg/dl and 123.03±15.11 to 55.00±0.86 mg/dl respectively (Rotimi et al., 2018 ▶). When naringin is administered to hyperglycaemic rats, there is significant decrease in glucose levels, an increase in insulin levels, a decrease in TBARS and H2O2 levels, and increase in overall antioxidant activity with increase in the activity of the antioxidant enzyme (CAT, GPx, SOD and paraoxonase) (Mamdouh and Monira, 2004 ▶).
Nephroprotective effect
The results of Singh and Chopra, 2004 has demosntrated that ROS play a degrative effect in renal injury caused by ischemia/reperfusion (I/R) and naringin probably exerts renoprotective effects via antioxidant and radical scavenging activities (Singh and Chopra, 2004 ▶). Histopathological and other studies revealed that the molecular and biochemical findings are relevant nephroprotective effect of naringin in cisplatin caused nephrotoxicity in rats (Abd Elmonem et al., 2018 ▶).
Hepatoprotective effect
The increase in Fas/FasL/caspase-3 protein expression and in the Bax/Bcl-2 ratio have shown that diabetes increased both pathways of apoptosis, effects which were abrogated by naringin treatment (Rodríguez et al., 2018 ▶). The hepatoprotective effects of naringin was evaluated in carbon tetrachloride (0.5 ml/kg; subcutaneous) induced liver damage in rats. The altered changes were significantly restored in rats pretreated with naringin (Badr et al., 2009 ▶).
Ulcer
The gastroprotective effect of naringin in rats with ethanol caused gastric-lesions has been studied. Naringin has been shown to have cytoprotective action toward ethanol injury in rats, however this property seems to be mediated by a non-prostaglandin dependent mechanisms (Martin et al., 1994 ▶).
Wound healing
Evaluation of the wound healing action of naringin ointment formulation (NOF) in experimental wound models showed a substantial decrease (p<0.05) in the wound surface area and in the epithelial duration, while the wound contraction rate significantly increased (p<0.05). Histological changes in wound skin were also restored by the NOF (Kandhare et al., 2016 ▶).
Neuroprotective effect
Neuroprotective activity of naringin in streptozotocin induced painful diabetic neuropathy in rats has been evaluated. The result suggest that chronic naringin therapy reduced the nociceptive threshold level, membrane-bound inorganic phosphate enzyme, endogenous antioxidant oxidative-nitrosative stress, neural cells apoptosis and inflammatory mediators. (Kandhare et al., 2012 ▶). Naringin therapy resulted in substantial reduction in attenuated oxidative damage and cognitive function as proved by lower levels of MDA and nitrite and decreased levels of glutathione and acetylcholinesterase compared with control levels (Kumar et al., 2010 ▶). The study demonstrates that by attenuating hyperammonemia, naringin effectively decreased neurotoxicity, indicating that it possess neuroprotective properties (Ramakrishnan et al., 2016 ▶). Neuroprotective activity of naringin in streptozotocin induced diabetic in rats significantly reduced MDA levels, elevated SOD levels and also increased TNF-α, IL-1β, and IL-6. PPARγ expression was also increased when pretreated with naringin (Liu et al., 2016 ▶).
Anti-depressant effect
Naringin therapy may be helpful to achieve functional behavioral effects through enhancing the cholinergic transmission pathways (Ben-Azu et al., 2019 ▶). The post-stroke depression action of naringin showed that modulation of nitric oxide is involved in naringin's protective effect against post-stroke depression induced by bilateral common carotid artery occlusion (BCCAO) (Aggarwal et al., 2010 ▶). In another study, antipsychotic effects of naringin in ketamine induced deficits in rats showed that naringin has likely therapeutic add-on activity against schizophrenia induced by ketamine (George et al., 2020 ▶).
The summary of pharmacological activities is mentioned in Table 2.
Table 2.
The summary of pharmacological activities of naringin
| Type of study | Subject | Dose/Route | Finding & Inferences | Reference |
|---|---|---|---|---|
| Anti-inflammatory | Rat | 20, 50 or100 mg/kg; per oral | Naringin can be a useful dietary supplement for reducing the risk of nephrotoxicity caused by anticancer drugs like cisplatin in cancer chemotherapy. Cisplatin-induced renal dysfunction can be mitigated with naringin supplementation. During cisplatin toxicity, naringin was able to restore redox equilibrium, suppressing inflammation, NF-kB activation, and apoptosis. | (Chtourou et al. 2016 ▶) |
| Rat | 20, 40 & 80 mg/kg; intragastric | Naringin dependently decreased cigarette smoke, caused inflammatory cell invasion, bronchial wall thickening, and average alveolar airspace expansion. | (Nie et al. 2012 ▶) | |
| Guinea pig | 9.2, 18.4 and 36.8 mg/kg; per oral | Naringin effectively reduced exposure to chronic smoke-induced enhanced cough, inflammation of the airways, AHR and suppressed the decline in SOD activity and LXA4 airway content in this guinea pig model. | (Luo et al. 2012 ▶) | |
| Mice | 150 mg/kg/0.3 ml; per oral | Oral administration of Naringin to mice by collagen-induced arthritis reduced the severity of clinical symptoms in knee joints. | (Awaguchi et al. 2011) | |
| Anti-oxidant activity | Wistar rats | 0.46–0.92 mg in 1 to 2 ml of water; per oral | The rise in plasma-lipid levels induced by cholesterol feeding was significantly reduced by diets supplemented with naringin. | (Gorinstein et al. 2007 ▶) |
| Male rabbit | Naringin- 0.5 gr/kg Probucol- 0.5 gr/kg; per oral |
Naringin showed a comparable antioxidant ability by increasing gene expression followed by overexpression of antioxidant enzymes. | (Jeon et al. 2002 ▶) | |
| Anti-apoptotic effect | Rat | 20, 50 or 100 mg/kg; per oral |
Naringin protected kidney function, reversed the decrease in the activity of antioxidant enzymes, and suppress increases in nitrite, TNF-α and TBARS levels. | (Chtourou et al. 2016 ▶) |
| Carcinogenesis | Rat | 10, 25 and 35 mg/kg; i.p. | Naringin, prolonged the tumor growth increased the survival rate and avoided cachexia. Naringin can be used as a potent antitumor agent as highlighted in these findings. | (Camargo CA et al. 2012 ▶) |
| Rat | 40 mg/kg; per oral | Treatment with naringin prevents lipid peroxidation, liver damage & protects the antioxidant protection mechanism from liver carcinogenesis. | (Thangavel et al. 2012 ▶) | |
| Anti-tussive effect | Guinea pig | 15, 30, and 60 mg/kg; i.v. | Naringin possess the anti-tussive effect probably by suppressing the cough center of the brain. | (Gao et al. 2011 ▶) |
| Anti-asthmatic | Mouse | 5 mg/kg and 10 mg/kg; per oral | Naringin possess anti-asthmatic effect by inhibiting IL-4, improved IFN-γ, and suppressed both the formation of eosinophils and mucus overproduction in mice with OVA-induced asthma. | (Guihua et al. 2016 ▶) |
| Myocardial effect | Mouse | 100 mg/kg; per oral | Naringin has cardioprotective effects by controlling inflammatory response, oxidative stress, and apoptotic reaction. | (Xianchu et al. 2016 ▶) |
| Rat | 10, 20 and 40 mg/kg; per oral | The biochemical and histopathological results obtained from the research indicate that naringin provides myocardium defense against oxidative stress induced by ISO in rats. | (Rajadurai and Prince, 2006 ▶) | |
| Male albino rats | 100 & 200 mg/kg; per oral | The naringin showed protective effects against myocardial injury. The treatment with naringin significantly reduced the development of free radicals, the generation of lipid peroxides and the leakage of cytosolic enzymes, characterized by decreased biomarker levels. | (Papasani et al. 2014 ▶) | |
| Antiatherogenic effect Antiatherogenic effect |
Rabbit | 500 mg/kg; per oral | Naringin, significantly reduced the development of fatty streaks and neo-intimal macrophages in filtration and suppressed the activation of ICAM-1 in endothelial cells. It also has a hepatoprotective effect. | (Choe et al. 2001 ▶) |
| Mice | High-fat/High cholesterol diet (−41%); per oral | The antiatherogenic effect of naringin displayed nutritionally achievable dose supplemented specifically for diet-induced atherosclerosis. | (Chanet et al. 2012 ▶) | |
| Anti-hypertensive Anti-hypertensive |
Rat | 80 mg/kg; per oral | Results suggest that naringin can be used as an antihypertensive agent | (Akintunde JK et al. 2020 ▶) |
| Rat | 20, 40 and 80 mg/kg; per oral | Naringin, through its antioxidant activity, exerts antihypertensive potential. | (Visnagri A et al. 2015 ▶) | |
| Anti-diabetic | Rat | 50, 100 and 200 mg/kg; per oral | Naringin could reverse T2DM-associated atherosclerosis by reducing dyslipidemia through HDL-mediated reverse cholesterol transport and protecting lipoprotein from oxidation by raising paraoxonase activity. | (Rotimi et al. 2018 ▶) |
| Rat | 0, 10, 20, 40, or 80 mg/kg; i.p | Multiple doses of Naringin significantly improved the hypoglycemic & antioxidant activity of diabetic rats caused by streptozotocin. | (Mamdouh and Monira, 2004 ▶) | |
| Nephroprotective effect | Rat | 400 mg/kg; per oral | The results suggest that the renal injury induced by I/R relates to its capacity to produce free radicals and that the ability of naringin to defend against this injury is possibly due to the improvement of this drug's antioxidant potential & free radical scavenging activity. | (Singh and Chopra, 2004 ▶) |
| Rat | 80 mg/kg; per oral | Naringin has shown a strong protective effect against cisplatin-caused nephrotoxicity through its antioxidant, anti-inflammatory and apoptotic activities. | (Abd Elmonem et al. 2018 ▶) | |
| Hepatoprotective effect | Rat | 40 mg/kg; Subcutaneous | Naringin preserves the liver from the damage caused by streptozotocin-induced diabetes and may be a novel clinical technique for the prevention of type-1 diabetes mellitus-related non-alcoholic liver fat disease. | (Rodríguez et al. 2018 ▶) |
| Rat | 300 mg/kg; per oral | Naringin exerts a preventive effect, likely through its antioxidant action, on CCl4-induced haematology and liver damage in rats. Thus the, supplementation therapy with naringin can effective in reducing tissue damage in patients exposed to toxic doses of CCl4. | (Badr et al. 2009 ▶) | |
| Gastroprotective effect | Rat | 400 mg/kg; per oral | Naringin showed a cytoprotective function against ethanol damage, but this effect tends to be mediated by pathways other than prostaglandins. | (Martin et al. 1994 ▶) |
| Wound healing | Rat | Topical | Naringin exerts its wound healing ability through the down-regulating expression of the inflammatory & apoptotic mediators while the up-regulating expression of the growth factors, thereby modulating the appearance of the collagen-1 gene to induce angiogenesis leading to wound healing. | (Kandhare et al. 2016 ▶) |
| Neuroprotective effect | Mice | 2.5, 5 and 10 mg/kg; i.p. | Naringin induced anxiolytic-like activity in mice and improved cognitive performance. Naringin substantially increased the activity of SOD, CAT, and GSH concentration and decreased nitrite levels and MDA and brain acetylcholinesterase activity. | (Ben-Azu et al. 2019 ▶) |
| Mice | 50 and 100 mg/kg; i.p. | Oxidative damage, neurobehavioral alterations and recovered mitochondrial enzyme complex actions were significantly attenuated when treated with naringin showing recovery from depression. | (Aggarwal et al. 2010 ▶) | |
| Rat | 100 mg/kg; per oral | Naringin possesses a strong add-on therapeutic activity against schizophrenia caused by ketamine. | (George et al. 2020 ▶) | |
| Rat | 20, 40 & 80 mg/kg; per oral | Naringin exhibit its neuroprotective impact by downregulation of free radical, cytokine including TNF-α thus preventing diabetes-induced neuropathic pain over modulation of endogenous biomarkers. | (Kandhare et al. 2012 ▶) | |
| Rat | 40 and 80 mg/kg; per oral | Naringin's multiple effects firmly support its neuroprotective effects toward colchicine-induced cognitive impairment and oxidative injury. | (Kumar et al. 2010 ▶) | |
| Rat | 80 mg/k; per oral | The results indicate that naringin exerts protective efficiency related to neuronal complications against hyperammonemic rats induced by NH4Cl. | (Ramakrishnan et al. 2016 ▶) | |
| Rat | 100 mg/kg; per oral | The study has shown that Naringin may be an effective drug to enhance learning and memory efficiency in DACD. | (Liu et al. 2016 ▶) |
NF-kB: Nuclear Factor kappa-light-chain-enhancer of activated B cells; AHR: Airway hyperresponsiveness; SOD: Superoxidase dismutase; LXA4: lipoxin A4; TBARS: Thiobarbituric acid reactive substances; TNF-α: Tumour necrosis factor α; IL-4: Interleukin 4; IFN-γ: Interferon gamma; OVA: Ovalbumin; ISO: Isoprenaline; ICAM-1: intercellular adhesion molecule 1; T2DM: Type 2 diabetes mellitus; HDL: high-density lipoprotein; CCl4: carbon tetrachloride; CAT: catalase; GSH: reduced glutathione; MDA: Malondialdehyde; NH4Cl: Ammonium chloride; DACD: Diabetes-associated cognitive decline
Last few years have emerged as the era of development of novel formulation process technology. Novel drug delivery made it possible to overcome various flaws associated with the herbal formulations as well as plant isolates. So far great efforts have been made by researchers to develop various novel formulations of naringin or naringin-containing extracts. Various formulations are listed in Table 3.
Table 3.
Novel formulations of naringin
| Purpose of study | Formulation approach | Objective | Method of preparation | Result | Reference |
|---|---|---|---|---|---|
| To prepare deformable liposomes of Naringin for improved anti-inflammatory activity | Deformable liposomes | For anti-inflammatory skin activity deformable liposomes of Naringin was made | Thin- film hydration technique | When compared to marketed preparation, the liposomes showed increased anti-inflammatory activity in an in-vitro assay | (Pleguezuelos-Villa et al. 2018 ▶) |
| To prevent development of resistance toward chemotherapeutic agents by combining Naringin and paclitaxel | Mixed micelles | To develop anticancer medicine with combining paclitaxel and Naringin | Solvent diffusion method | Naringin synergistically increased its intracellular intake and 65 % in-vitro cytotoxicity | (Jabri et al. 2019 ▶) |
| To develop formulation which may prevent Naringin release bursting and osteogenesis | Microspheres | To prepare Naringin-loaded microsphere/sucrose acetate isobutyrate hybrid depots and improve osteogenesis | Single-nozzle-electro-spraying setup | Microspheres showed effective biocompatibility and osteogenic potential in-vitro. Ng-m-SAIB may demonstrate promising for bone repair to be a sustained release carrier |
(Yang et al. 2019 ▶) |
| To incorporate into sunscreen creams which may increase protection against U.V. radiation | Ethosomes of Naringin | To improve the penetrating capacity and retention capacity of Naringin into sunscreen creams | Hot method and mechanical dispersion method | Ethosomes showed a pronounced skin penetration for Naringin across the skin and had a good skin retention and U.V. protection ability | (Gollavilli et al. 2020 ▶) |
| To prepare a dosage form in form of nano-capsule which have good bioavailability, bioavailability, biotransformation and distribution | Naringin-loaded Nano-capsules | To formulate nano-capsules of Naringin and to evaluate the toxicity | Interfacial- deformation technique | The ethosomes showed desired pharmacokinetic effect and there was no indication of toxicity by nano-capsules | (Budel et al. 2020 ▶) |
| To prepare a gum tragacanth stabilized green nanoparticles for increasing bactericidal activity | Naringin nanoparticles | To formulate green gold gum tragacanth loaded Naringin nanoparticles | Through magnetic stirring the color change was observed | Naringin's bactericidal potential was increased when it was loaded into AuNPs against different bacterial strains | (Rao et al. 2017 ▶) |
| To prepare a dosage form with increased drug release | Ternary nanoparticles containing amylose, alpha-linoleic acid, and beta-lactoglobulin complexed with Naringin | To formulate Naringin-nanoparticle inclusion complex for increased bio accessibility and thereby bioavailability | Through magnetic stirring the preparation of ternary nanoparticles and inclusion complex with Naringin was prepared | Naringin gradually released from the complex mixture and nanoparticles are promising carrier for increased bioavailability of Naringin | (Feng et al. 2017 ▶) |
| To prepare high catalytic properties of alpha-amalyse | Enzyme immobilized in magnetic nanoparticles of Naringin. | To formulate alpha-amalyse immobilized functionalized Magnetic NPs | Magnetite nanoparticles followed by immobilization of alpha-amalyse onto magnetic nanoparticle containing Naringin | Improvement in enzyme catalytic properties made nano-biocatalyst a good candidate in bio industrial applications | (Defaei et al. 2018 ▶) |
| To prepare a formulation having better anti-tumor activity of Naringin against hepatocellular carcinoma | Nanostructured lipid carrier with Naringin & coix seed oil. | To develop a Nanostructured lipid carrier containing Naringin and coix seed oil for the treatment of hepatocellular carcinoma | Ultrasonic- melt emulsification method. | The drug release and synergistic antitumor effect provides new insight against cancer | (Zhu et al. 2020 ▶) |
| To develop sustainable agriculture by using Naringin novel formulation | Naringin & citric acid in polycaprolactone microcapsules | Plant development and sustainable agriculture with polycaprolactone microcapsules containing Naringin & citric acid | Combination of a double emulsion method of water-in-oil-in-water and a solvent evaporation technique | The use of PCL 45000 Mw for the synthesis of MCs containing citric acid or Naringin may be a viable alternative to the current need for environmentally friendly agricultural practices. MCs containing Naringin have a 30-day slow release that is unaffected by pH, indicating that it should be used in soils with a variety of characteristics and promote the continuous supply (slow release) of nutrients to plants | (Cesari et al. 2020 ▶) |
| To prepare a dosage form in order to increase solubility of Naringin | Naringin loaded polycaprolactone microspheres. | Naringin loaded polycaprolactone microspheres for increased solubility of Naringin . |
Solvent evaporation method | Three-level Box-Behnken configuration can be used to configure a Naringin-loaded polycaprolactone microspheres based oral suspension, demonstrating that Naringin solubility is greatly improved as evidenced by the optimized suspension's particle size | (Ghosal et al. 2018 ▶) |
| To increase water solubility, permeability and Bioavailability of Naringin |
Naringin polymeric micelles | To make polymeric Naringin micelles based from pluronic F68 and test their antitumor activity in mice with Ehrlich ascites carcinoma | Thin film hydration technique | 1:50 polymeric micelles containing PF68 may be a promising nanocarrier for the phytopharmaceutical Naringin, with increased water solubility, permeability, and bioavailability, and also increased antitumor and antiulcer activities | (Mohamed et al. 2018 ▶) |
Ng-m-SAIB: Naringin-loaded microsphere/sucrose acetate isobutyrate; AuNPs: Gold nanoparticles; PCL: Polycaprolactone; Mw: Molecular weight; MCs: Microcapsules; PF68: Pluronic-F68
Discussion
Naringin, and other bioflavonoids, possess the properties to act as powerful antioxidants which have been often proven via in vitro experiments. Naringin, is a key component of flavonoid phytoconstituent, has the potential to assist in the treatment of a wide range of chronic degenerative diseases due to its diverse pharmacological properties. Further study is required to explain the activities of naringin inside the body and the rate and degree of absorption. The antioxidant potential of naringin metabolites and the pathways involved in metabolic translation need to be recognized and appraised to precisely determine the effect naringin in vivo and its efficiency in preventing diseases occurring due to oxidative damage. Low water solubility, less bioavailability and delayed release are major glitches to be worried. To overcome these questions various approaches are been tested such as formulation and evaluation of novel drug delivery form such as Nano formulations, liposomes, etc. These novel methods were utilized in improving the pharmacokinetic and pharmacological properties of naringin.
Conflicts of interest
The authors have declared that there is no conflict of interest.
Acknowledgment
Authors are grateful and show their sincere thanks to Prof. Syed Waseem Akhtar, Hon. Chancellor and Prof. Javed Musarrat, Hon. Vice-Chancellor, Integral University for providing an excellent research environment. The University has provided a manuscript communication number for further communication (IU/R&D/2021-MCN0001111).
References
- Abd Elmonem AT, Khalifa MM, Abdel-Salam MI. Nephroprotective role of naringin against cisplatin-induced nephrotoxicity. Malaysian J Med Res. 2018;29:139–147. [Google Scholar]
- Alam F, Kharya AK, Juber A, Khan MI. Naringin: sources, chemistry, toxicity, pharmacokinetics, pharmacological evidences, molecular docking and cell line study. Res J Pharm Technol. 2020;13:2507–2515. [Google Scholar]
- Aggarwal A, Gaur V, Kumar A. Nitric oxide mechanism in the protective effect of naringin against post-stroke depression (PSD) in mice. Life Sci. 2010;86:928–935. doi: 10.1016/j.lfs.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Khan H, Aschner M, Hasan MM, Hassan ST. Therapeutic potential of naringin in neurological disorders. Food Chem Toxicol. 2019;132:110646. doi: 10.1016/j.fct.2019.110646. [DOI] [PubMed] [Google Scholar]
- Akintunde JK, Akintola TE, Hammed MO, Amoo CO, Adegoke AM, Ajisafe LO. Naringin protects against bisphenol-A induced oculopathy as implication of cataract in hypertensive rat model. Biomed Pharmacother. 2020;126:110043. doi: 10.1016/j.biopha.2020.110043. [DOI] [PubMed] [Google Scholar]
- Alam MA, Subhan N, Rahman MM, Uddin SJ, Reza HM, Sarker SD. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv Nutr. 2014;5:404–417. doi: 10.3945/an.113.005603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arafa HM, Abd-Ellah MF, Hafez HF. Abatement by naringenin of doxorubicin-induced cardiac toxicity in rats. J Egypt Natl Canc Inst. 2005;17:291–300. [PubMed] [Google Scholar]
- Badr HM, Ashour AM, El-kott AF. Hepatoprotective activity of naringin against acute hepatotoxicity in rats, 25 – 27/4/2009, The 3rd Conference of Basic Science. Faculty of Science, Elgabel Algharby University, Libya: [Google Scholar]
- Bai Y, Peng W, Yang C, Zou W, Liu M, Wu H, Fan L, Li P, Zeng X, Su W. Pharmacokinetics and metabolism of naringin and active metabolite naringenin in rats, dogs, humans, and the differences between species. Front Pharmacol. 2020:364. doi: 10.3389/fphar.2020.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Azu B, Nwoke EE, Aderibigbe AO, Omogbiya IA, Ajayi AM, Olonode ET, Umukoro S, Iwalewa EO. Possible neuroprotective mechanisms of action involved in the neurobehavioral property of naringin in mice. Biomed Pharmacother. 2019;109:536–546. doi: 10.1016/j.biopha.2018.10.055. [DOI] [PubMed] [Google Scholar]
- Bhagwat S, Haytowitz DB, Holden JM. USDA database for the flavonoid content of selected foods release 3, pp 159-160. Beltsville, Department of Agriculture: 2011. [Google Scholar]
- Braverman JB. Citrus Products: chemical composition and chemical technology (No. BRA 634.3 (BR 532)) Geneva; 1949. pp. 112–113. [Google Scholar]
- Budel RG, da Silva DA, Moreira MP, Dalcin AJ, da Silva AF, Nazario LR, Majolo JH, Lopes LQ, Santos RC, Soares FA, da Silva RS. Toxicological evaluation of naringin-loaded nanocapsules in vitro and in vivo. Colloids Surf B. 2020;188:110754. doi: 10.1016/j.colsurfb.2019.110754. [DOI] [PubMed] [Google Scholar]
- Camargo CA, Gomes-Marcondes MC, Wutzki NC, Aoyama H. Naringin inhibits tumor growth and reduces interleukin-6 and tumor necrosis factor α levels in rats with Walker 256 carcinosarcoma. Anticancer Res. 2012;32:129–133. [PubMed] [Google Scholar]
- Cesari A, Loureiro MV, Vale M, Yslas EI, Dardanelli M, Marques AC. Polycaprolactone microcapsules containing citric acid and naringin for plant growth and sustainable agriculture: physico-chemical properties and release behavior. Sci Total Environ. 2020;703:135548. doi: 10.1016/j.scitotenv.2019.135548. [DOI] [PubMed] [Google Scholar]
- Chanet A, Milenkovic D, Manach C, Mazur A, Morand C. Citrus flavanones: what is their role in cardiovascular protection? J Agric Food Chem. 2012;60:8809–8822. doi: 10.1021/jf300669s. [DOI] [PubMed] [Google Scholar]
- Chanet A, Milenkovic D, Deval C, Potier M, Constans J, Mazur A, Bennetau-Pelissero C, Morand C, Bérard AM. Naringin, the major grapefruit flavonoid, specifically affects atherosclerosis development in diet-induced hypercholesterolemia in mice. J Nutr Biochem. 2012;23:469–477. doi: 10.1016/j.jnutbio.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Chen R, Qi QL, Wang MT, Li QY. Therapeutic potential of naringin: an overview. Pharm Biol. 2016;54:3203–3210. doi: 10.1080/13880209.2016.1216131. [DOI] [PubMed] [Google Scholar]
- Choe SC, Kim HS, Jeong TS, Bok SH, Park YB. Naringin has an antiatherogenic effect with the inhibition of intercellular adhesion molecule-1 in hypercholesterolemic rabbits. J Cardiovasc Pharmacol. 2001;38:947–955. doi: 10.1097/00005344-200112000-00017. [DOI] [PubMed] [Google Scholar]
- Choudhury R, Chowrimootoo G, Srai K, Debnam E, Rice-Evans CA. Interactions of the flavonoid naringenin in the gastrointestinal tract and the influence of glycosylation. Biochem Biophys Res Commun. 1999;265:410–415. doi: 10.1006/bbrc.1999.1695. [DOI] [PubMed] [Google Scholar]
- Chtourou Y, Aouey B, Aroui S, Kebieche M, Fetoui H. Anti-apoptotic and anti-inflammatory effects of naringin on cisplatin-induced renal injury in the rat. Chem Biol Interact. 2016;243:1–9. doi: 10.1016/j.cbi.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Cook NC, Samman S. Flavonoids—chemistry, metabolism, cardioprotective effects, and dietary sources. J Nutr Biochem. 1996;7:66–76. [Google Scholar]
- Croft KD. The chemistry and biological effects of flavonoids and phenolic acids A. Ann N Y Acad Sci. 1998;854:435–442. doi: 10.1111/j.1749-6632.1998.tb09922.x. [DOI] [PubMed] [Google Scholar]
- Defaei M, Taheri-Kafrani A, Miroliaei M, Yaghmaei P. Improvement of stability and reusability of α-amylase immobilized on naringin functionalized magnetic nanoparticles: A robust nanobiocatalyst. Int J Biol Macromol. 2018;113:354–360. doi: 10.1016/j.ijbiomac.2018.02.147. [DOI] [PubMed] [Google Scholar]
- Duarte J, Utrilla P, Jimenez J, Tamargo J, Zarzuelo A. Vasodilatory effects of flavonoids in rat aortic smooth muscle structure-activity relationships. Gen Pharmacol. 1993;24:857–862. doi: 10.1016/0306-3623(93)90159-u. [DOI] [PubMed] [Google Scholar]
- Fang T, Wang Y, Ma Y, Su W, Bai Y, Zhao P. A rapid LC/MS/MS quantitation assay for naringin and its two metabolites in rats plasma. J Pharm Biomed Anal. 2006;40:454–459. doi: 10.1016/j.jpba.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Felgines C, Texier O, Morand C, Manach C, Scalbert A, Régerat F, Rémésy C. Bioavailability of the flavanone naringenin and its glycosides in rats. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1148–G1154. doi: 10.1152/ajpgi.2000.279.6.G1148. [DOI] [PubMed] [Google Scholar]
- Feng T, Wang K, Liu F, Ye R, Zhu X, Zhuang H, Xu Z. Structural characterization and bioavailability of ternary nanoparticles consisting of amylose, α-linoleic acid and β-lactoglobulin complexed with naringin. Int J Biol Macromol. 2017;99:365–374. doi: 10.1016/j.ijbiomac.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Gao S, Li P, Yang H, Fang S, Su W. Antitussive effect of naringin on experimentally induced cough in Guinea pigs. Planta Med. 2011;77:16–21. doi: 10.1055/s-0030-1250117. [DOI] [PubMed] [Google Scholar]
- George MY, Menze ET, Esmat A, Tadros MG, El-Demerdash E. Potential therapeutic antipsychotic effects of naringin against ketamine-induced deficits in rats: involvement of akt/gsk-3β and wnt/β-catenin signaling pathways. Life Sci. 2020;249:117535. doi: 10.1016/j.lfs.2020.117535. [DOI] [PubMed] [Google Scholar]
- Ghosal K, Ghosh D, Das SK. Preparation and evaluation of naringin-loaded polycaprolactone microspheres based oral suspension using Box-Behnken design. J Mol Liq. 2018;256:49–57. [Google Scholar]
- Gollavilli H, Hegde AR, Managuli RS, Bhaskar KV, Dengale SJ, Reddy MS, Kalthur G, Mutalik S. Naringin nano-ethosomal novel sunscreen creams: development and performance evaluation. Colloids Surf B. 2020;193:111122. doi: 10.1016/j.colsurfb.2020.111122. [DOI] [PubMed] [Google Scholar]
- Gorinstein S, Leontowicz H, Leontowicz M, Krzeminski R, Gralak M, Jastrzebski Z, Park YS, Jung ST, Kang SG, Trakhtenberg S. Effect of hesperidin and naringin on the plasma lipid profile and plasma antioxidant activity in rats fed a cholesterol‐containing diet. J Sci Food Agric. 2007;87:1257–1262. [Google Scholar]
- Guihua X, Shuyin L, Jinliang G, Wang S. Naringin protects ovalbumin-induced airway inflammation in a mouse model of asthma. Inflamm. 2016;39:891–899. doi: 10.1007/s10753-016-0321-7. [DOI] [PubMed] [Google Scholar]
- Hackett AM. The metabolism of flavonoid compounds in mammals. Prog Clin Biol Res. 1986;213:177–194. [PubMed] [Google Scholar]
- Hanasaki Y, Ogawa S, Fukui S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic Biol Med. 1994;16:845–850. doi: 10.1016/0891-5849(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Horowitz RM, Gentili B. Taste and structure in phenolic glycosides. J Agric Food Chem. 1969;17:696–700. [Google Scholar]
- Hurst WJ, Finley JW, deMan JM. Principles of Food Chemistry. Cham, Springer; 2018. Additives and contaminants. InPrinciples of Food Chemistry; pp. 527–565. [Google Scholar]
- Jabri T, Imran M, Aziz A, Rao K, Kawish M, Irfan M, Malik MI, Simjee SU, Arfan M, Shah MR. Design and synthesis of mixed micellar system for enhanced anticancer efficacy of paclitaxel through its co-delivery with naringin. Drug Dev Ind Pharm. 2019;45:703–714. doi: 10.1080/03639045.2018.1550091. [DOI] [PubMed] [Google Scholar]
- Jagetia GC, Reddy TK. Modulation of radiation-induced alteration in the antioxidant status of mice by naringin. Life Sci. 2005;77:780–794. doi: 10.1016/j.lfs.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Jane JL. Chemical and functional properties of food saccharides . New York: Springer; 2004. Starch: structure and properties; pp. 82–96. [Google Scholar]
- Jeon SM, Bok SH, Jang MK, Kim YH, Nam KT, Jeong TS, Park YB, Choi MS. Comparison of antioxidant effects of naringin and probucol in cholesterol-fed rabbits. Clinica Chimica Acta. 2002;317:181–190. doi: 10.1016/s0009-8981(01)00778-1. [DOI] [PubMed] [Google Scholar]
- Jeon SM, Park YB, Choi MS. Antihypercholesterolemic property of naringin alters plasma and tissue lipids, cholesterol-regulating enzymes, fecal sterol and tissue morphology in rabbits. Clin Nutr. 2004;23:1025–1034. doi: 10.1016/j.clnu.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Jiao HY, Su WW, Li PB, Liao Y, Zhou Q, Zhu N, He LL. Therapeutic effects of naringin in a guinea pig model of ovalbumin-induced cough-variant asthma. Pulm Pharmacol Ther. 2015;33:59–65. doi: 10.1016/j.pupt.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Jin Y, Tian T, Ma Y, Xu H, Du Y. Simultaneous determination of ginsenoside Rb1, naringin, ginsenoside Rb2 and oridonin in rat plasma by LC–MS/MS and its application to a pharmacokinetic study after oral administration of weifuchun tablet. J Chromatogr B. 2015;1000:112–119. doi: 10.1016/j.jchromb.2015.06.027. [DOI] [PubMed] [Google Scholar]
- Joshi R, Kulkarni YA, Wairkar S. Pharmacokinetic, pharmacodynamic and formulations aspects of naringenin: An update. Life Sci. 2018;215:43–56. doi: 10.1016/j.lfs.2018.10.066. [DOI] [PubMed] [Google Scholar]
- Jung UJ, Kim HJ, Lee JS, Lee MK, Kim HO, Park EJ, Kim HK, Jeong TS, Choi MS. Naringin supplementation lowers plasma lipids and enhances erythrocyte antioxidant enzyme activities in hypercholesterolemic subjects. Clin Nutr. 2003;22:561–568. doi: 10.1016/s0261-5614(03)00059-1. [DOI] [PubMed] [Google Scholar]
- Kanaze FI, Gabrieli C, Kokkalou E, Georgarakis M, Niopas I. Simultaneous reversed-phase high-performance liquid chromatographic method for the determination of diosmin, hesperidin and naringin in different citrus fruit juices and pharmaceutical formulations. J Pharm Biomed Anal. 2003;33:243–249. doi: 10.1016/s0731-7085(03)00289-9. [DOI] [PubMed] [Google Scholar]
- Kanaze FI, Bounartzi MI, Georgarakis M, Niopas I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur J Clin Nutr. 2007;61:472–477. doi: 10.1038/sj.ejcn.1602543. [DOI] [PubMed] [Google Scholar]
- Kandhare AD, Alam J, Patil MV, Sinha A, Bodhankar SL. Wound healing potential of naringin ointment formulation via regulating the expression of inflammatory, apoptotic and growth mediators in experimental rats. Pharm Biol. 2016;54:419–432. doi: 10.3109/13880209.2015.1038755. [DOI] [PubMed] [Google Scholar]
- Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Bodhankar SL. Neuroprotective effect of naringin by modulation of endogenous biomarkers in streptozotocin induced painful diabetic neuropathy. Fitoterapia. 2012;83:650–659. doi: 10.1016/j.fitote.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Kawaguchi K, Maruyama H, Hasunuma R, Kumazawa Y. Suppression of inflammatory responses after onset of collagen-induced arthritis in mice by oral administration of the citrus flavanone naringin. Immunopharmacol Immunotoxicol. 2011;33:723–729. doi: 10.3109/08923973.2011.564186. [DOI] [PubMed] [Google Scholar]
- Kumar A, Dogra S, Prakash A. Protective effect of naringin, a citrus flavonoid, against colchicine-induced cognitive dysfunction and oxidative damage in rats. J Med Food. 2010;13:976–984. doi: 10.1089/jmf.2009.1251. [DOI] [PubMed] [Google Scholar]
- Li SQ, Dong S, Su ZH, Zhang HW, Peng JB, Yu CY, Zou ZM. Comparative pharmacokinetics of naringin in rat after oral administration of chaihu-shu-gan-san aqueous extract and naringin alone. Metabol. 2013;3:867–880. doi: 10.3390/metabo3040867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Liu M, Mo Y, Peng H, Gong J, Li Z, Chen J, Xie J. Naringin ameliorates cognitive deficits in streptozotocin-induced diabetic rats. Iran J Basic Med Sci. 2016;19:417. [PMC free article] [PubMed] [Google Scholar]
- Lo Piero AR. The state of the art in biosynthesis of anthocyanins and its regulation in pigmented sweet oranges [(Citrus sinensis) L. Osbeck]. J Agric Food Chem. 2015;63:4031–4041. doi: 10.1021/acs.jafc.5b01123. [DOI] [PubMed] [Google Scholar]
- Luo YL, Zhang CC, Li PB, Nie YC, Wu H, Shen JG, Su WW. Naringin attenuates enhanced cough, airway hyperresponsiveness and airway inflammation in a guinea pig model of chronic bronchitis induced by cigarette smoke. Int Immunopharmacol. 2012;13:301–307. doi: 10.1016/j.intimp.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Ma G, Zhang L, Sugiura M, Kato M. The Genus Citrus . Sawston, Woodhead Publishing; 2020. Citrus and health; pp. 495–511. [Google Scholar]
- Mamdouh MA, Monira AA. The influence of naringin on the oxidative state of rats with streptozotocin-induced acute hyperglycaemia. Z Naturforsch C. 2004;59:726–733. doi: 10.1515/znc-2004-9-1018. [DOI] [PubMed] [Google Scholar]
- Martin MJ, Marhuenda E, Perez-Guerrero C, Franco JM. Antiulcer effect of naringin on gastric lesions induced by ethanol in rats. Pharmacology. 1994;49:144–150. doi: 10.1159/000139228. [DOI] [PubMed] [Google Scholar]
- Middleton JE. The impact of plant flavonoids on mammalian biology: implications for immunity, inflammation and cancer. The Flavonoids: Adva Res Sin. 1993;3:337–370. [Google Scholar]
- Mohamed EA, Hashim II, Yusif RM, Shaaban AA, El-Sheakh AR, Hamed MF, Badria FA. Polymeric micelles for potentiated antiulcer and anticancer activities of naringin. Int J Nanomedicine. 2018;13:1009. doi: 10.2147/IJN.S154325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty S, Sahoo AK, Konkimalla VB, Pal A, Si SC. Naringin in combination with isothiocyanates as liposomal formulations potentiates the anti-inflammatory activity in different acute and chronic animal models of rheumatoid arthritis. ACS omega. 2020;5:28319–28332. doi: 10.1021/acsomega.0c04300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie YC, Wu H, Li PB, Luo YL, Long K, Xie LM, Shen JG, Su WW. Anti-inflammatory effects of naringin in chronic pulmonary neutrophilic inflammation in cigarette smoke-exposed rats. J Med Food. 2012;15:894–900. doi: 10.1089/jmf.2012.2251. [DOI] [PubMed] [Google Scholar]
- Papasani VM, Hanumantharayappa B, Annapurna A. Cardioprotective effect of naringin against doxorubicin induced cardiomyopathy in rats. Indo Am J Pharm. 2014;4:2593–2598. [Google Scholar]
- Pleguezuelos-Villa M, Mir-Palomo S, Díez-Sales O, Buso MO, Sauri AR, Nácher A. A novel ultradeformable liposomes of naringin for anti-inflammatory therapy. Colloids Surf B. 2018;162:265–270. doi: 10.1016/j.colsurfb.2017.11.068. [DOI] [PubMed] [Google Scholar]
- Rajadurai M, Prince PS. Preventive effect of naringin on lipid peroxides and antioxidants in isoproterenol-induced cardiotoxicity in Wistar rats: biochemical and histopathological evidences. Toxicol. 2006;228:259–268. doi: 10.1016/j.tox.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan A, Vijayakumar N, Renuka M. Naringin regulates glutamate-nitric oxide cGMP pathway in ammonium chloride induced neurotoxicity. Biomed Pharmacother. 2016;84:1717–1726. doi: 10.1016/j.biopha.2016.10.080. [DOI] [PubMed] [Google Scholar]
- Rangaswami S, Seshadri TR, Veeraraghaviah J. Constitution of naringin The position of the sugar group. J Proc Ind Acad Sci. 1939;9:328–332. [Google Scholar]
- Rani N, Bharti S, Manchanda M, Nag TC, Ray R, Chauhan SS, Kumari S, Arya DS. Regulation of heat shock proteins 27 and 70, p-Akt/p-eNOS and MAPKs by naringin dampens myocardial injury and dysfunction in vivo after ischemia/reperfusion. PLoS One. 2013;8:e82577. doi: 10.1371/journal.pone.0082577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K, Imran M, Jabri T, Ali I, Perveen S, Ahmed S, Shah MR. Gum tragacanth stabilized green gold nanoparticles as cargos for naringin loading: a morphological investigation through afm. Carbohydr Polym. 2017;174:243–252. doi: 10.1016/j.carbpol.2017.06.071. [DOI] [PubMed] [Google Scholar]
- Ratty AK, Das NP. Effects of flavonoids on nonenzymatic lipid peroxidation: structure-activity relationship. Biochem Med Metabol Biol. 1988;39:69–79. doi: 10.1016/0885-4505(88)90060-6. [DOI] [PubMed] [Google Scholar]
- Renugadevi J, Prabu SM. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicol. 2009;256:128–134. doi: 10.1016/j.tox.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Rodríguez V, Plavnik L, de Talamoni NT. Naringin attenuates liver damage in streptozotocin-induced diabetic rats. Biomed Pharmacother. 2018;105:95–102. doi: 10.1016/j.biopha.2018.05.120. [DOI] [PubMed] [Google Scholar]
- Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- Rotimi SO, Adelani IB, Bankole GE, Rotimi OA. Naringin enhances reverse cholesterol transport in high fat/low streptozocin induced diabetic rats. Biomed Pharmacother. 2018;101:430–437. doi: 10.1016/j.biopha.2018.02.116. [DOI] [PubMed] [Google Scholar]
- Rivoira MA, Rodriguez V, Talamoni G, de Talamoni NT. New perspectives in the pharmacological potential of naringin in medicine. Curr Med Chem. 2021;28:1987–2007. doi: 10.2174/0929867327666200604171351. [DOI] [PubMed] [Google Scholar]
- Shen W, Xu Y, Lu YH. Inhibitory effects of citrus flavonoids on starch digestion and antihyperglycemic effects in hepg2 cells. J Agric Food Chem. 2012;60:9609–9619. doi: 10.1021/jf3032556. [DOI] [PubMed] [Google Scholar]
- Silver HJ, Dietrich MS, Niswender KD. Effects of grapefruit, grapefruit juice and water preloads on energy balance, weight loss, body composition, and cardiometabolic risk in free-living obese adults. Nutr Metab. 2011;8:8. doi: 10.1186/1743-7075-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair WB. The grapefruit: its composition, physiology & products, pp. 21-22, California, university of California, agriculture and natural resources. 1972. [Google Scholar]
- Singh D, Chopra K. The effect of naringin, a bioflavonoid on ischemia-reperfusion induced renal injury in rats. Pharmacol Res. 2004;50:187–193. doi: 10.1016/j.phrs.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Surampalli GK, Nanjwade B, Patil PA. Corroboration of naringin effects on the intestinal absorption and pharmacokinetic behavior of candesartan cilexetil solid dispersions using in-situ rat models. Drug Dev Ind Pharm. 2015;41:1057–1065. doi: 10.3109/03639045.2014.925918. [DOI] [PubMed] [Google Scholar]
- Thangavel P, Muthu R, Vaiyapuri M. Antioxidant potential of naringin–a dietary flavonoid–in N-nitrosodiethylamine induced rat liver carcinogenesis. Biomed Prev Nutr. 2012;2:193–202. [Google Scholar]
- Tomasik P. Chemical and functional properties of food saccharides . Florida, CRC Press; 2003. pp. 209–210. [Google Scholar]
- Tsai YJ, Tsai TH. Mesenteric lymphatic absorption and the pharmacokinetics of naringin and naringenin in the rat. J Agric Food Chem. 2012;60:12435–12442. doi: 10.1021/jf301962g. [DOI] [PubMed] [Google Scholar]
- Visnagri A, Adil M, Kandhare AD, Bodhankar SL. Effect of naringin on hemodynamic changes and left ventricular function in renal artery occluded renovascular hypertension in rats. J Pharm Bioallied Sci. 2015;7:121. doi: 10.4103/0975-7406.154437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DM, Yang YJ, Zhang L, Zhang X, Guan FF, Zhang LF. Naringin enhances CaMKII activity and improves long-term memory in a mouse model of alzheimer’s disease. Int J Mol Sci. 2013;14:5576–5586. doi: 10.3390/ijms14035576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xianchu L, Lan Z, Qiufang L, Yi L, Xiangcheng R, Wenqi H, Yang D. Naringin protects against lipopolysaccharide-induced cardiac injury in mice. Environ Toxicol Pharm. 2016;48:1–6. doi: 10.1016/j.etap.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Yang X, Almassri HN, Zhang Q, Ma Y, Zhang D, Chen M, Wu X. Electrosprayed naringin-loaded microsphere/SAIB hybrid depots enhance bone formation in a mouse calvarial defect model. Drug Deliv. 2019;26:137–146. doi: 10.1080/10717544.2019.1568620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Cheng W, Qin Z, Yu H, Yu Z, Zhong M, Sun K, Zhang W. Effects of naringin on proliferation and osteogenic differentiation of human periodontal ligament stem cells in vitro and in vivo. Stem Cells Int. 2015;4:2015. doi: 10.1155/2015/758706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Huang Y, Zhang J, Feng Y, Shen L. Formulation, preparation and evaluation of nanostructured lipid carrier containing naringin and coix seed oil for anti-tumor application based on “unification of medicines and excipients”. Drug Des Devel Ther. 2020;14:1481. doi: 10.2147/DDDT.S236997. [DOI] [PMC free article] [PubMed] [Google Scholar]


