Abstract
Objective:
This study aimed to identify risk factors for recurrence after pancreatic resection for intraductal papillary mucinous neoplasm (IPMN).
Summary Background Data:
Long-term follow-up data on recurrence after surgical resection for IPMN are currently lacking. Previous studies have presented mixed results on the role of margin status in risk of recurrence after surgical resection.
Methods:
A total of 126 patients that underwent resection for noninvasive IPMN were followed for a median of 9.5 years. Dedicated pathological and radiological reviews were performed to correlate clinical and pathological features (including detailed pathological features of the parenchymal margin) with recurrence after surgical resection. In addition, in a subset of 32 patients with positive margins, we determined the relationship between the margin and original IPMN using driver gene mutations identified by next-generation sequencing.
Results:
Family history of pancreatic cancer and high-grade IPMN was identified as risk factors for recurrence in both uni- and multivariate analysis (adjusted hazard ratio 3.05 and 1.88, respectively). Although positive margin was not significantly associated with recurrence in our cohort, the size and grade of the dysplastic focus at the margin were significantly correlated with recurrence in margin-positive patients. Genetic analyses showed that the neoplastic epithelium at the margin was independent from the original IPMN in at least 9 of 32 cases (28%). The majority of recurrences (74%) occurred after 3 years, and a significant minority (32%) occurred after 5 years.
Conclusion:
Sustained postoperative surveillance for all patients is indicated, particularly those with risk factors such has family history and high-grade dysplasia.
Keywords: intraductal papillary mucinous neoplasm, pancreatic cancer, progression, recurrence, surveillance
The increased use of abdominal imaging and improved imaging techniques has increased the diagnosis of intraductal papillary mucinous neoplasms (IPMNs) of the pancreas in recent years.1–4 A subset of these patients undergo surgical resection to prevent malignant progression, particularly if main duct (MD) dilatation, solid component, jaundice, or other high-risk features is present. Although recurrence in the remnant pancreas has been reported in as many as 20% of surgically resected patients, recommendations for postoperative surveillance are controversial, and more data are needed to inform the best approach.5–10 Particularly, the time to recurrence and the risk factors predicting recurrence are important data for clinical decision-making.
Pancreatic parenchymal margin status may contribute to risk of recurrence and is typically reported in a binary fashion as either positive or negative. However, there is no standard definition for what constitutes a positive pancreatic transection margin. High-grade dysplasia or invasive carcinoma at the margin is universally reported, as this will result in additional surgical resection. However, low-grade dysplasia at the margin is inconsistently reported as it does not currently alter clinical care. These inconsistencies significantly complicate retrospective studies on margin status, which use variable definitions of margin positivity. Additionally, the origin of the neoplastic epithelium at the surgical margin is unclear, as it may represent extension of a single IPMN or multiple independent precancerous lesions. This is of importance to understand the biological behavior of these lesions, especially the extent of intra-pancreatic disease and recurrence after surgical resection.
In this study we set out to determine risk factors for IPMN recurrence after surgical resection. In a large cohort of surgically resected IPMNs, we performed a detailed pathological analysis of IPMN specimens with a particular emphasis on the pancreatic parenchymal surgical margin. We then correlated clinical and pathological features to the risk of clinically significant recurrence and radiological progression over long-term follow-up, with a median follow-up of almost 10 years. Furthermore, in a subset of margin-positive specimens, we performed targeted next-generation sequencing to determine the relationship of the neoplastic epithelium at the margin with the original resected IPMN.
METHODS
The study was approved by the Institutional Review Board (IRB) of The Johns Hopkins Hospital. This study is a follow-up retrospective review and re-analysis of the previously described cohort of resected IPMNs reported by He et al.5 Patients in the cohort underwent surgery for IPMN without associated invasive carcinoma at The Johns Hopkins Hospital between 1995 and 2009. Surgical procedures included pancreaticoduodenectomy, central pancreatectomy, and distal pancreatectomy. Patients who underwent total pancreatectomy (TP) at the initial surgery were excluded, as removal of the entire pancreas eliminates the potential for intra-pancreatic recurrence. Clinical patient information was retrieved from our institutional database; pathological slides and radiographic imaging were separately reviewed for this study by trained experts (L.A.A.B. and S.K., respectively). Family history was considered positive if any family history of pancreatic ductal adenocarcinoma (PDAC) was reported.
Pathological Examination
Detailed histopathological review of the formalin-fixed paraffin-embedded (FFPE) tissue slides of all available pancreatic resection specimens in the study cohort was performed by a single pancreatic pathologist (L.A.A.B.). Presence of any mucinous epithelium at the margin was considered a positive margin. If positive, additional features at the margin such as grade, size, number of dysplastic foci, and MD involvement were assessed. See Supplementary Methods, http://links.lww.com/SLA/C554 for additional details.
Follow-Up
Typically, follow-up imaging was performed every 6 months for 2 years and annually thereafter. Primary imaging modalities included computed tomography (CT) or magnetic resonance imaging (MRI) with cholangiopancreatography, but positron emission tomography (PET) or endoscopic ultrasound (EUS) was also performed as clinically indicated. CT, MRI, and PET/CT data were reviewed on a picture archiving and communication system (Emageon Workstation; Advanced Visualization, Version 5.30.7.26: Emageon Inc., Birmingham, AL). For 6 patients, follow-up imaging studies after surgery were performed but not available for review, and radiology (CT or MRI) reports were used for analysis.
Clinically significant recurrence was defined as diagnosis of IPMN requiring resection or PDAC during the follow-up period. Diagnosis of PDAC included cases with pathological diagnosis based on resection specimen or biopsy in unresected disease. Additionally, patients found to have died from PDAC based on social security death index, death certificates, and/or obituaries were included.
Radiographic progression was defined as (a) worsening (>5 mm increasing size of cyst or >2 mm increasing diameter of dilated (>5 mm) MD) compared to the initial postoperative imaging and/or (b) new cystic lesion (>5 mm) or new MD dilatation (>5 mm) or new solid mass in the remnant pancreas on cross-sectional follow-up imaging. The size of cysts and MD diameter were measured by the largest diameter on axial plane using an electronic caliper. Apparent postoperative MD dilatation (diffuse dilatation that started immediately after surgery) was not considered progression. Because imaging studies were not available for review, 15 patients had missing information on radiographic progression and were not included in this analysis.
Statistical Analysis
The data were processed using Excel (Microsoft Co., Redmond, WA) and all statistical analyses were compiled with R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria).
P values <0.05 were considered statistically significant, if not stated otherwise. Details are provided in the Supplementary Methods, http://links.lww.com/SLA/C554.
Analysis of Somatic Mutations in IPMN and Margin Samples
In a subset of 32 patients with positive margins on pathology review, the somatic mutations in the original resected IPMN and the corresponding margin were compared. Mutations in 11 known driver genes in pancreatic neoplasia were analyzed with next-generation sequencing using a previously established panel (KRAS, GNAS, TP53, SMAD4, CDKN2A, RNF43, TGFBR2, ARID1A, BRAF, MAP2K4, and PIK3CA).10 Details are provided in the Supplementary Methods, http://links.lww.com/SLA/C554. Based on these results, IPMN/margin pairs were categorized to assess the relatedness of the separate lesions. Lesions were categorized as “independent” if no shared somatic mutations were identified and categorized as “potentially related” if at least one mutation was shared.
RESULTS
Clinical and Pathological Features of Cohort
In total, 126 patients with a median age of 69 years (range 3690) were included in the study. The cohort showed an almost equal sex distribution (62 females, 49%). Patient characteristics are displayed in Table 1. Whipple’s procedure was performed in 90 cases (71%), distal pancreatectomy in 33 (26%), and central pancreatectomy in 3 (2%) cases. According to the preoperative imaging, 92 cases (73%) were classified as branch duct, 10 cases (8%) as MD, and 24 (19%) as mixed duct type. Dedicated pathological review determined a mean IPMN cyst size of 2.5 cm, and assessment of the IPMN lesions based on a 3-tiered grading system led to a diagnosis of low-grade dysplasia in 14 cases (11%), intermediate-grade in 69 (55%), and high-grade dysplasia in 41 cases (33%). Synchronous PanIN lesions were found in 102 cases (81%). Of these, 6 (5%) were high-grade PanINs, and 54 patients (43%) had ≥5 PanIN lesions.
TABLE 1.
Patient Characteristics and Clinical Features of Study Cohort
| Age, y, median (range) | 69 (36–90) |
| Sex, male/female | 64 (50.8%)/62 (49.2%) |
| Race, white/non-white | 110 (87.3%)/16 (12.7%) |
| BMI, median (range) | 25.7 (14.5–38.8) |
| Missing | 4 |
| Smoker | |
| Never | 48 (38.1%) |
| Former | 66 (52.4%) |
| Current | 12 (9.5%) |
| Alcohol | |
| Never/rare | 96 (76.2%) |
| Frequent/former | 27 (21.4%) |
| Missing | 3 |
| Diabetes (preop) | |
| No | 107 (84.9%) |
| Yes | 19 (15.1%) |
| Family history of PDAC | |
| absent | 104 (82.5%) |
| ≦ 2 First-degree relatives or germline mutation | 9 (7.1%) |
| BRCA2 | 2 |
| STK11 | 1 |
| ≦ 1 First-degree relative | 7 (5.6%) |
| any other FH of PDAC | 6 (4.8%) |
| Previous other malignancy or neoplasm | |
| No | 80 (63.5%) |
| Yes | 46 (36.5%) |
| Surgical procedure | |
| Whipple | 90 (71.4%) |
| Distal pancreatectomy | 33 (26.2%) |
| Central resection | 3 (2.4%) |
| Margin (original diagnostic pathology report) | |
| Negative | 95 (75.4%) |
| Positive | 31 (24.6%) |
| Margin (pathology review for study) | |
| Negative | 36 (28.6%) |
| Positive* | 90 (71.4%) |
BMI indicates body mass index.
For this study, margins were considered positive if any mucinous epithelium was identified on pathology review.
Review of the final resection margins showed presence of mucinous epithelium in 90 patients (71%), including 68 with low-, 17 with intermediate-, and 4 with high-grade dysplasia. A median of 2 (range 1–11) dysplastic foci were found at the margin and 43 (48%) patients had >1 focus. Median size of the largest dysplastic focus was 0.2 cm (range 0.05–1.5). Additional histopathological features are depicted in Table 2.
TABLE 2.
Clinicopathological Characteristics of Resected IPMNs and Positive Margins on Final Pathology
| IPMN | Margin | |
|---|---|---|
| No. of cases | 126 (100%) | 90 (71.4%) |
| Largest size, median (range) | 2.2 cm (0.2–10) | 0.2 cm (0.05–1.5) |
| Missing | 1 | 13 |
| Highest grade of dysplasia, count | ||
| Low (adeno) | 14 (11.1%) | 68 (75.6%) |
| Intermediate (border) | 69 (54.8%) | 17 (18.9%) |
| High (carcinoma in situ) | 41 (32.5%) | 4 (4.4%) |
| Missing | 2 | 1 |
| Predominant grade of dysplasia | — | |
| Low (adenoma) | 44 (34.9%) | |
| Intermediate (borderline) | 68 (54.0%) | |
| High (carcinoma in situ) | 4 (3.2%) | |
| Missing | 10 | |
| No. of lesions/multifocality | Multifocal (≧2 IPMN): yes 50 (39.7%) no 76 (60.3%) |
No. of dysplastic foci: median (range) 2 (1 – 11) missing 15 ≧ 2 dysplastic foci: 43 (47.8%) |
| Diffuse gland involvement (≧ 2/3 gland involvement) | ||
| Yes/no/missing | 15 (11.9%)/82 (65.1%)/29* | — |
| Main Duct involvement | 34 (27%)* | 18 (20%) |
| Histologic subtypes | ||
| Gastric | 83 (65.9%) | 73 (81.1%) |
| Intestinal | 9 (7.1%) | 1 (1.1%) |
| Pancreatobiliary | 1 (0.8%) | 1 (1.1%) |
| Oncocytic | 2 (1.6%) | 0 |
| Mixed | 19 (15.1%) | 0 |
| Missing | 12 | 15 |
Imaging data.
Interval, Pattern, and Features of Recurrence
A total of 124 patients (98%) had a follow-up of at least 1 year after primary surgery, with a maximum of >17 years. Median follow-up time from surgery to death or last follow-up date was 9.5 years in the entire cohort. Clinically significant recurrence was reported in 19 cases (15%), whereas radiographic progression was found in 30 cases (27%). The estimated median survivals were not reached for clinically significant recurrence-free survival (csRFS) and radiographic progression free survival (rPFS). The median time from surgery to the onset of clinically significant recurrence was 4.5 years (range 1.0 – 10.1), and the median time from surgery to the onset of radiographic progression was 4.0 years (range 0.5–9.7). Three-year csRFS was 95% [95% confidence interval (CI): 91%−99%], and 5-year csRFS was 86% (95% CI: 76%−94%). Notably, of the 19 patients with clinically significant recurrence, 14 (74%) recurred after 3 years and 6 (32%) after 5 years. Three-year rPFS was 87% (95% CI: 81%−94%), and 5-year rPFS was 73% (95% CI: 64%−84%). The corresponding Kaplan-Meier curves are displayed in Figure 1.
FIGURE 1.
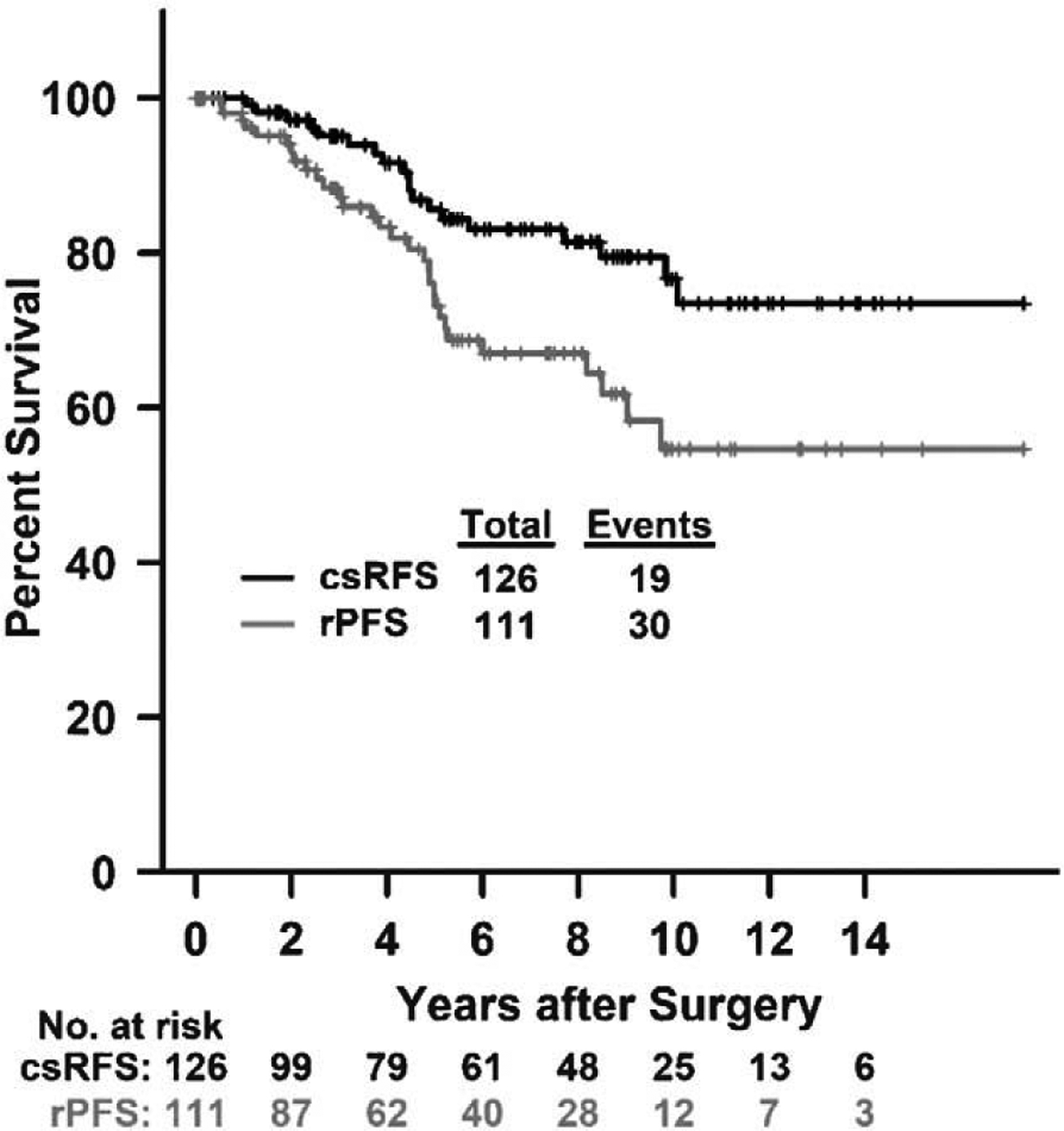
Kaplan-Meier curves of csRFS and rPFS. Fifteen patients had missing date of rPFS.
Clinically significant recurrence represented PDAC in 13 cases and IPMN in 6 cases. Intriguingly, none of the patients diagnosed with IPMN in the follow-up experienced symptoms and only 8 of 13 PDAC patients were symptomatic before diagnosis, with abdominal pain and weight loss as the most prevalent manifestations. Pancreatic re-resection was performed in 12 cases of clinically significant recurrence (6 IPMN, 6 PDAC) and led to removal of the entire remnant gland in all cases. Procedures of these completion pancreatectomies were distal pancreatectomy in 9 cases and pancreaticoduodenectomy in 3 cases, reflecting recurrence in the pancreatic body and/or tail in the majority of cases. Of the 6 cases with PDAC for which the pancreatic re-resection specimen was available, IPMN was synchronously found in 3 of them. The remaining cases of clinically significant recurrence as PDAC were identified by death certificates in 5 cases and extrapancreatic biopsy of metastatic disease in 2 cases. Thus, for these cases, information on pancreatic re-resection specimen was not available or secondary pancreatic surgery was not performed due to unresectable disease.
Of all patients that were diagnosed with radiographic progression in the follow-up, 15 patients (50%) developed a new disease (ie, new cyst, new MD dilatation, or new solid mass) in a pancreatic remnant that was free from disease (ie, no cyst or MD dilatation) on the initial postoperative imaging study. Radiographic progression occurred at the margin in 6 cases (20%), distant from the margin in 19 cases (63%), and involved both locations in 5 cases (17%). Fourteen cases with clinically significant recurrence met the criteria for radiographic progression.
Risk Factors for Recurrence
Univariate analyses revealed that family history of pancreatic cancer (P = 0.03) and high-grade dysplasia of the IPMN (P = 0.0156) were significantly associated with decreased csRFS. In patients with positive margin, the size of the largest dysplastic focus at the margin (P = 0.04) and high-grade dysplasia at the margin (P = 0.04) were statistically significant risk factors for csRFS. Patients with foci of mucinous epithelium measuring >0.5 cm at the margin had a significantly worse csRFS compared to those with foci <0.5 cm (Supplementary Figure 1, http://links.lww.com/SLA/C553). Interestingly, only body mass index (P = 0.007) was found to be a risk factor for rPFS in univariate analysis of the entire cohort. Results of multivariate analyses revealed 2 independent risk factors for csRFS. Patients with family history of PDAC were at risk of recurrence with an adjusted hazard ratio (aHR) of 3.05 (1.17–7.94; P = 0.023). Similarly, patients with high-grade dysplasia in their original IPMN were at increased risk of recurrence [aHR 1.88 (1.17–3); P = 0.008]. Of note, variables of the margin were not considered in multivariable analysis because this information was only available for margin positive patients. Results of uni- and multivariate analyses are shown in Tables 3 to 5.
TABLE 3.
Results of Univariate Analysis of csRFS and rPFS for Patient Characteristics, IPMN Variables, and PanIN Variables
| Variables | n (%) or Mean (SD) | csRFS | rPFS | ||||
|---|---|---|---|---|---|---|---|
| Events (n) | HR (95% Cl) | P | Events (n) | HR (95% Cl) | P | ||
| Patient characteristics (n = 126) | |||||||
| Total cohort | 126 (100) | 19 | — | — | 30 | — | — |
| Age, mean (SD) | 66.85 (11.78) | 19 | 1.01 (0.97–1.05) | 0.61 | 30 | 1.02 (0.99–1.05) | 0.21 |
| Sex | |||||||
| Male | 64 (50.79) | 13 | 1 | 18 | 1 | ||
| Female | 62 (49.21) | 6 | 0.45 (0.17–1.17) | 0.10 | 12 | 0.64 (0.31–1.32) | 0.23 |
| BMI, mean (SD) | 26.18 (4.58) | 19 | 1.07 (0.97–1.18) | 0.18 | 30 | 1.11 (1.03 −1.2) | 0.007 |
| Missing | 4 (3.17) | ||||||
| Smoker | |||||||
| Never | 48 (38.1) | 8 | 1 | 12 | 1 | ||
| Former/current | 78 (61.9) | 11 | 1.06 (0.42–2.63) | 0.91 | 18 | 1.33 (0.64–2.76) | 0.45 |
| Diabetes (preop) | |||||||
| No | 107 (84.92) | 16 | 1 | 24 | 1 | ||
| Yes | 19 (15.08) | 3 | 1.16 (0.34–3.97) | 0.82 | 6 | 1.95 (0.79–4.79) | 0.15 |
| Alcohol | |||||||
| Never/rare | 96 (76.19) | 15 | 1 | 25 | 1 | ||
| Frequent/former | 27 (21.43) | 3 | 0.69 (0.2–2.38) | 0.56 | 5 | 0.82 (0.31–2.15) | 0.68 |
| Missing | 3 (2.38) | ||||||
| Family history pancreatic cancer | |||||||
| No | 104 (82.54) | 11 | 1 | 24 | 1 | ||
| Yes | 22 (17.46) | 8 | 2.8 (1.12–6.96) | 0.03 | 6 | 0.89 (0.36–2.18) | 0.80 |
| Previous other malignancy or neoplasm | |||||||
| No | 80 (63.49) | 13 | 1 | 19 | 1 | ||
| Yes | 46 (36.51) | 6 | 0.94 (0.36–2.49) | 0.91 | 11 | 1.2 (0.57 −2.54) | 0.63 |
| Surgical procedure | |||||||
| Whipple | 90 (71.43) | 15 | 1 | 21 | 1 | ||
| distal/central | 36 (28.57) | 4 | 0.62 (0.21–1.88) | 0.40 | 9 | 1.02 (0.47–2.24) | 0.96 |
| Margin | |||||||
| Negative | 36 (28.57) | 2 | 1 | 7 | 1 | ||
| Positive | 90 (71.43) | 17 | 3.58 (0.83–15.49) | 0.09 | 23 | 1.6 (0.68–3.73) | 0.28 |
| IPMN variables (n = 126) | |||||||
| Largest size, cm, mean (SD) | 2.5 (1.58) | 19 | 0.95 (0.67–1.35) | 0.78 | 30 | 0.99 (0.75–1.3) | 0.94 |
| Missing | 1 (0.79) | ||||||
| Duct involvement (preop imaging) | |||||||
| Main/mixed | 34 (26.98) | 5 | 1 | 5 | 1 | ||
| Branch | 92 (73.02) | 14 | 0.75 (0.27–2.10) | 0.59 | 25 | 1.11 (0.42–2.92) | 0.83 |
| Papillary growth | |||||||
| No/focal | 68 (53.97) | 9 | 1 | 17 | 1 | ||
| Moderate/extensive | 45 (35.71) | 8 | 1.25 (0.48–3.25) | 0.64 | 8 | 0.68 (0.29–1.58) | 0.37 |
| Missing | 13 (10.32) | ||||||
| Predominant grade of dysplasia | |||||||
| Low/intermediate | 112 (88.89) | 16 | 1 | 26 | not performed | ||
| High | 4 (3.17) | 1 | 1.75 (0.23–13.32) | 0.59 | 0 | ||
| Missing | 10 (7.94) | ||||||
| Highest grade of dysplasia | |||||||
| Low/intermediate | 83 (65.87) | 8 | 1 | 19 | 1 | ||
| High | 41 (32.54) | 10 | 3.15 (1.24–7.98) | 0.02 | 10 | 1.44 (0.67–3.11) | 0.35 |
| Missing | 2 (1.59) | ||||||
| Prevalence of highest grade of dysplasia | |||||||
| Focal | 49 (38.89) | 7 | 1 | 13 | 1 | ||
| Multifocal/extensive | 68 (53.97) | 10 | 1.04 (0.4–2.74) | 0.93 | 13 | 0.6 (0.28–1.3) | 0.20 |
| Missing | 9 (7.14) | ||||||
| Multifocal (≧2 IPMN) | |||||||
| No | 76 (60.32) | 10 | 1 | 15 | 1 | ||
| Yes | 50 (39.68) | 9 | 1.37 (0.55–3.37) | 0.50 | 15 | 1.63 (0.79–3.35) | 0.18 |
| Diffuse gland involvement (≧ 2/3) | |||||||
| No | 82 (65.08) | 11 | 1 | 23 | 1 | ||
| Yes | 15 (11.9) | 2 | 1.17 (0.26–5.3) | 0.84 | 3 | 0.92 (0.27–3.07) | 0.89 |
| Missing | 29 (23.02) | ||||||
| PanIN variables (n = 126) | |||||||
| Synchronous PanIN lesion present | |||||||
| No | 24 (19.05) | 4 | 1 | 5 | 1 | ||
| Yes | 102 (80.95) | 15 | 0.78 (0.26–2.35) | 0.66 | 25 | 0.59 (0.23–1.56) | 0.29 |
| No. of lesions | |||||||
| <5 | 68 (53.97) | 10 | 1 | 15 | 1 | ||
| ≧5 | 54 (42.86) | 9 | 1.31 (0.53–3.24) | 0.55 | 14 | 1.31 (0.63–2.71) | 0.47 |
| Missing | 4 (3.17) | ||||||
| Highest grade of dysplasia | |||||||
| Low/intermediate | 95 (75.4) | 13 | 1 | 22 | 1 | ||
| High | 6 (4.76) | 2 | 2.95 (0.66–13.14) | 0.16 | 3 | 2.39 (0.71 −8.03) | 0.16 |
| Missing | 25 (19.84) | ||||||
BMI indicates body mass index; SD, standard deviation. Missing data were not included in the analysis.
TABLE 5.
Results of Multivariate Analysis
| csRFS | rPFS | |||
|---|---|---|---|---|
| Covariate | aHR (95% Cl) | P | aHR (95% Cl) | P |
| Family history pancreatic cancer | ||||
| No | 1 | — | ||
| Yes | 3.05 (1.17–7.94) | 0.02 | — | |
| Highest grade of dysplasia, IPMN | ||||
| Low/intermediate | 1 | — | ||
| High | 1.88 (1.17 −3) | 0.008 | — | |
Mutations at Margin
Of 90 patients (71%) with positive margin, we selected 32 cases to investigate somatic mutations at the margin. Selection was made after pathological review and was based on tissue availability for sequencing of primary IPMN, margin, and normal control tissue. The majority (n = 23; 72%) of this subset did not develop a recurrence during follow-up. However, 6 patients (19%) showed progression on imaging, 2 patients (6%) were diagnosed with IPMN, and 1 patient (3%) with PDAC. All 3 patients with pathologically proven IPMN or PDAC underwent reoperation. Targeted next-generation sequencing of the mucinous epithelium at the margin and the original IPMN lesion revealed KRAS mutations at the oncogenic hotspots at codons 12, 13, and 61 in 28 margin samples and 30 IPMNs samples. GNAS mutations at the oncogenic hotspot at codon 201 were found in 8 margin samples and 22 IPMN samples, and RNF43 mutations were found in 2 margin samples and 2 IPMN samples. In each IPMN sample at least one of the mutations targeted with our panel was present, whereas these mutations were absent at the margin in 4 cases.
Intriguingly, mutations in margin and corresponding IPMN matched entirely only in 2 cases. The majority showed at least some difference in the somatic mutations identified in the 2 separate lesions. To determine relatedness, we categorized the lesions according to the overlap of their somatic mutations. Lesions were called “independent” in 9 cases because there were no shared somatic mutations in the neoplastic epithelium at the margin and original IPMN lesion. The other 23 cases were “potentially related,” meaning they shared at least 1 somatic mutation. However, because many of the identified somatic mutations occurred in oncogenic hotspots, it is possible that some of these “potentially related” lesions were actually independent but shared hotspot alterations by chance. Detailed results of the genetic analyses are depicted in Table 6.
TABLE 6.
Results of Targeted Sequencing in a Subset of 32 Margin Positive Patients
| Case No. | IPMN | Margin | Relatedness | Recurrence (Type) |
|---|---|---|---|---|
| 1 |
KRAS: G12D (39.0) GNAS: R201C (27.9) |
KRAS: G12D (20.5) GNAS: wt |
Potentially related | PDAC |
| 2 |
KRAS: G12V (17.0), G12D (11.0), G12C (3.0) GNAS: wt |
KRAS: G12V (12.7), G13D (6.3) GNAS: wt |
Potentially related | IPMN |
| 3 |
KRAS: G12V (29.7) GNAS: R201C (23.4), R201H (1.9) |
KRAS: G12R (23.1), Q61H (18.1) GNAS: R201H (17.0), R201C (14.0) |
Potentially related | IPMN |
| 4 | KRAS: G12V (26.8) | wt | Independent | Progression on imaging |
| 5 |
KRAS: G12D (45.2), Q61H (5.0) GNAS: R201C (27.8) RNF43: wt |
KRAS: G12D (13.5), G12R (9.1) GNAS: R201C (7.8) RNF43: A136D (12.8) |
Potentially related | Progression on imaging |
| 6 |
KRAS: G12D (35.0), G12V (6.0) GNAS: R201H (2.6) |
KRAS: G12D (27.8) GNAS: wt |
Potentially related | Progression on imaging |
| 7 |
KRAS: G12D (32.0) GNAS: R201H (5.8) |
KRAS: G12V (13.8), G12D (6.5), G12R (18.8) GNAS: R201H (4.5) | Potentially related | Progression on imaging |
| 8 |
KRAS: Q61H (8.3), G12D (5.0) GNAS: R201H (3.1) |
KRAS: G12R (9.5) GNAS: wt |
Independent | Progression on imaging |
| 9 | KRAS: G12D (44.0) | KRAS: G12D (25.6) | Potentially related | Progression on imaging |
| 10 | KRAS: G12D (18.1), G12V (10.0), G12R (3.0) | KRAS: G12D (25.5), G12R (6.8) | Potentially related | — |
| 11 |
KRAS: G12V (8.9), Q61H (28.3) GNAS: R201H (19.9) |
KRAS: G12V (59.7) GNAS: R201H (37.5) |
Potentially related | — |
| 12 |
KRAS: G12D (5.9) GNAS: R201H (30.2) RNF43: R531H (6.4) |
KRAS: G12D (37.5) GNAS: wt RNF43: wt |
Potentially related | |
| 13 |
KRAS: Q61H (48.5) GNAS: R201H (50.2) |
KRAS: G12C (10.0) GNAS: wt |
Independent | — |
| 14 |
KRAS: G12V (33.2) GNAS: R201H (29.4), R201C (6.1) RNF43: G741E (6.4) |
KRAS: G12V (29.7), G12D (4.7), G12R (6.0), G12S (6.0)GNAS: wt RNF43: wt |
Potentially related | |
| 15 | KRAS: G12D (4.0), G12R (6.0), G12C (11.0) | KRAS: G12R (10.0) | Potentially related | — |
| 16 | KRAS: G12D (22.6) | KRAS: G12D (39.8) | Potentially related | — |
| 17 |
KRAS: G12V (5.9), G12R (3.0) GNAS: R201H (22.6), R201C (4.8) |
wt | Independent | — |
| 18 |
KRAS: G12D (30.3), G12V (9.3), Q61H (7.3) GNAS: R201H (6.8), R201C (6.0) |
KRAS: G12D (50.0) GNAS: - | Potentially related | |
| 19 | KRAS: G12D (16.7), G12V (29.1) | KRAS: G12D (3.5), Q61H (31.8) | Potentially related | — |
| 20 |
KRAS: G13D (36.1), G12V (6.0), Q61H (14.3) GNAS: R201H (15.3) RNF43: wt |
KRAS: G12R (35.3), G12C (5.4) GNAS: wt RNF43: A46G |
Independent | |
| 21 | KRAS: G12V (28.0) | KRAS: G12D (17.0), G12C (7.5) | Independent | — |
| 22 |
KRAS: G13D (2.0) GNAS: R201H (36.0) |
KRAS: G13D (14.0), G12R (14.0) GNAS: wt |
Potentially related | — |
| 23 | GNAS: R201H (30.0) | wt | Independent | — |
| 24 |
KRAS: G12D (39.0) GNAS: R201C (32.5), R201H (6.5) |
KRAS: G12D (27.0), G12V (13.0) GNAS: R201C (22.5), R201H (7.0) |
Potentially related | — |
| 25 |
KRAS: G12D (9.1), G12V (10.4) GNAS: R201C (11.3) |
KRAS: G12D (14.6), G12V (10.4), G12R (10.4) GNAS: | Potentially related | — |
| 26 | GNAS: R201H (28.0) | wt | Independent | — |
| 27 |
KRAS: G12V (42.2), G12R (3.5) GNAS: R201C (16.5) |
KRAS: G12D (26.0), G12R (6.0) GNAS: wt |
Potentially related | — |
| 28 |
KRAS: G12R (41.1), G12D (6.0) GNAS: R201H (6.4) |
KRAS: G12D (30.3) GNAS: R201H (2.8) |
Potentially related | — |
| 29 |
KRAS: G12C (6.0), G12V (13.0) GNAS: R201C (6.6) |
KRAS: G12C (18.4), G12D (6.8), G12V (3.4), Q61H (13.8) GNAS: R201C (3.0) |
Potentially related | — |
| 30 |
KRAS: G13D (33.3), G12D (5.0) GNAS: R201H (19.5) |
KRAS: G12V (4.0) GNAS: wt |
Independent | — |
| 31 |
KRAS: G12D (37.2), G12V (5.0) GNAS: R201H (39.3) |
KRAS: G12D (7.0), G12R (24.5) GNAS: R201H (3.5) |
Potentially related | — |
| 32 | KRAS: G12D (31.4), G12V (26.6) | KRAS: G12D (45.0) | Potentially related | — |
Somatic mutations in driver genes in IPMNs and at the corresponding margins were analyzed and their relatedness assessed. Wt indicates wild type.
DISCUSSION
Surgical resection of IPMN is recommended for a subgroup of patients to prevent progression to pancreatic cancer and is increasingly performed due to improvements in cross-sectional imaging, surgical technique, and postoperative care.11,12 Previous studies indicate that as many as 20% of patients develop recurrent disease in their remnant pancreas after IPMN resection.5–10 The majority of current guidelines recommend lifelong postoperative surveillance for as long as the patient is fit to undergo surgery.2,3,13 However, the underlying studies were hindered by limited length of the postoperative observation periods (median follow-up 2.2–4.8 years in previous studies, compared to 9.5 years in our study, see Table 7), and long-term results were based on predictions rather than on actual follow-up.5,6,9,14–22 Given that intraepithelial neoplastic disease may be left behind after surgical resection and progression to pancreatic canceris a slow process, sufficient data on long-term recurrence are of particular interest.23 With the present study, we provide these data by extending the previously investigated median follow-up by several years. Our results show a 5-year csRFS of 86% (95% CI: 76%−94%), indicating an ongoing risk of recurrence with 6 of 19 (32%) clinically significant recurrences diagnosed after 5 years. Of note, a considerable proportion of patients with clinically significant recurrence did not experience any symptoms before diagnosis of recurrent disease. These data underscore the importance of continued postoperative surveillance after resection. In contrast to the majority of recommendations, the AGA guidelines do not recommend postoperative surveillance of patients with resected lesions that are low- or intermediate-grade.1 However, we show that the 5-year csRFS was 91 % (95% CI: 84%−98%) for patients that had a resection of low- or intermediate-grade IPMN in their primary surgery, and 8 of the 19 patients (42%) in our cohort that developed recurrent IPMN or PDAC had a resection of low- or intermediate-grade IPMN in their primary surgery.
TABLE 7.
Previous Publications on IPMN Recurrence (Sort by Year)
| Authors | Year | Study Type | IPMN Cohort | No. of Cases | Definition of Margin Positivity | Margin Positive | Margin Positivity Risk Factor for Recurrence | Median Follow-Up |
|---|---|---|---|---|---|---|---|---|
| White et al14 | 2007 | SC | Noninvasive | 78 | Any grade of dysplasia; PanIN 3 (only) explicitly included | 29.5% (n = 23) | Yes | 40 months |
| Park et al15 | 2011 | SC | Unselected | 103 | Any grade of dysplasia including invasive carcinoma | 4.9% (n = 5) | No | 26.7 months |
| Mariya and Traverso16 | 2012 | SC | Unselected | 203 | Any grade of dysplasia including invasive carcinoma | 6.9% (n = 14) | No | 40 months |
| He et al5 | 2013 | SC | Noninvasive | 130 | Not explicitly stated | 20% (n = 26) | No | 38 months |
| Frankel et al9 | 2013 | SC | Noninvasive | 192 | Any grade of dysplasia; PanIN explicitly included | 45% (n = 86) | Yes | 46 months |
| Kang et al17 | 2014 | SC | Unselected | 366 | Any grade of dysplasia | 17.8% (n = 65) | No | 44.4 months |
| Marchegiani et al6 | 2015 | SC | Unselected | 381 | Any grade of dysplasia including invasive carcinoma | 15.5% (n = 59) | Yes | 58 months* |
| Marchegiani et al18 | 2015 | SC | Main duct | 173 | High-grade dysplasia or invasive carcinoma only | 9.8% (n = 17) | Yes | 56 months* |
| Yamaguchi et al19 | 2016 | SC | Unselected | 55 | Not explicitly stated | 29.1% (n = 16) | No | 2.3 years |
| Dhar et al20 | 2018 | MC (8) | Noninvasive | 330 | Any grade of dysplasia; PanIN explicity included | 19.7% (n = 65) | No | 36 months |
| Al Efishat et al22 | 2018 | SC | Noninvasive +microinvasive (≤10 mm of invasive component) | 319 | Any grade of dysplasia including invasive carcinoma; PanIN explicitly included | 50.8% (n = 162) | No | 42 months |
| Hirono et al21 | 2020 | MC (11) | Unselected | 1074 | High-grade dysplasia or invasive IPMC; PanIN 3 (only) explicitly included | Not reported | No | 54.2 months |
| Our study | 2020 | SC | Noninvasive | 126 | Any grade of dysplasia; PanIN explicitly included | 71.4% (n = 90) | No | 114 months |
MC indicates multicenter (number of institutions); SC, single-center.
Follow-up of survivors only.
Uni- and multivariate analyses of our long-term data both identified family history of pancreatic cancer and high-grade dysplasia of the resected IPMN lesion to be independent risk factors for recurrence. Family history of pancreatic cancer is an established risk factor for PDAC, and an association with recurrence following IPMN resection has been identified before.5,24,25 Interestingly, a recent study detected the prevalence of deleterious germline variants in sporadic IPMN patients to be similar to that in sporadic PDAC patients.26 Of the 315 tested patients with surgically resected IPMN in that study, 7.3% had a deleterious germline variant in a hereditary cancer predisposition gene and 2.9% in pancreatic cancer susceptibility genes. If these patients have a similarly increased risk of recurrence as those with a documented family history, germline testing in IPMN patients may have clinical utility for recurrence risk stratification.
Previous studies have reported mixed results with regards to grade of dysplasia in IPMN and its role as risk factor for recurrence. Although some authors identified presence of high-grade IPMN to be a risk factor for recurrence, others did not.5,8,10,17,22,27,28 The latter includes a previous study utilizing our cohort, which did not find statistical significance for this factor.5 Of note, the study had a shorter postoperative observation period with a median follow-up of 38 months (vs 114 months in ours). This may indicate that dysplastic grade of IPMN becomes more relevant for recurrence in the long-term following resection. Furthermore, our current results appear to be consistent with previous studies that have shown an increased risk of subsequent development of invasive cancer in patients with an IPMN with high-grade dysplasia as compared to patients with low/intermediate lesions.10,27 A recent study from the Mayo clinic confirmed this finding in MD-IPMNs.29 Therefore, it is reasonable to label patients with high-grade IPMN as group with a particularly high risk of postoperative recurrence. Of note, our analysis did not confirm other previously reported risk factors such as MD involvement.17 In addition, since type of surgery (Whipple vs distal) was not indicative of recurrence, location of the lesion did not seem to have played a major role for recurrence in our cohort.9,21,22
The results of our statistical analyses did not show a significant impact of margin status (defined as positive or negative for any mucinous epithelium) on recurrence, neither clinically significant (P = 0.09) nor radiographic (P = 0.28). However, although not statistically significant, margin-positive patients showed considerably higher recurrence rates, with clinically significant recurrence in 17 of 90 (19%) margin-positive cases, compared to 2 of 36 (6%) margin-negative cases. The lack of statistical significance may be due to the low number of events in our cohort, which limits the statistical power. Of note, although presence of any neoplastic epithelium at the margin was not significantly associated with recurrence, multiple features of the neoplastic epithelium at the margin were associated with recurrence. Specifically, both size and grade of dysplasia at the margin were associated with an increased risk of clinically significant recurrence (P = 0.04 and P = 0.04, respectively), with a size threshold of 0.5 cm identifying a subset of margin-positive patients with significantly increased risk of recurrence. These additional features may aid decision-making on postoperative surveillance interval or even consideration of completion pancreatectomy, if the margin is determined to be positive.
There is currently controversy in literature about the impact of margin status on IPMN recurrence. Although some studies previously reported increased risk of recurrence if margin is positive,9,18,19,30 others did not find such an association.7,8,16,20,22 This difference may be explained in part by various definitions of margin positivity among publications. Definition of margin positivity ranged from “any dysplasia”20,22 to “IPMN only,”16 whereas some authors included all PanINs20,22 and others excluded PanIN-1A and 1B30 from margin positivity. The large variation in the rate of margin positivity between studies (4.9%−71.4%, see Table 7) may not only be a result of these differing definitions, but may also be influenced by the source of information used to assess margin status. Although presence of high-grade dysplasia and invasive carcinoma at the margin are consistently reported in clinical pathology reports due to their impact on the surgical procedure, reporting of low-grade dysplasia is more variable because it does not currently impact clinical decision-making. Separate review of FFPE margin tissue blocks by trained experts, as in our study, is likely to be more accurate and consistent than assessment of margin status based on review of pathological reports only. Such reports are driven by clinical considerations and thus may not contain the required data to answer specific research questions. For example, in our study, mucinous epithelium was reported at the margin of 50 cases in the clinical pathology report, whereas dedicated pathological review revealed it in 90 cases, underscoring the potential inaccuracies in relying on the clinical pathology report for variables that do not drive clinical care. This highlights the importance of precise terminology and consistent definitions of “positive” margins to compare data between studies. Our study identified a trend toward increased recurrence based on the presence of mucinous epithelium at the margin. In addition, the size of the largest dysplastic focus at the margin (P = 0.04) and high-grade dysplasia at the margin (P = 0.04) were statistically significant risk factors for csRFS. Larger cohorts could confirm this finding, but only if a consistent definition of margin positivity (including size of lesions) is adopted across studies. Our data suggest that dysplastic foci >0.5 cm at the margin separate a group with increased risk of recurrence, which is in keeping with the size threshold used to distinguish PanIN from IPMN.31 Taken together, our results indicate that criteria beyond dichotomous assessment of the margin may be more helpful to determine recurrence risk.
For more insights into molecular relationship between the margin and the IPMN, we performed targeted next-generation sequencing in a subset of 32 margin positive patients. Comparison of driver gene mutations at the margin with those found in the synchronous IPMN lesion suggests that in a sizable number of cases (at least 9/32), the dysplastic focus at the margin is a second precancerous neoplasm, unrelated to the original IPMN. This interpretation of independent neoplasms in the absence of shared somatic mutations is supported by several previous studies.10,32,33 As most of the shared mutations in the remaining 23 cases are hotspot mutations, they could be shared in unrelated lesions by chance, and it is likely that some of these “potentially related” lesions are in fact independent. In a previous study, whole exome sequencing of IPMNs and co-occurring cancers sharing only KRAS mutations revealed that the majority shared no additional mutations, suggesting that chance sharing of KRAS hotspot mutations in unrelated pancreatic ductal neoplasms occurs commonly.33 In addition, the much lower prevalence of GNAS mutations in the sequenced margin samples (8/32) compared to IPMNs (22/32) suggests that in many cases the neoplastic epithelium at the margin represents PanIN rather than IPMN. Moreover, a high proportion (>80%) of the analyzed samples histologically had PanIN lesions in addition to the IPMN. These results, along with the clinical data discussed above, highlight the concept of IPMN and precancerous pancreatic neoplasia more generally as a multifocal disease affecting the entire organ. Previous work from our group indicated that IPMN recurrences are often genetically independent neoplasms.10 Thus, IPMN resection should be viewed as a risk-reducing rather than curative procedure, as recurrence often occurs remotely from the margin and could be a solid mass/cancer with no preceding cystic lesion. This concept is supported by our imaging data, in which radiographic progression was more likely to be found distant from the previous resection margin. Since TP eradicates the risk of local recurrence including the risk of pancreatic cancer and can nowadays be performed with similar outcomes as partial pancreatectomy, it has been proposed for surgical first-line management of IPMN lesions, particularly those with features suggestive of a high risk of postoperative recurrence.34–36
An additional finding of our sequencing analyses is genetic heterogeneity, which has been described in the context of IPMNs and pancreatic cancer development before.33,37,38 By extracting genomic DNA from 3 different blocks of each IPMN, we were able to obtain mutations from different areas of the lesion. Of the paired IPMN-margin lesions that were “potentially related,” only 2 cases had perfect concordance of these patterns and the majority presented with only partially shared mutation patterns. Although we cannot exclude that we may have missed some mutations by our tissue collection method, these findings likely indicate significant heterogeneity with respect to driver gene mutations in genetically related lesions, confirming the findings of previous studies.33,37,38
Although our study has several strengths, some limitations should be noted. First, imaging studies may lack accuracy of detecting recurrence, especially given that the entire organ may show alterations on imaging before surgery and assessment is additionally hampered by postoperative changes. To remove subjectivity and variability, a single radiologist reviewed all available imaging studies. Of note, the statistically significant features in our study referred to clinically significant recurrence and were not dependent on radiology. Second, our cohort comprises retrospective data from a single tertiary referral center, which could limit the generalizability of our results. However, pancreatic surgery is recommended in specialized centers, and many of our results are concordant with other studies, indicating general applicability. Third, we reported family history of PDAC in <20% of our cohort, and our retrospective study was limited to the data documented in the medical record. However, family history is routinely queried and recorded in the medical records at our institution, and detailed information on the individual’s relationship to family members previously affected by PDAC is typically included. Still, studies in larger cohorts with dedicated family history data along with analyses of germline mutations may be helpful to confirm the risk associated with specific patterns of family history and germline mutations.
Taken together, our data underscore the need for long-term surveillance for all patients after IPMN resection, as the median time to onset of recurrence in our cohort was 4.5 years, with about one-third of patients recurring after 5 years. In addition, we identified specific clinical and pathological features significantly associated with increased risk of recurrence, including family history of pancreatic cancer, high-grade dysplasia in the IPMN or at the margin, and size of neoplastic focus at the margin. Finally, we demonstrate using genetic data that neoplastic epithelium at the margin is frequently unrelated to the resected IPMN, highlighting prominent multifocality in premalignant pancreatic neoplasia. These results provide important insights into IPMN progression, with direct implications for the postoperative surveillance of IPMN patients.
Supplementary Material
TABLE 4.
Results of Univariate Analysis of csRFS and rPFS for Margin Variables
| csRFS | rPFS | ||||||
|---|---|---|---|---|---|---|---|
| Variables | n (%) or mean (SD) | Events (n) | HR (95% Cl) | P | Events (n) | HR (95% Cl) | P |
| Margin variables within positive margin (n = 90) | |||||||
| No. of dysplastic foci, mean (SD) | 2.47 (2.15) | 12 | 1.07 (0.84–1.35) | 0.58 | 15 | 1.16 (0.99–1.38) | 0.07 |
| Missing | 15 (16.67) | ||||||
| Size largest dysplasia, cm, mean (SD) | 0.31 (0.29) | 13 | 3.69 (1.09–12.46) | 0.04 | 16 | 2.41 (0.53–10.84) | 0.25 |
| Missing | 13 (14.44) | ||||||
| Main Duct involvement | |||||||
| No | 48 (53.33) | 7 | 1 | 8 | 1 | ||
| Yes | 18 (20) | 4 | 1.47 (0.43–5.06) | 0.54 | 4 | 1.61 (0.48–5.37) | 0.44 |
| Missing | 24 (26.67) | ||||||
| Highest grade of dysplasia | |||||||
| Low/intermediate | 85 (94.44) | 13 | 1 | 20 | 1 | ||
| High | 4 (4.44) | 3 | 3.68 (1.04–12.97) | 0.04 | 2 | 3.65 (0.83–15.98) | 0.09 |
| Missing | 1 (1.11) | ||||||
Patients with negative margin were not considered in the univariate analysis. Missing data were not included in the analysis.
SD indicates standard deviation.
ACKNOWLEDGMENTS
The authors thank Dr. Ralph Hruban for helpful discussions. The authors acknowledge the following sources of support: NIH/NCI P50 CA62924; NIH/NIDDK K08 DK107781; Sol Goldman Pancreatic Cancer Research Center; Buffone Family Gastrointestinal Cancer Research Fund; Carol S. and Robert M. Long Pancreatic Cancer Research Fund; Kaya Tuncer Career Development Award in Gastrointestinal Cancer Prevention; AGA-Bemard Lee Schwartz Foundation Research Scholar Award in Pancreatic Cancer; Sidney Kimmel Foundation for Cancer Research Kimmel Scholar Award; AACR-Incyte Corporation Career Development Award for Pancreatic Cancer Research; American Cancer Society Research Scholar Grant; Emerson Collective Cancer Research Fund; Rolfe Pancreatic Cancer Foundation; Joseph C Monastra Foundation; The Gerald O Mann Charitable Foundation (Harriet and Allan Wulfstat, Trustees); Susan Wojcicki and Denis Troper; Dutch Digestive Disease Foundation (MLDS CDG 14-02).
L.D.W. receives research funding from Applied Materials.
Footnotes
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Vege SS, Ziring B, Jain R, et al. Clinical Guidelines Committee, American Gastroenterology Association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neo-plastic pancreatic cysts. Gastroenterology. 2015;148:819–822. quize 812–813. [DOI] [PubMed] [Google Scholar]
- 2.Elta GH, Enestvedt BK, Sauer BG, et al. ACG clinical guideline: diagnosis and management of pancreatic cysts. Am J Gastroenterol. 2018;113:464–479. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka M, Femandez-Del Castillo C, Kamisawa T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738–753. [DOI] [PubMed] [Google Scholar]
- 4.Basturk O, Hong SM, Wood LD, et al. A revised classification system and recommendations from the baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol. 2015;39:1730–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He J, Cameron JL, Ahuja N, et al. Is it necessary to follow patients after resection of a benign pancreatic intraductal papillary mucinous neoplasm? J Am Coll Surg. 2013;216:657–665. discussion 665–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchegiani G, Mino-Kenudson M, Ferrone CR, et al. Patterns of recurrence after resection of IPMN: who, when, and how? Arm Surg. 2015;262:1108–1114. [DOI] [PubMed] [Google Scholar]
- 7.Miller JR, Meyer JE, Waters JA, et al. Outcome of the pancreatic remnant following segmental pancreatectomy for non-invasive intraductal papillary mucinous neoplasm. HPB (Oxford). 2011;13:759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamura K, Ohtsuka T, Ideno N, et al. Treatment strategy for main duct intraductal papillary mucinous neoplasms of the pancreas based on the assessment of recurrence in the remnant pancreas after resection: a retrospective review. Arm Surg. 2014;259:360–368. [DOI] [PubMed] [Google Scholar]
- 9.Frankel TL, LaFemina J, Bamboat ZM, et al. Dysplasia at the surgical margin is associated with recurrence after resection of non-invasive intraductal papillary mucinous neoplasms. HPB (Oxford). 2013;15:814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pea A, Yu J, Rezaee N, et al. Targeted DNA sequencing reveals patterns of local progression in the pancreatic remnant following resection of intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg. 2017;266:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg. 2015;220:530–536. [DOI] [PubMed] [Google Scholar]
- 12.Bollen TL, Wessels FJ. Radiological workup of cystic neoplasms of the pancreas. Vise Med. 2018;34:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Study Group on Cystic Tumours of the P. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White R, D’Angelica M, Katabi N, et al. Fate of the remnant pancreas after resection of noninvasive intraductal papillary mucinous neoplasm. J Am Coll Surg. 2007;204:987–993. discussion 993–985. [DOI] [PubMed] [Google Scholar]
- 15.Park J, Lee KT, Jang TH, et al. Risk factors associated with the postoperative recurrence of intraductal papillary mucinous neoplasms of the pancreas. Pancreas. 2011;40:46–51. [DOI] [PubMed] [Google Scholar]
- 16.Moriya T, Traverso W. Fate of the pancreatic remnant after resection for an intraductal papillary mucinous neoplasm: a longitudinal level II cohort study. Arch Surg. 2012;147:528–534. [DOI] [PubMed] [Google Scholar]
- 17.Kang MJ, Jang JY, Lee KB, et al. Long-term prospective cohort study of patients undergoing pancreatectomy for intraductal papillary mucinous neoplasm of the pancreas: implications for postoperative surveillance. Ann Surg. 2014;260:356–363. [DOI] [PubMed] [Google Scholar]
- 18.Marchegiani G, Mino-Kenudson M, Sahora K, et al. IPMN involving the main pancreatic duct: biology, epidemiology, and long-term outcomes following resection. Ann Surg. 2015;261:976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi J, Kaneoka Y, Maeda A, et al. Positive surgical margins in surgically treated unifocal and multifocal IPMN. Int J Surg. 2016;28:51–55. [DOI] [PubMed] [Google Scholar]
- 20.Dhar VK, Merchant NB, Patel SH, et al. Does surgical margin impact recurrence in noninvasive intraductal papillary mucinous neoplasms?: A multi-institutional study. Ann Surg. 2018;268:469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirono S, Shimizu Y, Ohtsuka T, et al. Recurrence patterns after surgical resection of intraductal papillary mucinous neoplasm (IPMN) of the pancreas; a multicenter, retrospective study of 1074 IPMN patients by the Japan Pancreas Society. J Gastroenterol. 2020;55:86–99. [DOI] [PubMed] [Google Scholar]
- 22.Al Efishat M, Attiyeh MA, Eaton AA, et al. Progression patterns in the remnant pancreas after resection of non-invasive or micro-invasive intraductal papillarymucinous neoplasms (IPMN). Ann Surg Oncol. 2018;25:1752–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canto MI, Almario JA, Schulick RD, et al. Risk of neoplastic progression in individuals at high risk for pancreatic cancer undergoing long-term surveillance. Gastroenterology. 2018;155:740–751. e742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen GM. Familial pancreatic cancer. Semin Oncol. 2016;43:548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein AP, Brune KA, Petersen GM, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634–2638. [DOI] [PubMed] [Google Scholar]
- 26.Skaro M, Nanda N, Gauthier C, et al. Prevalence of germline mutations associated with cancer risk in patients with intraductal papillary mucinous neoplasms. Gastroenterology. 2019;156:1905–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rezaee N, Barbon C, Zaki A, et al. Intraductal papillary mucinous neoplasm (IPMN) with high-grade dysplasia is a risk factor for the subsequent development of pancreatic ductal adenocarcinoma. HPB (Oxford). 2016;18:236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blackham AU, Doepker MP, Centeno BA, et al. Patterns of recurrence and long-term outcomes in patients who underwent pancreatectomy for intraductal papillary mucinous neoplasms with high grade dysplasia: implications for surveillance and future management guidelines. HPB (Oxford). 2017;19:603–610. [DOI] [PubMed] [Google Scholar]
- 29.Majumder S, Philip NA, Singh Nagpal SJ, et al. High-grade dysplasia in resected main-duct intraductal papillary mucinous neoplasm (MD-IPMN) is associated with an increased risk of subsequent pancreatic cancer. Am J Gastroenterol. 2019;114:524–529. [DOI] [PubMed] [Google Scholar]
- 30.Leng KM, Wang ZD, Zhao JB, et al. Impact of pancreatic margin status and lymph node metastases on recurrence after resection for invasive and noninvasive intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Dig Surg. 2012;29:213–225. [DOI] [PubMed] [Google Scholar]
- 31.Basturk O, Hong SM, Wood LD, et al. A revised classification system and recommendations from the Baltimore Consensus Meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol. 2015;39:1730–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Omori Y, Ono Y, Tanino M, et al. Pathways of progression from intraductal papillary mucinous neoplasm to pancreatic ductal adenocarcinoma based on molecular features. Gastroenterology. 2019;156:647–661. e642. [DOI] [PubMed] [Google Scholar]
- 33.Felsenstein M, Noe M, Masica DL, et al. IPMNs with co-occurring invasive cancers: neighbours but not always relatives. Gut. 2018;67:1652–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffin JF, Poruk KE, Wolfgang CL. Is it time to expand the role of total pancreatectomy for IPMN? Dig Surg. 2016;33:335–342. [DOI] [PubMed] [Google Scholar]
- 35.Poiraud C, El Amrani M, Barbier L, et al. Total pancreatectomy for presumed intraductal papillary mucinous neoplasms: a multicentric study of the French Surgical Association (AFC). Ann Surg. 2018;268:823–830. [DOI] [PubMed] [Google Scholar]
- 36.Crippa S, Tamburrino D, Partelli S, et al. Total pancreatectomy: indications, different timing, and perioperative and long-term outcomes. Surgery. 2011;149:79–86. [DOI] [PubMed] [Google Scholar]
- 37.Fischer CG, Beleva Guthrie V, Braxton AM, et al. intraductal papillary mucinous neoplasms arise from multiple independent clones, each with distinct mutations. Gastroenterology. 2019;157:1123–1137. e1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuboki Y, Fischer CG, Beleva Guthrie V, et al. Single-cell sequencing defines genetic heterogeneity in pancreatic cancer precursor lesions. J Pathol. 2019;247:347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


