Abstract
Oral cancer (OC) is a debilitating disease with a high mortality rate when diagnosed in advanced stage. Conversely, early-stage OC has a high survival rate, supporting a need for early detection programmes. A previous systematic review of clinical trials evaluating efficacy of screening for OC was inconclusive. This systematic review aimed to determine the impact of screening for oral lesions on reducing mortality and incidence of OC by looking at a broader spectrum of evidence.
The search for randomized controlled trials and observational studies with a control group was conducted in PubMed, OVID, Cochrane, CINAHL and grey literature sources. Risk of bias for included studies was assessed with the tools developed by the Cochrane collaboration.
Six out of two identified randomized trials and five observational studies had moderate to high risk of bias. Nevertheless, the predictions on impact of OC screening on incidence and mortality were similar across the majority of the studies. The meta-analysis concluded on a 26% decrease in OC mortality, and an 19% decrease in advanced OC cases as a result of OC screening in high-risk population. Three out of four studies did not identify an impact of screening on OC incidence. No positive impact of OC screening on incidence or mortality among general population was identified in the only available randomized trial. Consistency in the outcomes and the limitations of the few available studies suggest a need for real-life setting research to evaluate the overall effectiveness of screening for OC in high-risk population.
Abbreviations: CG, Control group; CI, Confidence interval; CINAHL, Cumulative Index to Nursing and Allied Health Literature; COE, Conventional oral examination; IG, Intervention group; ISRTCN, International Standard Randomised Controlled Trial Number; MSE, Mucosal self-examination; OC, Oral cancer; OR, Odds ratio; OSF, Oral submucous fibrosis; OPMD, Oral potentially malignant disorders; PYO, Person years of observation; RCT, Randomized clinical trial; ROB, Risk of bias; ROBINS-I, Risk of bias in non-randomized interventional studies; RR, Risk ratio/Relative risk; TB, Toluidine blue
Keywords: Oral cancer, Premalignant, Screening, Efficacy, Effectiveness, Systematic review
1. Introduction
Definition of oral cancer (OC) varies in the literature, but for the purpose of early detection, OC is usually considered as a malignant neoplasia which arises on the lip or oral cavity. OC accounted for 2 % of all cancers, as well as 1.9 % of all cancer related deaths resulting in almost 355 000 new cases diagnosed and over 177 000 associated deaths (Miranda-Filho and Bray, 2020). While OC presents a five-year overall survival around 50 %, early OC diagnosis may increase it to 85 %. This supports the rationale of early detection contributing to better outcomes (National Cancer Institute. Browse the SEER Cancer Statistics Review (CSR) (2014)), with survival rates directly linked to the cancer stage at diagnosis (Strome et al., 2018).
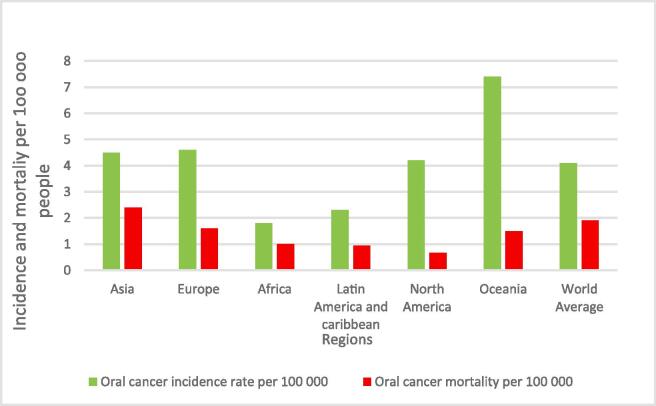
OC is the 16th most common cancer with OC incidence varying widely around the world. Asia, Europe, and Oceania have the highest incidence rate in the world, while Asia has the highest OC mortality (Fig. 1). The incidence of OC in populations depends on the prevalence of the attributable risk factors such as tobacco chewing and smoking, betel quid usage, and alcohol consumption (Petti, 2009, Kumar et al., 2016). For instance, a greater incidence is noted in males, who culturally exhibit higher exposure to the risk factors (Rao et al., 2013, Zain, 2001), with a world age standardized incidence rate of 6 versus 2.3 per 100 000 people respectively. Fig. 1.
Fig. 1.
The incidence and mortality of OC per 100 000 people in 2020 across various regions (International Agency for Research on Cancer, 2020, WHO, 2020).
Screening programmes for other cancers sites demonstrated clinical benefits through detecting pre-malignant and early stage cancer lesions (Mandrik et al., 2019, Peirson et al., 2013). Motivated by the disease burden and conventional approach of community screening, a potential of different OC screening approaches to detect oral potentially malignant disorders (OPMDs) and invasive cancer has been investigated in randomised trials and the following systematic review (Brocklehurst et al., 2013). From the investigated approaches, the current standard of screening is through conventional oral examination (COE) under direct light. This is usually performed by a general dentist or doctor, but other healthcare workers such as nurses or community health workers have been known to assist in screening examinations with high efficiency noted after appropriate training (Birur et al., 2019). The healthcare worker redirects the suspected positive case to the diagnostic pathway including biopsy and histopathological confirmation (Higgins, 2021).
Considering potential benefits of OC screening, a few countries with high incidence of OC implemented national or pilot OC screening programmes targeting high-risk population, for instance Cuba, Taiwan (China), Kerala (India) and Sri Lanka. Meanwhile, commissioned by the Cochrane collaboration a systematic review on effectiveness of OC screening identified and included only one randomised controlled trial (RCT). The authors’ conclusions were that screening is ineffective in the general population but may provide some benefit in high-risk population groups, though the evidence to support this is limited. This review will address the existing knowledge gap by looking at a wider range of evidence, including both experimental and observational studies, aiming to evaluate an impact of OC screening on OC incidence, advanced stage OC diagnosis, and mortality.
2. Methods
The protocol of this study, based on the recommendations of the Cochrane Handbook for Systematic reviews of interventions (Higgins, 2021), was registered with the International prospective register of systematic reviews (PROSPERO), registration number: CRD42021246383.
2.1. Search and eligibility
The search strategy was exhaustive, not restricted to a specific language or year of publication. Databases included Ovid, PubMed, CINAHL, ISRCTN and the Cochrane database of systematic reviews, from inception to August 12, 2021. We searched for RCT’s and observational studies that investigated the association between OC screening and OC mortality, as well as downstaging (Appendix 1). We further hand-searched the citations of the retrieved eligible papers to identify additional publications that might have been missed during the initial search. We also searched clinicaltrials.gov for non-published studies.
2.2. Inclusion and exclusion criteria
-
a)
Type of studies.
This review considered quasi-experimental, randomized controlled (cluster and individually), case-controlled, cohort and cross-sectional studies with a control group.
-
b)
Type of Participants.
Studies were considered eligible if included an adult population group (defined as anyone over 15 years of age) of any gender who attend OC screening programs. While the adult population is typically defined as over 18 years old, we extended the lower limit to 15-years old considering that the definition of adult population in the National Oral Cancer Screening programme in Cuba is 15 years and older (Birur et al., 2019). Symptomatic population, such as individuals with confirmed OC or a history of OC, were excluded. Studies on either general-risk or high-risk populations were included. High-risk population was broadly described as regular tobacco (any type or form – smoked or smokeless) and/or alcohol consumption.
-
c)
Type of Intervention.
Studies investigating any screening method for OC or OPMD were eligible. OC screening comparing types of examination including, but limited to, the conventional oral visual examination, chemical staining, auto-fluorescence, biomarker analysis and chemiluminescence versus no screening or placebo, were also eligible. Self-examinations, conducted by patients under direction and supervision by healthcare workers, were excluded, considering the suggested low test accuracy (Ghani et al., 2019, Lee et al., 2019).
-
d)
Comparator.
All studies considered eligible had to have a control group, including but not limiting to no screening, ‘usual care’ (e.g., opportunistic screening) or modified interventions. Studies which lacked a control group were excluded from this review.
-
e)
Outcomes.
In order to be considered eligible, at least one of the following primary outcomes was required:
-
•
OC mortality
-
•
OC incidence
-
•
Clinical stage at diagnosis
Studies identified in accordance with the above outcomes may also be subjected to extraction in terms of the following secondary outcomes:
-
•
Sensitivity and specificity of screening programs or diagnostic examinations
-
•
Overdiagnosis
-
•
Other clinical benefits, such as incidental findings: detection of dental issues, other cancers, or systematic health problems.
As per conventional definition, OPMD has been defined as "a group of lesions and conditions characterized by a variably increased risk of developing cancers of the lip and the oral cavity” (Warnakulasuriya et al., 2021) such as leukoplakia, erythroplakia, lichen planus, and oral submucous fibrosis.
3. Risk of bias in included studies
Cochrane’s Risk of Bias (ROB-2) in RCTs tool and Cochrane’s Risk of Bias in Non-randomised Studies – of Interventions (ROBINS-I) was used to assess the risk of bias in studies independently by two reviewers resolving any disagreements through discussion.
3.1. Data analysis
Review Manager software was applied to synthesise the outcomes. Dichotomous outcomes, such as OC mortality, OC detection, advanced stage OC detection and OPMD detection were used to estimate the effect of screening as expressed by risk ratios with a confidence interval (95 %).
We used the I2 statistic to assess a level of heterogeneity and so to define a possibility for quantitative synthesis. A meta-analysis, with a weighted random effects model was performed to report on the effect of OC screening on mortality and advanced OC cases. Only studies reporting similar outcomes were included in the meta-analysis.
4. Results
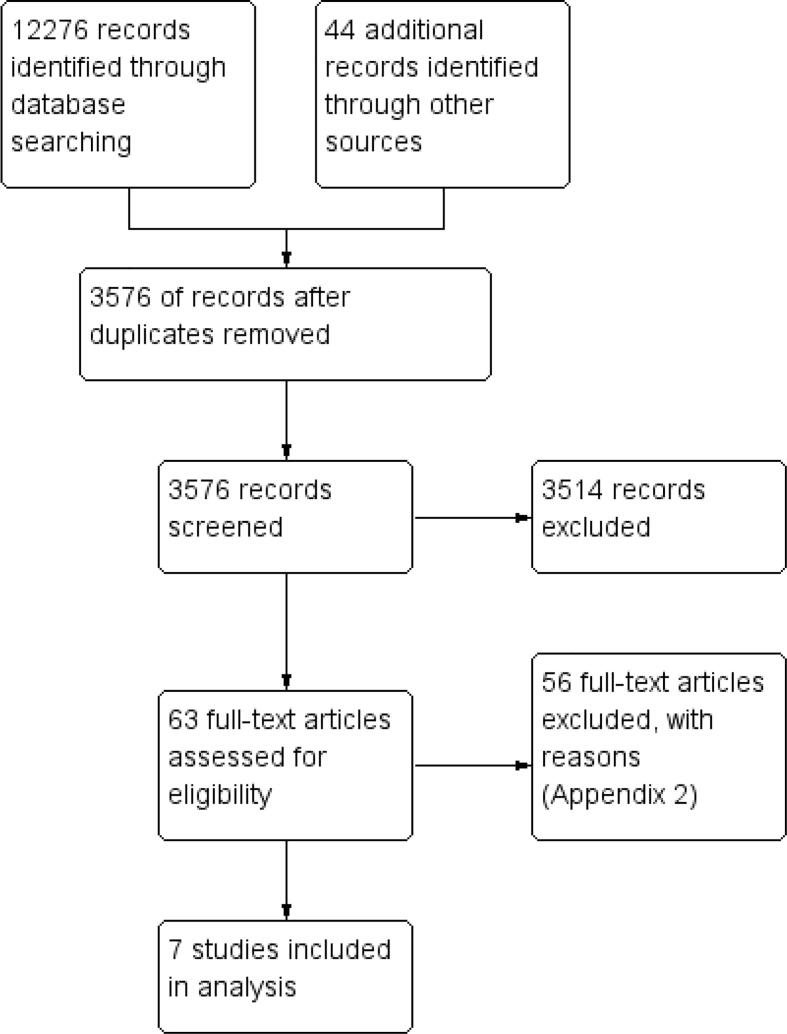
Of the initial 12,276 records identified, seven studies published between 1995 and 2021 were selected for inclusion (Fig. 2). The characteristics of the included studies are reported in Table 1.
Fig. 2.
PRISMA chart depicting the selection of studies.
Table 1.
Characteristics of included studies.
| Author/year/Country | Garrote et al. 1995 Cuba |
Sankaranarayanan et al. 2002 Cuba |
Pei-Shan Ho et al., 2019 Taiwan |
Chuang et al. 2017 Taiwan |
Morikawa et al. 2020 Japan |
Sankaranarayanan et al. 2013 India |
Su et al. 2010 Taiwan |
|
|---|---|---|---|---|---|---|---|---|
| Study design | Repeated cross-sectional | Case control study | Retrospective cohort study | Population based cohort study | Cohort study | Cluster RCT Clusters randomized at municipal level |
RCT | |
| Intervention | National screening program, conducted by dentists | Determination of screening history in advanced OC cases, part of the national screening program | Analysis of Taiwan Oral mucosal screening program | Invitational screening by medical healthcare workers | Countermeasure screening (Invitational) | House visit screening by trained healthcare workers | TB staining for detection of OC and OPMD | |
| Control | Routine care, data taken from national cancer registry | Three (3) healthy participants per each advanced OC case were recruited | Individuals without screening history who were reported to have OC | Data linked to National cancer registry used to identify cases in the control group, who did not attend screening | Opportunistic screening | Routine care | Placebo dye staining for detection of OC and OPMD | |
| Endpoints measured | OC incidence OC mortality |
OC late-stage incidence | OPMD incidence OC incidence OC late-stage incidence OC mortality |
OPMD incidence OC incidence OC late-stage incidence OC mortality |
OPMD incidence OC incidence |
OC incidence OC late-stage incidence OC mortality |
OMPD incidence OC incidence |
|
| Sample size | IG | 12 990 677 | 200 | 11 725 | 2 933 402 | 19 721 | 96 517 | 4 080 |
| CG | 84 228 675 | 600 | 6 900 | 1 900 094 | 29 912 | 93 355 | 3 895 | |
| Inclusion criteria | >=15 years | IG- late-stage OC CG- healthy individuals residing withing 200 m of the matched OC case |
>=30 years with risk factors (tobacco use) | >=18 years with risk factors (tobacco use) | >=40 years | >=35 years | >=15 years with risk factors (tobacco use) | |
| Compliance with intervention | Males- 11.9 % − 20.1 % Females- 19.9 % − 26.8 % | Not reported | Not reported | 55 % | Not reported | IG- 92 % CG for 1 round of screening- 46 % |
77.60 % | |
| Intervention period reported | IG | 1984–1990 | 1 January 1994 – 17 July 1997 | 2008–2015 | 2004–2012 | 1992–2018 | 1996–2008 | January 2000- December 2000 |
| CG | 1984–1990 | 1 January 1994 – 17 July1997 | 2008–2015 | 2004–2012 | 2006–2018 | Routine care-1996–2005 Screened from 2006 to 2008 |
January 2000- December 2000 | |
| Number of screening rounds | IG | Not reported | 0–2 | 1 -more than 3 | 3 | 1–3 times per year | 1–4 | 1 |
| CG | Not reported | 0–2 | 0 | 0 | Annually or as required | 0–1 | 1 | |
| Follow up for screen positive cases- definition | Referral to a specialist surgeon or oncologist | Referral to a specialist surgeon or oncologist | Referral to a specialist surgeon or oncologist | Referral to a specialist surgeon or oncologist | Referral to a specialist surgeon or oncologist | Referral to a specialist surgeon or oncologist | Referral to a specialist surgeon or oncologist | |
| Follow up compliance rate | 25–34 % | Not reported | Not reported | 91.10 % | Not reported | 59 % | IG- 82.3 % CG- 91 % | |
4.1. Quality of evidence
Five of the seven studies included in this review are judged to be at a high or serious risk of bias (16,20–23) (Table 2). One study was judged to be at a moderate risk of bias (Sankaranarayanan et al., 2013), and only one study was deemed to be at a low risk of bias (Su et al., 2010). Five of the included studies are observational studies, with a high risk of selection bias and confounding (Frenández Garrote et al., 1995, Chuang et al., 2017, Morikawa et al., 2021, Ho et al., 2019, Sankaranarayanan et al., 2002)(Appendix 3).
Table 2.
Risk of bias for included studies.
 |
The overall quality of the evidence found is poor and limited. The lack of high-quality studies precluded us from only analysing studies deemed to be at a low or moderate risk of bias. However, the results presented above are consistent, and show a positive effect of OC screening on OC mortality and OC downstaging at diagnosis.
4.2. Effect on OC mortality
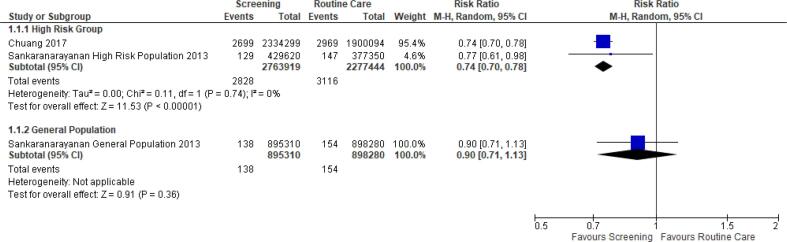
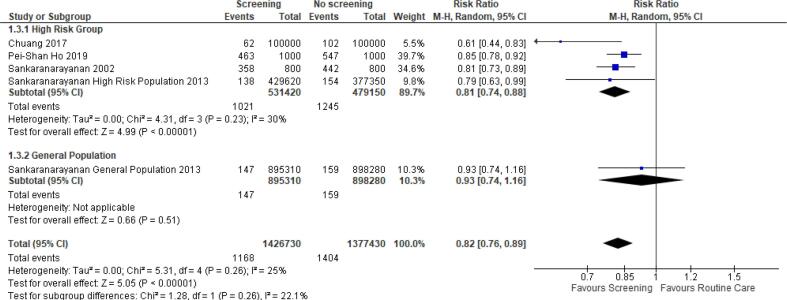
Three studies of different designs reported the effect of OC screening on OC mortality (Chuang et al., 2017, Ho et al., 2019, Sankaranarayanan et al., 2013). A meta-analysis of two studies reporting the number of deaths and the population size in the intervention and the comparator groups was performed based on the different subgroups as well as an overall statistic (Chuang et al., 2017, Sankaranarayanan et al., 2013). A mortality significant decrease of 26 % with minimal heterogeneity (I2 = 0 %) was noted when analysing the high-risk group, but no difference was observed among the general population (Fig. 3). The limited number of studies, and methodological differences included in this meta-analysis should be noted. Pei-Shan Ho et al. (Ho et al., 2019), also reported the hazard ratio for mortality associated with OC screening of 0.92 in screened vs non-screened population (95 % CI, 0.84–1.00) (Ho et al., 2019).
Fig. 3.
Relative risk of oral cancer mortality in screened versus non-screened groups (20,24).
4.3. Effect on OC incidence
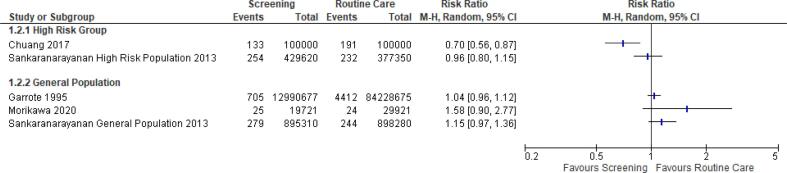
For OC detection in the general population, three studies (Frenández Garrote et al., 1995, Morikawa et al., 2021, Sankaranarayanan et al., 2013) were included, as well as two studies in the high-risk group (Chuang et al., 2017, Sankaranarayanan et al., 2013). None of the included studies reported a statistically significant decrease in OC incidence in the general population, whereas in the high-risk population one observational study (Chuang et al., 2017) found a statistically significant decrease in OC incidence of 30 % which decreased to 17 % after adjustment for self-selection bias (Fig. 4). No impact on incidence for OC screening was observed in randomised controlled trials (Sankaranarayanan et al., 2013). A meta-analysis could not be conducted for the effect of OC screening on OC incidence due to the high level of heterogeneity (I2= >70 %).
Fig. 4.
OC incidence in screened and non-screened groups (16,20,21,24).
4.4. Effect on OC stage at diagnosis
Three studies reported increased detection of early-stage OC through the screening programmes. Pei-Shan Ho et al. (Ho et al., 2019) reported an increase in early OC detection for confirmed OPMD’s (99 %), confirmed non OPMD’s (85 %) not referred (49 %) or did not comply with referral (25 %), which may lead to earlier treatment, resulting in lower advanced OC cases (Ho et al., 2019). Sankaranarayanan et al. (Sankaranarayanan et al., 2013) reported a higher percentage of early stage OC in the screened vs non-screened group (39.4 % vs 27 %) (Sankaranarayanan et al., 2013). Garrote et al. (1995) reported an increased proportion of early OC detection, 50 % in the assigned control year of 1983 compared to 64 % in 1989 (Frenández Garrote et al., 1995).
Four studies reported the reduction of advanced stage OC detected at diagnosis as outcome (defined as stage 3 and 4 by TNM classification- 8th edition). For illustrative purposes, the reported relative risk of late stage diagnoses is presented on Fig. 5 (Chuang et al., 2017, Ho et al., 2019, Sankaranarayanan et al., 2013).
Fig. 5 shows four studies reporting a statistically significant reduction in incidence of advanced stage OC in high-risk populations. Chuang et al. (Chuang et al., 2017); recorded a 21 % reduction in advanced stage presentation amongst screening participants after adjustment for self-selection bias 0.79 (95 % CI, 0.76–0.82) (Chuang et al., 2017). Sankaranarayanan et al. (Sankaranarayanan et al., 2013) recorded a 21 % decrease in advanced stage OC presentation amongst screening participants in India, RR = 0.79 (95 % CI, 0.68–0.91). Sankaranarayanan et al. (Sankaranarayanan et al., 2002) reported a 19 % decrease in advanced OC in screened individuals in Cuba. They also reported an OR of advanced OC from 0.67 (95 % CI, 0.46–0.95) after one screening to 0.41 (95 % CI, 0.24–0.68) in individuals who were screened two or more times, alluding to greater protection offered by subsequent screenings. The calculated RR based on reported distribution of advanced stage OC in the Pei-Shan Ho et al. (Ho et al., 2019) study, showed a statistically significant decrease in advanced stage OC presentation of 15 %, RR = 0.85 (95 % CI, 0.78–0.92) (Ho et al., 2019).
Fig. 5.
Detection of advanced stage OC in screened and non-screened populations (20,22,24).
A meta-analysis showed a reduction in advanced stage OC of 19 % (95 % CI, 0.74–0.88) in the high risk screened groups with a minimal heterogeneity of 30 %.
4.5. Effect on OPMD incidence
Outcomes on OPMD were reported by 4 studies (Su et al., 2010, Chuang et al., 2017, Morikawa et al., 2021, Ho et al., 2019). Morikawa et al. (2020) reported a statistically significant 14 % decreased risk of developing OPMD in the countermeasure group (Morikawa et al., 2021). Chuang et al. (Chuang et al., 2017), reported a statistically significant 33 % increase in OPMD detection for a subsequent screening (Chuang et al., 2017). Pei-Shan Ho et al. (Ho et al., 2019); reported a statistically significant hazard ratio of 0.72 (0.64, 0.81) for individuals with confirmed OPMD in the screening group. This equates to a decreased OC mortality of 28 % for individuals with confirmed OPMD detected during OC screening (Ho et al., 2019). Su et al. (Su et al., 2010); reported a OPMD and malignant lesion detection risk ratio of 1.05 (95 % CI, 0.74–1.41), however this result was not statistically significant (Su et al., 2010).
4.6. OC screening-related harms
None of the included studies explicitly reported any data or analysis relating to overdiagnosis. Overdiagnosis is commonly measured in studies with the long follow-up and may be interpreted as a higher incidence of OPMD or early-stage OC in the intervention versus control group. A statistically non-significant but higher (31.2 vs 27.2) OC incidence per 100 000 was noted by Sankaranarayanan et al. (Sankaranarayanan et al., 2013) in the screened vs non-screened group of general OC risk with 15 years of the follow-up and may be interpreted as a possibility for overdiagnosis related to OC screening. However, the authors mentioned mitigation of over-treatment, by conservative management of benign lesions and monitoring of OPMD (Sankaranarayanan et al., 2013).
5. Discussion
The objective of this systematic review was to determine the impact of OC screening on OC incidence, OC clinical stage at diagnosis, and OC mortality. Our meta-analysis demonstrated a risk reduction for OC mortality of 26% and for advanced OC cases of 19% among high-risk participants of the OC screening. Only one study (a RCT) assessed the impact of OC screening on advanced stage OC and mortality among general risk population and was not able to demonstrate a statistically significant impact of screening (Sankaranarayanan et al., 2013). To supplement these empirical findings, a recent analysis re-evaluated the data from Kerala trial using a Cox proportional hazards risk prediction model, assigning each person in the model the counterfactual hazard of OC mortality in the absence and presence of screening. The study concluded on 27% OC mortality reduction in the screening versus control arms (Cheung et al., 2021), providing proof of principle for risk-based OC screening.
Several studies reported on the effect of detection of OPMD through OC screening or a decrease in risk of developing OPMD when screened (Su et al., 2010, Chuang et al., 2017, Morikawa et al., 2021), though, considering that the natural history of OC is still not well explored, it is not clear how much OPMD detection will contribute to the final clinical endpoint (mortality decrement) or contribute to overdiagnosis. This issue may become especially important considering that three out of four included studies (two evaluating the same public screening programme in Taiwan) did not identify a statistically significant impact of OC screening on OC incidence. Overdiagnosis was not explicitly explored in the included studies and the risk and the magnitude of it needs to be addressed in further research.
The results from our systematic review strengthen the conclusions from the previous systematic review of (Brocklehurst et al., 2013) based only on one RCT (Kerala, India), that there is evidence of benefits of COE. In particular, the results from our review show that a screening program targeting individuals in a high-risk group decreases the OC mortality rate and incidence of late stage (stage 3 and 4) OC at diagnosis in the screened groups and improves detection of early-stage OC and OPMD, thus contributing to OC downstaging. While (Brocklehurst et al., 2013) concluded that there is insufficient evidence to recommend a population wide OC screening approach, our review, looking at the broader literature of different research designs, suggests the similarity in the risk reduction for clinical endpoints despite the methodological limitations and heterogeneity in designs and outcomes. Thus, we assume that implementation studies, i.e., well-designed pilot OC screening programmes in high-risk populations, will be necessary to support the existing evidence on effectiveness of OC screening. Our conclusion on usefulness of the available OC screening studies to inform policy questions albeit demonstrated weaknesses in their design, is aligned with the previous recent narrative reviews (D’Cruz and Vaish, 2021, Warnakulasuriya and Kerr, 2021). Despite the low quality of evidence and limited number of studies, we believe these results may inform public health policies, however, there is a significant need for further high-quality implementation research in this domain.
6. Limitations of the review
While the search strategy and databases used to conduct the search were comprehensive, it is possible that some studies may be missed considering that the abstracts were screened by one reviewer only. We did not have a language restriction, but no studies were found in languages other than English. While we were able to conduct a meta-analysis, the low number and lack of high-quality studies may reduce the impact of the findings in our review.
7. Research and information gaps
Pilot studies of prospective design with well-designed evaluation protocol should be conducted in countries with a high prevalence of tobacco and or alcohol consumption to provide local evidence for policy making in these nations. These studies should follow the structure of implementation research and supplement the assessments of screening benefits with evaluation of screening-related harms (overdiagnosis), and implementation outcomes, such as costs, acceptability and feasibility (Proctor et al., 2011). Due to the relatively low compliance rate and a rate of follow-up for the positive cases, reported in some of the current studies, incentives may be offered to improve compliance and uptake with these screening programs, as well as subsequent referrals. For instance, such motivational factors may include OC education, OC risk factor education, alcohol and tobacco use cessation therapy, and personalized or specific referral letters (Camilloni et al., 2013). More research is needed to conclude on efficacy of adjunctive technologies to COE.
8. Conclusion
OC screening via COE in high-risk populations results in a decrease in OC mortality (26%) and advanced OC cases at diagnosis (19%). There is no conclusive evidence on impact of OC screening on OC incidence as well as no evidence on impact of other OC screening methods on clinical outcomes. No studies were detected to assess a magnitude of overdiagnosis related to OC screening, ultimately motivating for more research to be done to address this issue.
Disclaimer: Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
All persons who have made substantial contributions to the work reported in the manuscript (e.g., technical help, writing and editing assistance, general support), but who do not meet the criteria for authorship, are named in the Acknowledgements and have given us their written permission to be named. If we have not included an Acknowledgements, then that indicates that we have not received substantial contributions from non-authors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2022.101987.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- Birur NPraveen, Gurushanth K., Patrick S., Sunny SumsumP, Raghavan ShubhasiniA, Gurudath S., Hegde U., Tiwari V., Jain V., Imran M., Rao P., Kuriakose MoniAbraham. Role of community health worker in a mobile health program for early detection of oral cancer. Indian J Cancer. 2019;56(2):107. doi: 10.4103/ijc.IJC_232_18. [DOI] [PubMed] [Google Scholar]
- Brocklehurst P., Kujan O., O’Malley L.A., Ogden G., Shepherd S., Glenny A.M. Screening programmes for the early detection and prevention of oral cancer. Cochrane Database Syst Rev. 2013;2013(11) doi: 10.1002/14651858.CD004150.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilloni L., Ferroni E., Cendales B.J., Pezzarossi A., Furnari G., Borgia P., Guasticchi G., Rossi P.G. Methods to increase participation in organised screening programs: A systematic review. BMC Public Health. 2013;13(1) doi: 10.1186/1471-2458-13-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung L.C., Ramadas K., Muwonge R., Katki H.A., Thomas G., Graubard B.I., Basu P., Sankaranarayanan R., Somanathan T., Chaturvedi A.K. Risk-Based Selection of Individuals for Oral Cancer Screening. J Clin Oncol. 2021;39(6):663–674. doi: 10.1200/JCO.20.02855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang S.-L., Su W.-Y., Chen S.-S., Yen A.-F., Wang C.-P., Fann J.-Y., Chiu S.-H., Lee Y.-C., Chiu H.-M., Chang D.-C., Jou Y.-Y., Wu C.-Y., Chen H.-H., Chen M.-K., Chiou S.-T. Population-based screening program for reducing oral cancer mortality in 2,334,299 Taiwanese cigarette smokers and/or betel quid chewers. Cancer. 2017;123(9):1597–1609. doi: 10.1002/cncr.30517. [DOI] [PubMed] [Google Scholar]
- D’Cruz A.K., Vaish R. Risk-based oral cancer screening — lessons to be learnt. Nat Rev Clin Oncol. 2021;18(8):471–472. doi: 10.1038/s41571-021-00511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenández Garrote L., Sankaranarayanan R., Lence Anta J.J., Rodriguez Salvá A., Maxwell P.D. An evaluation of the oral cancer control program in Cuba. Epidemiology. 1995;6(4):428–431. doi: 10.1097/00001648-199507000-00019. [DOI] [PubMed] [Google Scholar]
- Ghani W.M.N., Razak I.A., Doss J.G., Ramanathan A., Tahir Z., Ridzuan N.A., Edgar S., Zain R.B. Mouth self-examination as a screening tool for oral potentially malignant disorders among a high-risk Indigenous population. J Public Health Dent. 2019;79(3):222–230. doi: 10.1111/jphd.12313. [DOI] [PubMed] [Google Scholar]
- Higgins JPT GS (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021)Available from www.cochrane-handbook.org. The Cochrane Collaboration. 2021.
- Ho P.-S., Wang W.-C., Huang Y.-T., Yang Y.-H. Finding an oral potentially malignant disorder in screening program is related to early diagnosis of oral cavity cancer – Experience from real world evidence. Oral Oncology. 2019;89:107–114. doi: 10.1016/j.oraloncology.2018.12.007. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. Cancer Today. [Internet]. Vol. 418, International Agency for research. 2020 [cited 2021 Aug 10]. p. 1–2. Available from: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=population&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=1&type=1&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=.
- Kumar M., Nanavati R., Modi T., Dobariya C. Oral cancer: Etiology and risk factors: A review. J Cancer Res Ther. 2016;12(2):458–463. doi: 10.4103/0973-1482.186696. [DOI] [PubMed] [Google Scholar]
- Lee H., Ho P.-S., Wang W.-C., Hu C.-Y., Lee C.-H., Huang H.-L. Effectiveness of a health belief model intervention using a lay health advisor strategy on mouth self-examination and cancer screening in remote aboriginal communities: A randomized controlled trial. Patient Education and Counseling. 2019;102(12):2263–2269. doi: 10.1016/j.pec.2019.07.001. [DOI] [PubMed] [Google Scholar]
- Mandrik O., Zielonke N., Meheus F., Severens J.L.(., Guha N., Herrero Acosta R., Murillo R. Systematic reviews as a ‘lens of evidence’: Determinants of benefits and harms of breast cancer screening. Int J Cancer. 2019;145(4):994–1006. doi: 10.1002/ijc.32211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Filho A., Bray F. Global patterns and trends in cancers of the lip, tongue and mouth. Oral Oncol. 2020;102:104551. doi: 10.1016/j.oraloncology.2019.104551. [DOI] [PubMed] [Google Scholar]
- Morikawa T., Shibahara T., Takano M., Iwamoto M., Takaki T., Kasahara K., Nomura T., Takano N., Katakura A. 105047. Available from: 2021;112:105047. doi: 10.1016/j.oraloncology.2020.105047. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. Browse the SEER Cancer Statistics Review (CSR) 1975-2015 [Internet]. SEER Cancer Statistics Review. 2014 [cited 2021 Mar 14]. Available from: https://seer.cancer.gov/csr/1975_2017/browse_csr.php?sectionSEL=20&pageSEL=sect_20_table.10.
- Peirson L., Fitzpatrick-Lewis D., Ciliska D., Warren R. Screening for cervical cancer: A systematic review and meta-analysis. Syst Rev. 2013;2(1) doi: 10.1186/2046-4053-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petti S. Lifestyle risk factors for oral cancer. Oral Oncology. 2009;45(4-5):340–350. doi: 10.1016/j.oraloncology.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Proctor E., Silmere H., Raghavan R., Hovmand P., Aarons G., Bunger A., Griffey R., Hensley M. Outcomes for implementation research: Conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Heal Ment Heal Serv Res. 2011;38(2):65–76. doi: 10.1007/s10488-010-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S.V.K., Mejia G., Roberts-Thomson K., Logan R. Epidemiology of oral cancer in Asia in the past decade – An update (2000–2012) Asian Pacific J Cancer Prev. 2013;14(10):5567–5577. doi: 10.7314/apjcp.2013.14.10.5567. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan R., Fernandez Garrote L., Lence Anta J., Pisani P., Rodriguez S.A. Visual inspection in oral cancer screening in Cuba: A case-control study. Oral Oncol. 2002;38(2):131–136. doi: 10.1016/s1368-8375(01)00033-1. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan R., Ramadas K., Thara S., Muwonge R., Thomas G., Anju G., Mathew B. Long term effect of visual screening on oral cancer incidence and mortality in a randomized trial in Kerala, India. Oral Oncology. 2013;49(4):314–321. doi: 10.1016/j.oraloncology.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Strome A., Kossatz S., Zanoni D.K., Rajadhyaksha M., Patel S., Reiner T. Current Practice and Emerging Molecular Imaging Technologies in Oral Cancer Screening. Mol Imaging. 2018;17:1–11. doi: 10.1177/1536012118808644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W.W.Y., Yen A.M.F., Chiu S.Y.H., Chen T.H.H. A community-based RCT for oral cancer screening with toluidine blue. J Dent Res. 2010;89(9):933–937. doi: 10.1177/0022034510373763. [DOI] [PubMed] [Google Scholar]
- Warnakulasuriya S., Kerr A.R. Oral Cancer Screening: Past, Present, and Future. J Dent Res. 2021;100(12):1313–1320. doi: 10.1177/00220345211014795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnakulasuriya S., Kujan O., Aguirre‐Urizar J.M., Bagan J.V., González‐Moles M.Á., Kerr A.R., Lodi G., Mello F.W., Monteiro L., Ogden G.R., Sloan P., Johnson N.W. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021;27(8):1862–1880. doi: 10.1111/odi.13704. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Cancer fact sheets – lip, oral cavity. Cancer Fact Sheets [Internet]. 2020;0–5. Available from: http://gco.iarc.fr/today/data/factsheets/cancers/1-Lip-oral-cavity-fact-sheet.pdf.
- Zain R.B. Cultural and dietary risk factors of oral cancer and precancer – A brief overview. Oral Oncol. 2001;37(3):205–210. doi: 10.1016/s1368-8375(00)00133-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.