Abstract
Inflammatory bowel disease (IBD) includes Crohn’s disease and ulcerative colitis and is an idiopathic, chronic inflammatory disease of the colonic mucosa. The occurrence of IBD, causes irreversible damage to the colon and increases the risk of carcinoma. The routine clinical treatment of IBD includes drug treatment, endoscopic treatment and surgery. The vast majority of patients are treated with drugs and biological agents, but the complete cure of IBD is difficult. Mesenchymal stem cells (MSCs) have become a new type of cell therapy for the treatment of IBD due to their immunomodulatory and nutritional functions, which have been confirmed in many clinical trials. This review discusses some potential mechanisms of MSCs in the treatment of IBD, summarizes the experimental results, and provides new insights to enhance the therapeutic effects of MSCs in future applications.
Keywords: Mesenchymal stem cells, Inflammatory bowel diseases, Inflammation, Pretreatment, Gene editing
Core Tip: Mesenchymal stem cell (MSC) transplantation is a novel treatment method for inflammatory bowel disease (IBD) that has exhibits certain achievements in clinical trials. Here, we reviewed the developed strategies for enhancing the therapeutic effect of MSCs, and among these, pretreatment with MSCs is the most common method. The pretreatments include bioactive substances, hypoxia and modification of culture methods and are able to enhance the migration ability of MSCs to repair the damaged intestinal mucosa or upregulate the expression of cytokines. These methods provide new ideas for the future clinical application of MSCs in the treatment of IBD.
INTRODUCTION
Inflammatory bowel disease (IBD) is divided into three types: Chronic IBD, ulcerative colitis (UC) and Crohn’s disease (CD). Genetic susceptibility, environmental factors, the intestinal microbiota and the immune system play important roles in the pathogenesis of IBD[1], and immune factors may be the most direct and important factor[2]. In recent decades, the incidence rate of IBD has increased worldwide. The increase in the incidence of IBD has led to increased social and economic burdens[3]. IBD is traditionally considered common in the Western world. However, data from the last ten years showed that the IBD incidence rates in newly industrialized countries, including China and India, are increasing[4,5]. IBD has developed into a common disease of the digestive system in China. Patients with IBD may exhibit extracolonic manifestations, such as primary sclerosis cholangitis and arthritis, and are also more prone to complications, such as colon cancer, coronary artery disease, osteoporosis and venous thrombosis, than the general population[6,7]. The routine clinical treatment for IBD includes three categories: Traditional therapeutic drugs, biological agents and new small-molecule drugs. The traditional therapeutic drugs include 5-aminosalicylic acid (5-ASA), glucocorticoids such as budesonide and immunosuppressants such as azathioprine (AZA), 6-mercaptopurine (6-MP), ciclosporin and methotrexate. Anti-tumor necrosis factor (TNF)-α drugs, insulin receptor antagonists, and interleukin (IL) inhibitors, are biological agents widely used for the treatment of IBD. The new small-molecule drugs include selective Janus kinase inhibitors, and sphingosine-1-phosphate receptor modulators[8,9]. Although these drugs alleviate IBD, maintaining the effects is difficult, and the expected effect is not ideal. More importantly, these drugs may lead to various adverse reactions[10], such as a risk of increased mortality. The use of corticosteroids has been shown to be associated with skin effects, weight gain, hyperglycaemia, osteoporosis, adrenal insufficiency and cataracts. The use of immunosuppressants also increase the risk of opportunistic infection. Intolerance or the potential occurrence of bone marrow/liver toxicity caused by immunomodulators may result in treatment cessation in one quarter of patients[11]. Ileocolectomy is the most common surgical strategy used to treat patients with CD, but this treatment rarely cures the disease; new lesions usually develop rapidly at the anastomosis, and a risk of urinary incontinence has been documented[12,13]. Therefore, new treatments are needed to improve this condition without a risk of incontinence. The new era of cell-based therapy in stem cell biology has provided promising prospects and aroused great interest from scientists, clinicians and patients[14]. Mesenchymal stem cells (MSCs) are heterogeneous spindle-shaped cells with the ability to differentiate into osteoblasts, chondrocytes and adipocytes in vitro. MSCs originate from various tissues, including the bone marrow, umbilical cord, placenta, fat and tooth tissue[15]. Considering their immunoregulatory and nutritional characteristics, MSCs have become promising candidates for the treatment of autoimmune diseases and have promoted the development of regenerative medicine. Inflammatory signals stimulate bone marrow MSCs (BMMSCs) to produce a variety of growth factors, that accelerate tissue repair by promoting angiogenesis, extracellular matrix remodeling and tissue progenitor cell differentiation. BMMSCs effectively regulate immune cells in the inflammatory microenvironment. Interestingly, the immunomodulatory effects of BMMSCs are not inherent but are determined by the type and intensity of the inflammatory reaction[16]. In this review, we discuss the research progress and possible molecular mechanism of MSCs in the treatment of IBD, summarize the protocols and improved technical methods currently being developed to enhance the effect of MSCs on the treatment of IBD and provide a basis for more promising and safer prospects for MSC applications.
SEARCH STRATEGY
We conducted a comprehensive literature search of the following databases: PubMed, Google Scholar and SpiScholar, using Reference Citation Analysis (https://www.referencecitationanalysis.com). Free text words and database-specific index terms were combined with Boolean operators (“AND” and “OR“) to improve the sensitivity of our search. We searched for multiple combinations of the following keywords: mesenchymal stem cells, inflammatory bowel disease, hypoxia, inflammatory, pretreatment, preconditioning, stimulation, priming, regeneration, immunomodulation, secretome, conditioned medium (CM), paracrine, therapeutic, brain, nervous system, bone, and cartilage, among others. The identified studies were not constrained by the publication language or publication status. Most of the publications were published in the last five years, but some classical studies are also cited.
USE OF MSCS AND THEIR DERIVATIVES IN THE TREATMENT OF IBD
MSCs are pluripotent stem cells with the ability to self-renew and differentiate into various cell types. These cells play a key role in immune regulation and regenerative therapy[17]. According to the standard definition of MSCs, MSCs express CD73, CD90, CD105 and other markers, but do not express the haematopoietic markers CD45, CD34, CD14, CD19 and HLA-DR (≤ 2%) and differentiate into adipogenic, osteogenic and chondrogenic cells in vitro. The sources of MSCs include the bone marrow, fat, muscle, peripheral blood, umbilical cord, placenta, foetal tissue and amniotic fluid[18]. BMMSCs are the most widely used and studied stem cells, but their application is limited due to the possible pain and the incidence rate caused by bone marrow aspiration, and the limited number of BMSCs obtained[19]. Adipose-derived mesenchymal stem cells (AMSCs) and umbilical cord derived mesenchymal stem cells (UCMSCs) have recently received considerable attention. The procedures for collecting UCMSCs are painless, and these cells exhibit faster self-renewal characteristics[20]. Dental mesenchymal stem cells have a strong muscle regeneration ability. After combination with appropriate scaffold materials, these cells provide a favorable alternative treatment for muscle tissue engineering[21]. Human term placental tissue-derived MSCs and the conditioned medium remaining after cultures of these cells reportedly enhance angiogenesis[22]. MSCs derived from the amniotic fluid and amniotic membrane have been introduced as attractive and potent stem cell sources for clinical application due to their easy, safe, and painless collection procedures with minimal ethical issues[23].
MSCs influence the phenotype and function of innate immune cells (macrophages, dendritic cells, neutrophils, eosinophils, basophils, natural killer cells, natural killer T cells, and natural lymphocytes) and acquired immune cells (T and B lymphocytes) through paracrine signalling (secretion of soluble factors) or cell-cell contact[24] (Figure 1). Clinical trials of MSC treatments as a new cell therapy strategy for IBD have yielded certain results, but considerable room for improvement remains. According to a published study, more than 200 patients with refractory fistulas have received local injections of BMMSCs. More than half of the patients achieved complete remission, and approximately two-thirds of the patients achieved overall remission. Among patients with refractory luminal CD, 49 cases of systematic transplantation of MSCs have been recorded, and the results have shown that autologous BMMSCs generate reduced responses, whereas the use of allogeneic BMMSCs is promising because approximately 60% of patients exhibited a response, and approximately 40% achieved clinical remission[25]. In general, bone marrow mesenchymal stem cell transplantation (BMSCT) is presumed to be markedly safer than haematopoietic stem cell transplantation (HSCT). Although autologous BMSCT does not show higher efficacy than conventional treatment, allogeneic BMSCT appeared to be more effective in patients with intracranial CD in a phase II metacentre clinical trial[26]. In addition, clinical research on the treatment of CD patients with AMSCs has been performed. Panés et al[27] completed a phase III randomized double-blind trial involving the use of allogeneic AMSC transplantation to treat patients with complex anal fistulas and observed obvious curative effects. These experiments showed the prospects of MSCs in the treatment of IBD.
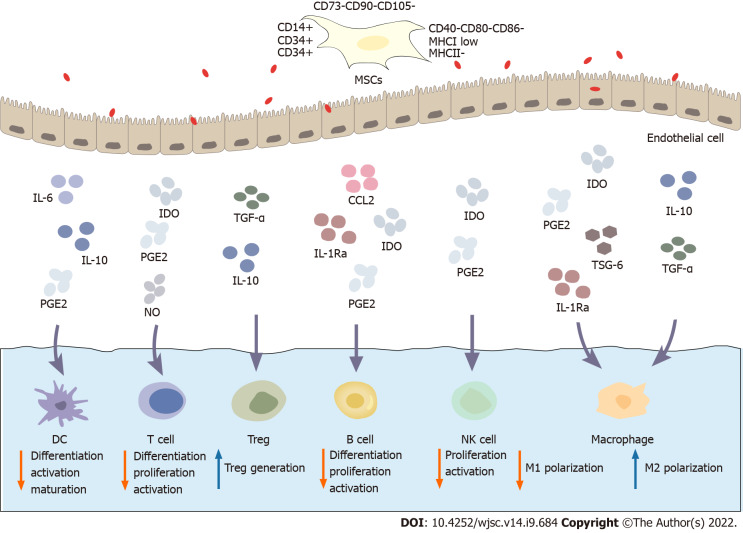
Figure 1.
Immunoregulatory mechanism of mesenchymal stem cells. Mesenchymal stem cells (MSCs) express CD73, CD90 and CD105 and do not express haematopoietic markers such as CD14, CD34 and CD45 or the costimulatory molecules CD40, CD80 and CD86. MSCs exhibit low expression of major histocompatibility complex class (MHC) I and do not express MHC II. MSCs possess a wide range of immunomodulatory properties. Activated MSCs secrete a variety of soluble factors, such as indoleamine 2,3-dioxygenase, prostaglandin E2, transforming growth factor-β, tumor necrosis factor-α stimulating gene 6, interleukin (IL)-1Ra, and IL-6. These factors inhibit the differentiation, proliferation and activation of various immune cell subsets, including T cells, B cells, dendritic cells, macrophages, and natural killer cells. Therefore, MSCs inhibit the immune response to inhibit inflammation. MSCs: Mesenchymal stem cells; MHC: Major histocompatibility complex; IDO: Indoleamine 2,3-dioxygenase; PGE2: Prostaglandin E2; TGF-β: Transforming growth factor-β; TSG-6: Tumor necrosis factor-α stimulating gene 6; IL: Interleukin; NK: Natural killer; CCL: CC chemokine ligand.
Although the results of clinical trials are promising in terms of safety and efficacy, many problems remain to be solved, such as the exact working mechanism, dose, mode of administration and optimal concentration. In a trinitrobenzene sulfonic acid (TNBS)-induced colitis model, intravenously injected MSCs were cleared nonspecifically by the innate immune system under physiological conditions. However, local administration may prevent MSCs from directly entering the blood circulation and thereby significantly reduce spleen and liver clearance, and local administration increases the concentration and duration of MSC engraftment in target organs. However, the disadvantage of local administration is that the operation is relatively complex[28]. Similarly, a dextran sulfate sodium (DSS)-induced colitis model was intravenously injected with human embryonic stem cell-derived MSCs (TMSCs), and CM-DiI was used for tracking for 12 and 24 h. The injected TMSCs mainly accumulate in the lung, and a small portion of TMSCs localized to the liver and spleen. However, MSCs expressing fluorescein were only detected at the injection site 12 and 24 h after intrapulmonary administration. No signal was detected after intravenous injection, indicating that most of the intravenously injected TMSCs migrated away from the injection site through the blood circulation, that the number of cells in the target organ was too low for bioluminescence imaging, and that the intravenously injected cells may no longer have been viable during the observation period[15]. Despite many unsolved problems, the direct injection of MSCs into target tissues has been indicated to improve the homing efficiency[29]. Arterial MSC infusion is another option, and the major limitation is the risk of intestinal ischaemia due to embolic events[30]. Consequently, MSCs may interact with resident cells by secreting paracrine factors or through intercellular communication[31]. In addition to the low survival rates of stem cells that are injected intravenously into the targeted area to treat intracranial diseases, the injected stem cells may also migrate to other sites and produce side effects. Therefore, some techniques have been developed to promote colonic mucosal healing through interventional radiology and an intra-arterial injection of MSCs into the ileum, and clinical trials have also suggested their safety and efficacy[32]. In a recent study, a temperature-responsive Petri dish was used to endoscopically transplant MSC sheets into the inflammatory area of mice with TNBS-induced colitis. The effect of MSC sheet transplantation on ulcer reduction was then confirmed, verifying that endoscopic MSC transplantation may be a new and effective method for the treatment of IBD[33]. Many inconsistencies regarding the location and persistence of MSCs after transplantation have been reported. Therefore, many recent studies have focused on the paracrine immunomodulatory effects of biological factors secreted by MSCs, particularly the immunomodulatory potential of soluble factors (cytokines, chemokines and growth factors)[31,34]. Cell-free therapy with these derivatives has been proposed as a treatment for IBD. An important advantage of these cell-free therapies is that they may reduce the risk of immune rejection.
MSCs have emerged as a new paradigm for IBD treatment, largely due to their multifaceted biological functions. MSCs secrete numerous factors that target immune cells and affect their functions[35] (Table 1), such as indoleamine 2,3-dioxygenase (IDO), canine urinary quinoline, prostaglandin E2 (PGE2), CD73, transforming growth factor-β (TGF-β), IL-6 and TNF-α stimulating gene 6 (TSG-6), and these effects enable MSCs to regulate T cells, B cells, macrophages, natural killer cells, and dendritic cells[36]. MSCs adjust their immune function in an inflammatory environment, particularly by stimulating the proinflammatory cytokines interferon-γ (IFN-γ) and TNF-α. Activated, MSCs upregulate the expression of IL-6, IL-10, IDO, TGF, PGE2, hepatocyte growth factor, nitric oxide and haem oxygenase-1 (HO-1)[31].
Table 1.
Common factors secreted by mesenchymal stem cells[35]
|
Type
|
Representative factors
|
| Immunomodulatory factor | HGF, TGF-β1, PGE2, IDO |
| Chemokine | RANTES, SDF-1α, MIP-1α, MCP-1 |
| Nutritional factors | HGF, NGF, FGF-2, PDGF-AA, PDGF-BB, EGF |
| Haematopoietic growth factor | G-CSF, M-CSF, GM-CSF, EPO |
| Vascular regeneration factor | VEGF165, FGF-2, EGF, PDGF |
| Scar inhibiting factor | HGF, FGF-2 |
| Anti-apoptotic factor | VEGF165, FGF-2, HGF |
HGF: Hepatocyte growth factor; TGF-β: Transforming growth factor-β; PGE2: Prostaglandin E2; IDO: Indoleamine 2,3-dioxygenase; SDF-1α: Stromal cell-derived factor-1α; MCP-1: Monocyte chemoattractant protein-1; NGF: Nerve growth factor; FGF: Fibroblast growth factor; PDGF: Platelet-derived growth factor; G-CSF: Colony stimulating factor 3; M-CSF: Colony stimulating factor 1; GM-CSF: Colony stimulating factor 2; EPO: Erythropoietin; VEGF165: Vascular endothelial growth factor-165.
In addition to MSCs, cytokines and extracellular vesicles (EVs) released by MSCs exert therapeutic effects on CD. According to the position statement of the International Society of Exosomes, EVs are particles with a lipid bilayer, that are naturally released from cells and cannot be replicated. MSC EVs perform their functions by transferring their contents, such as proteins, mRNAs and microRNAs (miRNAs), to target cells[37]. EVs have been proven to retain the therapeutic characteristics of MSCs and thereby stimulate tissue repair, limit oxidative stress, reduce inflammation and regulate the immune response. Therefore, an increasing number of studies have focused on the paracrine effects of MSCs[36,38]. In a previous study, researchers compared the effects of MSCs and MSC-derived EVs on IBD in a DSS-induced colitis model and found that MSCs and EVs exert similar immunosuppressive and anti-inflammatory effects by decreasing colonic lymphocyte infiltration and reducing disease severity in DSS-induced mice[39]. Some studies have performed in situ injection of EVs to treat colitis. The results show that EV injection regulates the balance of proinflammatory and anti-inflammatory cytokines in colon tissue[40]. As an important paracrine product of MSCs, EVs inhibit the nuclear factor-kappaB (NF-κB) p65 signaling pathway and TNBS-induced colonic oxidative stress in a TNBS-induced experimental colitis model, reduce the production of free radicals, enhance the enzyme defense system, maintain the cellular oxidation/antioxidant balance, and inhibit cell apoptosis through the exogenous death receptor signaling pathway and inherent mitochondrial signaling pathway, which play important roles in colon repair[41]. The experimental data show that EVs promote the M2 polarization of macrophages[42], increase the expression of IL-10, and inhibite IL-1β, TNF-α and IL-6 production. The treatment of RAW264.7 macrophages with EVs upregulates TGF-β1 expression and thereby increases the expression of miR-132. Based on these findings, EVs carry TGF-β1 and regulate the miR-132/mycbp2/TSC2 axis to promote M2 macrophage polarization[43].
ENHANCEMENT OF THE THERAPEUTIC EFFECTS OF MSCS ON IBD
MSCs are typically injected intravenously to treat IBD and may thus remain in blood-rich tissues (liver, lung and spleen) without reaching the target organs. Many animal studies have reported that the level of recruitment and persistence of MSCs in vivo is low[44]. Some infusion techniques can be modified or MSCs can be combined with conventional drugs to combat the problems of the low differentiation potential and homing of MSCs to the injured site; additionally, the homing to the target organ and anti-inflammatory effects of MSCs can be enhanced. The enhancement strategies include pretreatment, gene modification and combination with currently used drugs[45]. The pretreatment of MSCs before use comprises a large proportion of improvement strategies. The pretreatment reagents include bioactive substances (cytokines, growth factors and innate immune receptor agonists), hypoxia and modification of the culture medium (Figure 2).
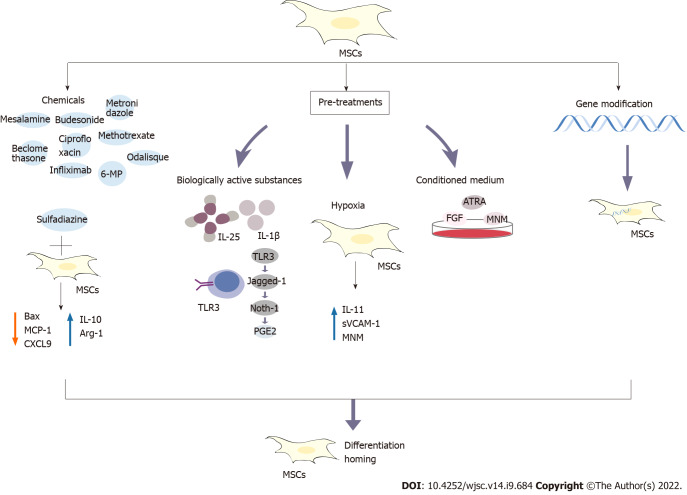
Figure 2.
The developed strategies to improve the efficacy of mesenchymal stem cells in the treatment of inflammatory bowel disease include combined treatment with conventional drugs, pretreatment and gene modification. The tested pretreatments include bioactive factors, hypoxia and medium modification. The conventional drugs include biological preparations of mesalazine, budesonide, beclomethasone, ciprofloxacin, metronidazole, 6-mercaptopurine, methotrexate, infliximab and adalimumab. For example, the combination of mesenchymal stem cells (MSCs) and the drug sulfadiazine inhibits the nuclear factor-kappaB pathway, reduces Bax expression, prevents loss of the B cell lymphoma-2 protein, reduces the levels of monocyte chemoattractant protein-1 and CXCL9, increases the levels of interleukin (IL)-10 and Arg-1, and transforms inflammatory M1 macrophages into anti-inflammatory M2 macrophages. Pretreatment with IL-25 and IL-1β enhances the immunosuppressive abilities of MSCs. MSCs pretreated with Toll-like receptor 3 (TLR3) for a short time in vitro produce prostaglandin E2 through the TLR3-Jagged-1-Notch-1 pathway. In response to hypoxia, the levels of IL-11, soluble vascular cell adhesion protein-1 and stromal cell-derived factor-1α are significantly upregulated. MSCs have also been pretreated by modifying the culture medium, such as the addition of fibroblast growth factor, all-trans retinoic acid and modified neuronal medium. In addition, genetically modified MSCs have been developed. These methods and strategies potentially improve the immunosuppressive abilities of MSCs by promoting their homing and differentiation abilities. PGE2: Prostaglandin E2; MSCs: Mesenchymal stem cells; IL: Interleukin; 6-MP: 6-mercaptopurine; MCP-1: Monocyte chemoattractant protein-1; TLR: Toll-like receptor; MNM: Modified neuronal medium; sVCAM-1: Soluble vascular cell adhesion protein-1; ATRA: All-trans retinoic acid; FGF: Fibroblast growth factor.
Combination therapy with MSCs and conventional drugs
Currently, patients with IBD are treated with 5-ASA compounds (mainly mesalamine), glucocorticoids (conventional and other forms, such as budesonide and beclomethasone), antibiotics (usually ciprofloxacin and metronidazole), immunomodulators (mainly AZA/6-MP or methotrexate) and biological agents[32,46]. The aim of these treatments is to inhibit intestinal inflammation and ultimately improve the quality of life of patients with IBD[47]. Biological agents such as infliximab, Odalisque, anti-TNF therapy, α4β7 integrin inhibitors and IL-12/23 inhibitors have changed the treatment of patients with IBD. However, up to 30% of patients with CD and UC do not show a response or do not receive clinical benefits after treatment. In addition, up to half of patients who initially attained clinical benefits lost the second response[48]. In addition to these biological treatments, HSCT has been used to treat some serious gastrointestinal diseases, including IBD[49]. The use of local immunomodulatory cell therapy is an alternative method to the current treatments for IBD. As mentioned above, a phase I-III clinical trial of MSCs to treat IBD has achieved good results, which raises the possibility of combining MSCs with conventional IBD drugs, such as immunosuppressants and anti-inflammatory drugs. However, this strategy remains questionable because whether MSCs and other conventional drugs will affect each other or reduce their functions is unclear. Although some studies suggest that these drugs may affect MSCs, another study showed that MSCs reduce the effects of several immunosuppressive drugs on T-cell subsets in mouse models[50]. A previous study analysed the interaction of immunosuppressive drugs with MSCs in the context of cell proliferation and function[51]. Another study demonstrated that the aggregation of MSCs into globules (when MSCs were injected into a narrow space) resulted in loss of their ability to inhibit T cells. Interestingly, the addition of budesonide to the pellet partially restored the inhibitory effect of MSCs on T-cell proliferation. Although globular MSCs do not inhibit the proliferation of T cells alone, when combined with budesonide, PGE2 produced by globular MSCs act synergistically with budesonide on EP2-/-EP4 receptors of T cells to inhibit T-cell proliferation[36]. The thiopurine analogues AZA and 6-MP were widely accepted in the early 1980s as agents for the treatment of IBD. The thiopurine metabolite 6-MP is a purine antagonist that inhibits the proliferation of T and B lymphocytes by interfering with DNA and RNA synthesis[47]. The differences in cell polarity and actin organization induced by AZA and dexamethasone (DEX) might reflect the different effects of immunosuppressive drugs on MSC migration. Because the clearance of allogeneic MSCs can be delayed, combined treatment with AZA may improve the homing of BMMSCs to the injured site, to achieve a better therapeutic effect[52]. The combination of AZA and MSCs does not alter their respective effects. In contrast, the combination may improve the therapeutic effects on IBD. A molecular analysis showed that steroids and TNF substantially increase vascular endothelial growth factor C (VEGF-C) production in MSCs, and VEGF-C in turn promote the CD8+ T-cell response to reverse the immunosuppressive effect of MSCs and provide new information for effective MSC therapy[53]. The T-cell subsets, and the Bax (proapoptotic protein) and B cell lymphoma-2 (Bcl-2) (anti-apoptotic protein) levels are unbalanced, which results in defects in immune cell apoptosis in the IBD microenvironment[54]. In a recent study, the NF-κB signaling pathway in the colons of rats with TNBS-induced colitis was effectively inhibited by the combination of AMSCs and sulfadiazine, and the loss of Bcl-2 protein expression was prevented by reduction of Bax expression. A decrease in NF-κB signaling reduces inflammatory, proliferative and proapoptotic activities[55,56]. In addition, the combination of AMSCs and sulfadiazine transforms inflammatory M1 macrophages into anti-inflammatory M2 macrophages by reducing the levels of monocyte chemoattractant protein-1 (MCP-1) and CXCL9 and increasing the levels of IL-10 and Arg-1. Therefore, AMSCs combined with conventional IBD drugs may be a more effective strategy to alleviate the progression of colitis by reducing the levels of inflammatory and apoptosis markers than individual treatments[57]. In addition to routine medication, some relevant studies have examined the efficacy of MSCs combined with other drugs as treatments for IBD. MIS416 is a novel immunomodulatory factor extracted from Propionibacterium acne that is composed of MDP and bacterial DNA, and activates the cytoplasmic receptors nucleotide-binding oligomerization domain 2 and Toll-like receptor 9 (TLR9)[58]. The effect of MIS416 on mice with 3% DSS-induced experimental colitis has been analysed. The retro-orbital administration of MIS416 has been followed by subsequent intrapulmonary injection of umbilical cord blood derived MSCs (human UCMSCs). Compared with single administration, the combination of MIS416 with UCMSCs significantly alleviate the symptoms of IBD to improve the treatment efficacy of stem cells. The therapeutic effect is mediated by inhibiting Th1 and Th17 cells, polarization of Th2 cells, and increases in the numbers of Treg and B cells; in particular, the combination of MIS416 and human UCMSCs shifted the balance from Th1/Th17 to a Treg-oriented response. In contrast, improper changes in the immune environment promote increases in the levels of cytokines such as IFN-γ, IL-6, and IL-12, and human UCMSCs are stimulated by these cytokines and subsequently inhibit proinflammatory cells in the inflamed colon. Moreover, MIS416-induced MCP-1 production increases the migration of human UCMSCs, leading to increased colonic infiltration. In conclusion, MIS416 enhances the therapeutic effect of human UCMSCs on experimental colitis by improving immunosuppression and regulating immune homeostasis in the intestine[59]. The combined use of BMMSCs and immunosuppressants may prolong the survival of transplanted BMMSCs and reduce the adverse reactions to drugs to improve the therapeutic effect. Importantly, the use of bioactive reagents to promote homeostasis of the immune balance in vivo stabilizes the effects of BMMSCs[54]. A study showed that combined transplantation of MSCs and tryptophan decarboxylases (TDCs) (dendritic cells) is more effective than single therapy in alleviating the clinical and histological manifestations of colitis, particularly compared with MSC transplantation alone. The protective effect of TDC-MSCs is accompanied by the induction of Treg cells and increased production of anti-inflammatory cytokines in the spleen and mesenteric lymph nodes (MLNs). Therefore, the combined transplantation of BMMSCs and TDCs may be a promising and effective method for treating IBD[60]. MSCs combined with new immune agents will be a more effective method for IBD therapy than conventional treatment, and studies exploring agents to enhance the therapeutic effect of MSCs on IBD are urgently needed.
Pretreatment of MSCs
Pretreatment with bioactive substances: Injured or inflamed tissue may release cytokines and growth factors, such as TGF-β1, TNF-α and IL. IL-1β is a member of the IL-1 family and plays a key role in innate immunity and inflammation in a variety of tissues and organs. A previous study showed that IL-1β increases leukocyte migration. Animal experiments have shown that IL-1β increases the migration of BMMSCs to the inflamed spleen, mesentery and colon, repairs the damaged intestinal mucosa and exerts an immunosuppressive effect by increasing the expression of chemokine receptor 3 (CXCR3) and CXCR4[54].
In colitis mice, IL-1β-treated MSCs regulate the balance of immune cells in the spleen and MLNs by increasing the expression of cyclooxygenase-2 (COX-2), IL-6 and IL-8 and altering the polarization of peritoneal macrophages. Importantly, IL-1β-induced MSCs exhibit upregulates CXCR4 expression and better engraftment at the site of intestinal inflammation, which increases the efficacy of IL-1β-induced MSCs in the treatment of DSS-induced colitis[61]. INF-γ induction maintains the classic phenotype of AMSCs without significantly changing the proliferation or migration of AMSCs. However, compared with untreated AMSCs, IFN-γ treated AMSCs produce significantly increased levels of IDO, exhibited higher expression of adhesion molecule family-1 (ICAM-1), and inhibit the proliferation of activated T cells[62]. Therefore, an experiment using IL-1β and IFN-γ combined with human UCMSCs showed that the pretreated MSCs significantly reduce the proliferation of peripheral blood mononuclear cells, indicating that their immunosuppressive activity is enhanced. Compared with untreated human UCMSCs, PGE2 secretion and the expression of COX-2 and IDO are significantly increased in pretreated human UCMSCs. Thus, DSS-induced colitis might be alleviated by pretreatment with human UCMSCs[63]. In addition, IL-25, which is a member of the IL-17 cytokine family, stimulates the Th2 cell-mediated immune response, and increases the recruitment of inflammatory cells to damaged tissues by affecting epithelial cells[64]. The pretreatment of MSCs with IL-25 may alleviate destructive inflammation in several autoimmune diseases by inhibiting the Th1 or Th17 immune response[65]. Recently, Wang et al[66] showed that knocking out IL-25 expression in MSCs eliminates the inhibitory effects of Th17 cells. In addition, MSCs have been manipulated to express CX3CR1 and IL-25 to promote their delivery to the inflamed colon and enhance their immunosuppressive activity[67]. These results help to better clarify the inhibitory potential of AMSCs and their products, and build a foundation for the development of new therapeutic methods to control the immune response. IL-37 exerts a potent immunosuppressive effect on both innate and adaptive immunity. The expression of IL-37 in macrophages or epithelial cells almost completely inhibits the production of proinflammatory cytokines[68]. IL-37-treated MSCs attenuate the histological damage in mice with DSS-induced colitis by inducing the production of Th2-related cytokines and inhibiting splenic production of Th1-related cytokines by CD4+ cells[69]. TLR pathway activation in BMSCs changes their inflammatory characteristics and immunomodulatory effects on cells in the innate and adaptive immune systems[70]. This stimulation of receptors on the cell surface or cytoplasm with corresponding ligands activates the TLR pathway, which involves various adaptor molecules and the transcription factors NF-κB and interferon regulatory factor, resulting in a cytokine response. MSCs express a variety of functional TLRs at high levels, including TLR3 and TLR4, which change the phenotype and immunophenotype of cells after activation[71]. The activation of TLR3 enhances the immunosuppressive activity of MSCs. If human UCMSCs are pretreated with TLR3 for a short time in vitro, they produce PGE2 through the TLR3-Jagged-1-Notch-1 pathway and enhance the protective effect of MSCs on TNBS induced colitis in mice[72]. Granulocyte colony stimulating factor (G-CSF) is a glycoprotein that is mainly produced by monocytes and macrophages. G-CSF plays an important role in promoting the differentiation and maturation of haematopoietic cells and the release of mature blood cells[73]. Clinically, G-CSF is mainly used to treat patients with chemotherapy-induced leukopenia and patients with poor responses to peripheral blood stem cell transplantation. The combination of BMSCs and G-CSF in rats increases the number of transplanted MSCs, enhances the immunosuppressive ability of MSCs, inhibits inflammation and reduces leukocyte activation in the intestinal mucosa during UC therapy[74,75]. TSG-6 possesses anti-inflammatory, secretory and tissue-protective properties[76]. The injection of TSG-6 derived from MSC exosomes inhibits the immune response and repairs the damaged tissue, resulting in the alleviation of IBD in mice[77]. In summary, stimulation and pretreatment with these factors may enhance the differentiation and migration of MSCs and exert some immunosuppressive and anti-inflammatory effects. Other researchers have shown that stem cells responding to different types of injury signals will actively secrete endogenous CSF-2, which stimulates MSCs through the PI3K/Akt or FAK/ERK1/2 signaling pathway to increase the differentiation and migration of MSCs. This enhanced therapeutic effect has been proven in an animal model of endometrial ablation[78]. Experiments must be performed to verify whether these factors stimulate MSCs to exert enhanced therapeutic effects and whether they are applicable to IBD. Studies have shown that the intestinal microbiota plays a critical role in IBD[79]. Thus, some experimental models and clinical trials have attempted to correct changes in the gut microbiota (FMT) using various approaches, including microbiota transplantation and probiotic administration[80]. As a treatment for IBD, FMT-MSC transplantation improves the clinical remission rate, enhances the efficacy of radiation therapy against pathogenic bacteria and ultimately restores the intestinal health of patients with IBD[81].
Hypoxic preconditioning of MSCs: MSCs located in inflammatory tissue release many chemokines, which upregulate the expression of cell adhesion molecules, such as ICAM-1 and vascular cell adhesion protein-1 (VCAM-1)[78,82]. These chemokines induce the accumulation of large numbers of CD4+ T cells and Treg cells in the lesion site and enhance the ability of MSCs to regulate the imbalance in immune cells. In recent years, hypoxia has become an effective method to control the proliferation, differentiation and multidirectional differentiation of BMSCs. Hypoxia-treated conditioned medium (HCM) has been proven to exert numerous beneficial effects on tissue regeneration, such as cell recruitment, wound healing, angiogenesis and reconstruction[83]. In long-term culture, the proliferation potential of MSCs cultured under hypoxic conditions is higher than the effect of normobaric oxygen. An oxygen concentration of 1%-5% has been proven to significantly increase the proliferation of MSCs while maintaining their normal morphology[84]. IL-11 is a member of the IL-6 cytokine family and has a structure and function similar to those of IL-6. In recent years, the interest in IL-11 has been renewed due to its unique biological effects on epithelial cancers and inflammatory diseases[85]. IL-11 plays a key role in promoting cell proliferation and protecting cells from oxidative stress[86]. The proteolytic shedding of VCAM-1 also produces soluble VCAM-1 (sVCAM-1), which is present in many cell types of the haematopoietic lineage, including B and T lymphocytes, monocytes, eosinophils and basophils. This soluble protein plays an important role by mediating leukocyte adhesion and endothelial cell migration during inflammation[87]. Stromal cell-derived factor-1α (SDF1α) is a widely characterized small proinflammatory chemokine that binds to the transmembrane receptor CXCR4[88]. The binding of SDF-1α to CXCR4 induces not only the migration of stem cells but also the expression of adhesion molecules in stem cells. Compared with those in the normobaric oxygen control group, IL-11, the sVCAM-1 and SDF-1α levels are significantly upregulated by hypoxia, and this upregulation increases chemotaxis and reveals their key role in human BMMSC migration and in characterizing the HCM chemotactic components[89,90].
Another experiment showed that BMSCs coated with VCAM1 antibodies (V-MSCs) can be successfully obtained. The analysis showed that V-MSCs and uncoated MSCs had similar surfaces and differentiation potentials. A transwell analysis showed that V-MSCs exhibit higher mobility than uncoated MSCs. The injection of V-MSCs increases the expression of the SRY gene in the diseased colon and resulted in rapid recovery of all disease indices [including weight change, Drug Attitude Inventory (DAI) scores, histological changes and the expression of Ki67 and claudin 1]. The treatment decreases the proportions of proinflammatory Th1 and Th17 cells and increases the proportions of anti-inflammatory Th2 and Treg cells. V-MSCs show enhanced homing and regulate the immune balance in experimental colitis models, which suggests that these cells may be useful in the treatment of IBD or other immune diseases[91]. Similarly, an intravenous injection of MSCs overexpressing ICAM-1 (C3 cells) into mice with DSS-induced IBD decreases the numbers of Th1 and Th17 cells in the spleen while increasing the number of Tregs. A quantitative polymerase chain reaction analysis showed that the infusion of ICAM-1-overexpressing MSCs significantly reduces the IFN-γ and IL-17 mRNA levels and increases the Foxp3 mRNA levels. These cells reduce inflammatory damage by promoting MSC homing to target organs and immune organs to significantly enhance the beneficial effects of MSC treatment[92].
Modification of the culture medium: CM preparations differ from other biological preparations because they represent a mixture of different factors secreted by cells, including growth factors, cytokines, enzymes, nucleic acids and bioactive lipids[93]. Various studies have shown that MSC-derived exosomes or CM exert similar effects on repairing damaged tissues, inhibiting the inflammatory response and regulating the immune response[36]. CM helps to maintain the increased paracrine factor gradient between the diseased organ and the stem cell niche to accelerate the recovery process[94]. Kang et al[95] and Wu et al[96] showed that using different culture media without foetal bovine serum during the in vitro expansion of BMMSCs enhances the immunomodulatory effect of MSCs in an in vivo model of IBD. Other researchers then supplemented culture media with different compounds, such as a combination of aspirin, b-fibroblast growth factor (b-FGF), all-trans retinoic acid and modified neuronal medium (MNM) or the combination of activin A, b-FGF and platelet lysates. Studies have been designed to verify whether the therapeutic effect of MSCs in vivo could be enhanced through this modification[32,97], and the results showed that culturing MSCs with specific pretreated culture media increase cell survival, migration, differentiation and secretory functions[98]. Yang et al[99] pretreated human UCMSCs with MNM for 24 h, washed off the MNM and replaced the culture medium with minimum essential medium. After two days of growth, the cells were labelled deadapted MSCs (De-hUCMSCs). Compared with human UCMSC treatment, De-hUCMSC treatment resulted in less weight loss in mice with colitis; specifically, this treatment significantly reduces the ulceration, expansion and DAI score of the mice with colitis and significantly decreases crypt damage and inflammatory cell infiltration in the mouse colon. Based on these results, De-hUCMSCs have obvious therapeutic advantages in the treatment of IBD and better improve the symptoms of colitis than unmodified cells.
Genetic modification enhances the therapeutic effects of MSCs
Genetic or particle modification of BMMSCs potentially improves their immunosuppressive abilities[100]. IFN-γ enhances the immunosuppressive characteristics of MSCs[101], and the basic characteristics of MSCs are not changed after the transfection of pcDNA3 carrying 1-IFN-γ. The transfection of MSCs with IFN-γ induces the overexpression of IFN-γ to balance immunity, upregulation of IDO expression and inhibition of the production of cytokines in subjects with intestinal mucositis to ameliorate intestinal inflammation in a DSS-induced colitis model[102]. Based on accumulating evidence, miR-146a is an anti-inflammatory miRNA and a negative regulator of the innate immune response[103]. BMMSCs were transfected with a lentivirus expressing miR-146a in one study. EVs has been isolated from BMMSCs after gene modification and then delivered via tail vein injection to the target tissue of TNBS-induced IBD mice. MiR-146a-carrying EVs significantly inhibite the expression of TNF receptor-related factor 6 (TRAF6) and IL-1 receptor-related kinase 1 (IRAK1) in rats with TNBS-induced colitis. Thus, EVs containing miR-146a improve TNBS-induced experimental colitis caused by targeting TRAF6 and IRAK1[104]. 15-LOX-1 is a key regulator of the inflammatory response in the colon and other tissues and is mainly expressed in macrophages[105]. Notably, miR-148b-5p complementarily binds to the 3’ untranslated region of 15-LOX-1 mRNA, and human UCMSCs transfected with miR148b-5p relieve IBD by downregulating the expression of 15-LOX-1 in macrophages[106]. SDF-1 has been recognized as one of the most critical factors for stem cell homing to the bone marrow and other damaged tissues[107]. The CXCR4 gene has been transfected into BMMSCs with a lentiviral vector, and overexpression of the CXCR4 gene does not alter the biological characteristics or viability of BMMSCs but increases the migration and homing of BMMSCs in vitro and in vivo. The overexpression of CXCR4 may promote the homing of BMMSCs to the damaged intestinal mucosa and improve its therapeutic effect on colitis[108]. Overexpression of CXCR4 also exerts a more obvious antitumor effect, which is helpful for preventing and treating the most serious complications of IBD, such as colitis-associated cancer[109]. COX2 is an enzyme involved in arachidonic acid metabolism that is responsible for the production of PGE2. This major inflammatory regulator maintains the immune balance and has been proven to play a vital role in the treatment of IBD with MSCs[110]. COX2 increases the expression of insulin-like growth factor-1 (IGF-1) in the skeletal muscle of MSC-transplanted mice[111,112]. CXCR3 is a G-protein-coupled seven transmembrane receptor that is expressed in damaged parenchymal cells in lesions of multiple organs and inflammatory cells, including activated lymphocytes, macrophages and dendritic cells[113]. HO-1 and its metabolites exert antioxidant, anti-inflammatory, antiproliferative and immunomodulatory effects[114]. The transfection of HO-1 improves the transformation and antioxidant capacity of BMMSCs. A study also showed that the number of BMMSCs modified by CXCR3 and HO-1 are significantly increased at the site of injury and that the damage to intestinal function exhibited rapid recovery. BMMSCs modified with the CXCR3 and/or HO-1 genes were transplanted into the intestinal epithelial recess cell line-6 injury model, and CXCR3 overexpression improves BMMSC chemotaxis to induce the early and rapid recruitment of BMMSCs to damaged intestinal epithelial cells[115,116]. Other experiments have shown that the exogenous or endogenous overexpression of heparin binding epidermal growth factor-like growth factor (HBEGF) promotes the proliferation and migration of MSCs, and the synergistic effect of HBEGF and MSCs further restores the function of the intestinal barrier[117,118]. The transfection of MSCs with an IL-33 overexpression plasmid reduces apoptosis in early MSCs and further improves the therapeutic effects of MSCs on myocardial infarction (MI) through the polarization of macrophages and T cells[119]. Similarly, in an animal model of MI, BMMSCs overexpressing VEGF and Bcl-2, which inhibit apoptosis and autophagy and enhance paracrine signaling, significantly improves cardiac function by improving the survival rate and angiogenesis, and these modified MSCs are more resistant to harsh environments than unmodified cells[120]. In addition, pioglitazone combined with BMMSC transplantation further enhances the protective effect of BMMSCs on diabetes and heart damage[121]. These studies showed the improved abilities of MSCs to repair damaged tissues, but unfortunately, a study of IBD therapy has not been performed. Research on MSC gene editing in other disease models also provides some insights into the future treatment of IBD with MSCs.
CONCLUSION
MSCs exist widely in human tissues and organs. They not only exhibit multidirectional differentiation and proliferation potential but also show a wide range of prospects and applications in medicine because of their immunoregulatory activity. MSCs represent a safe and effective treatment for IBD. Although MSC therapy has shown great potential in a large number of animal experiments and clinical trials, a consensus on the effectiveness of intravenous MSCs in IBD-focused clinical trials has not been achieved. Many animal studies have suggested that the level of recruitment and persistence of mesenchymal stem cells in vivo is low, and thus we must improve strategies to enhance their therapeutic effects. We summarized the strategies to improve the efficacy of MSCs in the treatment of IBD. MSCs have been combined with current drugs, cultured in different media or pretreated with cytokines and biological factors, combined with current IBD treatments and subjected to genetic engineering. These new strategies may increase the efficacy of MSC-based treatments, and further research is ongoing. The goal of IBD treatment remains the same: To achieve prolonged remission and halt any ongoing disease progression. In future research, we must strive to improve the safety and feasibility of MSCs in IBD therapy, improve patient quality of life and maximize the utilization of MSCs.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: May 18, 2022
First decision: June 23, 2022
Article in press: September 8, 2022
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Amin A, United Arab Emirates; Hassaan NA, Egypt; Kamalabadi-Farahani M, Iran; Prasetyo EP, Indonesia; Song BW, South Korea S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
Contributor Information
Meng-Yue Shi, School of Medicine, Yangtze University, Jingzhou 434023, Hubei Province, China.
Lian Liu, Department of Pharmacology, Medical School of Yangtze University, Yangtze University, Jingzhou 434023, Hubei Province, China.
Fu-Yuan Yang, Health Science Center, Yangtze University, Jingzhou 434020, Hubei Province, China. ivansblue@sina.com.
References
- 1.Ramos GP, Papadakis KA. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin Proc. 2019;94:155–165. doi: 10.1016/j.mayocp.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pei XF, Cao LL, Huang F, Qiao X, Yu J, Ye H, Xi CL, Zhou QC, Zhang GF, Gong ZL. Role of miR-22 in intestinal mucosa tissues and peripheral blood CD4+ T cells of inflammatory bowel disease. Pathol Res Pract. 2018;214:1095–1104. doi: 10.1016/j.prp.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17–30. doi: 10.1016/S2468-1253(19)30333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui G, Yuan A. A Systematic Review of Epidemiology and Risk Factors Associated With Chinese Inflammatory Bowel Disease. Front Med (Lausanne) 2018;5:183. doi: 10.3389/fmed.2018.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mak WY, Zhao M, Ng SC, Burisch J. The epidemiology of inflammatory bowel disease: East meets west. J Gastroenterol Hepatol. 2020;35:380–389. doi: 10.1111/jgh.14872. [DOI] [PubMed] [Google Scholar]
- 6.Rogler G, Singh A, Kavanaugh A, Rubin DT. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology. 2021;161:1118–1132. doi: 10.1053/j.gastro.2021.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Argollo M, Gilardi D, Peyrin-Biroulet C, Chabot JF, Peyrin-Biroulet L, Danese S. Comorbidities in inflammatory bowel disease: a call for action. Lancet Gastroenterol Hepatol. 2019;4:643–654. doi: 10.1016/S2468-1253(19)30173-6. [DOI] [PubMed] [Google Scholar]
- 8.Iskandar HN, Dhere T, Farraye FA. Ulcerative Colitis: Update on Medical Management. Curr Gastroenterol Rep. 2015;17:44. doi: 10.1007/s11894-015-0466-9. [DOI] [PubMed] [Google Scholar]
- 9.Misselwitz B, Juillerat P, Sulz MC, Siegmund B, Brand S Swiss IBDnet, an official working group of the Swiss Society of Gastroenterology. Emerging Treatment Options in Inflammatory Bowel Disease: Janus Kinases, Stem Cells, and More. Digestion. 2020;101 Suppl 1:69–82. doi: 10.1159/000507782. [DOI] [PubMed] [Google Scholar]
- 10.Troncone E, Monteleone G. The safety of non-biological treatments in Ulcerative Colitis. Expert Opin Drug Saf. 2017;16:779–789. doi: 10.1080/14740338.2017.1340936. [DOI] [PubMed] [Google Scholar]
- 11.Shi X, Chen Q, Wang F. Mesenchymal stem cells for the treatment of ulcerative colitis: a systematic review and meta-analysis of experimental and clinical studies. Stem Cell Res Ther. 2019;10:266. doi: 10.1186/s13287-019-1336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joustra V, Duijvestein M, Mookhoek A, Bemelman W, Buskens C, Koželj M, Novak G, Hindryckx P, Mostafavi N, D'Haens G. Natural History and Risk Stratification of Recurrent Crohn's Disease After Ileocolonic Resection: A Multicenter Retrospective Cohort Study. Inflamm Bowel Dis. 2022;28:1–8. doi: 10.1093/ibd/izab044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lightner AL, Faubion WA. Mesenchymal Stem Cell Injections for the Treatment of Perianal Crohn's Disease: What We Have Accomplished and What We Still Need to Do. J Crohns Colitis. 2017;11:1267–1276. doi: 10.1093/ecco-jcc/jjx046. [DOI] [PubMed] [Google Scholar]
- 14.Nagaishi K, Arimura Y, Fujimiya M. Stem cell therapy for inflammatory bowel disease. J Gastroenterol. 2015;50:280–286. doi: 10.1007/s00535-015-1040-9. [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Wang X, Chen J, Chen S, Li Z, Liu H, Bai Y, Zhi F. Embryonic stem cell-derived mesenchymal stem cells promote colon epithelial integrity and regeneration by elevating circulating IGF-1 in colitis mice. Theranostics. 2020;10:12204–12222. doi: 10.7150/thno.47683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14:493–507. doi: 10.1038/s41581-018-0023-5. [DOI] [PubMed] [Google Scholar]
- 17.Jasim SA, Yumashev AV, Abdelbasset WK, Margiana R, Markov A, Suksatan W, Pineda B, Thangavelu L, Ahmadi SH. Shining the light on clinical application of mesenchymal stem cell therapy in autoimmune diseases. Stem Cell Res Ther. 2022;13:101. doi: 10.1186/s13287-022-02782-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown C, McKee C, Bakshi S, Walker K, Hakman E, Halassy S, Svinarich D, Dodds R, Govind CK, Chaudhry GR. Mesenchymal stem cells: Cell therapy and regeneration potential. J Tissue Eng Regen Med. 2019;13:1738–1755. doi: 10.1002/term.2914. [DOI] [PubMed] [Google Scholar]
- 19.Mohamed-Ahmed S, Fristad I, Lie SA, Suliman S, Mustafa K, Vindenes H, Idris SB. Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res Ther. 2018;9:168. doi: 10.1186/s13287-018-0914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding DC, Chang YH, Shyu WC, Lin SZ. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24:339–347. doi: 10.3727/096368915X686841. [DOI] [PubMed] [Google Scholar]
- 21.Ansari S, Chen C, Xu X, Annabi N, Zadeh HH, Wu BM, Khademhosseini A, Shi S, Moshaverinia A. Muscle Tissue Engineering Using Gingival Mesenchymal Stem Cells Encapsulated in Alginate Hydrogels Containing Multiple Growth Factors. Ann Biomed Eng. 2016;44:1908–1920. doi: 10.1007/s10439-016-1594-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komaki M, Numata Y, Morioka C, Honda I, Tooi M, Yokoyama N, Ayame H, Iwasaki K, Taki A, Oshima N, Morita I. Exosomes of human placenta-derived mesenchymal stem cells stimulate angiogenesis. Stem Cell Res Ther. 2017;8:219. doi: 10.1186/s13287-017-0660-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Testrow S, McGovern R, Tully V. Secondary care interface: optimising communication between teams within secondary care to improve the rehabilitation journey for older people. BMJ Open Qual. 2021;10 doi: 10.1136/bmjoq-2020-001274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrell CR, Volarevic V. Apoptosis: A friend or foe in mesenchymal stem cell-based immunosuppression. Adv Protein Chem Struct Biol. 2021;126:39–62. doi: 10.1016/bs.apcsb.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Grégoire C, Lechanteur C, Briquet A, Baudoux É, Baron F, Louis E, Beguin Y. Review article: mesenchymal stromal cell therapy for inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;45:205–221. doi: 10.1111/apt.13864. [DOI] [PubMed] [Google Scholar]
- 26.Forbes GM, Sturm MJ, Leong RW, Sparrow MP, Segarajasingam D, Cummins AG, Phillips M, Herrmann RP. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn's disease refractory to biologic therapy. Clin Gastroenterol Hepatol. 2014;12:64–71. doi: 10.1016/j.cgh.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 27.Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, Nachury M, Ferrante M, Kazemi-Shirazi L, Grimaud JC, de la Portilla F, Goldin E, Richard MP, Leselbaum A, Danese S ADMIRE CD Study Group Collaborators. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281–1290. doi: 10.1016/S0140-6736(16)31203-X. [DOI] [PubMed] [Google Scholar]
- 28.Cao X, Duan L, Hou H, Liu Y, Chen S, Zhang S, Wang C, Qi X, Liu N, Han Z, Zhang D, Han ZC, Guo Z, Zhao Q, Li Z. IGF-1C hydrogel improves the therapeutic effects of MSCs on colitis in mice through PGE2-mediated M2 macrophage polarization. Theranostics. 2020;10:7697–7709. doi: 10.7150/thno.45434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liesveld JL, Sharma N, Aljitawi OS. Stem cell homing: From physiology to therapeutics. Stem Cells. 2020;38:1241–1253. doi: 10.1002/stem.3242. [DOI] [PubMed] [Google Scholar]
- 30.Lightner AL. Stem Cell Therapies for Inflammatory Bowel Disease. Curr Gastroenterol Rep. 2019;21:16. doi: 10.1007/s11894-019-0672-y. [DOI] [PubMed] [Google Scholar]
- 31.da Costa Gonçalves F, Paz AH. Cell membrane and bioactive factors derived from mesenchymal stromal cells: Cell-free based therapy for inflammatory bowel diseases. World J Stem Cells. 2019;11:618–633. doi: 10.4252/wjsc.v11.i9.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez-Santalla M, Garin MI. Improving the Efficacy of Mesenchymal Stem/Stromal-Based Therapy for Treatment of Inflammatory Bowel Diseases. Biomedicines. 2021;9 doi: 10.3390/biomedicines9111507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pak S, Hwang SW, Shim IK, Bae SM, Ryu YM, Kim HB, Do EJ, Son HN, Choi EJ, Park SH, Kim SY, Ye BD, Yang SK, Kanai N, Maeda M, Okano T, Yang DH, Byeon JS, Myung SJ. Endoscopic Transplantation of Mesenchymal Stem Cell Sheets in Experimental Colitis in Rats. Sci Rep. 2018;8:11314. doi: 10.1038/s41598-018-29617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crivelli B, Chlapanidas T, Perteghella S, Lucarelli E, Pascucci L, Brini AT, Ferrero I, Marazzi M, Pessina A, Torre ML Italian Mesenchymal Stem Cell Group (GISM) Mesenchymal stem/stromal cell extracellular vesicles: From active principle to next generation drug delivery system. J Control Release. 2017;262:104–117. doi: 10.1016/j.jconrel.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 35.Samsonraj RM, Rai B, Sathiyanathan P, Puan KJ, Rötzschke O, Hui JH, Raghunath M, Stanton LW, Nurcombe V, Cool SM. Establishing criteria for human mesenchymal stem cell potency. Stem Cells. 2015;33:1878–1891. doi: 10.1002/stem.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burand AJ Jr, Di L, Boland LK, Boyt DT, Schrodt MV, Santillan DA, Ankrum JA. Aggregation of Human Mesenchymal Stromal Cells Eliminates Their Ability to Suppress Human T Cells. Front Immunol. 2020;11:143. doi: 10.3389/fimmu.2020.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiu G, Zheng G, Ge M, Wang J, Huang R, Shu Q, Xu J. Functional proteins of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther. 2019;10:359. doi: 10.1186/s13287-019-1484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez-Pujana A, Igartua M, Santos-Vizcaino E, Hernandez RM. Mesenchymal stromal cell based therapies for the treatment of immune disorders: recent milestones and future challenges. Expert Opin Drug Deliv. 2020;17:189–200. doi: 10.1080/17425247.2020.1714587. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Altemus J, Lightner AL. Mesenchymal stem cells and acellular products attenuate murine induced colitis. Stem Cell Res Ther. 2020;11:515. doi: 10.1186/s13287-020-02025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duan L, Huang H, Zhao X, Zhou M, Chen S, Wang C, Han Z, Han ZC, Guo Z, Li Z, Cao X. Extracellular vesicles derived from human placental mesenchymal stem cells alleviate experimental colitis in mice by inhibiting inflammation and oxidative stress. Int J Mol Med. 2020;46:1551–1561. doi: 10.3892/ijmm.2020.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang J, Liu XX, Fan H, Tang Q, Shou ZX, Zuo DM, Zou Z, Xu M, Chen QY, Peng Y, Deng SJ, Liu YJ. Extracellular Vesicles Derived from Bone Marrow Mesenchymal Stem Cells Protect against Experimental Colitis via Attenuating Colon Inflammation, Oxidative Stress and Apoptosis. PLoS One. 2015;10:e0140551. doi: 10.1371/journal.pone.0140551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells. 2019;8 doi: 10.3390/cells8121605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Han B, Wang Y, Wang C, Zhang H, Xue J, Wang X, Niu T, Niu Z, Chen Y. Mesenchymal stem cell-secreted extracellular vesicles carrying TGF-β1 up-regulate miR-132 and promote mouse M2 macrophage polarization. J Cell Mol Med. 2020;24:12750–12764. doi: 10.1111/jcmm.15860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo YC, Chiu YH, Chen CP, Wang HS. Interleukin-1β induces CXCR3-mediated chemotaxis to promote umbilical cord mesenchymal stem cell transendothelial migration. Stem Cell Res Ther. 2018;9:281. doi: 10.1186/s13287-018-1032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu C, Wu Z, Li L. Pre-treatments enhance the therapeutic effects of mesenchymal stem cells in liver diseases. J Cell Mol Med. 2020;24:40–49. doi: 10.1111/jcmm.14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magro F, Cordeiro G, Dias AM, Estevinho MM. Inflammatory Bowel Disease - Non-biological treatment. Pharmacol Res. 2020;160:105075. doi: 10.1016/j.phrs.2020.105075. [DOI] [PubMed] [Google Scholar]
- 47.Grevenitis P, Thomas A, Lodhia N. Medical Therapy for Inflammatory Bowel Disease. Surg Clin North Am. 2015;95:1159–1182, vi. doi: 10.1016/j.suc.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 48.Papamichael K, Cheifetz AS. Therapeutic drug monitoring in inflammatory bowel disease: for every patient and every drug? Curr Opin Gastroenterol. 2019;35:302–310. doi: 10.1097/MOG.0000000000000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cassinotti A, Passamonti F, Segato S. CELL THERAPY IN INFLAMMATORY BOWEL DISEASE. Pharmacol Res. 2021;163:105247. doi: 10.1016/j.phrs.2020.105247. [DOI] [PubMed] [Google Scholar]
- 50.Hajkova M, Hermankova B, Javorkova E, Bohacova P, Zajicova A, Holan V, Krulova M. Mesenchymal Stem Cells Attenuate the Adverse Effects of Immunosuppressive Drugs on Distinct T Cell Subopulations. Stem Cell Rev Rep. 2017;13:104–115. doi: 10.1007/s12015-016-9703-3. [DOI] [PubMed] [Google Scholar]
- 51.Javorkova E, Vackova J, Hajkova M, Hermankova B, Zajicova A, Holan V, Krulova M. The effect of clinically relevant doses of immunosuppressive drugs on human mesenchymal stem cells. Biomed Pharmacother. 2018;97:402–411. doi: 10.1016/j.biopha.2017.10.114. [DOI] [PubMed] [Google Scholar]
- 52.Schneider N, Gonçalves Fda C, Pinto FO, Lopez PL, Araújo AB, Pfaffenseller B, Passos EP, Cirne-Lima EO, Meurer L, Lamers ML, Paz AH. Dexamethasone and azathioprine promote cytoskeletal changes and affect mesenchymal stem cell migratory behavior. PLoS One. 2015;10:e0120538. doi: 10.1371/journal.pone.0120538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gan Y, Zhang T, Chen X, Cao W, Lin L, Du L, Wang Y, Zhou F, He X, He Y, Gan J, Sheng H, Sorokin L, Shi Y. Steroids Enable Mesenchymal Stromal Cells to Promote CD8+ T Cell Proliferation Via VEGF-C. Adv Sci (Weinh) 2021;8:2003712. doi: 10.1002/advs.202003712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee BC, Kang KS. Functional enhancement strategies for immunomodulation of mesenchymal stem cells and their therapeutic application. Stem Cell Res Ther. 2020;11:397. doi: 10.1186/s13287-020-01920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamza AA, Lashin FM, Gamel M, Hassanin SO, Abdalla Y, Amin A. Hawthorn Herbal Preparation from Crataegus oxyacantha Attenuates In Vivo Carbon Tetrachloride -Induced Hepatic Fibrosis via Modulating Oxidative Stress and Inflammation. Antioxidants (Basel) 2020;9 doi: 10.3390/antiox9121173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamza AA, Heeba GH, Hamza S, Abdalla A, Amin A. Standardized extract of ginger ameliorates liver cancer by reducing proliferation and inducing apoptosis through inhibition oxidative stress/ inflammation pathway. Biomed Pharmacother. 2021;134:111102. doi: 10.1016/j.biopha.2020.111102. [DOI] [PubMed] [Google Scholar]
- 57.Yousefi-Ahmadipour A, Rashidian A, Mirzaei MR, Farsinejad A, PourMohammadi-Nejad F, Ghazi-Khansari M, Ai J, Shirian S, Allahverdi A, Saremi J, Ebrahimi-Barough S. Combination therapy of mesenchymal stromal cells and sulfasalazine attenuates trinitrobenzene sulfonic acid induced colitis in the rat: The S1P pathway. J Cell Physiol. 2019;234:11078–11091. doi: 10.1002/jcp.27944. [DOI] [PubMed] [Google Scholar]
- 58.Mainini F, Larsen DS, Webster GA, Young SL, Eccles MR. MIS416 as a siRNA Delivery System with the Ability to Target Antigen-Presenting Cells. Nucleic Acid Ther. 2018;28:225–232. doi: 10.1089/nat.2017.0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee BC, Shin N, Lee JY, Kang I, Kim JJ, Lee SE, Choi SW, Webster GA, Kang KS. MIS416 Enhances Therapeutic Functions of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Against Experimental Colitis by Modulating Systemic Immune Milieu. Front Immunol. 2018;9:1078. doi: 10.3389/fimmu.2018.01078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abbasi-Kenarsari H, Heidari N, Baghaei K, Amani D, Zali MR, Gaffari Khaligh S, Shafiee A, Hashemi SM. Synergistic therapeutic effect of mesenchymal stem cells and tolerogenic dendritic cells in an acute colitis mouse model. Int Immunopharmacol. 2020;88:107006. doi: 10.1016/j.intimp.2020.107006. [DOI] [PubMed] [Google Scholar]
- 61.Fan H, Zhao G, Liu L, Liu F, Gong W, Liu X, Yang L, Wang J, Hou Y. Pre-treatment with IL-1β enhances the efficacy of MSC transplantation in DSS-induced colitis. Cell Mol Immunol. 2012;9:473–481. doi: 10.1038/cmi.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serejo TRT, Silva-Carvalho AÉ, Braga LDCF, Neves FAR, Pereira RW, Carvalho JL, Saldanha-Araujo F. Assessment of the Immunosuppressive Potential of INF-γ Licensed Adipose Mesenchymal Stem Cells, Their Secretome and Extracellular Vesicles. Cells. 2019;8 doi: 10.3390/cells8010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu Y, Yoo SM, Park HH, Baek SY, Kim YJ, Lee S, Kim YL, Seo KW, Kang KS. Preconditioning with interleukin-1 beta and interferon-gamma enhances the efficacy of human umbilical cord blood-derived mesenchymal stem cells-based therapy via enhancing prostaglandin E2 secretion and indoleamine 2,3-dioxygenase activity in dextran sulfate sodium-induced colitis. J Tissue Eng Regen Med. 2019;13:1792–1804. doi: 10.1002/term.2930. [DOI] [PubMed] [Google Scholar]
- 64.Bredo G, Storie J, Shrestha Palikhe N, Davidson C, Adams A, Vliagoftis H, Cameron L. Interleukin-25 initiates Th2 differentiation of human CD4(+) T cells and influences expression of its own receptor. Immun Inflamm Dis. 2015;3:455–468. doi: 10.1002/iid3.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Su J, Xie C, Fan Y, Cheng W, Hu Y, Huang Q, Shi H, Wang L, Ren J. Interleukin-25 enhances the capacity of mesenchymal stem cells to induce intestinal epithelial cell regeneration. Am J Transl Res. 2017;9:5320–5331. [PMC free article] [PubMed] [Google Scholar]
- 66.Wang WB, Yen ML, Liu KJ, Hsu PJ, Lin MH, Chen PM, Sudhir PR, Chen CH, Sytwu HK, Yen BL. Interleukin-25 Mediates Transcriptional Control of PD-L1 via STAT3 in Multipotent Human Mesenchymal Stromal Cells (hMSCs) to Suppress Th17 Responses. Stem Cell Reports. 2015;5:392–404. doi: 10.1016/j.stemcr.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fu Y, Ni J, Chen J, Ma G, Zhao M, Zhu S, Shi T, Zhu J, Huang Z, Zhang J. Dual-Functionalized MSCs that Express CX3CR1 and IL-25 Exhibit Enhanced Therapeutic Effects on Inflammatory Bowel Disease. Mol Ther. 2020;28:1214–1228. doi: 10.1016/j.ymthe.2020.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Su Z, Tao X. Current Understanding of IL-37 in Human Health and Disease. Front Immunol. 2021;12:696605. doi: 10.3389/fimmu.2021.696605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang WQ, Dong K, Zhou L, Jiao GH, Zhu CZ, Li WW, Yu G, Wu WT, Chen S, Sun ZN, Wang YM, Liu WT, Zhang J, Wang BM, Feng XM. IL-37b gene transfer enhances the therapeutic efficacy of mesenchumal stromal cells in DSS-induced colitis mice. Acta Pharmacol Sin. 2015;36:1377–1387. doi: 10.1038/aps.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kordjazy N, Haj-Mirzaian A, Rohani MM, Gelfand EW, Rezaei N, Abdolghaffari AH. Role of toll-like receptors in inflammatory bowel disease. Pharmacol Res. 2018;129:204–215. doi: 10.1016/j.phrs.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 71.Abdi J, Rashedi I, Keating A. Concise Review: TLR Pathway-miRNA Interplay in Mesenchymal Stromal Cells: Regulatory Roles and Therapeutic Directions. Stem Cells. 2018;36:1655–1662. doi: 10.1002/stem.2902. [DOI] [PubMed] [Google Scholar]
- 72.Qiu Y, Guo J, Mao R, Chao K, Chen BL, He Y, Zeng ZR, Zhang SH, Chen MH. TLR3 preconditioning enhances the therapeutic efficacy of umbilical cord mesenchymal stem cells in TNBS-induced colitis via the TLR3-Jagged-1-Notch-1 pathway. Mucosal Immunol. 2017;10:727–742. doi: 10.1038/mi.2016.78. [DOI] [PubMed] [Google Scholar]
- 73.Lotfi N, Thome R, Rezaei N, Zhang GX, Rezaei A, Rostami A, Esmaeil N. Roles of GM-CSF in the Pathogenesis of Autoimmune Diseases: An Update. Front Immunol. 2019;10:1265. doi: 10.3389/fimmu.2019.01265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tang Y, Chen Y, Wang X, Song G, Li Y, Shi L. Combinatorial Intervention with Mesenchymal Stem Cells and Granulocyte Colony-Stimulating Factor in a Rat Model of Ulcerative Colitis. Dig Dis Sci. 2015;60:1948–1957. doi: 10.1007/s10620-015-3655-3. [DOI] [PubMed] [Google Scholar]
- 75.Castro-Dopico T, Fleming A, Dennison TW, Ferdinand JR, Harcourt K, Stewart BJ, Cader Z, Tuong ZK, Jing C, Lok LSC, Mathews RJ, Portet A, Kaser A, Clare S, Clatworthy MR. GM-CSF Calibrates Macrophage Defense and Wound Healing Programs during Intestinal Infection and Inflammation. Cell Rep. 2020;32:107857. doi: 10.1016/j.celrep.2020.107857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Day AJ, Milner CM. TSG-6: A multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. 2019;78-79:60–83. doi: 10.1016/j.matbio.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 77.Yang S, Liang X, Song J, Li C, Liu A, Luo Y, Ma H, Tan Y, Zhang X. A novel therapeutic approach for inflammatory bowel disease by exosomes derived from human umbilical cord mesenchymal stem cells to repair intestinal barrier via TSG-6. Stem Cell Res Ther. 2021;12:315. doi: 10.1186/s13287-021-02404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park SR, Cho A, Kim JW, Lee HY, Hong IS. A Novel Endogenous Damage Signal, CSF-2, Activates Multiple Beneficial Functions of Adipose Tissue-Derived Mesenchymal Stem Cells. Mol Ther. 2019;27:1087–1100. doi: 10.1016/j.ymthe.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weingarden AR, Vaughn BP. Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes. 2017;8:238–252. doi: 10.1080/19490976.2017.1290757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aggeletopoulou I, Konstantakis C, Assimakopoulos SF, Triantos C. The role of the gut microbiota in the treatment of inflammatory bowel diseases. Microb Pathog. 2019;137:103774. doi: 10.1016/j.micpath.2019.103774. [DOI] [PubMed] [Google Scholar]
- 81.Ocansey DKW, Wang L, Wang J, Yan Y, Qian H, Zhang X, Xu W, Mao F. Mesenchymal stem cell-gut microbiota interaction in the repair of inflammatory bowel disease: an enhanced therapeutic effect. Clin Transl Med. 2019;8:31. doi: 10.1186/s40169-019-0251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rubtsov Y, Goryunov К, Romanov А, Suzdaltseva Y, Sharonov G, Tkachuk V. Molecular Mechanisms of Immunomodulation Properties of Mesenchymal Stromal Cells: A New Insight into the Role of ICAM-1. Stem Cells Int. 2017;2017:6516854. doi: 10.1155/2017/6516854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qiu X, Liu J, Zheng C, Su Y, Bao L, Zhu B, Liu S, Wang L, Wang X, Wang Y, Zhao W, Zhou J, Deng Z, Jin Y. Exosomes released from educated mesenchymal stem cells accelerate cutaneous wound healing via promoting angiogenesis. Cell Prolif. 2020;53:e12830. doi: 10.1111/cpr.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Choudhery MS. Strategies to improve regenerative potential of mesenchymal stem cells. World J Stem Cells. 2021;13:1845–1862. doi: 10.4252/wjsc.v13.i12.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Negahdaripour M, Nezafat N, Ghasemi Y. A panoramic review and in silico analysis of IL-11 structure and function. Cytokine Growth Factor Rev. 2016;32:41–61. doi: 10.1016/j.cytogfr.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 86.Yang W, Zhang S, Ou T, Jiang H, Jia D, Qi Z, Zou Y, Qian J, Sun A, Ge J. Interleukin-11 regulates the fate of adipose-derived mesenchymal stem cells via STAT3 signalling pathways. Cell Prolif. 2020;53:e12771. doi: 10.1111/cpr.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Troncoso MF, Ortiz-Quintero J, Garrido-Moreno V, Sanhueza-Olivares F, Guerrero-Moncayo A, Chiong M, Castro PF, García L, Gabrielli L, Corbalán R, Garrido-Olivares L, Lavandero S. VCAM-1 as a predictor biomarker in cardiovascular disease. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166170. doi: 10.1016/j.bbadis.2021.166170. [DOI] [PubMed] [Google Scholar]
- 88.Jarrah AA, Schwarskopf M, Wang ER, LaRocca T, Dhume A, Zhang S, Hadri L, Hajjar RJ, Schecter AD, Tarzami ST. SDF-1 induces TNF-mediated apoptosis in cardiac myocytes. Apoptosis. 2018;23:79–91. doi: 10.1007/s10495-017-1438-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gabrielyan A, Quade M, Gelinsky M, Rösen-Wolff A. IL-11 and soluble VCAM-1 are important components of Hypoxia Conditioned Media and crucial for Mesenchymal Stromal Cells attraction. Stem Cell Res. 2020;45:101814. doi: 10.1016/j.scr.2020.101814. [DOI] [PubMed] [Google Scholar]
- 90.Gabrielyan A, Neumann E, Gelinsky M, Rösen-Wolff A. Metabolically conditioned media derived from bone marrow stromal cells or human skin fibroblasts act as effective chemoattractants for mesenchymal stem cells. Stem Cell Res Ther. 2017;8:212. doi: 10.1186/s13287-017-0664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen Q, Li Y, Chen Z, Du H, Wan J. Anti-VCAM 1 Antibody-Coated Mesenchymal Stromal Cells Attenuate Experimental Colitis via Immunomodulation. Med Sci Monit. 2019;25:4457–4468. doi: 10.12659/MSM.914238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li X, Wang Q, Ding L, Wang YX, Zhao ZD, Mao N, Wu CT, Wang H, Zhu H, Ning SB. Intercellular adhesion molecule-1 enhances the therapeutic effects of MSCs in a dextran sulfate sodium-induced colitis models by promoting MSCs homing to murine colons and spleens. Stem Cell Res Ther. 2019;10:267. doi: 10.1186/s13287-019-1384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bogatcheva NV, Coleman ME. Conditioned Medium of Mesenchymal Stromal Cells: A New Class of Therapeutics. Biochemistry (Mosc) 2019;84:1375–1389. doi: 10.1134/S0006297919110129. [DOI] [PubMed] [Google Scholar]
- 94.Pouya S, Heidari M, Baghaei K, Asadzadeh Aghdaei H, Moradi A, Namaki S, Zali MR, Hashemi SM. Study the effects of mesenchymal stem cell conditioned medium injection in mouse model of acute colitis. Int Immunopharmacol. 2018;54:86–94. doi: 10.1016/j.intimp.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 95.Kang JY, Oh MK, Joo H, Park HS, Chae DH, Kim J, Lee HR, Oh IH, Yu KR. Xeno-Free Condition Enhances Therapeutic Functions of Human Wharton's Jelly-Derived Mesenchymal Stem Cells against Experimental Colitis by Upregulated Indoleamine 2,3-Dioxygenase Activity. J Clin Med. 2020;9 doi: 10.3390/jcm9092913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu X, Wu D, Mu Y, Zhao Y, Ma Z. Serum-Free Medium Enhances the Therapeutic Effects of Umbilical Cord Mesenchymal Stromal Cells on a Murine Model for Acute Colitis. Front Bioeng Biotechnol. 2020;8:586. doi: 10.3389/fbioe.2020.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jin S, Yang C, Huang J, Liu L, Zhang Y, Li S, Zhang L, Sun Q, Yang P. Conditioned medium derived from FGF-2-modified GMSCs enhances migration and angiogenesis of human umbilical vein endothelial cells. Stem Cell Res Ther. 2020;11:68. doi: 10.1186/s13287-020-1584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sagaradze G, Grigorieva O, Nimiritsky P, Basalova N, Kalinina N, Akopyan Z, Efimenko A. Conditioned Medium from Human Mesenchymal Stromal Cells: Towards the Clinical Translation. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20071656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang FY, Chen R, Zhang X, Huang B, Tsang LL, Li X, Jiang X. Preconditioning Enhances the Therapeutic Effects of Mesenchymal Stem Cells on Colitis Through PGE2-Mediated T-Cell Modulation. Cell Transplant. 2018;27:1352–1367. doi: 10.1177/0963689718780304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pawitan JA, Bui TA, Mubarok W, Antarianto RD, Nurhayati RW, Dilogo IH, Oceandy D. Enhancement of the Therapeutic Capacity of Mesenchymal Stem Cells by Genetic Modification: A Systematic Review. Front Cell Dev Biol. 2020;8:587776. doi: 10.3389/fcell.2020.587776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang R, Huang H, Cui S, Zhou Y, Zhang T. IFN-γ promoted exosomes from mesenchymal stem cells to attenuate colitis via miR-125a and miR-125b. Cell Death Dis. 2020;11:603. doi: 10.1038/s41419-020-02788-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen Y, Song Y, Miao H, Xu Y, Lv M, Wang T, Hou Y. Gene delivery with IFN-γ-expression plasmids enhances the therapeutic effects of MSCs on DSS-induced mouse colitis. Inflamm Res. 2015;64:671–681. doi: 10.1007/s00011-015-0845-6. [DOI] [PubMed] [Google Scholar]
- 103.Shahriar A, Ghaleh-Aziz Shiva G, Ghader B, Farhad J, Hosein A, Parsa H. The dual role of mir-146a in metastasis and disease progression. Biomed Pharmacother. 2020;126:110099. doi: 10.1016/j.biopha.2020.110099. [DOI] [PubMed] [Google Scholar]
- 104.Wu H, Fan H, Shou Z, Xu M, Chen Q, Ai C, Dong Y, Liu Y, Nan Z, Wang Y, Yu T, Liu X. Extracellular vesicles containing miR-146a attenuate experimental colitis by targeting TRAF6 and IRAK1. Int Immunopharmacol. 2019;68:204–212. doi: 10.1016/j.intimp.2018.12.043. [DOI] [PubMed] [Google Scholar]
- 105.Mao F, Wang M, Wang J, Xu WR. The role of 15-LOX-1 in colitis and colitis-associated colorectal cancer. Inflamm Res. 2015;64:661–669. doi: 10.1007/s00011-015-0852-7. [DOI] [PubMed] [Google Scholar]
- 106.Kang J, Zhang Z, Wang J, Wang G, Yan Y, Qian H, Zhang X, Xu W, Mao F. hucMSCs Attenuate IBD through Releasing miR148b-5p to Inhibit the Expression of 15-lox-1 in Macrophages. Mediators Inflamm. 2019;2019:6953963. doi: 10.1155/2019/6953963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jiang Q, Huang K, Lu F, Deng S, Yang Z, Hu S. Modifying strategies for SDF-1/CXCR4 interaction during mesenchymal stem cell transplantation. Gen Thorac Cardiovasc Surg. 2022;70:1–10. doi: 10.1007/s11748-021-01696-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen Z, Chen Q, Du H, Xu L, Wan J. Mesenchymal stem cells and CXC chemokine receptor 4 overexpression improved the therapeutic effect on colitis via mucosa repair. Exp Ther Med. 2018;16:821–829. doi: 10.3892/etm.2018.6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zheng XB, He XW, Zhang LJ, Qin HB, Lin XT, Liu XH, Zhou C, Liu HS, Hu T, Cheng HC, He XS, Wu XR, Chen YF, Ke J, Wu XJ, Lan P. Bone marrow-derived CXCR4-overexpressing MSCs display increased homing to intestine and ameliorate colitis-associated tumorigenesis in mice. Gastroenterol Rep (Oxf) 2019;7:127–138. doi: 10.1093/gastro/goy017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cui J, Jia J. Natural COX-2 Inhibitors as Promising Anti-inflammatory Agents: An Update. Curr Med Chem. 2021;28:3622–3646. doi: 10.2174/0929867327999200917150939. [DOI] [PubMed] [Google Scholar]
- 111.Silva DN, Souza BSF, Azevedo CM, Vasconcelos JF, de Jesus PG, Feitoza MS, Meira CS, Carvalho GB, Cavalcante BR, Ribeiro-Dos-Santos R, Soares MBP. IGF-1-Overexpressing Mesenchymal Stem/Stromal Cells Promote Immunomodulatory and Proregenerative Effects in Chronic Experimental Chagas Disease. Stem Cells Int. 2018;2018:9108681. doi: 10.1155/2018/9108681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dave M, Hayashi Y, Gajdos GB, Smyrk TC, Svingen PA, Kvasha SM, Lorincz A, Dong H, Faubion WA Jr, Ordog T. Stem cells for murine interstitial cells of cajal suppress cellular immunity and colitis via prostaglandin E2 secretion. Gastroenterology. 2015;148:978–990. doi: 10.1053/j.gastro.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Karin N. CXCR3 Ligands in Cancer and Autoimmunity, Chemoattraction of Effector T Cells, and Beyond. Front Immunol. 2020;11:976. doi: 10.3389/fimmu.2020.00976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nitti M, Ivaldo C, Traverso N, Furfaro AL. Clinical Significance of Heme Oxygenase 1 in Tumor Progression. Antioxidants (Basel) 2021;10 doi: 10.3390/antiox10050789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yin M, Shen Z, Yang L, Zheng W, Song H. Protective effects of CXCR3/HO1 genemodified BMMSCs on damaged intestinal epithelial cells: Role of the p38MAPK signaling pathway. Int J Mol Med. 2019;43:2086–2102. doi: 10.3892/ijmm.2019.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sun D, Cao H, Yang L, Lin L, Hou B, Zheng W, Shen Z, Song H. MiR-200b in heme oxygenase-1-modified bone marrow mesenchymal stem cell-derived exosomes alleviates inflammatory injury of intestinal epithelial cells by targeting high mobility group box 3. Cell Death Dis. 2020;11:480. doi: 10.1038/s41419-020-2685-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Watkins DJ, Zhou Y, Matthews MA, Chen L, Besner GE. HB-EGF augments the ability of mesenchymal stem cells to attenuate intestinal injury. J Pediatr Surg. 2014;49:938–44; discussion 944. doi: 10.1016/j.jpedsurg.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wei J, Zhou Y, Besner GE. Heparin-binding EGF-like growth factor and enteric neural stem cell transplantation in the prevention of experimental necrotizing enterocolitis in mice. Pediatr Res. 2015;78:29–37. doi: 10.1038/pr.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen Y, Zuo J, Chen W, Yang Z, Zhang Y, Hua F, Shao L, Li J, Chen Y, Yu Y, Shen Z. The enhanced effect and underlying mechanisms of mesenchymal stem cells with IL-33 overexpression on myocardial infarction. Stem Cell Res Ther. 2019;10:295. doi: 10.1186/s13287-019-1392-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ni X, Ou C, Guo J, Liu B, Zhang J, Wu Z, Li H, Chen M. Lentiviral vector-mediated co-overexpression of VEGF and Bcl-2 improves mesenchymal stem cell survival and enhances paracrine effects in vitro. Int J Mol Med. 2017;40:418–426. doi: 10.3892/ijmm.2017.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hamza AA, Fikry EM, Abdallah W, Amin A. Mechanistic insights into the augmented effect of bone marrow mesenchymal stem cells and thiazolidinediones in streptozotocin-nicotinamide induced diabetic rats. Sci Rep. 2018;8:9827. doi: 10.1038/s41598-018-28029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]