Abstract
We hypothesized that bone marrow-derived mesenchymal stem cells (BM-MSCs) would have a possible role in the treatment of acute respiratory distress syndrome (ARDS). ARDS disease model was developed in Wistar albino male rats by intratracheal instillation of physiological saline solution. Anesthezied and tracheotomized rats (n = 8) with ARDS were pressure-controlled ventilated. Isolated and characterized rat (r-) BM-MSCs were labeled with GFP gene, and introduced in the lungs of the ARDS rat-model. After applying of MSCs, the life span of each rat was recorded. When rats died, their lung tissues were removed for histopathological examination. Also the tissue sections were analyzed for GFP labeled rBM-MSCs and stained for vimentin, CK19, proinflammatory (MPO, IL-1β, IL-6 and MIP-2) and anti-inflammatory [IL-1ra and prostaglandin E2 receptor (EP3)] cytokines. The histopathological signs of rat-model ARDS were similar to the acute phase of ARDS in humans. rBM-MSCs were observed to home in lung paranchyma. Although the infiltration of neutrophils slightly decreased in the interalveolar, peribronchial and perivascular area, a notable improvement was determined in the degree of hemorrhage, edema and hyaline membrane formation in rats treated with rBM-MSCs. Also decreased proinflammatory cytokines levels and increased the intensity of anti-inflammatory cytokines were established. Therefore MSCs could promote alveoar epithelial repair by mediating of cytokines from a proinflammatory to an anti-inflammatory response. As a novel therapeutic approach, mesenchymal stem cell treatment with intratracheal injection could be helpful in the management of critically ill patients with ARDS.
Keywords: Acute respiratory distress syndrome, Mesenchymal stem cell, Cytokine, Inflammation, Critically ill
Introduction
The acute respiratory distress syndrome (ARDS) is characterized by diffuse inflammation of the lung’s alveolar-capillary membrane in response to various pulmonary and extrapulmonary insults [1]. Pulmonary injury is occured by directly gastric aspiration, pneumonia, inhalational injury, pulmonary contusion or indirectly sepsis, trauma, pancreatitis, multiple transfusions of blood products mechanisms [2]. The pathologic features of ARDS, infiltration of polymorphonuclear neutrophil leukocytes (PMNL) into the lung alveoli, interstitial/intraalveolar edema, perivascular and/or intraalveolar hemorrhage and hyaline membrane (HM) formation are consistent with the effects of the complex interaction of inflammatory mediators on alveolar epithelial and capillary endothelial cells [3].
A recent systematic review suggests that the mortality for ARDS is ranging from 36 to 44 %. The current treatment modalities and new therapeutic approaches primarily focused on the resolution of pathologic features in ARDS. These treatments include glucocorticoids, surfactants, inhaled nitric oxide, antioxidants, protease inhibitors, and a variety of other antiinflammatory treatments. Unfortunately, to date none of these pharmacologic treatments has proven to be effective [4–7]. Recent experimental studies have demonstrated that rat bone marrow-derived mesenchymal stem cells (rBM-MSC) have the ability to participate in the repair of lung injury [8]. Mesenchymal stem cells, also called multi-potent mesenchymal stromal cells, can be isolated from a variety of human tissues including the bone marrow, adipose tissue and placenta. BM-MSCs are adult stem cells that are capable of differentiating into mesenchymal lineages including chondroblasts, osteoblasts, adipocytes, fibroblasts, and myofibroblasts [9]. BM-MSCs have been detected as type I and type II in alveolar epithelial cells, endothelial cells, fibroblasts, and bronchial epithelial cells [10]. Mesenchymal stem cells must express certain cell surface markers such as CD73, CD90, and CD105, but not express other markers including CD45, CD34, CD14, or CD11b [11].
In this study, we examined the effects of intratracheal delivery of rat bone marrow-derived MSCs on histology and lung inflammation in a rat model of ARDS with induced by intratracheal instillation of saline solution. We hypothesized that the administration of BM-MSCs might have beneficial functional effects related to the immunomodulatory properties of mesenchymal stem cells. Histological injury scores and proinflammatory cytokines included interleukin 6 (IL-6), macrophage inflammatory protein (MIP-2), MPO, IL-1β and antiinflammatory cytokines included IL-1ra and EP3, a prostoglandin E2 receptör, activity in lung homogenates were evaluated. The intratracheally route of administration was used to maximize the efficiency of delivery of rBM-MSC to the damaged lung tissue. The present results indicated that intrapulmonary treatment with MSC conferred a functional advantage in the rat model of ARDS, and the protective mechanism might be primarily mediated by antiinflammatory effects.
Material and Methods
Animals
Wistar albino male rats, weighing 250–300 g, were obtained from the Experimental Animal Research Center, Cukurova University Medical Faculty. The animals were kept in a temperature (21 ± 2 °C) and humidity (60 ± 5 %) controlled room in which a 12–12 h light–dark cycle was maintained. Animals were fed a standard rat chow diet, had access to water ad libitum, and were synchronized by the maintenance of controlled environmental conditions (light, temperature, feeding time, etc.). The experiments were performed in accordance with the guidelines for Animal Research from the National Institute of Health and were approved by the Committee on Animal Research at Cukurova University, Turkey.
Isolation and Culture of Mesenchymal Stem Cells
BM-MSC were generated from both femurs and tibiae of sacrificed rats under anesthesia. A 21-gauge needle inserted into the shaft of the bone and marrow was extruded by flushing with Dulbecco’s modified Eagle’s medium, DMEM (Biochrom, Berlin, Germany), supplemented with 10 % fetal bovine serum (GIBCO, Life Technologies, Paisley, UK), as well as 100 IU/ml penicilin and 100 μg/ml streptomycin (GIBCO, Life Technologies). The marrow suspension was dispersed by pipetting, successively filtered with a 70-μm mesh nylon filter (BD Biosciences, Bedford, MA, USA), and centrifuged at 200 × g for 10 min. The supernatant containing thrombocytes and erythrocytes was removed, and the cell pellet was resuspended in the medium. The cells from one rat were seeded onto two 25-cm2 plastic tissue culture flasks (BD Biosciences) and incubated at 37 °C in a humidified atmosphere containing 5 % CO2 for 3 days.
The mesenchymal stem cells were isolated on the basis of their ability to adhere to the culture plates. On the third day, red blood cells and other non-adherent cells were removed and fresh medium was added to allow further growth. The adherent cells were grown to 70 % confluency and were defined as passage zero (P0) cells. The P0 MSCs were washed with Ca2+–Mg2+ free phosphate-buffered saline (PBS) (GIBCO, Life Technologies) and detached by incubating with 0.25 % trypsin-EDTA solution (GIBCO, Life Technologies) for 5–10 min at 37 °C. Complete medium was added to inactivate the trypsin. The cells were centrifuged at 200 × g for 10 min, resuspended in 1 ml complete medium, counted manually in duplicate using a Thoma chamber, then plated as P1 in 75-cm2 flasks (BD Biosciences) at a density of 1 × 106 cells/flask. Complete medium was replaced every third day over a 10–14-day period. For each passage, the cells were plated similarly and grown to 70 % confluency.
Flow Cytometry
Undifferentiated rBM-MSCs were subjected to flow cytometry analysis. After 3rd passage (P3), stem cells were harvested. Flow cytometry was performed using a FACSCalibur (BD Biosciences, San Jose, CA). Immunophenotyping of rBMDMSCs was performed with antibodies against rat antigens CD29, CD45 and CD90 (BD Biosciences, San Diego, USA).
GFP Labeling of rBM-MSCs
The plasmid was supplied from Clontech (Palo Alto, CA), amplified in E.coli strain XL-1 and purified by endotoxin free plasmid isolation kit (Qiagen Endofree Plasmid Maxi kit, Hilden, Germany). On plasmid GFP coding gene was inserted downstream of the CMV (murine leukaemia virus) constitutive promoter.
The plasmid was transfected by Neon Transfection System (Invitrogen/Life Technologies). 2 × 105 cells were mixed with 1 μg plasmid DNA in 10 μl transfer buffer. Following transfection parameters were used: 990 V, 40 ms, 2 pulse. The transformed cells were cultured in DMEM-medium supplied with 10 % FBS. After 48 h, the cells were selected according to their resistance toward G418 (400 μg/ml) for 6 weeks. After that the cells were observed for their GFP stability by continuous culturing for 4 passages, and the number of GFP+ cells was counted in flow cytometer. The integration of GFP gene into genome was checked by Real-Time PCR, and copy number of GFP in genome was determined.
In Vivo Experimental Design and Cell Transplantation
Rats were anesthezied with 1.25 mg/kg body weight xylazine (Rompum, Bayer, Brazil, 2 % solution) and 80 mg/kg body weight ketamine (Ketalar, Pfizer, Luleburgaz, Turkey, 50 mg/ml) intraperitoneally and instrumented in a manner previously described by German and Häfner et al. [12, 13]. A catheter was placed into one carotid artery for blood gas parameters, partial arterial oxygen pressure (PaO2) and partial arterial carbon dioxide pressure (PaCO2). A tracheostomy was performed, and the trachea was canullated with polyethylene tubing, ID 1.5 mm. They were pressure-controlled ventilated (Evita 4 Neoflow, Dräger, Germany) with 100 % oxygen at a respiratory rate of 30 breaths/min, inspiration–expiration ratio of 1:2, peak inspiratory pressure of 15 cmH2O at positive end-expiratory pressure (PEEP) of 2 cmH2O. At the starting of the experiment, PaO2 and PaCO2 were evaluated under the described ventilatory settings. Before lavage, the peak inspiration pressure (PIP) was raised to 28 cmH2O and PEEP to 8cmH2O. Rat lung parenchyma lavage was applied with 5 × 6 ml of physiological saline solution per animal every half an hour through 210 min (10,11). The ventilation setting was not changed during the whole experimental period. After the development of the disease model, the animals were rested for 2 h more at room temperature while they were still connected to ventilator. All rats with ARDS were assigned to two groups: Group 1 (n = 10) control group: Given no treatment, group 2 (n = 8): rBM-MSCs group. 750,000 cells in 0.2 ml of DMEM (without phenol red, FBS, or any antibiotics) were transplanted intratracheally. Rats were kept on the ventilator as long as they survived and the life span of each rat was recorded. When they died, their both lungs were excised and examined histopathologically.
Light Microscopic Examination
Lung tissues were fixed in 10 % neutral buffered formalin and embedded in paraffin. Sections of tissue were cut at 5 μm, mounted on slides, stained with hematoxylin-eosin (H-E). The sections were examined by Olympus conventional CX21 light microscope. Grading was performed with respect to the severity of the histopathological features as grade 0 (clear lung paranchyma), grade 1 (25 % of lung paranchyma), grade 2 (50 % of lung paranchyma), grade 3 (75 % of lung paranchyma) and grade 4 (100 % of lung paranchyma) [12]: Hyaline membrane (HM) formation, margination and infiltration of polymorphonuclear neutrophil leukocytes (PMNL) into the lung alveoli, interstitial/intraalveolar edema and perivascular and/or intraalveolar hemorrhage. Slides were coded and evaluated without any knowledge of the sacrifice time.
Immunohistochemical Examination
To localize the GFP labeled rBM-MSCs after injection, immunofluorescence double staining protocol was performed on the paraffin-embedded tissues. Slides were deparaffinized with two changes of xylene for 5 min each and rehydrated in a series of graded alcohol solutions. Endogenous peroxidases were inhibited by incubation with fresh 3 % H2O2 in PBS buffer. The sections were then treated with a trypsin solution in a moist chamber at 37 °C for 10 min. DNA was denatured by incubation with the denaturing solution. Nonspecific staining was blocked with the mixture of two different sera at 1.5 % in PBS for 30 min at room temperature (RT). Afterwards, the sections were incubated in a mixture of two primary antibodies in a pairwise fashion. For the immunostaining, the following antibodies were supplied from Santa Cruz Biotechnology (Heidelberg, Germany): GFP (sc-9996), Vimentin (sc-7557), CK19 (sc-33119), EP3 (sc-16019), MPO (sc-34161), IL1ra (sc-25444), IL1b (sc-23460), IL6 (sc-1265) and MIP2 (sc-1388). After 2 h of incubation with antibody mixture at RT, the sections were later incubated with appropriate secondary antibodies for 30 min at RT and were mounted with mounting medium containing DAPI (Santa Cruz Biotechnology). The cells were investigated under fluoresans microscope (Leica DMI 4000B, Wetzlar, Germany). The number of positive cells in samples were determined by counting the stained cells per area (294 mm2 each) in 5 randomly selected sections on slide and normalized to total area.
Electron Microscopic Examination
The tissues of 1 mm3 thickness were immediately placed in 5 % glutaraldehyde buffered at pH 7.4 with Millonig phosphate buffer for 4 h. Samples were subsequently fixed in 1 % osmic acid for 2 h. After dehydration in acetone, they were embedded in araldite. Ultrathin sections were stained with uranyl acetate and lead citrate and were examined in a Jeol JEM 1400 electron microscope.
Statistical Analysis
A computer program (SPSS 11.0) was used for statistical analysis. Histopathological grading were expressed as means ± standard deviation (SD). While differences among the groups were detected, group means were compared with the using of the Mann–Whitney U-test. The statistical analysis of cells expressing proinflammatory cytokines was performed using paired t-test. Differences between the experimental and control groups were regarded as statistically significant when p < 0.05 and highly significant when p < 0.01.
Results
Culture of rBM-MSC
MSCs attached to the culture flasks sparsely and displayed a fibroblast-like, spindle-shaped morphology during the initial days of incubation. Following 3–4 days of incubation, proliferation started and the cells gradually grew into small colonies (Fig. 1a). During culture, adjacent colonies interconnected with each other, and a monolayer confluence was obtained after 12–15 days of incubation. In later passages, MSCs exhibited large, flattened or fibroblast-like morphology (Fig. 1b–d) and did not change throughout 25 passages. Tests for bacterial and mycoplasm contamination were negative and the viability was higher than 95 %. rBM-MSCs expressed CD29, CD54 and CD90, but not CD45, CD106 (Fig. 1e) and maintained their phenotype in the following passages.
Fig. 1.
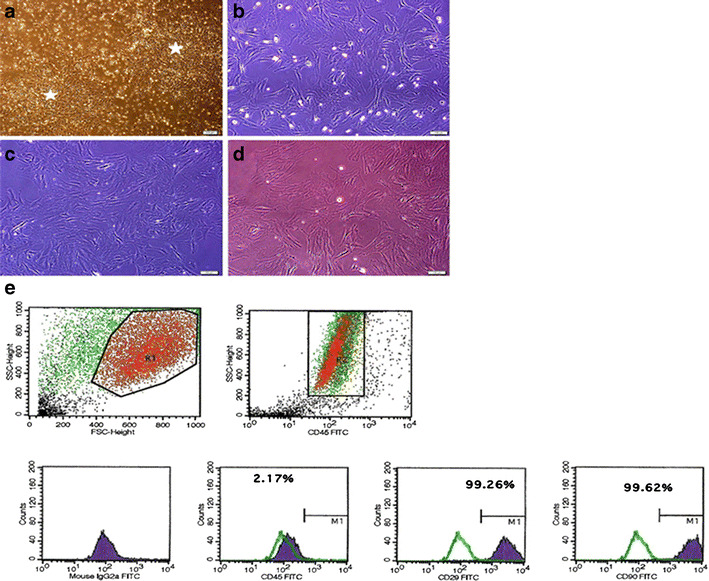
Morphological and phenotypic characteristics of rBM-MSCs. The cells isolated from rat bone-marrow exhibited large, flat fibroblast-like morphology during the onset of culture: a (P0)—7th day, b P1—3rd day, c P2—4th day and d P3—5th day. A representative flow cytometric analysis of cell-surface markers of rBM-MSCs at P3; e cells were labeled with antibodies against hematopoietic (CD45) and MSC markers (CD29, CD90). (Green line: histogram of isotype control immunoglobulin)
Functionality Tests
At the beginning of the experiment, the mean blood PaO2 and PaCO2 levels were 350.34 ± 75.69 and 52.16 ± 5.89 mmHg before lavage, respectively. Directly after lavage (210 min after lavage), PaO2 decreased to 57.55 ± 12.10 mmHg and PaCO2 increased to 73.27 ± 5.60 mmHg (Table 1).
Table 1.
Arterial blood gas parameters of control group
| Before beginning of the experiment | 210 min after lavage | |
|---|---|---|
| PaO2/PaCO2 | PaO2/PaCO2 | |
| Minimum | 255.40/40.80 | 35.40/62.70 |
| Maximum | 515.80/59.60 | 71.20/78.90 |
| Mean ± SD | 350.34 ± 75.69/52.16 ± 5.89 | 57.55 ± 12.10/73.27 ± 5.60 |
Histological Examination
In light microscopic examination, control group had similar histopathological variables as infiltration of polymorphonuclear neutrophil leukocytes, interstitial/intraalveolar edema, perivascular and/or intraalveolar hemorrhage and hyaline membrane formation which is occuring during the acute phase of ARDS in humans (Fig. 2, control group). Electron microscope (EM) examination depicted thick basal lamina and increased intracytoplasmic vacualization of type II pneumocyte (Fig. 3). The grading of histopathological variables, median and means ± standard deviation (SD) values were presented in Table 2.
Fig. 2.
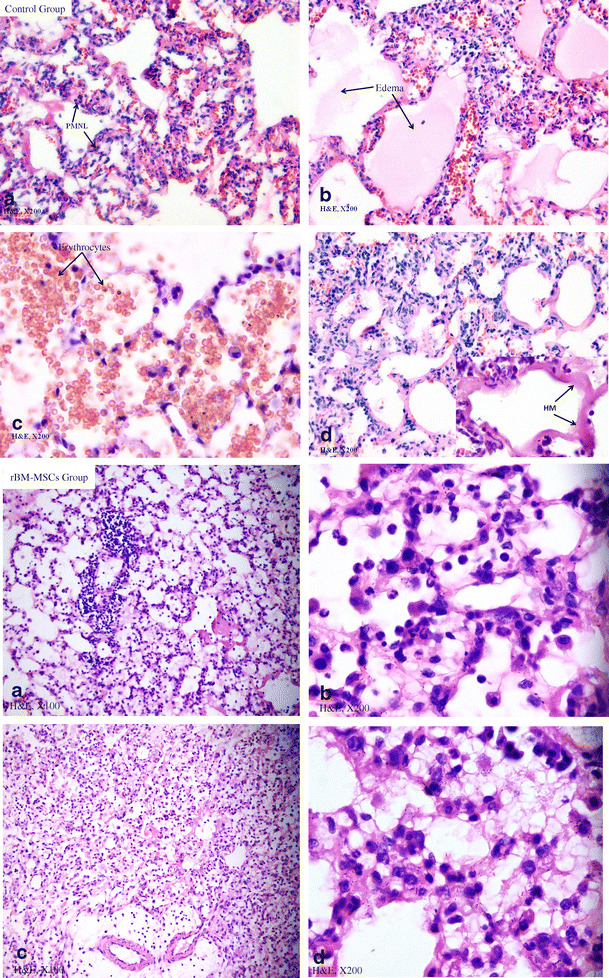
Light misroscopic examination. Control Group (H&E ×200): a Infiltration of polymorphonuclear neutrophil leukocytes into the lung alveoli. b Interstitial and intraalveolar edema. c Perivascular and/or intraalveolar hemorrhage. d Hyaline membrane formation. rBM-MSCs Group: a, c (H&E ×100); b, d (H&E ×200), showing histopathological signs as prominently decreased interstitial/intra-alveolar edema, perivascular and/or intra-alveolar hemorrhage and hyaline membrane formation. In contrast, the infiltration of PMNL was slightly diminished
Fig. 3.
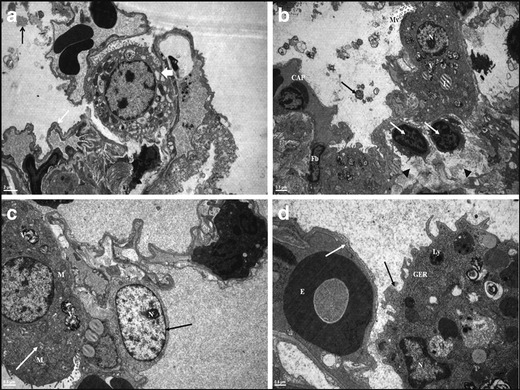
Electron microscopic examination: in control group: a Granular and fibrous dens stractures are seen in alvoalar space (black arrow). Notice thick basal lamina on blood–air barrier area (white arrow) and increased intracytoplasmic vacualization of type II pneumocyte (thick white arrow). In rBM-MSCs study group: (b, c, d). b Electron dense lameller bodies in alveolar space (black arrow). Short and thick microvilli (Mv) on the apical surface of type II pneumocyte, fibroblast (Fb), type I capillary (CAP), increased lymphocytes (white arrows) and edema (black arrow head) in the interalveolar septum are displayed. c The normal structure of type I (black arrow) and type II pneumocyte (white arrow). d Thick basal lamina (white arrow). Macrophage in the alveolar space located nearby the alveolar wall (black arrow). Hyaline membrane (HM), Alveolar space (A), Erytrocyte (E), Nucleus (N), Granular Endoplasmic Reticulum (GER), Mitocondrium (M), Lysosome (Ly), Vesicule (V), Lipid droplets (L)
Table 2.
Median, mean ± SD values and scores of histopatological signs in acute phase of ARDS
| Groups | Grading | Median (range) | Mean ± SD | |||||
|---|---|---|---|---|---|---|---|---|
| 0 (right/left) | 1 (right/left) | 2 (right/left) | 3 (right/left) | 4 (right/left) | n | |||
| Control group | ||||||||
| PMNL | −/− | −/− | 2/1 | 6/7 | 2/2 | 10/10 | 3.0 | 3.0 ± 0.6 |
| Edema | −/− | −/− | 0/1 | 8/4 | 2/5 | 10/10 | 3.0 | 3.3 ± 0.5 |
| Hemorrhage | −/− | −/− | −/− | 4/5 | 6/5 | 10/10 | 4.0 | 3.5 ± 0.5 |
| HM | −/− | −/− | 2/3 | 5/3 | 3/4 | 10/10 | 3.0 | 3.1 ± 0.7 |
| Group 2 | ||||||||
| PMNL | 0/0 | 1/0 | 2/0 | 4/6 | 1/2 | 8/8 | 3.0 | 2.9 ± 0.7 |
| Edema | 1/2 | 1/1 | 5/5 | 1/0 | 0/0 | 8/8 | 2.0 | 1.5 ± 0.8a |
| Hemorrhage | 8/8 | 0/0 | 0/0 | 0/0 | 0/0 | 8/8 | 0.0 | 0.0 ± 0.0a |
| HM | 1/0 | 1/1 | 5/5 | 0/1 | 1/1 | 8/8 | 2.0 | 2.0 ± 0.9b |
a p < 0.001 compared to control group
b p = 0.002 compared to control group
The life spans of rats were 3, 4, 5, 12 and 24 h in 2, 2, one, one and 2 rats, respectively in rBM-MSCs group. Histopathologic examinations showed that rBM-MSCs-treated rats had significantly less injury compared with control group. Although the mean severity of infiltration of PMNL in rats received rBM-MSCs was lower than that of control group, we did not found significant difference between control and rBM-MSCs group according to the infiltration of PMNL (p = 0.766). Rats injected with rBM-MSCs also had a significant improvement in the degree of hemorrhage and edema as assessed by the grading of lung injury. The mean severity of interstitial/intraalveolar edema (1.5 ± 0.8) decreased in cell treated rats statistically. There was a significant difference in rats with rBM-MSCs according to the interstitial/intra-alveolar edema (p < 0.001). The disappearing of hemorrhage was noticed in MSCs group. The perivascular and/or intra-alveolar hemorrhage were not detected in this group (p < 0.001). In addition, the mean severity of hyaline membrane formation was decreased from 3.1 ± 0.7 to 2.0 ± 0.9 in group 2 (Table 2). After administration of rBM-MSCs, we found a noticable difference between control and mesenchymal stem cell injected group for decreasing of HM (p = 0.002) (Fig. 2, rBM-MSCs group).
Detection of GFP+ Cells in Lung Parenchyma
In the delivery of stem cells, the intratracheal route was of choice due to the lung’s unique accessibility via the airways. This method provided piovital advantage of local administration, enhancing the number of cells that reach the target site. Tissues were subsequently screened for the presence of GFP+ MSCs focusing on the homing and differentiation events by immunohistochemistry. MSCs expressing GFP were seen scattered through the lungs of all rats treated with MSCs, and the cells were discovered in interalveolar, peribronchial and perivascular area (Fig. 4, upper). Additionaly the localization of rBM-MSCs of different rats was seen in the interalveolar septae (Fig. 4, middle). We also detected GFP-expressing cells with epithelial morphology in the lung of recipient animals, which are expressing CK19 (Fig. 4, bottom).
Fig. 4.
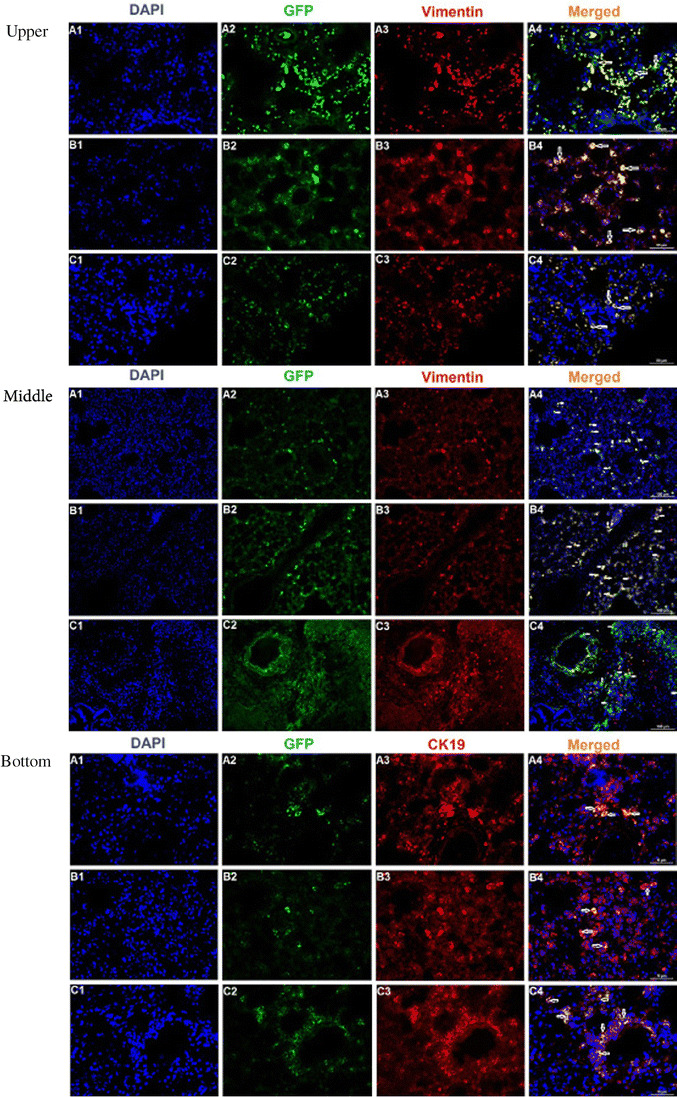
Upper: GFP labeled rBM-MSCs in rat lung tissue after transplantation. rBM-MSCs were labeled with GFP and injected intratracheally into rats. Immunofluorescence of paraffin lung sections examined by flouresans microscopy revealing colocalization of BM-MSCs (green) and the MSCs marker vimentin (red) together with nuclear staining (4′,6-diamidino-2-phenylindole [DAPI], blue). GFP+ MSCs (green) were located in perivascular, peribronchial and interalveolar area (indicated by arrows) (A4–C4). Those cells were also positive for MSC marker, vimentin (red) (Scale Bars: 100 μm). Middle: Localization of GFP + rBM-MSCs in lung tissue samples of different rats. GFP+ cells (white arrows; green fluorescence) could be observed in the interalveolar septae (A2, B2, C2, A4, B4, C4). The labeled cells (white arrows) could also be located in both peribronchial and perivascular areas of the lung. Some of these cells (red arrow) were observed positive for vimentin (red fluorescence) (C4) (Scale bars: 100 μm). Bottom: Immunocytochemical expression patterns for GFP (green) and CK19 (red) were colocalized in the cytoplasm of epithelial cells (visualized as orange/yellow fluorescence in merge panel). These GFP+ cells had not only acquired proper epithelial morphology, but it also stained positive for specific antigens such as CK19. In addition, specific GFP+ cells were observed within bronchiolar epithelial cells (Scale bars: 100 μm)
Protective Effects of rBM-MSC In Vivo
Because of the discrepancy between the therapeutic benefit and the low rate of MSC engraftment and accumulating evidence in the literature suggesting a paracrine activity of MSCs, we explored the potential protective effect of rBM-MSCs in in vivo assays. After rBM-MSC transplantation into the tissue, the reduction in the immune response was observed, assesed by the immunofluoresence staining. For these purpose, the lung paraffin sections were analyzed for the expression of anti-inflammatory (EP3, IL1ra) and proinflammatory (ILβ1, IL-6, MIP-2, and MPO) cytokines. The expression EP3 and IL1ra were significantly increased in the tissue of MSCs injected group, whereas the same expression in control group was weak. The level of these membrane proteins was assessed by the staining intensity of the MSCs. The immune regulation of rBM-MSC was not limited with the expression of anti-inflammatory cytokines, but also the proinflammatory cytokines’ expression was also suppressed by the activity of rBM-MSC. The reduction in ILβ1, IL-6, MIP-2 and MPO expressions was analyzed by counting the positive cells on the randomly selected sections on paraffin sections. Increased cytokine levels in rats with ARDS were significantly attenuated by MSCs administration (Fig. 5, a). The average ratios of proinflammatory cytokines, IL-1β and IL-6, positive cells were increased considerably in ARDS rat-models, 61.3 % and 31.3 % respectively. After the administration of MSCs, those ratios were significantly decreased in both left (26.3 % and 5.6 %, respectively) and right (12.6 % and 6.6 %, respectively) lungs (Fig. 9). The production of MIP-2 and MPO was observed to increase in the sections of rat-models without cell injection, 41.6 % and 78.6 % respectively. Those levels were reduced after treatment with MSCs to 13.6 % & 15 % for left lungs and 5 % & 10.6 % for right lungs, respectively (Fig. 5, b).
Fig. 5.
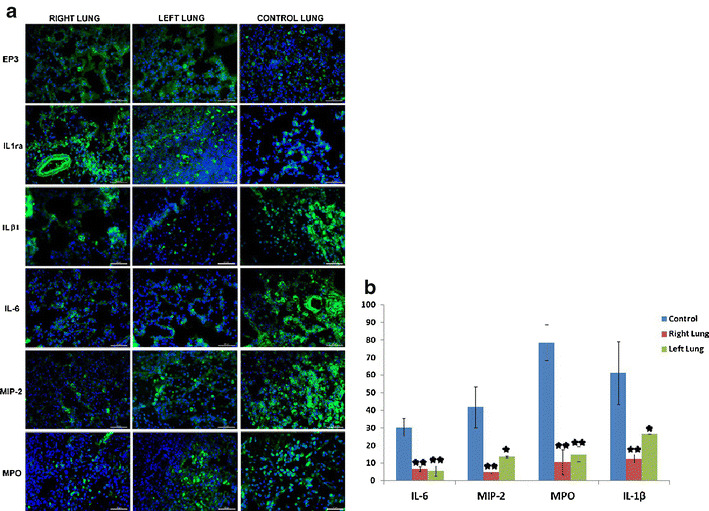
a: The expression of anti-inflammatory (EP3, IL-1ra) and proinflammatory (IL-1β, IL-6, MIP-2, and MPO) cytokines in control and MSCs transplanted lung parafin sections. The expression levels of EP3 and IL1ra (membranous) were increased in the tissues of rBM-MSCs group with respect to those of the control group. In contrast, the expressions of proinflammatory cytokins, IL-1β, IL-6, MIP-2 and MPO were reduced in rBM-MSCs group. b: The number of cells expressing proinflammatory cytokines (IL6, MIP-2, MPO and IL-1β) decreased in the group transferred with MSCs compared with control group (*p < 0.05, **p < 0.01)
Discussion
ARDS is a clinically important complication of severe acute lung injury (ALI) and a significant cause of morbidity and mortality in critically ill patients. Previous studies indicated that MSCs may be valuable as a new and investigational therapy in the treating of acute lung injury [8–15]. Our aim was to investigate the effects of MSCs in rat-model ARDS. In our rat-model ARDS performed by instillation of saline solution into rat lungs, the severity of inflammatory responses presented with the infiltration of polymorphonuclear neutrophil leukocytes (PMNL) into the lung alveoli, interstitial/intraalveolar edema, perivascular and/or intraalveolar hemorrhage and hyaline membrane (HM) formation was similar to that of human ARDS. We found two remakable findings related to the beneficial effect of MSC delivered by intratracheally. One important finding of our study was that the reduced intensity of pathological findings was observed with histopathologic examination.
The releasing of cytokines caused by neutrophils contributes to alveolar capillary endothelial injury resulted in the leakage of fluid and macromolecules. Therefore increased vascular permeability is the most important initial cause of ALI/ARDS and related to the outcome [4, 16]. The infiltration of neutrophils into the lung alveoli was slightly decreased in rBM-MSC group compare with rats given any treatment in our study. But we focused on rat-model of ARDS that intratracheal administration of rBM-MSCs was effective at reducing alveolar edema. Gupta et al. reported that MSCs improved significantly alveolar edema and hemorrhage in mice with ALI [14]. Moreover, we found that rBM-MSCs attenuated prominently perivascular and/or intraalveolar hemorrhage. Xu et al. found that intravenous administration of syngeneic MSC prevented endotoxin-induced pulmonary inflammation, injury and edema as well as the influx of neutrophils into the injured alveoli [17]. Another study showed that MSCs blocked the recruitment of lymphocytes and neutrophils into the injured lung [10]. Although reduced alveolar edema and erythrocyte extravasation to perivascular and/or intraalveolar area were seen in rats with different life spans, the prominent reduction of alveolar edema and hemorrhage was found mostly in rats survived 12 and 24 h after delivering of MSC intratracheally. Attenuated alveolar edema could be explained that the epithelial sodium channel and Na-K ATPase in type II pneumocytes as candidate formations for endothelial permeability were modulated with keratinocyte growth factor secreted by MSCs [18]. The certain function of MSCs on alveolar barrier integrity needs to be further investigated.
Despite MSCs engraftment had a high opinion of importance in the past, the growth factors and antimicrobial peptides secreted by MSC have been emphasizing as the modulating of immune responses in the injuried endothelium or epithelium in recent studies [10, 15, 19]. In our observation, different engraftment levels of rBM-MSCs provided the same favorable result in the decreasing of alveolar edema and hemorrhage. In different studies, the significant histological improvement was found following MSC administration in mice with ALI despite a level of <5 % of MSC engraftment [10, 14]. It could be thought that the engraftment level of MSCs might be independent of their ability to have an influence in damaged alveolar area. In acute phase of ARDS, prominent hyaline membranes caused by dysregulation of surfactant production is occured in the alveoli as well as alveolar edema [1, 4]. After applying of MSCs, the ameliorating of lung injury in terms of diminished hyaline membrane formation was noteworthy in our study. Taken together, these published findings supported our current findings that MSCs led to an affirmative result with the reduction of alveolar edema, hemorrhage and hyaline membrane formation in rBM-MSCs group.
The second finding of our investigation was that rBM-MSCs could be effective in the modulating of inflammatory conditions. Various studies demonstrated that MSC displayed their therapeutic benefits by paracrine regulation with growth factors and cytokines for promoting of vascular repair in the disease-associated situation [20–24]. Two hours after the performing of ARDS, the injection of MSCs was likely to prevent immune cell activation and in particular, to reduce the secretion of proinflammatory cytokines included IL-6, MIP-2, MPO and IL-1β as possible direct markers of lung inflammation in our study. IL-6 induced by PGE2 has a major role in the inflammatory conditions [25]. The present study notified that the levels of pro-inflammatory cytokines including IL-6 and MIP-2 were consistently attenuated by rBM-MSCs transplantation in rats with ARDS. Here, we also demonstrated reduced lung MPO activity, which is an indicator of neutrophil accumulation or activity, resulting from intratracheal rBM-MSCs transplantation into rats with ARDS. Our results clearly stated an association between the attenuation of pathological findings and anti-inflammatory effects of rBM-MSCs. It was shown that MSCs administration significantly normalized lung edema, the levels of TNF-α, IL-6, IL-1β, MIP-2 and neutrophil count in the bronchoalveolar lavage and MPO content in the lung tissue in rats treated with MSCs given by intravenously or intratracheally [27–29]. In addition we found that the number of cells expressing IL-1β was decreased prominently in rats given MSCs compare with those of receiving any treatment. Geiser et al. stated that IL-1β is one of the major inflammatory cytokines in pulmonary edema fluid in patients with ALI/ARDS [26]. In a model of endotoxin induced lung injury, the study indicated that intrapulmonary MSC improved survival and lung injury in association with a decrease in MIP-2 and TNFα levels in the bronchoalveolar lavage fluid [14, 17]. Despite lower levels of MIP-2 in the rBM-MSCs treated rats, the infiltration of neutrophils in the interalveolar, peribronchial and perivascular area was not attenuated by MSCs in our investigation. It could be explained that MSCs was not dependent on the reduction in neutrophil migration to the lung. Moreover rBM-MSCs delivered 2 h after the occurence of ARDS model could not affect the neutrophil movement. In our investigation, rats survived in a different period of time showed that rBM-MSCs lessened apparently the levels of IL-6, MIP-2, MPO and IL-1β in the lung tissue. These findings indicated that MSCs could be a therapeutic approach as a potent immunosuppressive for the attenuating of histopathological signs of ARDS.
In the guiding of these results, it could be made a sensation whether MSCs led to increase the anti-inflammatory cytokines. Nemeth et al. demonstrated that anti-inflammatory mediators like IL-10, IL-1ra and IL-13 increased after MSC treatment [30]. IL-1ra inhibits IL-1 that attracts neutrophils, macrophages, and lymphocytes resulting in tissue inflammation [31] and directly enhances epithelial cell survival [32]. Our results showed that rBM-MSCs administration was found to be effective for reducing of the intensity of IL-1ra compare with rats did not have any treatment. Ortiz et al. found that a subpopulation of mouse MSC produced IL-1ra that was capable of attenuating the severity of bleomycin-induced lung injury [10]. It was recently found that MSCs constitutively produce prostoglandin E2 (PGE2). PGE-2 is synthesized from arachidonic acid by cyclooxygenase (COX) enzymes COX-1 and COX-2. Four different PGE2 receptors exist, EP1, EP2, EP3 and EP4 [33, 34]. Bouffi et al. demonstrated that IL-6-dependent PGE2 might act locally by inhibiting the proliferation of immune cells in the synovium thereby reducing local inflammation [23]. In a different study performed on motor neurons, Lu et al. found that EP3 protected motor neurons from chronic glutamate toxicity [35]. In rats received MSCs, we demonstrated that the increased intensity of EP3 was correlated with the immunosuppressive activity of MSCs. Our results were consistent with resent investigations reported that MSCs induced anti-inflammatory effect. MSC-derived PGE2 was reported to act on macrophages, increasing their IL-10 secretion and reducing inflammation. IL-10 seemed to prevent neutrophils from migrating into tissues and causing oxidative damage, thus mitigating multiorgan damage [33]. Attenuated pathological signs of ARDS could be attributed to this immunosuppressive effect of MSCs in our study. Results from our analysis presented that hyaline membrane formation was decreased by MSCs transplantation. Recent data from both animal and human studies suggest that the reduced levels of critical surfactant apoproteins are present in the lung with ARDS. Surfactant protein A levels were decreased by inflammatory reaction in the injured animals and the pulmonary COX-2 expression is increased. Although our available data do not allow for a complete understanding of how hyaline membrane formation was attenuated by rBM-MSCs, it could be explained that increased of EP3 receptor expression caused by MSCs had a beneficial effect in the injured lung tissue. Also PGE2 has been shown to inhibit fibroblast proliferation and collagen secretion [36]. Also it could be inferred from latter study that MSCs might be effective in chronic phase of ARDS. Taken all together, the anti-inflammatory effect of MSCs should be pointed out by potential decrease of proinflammatory and increase of anti-inflammatory cytokines.
The different routes of MSC administration were presented in various studies. A beneficial effect of local administration of stem cells has been shown in a study of myocardial infarction [37]. A study performed in mice with endotoxin-induced ALI reported that intrapulmonary (via the trachea) treatment with MSCs improved survival and reduced pulmonary edema formation, although the exact mechanisms of benefit were not identified in the study [17]. In other studies, it was shown that the intravenous administration of MSC ameliorated the lung inflammation in a mouse model of bleomycin-induced lung injury and fibrosis [10, 11]. In our study, the intratraceal administration of rBM-MSCs was found a promising alternative treatment in ARDS. Although the underlying mechanisms need to be investigated, a growing body of data suggests that systemic infusion of MSCs may be used as an immunosuppressive treatment in inflammatory diseases.
Our study has some limitations. The present experiment could not be designed to test the efficacy of MSCs with considering of the dose, administration time and underlying pulmonary or extrapulmonary insults in the inflammatory lung tissue. Also the difference between intravenous and intratracheally routes could not be addressed in our study. In addition, since all tracheotomized rats had to be on ventilator to survive during the whole experimental period, rats with ARDS could not be followed in their natural environmental conditions after MSCs transplantation.
Conclusion
The result achieved from our study demonstrated a significant beneficial effect of intratracheal delivery of MSCs by mediating of cytokines from a proinflammatory to an anti-inflammatory response in spite of different life spans of rats and engraftment levels of MSCs. Even though many questions remain to be addressed, MSCs could provide better ways to control and optimize the immune response for inflammation-induced injuries. Therefore MSCs could permit the good clinical management in the acute phase of ARDS and probably could improve outcome. Moreover MSC therapy may potentially increase the quality of life in terms of decreasing the duration of mechanical ventilation and hospitalization in critically ill patients with ARDS. To confirm this hypothesis, there is a need to provide further study for the interpretation of results and the manner in which research should be conducted in clinical practice.
Acknowledgment
This work was supported by the Cukurova University Research (TF2011BAP20) Fund. The authors thank Kezban Bostaner, Recep Mutlu and Gizem Turaç for their technical assistance. We also express our gratitude to Dr. Gökhan Duruksu for editing of this manuscript.
Conflict of Interest Statement
None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Contributor Information
Sema Yilmaz, Email: semayilmaz@hotmail.com.
Nihal Inandiklioglu, Email: nihal.inan@hotmail.com.
Dincer Yildizdas, Email: dyildizdas@cu.edu.tr.
Cansu Subasi, Email: cansusbs@hotmail.com.
Arbil Acikalin, Email: arbilavci@yahoo.com.
Yurdun Kuyucu, Email: yurdunkuyucu01@yahoo.com.tr.
Ibrahim Bayram, Email: miharbibay@hotmail.com.
Ali Topak, Email: flashgordon_83@hotmail.com.
Atila Tanyeli, Email: atanyeli@gmail.com.
Gokhan Duruksu, Email: gokhanduruksu@gmail.com.
Erdal Karaoz, Email: ekaraoz@hotmail.com.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. The New England Journal of Medicine. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Bernard GR, Artigas A, Brigham KL, et al. Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee. Intensıve Care Medicine. 1994;20(3):225–232. doi: 10.1007/BF01704707. [DOI] [PubMed] [Google Scholar]
- 3.Tomashefski JF., Jr Pulmonary pathology of acute respiratory distress syndrome. Clinics in Chest Medicine. 2000;21(3):435–466. doi: 10.1016/S0272-5231(05)70158-1. [DOI] [PubMed] [Google Scholar]
- 4.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annual Review of Pathology. 2011;6:147–163. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dushianthan A, Grocott MP, Postle AD, Cusack R. Acute respiratory distress syndrome and acute lung injury. Postgraduate Medical Journal. 2011;87(1031):612–622. doi: 10.1136/pgmj.2011.118398. [DOI] [PubMed] [Google Scholar]
- 6.Calfee CS, Matthay MA. Nonventilatory treatments for acute lung injury and ARDS. Chest. 2007;131(3):913–920. doi: 10.1378/chest.06-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cepkova M, Matthay MA. Pharmacotherapy of acute lung injury and the acute respiratory distress syndrome. Journal of Intensive Care Medicine. 2006;21(3):119–143. doi: 10.1177/0885066606287045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rojas M, Xu J, Woods CR, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. American Journal of Respiratory Cell and Molecular Biology. 2005;33(2):145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 10.Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proceedings of the National Academy of Sciences. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel SA, Sherman L, Munoz J, Rameshwar P. Immunological properties of mesenchymal stem cells and clinical implications. Archivum Immunologiae et Therapiae Experimentalis. 2008;56(1):1–8. doi: 10.1007/s00005-008-0001-x. [DOI] [PubMed] [Google Scholar]
- 12.Germann PG, Häfner D. A rat model of acute respiratory distress syndrome (ARDS): part 1. Time dependency of histological and pathological changes. Journal of Pharmacological and Toxicological Methods. 1998;40(2):101–107. doi: 10.1016/S1056-8719(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 13.Häfner D, Germann PG. A rat model of acute respiratory distress syndrome (ARDS) part 2, influence of lavage volume, lavage repetition, and therapeutic treatment with rSP-C surfactant. Journal of Pharmacological and Toxicological Methods. 1999;41(2–3):97–106. doi: 10.1016/S1056-8719(99)00025-8. [DOI] [PubMed] [Google Scholar]
- 14.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. Journal of Immunology. 2007;179(3):1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 15.Lee JW, Gupta N, Serikov V, Matthay MA. Potential application of mesenchymal stem cells in acute lung injury. Expert Opinion on Biological Therapy. 2009;9(10):1259–1270. doi: 10.1517/14712590903213651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinclair DG, Braude S, Haslam PL, Evans TW. Pulmonary endothelial permeability in patients with severe lung injury. Clinical correlates and natural history. Chest. 1994;106(2):535–539. doi: 10.1378/chest.106.2.535. [DOI] [PubMed] [Google Scholar]
- 17.Xu J, Qu J, Cao L, et al. Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. The Journal of Pathology. 2008;214:472–481. doi: 10.1002/path.2302. [DOI] [PubMed] [Google Scholar]
- 18.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proceedings of the National Academy of Sciences. 2009;106(38):16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. Journal of Biological Chemistry. 2010;285(34):26211–26222. doi: 10.1074/jbc.M110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart DJ, Mei SH. Cell-based therapies for lung vascular diseases: lessons for the future. Proceedings of the American Thoracic Society. 2011;8(6):535–540. doi: 10.1513/pats.201105-035MW. [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 22.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 23.Bouffi C, Bony C, Courties G, Jorgensen C, Noël D. IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS One. 2010;5(12):e14247. doi: 10.1371/journal.pone.0014247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA. Concise review: mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells. 2011;29(6):913–919. doi: 10.1002/stem.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takaoka Y, Niwa S, Nagai H. Interleukin-1beta induces interleukin-6 production through the production of prostaglandin E (2) in human osteoblasts, MG-63 cells. Journal of Biochemistry. 1999;126(3):553–558. doi: 10.1093/oxfordjournals.jbchem.a022485. [DOI] [PubMed] [Google Scholar]
- 26.Geiser T, Atabai K, Jarreau PH, Ware LB, Pugin J, Matthay MA. Pulmonary edema fluid from patients with acute lung injury augments in vitro alveolar epithelial repair by an il-1beta-dependent mechanism. American Journal of Respiratory and Critical Care Medicine. 2001;163(6):1384–1388. doi: 10.1164/ajrccm.163.6.2006131. [DOI] [PubMed] [Google Scholar]
- 27.Chimenti, L., Luque, T., Bonsignore, M. R., Ramírez, J., Navajas, D., & Farré, R. (2012). Pre-treatment with mesenchymal stem cells reduces ventilator-induced lung injury. European Respiratory Journal. doi:10.1183/09031936.00153211. [DOI] [PubMed]
- 28.Raffaghello L, Bianchi G, Bertolotto M, et al. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells. 2008;26:151–162. doi: 10.1634/stemcells.2007-0416. [DOI] [PubMed] [Google Scholar]
- 29.Tai WL, Dong ZX, Zhang DD, Wang DH. Therapeutic effect of intravenous bone marrow-derived mesenchymal stem cell transplantation on early-stage LPS-induced acute lung injury in mice. Nan Fang Yi Ke Da Xue Xue Bao. 2012;32(3):283–290. [PubMed] [Google Scholar]
- 30.Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E (2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nature Medicine. 2009;15(1):42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volarevic V, Al-Qahtani A, Arsenijevic N, Pajovic S, Lukic ML. Interleukin-1 receptor antagonist (IL-1Ra) and IL-1Ra producing mesenchymal stem cells as modulators of diabetogenesis. Autoimmunity. 2010;43(4):255–263. doi: 10.3109/08916930903305641. [DOI] [PubMed] [Google Scholar]
- 32.Ortiz LA, Dutreil M, Fattman C, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proceedings of the National Academy of Sciences. 2007;104(26):11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kleiveland CR, Kassem M, Lea T. Human mesenchymal stem cell proliferation is regulated by PGE2 through differential activation of cAMP-dependent protein kinase isoforms. Experimental Cell Research. 2008;314(8):1831–1838. doi: 10.1016/j.yexcr.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Bunnell BA, Betancourt AM, Sullivan DE. New concepts on the immune modulation mediated by mesenchymal stem cells. Stem Cell Research and Therapy. 2010;1(5):34. doi: 10.1186/scrt34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu L, Wang Q, Liang X, Andreasson K. Divergent effects of prostaglandin receptor signaling on neuronal survival. Neuroscience Letters. 2007;421(3):253–258. doi: 10.1016/j.neulet.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore BB, Ballinger MN, White ES, et al. Bleomycin-induced E prostanoid receptor changes alter fibroblast responses to prostaglandin E2. Journal of Immunology. 2005;174(9):5644–5649. doi: 10.4049/jimmunol.174.9.5644. [DOI] [PubMed] [Google Scholar]
- 37.Barbash IM, Chouraqui P, Baron J, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108(7):863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]


