Abstract
Drugs used to treat psychotic disorders (‘antipsychotics’) have been widely used in psychiatry since the introduction of chlorpromazine in the mid-1950s. The categorization of these drugs evolved in a piecemeal way, relying initially on grouping by chemical structure (e.g. phenothiazines, butyrophenones), then by epoch of introduction (e.g. first generation (‘conventional’) vs second generation (‘atypical’)). As psychopharmacological expertise has advanced, it has become possible to quantify affinities for each drug in this class for relevant receptors including dopamine D2, 5HT2A, 5HT2C, histamine H1 and others. However, until the recent emergence of a new generation of agents known collectively as dopamine D2 receptor partial agonists (e.g. aripiprazole, brexpiprazole and cariprazine), there had been little reference in drug classification to specific pharmacological properties. An overview of data on receptor affinities across multiple drugs and receptor types would permit categorization according to binding affinities and putative pharmacological mechanisms. In this paper, we have attempted to construct a ‘subway map’ of 32 drugs used for treatment of psychotic disorders. This design allows a visualization of both the historical classifications by structure and epoch of introduction, and of the binding affinities for key receptors based on appraisal of scientific literature. The map represents a step towards categorization by mechanism, allowing prescribers and patients to understand which drugs share common biological features and the extent to which drugs may have similarities and differences in their mechanisms. In addition, this approach may encourage more logical groupings of drugs to be used in systematic reviews and meta-analyses.
Keywords: Antipsychotics, drugs for psychosis, classification, dopamine, psychopharmacology
Background
Drugs used in psychotic disorders, commonly referred to as ‘antipsychotics’ or by the older term ‘neuroleptics’, first became available in the 1950s with the introduction of chlorpromazine (McPherson, 2007). Throughout the next 30 years, new compounds were developed and brought to market. Almost all of the drugs which entered clinical use for psychotic disorders in this era were strong or moderate dopamine D2 receptor antagonists, and a seminal paper (Seeman and Lee, 1975) illustrated that their potency was linked to affinity for this receptor. However, clozapine, first made available in the 1960s, appeared to differ from the rest in both its mechanism (as it had only weak affinity for the dopamine D2 receptor) and its superior efficacy in treatment-resistant schizophrenia (Remington et al., 2016). Although clozapine was initially withdrawn due to concerns about agranulocytosis (only to return later with strict requirements for haematological monitoring), the observation of its enhanced efficacy was a catalyst for the development of many new drugs.
Until recently, classification of drugs used in psychosis was piecemeal, relying on a combination of grouping by chemical structure (e.g. phenothiazines, butyrophenones) and by epoch of introduction (e.g. first generation vs second generation), with limited reference to pharmacological mechanisms. Some acknowledgement of the desire to classify by mechanism is present through the distinction between the terms ‘conventional’ antipsychotics, whose action was thought to be mainly dependent on dopamine D2 antagonism, versus ‘atypical’ antipsychotics, which were understood to have a broader range of mechanisms. However, several ‘atypicals’ such as risperidone, lurasidone and asenapine still have strong affinity for the D2 receptor, while many ‘conventional’ drugs have appreciable affinity for other non-dopaminergic receptors (e.g. loxapine for 5HT2A, 5HT2C and histamine H1 receptors). Thus, the ‘conventional versus atypical’ distinction offers only limited insight into putative antipsychotic treatment mechanisms. The problem may stem from the fact that the original development of drugs for psychosis predates an understanding of the mechanisms involved. There was a gap of more than 20 years between the approval of the first agents and Seeman’s elucidation of the role of the dopamine D2 receptor. A further 20 years elapsed before the technology was widely available to ascertain affinities for many of the other receptor systems that may be related or even integral to drug action. These receptors include serotonin 5HT2A and 5HT2C receptors, alpha 2 adrenoceptors, histamine H1 receptors and cholinergic M1 and M4 receptors.
We will briefly discuss some of the functions of these receptors. The 5HT2A receptor is implicated in modulation of dopamine transmission and blockade leads to decreased dopamine transmission in the mesolimbic system (Aringhieri et al., 2018). 5HT2C has a role in inhibition of dopamine transmission and modulating acetylcholine transmission (Zhelyazkova-Savova et al., 1999). Alpha 2 adrenergic receptor antagonism has a role in treatment of negative and cognitive symptoms of schizophrenia through actions on cortical noradrenaline release, dopaminergic mesocorticolimbic circuitry and direct or indirect facilitation of central serotonergic neurotransmission (Svensson, 2003). The histamine H1 receptor is involved in wakefulness (among other functions), and medications which are antihistaminergic are generally quite sedating. Acetylcholine M1 receptors have a role in executive function and episodic memory (López-Álvarez et al., 2019). M4 receptors regulate the levels of acetylcholine (López-Álvarez et al, 2019). Blockade of M1 and M4 receptors mediates anticholinergic side effects and helps offset extrapyramidal side effects produced by D2 receptor blockade. M1 and M4 receptors may constitute a therapeutic target in schizophrenia and other psychotic disorders as they have been proposed to contribute to an imbalance between central cholinergic and dopaminergic systems (Yohn and Conn, 2018). Each receptor listed above has more functions than outlined here. Several other receptors that may have a role in drug action were not included in this paper due to limited space, although we noted the putative importance of the dopamine D3 receptor in the action of cariprazine, a D3 partial agonist (Girgis et al., 2016), whose mechanism is still being explored.
Faced with overlapping and sometimes conflicting layers of classification, there have been calls for improved categorization of drugs used in psychotic disorders (Nutt, 2009; Zohar et al., 2014). Many experts believe that categorization should be primarily based on pharmacological mechanism. Mechanism-based categorization such as NBN-2R (Zohar et al., 2019) would allow prescribers and users to better identify which drugs may share common pharmacological effects, both in terms of efficacy and side effect profiles. A further benefit of mechanism-based classification is guidance towards more logical drug groupings for those undertaking systematic reviews and meta-analyses. For example, a system of classification paying close attention to drug mechanisms may more intuitively allow analyses of the efficacy of all drugs that share a specific property (e.g. high affinity for a certain receptor). Nutt (2017) has attempted to produce a visual characterization of drug actions, including most commonly prescribed agents used for psychotic disorders through the visualizing psychotropic medicine (VPM) library project (https://www.vpmlibrary.com/Browser/Show). This body of work allows the mechanisms of individual drugs to be viewed according to a predefined schema of actions on receptors, transporters, ion channels and enzymes and can be seen as constituting the building blocks of information on which categorization by mechanism might rely.
In this paper, we set out to design a graphical representation of antipsychotic drugs in the style of a ‘subway map’ or a ‘tube map’ (Garland, 1994) as we have previously designed for drugs that treat depression (Davies et al., 2014). Unlike the case with VPM, which dedicates a separate slide to each drug included, we attempted to represent the majority of drugs used to treat psychotic disorders together on a single slide. The aim was to produce a map that would allow categorization by (a) molecular structure, (b) epoch of introduction, (c) detailed pharmacological mechanisms to be visualized and (d) acknowledges other existing non mechanism-based (or loosely mechanism based) categorizations.
Methods
We selected 32 drugs recognized for the treatment of psychotic disorders to construct a map representing a visual representation of classification. In all, 27 are currently available for this indication and a further five are no longer used in most jurisdictions but were included due to historical or mechanistic interest. Some drugs have never been marketed in key regions of the world (e.g. amisulpride was never approved in the United States or Canada but is commonly used in Europe, and blonanserin is marketed in Japan and Korea but is not available in Europe or North America).
Map design
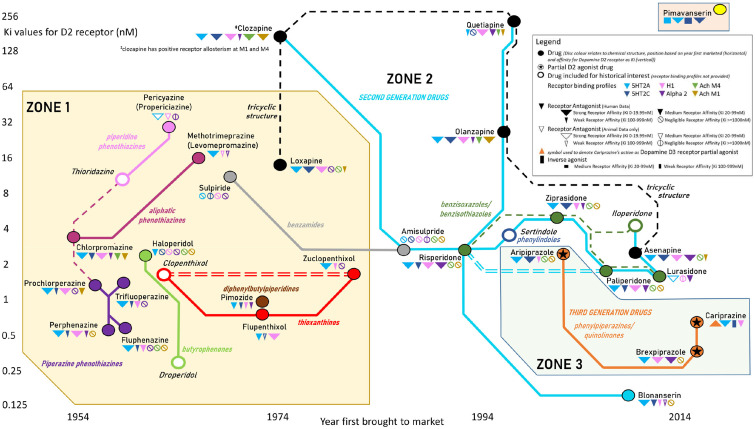
Table 1 provides a reference guide for the design principles of the simplified (Figure 1) and full (Figure 2) versions of the map. The principles determining drug locations on the east–west and north–south axes, colours, groupings (into zones corresponding to first-generation (conventional), second-generation (atypical) and third-generation (dopamine D2 partial agonist) agents; Virani et al., 2012), connections between drugs and additional mechanistic information are described. As described in Table 1, the position of each drug on the map’s east–west axis was established by the year in which they were first brought to market (in any country as recorded in McPherson (2007) or else from other sources such as regulatory approval documentation) ranging from the oldest which appeared in the 1950s to those which have arrived only in the last decade.
Table 1.
Map design principles.
| The following principles determined drug locations, colours, groupings,
connections and additional mechanistic information for the simplified (Figure 1) and full (Figure 2) versions of the
map. All symbols described in this table are illustrated in the figure
legends. Drug locations and colours • Drugs were represented by coloured discs (akin to a ‘station’ of a subway map), with disc colour determined by the chemical structure. Five drugs no longer available in most or all markets were distinguished by their discs having a white centre and appear in italic script. • Position on the east–west axis was established by the year first brought to market. • Position on the north–south axis was determined by the affinity for the dopamine D2 receptor, inversely proportional to the inhibition constant (Ki) – that is, lower affinity at the top of the page. Ki values were sourced from human studies of D2 receptor affinity listed in the Psychoactive Drug Screening Project (PDSP) database or if not listed in PDSP from published literature. Drug groupings: zones and interconnections between drugs • Drugs were grouped into zones corresponding to (1) first-generation (conventional), (2) second-generation (atypical) and (3) third-generation (dopamine D2 partial agonist) agents. • Solid coloured lines were employed to indicate commonly used groupings irrespective of their basis. ◦ For first-generation agents (zone 1), this was based on long-established structure-based categories, for example, phenothiazines (subdivided into aliphatic phenothiazines (maroon) connected by dashed lines to the related piperidine (pink) and piperidine (purple) phenothiazine types), butyrophenones (light green), thioxanthenes (red), benzamides (silver) and diphenylbutylpiperidines (brown). ◦ Clozapine and most drugs brought to market after 1985 are commonly grouped together in a ‘second-generation’ or an ‘atypical’ (zone 2) category and were connected by pale blue solid lines. As classification by structure is possible (but rarely used), we denoted second-generation drugs as benzisoxazoles/benzisothiazoles (green), benzamides (silver) and phenylindoles (dark blue), while some second-generation drugs and the first-generation drug loxapine can be described as having a tricyclic structure (black). Here, drugs sharing similar chemical structures were connected to each other with the weaker dashed lines. ◦ Dopamine D2 partial agonist drugs, ‘third-generation’ drugs, were depicted in orange (also representing the rarely used structural description of phenylpiperazines/quinolinones) and form the map’s zone 3. ◦ Pimavanserin, a serotonin-receptor modulator which does not belong to any of the three drugs groups described, was placed in an inset box at the top of the map due to negligible affinity for the dopamine D2 receptor. • Pairs of closely related drugs differing only by stereoisomer content or by one being an active metabolite of another were connected by double lines to emphasize their close connections. Additional mechanistic information • D2 partial agonists (aripiprazole and related ‘third-generation’ drugs) were marked with a star symbol on the station disc to emphasize the difference in their mechanism from D2 antagonist first-generation and second-generation drugs (both figures). • Symbols adjacent to each drug name provide additional information on drug mechanisms (Figure 2 only, with exception of cariprazine’s D3 affinity as a partial agonist being illustrated on both figures by an inverted triangle). • For six further receptors of interest (5HT2A, 5HT2C, H1, alpha 2 and M1/M4 cholinoceptors), additional symbols adjacent to the drug name were added (Figure 2 only). The final median Ki value from PDSP was represented by a solid triangle; large, medium or small size depending on affinity (strong: 0–19.99 nM, medium: 20–99.9 nM and weak: 100–999.9 nM). Where the Ki was ⩾1000 nM, a small circle containing an oblique line similar to a ‘zero’ was used. • Where no human data were available in PDSP but animal data were listed, hollow triangles of comparable size to those described above or for Ki ⩾ 1000 nM a small circle containing a vertical line were used to represent affinities. • Inverse agonist properties were denoted by a rectangle. |
Figure 1.
Map of 32 drugs used in treatment of psychosis (simplified version). As described in Table 1, drug locations are determined by affinity for the dopamine D2 receptor (vertical) and year first came to market (horizontal). Drugs are grouped by zone (‘conventional’/first generation vs ‘atypical’/second generation vs D2 receptor partial agonists/third generation). Solid connecting lines represent grouping by chemical structure (first-generation drugs) versus second or third generation. Dashed connecting lines represent potential (but rarely used) grouping by chemical structure for second-generation drugs. Double lines connect drugs closely related through chirality or metabolism.
Figure 2.
Map of 32 drugs used in treatment of psychosis (full version). This figure contains all of the information described for Figure 1. In addition, for 27 drugs remaining in common use (excluding the five shown in italics), affinities for six additional receptors: 5HT2A and 5HT2C receptors, alpha 2 adrenoceptor, histamine H1 receptor and acetyl choline M4/M1 receptors are illustrated by the appropriate symbols.
Acquisition of receptor affinity data
The position of drugs on the north–south axis was determined by the affinity for the dopamine D2 receptor which is inversely proportional to the inhibition constant (Ki), that is, lower affinity at the top of the page. Ki values were sourced from human studies of D2 receptor affinity listed in the Psychoactive Drug Screening Project (PDSP) database (Roth and Driscoll, 1999-present: https://pdsp.unc.edu/databases/pdsp.php, Roth et al., 2000), taking the median value where more than one human study was recorded. Since PDSP lists numerous similarly named receptors, we used only those studies listed in PDSP as relating to the ‘dopamine D2 receptor’ with receptor ID number = 20. In any instances where a drug was not listed in PDSP, we sought alternative sources from published literature to establish Ki values for the dopamine D2 receptor.
For the 27 currently available drugs, receptor Ki was determined for six further receptors. Where possible data were obtained from human studies listed in the PDSP database (Roth and Driscoll, 1999-present; Roth et al., 2000), taking the median value where data from more than one study was described. The receptor ID numbers used in PDSP were as follows: serotonin 5HT2A = 9, serotonin 5HT2C = 11, histamine H1 = 31, adrenaline alpha 2 = 25, acetylcholine M1 = 50 and acetylcholine M4 = 73. Final median Ki values were represented by triangular symbols with size depending on the range (strong: 0–19.99 nM, medium: 20–99.9 nM and weak: 100–999.9 nM) in which they fell. Where the Ki was ⩾1000 nM, indicating very little affinity for a given drug at the receptor, instead of a triangle a ‘zero’ symbol was used. Where no human data were available in PDSP but animal data were listed, we took the median value from the animal studies and used adapted map symbols (e.g. hollow triangles or a rotated ‘zero’ containing a vertical line) to represent the affinity value. Inverse agonists were denoted by rectangular symbols. For reasons of space, we did not provide any additional mechanistic data for the five drugs which are no longer available in most markets.
Results
The final map providing a visual representation of 32 drugs used in treatment of psychotic disorders is presented in Figure 1. Data relating to dopamine D2 receptor affinity were available in the PDSP database for 27 of the drugs selected (Table 2). Four (blonanserin, clopenthixol/zuclopenthixol and pimavanserin) did not have any data for Ki for dopamine D2 from human receptor studies referenced in PDSP. The alternative sources used to ascertain D2 affinity values for these compounds are listed in Table 2 (Christensen et al., 1984; Kitten et al., 2018; Tenjin et al., 2013). Brexpiprazole had data in PDSP referring to a dopamine D2L receptor but on consulting the source (Maeda et al., 2014), this was judged to be an acceptable proxy for D2. By design, drugs high on the vertical axis (e.g. clozapine and olanzapine) have the highest Ki and lowest affinity for the dopamine D2 receptor, while conversely, those lowest on the vertical axis (e.g. droperidol and blonanserin) have the highest affinity for dopamine D2 and the lowest Ki. The horizontal axis allows the reader to visualize the order in which the drugs came to market ranging from the earliest, chlorpromazine, to the most recent, pimavanserin and cariprazine.
Table 2.
Receptors and ligands searched in PDSP and where necessary from other sources as specified.
| Receptors* | Ligands where Ki for dopamine D2 receptor listed in PDSP | Ligands where alternative sources were required for Ki for dopamine D2 receptor |
|---|---|---|
| Dopamine D2 Serotonin 5-HT2A Serotonin 5-HT2C Alpha 2 adrenoceptor Histamine H1 Acetyl choline M1 Acetyl choline M4 *Dopamine D3 receptor was considered for cariprazine only |
Amisulpride Aripiprazole Asenapine (listed as ORG-5222) Cariprazine Chlorpromazine Clozapine Droperidol† Flupenthixol, CIS Fluphenazine Haloperidol Iloperidone† Levomepromazine (methotrimeprazine) Loxapine Lurasidone (also listed as SM-13496) Olanzapine 9-OH-risperidone (Paliperidone) Perphenazine Pimozide Prochlorperazine Propericiazine (pericyazine) Quetiapine Risperidone Sertindole† Sulpiride Thioridazine† Trifluoperazine Ziprasidone |
Blonanserin (Tenjin et al.,
2013) Brexpiprazole (PDSP listed data for a ‘D2L’ receptor which was included in place of D2 after consulting the source (Maeda et al., 2014) Clopenthixol† (extrapolated from Christensen et al. (1984)) Pimavanserin (Kitten et al., 2018) Zuclopenthixol (Christensen et al., 1984) |
For drugs of historical interest which are no longer available in most markets (clopenthixol, droperidol, iloperidone, sertindole and thioridazine) only dopamine D2 was searched.
PDSP: Psychoactive Drug Screening Project.
The map illustrates connections between drugs both in terms of the early classifications based on chemical structure and the later terminology which divides drugs into first-generation (conventional), second-generation (atypical), third-generation (Dopamine D2 partial agonist) agents as well as pimavanserin which belongs to none of these categories. For the 16 ‘conventional’ agents depicted in zone 1, the map reveals the traditional subdivision by chemical structure. Eight are known as phenothiazines, further divided into aliphatic, piperidine and piperazine groups and the remaining eight belong to six additional structure-based categories. Meanwhile, the 12 agents making up zone 2 are most commonly referred to merely as ‘atypical’ drugs, without further categorization, despite their wide-ranging mechanistic differences. Classification by chemical structure would be possible for almost all of these second-generation drugs, with five being benzisoxazoles/benzisothiazoles, one a benzamide (in common with the first-generation drug sulpiride) and one a phenylindole. A further four second-generation drugs are best described as having a tricylic structure, as is the first-generation drug loxapine, although this term is used with the caveat that it is non-specific and may be used to refer to numerous other psychotropic drugs that possess a similar three-ringed structure. However, with interest in structure-based classification waning by the time these drugs were introduced, these terms are very rarely employed for categorization. Three dopamine D2 partial agonists (third-generation drugs) make up zone 3. As anticipated, all three share a common phenylpiperazines/quinolinone structure, meaning that the dopamine D2 partial agonists and phenylpiperazines/quinolinones are rare examples of the mechanism (at least with regard to the dopamine D2 receptor) and the chemical structure being uniform within a drug group. As anticipated, the final medication, pimavanserin, appears in an insert box in the top right corner of the map since it has negligible affinity for the dopamine D2 receptor and its approval by the United States Food and Drug Administration came as recently as 2016.
Two pairs of drugs were found to be very closely related; zuclopenthixol/clopenthixol which contain the same cis isomers but differ in the presence or ratio of the trans-isomer, and risperidone/paliperidone – the latter being a hydroxy metabolite of the former.
The map provides information for the 27 more widely available drugs on additional serotonergic, alpha-adrenergic, histaminergic and cholinergic receptor affinities by virtue of the symbols adjacent to each station disc. Where data exist in PDSP (or additional sources as described in the Methods), affinity stronger than a Ki threshold of 1000 nM is indicated by triangles of increasing size depending on the magnitude of affinity. There were a small number of examples of inverse agonists: cariprazine at the 5HT2C receptor and potentially pimavanserin at both 5HT2A and 5HT2C receptors. We chose to highlight the D3 partial agonist action of cariprazine with a distinctive symbol as the 10-fold greater affinity of the D3 receptor over the D2 is unique to this drug and may be essential understanding its therapeutic action (Girgis et al., 2016).
We encountered a small number of situations whereby the evolution of receptor nomenclature generated issues in ascertaining the data on receptor affinities. For levomepromazine and zuclopenthixol, data listed as relating to ‘5HT2’ were assumed to relate to 5HT2A as the modern nomenclature of 5HT2A, 5HT2C and other 5HT2 subtypes (Saxena, 1995) had not yet been adopted at the time of publication of papers providing affinity data, the ‘5HT2’ receptors described in that era subsequently being relabelled ‘5HT2A’. For aripiprazole, brexpiprazole and lurasidone, no data for alpha 2 adrenoceptor affinity were listed in the PDSP database. For each drug, however, there were several studies yielding data for one or more related receptor subtypes, that is, alpha 2A, 2B and/or 2C receptors. We therefore took the median value of Ki values across all three of these receptor subtypes to ascertain the value for alpha 2 for aripiprazole, and across the alpha 2A and 2C values available for lurasidone, while for brexpiprazole we used the value for alpha 2C receptors which was the only receptor subtype which had data available in PDSP. We noted a small disparity between PDSP data listed for lurasidone and the original data in the corresponding cited publication (Ishibashi et al., 2010) for 5HT2A and H1 affinities, and chose to use the source data from the paper.
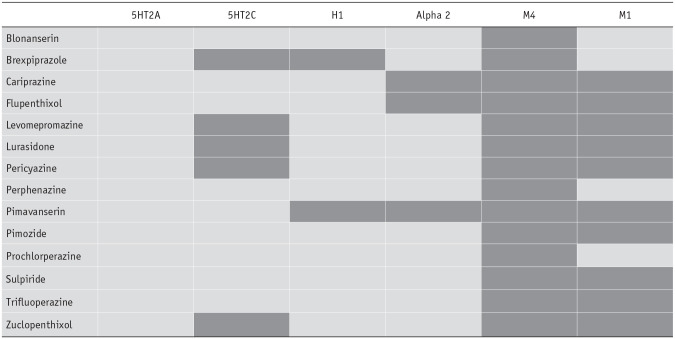
In addition to the cases described above where no data for the affinity of certain drugs for dopamine D2 receptors were listed in PDSP, there were also instances where no data were available in the PDSP database for other drug/receptor combinations from binding affinity studies in either humans or animal studies. In such instances, we did not attempt to undertake any further searches beyond PDSP. The one exception was that for the drugs where D2 affinities were not listed in PDSP, we included any data on affinity for other receptors presented in the same papers as those which provided our sources for D2 affinity (Table 2). Overall, the number of missing data points was 34, 17.5% of the 194 drug–receptor pairs (e.g. haloperidol-5HT2A) examined. M1 and M4 receptor affinities accounted for 24 instances of missing data. We have tabulated all instances where data were lacking in Table 3.
Table 3.
Missing data for drug/receptor pairs from PDSP Ki database or alternative sources referenced in Table 2, illustrated by dark grey rectangles.
|
Discussion
We have constructed a schematic map of 32 drugs currently or previously used in treatment of psychotic disorders according to the principles outlined above. This map allows simultaneous visualization both of historical classification systems (e.g. those relying on chemical structure, those related to some aspect of the pharmacological mechanism and those describing the era in which drugs came to market) and of more robust mechanism-based classifications that may be potentially used in the future. The map clearly illustrates how the original drugs were classified only by their chemical structure, but once the means existed to appraise newly developed compounds by their biological mechanisms, drug categorization has been increasingly influenced by pharmacology, initially informally with the introduction of terms such as ‘atypical’ and then more specifically with aripiprazole and similar agents being described as ‘dopamine D2 partial agonists’. However, we have still provided structural information for more recently introduced medications as well as the more commonly used structure-based groupings for first-generation drugs. Knowledge of which drugs have related structures may be of value in anticipating that drugs may share propensity to hypersensitivity-based reactions which are commonly linked to chemical structure. This might allow prescribers to decrease the likelihood of further such reactions after hypersensitivity to a drug with a specific structure has occurred or to reduce the risk of cross-reactivity between co-prescribed drugs.
Whether the ideal mechanism-based classification should be predominantly based on the affinity and type of action (e.g. antagonism vs partial agonism) at dopamine D2 receptors or should take account of interactions with other receptor systems (e.g. 5HT2A, 5HT2C and alpha 2) is not currently clear. However, both the NBN-2R system (Zohar et al., 2019) and VPM (Nutt, 2017) attempt to incorporate information on receptor systems beyond dopamine D2. While interactions with certain receptors (e.g. histamine H1) may not be central to drugs’ efficacy in treating psychosis, these actions may contribute indirectly to the overall therapeutic effect and/or side effects (e.g. H1 antagonism and sedation or weight gain).
A classification system with more information on drug mechanisms offers potential to perform better targeted systematic reviews or meta-analysis. For instance, once ‘second-generation’ drugs for psychosis became established in prescribing practice, numerous meta-analyses were performed to examine differences in efficacy and side effect profile between second-generation and first-generation drugs. As discussed previously, the first-generation versus second-generation divide is based to some extent on affinities for the D2 receptor, but is not entirely representative. A more targeted approach would be to group together drugs sharing specific mechanisms based on affinities for one or more receptor system (e.g. a meta-analysis comparing various medications based on high–intermediate–low affinity for 5HT2A for efficacy on a specific symptom or incidence of a specific side effect). The map will facilitate mechanism-based approaches to drug categorization such as these.
As we have found with our previous mechanism-based map of antidepressants, the map may also have a utility in clinical and educational settings. It may assist prescribers in explaining to patients and learners the similarities and difference between the various drugs depicted and how a proposed medication’s pharmacology may differ from or resemble that of drugs already trialled. One example of a scenario we encountered clinically where the map proved to be valuable was the case of a consultation with an elderly patient who had experienced a brief psychotic episode many years earlier which had resolved on taking trifluoperazine. She continued to take a small dose of this medication as a prophylaxis and was concerned at reports of the possibility that it might cease to be available at some point in the future. She therefore asked for our recommendations as to which of the newer (and more readily available) medications would be an alternative if access to trifluoperazine became problematic. Inspection of the map revealed the pharmacological similarities between trifluoperazine and risperidone, with their comparable affinities for dopamine D2 and 5HT2A receptors, while risperidone has slightly greater affinities for 5HT2C and histamine H1 receptors. Asenapine, paliperidone and ziprasidone might be other alternatives with some affinities in common. Certain other second-generation drugs such as quetiapine and amisulpride appear to have few similarities to trifluoperazine in terms of receptor affinities and accounting for the impact of the higher dose ranges usually employed, these drugs would be less logical choices to be its direct replacement. To facilitate both education opportunities and clinical discussion, we will aim to incorporate this work into a web or smartphone-based app format.
There are a number of limitations to this work. First, and as discussed earlier, for some drugs, affinities for certain receptors have yet to be studied or have only been studied in animals rather than in humans. Second, receptor affinity allows us to understand the degree to which drugs may bind to a receptor at numerically comparable doses, but disparities between drugs may be offset when they are typically given at markedly differing doses, while pharmacokinetic factors also need to be considered. Third, receptor binding (based on the affinities illustrated) is not always correlated with clinical effects. Fourth, while we selected seven receptors to include on this map, a number of others (e.g. dopamine D3, histamine H3, acetyl choline M2 and M3, and alpha 1) were judged to be less important in terms of impact on clinical efficacy and side effects. These were omitted from the map in the interest of clarity, except to emphasize the importance of the D3 receptor in the mechanism of cariprazine. Finally, while the third-generation dopamine D2 partial agonists (aripiprazole, brexpiprazole and cariprazine) are shown in the same plane as the remaining drugs which are D2 antagonists, the reader should be aware that equivalent D2 affinities may be associated with biological effects which differ systematically between the former and latter groups as suggested by the symbols demarcating the third-generation drugs.
Conclusions
This map allows simultaneous visualization of different means to conceptualize drugs developed for the treatment of psychosis. The map illustrates the historical development of the drugs and the classification of earlier compounds by their chemical structure. As newer drugs have been developed along with the technology to understand and quantify affinities for key receptors, the focus has begun to shift to classification by mechanism. The present map provides an easily accessible schema for receptor binding properties and affinities for the drugs included, offering prescribers and users a simultaneously quicker and more detailed method to appreciate similarities and differences between medications compared to existing classification systems. This schema may also have utility in allowing drugs which share specific pharmacological properties to be grouped together more logically in a systematic review or in a meta-analysis.
Acknowledgments
We are grateful to Dr Sian McIver and Professor Marco Battaglia for their comments and to Mr Ian Golden for technical assistance with map design. Ki determinations and receptor binding profiles were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program, Contract # HHSN-271-2018-00023-C (NIMH PDSP). The NIMH PDSP is Directed by Bryan L. Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscoll at NIMH, Bethesda MD, USA.
Footnotes
Declaration of conflicting interests: The authors declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: CZ and SJCD have no conflicts of interest to report. DJN states ‘In a career as a psychopharmacologist spanning over 40 years I have had associations with numerous drug companies relating to every class of drugs used in the treatment of psychiatric disorders, however no commercial company had any role in or influence on the present manuscript’.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Simon J C Davies  https://orcid.org/0000-0003-0095-5993
https://orcid.org/0000-0003-0095-5993
References
- Aringhieri S, Carli M, Kolachalam S, et al. (2018) Molecular targets of atypical antipsychotics: From mechanism of action to clinical differences. Pharmacol Ther 192: 20–41. [DOI] [PubMed] [Google Scholar]
- Christensen AV, Arnt J, Hyttel J, et al. (1984) Pharmacological effects of a specific dopamine D-1 antagonist SCH 23390 in comparison with neuroleptics. Life Sci 34: 1529–1540. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Champion C, Dawson S, et al. (2014) The subway map - a new way to visualize antidepressant classification (a Cochrane CCDAN initiative). In: 14th ICGP and 19th JSNP joint congress, Tsukuba, Japan, p. 78. [Google Scholar]
- Garland K. (1994). Mr Beck’s Underground Map. Harrow Weald, Middlesex: Capital Transport. [Google Scholar]
- Girgis RR, Slifstein M, D’Souza D, et al. (2016) Preferential binding to dopamine D3 over D2 receptors by cariprazine in patients with schizophrenia using PET with the D3/D2 receptor ligand [(11)C]-(+)-PHNO. Psychopharmacology 233: 3503–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T, Horisawa T, Tokuda K, et al. (2010) Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J Pharmacol Exp Ther 334: 171–181. [DOI] [PubMed] [Google Scholar]
- Kitten AK, Hallowell SA, Saklad SR, et al. (2018) Pimavanserin: A novel drug approved to treat Parkinson’s disease psychosis. Innov Clin Neurosci 15: 16–22. [PMC free article] [PubMed] [Google Scholar]
- López-Álvarez J, Sevilla-Llewellyn-Jones J, Agüera-Ortiz L. (2019) Anticholinergic drugs in geriatric psychopharmacology. Front Neurosci 13: 1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Sugino H, Akazawa H, et al. (2014) Brexpiprazole I: In vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther 350: 589–604. [DOI] [PubMed] [Google Scholar]
- McPherson EM. (2007) Pharmaceutical Manufacturing Encyclopedia, 3rd edn. Burlington: Elsevier. [Google Scholar]
- Nutt DJ. (2017) VPM (Visualising Psychotropic Medicine) library project. Available at: https://www.vpmlibrary.com/Browser/Show (accessed 14 November 2021).
- Nutt DJ. (2009) Beyond psychoanaleptics – can we improve antidepressant drug nomenclature? J Psychopharmacol 23: 343–345. [DOI] [PubMed] [Google Scholar]
- Remington G, Lee J, Agid O, et al. (2016). Clozapine’s critical role in treatment resistant schizophrenia: ensuring both safety and use. Expert Opin Drug Saf 5: 1193–1203. [DOI] [PubMed] [Google Scholar]
- Roth BL, Driscoll J. (1999-present) PDSP Ki database. Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Available at: https://pdsp.unc.edu/databases/pdsp.php (accessed 17 April 2021). [Google Scholar]
- Roth BL, Kroeze WK, Patel S, et al. (2000) The multiplicity of serotonin receptors: Uselessly diverse molecules or an embarrassment of riches? Neuroscientist 6: 252–262. [Google Scholar]
- Saxena PR. (1995) Serotonin receptors: Subtypes, functional responses and therapeutic relevance. Pharmacol Ther 66: 339–368. [DOI] [PubMed] [Google Scholar]
- Seeman P, Lee T. (1975). Antipsychotic drugs: Direct correlation between clinical potency and presynaptic action on dopamine neurons. Science 188: 1217–1219. [DOI] [PubMed] [Google Scholar]
- Svensson TH. (2003) Alpha-adrenoceptor modulation hypothesis of antipsychotic atypicality. Prog Neuro-Psychopharmacol Biol Psychiatry 27: 1145–1158. [DOI] [PubMed] [Google Scholar]
- Tenjin T, Miyamoto S, Ninomiya Y, et al. (2013) Profile of blonanserin for the treatment of schizophrenia. Neuropsychiatr Dis Treat 9: 587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virani A, Bezchlibnyk-Butler K, Jeffries JJ, et al. (eds) (2012) Clinical Handbook of Psychotropic Drugs, 19th Revised edn. Göttingen: Hogrefe Publishing. [Google Scholar]
- Yohn SE, Conn PJ. (2018) Positive allosteric modulation of M1 and M4 muscarinic receptors as potential therapeutic treatments for schizophrenia. Neuropharmacology 136: 438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhelyazkova-Savova M, Giovannini MG, Pepeu G. (1999) Systemic chlorophenylpiperazine increases acetylcholine release from rat hippocampus implication of 5-HT2C receptors. Pharmacol Res 40: 165–170. [DOI] [PubMed] [Google Scholar]
- Zohar J, Blier P, Stahl SM, et al. (2019) NbN2R (Neuroscience-Based Nomenclature), 2nd edn. Utrecht: ECNP. [Google Scholar]
- Zohar J, Nutt DJ, Kupfer DJ, et al. (2014) A proposal for an updated neuropsychopharmacological nomenclature. Eur Neuropsychopharmacol 24: 1005–1014. [DOI] [PubMed] [Google Scholar]