Abstract
About one-fourth of adults globally suffer from nonalcoholic fatty liver disease (NAFLD), which is becoming a leading cause of chronic liver disease worldwide. Its prevalence has rapidly increased in recent years, and is projected to increase even more. NAFLD is a leading cause of hepatocellular carcinoma (HCC), the sixth-most prevalent cancer worldwide and the fourth most common cause of cancer-related death. Although the molecular basis of HCC onset in NAFLD is not completely known, inflammation is a key player. The tumor microenvironment (TME) is heterogeneous in patients with HCC, and is characterized by complex interactions between immune system cells, tumor cells and other stromal and resident liver cells. The etiology of liver disease plays a role in controlling the TME and modulating the immune response. Markers of immune suppression in the TME are associated with a poor prognosis in several solid tumors. Immunotherapy with immune checkpoint inhibitors (ICIs) has become the main option for treating cancers, including HCC. However, meta-analyses have shown that patients with NAFLD-related HCC are less likely to benefit from therapy based on ICIs alone. Conversely, the addition of an angiogenesis inhibitor showed better results regarding the objective response rate and progression-free survival. Adjunctive diagnostic and therapeutic strategies, such as the application of novel biomarkers and the modulation of gut microbiota, should be considered in the future to guide personalized medicine and improve the response to ICIs in patients with NAFLD-related HCC.
Keywords: Hepatocellular carcinoma, Immunotherapy, Liver cancer, Nonalcoholic fatty liver disease, Metabolic dysfunction-associated fatty liver disease, Obesity
Core tip: Complex interactions involving the immune system, angiogenesis and inflammation are associated with the pathogenesis of hepatocellular carcinoma (HCC). Recent reviews suggested lower efficacy of immunotherapy in patients with nonviral HCC. This calls into question the need to stratify patients to maximize the effectiveness of immunotherapeutic agents. In this study, we provided the latest report on the tumor microenvironment structure and its implications in response to immunotherapy in nonalcoholic fatty liver disease-related HCC and also discussed the efficacy of first-line systemic treatment in this patient population.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) includes a wide spectrum of hepatic abnormalities ranging from simple hepatic steatosis to nonalcoholic steatohepatitis (NASH)[1]. About 24% of adults globally suffer from NAFLD[2,3]. This percentage has considerably increased in recent years, almost doubling between 2005 and 2010[3]. NAFLD starts developing with the accumulation of lipids in hepatocytes, while progression to steatohepatitis occurs in 20%–30% of the cases[4,5]. Cirrhosis occurs in 10%–20% of the cases due to the deposition of fibrous tissue (fibrosis) and alterations in the regeneration of hepatocytes[4,5]. Although it is difficult to precisely determine the prevalence of NAFLD-related cirrhosis, NAFLD is one of the leading causes of cirrhosis worldwide[6,7], and it is currently the second most common indication for liver transplantation[2]. As NAFLD is associated with metabolic disorders in almost all patients, a change in the terminology from NAFLD to metabolic dysfunction-associated fatty liver disease (MAFLD) was proposed[8]. This new classification might lead to a further increase in the prevalence of this disease, but there is still a lack of complete agreement among experts about redefining NAFLD as MAFLD.
Besides increasing in prevalence, NAFLD is becoming one of the leading causes of hepatocellular carcinoma (HCC), being responsible for 1%–38% of HCCs globally[9-12]. The high variation in estimating the prevalence of NAFLD-related HCC is due to the heterogeneous definition of NAFLD used in different studies (histological vs radiological vs clinical)[9].
Chronic viral hepatitis, caused by hepatitis B virus (HBV) and hepatitis C virus (HCV) infections, accounts for 80% of HCC cases globally[13]. However, some studies suggest that NAFLD is the main cause of HCC in some areas of Europe, while in the USA, the number of NAFLD-related HCC cases is steadily increasing[10]. Considering the growing prevalence of NAFLD and the progressive reduction in viral-hepatitis-related HCC (due to vaccination and effective antiviral treatments)[14-16], NAFLD/MAFLD might become the main cause of HCC in the next 10 years[10,14]. Although most cases of NAFLD-related HCC occur in a cirrhotic liver, retrospective studies have shown that NAFLD-driven HCC can occur even in the absence of cirrhosis in 20%–50% of the cases, especially when NASH is present[10,13,17,18].
Many factors contribute to making NAFLD a leading cause of HCC, even in the absence of cirrhosis. Specifically, type 2 diabetes mellitus and obesity increase the risk of HCC in NAFLD patients with or without cirrhosis[19-23]. Moreover, NAFLD-related HCC has higher mortality than HCC associated with viral hepatitis[11]. This is probably because HCC in patients with NAFLD is generally diagnosed in more advanced stages and mostly outside surveillance programs[17].
Recently, immunotherapy with immune checkpoint inhibitors (ICIs) was found to be a good therapeutic option for advanced HCC, either as an alternative to tyrosine kinase inhibitors (TKIs) or along with them[24]. Patients need to be categorized according to their likelihood of response, considering that ICIs were found to be less effective in certain patient subpopulations, particularly those with NAFLD. In this review, we describe the mechanism behind the progression of NAFLD to HCC and discuss the efficacy of ICIs in patients with NAFLD to determine the factors that might elucidate the best therapeutic choice for this patient population.
HCCIMMUNOBIOLOGY AND LIVER MICROENVIRONMENT
Many factors contribute to the progression of NAFLD to HCC, including individual (i.e., genetics, epigenetics and gut microbiota) and environmental (i.e., diet) factors[25]. Although the molecular basis of this process is not completely known[26], most authors agree that chronic inflammation and immune system disorders play a crucial role[25,27-30]. These interactions occur in the tumor microenvironment (TME) (Figure 1).
Figure 1.
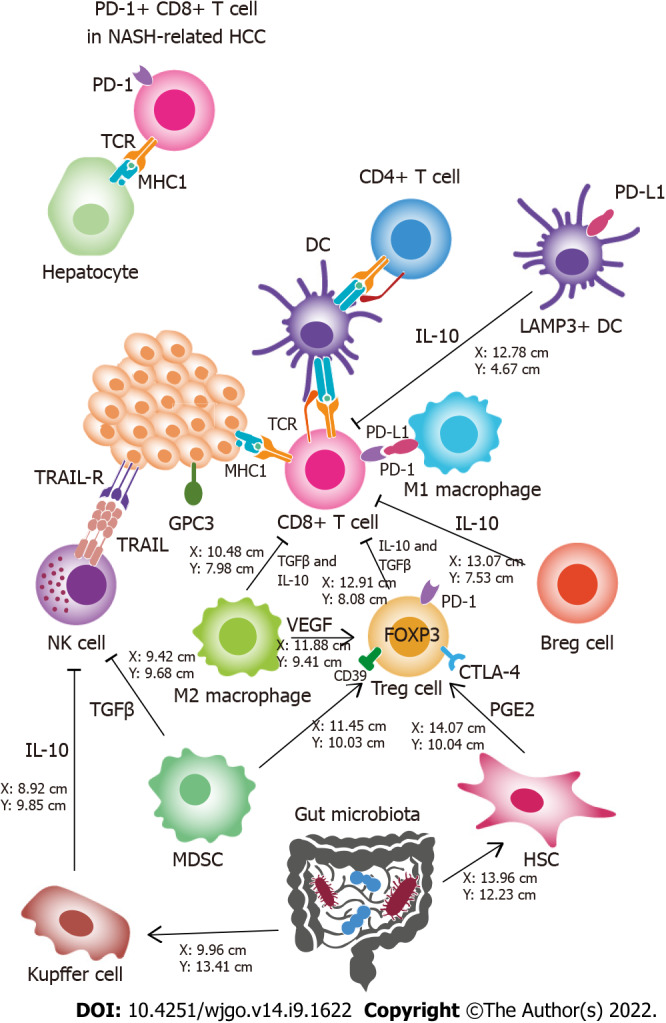
Hepatocellular carcinoma immunological microenvironment. Different elements that contribute to the antitumor activity or limit antitumor immunity are illustrated schematically. The main effectors against tumor cells are CD8+ T cells and natural killer (NK) cells. Dendritic cells (DCs), CD4+ cells, and M1 macrophages enhance CD8+ T cell cytotoxicity. Regulatory T cells, regulatory B cells, LAMP3+ DCs, and M2 macrophages inhibit CD8+ T cells and induce an immunosuppressive environment. Tumor cells attract M2 macrophages by expressing glypican-3 (GPC3). Myeloid-derived suppressor cells and Kupffer cells produce immunosuppressive cytokines in the tumor microenvironment and inhibit NK cells. The gut microbiota might play an indirect role in immunosuppression through persistent inflammation or other mechanisms leading to immune cell exhaustion. PD-1+ CD8+ T cells in NASH-related hepatocellular carcinoma show cytotoxic activity against hepatocytes, instead of exhibiting antitumor function. Breg: Regulatory B cells; DC: Dendritic cell; HSC: Hepatic stellate cell; MDSC: Myeloid-derived suppressor cell; MHC I: Major histocompatibility complex class I; NASH: Nonalcoholic steatohepatitis; NK: Natural killer; TCR: T cell receptor; TME: Tumor microenvironment; Treg: Regulatory T cells; TRAIL: Tumor necrosis factor-related apoptosis-inducing ligand; TRAIL-R: Tumor necrosis factor-related apoptosis-inducing ligand receptor; VEGF: Vascular endothelial growth factor.
The immune system: ally or enemy in the TME?
Based on the complex interactions with other cell types and cytokines, each cell in the TME has a different function. To provide an overview of the TME, a classification of HCCs based on immunological features was proposed for determining prognostic phenotypes and predicting therapeutic responses[29-31]. Two main types of HCC can be identified: the inflamed class (high immune infiltration, increased PD-1/PD-L1 signaling, and markers of CD8+ T cell cytotoxic activity) and the noninflamed class (low abundance of tumor-infiltrating lymphocytes, low expression of immune checkpoints, and markers of CD8+ T cell cytotoxic activity)[32]. The inflamed class can be divided into two subclasses[29,31]: (1) The active immune class, characterized by a high abundance of CD8+ T cells, M1-phenotype macrophages, and overexpression of T cell effector genes, such as CXCL9, CXCL10 and IFNγ, and granzymes, shows a favorable prognosis compared to the other classes; and (2) The exhausted immune class, characterized by an increase in M2 tumor-associated macrophages and T-cell exhaustion markers[29]. Transforming growth factor (TGF)-β signaling plays a key role in inducing T-cell exhaustion[33,34] and is associated with an increase in the expression of programmed cell death protein (PD)-1, TIM-3, LAG3 and TIGIT[35]. Another molecule involved in CD8+ T-cell exhaustion is the thymocyte selection-associated high mobility group box (TOX) transcription factor, whose expression is induced by the vascular endothelial growth factor (VEGF) signaling pathway[35]. Noninflamed subclasses include: (1) The intermediate class, in which immune infiltration is lower than that in the inflamed class; and (2) The immune excluded class, characterized by immunosuppressive gene upregulation in tissues surrounding the tumor, which leads to immunological desertification[32,36]. The immune excluded class has the worst prognosis and is unlikely to respond to immunotherapy[36,37].
Another classification considers immune cell tumor infiltration, with three subtypes of HCC phenotype: (1) The immune-high subtype, which has a high rate of T-cell, B-cell and plasma cell infiltration; (2) The immune-mid subtype, which has a moderate rate of immune cell infiltration; and (3) The immune-low subtype, which has a low rate of immune cell infiltration[30]. As in the active immune class, the high-immune subtype is associated with an increase in T helper 1 cells and CD8+ cell cytokines[30,32]. The high-immune subtype has a better prognosis than the other subtypes[30], particularly in poorly differentiated HCCs[30]. Furthermore, alterations in the T-cell count occur mostly during the shift from moderately to poorly differentiated HCC, resulting in immunological subtype differentiation in this phase[30].
Different roles of T cells in the TME
T cells play an important role in the progression of liver diseases to HCC. In an inflammatory setting, such as NASH, regulatory T (Treg) cells decrease while T-helper 17 cells increase[38]. IL-17 released by T-helper 17 exacerbates liver inflammation and promotes hepatocarcinogenesis[39]. After the development of HCC, the number of Foxp3+ GARP+ CTLA-4+ Treg cells increases in the TME, inhibiting the cytotoxic action of CD8+ T cells against tumor cells[40,41]. The infiltration of Treg cells in the TME is associated with the immune excluded class[29].
CD8+ T cells are directly cytotoxic to tumor cells. Two distinct phenotypes of CD8+ T cells in HCC were described: one with low cytotoxic activity, associated with an upregulation of the KLRB gene and a poor prognosis[42]; the other, with a high cytotoxic capacity, associated with the overexpression of the XCL1 gene and a better prognosis[43]. The participation of T cells in the TME is strongly determined by the etiology of liver disease. In NASH-related HCC, there is an excess of CD8+ T cells expressing PD-1[44,45]. These cells develop major-histocompatibility-complex-I-independent cytotoxicity against hepatocytes and lose tumor surveillance functions. This might be due to the metabolic dysregulation of immune system cells, which occurs in NASH[44,45]. In HBV-related HCC, the number of Treg cells increases with the overexpression of PD-1[46]. Furthermore, T cells are susceptible to Bcl-2-like protein 11-mediated apoptosis, which contributes to the tolerogenic milieu of HBV infection[47]. The number of CD8+ T resident memory cells, which probably have cytotoxic activity[46,48], is also enhanced in HBV-related HCC, and they are associated with a favorable prognosis[46]. However, these cells overexpress PD-1, suggesting an immune-exhausted microenvironment[46]. Chronic HCV infection also induces an exhausted phenotype in CD8+ T cells, causing a decrease in the production of interferon (IFN)γ, reduction in the expression of CD127, and overexpression of PD-1 and TIM[49]. Although the number of PD-1+ CD8+ T cells increases in both virus-related and NASH-related HCC, the response to anti-PD-1 ICIs is different in these two scenarios, suggesting that PD-1+ CD8+ T cells play a distinct function[44]. This is dependent on the type of cells with which PD-1+ CD8+ T cells interact. In the high-immune HCC subtype, PD ligand (PD-L)1 is mainly expressed by macrophages, which suggests that the PD-1/PD-L1 interaction plays a role in T cell–macrophage crosstalk rather than in T-cell inhibition by the tumor[30]. Furthermore, macrophages from high-immune subtypes overexpress CD169[30], which is an M1 phenotype marker associated with macrophage-dependent T-cell activation and favorable prognosis in several cancers[50]. Other T cells, such as - T cells, might also be involved in antitumor surveillance, considering that their depletion in tumor tissues is associated with a higher incidence of postoperative recurrence[51].
B cells play an ambivalent role in the TME
B cells play a dual role in HCC, depending on their interaction with other components of the TME. B-cell infiltration occurs in high-immune subtypes and is associated with a better prognosis[30]. On the contrary, B-cell infiltration, when associated with elevated interleukin (IL)-17 production, is a poor prognostic marker[52]. Moreover, regulatory B cells inhibit the activation and cytotoxic activity of CD8+ T cells in NASH-related HCC by expressing PD-L1 and producing IL-10[53].
Role of innate immunity in HCC TME
Along with CD8+ T cells, natural killer (NK) cells act as the main cytotoxic effectors in HCC, contributing to innate immune system tumor surveillance[54]. However, due to the abundance of Treg cells in the TME, the number of intratumoral NK cells decreases and impairs their cytotoxic activity, as well as the production of IFNγ[55,56]. NK T cells play a role in the development of HCC in patients with NASH by triggering the transformation of hepatic stellate cells (HSCs) into fibroblasts[28] and promoting liver inflammation and injury via nuclear factor-B signaling and the production of cytokines[57]. NK T cells show antitumor activity and cooperate with CD4+ T cells to remove senescent hepatocytes from the liver following a chemically induced liver injury[58]. This antitumor property is associated with the chemokine CXC ligand (CXCL)16/CXCR6 hepatic chemokine pathway[58]. The interaction between bile acids and the gut microbiotaregulates CXCL16 expression in the liver sinusoidal endothelial cells[59], suggesting that microbiota the plays a role in the development of HCC.
Other innate immune cells, such as M2-phenotype tumor-associated macrophages and myeloid-derived suppressor cells, might suppress CD8+ T cells by releasing TGF-β and IL-10[39,60,61]. Tumor-associated macrophage abundance in the HCC tissue is associated with a poor prognosis[62,63]. Dendritic cells play an important role in PD-1/PD-L1 crosstalk. In the TME and lymph nodes, PD-L1+ LAMP3+ dendritic cells inhibit circulating CD8+ T cells activated by tumor antigens[64]. This immunoregulatory function primarily occurs through the secretion of IL-10 and the recruitment of Treg cells[65,66].
Tumor cells modulate the immune response
Tumor cells can effectively control the HCC TME. The overexpression of the MYC proto-oncogene in tumor cells is associated with upregulation of PD-L1 on the cell surface[67]. Furthermore, alterations in the WNT–β-catenin signaling pathway decrease the secretion of chemokine CC ligand 5, which affects the recruitment of dendritic cells[68]. The immune-excluded HCC class shows a higher rate of WNT/CTNNB1 gene mutation[36]. Tumor cells in the HCC TME directly recruit immunosuppressive neutrophils by upregulating the chemokine CXCL5[69].
The gut–liver axis influences the TME in HCC
The inflammatory condition associated with the progression of HCC is not limited to the liver. Intestinal inflammation acts as a cofactor in the pathogenesis of HCC[70,71]. This was confirmed by finding a higher fecal calprotectin concentration in patients with cirrhosis and HCC compared to that in healthy subjects or in patients affected by cirrhosis without HCC[70]. The gut microbiota might play an important role in modulating intestinal inflammation with noticeable effects on hepatocarcinogenesis[70-72]. The dominant phyla associated with HCC include Bacteroidetes, Firmicutes and Proteobacteria[73]. Changes in the gut microbiota occur commonly in patients with NAFLD-related cirrhosis, where the microbial diversity decreases compared to that in healthy people[74]. Other proinflammatory bacteria, such as Bacteroides and Ruminococcaceae, are overabundant in individuals with NAFLD-related cirrhosis and HCC[70]. In contrast, depletion of bacteria such as Akkermansia and Bifidobacterium, which have an anti-inflammatory effect, might also occur[70]. The integrity of the gut epithelial and vascular barrier plays an important role in preventing bacteria from entering the portal circulation[75]. Alterations in the gut–liver axis that occur in cirrhosis (such as changes in the gut microbiota composition and impaired bile acid production) might result in the disruption of the gut barrier, thus, increasing intestinal permeability[76]. Hence, intestinal bacteria, along with their antigens and products, such as lipopolysaccharides, can easily reach the liver via the portal system[77]. By binding Toll-like receptors, bacterial antigens activate Kupffer cells and HSCs[78]. This interaction enhances liver inflammation and plays a crucial role in hepatocarcinogenesis[71]. For example, Toll-like receptor 4 activation in Kupffer cells can increase IL-10 production, which suppresses the cytotoxic functions of NK cells[79]. Gram-positive bacteria might also play an important role in NAFLD-related HCC. Their metabolic products, such as deoxycholic acid and lipoteichoic acid, cause senescence of HSCs[80]. Senescent HSCs have a distinct phenotype characterized by higher production of cytokines, chemokines, and matrix-remodeling proteins[81]. In a preclinical mouse model, prostaglandin E2 generated by senescent HSCs was shown to interfere with the TME through the prostaglandin E receptor 4 signaling pathway[82]. Prostaglandin E receptor 4 is a G-protein-coupled receptor that is mostly expressed by immune cells[83]. Its activation may have an immunosuppressive effect by enhancing the infiltration of Tregs and PD1+ CD8+ T cells in the TME[82].
IMMUNOTHERAPY IN NAFLD-RELATED HCC: EVIDENCE AND CONCERNS
Systemic therapies represent the standard of care for unresectable HCC, either in an advanced or intermediate stage, that is unsuitable for further treatment[84]. Over the last few years, ICIs have shown positive therapeutic results, leading researchers to shift their focus from tyrosine kinase inhibitors (TKIs) to ICIs. Several agents have been approved, either alone or in combination, as first-line or second-line treatments for HCC, with some variations in the treatment regimen found in different countries[85] (Table 1).
Table 1.
The main immune checkpoint inhibitors approved for treatment of advanced hepatocellular carcinoma
|
Drug
|
Mechanism of action
|
Efficacy
|
Safety
|
Approval
|
| First-line | ||||
| Atezolizumab (1200 mg, IV) plus bevacizumab (15 mg/kg, IV) every 3 wk | ICI, anti-PD-L1 antibody (atezolizumab) plus antiangiogenic, anti-VEGF-A antibody (bevacizumab) | Improved OS, PFS, ORR vs sorafenib (IMbrave-150 phase III trial[93]) | irAEs1, hypertension, fatigue, proteinuria, pruritus, gastrointestinal bleeding | Approved by FDA and EMA for patients with advanced HCC |
| Tremelimumab (300 mg, IV) plus durvalumab (1500 mg, IV) once, followed by durvalumab (1500 mg, IV) every 4 wk | ICI, anti-CTLA-4 antibody (tremelimumab) plus ICI, anti-PD-L1 antibody (durvalumab) | Improved OS vs sorafenib and favorable benefit-risk ratio (HIMALAYA phase III trial[89]) | Pruritus, irAEs1 | Under evaluation for approval. Granted orphan drug designation by FDA for HCC treatment (2020) |
| Sintilimab (200 mg, IV) plus IBI305 (bevacizumb biosimilar; 15 mg/kg, IV) every 3 wk | ICI, anti-PD-1 antibody (sintilimab) plus antiangiogenic, anti-VEGF-A antibody (IBI305) | Better OS and PFS in HBV-related advanced HCC vs sorafenib (ORIENT-32 phase II/III trial[113]) | Proteinuria, irAEs1, thrombocytopenia, leukopenia, hypertension, fatigue | Approved by NMPA in China for patients with advanced HCC (2021) |
| Second-line | ||||
| pembrolizumab (200 mg, IV) every 3 wk plus best supportive care | ICI, anti-PD-1 monoclonal antibody | Better OS, PFS and ORR in patients post-sorafenib vs placebo (KEYNOTE-394 phase III trial[114] and KEYNOTE-224 phase II trial[115]) | irAEs1, fatigue, pruritus, anorexia | Approved by FDA for advanced HCC post-sorafenib (2018) |
| Nivolumab (1 mg/kg, IV) plus ipilimumab (3 mg/kg, IV) every 3 wk for 4 cycles, followed by nivolumab (240 mg, IV) every 2 wk | ICI, anti-PD-1 monoclonal antibody (nivolumab) plus ICI, anti-CTLA-4 antibody (ipilimumab) | Promising OS and durable response post-sorafenib (cohort 4 of CheckMate-040 phase I/II trial[90]). CheckMate 9DW phase III trial ongoing[116] | Pruritus, irAEs1 | Approval by FDA for advanced HCC post-sorafenib (2020) |
Immune-related adverse events include hepatitis, colitis, pneumonia, endocrinopathy, skin rash, neurological disorders.
IV: Intravenous administration; ICI: Immune checkpoint inhibitor; VEGF-A: Vascular endothelial growth factor-A; OS: Overall survival; PFS: Progression free survival; ORR: Objective response rate; irAEs: Immune-related adverse events; FDA: Food and Drug Administration; EMA: European Medicines Agency; HCC: Hepatocellular carcinoma; HBV: Hepatitis B virus; NMPA: National Medical Products Administration.
Effectiveness of immunotherapy in NAFLD-related HCC
Subgroup analyses of survival outcomes based on trials evaluating the efficacy of ICIs as first-line treatment revealed a discrepancy between HCC associated with HBV or HCV infection (viral HCC) compared to liver disease of other etiology (nonviral HCC), including NASH-related HCC (Table 2). To our knowledge, none of the clinical trials that evaluated the efficacy of immunotherapy for the treatment of HCC differentiated the nonviral HCC subgroup of patients, thus including cases of HCC associated with NASH, alcohol use disorder, autoimmune hepatitis, primary biliary cholangitis, or sclerosing cholangitis.
Table 2.
Overall survival of patients with hepatocellular carcinoma receiving first-line immunotherapy alone or in combination, based on the etiology of the liver disease
|
Treatment
|
HCC etiology
|
HR (95%CI)
|
Trial
|
Phase
|
| Atezolizumab plus bevacizumab vs sorafenib in first-line | Nonviral HCC | 1.05 (0.68-1.63) | IMbrave150[93] | III |
| HBV-HCC | 0.58 (0.40-0.83) | |||
| HCV-HCC | 0.43 (0.25-0.73) | |||
| Nivolumab vs sorafenib in first-line | Nonviral HCC | 0.91 (0.72-1.16) | CheckMate-459[86] | III |
| HBV-HCC | 0.79 (0.59-1.07) | |||
| HCV-HCC | 0.72 (CI 0.51-1.02) | |||
| Atezolizumab plus cabozantinib vs sorafenib in first-line | Nonviral HCC | 1.18 (0.78–1.79) | COSMIC-312[99] | III |
| HBV-HCC | 0.53 (0.33-0.87) | |||
| HCV-HCC | 1.10 (0.72-1.68) | |||
| Tremelimumab 300 mg × 1 dose + Durvalumab 1500 mg vs sorafenib in first-line | Nonviral HCC | 0.74 (0.57-0.95) | HIMALAYA[89] | III |
| HBV-HCC | 0.64 (0.48-0.86) | |||
| HCV-HCC | 1.06 (0.76-1.49) |
HCC: Hepatocellular carcinoma; OS: Overall survival; HBV: Hepatitis B virus; HCV: Hepatitis C virus.
In the CheckMate-459 trial, nivolumab treatment was found to be associated with slightly lower median overall survival (OS) than sorafenib in the nonviral HCC group (16 mo vs 17.4 mo; HR: 0.91; 95%CI: 0.72–1.16), while the best results were obtained in the viral HCC group (HCV-HCC patients: 17.5 vs 12.7 mo; HBV-HCC patients: 16.1 vs 10.4 mo)[86]. In the KEYNOTE-240 trial, pembrolizumab showed higher OS in HCC of any etiology compared to placebo, but better results were reported in patients with HBV-HCC (HR: 0.57; 95%CI: 0.35–0.94) than in those with nonviral HCC (HR: 0.88; 95%CI: 0.64–1.20)[87]. In the study 22 phase 1/2 trial that evaluated the effectiveness of the combination of tremelimumab plus durvalumab, HBV-HCC and nonviral HCC patients showed comparable OS results (14.4 and 13.8 mo, respectively), which differed from the results of the HCV-HCC patients (22.3 mo)[88]. In the subsequent HIMALAYA phase 3 study[89], tremelimumab plus durvalumab showed longer OS than sorafenib in HBV-HCC patients (HR: 0.64; 95%CI: 0.48–0.86) and in nonviral HCC patients (HR: 0.74; 95%CI: 0.57–0.95), which was opposite to that found in the HCV-HCC patients (HR: 1.06; 95%CI: 0.76–1.49).
Regarding second-line regimens, in cohort 4 of the CheckMate-040 trial, which investigated the therapeutic efficacy of three different dosing regimens of nivolumab plus ipilimumab, the OS benefit was similar in the nonviral HCC (14.7 mo) and HBV-HCC (15.2 mo) groups, while the OS in the HCV-HCC group was significantly higher (21.9 mo)[90].
Thus, the results of several studies supported the hypothesis that the underlying etiology might influence tumor response to immunotherapy. As shown by Foerster et al[91], this is particularly relevant in the case of NASH-related HCC, which is frequently identified at an advanced stage when systemic therapy becomes necessary. Regarding this, the authors highlighted some clinical issues. First, there are no effective strategies to prevent the development of HCC in NASH. Second, many cases of NASH-related HCCs arise in the absence of cirrhosis, but it is not known which subgroup of NASH patients might have a higher oncogenic risk and benefit from a surveillance program. Third, the low efficacy of ICIs in NASH-related HCC was concluded from post hoc analyses of phase III studies, which prevented definitive inferences from being drawn.
Tumor immune surveillance in NAFLD-related HCC and its association with efficacy of immunotherapy
Pfister et al[44] investigated the role of adaptive immunity in both NASH and NASH-related HCC and its effects on the efficacy of ICIs. In mice with steatohepatitis, an increase in hepatic resident-like CD8+ PD1+ T cells with characteristics such as exhaustion and effector functions were observed. Moreover, NASH severity was correlated with PD-L1 expression in hepatocytes and nonparenchymal cells. These results suggested that steatohepatitis-related HCC might significantly benefit from treatment with ICIs. However, when mice were administered anti-PD-1 immunotherapy, they did not show any tumor regression; instead, liver fibrosis increased. Conversely, mice affected by HCC of other origin had a positive response to anti-PD-1 immunotherapy. Furthermore, in NASH mice without HCC, the pre-emptive depletion of CD8+ PD1+ T cells significantly decreased liver damage and, consequently, the incidence of HCC. Pre-emptive treatment with ICIs increased PD1+ CD8+ T-cell infiltration in the liver and the incidence of HCC. When anti-tumor necrosis factor (TNF) or anti-CD8 antibodies were administered together with anti-PD-1 antibodies, liver damage and HCC incidence decreased compared to the reduction in liver damage after anti-PD-1 treatment alone. In humans, PD1+ T cells were absent in healthy livers, but they were abundant in NASH livers and expressed the same gene profile as PD1+ T cells found in mice. In summary, CD8+ PD1+ T cells failed to provide adequate antitumor immune surveillance in NASH-related HCC after immunotherapy. Instead, they triggered the transition to liver cancer through a TNF-dependent mechanism that was further enhanced by anti-PD-1 treatment. Although the study included data from patients with NASH, further investigations need to be conducted to elucidate the true effect of ICIs on NASH-related HCC in the clinical setting.
Benefits of antiangiogenic drugs in NAFLD-related HCC
Retrospective studies analyzed the response of tumors to lenvatinib and sorafenib, two TKIs with anti-VEGF activity, associated with the etiology of HCC. While some studies[92-94] did not find significant differences, Shimose et al[95] reported that NAFLD/NASH etiology was associated with greater survival of the patients treated with sorafenib. This was also confirmed by the REACH-2 trial, which showed that second-line treatment with ramucirumab (an inhibitor of VEGF receptor 2) achieved higher OS in nonviral than in viral HCC patients compared to placebo (HR: 0.633; 95%CI: 0.379–1.057 vs HR: 0.762; 95%CI: 0.435–1.334 vs HR: 0.838; 95%CI: 0.522–1.347, respectively)[96]. However, another study did not find any difference in the OS of patients with NAFLD/NASH who received sequential therapy after sorafenib treatment compared to those with viral or alcohol-related etiology of liver disease[95]. The favorable effect of angiogenesis inhibition on nonviral HCC was confirmed by administering combination therapies. In phase 3 IMbrave150 trial, the combination of atezolizumab plus bevacizumab showed considerable improvement in the OS compared to the OS after treatment with sorafenib in the HCV-HCC group (24.6 vs 12.6 mo; HR: 0.43; 95%CI: 0.25–0.73) and in the HBV-HCC group (19.0 vs 12.4 mo; HR: 0.58; 95%CI: 0.40–0.83). However, no improvement in the OS was observed in the nonviral HCC group (17.0 vs 18.1 mo; HR: 1.05; 95%CI: 0.68–1.63)[97]. There was also a significant improvement in the objective response rate (ORR) (26.5% vs 9.4%; HR: 3.47; 95%CI: 1.24–9.65) and the progression-free survival (PFS) (7.1 vs 5.6 mo; HR: 0.80; 95%CI: 0.55–1.17) compared to the Sorafenib in the nonviral HCC group. Moreover, upon comparing the effectiveness of atezolizumab plus bevacizumab treatment with that of atezolizumab treatment, it became clear that the improvement in the PFS was related to the addition of the anti-VEGF drug (6.3 vs 3.4 mo; HR: 0.49; 80%CI: 0.26–0.92)[98]. Cabozantinib is another TKI featuring an anti-VEGF effect. The phase 3 COSMIC-312 trial[99] investigated its efficacy in combination with the anti-PD-L1 atezolizumab versus sorafenib in the first-line treatment of advanced HCC. The PFS and preliminary interim OS results were assessed, while follow-up for the final OS analysis is ongoing. Overall, cabozantinib plus atezolizumab did not show any improvement in the OS compared to sorafenib at the interim analysis (15.4 vs 15.5 mo; HR: 0.90; 96%CI: 0.69–1.18), while the PFS was significantly higher in the subgroup treated with the combination therapy (6.8 vs 4.2 mo; HR: 0.63; 99%CI: 0.44–0.91). Specifically, compared to sorafenib, atezolizumab plus cabozantinib showed the best results in the HBV-HCC patients (PFS: 6.7 vs 2.7 mo; HR: 0.46, 95%CI: 0.29–0.73; OS: 18.2 vs 14.9 mo; HR: 0.53, 95%CI: 0.33–0.87), whereas, modest improvements were observed in the HCV-HCC patients (PFS: 7.9 vs 5.6 mo; HR: 0.64, 95%CI: 0.38–1.09; OS: 13.6 vs 14.0 mo; HR: 1.1, 95%CI: 0.72–1.68) and no benefit was found in the nonviral HCC subgroup (PFS: 5.8 vs 7.0 mo; HR: 0.92, 95%CI: 0.60–1.41; OS: 15.2 mo vs not reached; HR: 1.18, 95%CI: 0.78–1.79). Two meta-analyses of the CheckMate-459, KEYNOTE-240, and IMbrave150 phase 3 trials[44,100] confirmed that anti-PD-(L)1 therapy resulted in lower OS in nonviral HCC compared to that in viral HCC. Haber et al[100] also conducted a meta-analysis of five phase 3 trials to assess TKIs or anti-VEGF in the second-line setting (REACH, REACH-2, METIV-HCC, CELESTIAL, and JET-HCC) and showed that survival outcomes were not influenced by the HCC etiology (viral HCC-pooled HR: 0.81; 95%CI: 0.71–0.92; nonviral HCC-pooled HR: 0.82; 95%CI: 0.67–1.01). These results highlighted the synergistic effect of anti-VEGF and anti-PD-(L)1 agents for the treatment of HCC associated with liver disease of any etiology, especially in the nonviral setting.
Potential applications of biomarkers in immunotherapy for HCC
So far, PD-L1 expression assessed by immunohistochemistry on tumor tissue is the only approved biomarker to identify patients with higher probability to respond to ICIs[101]. However, as discussed above, PD-1/PD-L1 signaling is involved in complex and partially unclear molecular pathways that could limit the role of PD-L1 tissue expression as a reliable predictive factor. Some PD-L1-negative patients benefit from immunotherapy, whereas some PD-L1-positive patients do not. Hence, biomarkers identified through liquid biopsy, such as circulating tumor DNA[102], miRNAs[103], tumor cells[104], and extracellular vesicles[105], have been considered. However, no biological marker has demonstrated a strong predictive value in patients with HCC[106]. Scheiner et al[107] developed and proposed the C-reactive protein and -fetoprotein in immunotherapy (CRAFITY) score as an easily applicable clinical tool to predict response to ICIs in patients with HCC. The score ranges from 0 (C-reactive protein < 1 mg/dL and -fetoprotein < 100 ng/mL) to 2 (C-reactive protein ≥ 1 mg/dL and -fetoprotein ≥ 100 ng/mL). The authors found that higher scores indicated shorter OS and a worse radiological response. The gut microbiota might play a significant role in predicting the response to ICIs and modulating the immune response, thus, affecting the effectiveness of immunotherapy. Microbiological changes and intestinal permeability that occur in cirrhosis increase the interaction between hepatic cells and proinflammatory intestinal bacteria, enhancing inflammation in the liver[70]. Anti-inflammatory bacteria, such as Akkermansia and Bifidobacterium, are usually scarce in NASH-related HCC patients and might lead to a persisting inflammatory response with the suppression of immune system surveillance in the long term. An increase in the abundance of Akkermansia along with a reduction in Enterobacteriaceae occurs in patients who respond to ICI therapy[108,109]. Furthermore, the composition of the gut microbiota changes over time during immunotherapy, which might be associated with a modification in the expression of immunomodulating pathways or vice versa may be a result of the modulating effect of the immune system on the gut microenvironment. Whether these modifications can predict responses that are essential for making further treatment decisions[106] needs further confirmation. Based on these findings, the oral administration of Akkermansia muciniphila was suggested to enhance the effect of ICIs[110]. Similar findings were observed in patients affected by epithelial cancer[110], colorectal cancer[111], and lung cancer[112].
CONCLUSION
In patients with HCC related to NASH, antitumor immune surveillance is impaired. The weaker efficacy of ICIs in NASH-related HCC contradicts the obesity paradox, in which mild obesity predicts a better response in patients with melanoma and other cancers treated with immunotherapy. Tremelimumab plus durvalumab was the only combination of two ICIs tested against sorafenib in the first-line setting and the only one that showed a relatively higher OS in the nonviral and viral HCC subgroups. Some studies showed similar efficacy in the treatment of viral versus nonviral HCC for the combination of nivolumab plus ipilimumab. Thus, regimens based on the combination of two ICIs rather than ICI monotherapy are promising for HCC treatment, regardless of the etiology of the liver disease. Similar results were reported for TKIs with anti-VEGF activity, including anti-VEGF and anti-VEGF receptor agents, and some studies found better results in NAFLD/NASH patients. Therefore, the addition of antiangiogenic agents might increase the efficacy of immunotherapy and improve responses in patients who have an impaired immune system, such as patients with nonviral HCC. This was confirmed by the effectiveness of atezolizumab plus bevacizumab in improving survival regardless of the liver disease etiology, along with a higher PFS in nonviral HCC. However, this preliminary evidence generated by post hoc analyses or meta-analyses needs validation. Indeed, no clinical trial could differentially address the outcome of NASH-driven HCC compared to HCC of other nonviral etiology, such as alcohol-related HCC. Future studies should distinguish patient populations based on the underlying liver disease for a specific analysis of clinical outcomes and a better understanding of the mechanisms that trigger tumor immune escape in these patients.
Overall, these findings suggest that changes are required in the current algorithm of advanced HCC treatment toward a strategy that involves the administration of highly specific and optimized therapies based on the etiology of liver disease. The stratification of the patients is hampered by intragroup molecular heterogeneity. Thus, a model based on histological or circulating biomarkers might be critical for predicting responses to immunotherapy and defining a personalized strategy. However, biomarkers that can predict the outcome of immunotherapy have not been identified yet; the CRAFITY score provided encouraging results but required prospective validation. Despite compelling evidence regarding the role of the gut–liver axis in NAFLD-associated HCC, putting the theoretical knowledge into practice, either to categorize patients or enhance the response to treatment, is still a work in progress.
ACKNOWLEDGMENTS
Thanks to Fondazione Roma for continuous support to our scientific research.
Footnotes
Conflict-of-interest statement: The authors have no conflict of interest to declare.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 18, 2022
First decision: April 17, 2022
Article in press: July 5, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hatanaka T, Japan; Oura S, Japan S-Editor: Zhang H L-Editor: Kerr C P-Editor: Zhang H
Contributor Information
Federico Costante, Internal Medicine and Gastroenterology-Hepatology Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Roma 00168, Italy.
Carlo Airola, Internal Medicine and Gastroenterology-Hepatology Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Roma 00168, Italy.
Francesco Santopaolo, Internal Medicine and Gastroenterology-Hepatology Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Roma 00168, Italy.
Antonio Gasbarrini, Internal Medicine and Gastroenterology-Hepatology Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Roma 00168, Italy; Catholic University, Largo Francesco Vito 1, 00168 Roma, Italy.
Maurizio Pompili, Internal Medicine and Gastroenterology-Hepatology Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Roma 00168, Italy; Catholic University, Largo Francesco Vito 1, 00168 Roma, Italy.
Francesca Romana Ponziani, Internal Medicine and Gastroenterology-Hepatology Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Roma 00168, Italy; Catholic University, Largo Francesco Vito 1, 00168 Roma, Italy. francesca.ponziani@gmail.com.
References
- 1.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed A, Wong RJ, Harrison SA. Nonalcoholic Fatty Liver Disease Review: Diagnosis, Treatment, and Outcomes. Clin Gastroenterol Hepatol. 2015;13:2062–2070. doi: 10.1016/j.cgh.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 5.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 6.Li B, Zhang C, Zhan YT. Nonalcoholic Fatty Liver Disease Cirrhosis: A Review of Its Epidemiology, Risk Factors, Clinical Presentation, Diagnosis, Management, and Prognosis. Can J Gastroenterol Hepatol. 2018;2018:2784537. doi: 10.1155/2018/2784537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188–2195. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A. MAFLD vs NAFLD: Where are we? Dig Liver Dis. 2021;53:1368–1372. doi: 10.1016/j.dld.2021.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223–238. doi: 10.1038/s41575-020-00381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ioannou GN. Epidemiology and risk-stratification of NAFLD-associated HCC. J Hepatol. 2021;75:1476–1484. doi: 10.1016/j.jhep.2021.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, Hunt S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723–1730. doi: 10.1002/hep.28123. [DOI] [PubMed] [Google Scholar]
- 12.Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149:1471–1482.e5; quiz e17. doi: 10.1053/j.gastro.2015.07.056. [DOI] [PubMed] [Google Scholar]
- 13.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73 Suppl 1:4–13. doi: 10.1002/hep.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guarino M, Viganò L, Ponziani FR, Giannini EG, Lai Q, Morisco F Special Interest Group on Hepatocellular carcinoma and new anti-HCV therapies” of the Italian Association for the Study of the Liver. Recurrence of hepatocellular carcinoma after direct acting antiviral treatment for hepatitis C virus infection: Literature review and risk analysis. Dig Liver Dis. 2018;50:1105–1114. doi: 10.1016/j.dld.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, Bellentani S HCC-NAFLD Italian Study Group. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology. 2016;63:827–838. doi: 10.1002/hep.28368. [DOI] [PubMed] [Google Scholar]
- 18.White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342–1359.e2. doi: 10.1016/j.cgh.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang JD, Ahmed F, Mara KC, Addissie BD, Allen AM, Gores GJ, Roberts LR. Diabetes Is Associated With Increased Risk of Hepatocellular Carcinoma in Patients With Cirrhosis From Nonalcoholic Fatty Liver Disease. Hepatology. 2020;71:907–916. doi: 10.1002/hep.30858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanwal F, Kramer JR, Li L, Dai J, Natarajan Y, Yu X, Asch SM, El-Serag HB. Effect of Metabolic Traits on the Risk of Cirrhosis and Hepatocellular Cancer in Nonalcoholic Fatty Liver Disease. Hepatology. 2020;71:808–819. doi: 10.1002/hep.31014. [DOI] [PubMed] [Google Scholar]
- 21.Kawamura Y, Arase Y, Ikeda K, Seko Y, Imai N, Hosaka T, Kobayashi M, Saitoh S, Sezaki H, Akuta N, Suzuki F, Suzuki Y, Ohmoto Y, Amakawa K, Tsuji H, Kumada H. Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol. 2012;107:253–261. doi: 10.1038/ajg.2011.327. [DOI] [PubMed] [Google Scholar]
- 22.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Nair S, Mason A, Eason J, Loss G, Perrillo RP. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology. 2002;36:150–155. doi: 10.1053/jhep.2002.33713. [DOI] [PubMed] [Google Scholar]
- 24.Sangro B, Sarobe P, Hervás-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:525–543. doi: 10.1038/s41575-021-00438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dongiovanni P, Meroni M, Longo M, Fargion S, Fracanzani AL. Genetics, Immunity and Nutrition Boost the Switching from NASH to HCC. Biomedicines. 2021;9 doi: 10.3390/biomedicines9111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Younossi ZM, Rinella ME, Sanyal AJ, Harrison SA, Brunt EM, Goodman Z, Cohen DE, Loomba R. From NAFLD to MAFLD: Implications of a Premature Change in Terminology. Hepatology. 2021;73:1194–1198. doi: 10.1002/hep.31420. [DOI] [PubMed] [Google Scholar]
- 27.Wree A, Broderick L, Canbay A, Hoffman HM, Feldstein AE. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol. 2013;10:627–636. doi: 10.1038/nrgastro.2013.149. [DOI] [PubMed] [Google Scholar]
- 28.Koo SY, Park EJ, Lee CW. Immunological distinctions between nonalcoholic steatohepatitis and hepatocellular carcinoma. Exp Mol Med. 2020;52:1209–1219. doi: 10.1038/s12276-020-0480-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sia D, Jiao Y, Martinez-Quetglas I, Kuchuk O, Villacorta-Martin C, Castro de Moura M, Putra J, Camprecios G, Bassaganyas L, Akers N, Losic B, Waxman S, Thung SN, Mazzaferro V, Esteller M, Friedman SL, Schwartz M, Villanueva A, Llovet JM. Identification of an Immune-specific Class of Hepatocellular Carcinoma, Based on Molecular Features. Gastroenterology. 2017;153:812–826. doi: 10.1053/j.gastro.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurebayashi Y, Ojima H, Tsujikawa H, Kubota N, Maehara J, Abe Y, Kitago M, Shinoda M, Kitagawa Y, Sakamoto M. Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification. Hepatology. 2018;68:1025–1041. doi: 10.1002/hep.29904. [DOI] [PubMed] [Google Scholar]
- 31.Giraud J, Chalopin D, Blanc JF, Saleh M. Hepatocellular Carcinoma Immune Landscape and the Potential of Immunotherapies. Front Immunol. 2021;12:655697. doi: 10.3389/fimmu.2021.655697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, Pikarsky E, Zhu AX, Finn RS. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19:151–172. doi: 10.1038/s41571-021-00573-2. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Gingold JA, Su X. Immunomodulatory TGF-β Signaling in Hepatocellular Carcinoma. Trends Mol Med. 2019;25:1010–1023. doi: 10.1016/j.molmed.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Chen J, Zaidi S, Rao S, Chen JS, Phan L, Farci P, Su X, Shetty K, White J, Zamboni F, Wu X, Rashid A, Pattabiraman N, Mazumder R, Horvath A, Wu RC, Li S, Xiao C, Deng CX, Wheeler DA, Mishra B, Akbani R, Mishra L. Analysis of Genomes and Transcriptomes of Hepatocellular Carcinomas Identifies Mutations and Gene Expression Changes in the Transforming Growth Factor-β Pathway. Gastroenterology. 2018;154:195–210. doi: 10.1053/j.gastro.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blank CU, Haining WN, Held W, Hogan PG, Kallies A, Lugli E, Lynn RC, Philip M, Rao A, Restifo NP, Schietinger A, Schumacher TN, Schwartzberg PL, Sharpe AH, Speiser DE, Wherry EJ, Youngblood BA, Zehn D. Defining 'T cell exhaustion'. Nat Rev Immunol. 2019;19:665–674. doi: 10.1038/s41577-019-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinyol R, Sia D, Llovet JM. Immune Exclusion-Wnt/CTNNB1 Class Predicts Resistance to Immunotherapies in HCC. Clin Cancer Res. 2019;25:2021–2023. doi: 10.1158/1078-0432.CCR-18-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599–616. doi: 10.1038/s41571-018-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rau M, Schilling AK, Meertens J, Hering I, Weiss J, Jurowich C, Kudlich T, Hermanns HM, Bantel H, Beyersdorf N, Geier A. Progression from Nonalcoholic Fatty Liver to Nonalcoholic Steatohepatitis Is Marked by a Higher Frequency of Th17 Cells in the Liver and an Increased Th17/Resting Regulatory T Cell Ratio in Peripheral Blood and in the Liver. J Immunol. 2016;196:97–105. doi: 10.4049/jimmunol.1501175. [DOI] [PubMed] [Google Scholar]
- 39.Ringelhan M, Pfister D, O'Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19:222–232. doi: 10.1038/s41590-018-0044-z. [DOI] [PubMed] [Google Scholar]
- 40.Yang XH, Yamagiwa S, Ichida T, Matsuda Y, Sugahara S, Watanabe H, Sato Y, Abo T, Horwitz DA, Aoyagi Y. Increase of CD4+ CD25+ regulatory T-cells in the liver of patients with hepatocellular carcinoma. J Hepatol. 2006;45:254–262. doi: 10.1016/j.jhep.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 41.Kalathil S, Lugade AA, Miller A, Iyer R, Thanavala Y. Higher frequencies of GARP(+)CTLA-4(+)Foxp3(+) T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res. 2013;73:2435–2444. doi: 10.1158/0008-5472.CAN-12-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Y, Wu L, Zhong Y, Zhou K, Hou Y, Wang Z, Zhang Z, Xie J, Wang C, Chen D, Huang Y, Wei X, Shi Y, Zhao Z, Li Y, Guo Z, Yu Q, Xu L, Volpe G, Qiu S, Zhou J, Ward C, Sun H, Yin Y, Xu X, Wang X, Esteban MA, Yang H, Wang J, Dean M, Zhang Y, Liu S, Yang X, Fan J. Single-cell landscape of the ecosystem in early-relapse hepatocellular carcinoma. Cell. 2021;184:404–421.e16. doi: 10.1016/j.cell.2020.11.041. [DOI] [PubMed] [Google Scholar]
- 43.Song G, Shi Y, Zhang M, Goswami S, Afridi S, Meng L, Ma J, Chen Y, Lin Y, Zhang J, Liu Y, Jin Z, Yang S, Rao D, Zhang S, Ke A, Wang X, Cao Y, Zhou J, Fan J, Zhang X, Xi R, Gao Q. Global immune characterization of HBV/HCV-related hepatocellular carcinoma identifies macrophage and T-cell subsets associated with disease progression. Cell Discov. 2020;6:90. doi: 10.1038/s41421-020-00214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, Gupta R, Qiu M, Deczkowska A, Weiner A, Müller F, Sinha A, Friebel E, Engleitner T, Lenggenhager D, Moncsek A, Heide D, Stirm K, Kosla J, Kotsiliti E, Leone V, Dudek M, Yousuf S, Inverso D, Singh I, Teijeiro A, Castet F, Montironi C, Haber PK, Tiniakos D, Bedossa P, Cockell S, Younes R, Vacca M, Marra F, Schattenberg JM, Allison M, Bugianesi E, Ratziu V, Pressiani T, D'Alessio A, Personeni N, Rimassa L, Daly AK, Scheiner B, Pomej K, Kirstein MM, Vogel A, Peck-Radosavljevic M, Hucke F, Finkelmeier F, Waidmann O, Trojan J, Schulze K, Wege H, Koch S, Weinmann A, Bueter M, Rössler F, Siebenhüner A, De Dosso S, Mallm JP, Umansky V, Jugold M, Luedde T, Schietinger A, Schirmacher P, Emu B, Augustin HG, Billeter A, Müller-Stich B, Kikuchi H, Duda DG, Kütting F, Waldschmidt DT, Ebert MP, Rahbari N, Mei HE, Schulz AR, Ringelhan M, Malek N, Spahn S, Bitzer M, Ruiz de Galarreta M, Lujambio A, Dufour JF, Marron TU, Kaseb A, Kudo M, Huang YH, Djouder N, Wolter K, Zender L, Marche PN, Decaens T, Pinato DJ, Rad R, Mertens JC, Weber A, Unger K, Meissner F, Roth S, Jilkova ZM, Claassen M, Anstee QM, Amit I, Knolle P, Becher B, Llovet JM, Heikenwalder M. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592:450–456. doi: 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dudek M, Pfister D, Donakonda S, Filpe P, Schneider A, Laschinger M, Hartmann D, Hüser N, Meiser P, Bayerl F, Inverso D, Wigger J, Sebode M, Öllinger R, Rad R, Hegenbarth S, Anton M, Guillot A, Bowman A, Heide D, Müller F, Ramadori P, Leone V, Garcia-Caceres C, Gruber T, Seifert G, Kabat AM, Mallm JP, Reider S, Effenberger M, Roth S, Billeter AT, Müller-Stich B, Pearce EJ, Koch-Nolte F, Käser R, Tilg H, Thimme R, Boettler T, Tacke F, Dufour JF, Haller D, Murray PJ, Heeren R, Zehn D, Böttcher JP, Heikenwälder M, Knolle PA. Auto-aggressive CXCR6+ CD8 T cells cause liver immune pathology in NASH. Nature. 2021;592:444–449. doi: 10.1038/s41586-021-03233-8. [DOI] [PubMed] [Google Scholar]
- 46.Lim CJ, Lee YH, Pan L, Lai L, Chua C, Wasser M, Lim TKH, Yeong J, Toh HC, Lee SY, Chan CY, Goh BK, Chung A, Heikenwälder M, Ng IO, Chow P, Albani S, Chew V. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma. Gut. 2019;68:916–927. doi: 10.1136/gutjnl-2018-316510. [DOI] [PubMed] [Google Scholar]
- 47.Maini MK, Pallett LJ. Defective T-cell immunity in hepatitis B virus infection: why therapeutic vaccination needs a helping hand. Lancet Gastroenterol Hepatol. 2018;3:192–202. doi: 10.1016/S2468-1253(18)30007-4. [DOI] [PubMed] [Google Scholar]
- 48.Amsen D, van Gisbergen KPJM, Hombrink P, van Lier RAW. Tissue-resident memory T cells at the center of immunity to solid tumors. Nat Immunol. 2018;19:538–546. doi: 10.1038/s41590-018-0114-2. [DOI] [PubMed] [Google Scholar]
- 49.Heim MH, Thimme R. Innate and adaptive immune responses in HCV infections. J Hepatol. 2014;61:S14–S25. doi: 10.1016/j.jhep.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 50.Kong W, Wei M, Liu R, Zhang J, Wang X. Prognostic value of CD169-positive macrophages in various tumors: a meta-analysis. Bioengineered. 2021;12:8505–8514. doi: 10.1080/21655979.2021.1985857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cai XY, Wang JX, Yi Y, He HW, Ni XC, Zhou J, Cheng YF, Jin JJ, Fan J, Qiu SJ. Low counts of γδ T cells in peritumoral liver tissue are related to more frequent recurrence in patients with hepatocellular carcinoma after curative resection. Asian Pac J Cancer Prev. 2014;15:775–780. doi: 10.7314/apjcp.2014.15.2.775. [DOI] [PubMed] [Google Scholar]
- 52.Liu RX, Wei Y, Zeng QH, Chan KW, Xiao X, Zhao XY, Chen MM, Ouyang FZ, Chen DP, Zheng L, Lao XM, Kuang DM. Chemokine (C-X-C motif) receptor 3-positive B cells link interleukin-17 inflammation to protumorigenic macrophage polarization in human hepatocellular carcinoma. Hepatology. 2015;62:1779–1790. doi: 10.1002/hep.28020. [DOI] [PubMed] [Google Scholar]
- 53.Shalapour S, Lin XJ, Bastian IN, Brain J, Burt AD, Aksenov AA, Vrbanac AF, Li W, Perkins A, Matsutani T, Zhong Z, Dhar D, Navas-Molina JA, Xu J, Loomba R, Downes M, Yu RT, Evans RM, Dorrestein PC, Knight R, Benner C, Anstee QM, Karin M. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature. 2017;551:340–345. doi: 10.1038/nature24302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruf B, Heinrich B, Greten TF. Immunobiology and immunotherapy of HCC: spotlight on innate and innate-like immune cells. Cell Mol Immunol. 2021;18:112–127. doi: 10.1038/s41423-020-00572-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S, Shi M, Zhang H, Yang Y, Wu H, Tien P, Wang FS. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol. 2008;129:428–437. doi: 10.1016/j.clim.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 56.Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten TF, Korangy F. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50:799–807. doi: 10.1002/hep.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolf MJ, Adili A, Piotrowitz K, Abdullah Z, Boege Y, Stemmer K, Ringelhan M, Simonavicius N, Egger M, Wohlleber D, Lorentzen A, Einer C, Schulz S, Clavel T, Protzer U, Thiele C, Zischka H, Moch H, Tschöp M, Tumanov AV, Haller D, Unger K, Karin M, Kopf M, Knolle P, Weber A, Heikenwalder M. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26:549–564. doi: 10.1016/j.ccell.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 58.Mossanen JC, Kohlhepp M, Wehr A, Krenkel O, Liepelt A, Roeth AA, Möckel D, Heymann F, Lammers T, Gassler N, Hermann J, Jankowski J, Neumann UP, Luedde T, Trautwein C, Tacke F. CXCR6 Inhibits Hepatocarcinogenesis by Promoting Natural Killer T- and CD4+ T-Cell-Dependent Control of Senescence. Gastroenterology. 2019;156:1877–1889.e4. doi: 10.1053/j.gastro.2019.01.247. [DOI] [PubMed] [Google Scholar]
- 59.Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, Ritz T, Longerich T, Theriot CM, McCulloch JA, Roy S, Yuan W, Thovarai V, Sen SK, Ruchirawat M, Korangy F, Wang XW, Trinchieri G, Greten TF. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360 doi: 10.1126/science.aan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan QM, Jing YY, Yu GF, Kou XR, Ye F, Gao L, Li R, Zhao QD, Yang Y, Lu ZH, Wei LX. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2014;352:160–168. doi: 10.1016/j.canlet.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 61.Zhou J, Ding T, Pan W, Zhu LY, Li L, Zheng L. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer. 2009;125:1640–1648. doi: 10.1002/ijc.24556. [DOI] [PubMed] [Google Scholar]
- 62.Yeung OW, Lo CM, Ling CC, Qi X, Geng W, Li CX, Ng KT, Forbes SJ, Guan XY, Poon RT, Fan ST, Man K. Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma. J Hepatol. 2015;62:607–616. doi: 10.1016/j.jhep.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 63.Li Z, Wu T, Zheng B, Chen L. Individualized precision treatment: Targeting TAM in HCC. Cancer Lett. 2019;458:86–91. doi: 10.1016/j.canlet.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Q, He Y, Luo N, Patel SJ, Han Y, Gao R, Modak M, Carotta S, Haslinger C, Kind D, Peet GW, Zhong G, Lu S, Zhu W, Mao Y, Xiao M, Bergmann M, Hu X, Kerkar SP, Vogt AB, Pflanz S, Liu K, Peng J, Ren X, Zhang Z. Landscape and Dynamics of Single Immune Cells in Hepatocellular Carcinoma. Cell. 2019;179:829–845.e20. doi: 10.1016/j.cell.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 65.Han Y, Chen Z, Yang Y, Jiang Z, Gu Y, Liu Y, Lin C, Pan Z, Yu Y, Jiang M, Zhou W, Cao X. Human CD14+ CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology. 2014;59:567–579. doi: 10.1002/hep.26694. [DOI] [PubMed] [Google Scholar]
- 66.Ormandy LA, Farber A, Cantz T, Petrykowska S, Wedemeyer H, Horning M, Lehner F, Manns MP, Korangy F, Greten TF. Direct ex vivo analysis of dendritic cells in patients with hepatocellular carcinoma. World J Gastroenterol. 2006;12:3275–3282. doi: 10.3748/wjg.v12.i20.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu Y, Poggio M, Jin HY, Shi Z, Forester CM, Wang Y, Stumpf CR, Xue L, Devericks E, So L, Nguyen HG, Griselin A, Gordan JD, Umetsu SE, Reich SH, Worland ST, Asthana S, Barna M, Webster KR, Cunningham JT, Ruggero D. Translation control of the immune checkpoint in cancer and its therapeutic targeting. Nat Med. 2019;25:301–311. doi: 10.1038/s41591-018-0321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruiz de Galarreta M, Bresnahan E, Molina-Sánchez P, Lindblad KE, Maier B, Sia D, Puigvehi M, Miguela V, Casanova-Acebes M, Dhainaut M, Villacorta-Martin C, Singhi AD, Moghe A, von Felden J, Tal Grinspan L, Wang S, Kamphorst AO, Monga SP, Brown BD, Villanueva A, Llovet JM, Merad M, Lujambio A. β-Catenin Activation Promotes Immune Escape and Resistance to Anti-PD-1 Therapy in Hepatocellular Carcinoma. Cancer Discov. 2019;9:1124–1141. doi: 10.1158/2159-8290.CD-19-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou SL, Dai Z, Zhou ZJ, Wang XY, Yang GH, Wang Z, Huang XW, Fan J, Zhou J. Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma. Hepatology. 2012;56:2242–2254. doi: 10.1002/hep.25907. [DOI] [PubMed] [Google Scholar]
- 70.Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, Sanguinetti M, Morelli D, Paroni Sterbini F, Petito V, Reddel S, Calvani R, Camisaschi C, Picca A, Tuccitto A, Gasbarrini A, Pompili M, Mazzaferro V. Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology. 2019;69:107–120. doi: 10.1002/hep.30036. [DOI] [PubMed] [Google Scholar]
- 71.Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R, Lefkowitch JH, Bower M, Friedman R, Sartor RB, Rabadan R, Schwabe RF. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu LX, Yan HX, Liu Q, Yang W, Wu HP, Dong W, Tang L, Lin Y, He YQ, Zou SS, Wang C, Zhang HL, Cao GW, Wu MC, Wang HY. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology. 2010;52:1322–1333. doi: 10.1002/hep.23845. [DOI] [PubMed] [Google Scholar]
- 73.Komiyama S, Yamada T, Takemura N, Kokudo N, Hase K, Kawamura YI. Profiling of tumour-associated microbiota in human hepatocellular carcinoma. Sci Rep. 2021;11:10589. doi: 10.1038/s41598-021-89963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L, Zhou J, Ni S, Liu L, Pons N, Batto JM, Kennedy SP, Leonard P, Yuan C, Ding W, Hu X, Zheng B, Qian G, Xu W, Ehrlich SD, Zheng S, Li L. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59–64. doi: 10.1038/nature13568. [DOI] [PubMed] [Google Scholar]
- 75.Spadoni I, Fornasa G, Rescigno M. Organ-specific protection mediated by cooperation between vascular and epithelial barriers. Nat Rev Immunol. 2017;17:761–773. doi: 10.1038/nri.2017.100. [DOI] [PubMed] [Google Scholar]
- 76.Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020;72:558–577. doi: 10.1016/j.jhep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 77.Achiwa K, Ishigami M, Ishizu Y, Kuzuya T, Honda T, Hayashi K, Hirooka Y, Katano Y, Goto H. DSS colitis promotes tumorigenesis and fibrogenesis in a choline-deficient high-fat diet-induced NASH mouse model. Biochem Biophys Res Commun. 2016;470:15–21. doi: 10.1016/j.bbrc.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 78.Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043–1055. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- 79.Tu Z, Bozorgzadeh A, Pierce RH, Kurtis J, Crispe IN, Orloff MS. TLR-dependent cross talk between human Kupffer cells and NK cells. J Exp Med. 2008;205:233–244. doi: 10.1084/jem.20072195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K, Ishikawa Y, Hara E, Ohtani N. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 81.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Loo TM, Kamachi F, Watanabe Y, Yoshimoto S, Kanda H, Arai Y, Nakajima-Takagi Y, Iwama A, Koga T, Sugimoto Y, Ozawa T, Nakamura M, Kumagai M, Watashi K, Taketo MM, Aoki T, Narumiya S, Oshima M, Arita M, Hara E, Ohtani N. Gut Microbiota Promotes Obesity-Associated Liver Cancer through PGE2-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2017;7:522–538. doi: 10.1158/2159-8290.CD-16-0932. [DOI] [PubMed] [Google Scholar]
- 83.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 84.Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bruix J, Chan SL, Galle PR, Rimassa L, Sangro B. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J Hepatol. 2021;75:960–974. doi: 10.1016/j.jhep.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 86.Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J. CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs. sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC) Ann Oncol. 2019;30 Suppl 5:v874–v875. [Google Scholar]
- 87.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL KEYNOTE-240 investigators. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 88.Kelley RK, Sangro B, Harris W, Ikeda M, Okusaka T, Kang YK, Qin S, Tai DW, Lim HY, Yau T, Yong WP, Cheng AL, Gasbarrini A, Damian S, Bruix J, Borad M, Bendell J, Kim TY, Standifer N, He P, Makowsky M, Negro A, Kudo M, Abou-Alfa GK. Safety, Efficacy, and Pharmacodynamics of Tremelimumab Plus Durvalumab for Patients With Unresectable Hepatocellular Carcinoma: Randomized Expansion of a Phase I/II Study. J Clin Oncol. 2021;39:2991–3001. doi: 10.1200/JCO.20.03555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Abou-Alfa GK, Chan SL, Kudo M, Lau G, Kelley RK, Furuse J. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40:379–379. [Google Scholar]
- 90.Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, Tovoli F, Knox JJ, Ruth He A, El-Rayes BF, Acosta-Rivera M, Lim HY, Neely J, Shen Y, Wisniewski T, Anderson J, Hsu C. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020;6:e204564. doi: 10.1001/jamaoncol.2020.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Foerster F, Gairing SJ, Müller L, Galle PR. NAFLD-driven HCC: Safety and efficacy of current and emerging treatment options. J Hepatol. 2022;76:446–457. doi: 10.1016/j.jhep.2021.09.007. [DOI] [PubMed] [Google Scholar]
- 92.Hiraoka A, Kumada T, Tada T, Tani J, Kariyama K, Fukunishi S, Atsukawa M, Hirooka M, Tsuji K, Ishikawa T, Takaguchi K, Itobayashi E, Tajiri K, Shimada N, Shibata H, Ochi H, Kawata K, Yasuda S, Toyoda H, Aoki T, Tanaka T, Ohama H, Nouso K, Tsutsui A, Nagano T, Itokawa N, Arai T, Okubo T, Imai M, Koizumi Y, Nakamura S, Joko K, Hiasa Y, Kudo M Real-life Practice Experts for HCC (RELPEC) Study Group and HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan) Efficacy of lenvatinib for unresectable hepatocellular carcinoma based on background liver disease etiology: multi-center retrospective study. Sci Rep. 2021;11:16663. doi: 10.1038/s41598-021-96089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Howell J, Samani A, Mannan B, Hajiev , Aval LM, Abdelmalak R. Impact of NAFLD on clinical outcomes in hepatocellular carcinoma treated with sorafenib: An international cohort study. J Clin Oncol. 2021;39:289. doi: 10.1177/17562848221100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hatanaka T, Kakizaki S, Nagashima T, Namikawa M, Ueno T, Tojima H, Takizawa D, Naganuma A, Arai H, Harimoto N, Shirabe K, Uraoka T. Lenvatinib for Hepatocellular Carcinoma Patients with Nonviral Infection Who Were Unlikely to Respond to Immunotherapy: A Retrospective, Comparative Study. Oncology. 2021;99:641–651. doi: 10.1159/000517494. [DOI] [PubMed] [Google Scholar]
- 95.Shimose S, Hiraoka A, Nakano M, Iwamoto H, Tanaka M, Tanaka T, Noguchi K, Aino H, Ogata K, Kajiwara M, Itano S, Yokokura Y, Yamaguchi T, Kawano H, Matsukuma N, Suga H, Niizeki T, Shirono T, Noda Y, Kamachi N, Okamura S, Kawaguchi T, Koga H, Torimura T. First-line sorafenib sequential therapy and liver disease etiology for unresectable hepatocellular carcinoma using inverse probability weighting: A multicenter retrospective study. Cancer Med. 2021;10:8530–8541. doi: 10.1002/cam4.4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 97.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY. IMbrave150: updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC) J Clin Oncol. 2021;39:267. [Google Scholar]
- 98.Lee MS, Ryoo BY, Hsu CH, Numata K, Stein S, Verret W, Hack SP, Spahn J, Liu B, Abdullah H, Wang Y, He AR, Lee KH GO30140 investigators. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21:808–820. doi: 10.1016/S1470-2045(20)30156-X. [DOI] [PubMed] [Google Scholar]
- 99.Kelley RK, Yau T, Cheng AL, Kaseb A, Qin S, Zhu A. VP10-2021: Cabozantinib (C) plus atezolizumab (A) vs sorafenib (S) as first-line systemic treatment for advanced hepatocellular carcinoma (aHCC): Results from the randomized phase III COSMIC-312 trial. Ann Oncol. 2022;33:114–116. [Google Scholar]
- 100.Haber PK, Puigvehí M, Castet F, Lourdusamy V, Montal R, Tabrizian P, Buckstein M, Kim E, Villanueva A, Schwartz M, Llovet JM. Evidence-Based Management of Hepatocellular Carcinoma: Systematic Review and Meta-analysis of Randomized Controlled Trials (2002-2020) Gastroenterology. 2021;161:879–898. doi: 10.1053/j.gastro.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Mattos-Arruda L, Siravegna G. How to use liquid biopsies to treat patients with cancer. ESMO Open. 2021;6:100060. doi: 10.1016/j.esmoop.2021.100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alunni-Fabbroni M, Rönsch K, Huber T, Cyran CC, Seidensticker M, Mayerle J, Pech M, Basu B, Verslype C, Benckert J, Malfertheiner P, Ricke J. Circulating DNA as prognostic biomarker in patients with advanced hepatocellular carcinoma: a translational exploratory study from the SORAMIC trial. J Transl Med. 2019;17:328. doi: 10.1186/s12967-019-2079-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yamamoto Y, Kondo S, Matsuzaki J, Esaki M, Okusaka T, Shimada K, Murakami Y, Enomoto M, Tamori A, Kato K, Aoki Y, Takizawa S, Sakamoto H, Niida S, Takeshita F, Ochiya T. Highly Sensitive Circulating MicroRNA Panel for Accurate Detection of Hepatocellular Carcinoma in Patients With Liver Disease. Hepatol Commun. 2020;4:284–297. doi: 10.1002/hep4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.von Felden J, Schulze K, Krech T, Ewald F, Nashan B, Pantel K, Lohse AW, Riethdorf S, Wege H. Circulating tumor cells as liquid biomarker for high HCC recurrence risk after curative liver resection. Oncotarget. 2017;8:89978–89987. doi: 10.18632/oncotarget.21208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang Y, Zhang C, Zhang P, Guo G, Jiang T, Zhao X, Jiang J, Huang X, Tong H, Tian Y. Serum exosomal microRNAs combined with alpha-fetoprotein as diagnostic markers of hepatocellular carcinoma. Cancer Med. 2018;7:1670–1679. doi: 10.1002/cam4.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maravelia P, Silva DN, Rovesti G, Chrobok M, Stål P, Lu YC, Pasetto A. Liquid Biopsy in Hepatocellular Carcinoma: Opportunities and Challenges for Immunotherapy. Cancers (Basel) 2021;13 doi: 10.3390/cancers13174334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Scheiner B, Pomej K, Kirstein MM, Hucke F, Finkelmeier F, Waidmann O, Himmelsbach V, Schulze K, von Felden J, Fründt TW, Stadler M, Heinzl H, Shmanko K, Spahn S, Radu P, Siebenhüner AR, Mertens JC, Rahbari NN, Kütting F, Waldschmidt DT, Ebert MP, Teufel A, De Dosso S, Pinato DJ, Pressiani T, Meischl T, Balcar L, Müller C, Mandorfer M, Reiberger T, Trauner M, Personeni N, Rimassa L, Bitzer M, Trojan J, Weinmann A, Wege H, Dufour JF, Peck-Radosavljevic M, Vogel A, Pinter M. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy - development and validation of the CRAFITY score. J Hepatol. 2022;76:353–363. doi: 10.1016/j.jhep.2021.09.035. [DOI] [PubMed] [Google Scholar]
- 108.Zheng Y, Wang T, Tu X, Huang Y, Zhang H, Tan D, Jiang W, Cai S, Zhao P, Song R, Li P, Qin N, Fang W. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer. 2019;7:193. doi: 10.1186/s40425-019-0650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ponziani FR, De Luca A, Picca A, Marzetti E, Petito V, Del Chierico F, Reddel S, Paroni Sterbini F, Sanguinetti M, Putignani L, Gasbarrini A, Pompili M. Gut Dysbiosis and Fecal Calprotectin Predict Response to Immune Checkpoint Inhibitors in Patients With Hepatocellular Carcinoma. Hepatol Commun. 2022;6:1492–1501. doi: 10.1002/hep4.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragón L, Jacquelot N, Qu B, Ferrere G, Clémenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 111.Xu X, Lv J, Guo F, Li J, Jia Y, Jiang D, Wang N, Zhang C, Kong L, Liu Y, Zhang Y, Li Z. Gut Microbiome Influences the Efficacy of PD-1 Antibody Immunotherapy on MSS-Type Colorectal Cancer via Metabolic Pathway. Front Microbiol. 2020;11:814. doi: 10.3389/fmicb.2020.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Botticelli A, Vernocchi P, Marini F, Quagliariello A, Cerbelli B, Reddel S, Del Chierico F, Di Pietro F, Giusti R, Tomassini A, Giampaoli O, Miccheli A, Zizzari IG, Nuti M, Putignani L, Marchetti P. Gut metabolomics profiling of non-small cell lung cancer (NSCLC) patients under immunotherapy treatment. J Transl Med. 2020;18:49. doi: 10.1186/s12967-020-02231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, Li Q, Lu Y, Chen Y, Guo Y, Chen Z, Liu B, Jia W, Wu J, Wang J, Shao G, Zhang B, Shan Y, Meng Z, Gu S, Yang W, Liu C, Shi X, Gao Z, Yin T, Cui J, Huang M, Xing B, Mao Y, Teng G, Qin Y, Xia F, Yin G, Yang Y, Chen M, Wang Y, Zhou H, Fan J ORIENT-32 study group. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 2021;22:977–990. doi: 10.1016/S1470-2045(21)00252-7. [DOI] [PubMed] [Google Scholar]
- 114.Qin S, Chen Z, Fang W, Ren Z, Xu R, Ryoo BY. Pembrolizumab plus best supportive care vs placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): Phase 3 KEYNOTE-394 study. J Clin Oncol. 2022;40:383. [Google Scholar]
- 115.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 116. A Study of Nivolumab in Combination With Ipilimumab in Participants With Advanced Hepatocellular Carcinoma (CheckMate 9DW). [accessed 2022 Jan 22]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04039607. ClinicalTrials.gov Identifier: NCT04039607.


