Abstract

Post-translational glycosylation of proteins results in complex mixtures of heterogeneous protein glycoforms. Glycoproteins have many potential applications from fundamental studies of glycobiology to potential therapeutics, but generating homogeneous recombinant glycoproteins using chemical or chemoenzymatic reactions to mimic natural glycoproteins or creating homogeneous synthetic neoglycoproteins is a challenging synthetic task. In this work, we use a site-specific bioorthogonal approach to produce synthetic homogeneous glycoproteins. We develop a bifunctional, bioorthogonal linker that combines oxime ligation and strain-promoted azide–alkyne cycloaddition chemistry to functionalize reducing sugars and glycan derivatives for attachment to proteins. We demonstrate the utility of this minimal length linker by producing neoglycoprotein inhibitors of cholera toxin in which derivatives of the disaccharide lactose and GM1os pentasaccharide are attached to a nonbinding variant of the cholera toxin B-subunit that acts as a size- and valency-matched multivalent scaffold. The resulting neoglycoproteins decorated with GM1 ligands inhibit cholera toxin B-subunit adhesion with a picomolar IC50.
Keywords: biorthogonal, conjugation, glycosylation, glycoprotein, bacterial toxin
Introduction
It is estimated that half of all proteins undergo glycosylation making it the most common post-translational modification.1,2 The complex, nontemplated biosynthetic pathways that introduce N- and O-linked glycans lead to high glycoprotein diversity, incorporating large numbers of highly complex oligosaccharides.3−5 While total chemical synthesis of glycoproteins has recently gained in popularity,6−9 methods for non-native glycan conjugation to make neoglycoproteins (a glycoprotein with a non-native linkage)10 remain important for glycoscience and glycotechnology.11 For many years, nucleophilic lysine side chains have been exploited through reductive amination12 for the production of glycoconjugate vaccines.13−15 However, this approach offers little control over the site or abundance of glycosylation due to the high prevalence of lysine on the surface of proteins.16 Cysteine residues have instead been used to gain more control, as their natural low surface abundance17 makes them useful reactive handles in site-specific protein engineering. Davis and co-workers, among others, have developed many ways in which cysteine can be exploited for the production of synthetic glycoproteins, including disulfide formation and chemical mutagenesis via a dehydroalanine intermediate.18−20 However, adding additional cysteine residues into a protein can introduce potential challenges, including disruption of protein folding, lowering expression levels.21
Advances in expanding the genetic code to introduce noncanonical amino acids into proteins22−25 have opened up new chemistries for protein modification, including copper-catalyzed azide–alkyne cycloaddition (CuAAC).26,27 However, despite the widespread application of the CuAAC reaction, including in the glycosylation of engineered proteins,19,28−31 the presence of copper catalyst used in CuAAC is not without its pitfalls.32 The use of cyclooctynes, rather than unstrained alkynes, in azide–alkyne reactions offers an alternative, copper-free biorthogonal route to covalent protein modification reactions.33,34 Such strain-promoted alkyne–azide cycloaddition (SPAAC) reactions33,34 have proved effective for the synthesis of neoglycoproteins through genetic incorporation of a bicyclononyne amino acid35 or through the use of cyclooctyne groups in complex multistep linker systems.36
Another important objective for neoglycoprotein preparation is the derivatization of unprotected glycans with suitable reactive groups for their attachment to the proteins.37,38 In this regard, oxime chemistry allows the latent aldehyde function of reducing sugars to be exploited to make a hydrolytically stable linker.39,40 The reaction can be catalyzed by anilines,41−43 and is also applicable to the modification of proteins containing aldehyde groups, for example, through N-terminal oxidation.44−47 We have previously used oxime ligation to a protein-derived aldehyde in the preparation of neoglycoproteins,44 in a case where CuAAC ligations with the protein proved problematic. However, this approach required extensive chemoenzymatic synthesis to generate complex derivatized glycans with a reactive handle.
In the work that we report here, our aim was to develop a simpler, flexible approach to neoglycoprotein synthesis that could be used for the functionalization of either chemically derivatized or reducing sugars. Our approach is to use a heterobifunctional linker, which combines an alkoxyamine for oxime formation with a strained alkyne for SPAAC conjugation (Figure 1). Thus, a single linker could be used either to connect a reducing sugar to an azide-functionalized protein or an azido-sugar to a protein with an N-terminal serine or threonine residue (following oxidation to generate an N-terminal glyoxyl residue).
Figure 1.

Cartoon representation of a bifunctional linker containing cyclooctyne and oxyamine functionalities that can be used to attach carbohydrates (yellow and blue circles) to proteins (orange) through oxime and SPAAC ligations.
Our strategy is exemplified through the synthesis of neoglycoprotein inhibitors of cholera toxin adhesion. Cholera toxin produced by Vibrio cholerae is the archetypal example of the AB5 bacterial toxin family that also includes E. coli heat-labile toxins and shiga-like toxins that cause severe diarrheal diseases.48 Cholera toxin has a single toxic A-subunit, which is an ADP-ribosyl transferase that is delivered into cells by a pentameric B-subunit (CTB) that is a sugar-binding protein that recognizes the glycolipid ganglioside GM1 (monosialotetrahexosylganglioside) and fucosylated structures.49−51 Inhibition of these protein–carbohydrate interactions can prevent the toxin from entering cells and thus prevent its toxic effects. Multivalent glycoconjugates have frequently been investigated as inhibitors of CTB adhesion.52−55 We have previously reported that neoglycoproteins based on a nonbinding mutant of the CTB (W88E, Figure 1) that have glycans matching the spacing and valency of the CTB binding sites are potent inhibitors of CT adhesion.44 Here, we investigate the effect of linker length and site of attachment to the protein scaffold on the activity of such inhibitors.
Results and Discussion
Synthesis of a New Bifunctional Linker
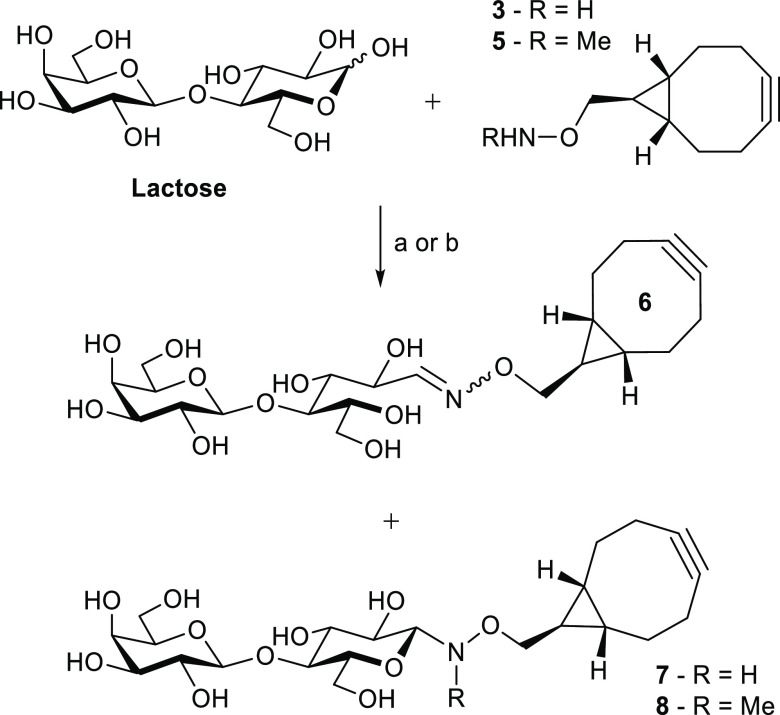
Among the many strained alkynes that have been extensively reviewed,24,56,57 bicyclo-[3.0.0]-nonyne (BCN) reported by Dommerholt et al.58 offered a desirable combination of symmetry and short linker length while maintaining a good balance between reaction kinetics and hydrophobicity.34 The known exo- and endo-isomers of bicyclononyne alcohol 1(58) were each converted to phthalimide-protected oxyamines 2 under Mitsunobu conditions in 71–86% yield (Scheme 1). The free oxyamines 3 could be accessed following deprotection of 2 by MeNH2 in anhydrous methanol. Oxyamines 3 were found to be particularly susceptible to reaction with any traces of aldehydes or ketones present within the laboratory, and so all further reactions involving 3 were performed following in situ deprotection of 2 in laboratories in which acetone was not in use.
Scheme 1. Synthesis of Novel Bifunctional Linkers 3 and 5 Starting with Either 1-Exo Or 1-Endo.
(a) N-hydroxyphthalimide, DIAD, PPh3, DCM, room temperature (rt), 5 h, 2-exo 71%; 2-endo 86%. (b) Methanolic methylamine, rt, 5 min; 3-endo was isolated in 64%; 3-exo was always used directly in subsequent reactions without isolation. (c) Only the exo-isomer of 2 was used for this reaction (i) NaOMe, MeOH, 30 min. (ii) MeI, MeOH, 1 h, quantitative. (d) iPrMgBr, toluene, rt, 2 h, 60% yield.
While condensation of sugars with primary O-alkyloxyamines typically leads to products with the open-chain oxime configuration, condensation with N,O-dialkyloxyamines will lead to glycosylamines that preserve the pyranose ring of the original sugar.59 Therefore, the N-methyl derivative 5 was synthesized from the exo-isomer of intermediate 2 using a different deprotection strategy. The phthalimide protecting group was first subjected to ring-opening with excess sodium methoxide followed by in situ methylation of the nitrogen using methyl iodide to yield the Weinreb amide 4. Cleavage of Weinreb amide 4 using isopropyl Grignard reagent (step d in Scheme 1) yielded linker 5 in 60% yield. The development of two differentiating deprotection methods provides a facile route to both linkers 3 and 5 from the same intermediate.
BCN Derivatization of Reducing Sugars
Using lactose as a model oligosaccharide (Scheme 2), oxime ligation was carried out with linker 3-exo using either a methanol/chloroform solvent mixture60 or sodium acetate buffer (pH 5).61 In line with previous reports, reactant concentrations of at least 250 mM were required for effective conversion to oxime products.62 The oxime products were isolated in 69 and 73% yield, respectively. Conveniently, the hydrophobic properties of the BCN group allow it to be exploited as a “purification tag” for reverse-phase chromatography of the conjugation product, thus separating it from the excess lactose. NMR analysis of the glycoconjugate resulting from a reaction of lactose and linker 3-exo showed it exists predominately in the ring-open form with a ratio of 20:5:14 for isomers 6(E)/6(Z)/7 (Lac-BCN). Reaction of methylaminooxy linker 5 with lactose was also achieved under the same conditions to produce exclusively the ring-closed isomer 8 in 40% yield.
Scheme 2. Oxime Ligation of Exo-Linker 3 or 5 to Unprotected Lactose.
Conditions: (a) CHCl3–MeOH (1:1), 50 °C, 24 h (69% 6 + 7); (b) 1 M sodium acetate buffer, pH 5, 50 °C, 24–48 h (73% 6 + 7; 40% 8).
Site-Specific SPAAC Glycosylation of Azido-CTB
We next sought to use the strained alkyne derivative 6, generated from lactose, to generate a neoglycoprotein. We used a nonbinding CTB variant containing azidohomoalanine (Aha) in place of lysine 43 (Figure 2A, cyan). We have previously reported an azido-CTB protein,63 in which Aha was introduced through isosteric replacement of methionine. In this protein, all native methionine residues had been mutated to leucine, and a single methionine residue introduced in place of lysine 43 (M37L-M68L-M101L-K43M). For this work, an additional mutation of tryptophan 88 (Figure 2A,B, blue) to glutamate was required to remove the protein’s native GM1 binding capability.64 Methionine-auxotrophic E. coli (B834) were transformed with a plasmid (pSAB2.4) harboring the gene for M37L-M68L-M101L-K43M-W88E CTB (Met-W88E). The protein was overexpressed in a defined growth medium containing Aha in place of methionine so that the expressed protein contained Aha at position 43 (M37L-M68L-M101L-K43Aha-W88E CTB; N3-W88E).65,66 Comparison of the deconvolved electrospray ionization (ESI) mass spectra with simulated isotope patterns for the Aha and Met variants of the protein confirmed successful incorporation of Aha over methionine in approximately 95% of the CTB monomers (N3-W88E) (Figure S5). Circular dichroism spectroscopy confirmed N3-W88E was identical to wild-type CTB, indicating that the protein was correctly folded (Figure S6).
Figure 2.

(A) Cartoon (side view) and (B) surface representation (bottom view) of the cholera toxin B-subunit (CTB) showing lysine 43 (cyan), which is the site for introduction of azidohomoalanine (Aha); also highlighted are tryptophan 88 (blue), which is mutated to glutamate to provide a nonbinding CTB variant CTB-W88E, and threonine 1 (green), which can be oxidized by periodate to introduce an aldehyde for oxime ligation. (C) Generation of N3-W88E by site-directed mutagenesis of azido-CTB, followed by SPAAC ligation of lactose derivative 6 and N3-W88E. (D) Electrospray ionization mass spectrometry (ESI-MS) time course of SPAAC ligation of N3-W88E and lactose derivative 6 over first 4 h shows complete conversion of the azido-CTB to leave a mass spectrometry signal matching that for the Met-W88E protein.
Modification of N3-W88E with strained alkyne 6 by SPAAC (Figure 2C) under optimized conditions (500 μM N3-W88E, 10 equiv of 6, 37 °C, phosphate-buffered saline (PBS) (pH 7.2)) led to the quantitative conversion of Aha residues to the triazole derivative within 4 h ((Lac)N3-W88E) (Figure 2D). Analysis by ESI-MS also revealed a peak corresponding to the mass of Met-W88E, consistent with the estimated 5% proportion of the unmodified protein substrate (Figure 2D). Analysis by sodium dodecyl sulfate poly(acrylamide) gel electrophoresis (SDS-PAGE) showed only a single band for the neoglycoprotein (Figure S15). Quantitative modification of the Aha residues could also be achieved at lower protein concentrations of N3-W88E (100 μM) and 1.5 equiv of 6; however, reaction times of 10–24 h were required.
Site-Specific N-Terminal Glycosylation of CTB
Having demonstrated that linker 3 can be used to attach a reducing sugar to an azide-functionalized protein, we then sought to apply the linker for attaching an azide-functionalized sugar to a protein aldehyde. For this study, we first functionalized CTB(W88E) with the endo isomer of oxyamine 3, which could then be labeled using SPAAC ligation with a glycosyl azide (Figure 3A). The N-terminal threonine residue of CTB-W88E (Figure 2A, green) was oxidized using 5 equiv of sodium periodate in sodium phosphate buffer. This reaction typically reaches completion within 5 min in sodium phosphate, whereas the reaction does not reach completion in phosphate-buffered saline containing potassium ions, as these are known to hinder periodate reactivity.67 Oxime ligation of the oxidized-W88E (450 μM) with 10 equiv linker 3-endo in the presence of aniline (1% v/v) at 37 °C reached completion within 16 h (Figure 3C). When the oxidized-W88E was used at a concentration below 200 μM, a mixture of labeled product and N-terminal cyclization was observed.68 For optimal oxime ligation reactions, an oxidized-W88E concentration between 400 and 500 μM was required to obtain quantitative labeling while minimizing precipitation of the product BCN-W88E.
Figure 3.

(A) Generation of BCN-W88E by N-terminal oxime ligation of endo-BCN-ONH23 to W88E, followed by SPAAC ligation of lactosyl azide 9 and BCN-W88E. (B) ESI-MS of W88E before treatment with NaIO4. (C) ESI-MS of W88E following oxidation with periodate, desalting and treatment of oxidized-W88E with endo-3 and aniline, showing the quantitive formation of BCN-W88E. (D) ESI-MS of SPAAC ligation of BCN-W88E and lactosyl azide 9 after 8 h. Formation of triazole-W88E (11 730.0 Da) and (Glc)BCN-W88E (11 893.1 Da) as a result of fragmentation within the MS.
Lactosyl azide 9 was prepared from lactose through peracetylation and α-bromination at its reducing terminus,69 followed by SN2 displacement with sodium azide. After removal of the acetyl groups with sodium methoxide, lactosyl azide 9 was conjugated to BCN-W88E using a SPAAC reaction to give (Lac)BCN-CTB in quantitative yield in under 8 h. Analysis post-reaction by ESI-MS showed fragmentation peaks corresponding to the loss of terminal galactose and lactose (Figure 3D). The reaction of BCN-W88E in the presence of sodium azide (10 equiv) confirmed that no reaction occurs over the same time scale (Figure S12), demonstrating that the signal at 11 730 Da (Figure 3D) was the result of ion fragmentation rather than degradation of the lactosyl azide prior to reaction with the protein. Furthermore, the observation of a single band for the product in SDS-PAGE (Figure S16) was also consistent with the complete reaction of the protein to give (Lac)BCN-W88E.
SPAAC Glycosylation for Synthesis of Neoglycoprotein Inhibitors of Cholera Toxin Adhesion
Having shown that the nonbinding CTB variant W88E could be functionalized with simple disaccharides using linker 3, these glycosylation methods were applied to the synthesis of a neoglycoprotein inhibitor of the cholera toxin. Branson et al. used the W88E nonbinding variant of CTB as a protein scaffold to develop the first neoglycoprotein-based inhibitor of CTB that matched the geometry of each GM1 ligand with the pentagonal symmetry of the target protein (Figure 4A).44 The neoglycoprotein was determined to be a highly potent inhibitor of cholera toxin adhesion, with an IC50 of 104 pM. This evidence supports the idea that the optimal design for multivalent inhibitors is to match the distance and geometry of the target binding sites, a hypothesis that also has support from theoretical studies on enhancing potency.70 However, we were curious to investigate if changing the site of glycosylation on the scaffold and length of linker might affect the activity of such neoglycoproteins. Therefore, we sought to attach the GM1 oligosaccharide to different sites on the CTB-W88E variants using bioorthogonal linker 3 (Figure 4B,C).
Figure 4.

(A) Synthetic approach to a protein-based pentavalent neoglycoprotein inhibitor by Branson et al. involved oxime ligation of a GM1 derivative to the N-terminus of a nonbinding CTB variant.44 (B) SPAAC ligation of a BCN-GM1 11 to the nonbinding CTB variant with azidohomoalanine incorporated at position 43. (C) SPAAC ligation of β-GM1 azide 13 to the BCN-W88E where linker 3 was site-specifically ligated to the N-terminus of W88E using oxime ligation.
GM1os pentasaccharide 10 was produced by enzymatic hydrolysis of GM1 ganglioside in a 77% yield using Rhodococcus sp. endoglycoceramidase II (EGCase II, Scheme 3).71 Enzymatic hydrolysis of GM1 ganglioside avoided the lengthy chemoenzymatic synthesis used previously.44,72 GM1os 10 was then derivatized with linker 3-exo by oxime ligation at 50 °C for 48 h, to make GM1-BCN 11 (Scheme 3). The 1H NMR spectra for GM1-BCN 11 indicated the presence of several isomers corresponding to the E- and Z-oximes and β-N-glycoside. Additional oxime signals increased in size over a period of 2 months, which we tentatively assign as the manno-configured C-2 epimer of GM1-BCN 11 (see Supporting Information Figure S0).
Scheme 3. Enzymatic Hydrolysis of GM1 Ganglioside and Derivatization of GM1 Oligosaccharide 10.
(A) EGCase II, 50 mM NaOAc buffer (pH 5), triton X-100 (0.2%), 37 °C, 5 days, 77% yield. (B) 3-exo, 1 M NaOAc buffer (pH 5), 50 °C, 48 h, 24% yield. (C) NaN3, TEA, H2O, 37 °C, 48 h, ca. 90% conversion to glycosyl azide.
Once again, the BCN group acted as a purification tag, allowing simple purification by reverse-phase chromatography and easy recovery of the unreacted starting oligosaccharide. While the presence of the ring-open form of reducing glucose unit differs from the structure of native GM1, it is unlikely to have any impact on inhibition as the glucose residue is not engaged in any interactions in the GM1 binding site.73,74 Furthermore, multivalent glycoconjugates of GM1 prepared by reductive amination, and thus fixed in the ring-open form, are known to be effective inhibitors.75−77 Nevertheless, even if the ring-closed form were advantageous for binding, the oxime derivatives are in equilibrium with the ring-closed glycosyloxyamines and so the system could adopt that configuration if preferable.
BCN-derivatized GM1 11 (10 equiv) was attached to N3-W88E (500 μM), and ESI-HRMS confirmed quantitative labeling of the Aha residues in 5 h to give neoglycoprotein (GM1)N3-W88E (Figure 4B). SDS-PAGE analysis was similar to that for (Lac)N3-W88E (Figure S15) confirming that this ligation method is unaffected by increasing glycan complexity from a simple disaccharide to a branched pentasaccharide.
Complex glycosyl azides can be prepared from their reducing sugars using 2-chloro-1,3-dimethylimidazolinium chloride (DMC, 12).35,78 DMC has previously been reported for derivatization of sialylated N-linked glycans, but not apparently for ganglioside-derived glycans. One-step conversion of GM1os pentasaccharide 10 to β-GM1 azide 13 was performed using DMC 12 (10 equiv) and sodium azide (44 equiv) in the presence of TEA (18 equiv) at 37 °C (Scheme 3). NMR and thin-layer chromatography (TLC) indicated that the reaction stopped after 90% conversion. Extending reaction time to over 48 h and adding further equivalents of sodium azide, DMC, or TEA had no further effect on the progress of the reaction. Therefore, the crude β-GM1 azide 13 was used for conjugation to BCN-W88E, resulting in complete conversion of the protein to (GM1)BCN-W88E using the optimized SPAAC conditions previously used for lactosyl azide 9 (Figure 4C).
Inhibitor Potency against CTB Binding to GM1
An enzyme-linked lectin assay (ELLA) was used to determine the IC50 of neoglycoproteins (GM1)N3-W88E and (GM1)BCN-W88E for the inhibition of wild-type CTB adhering to ganglioside GM1-coated microtiter plates. The (GM1)N3-W88E and (GM1)BCN-W88E neoglycoproteins were determined to have an IC50 of 457 and 924 pM, respectively (Figure 5 and Table 1). The analogous pentavalent lactose neoglycoprotein, (Lac)N3-W88E, produced by SPAAC of compound 6 and N3-W88E, demonstrated no inhibition of wild-type CTB binding to GM1 at concentrations up to 50 μM (data not shown).
Figure 5.

Inhibition of CTB–HRP conjugate binding to GM1-coated microtiter plates by GM1os (data reproduced from ref (44) under the terms of a Creative Commons Attribution License) and neoglycoproteins (GM1)N3-W88E and (GM1)BCN-W88E, determined by enzyme-linked lectin assay (ELLA). Error bars indicate the standard error of three measurements.
Table 1. Inhibitory Potential of the (GM1)N3-W88E and (GM1)BCN-W88E Neoglycoproteins in Comparison to GM1os, as Determined by ELLA.
| inhibitor | valency | log(IC50) | IC50 (nM)a | relative potency (per GM1)b |
|---|---|---|---|---|
| GM1osc | 1 | –6.27 ± 0.04 | 530 | 1 (1) |
| GM1(CH2)11-W88Ec | 5 | –9.98 ± 0.08 | 0.104 | 5096 (1019) |
| (GM1)N3-W88E | 5 | –9.34 ± 0.02 | 0.457 | 1160 (231) |
| (GM1)BCN-W88E | 5 | –9.03 ± 0.07 | 0.924 | 574 (115) |
As curve fitting was performed using log(IC50) as x values, calculated uncertainties in IC50 are asymmetric about the mean and have thus been omitted.
Relative potency values are quoted compared to monovalent GM1os.
Data reported by Branson et al.44 for monovalent GM1os and the previous neoglycoprotein inhibitor GM1(CH2)11-W88E.
Both GM1os-based neoglycoprotein inhibitors demonstrate over 100-fold enhancement in potency compared to monovalent GM1os (based on equivalent GM1os concentrations). Both neoglycoproteins were comparable in potency to the GM1(CH2)11-W88E reported previously (Table 1).44 Maintaining the same site of attachment while decreasing the length of the linker between the pentavalent scaffold and glycan or changing the site of glycosylation to the opposite face of the protein scaffold led to IC50 values that were within an order of magnitude of the GM1(CH2)11-W88E neoglycoprotein-based inhibitor. The fact that sub-nM IC50 values can be achieved across a range of neoglycoprotein designs demonstrates the robustness of this strategy for the preparation of multivalent inhibitors; the difference in potency between the neoglycoproteins presented in this work and the previously reported neoglycoprotein is minimal compared to the overall improvement observed over monovalent GM1os (Table 1). Nonetheless, the strategy for modification of GM1os presented here was performed in a single step prior to protein conjugation, compared to the lengthy chemoenzymatic synthesis of the inhibitor utilizing 11-azidoundecyl GM1.44,72
Conclusions
Here, we have developed a dual biorthogonal glycosylation strategy for the site-specific engineering of synthetic glycoproteins. The combination of two bioorthogonal reactive functional groups, oxyamine and cyclooctyne, into a single linker has demonstrated this to be a powerful method for synthetic glycosylation of proteins. The use of a divergent deprotection strategy in the synthesis of the bifunctional linkers 3 and 5 provides the opportunity to access both ring-open and ring-closed glycoconjugates. Functionalization of reducing oligosaccharides with linker 3 also facilitates efficient reverse-phase purification of the products. While CuAAC has often been used for the preparation of neoglycoproteins,28−31 SPAAC has been rarely used for this application; with fast reaction kinetics and high yields achieved, it offers a very convenient method for the site-specific production of homogeneous glycoproteins. SPAAC ligation of the BCN-glycan derivatives to azido-CTB(W88E) or functionalization of CTB(W88E) with linker 3via oxime ligation followed by SPAAC reaction with glycosyl azides both provide efficient routes to the preparation of neoglycoproteins that are potent multivalent inhibitors of cholera toxin adhesion. Altering the glycan attachment sites on the protein scaffold leads to only small variations in activity, demonstrating the robustness of a neoglycoprotein-based inhibitor strategy for effective inhibition of lectin adhesion. The bifunctional linkers described here could also be applied to the preparation of other synthetic protein conjugates, e.g., antibody–drug conjugates, or for glycosylation of other biomolecules, e.g., lipids or nucleic acids.
Methods
Synthesis of O-((1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyl)hydroxylamine (3-endo) and Ganglioside GM1 Conjugates
2-((1R,8S,9r)-Bicyclo[6.1.0]non-4-yn-9-ylmethoxy) Isolindine-1-3-dione (2-endo)
Compound 1-endo ((1R,8S,9s)-bicyclo[6.1.0]non-4-yn-9-ylmethanol (20 mg, 0.13 mmol)), triphenylphosphine (38 mg, 0.15 mmol), and N-hydroxyphthalimide (24 mg, 0.15 mmol) were dissolved in anhydrous CH2Cl2 (1.2 mL) under a N2 atmosphere and chilled to 0 °C. Diisopropylazodicarboxylate (29 μL, 0.15 mmol) was added dropwise, and the solution was stirred for 10 min at 0 °C, after which the reaction was allowed to warm at room temperature over 4 h. The reaction mixture was concentrated in vacuo to yield a yellow residue. Purification by flash column chromatography (1:9 EtOAc/hexane) yielded the title compound (2-endo) as a white crystalline solid (37 mg, 94%). Rf 0.58 (3:7 EtOAc/hexane); 1H NMR (500 MHz; CDCl3) δ 1.09–1.02 (m, 2H), 1.56 (app. pent, J = 8.5 Hz, 1H), 1.69–1.60 (m, 2H), 2.25–2.18 (m, 2H), 2.34–2.26 (m, 4H), 4.31 (d, J = 8.0 Hz, 2H), 7.75 (dd, J = 5.4, 3.1 Hz, 2H), 7.83 (dd, J = 5.4, 3.1, Hz, 2H); 13C NMR (125 MHz; CDCl3) δ 17.3, 20.8, 21.5, 29.3, 76.4, 99.0, 123.6, 129.1, 134.6, 163.8; high-resolution mass spectrometry (HRMS) [ES+] C18H17NO3Na requires 318.1101. Found [M + Na]+ = 318.1099.
O-((1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyl)hydroxylamine (3-endo)
Compound 2-endo (20 mg, 68 μmol) was added to anhydrous 2 M methanolic methylamine (0.169 mL, 339 μmol) under nitrogen in an oven-dried flask. The reaction was monitored by TLC and was found to be complete within 2 min. The product was diluted in 1:9 EtOAc/hexane and purified by flash column chromatography (1:9 EtOAc/hexane) to yield a colorless oil (7.2 mg, 64%). Rf 0.25 (3:7 EtOAc/hexane); 1H NMR (500 MHz; CDCl3) δ 0.96–0.86 (m, 2H), 1.35–1.25 (m, 1H), 1.62–1.52 (m, 2H), 2.34–2.16 (m, 6H), 3.75 (d, J = 7.7 Hz, 2H), 5.41 (br. s, 2H); 13C NMR (100 MHz; CDCl3) δ 17.6, 20.0, 21.6, 29.4, 73.2, 99.1; HRMS [ES+] C10H16NO requires 166.1226. Found [M + H]+ 166.1225.
β-d-Galactopyranosyl-(1→3)-2-acetamido-2-deoxy-β-d-galactopyranosyl-(1→4)-[(5-acetamido-3,5-dideoxy-d-glycero-α-d-galacto-non-2-ulopyranosylonic acid)-(2→3)]-β-d-galactopyranosyl-(1→4)-d-glucopyranose (1R,8S,9r)-bicyclo[6.1.0]non-4-yn-9-ylmethyloxime (GM1-BCN Oxime, 11)
Method 1
β-d-Galactopyranosyl-(1→3)-2-acetamido-2-deoxy-β-d-galactopyranosyl-(1→4)-[(5-acetamido-3,5-dideoxy-d-glycero-α-d-galacto-non-2-ulopyranosylonic acid)-(2→3)]-β-d-galactopyranosyl-(1→4)-d-glucopyranose (GM1os, 1 mg, 1 μmol) was suspended in 1:1 CHCl3/MeOH (2 μL) in a 100 μL PCR tube. Linker 3-exo (3 μL of 390 mM stock solution in 1:1 CHCl3/MeOH) was added to this suspension, and the reaction was heated at 50 °C for 48 h using a PCR thermocycler with a heated lid (105 °C). The crude mixture was diluted to 100 μL with water, and the product was isolated by reverse-phase extraction using a C18 SPE cartridge: excess GM1os was removed by extensive washing with water before elution of the product in 20% aq methanol. The title compound 11, comprising a mixture of open-chain and cyclized isomers, was obtained as a white solid by lyophilization (0.38 mg, 33%).
Method 2
GM1os (1 mg, 1 μmol) was dissolved in H2O (2 μL) in a 100 μL PCR tube, to which NaOAc buffer (1 μL of a 5 M stock solution, pH 5) and linker 3-exo (2 μL of a 390 mM stock solution in 1:1 CHCl3/MeOH) were added, and the reaction was heated at 50 °C for 48 h using a PCR thermocycler with a heated lid (105 °C). Completion of the reaction was confirmed by TLC (2:2:1 BuOH/MeOH/H2O). The crude mixture was diluted to 100 μL with water, and the product was isolated by reverse-phase extraction using a C18 SPE cartridge: excess GM1os was removed by extensive washing with water before elution of the product in 20% aq methanol. The title compound 11, comprising a mixture of open-chain and cyclized isomers, was obtained as a white solid by lyophilization (0.28 mg, 24%).
Rf 0.63 (2:2:1 BuOH/MeOH/H2O); 1H NMR (500 MHz; D2O) δ 0.71–0.84 (m), 1.36–1.46 (m), 1.93 (t, 11.8 Hz), 2.00–2.05 (m), 2.12–2.21 (m), 2.25–2.35 (m), 2.40–2.47 (m), 2.64–2.68 (m), 3.29–4.19 (m), 4.30–4.32 (m, H1N-glycoside), 4.48–4.59 (m, H1″,1⁗, H2E-oxime), 4.74 (m), 4.93–4.99 (m, H2Z-oxime) 6.93 (d, J = 5.4 Hz, H1Z-oxime), 6.97 (d, J = 4.9 Hz, H1epZ-oxime), 7.48 (d, J = 6.9 Hz, H1epE-oxime), 7.59 (d, J = 5.9 Hz, H1E-oxime); HRMS C47H75N3O29 + H requires 1146.4564. Found m/z [M + H]+ = 1146.4554.
β-d-Galactopyranosyl-(1→3)-2-acetamido-2-deoxy-β-d-galactopyranosyl-(1→4)-[(5-acetamido-3,5-dideoxy-d-glycero-α-d-galacto-non-2-ulopyranosylonic acid)-(2→3)]-β-d-galactopyranosyl-(1→4)-β-d-glucopyranosyl azide (GM1 azide, 13)
GM1os (9 mg, 9 μmol), 2-chloro-1,3-dimethylimidazolium (DMC, 15 mg, 90 μmol), and NaN3 (25 mg, 400 μmol) were dissolved in H2O (0.1 mL) in a 1.5 mL Eppendorf tube. Triethylamine (22.5 μL, 161 μmol) was added, mixed by vortex, and the solution was incubated at 37 °C for 48 h. The product was isolated by size exclusion chromatography (Biogel P2) eluting with 20 mM ammonium formate to yield the title compound 13 as a white foam after lyophilization (8.6 mg, ca. 90% conversion to azide product with ca. 10% hemiacetal remaining). Rf 0.58 (2:2:1 n-BuOH/MeOH/H2O); 1H NMR (500 MHz; D2O; 275 K) δ 1.94 (t, J = 12.3 Hz, 1H), 1.99 (s, 3H), 2.01 (s, 3H), 2.63 (dd, J = 12.3, 4.5 Hz, 1H), 3.34 (dd, J = 9.3, 8.2 Hz, 1H), 3.53–3.45 (m, 2H), 3.99–3.54 (m, 24H), 4.01 (dd, J = 10.8, 8.7 Hz, 1H), 4.19–4.11 (m, 3H), 4.52 (d, J = 7.8 Hz, 1H), 4.53 (d, J = 7.7 Hz, 1H), 4.75 (d, J = 8.8 Hz, 1H), 4.78 (d, J = 8.8 Hz, 1H); HRMS [ES+]: C37H61N5O28Na requires 1046.3401. Found [M + Na]+ = 1046.3417.
Acknowledgments
The authors thank the U.K. Engineering and Physical Sciences Research Council (1799721), Biotechnology and Biological Research Council (BB/M011151/1), Iceni Diagnostics, and the Wellcome Trust (109154/Z/15/A) for PhD studentships and funding. The authors thank the Wellcome Trust for support for equipment used during the project (097827/Z/11/A, WT094232MA, 094232/Z/10/Z). The authors would also like to thank Dr. M. Howard (University of Leeds) and Mr. G. N. Kahn (University of Leeds) for their assistance and support for NMR and circular dichroism spectroscopy, respectively. For the purpose of open access, the authors have applied a CC-BY public copyright license to any Author Accepted Manuscript version arising from this submission.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.2c00312.
Full experimental procedures for the preparation of compounds 2–9, 11, and 13; experimental procedures for the preparation of proteins and protein conjugates and experimental procedures for plate-based assay of candidate inhibitors; 1H and 13C NMR spectra for organic compounds; and mass spectrometry data for modified proteins (PDF)
Author Present Address
§ Crick-GSK Biomedical LinkLabs, GlaxoSmithKline, Gunnel Wood Road, Stevenage SG1 2NY, United Kingdom
Author Present Address
‡ School of Chemical and Physical Sciences & Centre for Glycoscience Research and Training, Keele University, Keele, Staffordshire ST5 5BG, United Kingdom.
Author Present Address
† Department of Chemistry & Manchester Institute of Biotechnology, The University of Manchester, 131 Princess Street, Manchester M1 7DN, United Kingdom
Author Contributions
R.M.: conceptualization, investigation, data curation, formal analysis, methodology, visualization, writing—original draft, and writing—review & editing. J.P.D.: conceptualization, investigation, data curation, formal analysis, methodology, visualization, writing—original draft, and writing—review & editing. E.E.C.: investigation, data curation, and writing—review & editing. M.E.W.: conceptualization, funding acquisition, project administration, supervision, and writing—review & editing. W.B.T.: conceptualization, funding acquisition, project administration, supervision, and writing—review & editing. CRediT: Ryan McBerney conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing-original draft, writing-review & editing; Jonathan Peter Dolan conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing-original draft, writing-review & editing; Emma E Cawood data curation, investigation, writing-review & editing; Michael E. Webb conceptualization, funding acquisition, project administration, supervision, writing-review & editing; W. Bruce Turnbull conceptualization, funding acquisition, project administration, supervision, writing-review & editing.
The authors declare no competing financial interest.
Notes
The raw data associated with this paper including NMR and mass spectra are openly available from the University of Leeds data repository. https://doi.org/10.5518/1195
Supplementary Material
References
- Apweiler R.; Hermjakob H.; Sharon N. On the Frequency of Protein Glycosylation, as Deduced from Analysis of the SWISS-PROT Database. Biochim. Biophys. Acta, Gen. Subj. 1999, 1473, 4–8. 10.1016/S0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- Khoury G. A.; Baliban R. C.; Floudas C. A. Proteome-Wide Post-Translational Modification Statistics: Frequency Analysis and Curation of the Swiss-Prot Database. Sci. Rep. 2011, 1, 90 10.1038/srep00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebi M. N-Linked Protein Glycosylation in the ER. Biochim. Biophys. Acta, Mol. Cell Res. 2013, 1833, 2430–2437. 10.1016/j.bbamcr.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Moremen K. W.; Tiemeyer M.; Nairn A. V. Vertebrate Protein Glycosylation: Diversity, Synthesis and Function. Nat. Rev. Mol. Cell Biol. 2012, 13, 448–462. 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen P. V.; Rudd P. M.; Dwek R. A.; Opdenakker G. Concepts and Principles of O-Linked Glycosylation. Crit. Rev. Biochem. Mol. Biol. 1998, 33, 151–208. 10.1080/10409239891204198. [DOI] [PubMed] [Google Scholar]
- Fairbanks A. J. The ENGases: Versatile Biocatalysts for the Production of Homogeneous N-Linked Glycopeptides and Glycoproteins. Chem. Soc. Rev. 2017, 46, 5128–5146. 10.1039/C6CS00897F. [DOI] [PubMed] [Google Scholar]
- Li C.; Wang L.-X. Chemoenzymatic Methods for the Synthesis of Glycoproteins. Chem. Rev. 2018, 118, 8359–8413. 10.1021/acs.chemrev.8b00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlo U.; Yasuhiro K. Recent Advances in the Chemical Synthesis of N-Linked Glycoproteins. Curr. Opin. Chem. Biol. 2018, 46, 130–137. 10.1016/j.cbpa.2018.07.004. [DOI] [PubMed] [Google Scholar]
- Marqvorsen M. H. S.; Araman C.; van Kasteren S. I. Going Native: Synthesis of Glycoproteins and Glycopeptides via Native Linkages To Study Glycan-Specific Roles in the Immune System. Bioconjugate Chem. 2019, 30, 2715–2726. 10.1021/acs.bioconjchem.9b00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz M. J.; Holtzman N. A.; Stowell C. P.; Lee Y. C.; Weiner J. W.; Liu H. H. Attachment of Thioglycosides to Proteins: Enhancement of Liver Membrane Binding. Biochemistry 1976, 15, 3963–3968. 10.1021/bi00663a009. [DOI] [PubMed] [Google Scholar]
- Pergolizzi G.; Dedola S.; Field R. A.. Contemporary Glycoconjugation Chemistry. In Carbohydrate Chemistry; The Royal Society of Chemistry, 2017; Vol. 42, pp 1–46. [Google Scholar]
- Gray G. R. The Direct Coupling of Oligosaccharides to Proteins and Derivatized Gels. Arch. Biochem. Biophys. 1974, 163, 426–428. 10.1016/0003-9861(74)90495-0. [DOI] [PubMed] [Google Scholar]
- Paoletti L. C.; Wessels M. R.; Michon F.; DiFabio J.; Jennings H. J.; Kasper D. L. Group B Streptococcus Type II Polysaccharide-Tetanus Toxoid Conjugate Vaccine. Infect. Immun. 1992, 60, 4009–4014. 10.1128/iai.60.10.4009-4014.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti L. C.; Wessels M. R.; Rodewald A. K.; Shroff A. A.; Jennings H. J.; Kasper D. L. Neonatal Mouse Protection against Infection with Multiple Group B Streptococcal (GBS) Serotypes by Maternal Immunization with a Tetravalent GBS Polysaccharide-Tetanus Toxoid Conjugate Vaccine. Infect. Immun. 1994, 62, 3236–3243. 10.1128/iai.62.8.3236-3243.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein L. J.; García-Ojeda P. A.; Michon F.; Jennings H. J.; Stein K. E. Murine Immune Responses to Neisseria Meningitidis Group C Capsular Polysaccharide and a Thymus-Dependent Toxoid Conjugate Vaccine. Infect. Immun. 1998, 66, 5450–5456. 10.1128/IAI.66.11.5450-5456.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb M. E.; Bon R. S.; Wright M. H.. Site-Specific Protein Modification and Bio-Orthogonal Chemistry. In Chemical and Biological Synthesis: Enabling Approaches for Understanding Biology; Westwood N.; Nelson A., Eds.; The Royal Society of Chemistry, 2018; pp 313–356. [Google Scholar]
- Miseta A.; Csutora P. Relationship Between the Occurrence of Cysteine in Proteins and the Complexity of Organisms. Mol. Biol. Evol. 2000, 17, 1232–1239. 10.1093/oxfordjournals.molbev.a026406. [DOI] [PubMed] [Google Scholar]
- Gamblin D. P.; Scanlan E. M.; Davis B. G. Glycoprotein Synthesis: An Update. Chem. Rev. 2009, 109, 131–163. 10.1021/cr078291i. [DOI] [PubMed] [Google Scholar]
- Chalker J. M.; Bernardes G. J. L.; Davis B. G. A “Tag-and-Modify” Approach to Site-Selective Protein Modification. Acc. Chem. Res. 2011, 44, 730–741. 10.1021/ar200056q. [DOI] [PubMed] [Google Scholar]
- Dadová J.; Galan S. R.; Davis B. G. Synthesis of Modified Proteins via Functionalization of Dehydroalanine. Curr. Opin. Chem. Biol. 2018, 46, 71–81. 10.1016/j.cbpa.2018.05.022. [DOI] [PubMed] [Google Scholar]
- O’Dowd A. M.; Botting C. H.; Precious B.; Shawcross R.; Randall R. E. Novel Modifications to the C-Terminus of LTB That Facilitate Site-Directed Chemical Coupling of Antigens and the Development of LTB as a Carrier for Mucosal Vaccines. Vaccine 1999, 17, 1442–1453. 10.1016/S0264-410X(98)00375-2. [DOI] [PubMed] [Google Scholar]
- Johnson J. A.; Lu Y. Y.; Van Deventer J. A.; Tirrell D. A. Residue-Specific Incorporation of Non-Canonical Amino Acids into Proteins: Recent Developments and Applications. Curr. Opin. Chem. Biol. 2010, 14, 774–780. 10.1016/j.cbpa.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. C.; Schultz P. G. Adding New Chemistries to the Genetic Code. Annu. Rev. Biochem. 2010, 79, 413–444. 10.1146/annurev.biochem.052308.105824. [DOI] [PubMed] [Google Scholar]
- Lang K.; Chin J. W. Cellular Incorporation of Unnatural Amino Acids and Bioorthogonal Labeling of Proteins. Chem. Rev. 2014, 114, 4764–4806. 10.1021/cr400355w. [DOI] [PubMed] [Google Scholar]
- Wiltschi B. Incorporation of Non-Canonical Amino Acids into Proteins in Yeast. Fungal Genet. Biol. 2016, 89, 137–156. 10.1016/j.fgb.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Rostovtsev V. V.; Green L. G.; Fokin V. V.; Sharpless K. B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. 2002, 114, 2708–2711. . [DOI] [PubMed] [Google Scholar]
- Wojnar J. M.; Lee D. J.; Evans C. W.; Mandal K.; Kent S. B. H.; Brimble M. A.. Neoglycoprotein Synthesis Using the Copper-Catalyzed Azide-Alkyne Click Reaction and Native Chemical Ligation. In Click Chemistry in Glycoscience; Witczak Z.; Bielski R., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp 251–270. [Google Scholar]
- van Kasteren S. I.; Kramer H. B.; Jensen H. H.; Campbell S. J.; Kirkpatrick J.; Oldham N. J.; Anthony D. C.; Davis B. G. Expanding the Diversity of Chemical Protein Modification Allows Post-Translational Mimicry. Nature 2007, 446, 1105–1109. 10.1038/nature05757. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Viana R.; Sánchez-Navarro M.; Luczkowiak J.; Koeppe J. R.; Delgado R.; Rojo J.; Davis B. G. Virus-like Glycodendrinanoparticles Displaying Quasi-Equivalent Nested Polyvalency upon Glycoprotein Platforms Potently Block Viral Infection. Nat. Commun. 2012, 3, 1303 10.1038/ncomms2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauster D.; Klenk S.; Ludwig K.; Nojoumi S.; Behren S.; Adam L.; Stadtmüller M.; Saenger S.; Zimmler S.; Hönzke K.; Yao L.; Hoffmann U.; Bardua M.; Hamann A.; Witzenrath M.; Sander L. E.; Wolff T.; Hocke A. C.; Hippenstiel S.; De Carlo S.; Neudecker J.; Osterrieder K.; Budisa N.; Netz R. R.; Böttcher C.; Liese S.; Herrmann A.; Hackenberger C. P. R. Phage Capsid Nanoparticles with Defined Ligand Arrangement Block Influenza Virus Entry. Nat. Nanotechnol. 2020, 15, 373–379. 10.1038/s41565-020-0660-2. [DOI] [PubMed] [Google Scholar]
- Kaltgrad E.; O’Reilly M. K.; Liao L.; Han S.; Paulson J. C.; Finn M. G. On-Virus Construction of Polyvalent Glycan Ligands for Cell-Surface Receptors. J. Am. Chem. Soc. 2008, 130, 4578–4579. 10.1021/ja077801n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens C. J.; Johnson S. N.; Pressnall M. M.; Leon M. A.; Berkland C. J. Practical Considerations, Challenges, and Limitations of Bioconjugation via Azide–Alkyne Cycloaddition. Bioconjugate Chem. 2018, 29, 686–701. 10.1021/acs.bioconjchem.7b00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sletten E. M.; Bertozzi C. R. From Mechanism to Mouse: A Tale of Two Bioorthogonal Reactions. Acc. Chem. Res. 2011, 44, 666–676. 10.1021/ar200148z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debets M. F.; van Berkel S. S.; Dommerholt J.; Dirks A. (Ton) J.; Rutjes F. P. J. T.; van Delft F. L. Bioconjugation with Strained Alkenes and Alkynes. Acc. Chem. Res. 2011, 44, 805–815. 10.1021/ar200059z. [DOI] [PubMed] [Google Scholar]
- Machida T.; Lang K.; Xue L.; Chin J. W.; Winssinger N. Site-Specific Glycoconjugation of Protein via Bioorthogonal Tetrazine Cycloaddition with a Genetically Encoded Trans -Cyclooctene or Bicyclononyne. Bioconjugate Chem. 2015, 26, 802–806. 10.1021/acs.bioconjchem.5b00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanetti G.; Hu Q.; Usera A.; Robinson Z.; Allan M.; Singh A.; Imase H.; Cobb J.; Zhai H.; Quinn D.; Lei M.; Saul A.; Adamo R.; MacLennan C. A.; Micoli F. Sugar–Protein Connectivity Impacts on the Immunogenicity of Site-Selective Salmonella O-Antigen Glycoconjugate Vaccines. Angew. Chem. 2015, 127, 13396–13401. 10.1002/ange.201506112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villadsen K.; Martos-Maldonado M. C.; Jensen K. J.; Thygesen M. B. Chemoselective Reactions for the Synthesis of Glycoconjugates from Unprotected Carbohydrates. ChemBioChem 2017, 18, 574–612. 10.1002/cbic.201600582. [DOI] [PubMed] [Google Scholar]
- Hang H. C.; Yu C.; Kato D. L.; Bertozzi C. R. A Metabolic Labeling Approach toward Proteomic Analysis of Mucin-Type O-Linked Glycosylation. Proc. Natl. Acad. Sci. 2003, 100, 14846–14851. 10.1073/pnas.2335201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia J.; Raines R. T. Hydrolytic Stability of Hydrazones and Oximes. Angew. Chem., Int. Ed. 2008, 47, 7523–7526. 10.1002/anie.200802651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendiak B. Preparation, Conformation, and Mild Hydrolysis of 1-Glycosyl-2-Acetylhydrazines of the Hexoses, Pentoses, 2-Acetamido-2-Deoxyhexoses, and Fucose. Carbohydr. Res. 1997, 304, 85–90. 10.1016/S0008-6215(97)00213-9. [DOI] [PubMed] [Google Scholar]
- Østergaard M.; Christensen N. J.; Hjuler C. T.; Jensen K. J.; Thygesen M. B. Glycoconjugate Oxime Formation Catalyzed at Neutral PH: Mechanistic Insights and Applications of 1,4-Diaminobenzene as a Superior Catalyst for Complex Carbohydrates. Bioconjugate Chem. 2018, 29, 1219–1230. 10.1021/acs.bioconjchem.8b00019. [DOI] [PubMed] [Google Scholar]
- Thygesen M. B.; Munch H.; Sauer J.; Cló E.; Jørgensen M. R.; Hindsgaul O.; Jensen K. J. Nucleophilic Catalysis of Carbohydrate Oxime Formation by Anilines. J. Org. Chem. 2010, 75, 1752–1755. 10.1021/jo902425v. [DOI] [PubMed] [Google Scholar]
- Cló E.; Blixt O.; Jensen K. J. Chemoselective Reagents for Covalent Capture and Display of Glycans in Microarrays. Eur. J. Org. Chem. 2010, 2010, 540–554. 10.1002/ejoc.200901234. [DOI] [Google Scholar]
- Branson T. R.; McAllister T. E.; Garcia-Hartjes J.; Fascione M. A.; Ross J. F.; Warriner S. L.; Wennekes T.; Zuilhof H.; Turnbull W. B. A Protein-Based Pentavalent Inhibitor of the Cholera Toxin B-Subunit. Angew. Chem. 2014, 126, 8463–8467. 10.1002/ange.201404397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Zeng W.; Offord R.; Rose K. A Novel Method for the Rational Construction of Well-Defined Immunogens: The Use of Oximation To Conjugate Cholera Toxin B Subunit to a Peptide–Polyoxime Complex. Bioconjugate Chem. 2003, 14, 614–618. 10.1021/bc025651u. [DOI] [PubMed] [Google Scholar]
- Geoghegan K. F.; Stroh J. G. Site-Directed Conjugation of Nonpeptide Groups to Peptides and Proteins via Periodate Oxidation of a 2-Amino Alcohol. Application to Modification at N-Terminal Serine. Bioconjugate Chem. 1992, 3, 138–146. 10.1021/bc00014a008. [DOI] [PubMed] [Google Scholar]
- Rose K. Facile Synthesis of Homogeneous Artificial Proteins. J. Am. Chem. Soc. 1994, 116, 30–33. 10.1021/ja00080a004. [DOI] [Google Scholar]
- Beddoe T.; Paton A. W.; le Nours J.; Rossjohn J.; Paton J. C. Structure, Biological Functions and Applications of the AB5 Toxins. Trends Biochem. Sci. 2010, 35, 411–418. 10.1016/j.tibs.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan E.; Merritt E. A.; Verlinde C. L.; Hol W. G. AB5 Toxins: Structures and Inhibitor Design. Curr. Opin. Struct. Biol. 2000, 10, 680–686. 10.1016/S0959-440X(00)00152-4. [DOI] [PubMed] [Google Scholar]
- Heggelund J. E.; Burschowsky D.; Bjørnestad V. A.; Hodnik V.; Anderluh G.; Krengel U. High-Resolution Crystal Structures Elucidate the Molecular Basis of Cholera Blood Group Dependence. PLoS Pathog. 2016, 12, e1005567 10.1371/journal.ppat.1005567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervin J.; Wands A. M.; Casselbrant A.; Wu H.; Krishnamurthy S.; Cvjetkovic A.; Estelius J.; Dedic B.; Sethi A.; Wallom K.-L.; Riise R.; Bäckström M.; Wallenius V.; Platt F. M.; Lebens M.; Teneberg S.; Fändriks L.; Kohler J. J.; Yrlid U. GM1 Ganglioside-Independent Intoxication by Cholera Toxin. PLoS Pathog. 2018, 14, e1006862 10.1371/journal.ppat.1006862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wands A. M.; Cervin J.; Huang H.; Zhang Y.; Youn G.; Brautigam C. A.; Matson Dzebo M.; Björklund P.; Wallenius V.; Bright D. K.; Bennett C. S.; Wittung-Stafshede P.; Sampson N. S.; Yrlid U.; Kohler J. J. Fucosylated Molecules Competitively Interfere with Cholera Toxin Binding to Host Cells. ACS Infect. Dis. 2018, 4, 758–770. 10.1021/acsinfecdis.7b00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haksar D.; Quarles van Ufford L.; Pieters R. J. A Hybrid Polymer to Target Blood Group Dependence of Cholera Toxin. Org. Biomol. Chem. 2020, 18, 52–55. 10.1039/C9OB02369K. [DOI] [PubMed] [Google Scholar]
- Haksar D.; de Poel E.; van Ufford L. Q.; Bhatia S.; Haag R.; Beekman J.; Pieters R. J. Strong Inhibition of Cholera Toxin B Subunit by Affordable, Polymer-Based Multivalent Inhibitors. Bioconjugate Chem. 2019, 30, 785–792. 10.1021/acs.bioconjchem.8b00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V.; Turnbull W. B. Carbohydrate Inhibitors of Cholera Toxin. Beilstein J. Org. Chem. 2018, 14, 484–498. 10.3762/bjoc.14.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett J. C.; Bertozzi C. R. Cu-Free Click Cycloaddition Reactions in Chemical Biology. Chem. Soc. Rev. 2010, 39, 1272. 10.1039/b901970g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debets M. F.; van der Doelen C. W. J.; Rutjes F. P. J. T.; van Delft F. L. Azide: A Unique Dipole for Metal-Free Bioorthogonal Ligations. ChemBioChem 2010, 11, 1168–1184. 10.1002/cbic.201000064. [DOI] [PubMed] [Google Scholar]
- Dommerholt J.; Schmidt S.; Temming R.; Hendriks L. J. A.; Rutjes F. P. J. T.; van Hest J. C. M.; Lefeber D. J.; Friedl P.; van Delft F. L. Readily Accessible Bicyclononynes for Bioorthogonal Labeling and Three-Dimensional Imaging of Living Cells. Angew. Chem., Int. Ed. 2010, 49, 9422–9425. 10.1002/anie.201003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudden A. R.; Chinoy Z. S.; Wolfert M. A.; Boons G.-J. A Multifunctional Anomeric Linker for the Chemoenzymatic Synthesis of Complex Oligosaccharides. Chem. Commun. 2014, 50, 7132–7135. 10.1039/C4CC02222J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Feizi T.; Campanero-Rhodes M. A.; Childs R. A.; Zhang Y.; Mulloy B.; Evans P. G.; Osborn H. M. I.; Otto D.; Crocker P. R.; Chai W. Neoglycolipid Probes Prepared via Oxime Ligation for Microarray Analysis of Oligosaccharide-Protein Interactions. Chem. Biol. 2007, 14, 847–859. 10.1016/j.chembiol.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Godula K.; Rabuka D.; Nam K. T.; Bertozzi C. R. Synthesis and Microcontact Printing of Dual End-Functionalized Mucin-like Glycopolymers for Microarray Applications. Angew. Chem. 2009, 121, 5073–5076. 10.1002/ange.200805756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsdottir A. V.; Paul C. E.; Nitz M. Stability Studies of Hydrazide and Hydroxylamine-Based Glycoconjugates in Aqueous Solution. Carbohydr. Res. 2009, 344, 278–284. 10.1016/j.carres.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Haigh J. L.; Williamson D. J.; Poole E.; Guo Y.; Zhou D.; Webb M. E.; Deuchars S. A.; Deuchars J.; Turnbull W. B. A Versatile Cholera Toxin Conjugate for Neuronal Targeting and Tracing. Chem. Commun. 2020, 56, 6098–6101. 10.1039/D0CC01085E. [DOI] [PubMed] [Google Scholar]
- Jobling M. G.; Holmes R. K. Analysis of Structure and Function of the B Subunit of Cholera Toxin by the Use of Site-Directed Mutagenesis. Mol. Microbiol. 1991, 5, 1755–1767. 10.1111/j.1365-2958.1991.tb01925.x. [DOI] [PubMed] [Google Scholar]
- Wiltschi B.Expressed Protein Modifications: Making Synthetic Proteins. In Synthetic Gene Networks: Methods and Protocols; Weber W.; Fussenegger M., Eds.; Humana Press: Totowa, NJ, 2012; pp 211–225. [DOI] [PubMed] [Google Scholar]
- Ngo J. T.; Tirrell D. A. Noncanonical Amino Acids in the Interrogation of Cellular Protein Synthesis. Acc. Chem. Res. 2011, 44, 677–685. 10.1021/ar200144y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabham R. L.; Keenan T.; Husken A.; Bilsborrow J.; McBerney R.; Kumar V.; Turnbull W. B.; Fascione M. A. Rapid Sodium Periodate Cleavage of an Unnatural Amino Acid Enables Unmasking of a Highly Reactive α-Oxo Aldehyde for Protein Bioconjugation. Org. Biomol. Chem. 2020, 18, 4000–4003. 10.1039/D0OB00972E. [DOI] [PubMed] [Google Scholar]
- Rose K.; Chen J.; Dragovic M.; Zeng W.; Jeannerat D.; Kamalaprija P.; Burger U. New Cyclization Reaction at the Amino Terminus of Peptides and Proteins. Bioconjugate Chem. 1999, 10, 1038–1043. 10.1021/bc9900587. [DOI] [PubMed] [Google Scholar]
- Kartha K. P. R.; Jennings H. J. A Simplified, One-Pot Preparation of Acetobromosugars from Reducing Sugars. J. Carbohydr. Chem. 1990, 9, 777–781. 10.1080/07328309008543873. [DOI] [Google Scholar]
- Liese S.; Netz R. R. Quantitative Prediction of Multivalent Ligand–Receptor Binding Affinities for Influenza, Cholera, and Anthrax Inhibition. ACS Nano 2018, 12, 4140–4147. 10.1021/acsnano.7b08479. [DOI] [PubMed] [Google Scholar]
- Vaughan M. D.; Johnson K.; DeFrees S.; Tang X.; Warren R. A. J.; Withers S. G. Glycosynthase-Mediated Synthesis of Glycosphingolipids. J. Am. Chem. Soc. 2006, 128, 6300–6301. 10.1021/ja058469n. [DOI] [PubMed] [Google Scholar]
- Pukin A. V.; Weijers C. A. G. M.; van Lagen B.; Wechselberger R.; Sun B.; Gilbert M.; Karwaski M.-F.; Florack D. E. A.; Jacobs B. C.; Tio-Gillen A. P.; van Belkum A.; Endtz H. P.; Visser G. M.; Zuilhof H. GM3, GM2 and GM1 Mimics Designed for Biosensing: Chemoenzymatic Synthesis, Target Affinities and 900MHz NMR Analysis. Carbohydr. Res. 2008, 343, 636–650. 10.1016/j.carres.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Turnbull W. B.; Precious B. L.; Homans S. W. Dissecting the Cholera Toxin–Ganglioside GM1 Interaction by Isothermal Titration Calorimetry. J. Am. Chem. Soc. 2004, 126, 1047–1054. 10.1021/ja0378207. [DOI] [PubMed] [Google Scholar]
- Merritt E. A.; Sarfaty S.; Van Den Akker F.; L’Hoir C.; Martial J. A.; Hol W. G. J. Crystal Structure of Cholera Toxin B-Pentamer Bound to Receptor G M1 Pentasaccharide. Protein Sci. 1994, 3, 166–175. 10.1002/pro.5560030202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. P.; Schengrund C.-L. Inhibition of the Adherence of Cholera Toxin and the Heat-Labile Enterotoxin of Escherichia Coli to Cell-Surface GM1 by Oligosaccharide-Derivatized Dendrimers. Biochem. Pharmacol. 1998, 56, 591–597. 10.1016/S0006-2952(98)00198-1. [DOI] [PubMed] [Google Scholar]
- Thompson J. P.; Schengrund C.-L. Oligosaccharide-Derivatized Dendrimers: Defined Multivalent Inhibitors of the Adherence of the Cholera Toxin B Subunit and the Heat Labile Enterotoxin of E. Coli to GM1. Glycoconjugate J. 1997, 14, 837–845. 10.1023/A:1018590021762. [DOI] [PubMed] [Google Scholar]
- Schengrund C. L.; Ringler N. J. Binding of Vibrio Cholera Toxin and the Heat-Labile Enterotoxin of Escherichia Coli to GM1, Derivatives of GM1, and Nonlipid Oligosaccharide Polyvalent Ligands. J. Biol. Chem. 1989, 264, 13233–13237. 10.1016/S0021-9258(18)51619-7. [DOI] [PubMed] [Google Scholar]
- Tanaka T.; Nagai H.; Noguchi M.; Kobayashi A.; Shoda S. One-Step Conversion of Unprotected Sugars to β-Glycosyl Azides Using 2-Chloroimidazolinium Salt in Aqueous Solution. Chem. Commun. 2009, 23, 3378–3379. 10.1039/b905761g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.