Abstract
Objective
This article reviews childhood cancers and the treatments that affect bone integrity. Interventions related to exercise, hormone replacement, vitamin D and calcium supplementation, and bisphosphonate use are addressed.
Data Sources
Literature was reviewed related to childhood cancers, treatment and side effects, assessment, and management to optimize bone health.
Conclusions
Cure rates of childhood cancer have dramatically improved due to new therapeutic advances allowing children diagnosed with cancer to live longer. Unfortunately, the cost of cure can be the increased development of chronic health issues. Since many children receive their treatment including antineoplastic agents, radiation, surgery, and corticosteroid therapy during time of active skeletal maturation and growth, their bone health may be negatively impacted. The development of bone mass can be impaired, bone density may be decreased, fractures may occur, growth may be restricted and there may be poor bone repair. Review of the data indicates that more research is needed to understand what is necessary for optimal bone health in the pediatric population in general, specifically for the child affected by cancer.
Implications for Nursing Practice
Nurses are integral to the development of comprehensive understanding of the bone health needs of the pediatric oncology patient population and educating the patient and family about the importance of bone health. Children and young adults survivors would benefit from collaborative care between all their health care providers. Steps to improve bone health before diagnosis, on treatment and through to survivorship remain to be addressed by future research.
Keywords: Child, Bone Density, Neoplasms, Survivorship, Antineoplastic Agents
Introduction
Cure rates of childhood cancer have dramatically improved due to new advances in treatment. Children with cancer are living longer with the five-year survival rate now close to 80%1. Unfortunately, the cost of cure is the increased development of chronic health issues. Since many children receive their treatment during time of active skeletal maturation and growth, they may have a significant impact on their bone health. Negative effects on bone can come from direct insult from surgery, radiation, chemotherapy, or corticosteroids which are the mainstay of most cancer treatment protocols. One of the most common late effects from cancer treatment in children is endocrine dysfunction, with associated hormonal alterations having a negative impact on bone health. It is important to understand the impact of current treatments prior to, during and after the cancer treatment period on children’s bone health so clinicians can intervene early to prevent negative outcomes, such as bone mass impairment, decreased bone mineral density, growth restriction, poor bone repair and fractures. Therefore, this article aims to increase awareness of bone health issues related to pediatric oncology treatment to optimizing bone health across the cancer care continuum.
Normal bone formation
Bone growth occurs during childhood and adolescence, as seen in increased lengthening and width of bones. Bone development continues throughout adulthood even after final height is achieved. Bone growth is completed at the end puberty when the end of long bones fuse. Typically, bone growth is completed at age 14-18 for girls and 16–20 for boys, but heredity, ethnic background and other factors can extend the age for some individuals2. There are three types of cells involved in the development, growth and remodeling of bones (osteoblasts, osteocytes and osteoclasts). Osteoblasts are responsible for synthesis and mineralization of bone. Osteocytes are derived from osteoblasts and are the longest living bone cell. Osteoclasts are the cells that degrade bone to initiate normal bone remodeling and mediate bone loss. In normal bone, formation occurs dynamically as modeling and remodeling throughout life. Modeling occurs when bones change in response to mechanical forces or physiological forces. Wolff’s law describes the observation that bones change shape to accommodate stresses placed on them3. The remodeling process maintains strength and mineral homeostasis by continually resorbing old bone and forming new bone structure throughout fetal development and life. Bone mass gradually increases during childhood and adolescence while bone resorption dominates after age 404.
Bone Tumors in Childhood: Benign or Malignant
Tumors that develop in childhood can be classified as benign or malignant. Malignant tumors are more aggressive, grow quickly and spread throughout the body. Childhood bone tumors may arise from the bone itself, the surrounding soft tissue, or from surrounding muscle. Clinical presentation of many malignant childhood bone tumors can be subtle and can be easily mistaken for a more common benign conditions, such as a painful injury which worsens or fails to improve over a few weeks. Peritumoral edema can be misleading and can be a symptom of a bone tumor so that any evidence of edema should be fully investigated to rule out a malignant process. Serum inflammation markers including C reactive protein and erythrocyte sedimentation rate may be helpful as a nonspecific diagnostic tool. On initial exam, a child is evaluated with a plain x-ray. If a questionable mass is seen, a dedicated MRI with contrast is needed6. General labs including a complete blood count, comprehensive metabolic panel and lactate dehydrogenase are drawn, and as previously stated inflammation markers are useful to evaluate surrounding tissue involvement.
Pain is a critical indicator and should help drive the clinician during the diagnostic evaluation6. Some children have intermittent or persistent localized pain, but pain that wakes a child from sleep can be a predictor of a more ominous process. Sometimes a child suffers a fracture from which the force seems insignificant to cause the severity of the injury and this is due to the underlying weakness created by the bone tumor. Even if there is no evidence of fracture at diagnosis, the risk of a pathologic fracture is high. For this reason, bracing of the extremity is essential as well as limiting the child to non-weight bearing status.
The most common bone tumors in childhood are benign and include non-ossifying fibroma, osteochondroma, Langerhans cell histiocytosis, unicameral bone cyst, and aneurysmal bone cyst. The most common malignant bone tumors include osteosarcoma and Ewing sarcoma6. Rhabdomyosarcoma is a type of tumor that affect bone and muscle and are less common than osteosarcoma and Ewing Sarcoma. Primary bone tumors are not the only malignancies that impact bone health. It is common for pediatric malignancies such as neuroblastoma, hepatoblastoma, leukemia, lymphoma and brain tumors to metastasize to the bone, disrupting bone integrity and requiring direct radiation, the effects of which continue for years after treatment is completed.
Treatment
Pediatric cancer treatment can include surgery, chemotherapy, radiation, stem cell transplantation, hormonal deprivation, glucocorticoids, immunotherapy, targeted agents, or a combination of the above. It is important to know the type and total dose of treatment received since the main contributing factor of bone mass impairment in childhood cancer survivors is the treatment itself. As scientific advances build exponentially with immunotherapy and targeted agents that may be combined with conventional therapies, it is important to be cognizant about the potential impact of these agents on the developing bone.
Chemotherapy
Chemotherapy is a combination of medications to destroy cancer cells throughout the body and blood stream, but unfortunately destroys healthy cells as well. The most common agents used to treat childhood cancer includes alkylating agents, antibiotics, and antimetabolites1. The goal of chemotherapy is to achieve systemic control of disease at a microscopic level before cells can metastasize to other areas of the body. Treatment effects are based on cumulative dose, schedule, route of administration, and the sex and age of a patient1. As per the NIH , up to 90 percent of peak bone mass is acquired at an earlier age in females than males which makes childhood illness detrimental to bone health2.
Chemotherapy schedules can lead to extended time of myelosuppression. Children who are myelosuppressed tend to feel weak and tired with reduction in physical activity, increased bed rest and decreased exposure to sunshine, resulting in less vitamin D production. Decreased activity with limited weight bearing decreases opportunity for adequate bone building1.
Radiation Therapy
Radiotherapy is utilized for treatment for several childhood cancers including leukemia, neuroblastoma, lymphoma, brain tumors, Wilms tumor, and most sarcoma’s excluding osteosarcoma as osteosarcoma cells are resistant to radiation1. Radiation destroys the DNA of cancer cells by making small breaks in the cells, preventing them from growing and dividing. Radiation provides local control that cannot be achieved with surgery alone. When there are multiple sites of disease or if surgery is not an option, radiation is indicated to attempt to eradicate residual cancer cells.
The risk of radiation induced late effects depend on the radiation source, field, cumulative dose, volume, and fractionation, as well as age and sex at the time of treatment1. Radiation to any area that includes the spine received during the growing years may cause a reduction in vertebral height7. Due to the absorbed dose of the irradiated bones, patients may be at increased risk for fractures, avascular necrosis, and alterations in bone growth. Children are more susceptible to radiation than adults8. Radiation to the brain can cause disruption of the hypothalamic pituitary axis leading to growth hormone deficiency and central hypogonadism with a subsequent reduction in androgen and estrogen production which has a negative impact on bone health. Pelvic, whole abdomen and lumbar/sacral spine radiation can directly impact the gonads causing endocrine alterations that can affect bone growth, bone mass acquisition and low bone mineral density1.
Surgery
Surgical intervention with wide excision is needed for tumor removal and or tumor debulking. Ideally, surgical intervention will achieve local control for solid tumors. When the whole tumor cannot be removed and or clean margins cannot be achieved, a combination of surgery and radiation may be needed for tumor control. Preoperative chemotherapy is aimed at controlling growth of primary tumors and symptoms. Decreased pain may be an indication of improvement. Overall, the aim of surgery is a reduction in tumor size and neurovascular bundle involvement reduction6. Full surgical resection is always the goal for bone tumors and is aimed to achieve the best function available to the patient. Joint preservation may or may not be possible. Prosthetic limbs with magnetic growth options are available and new procedures are being developed9. The success of limb lengthening procedures depends on the successful development of new bone. As some surgeries will disrupt the growth plate in long bone, bone arrest may be an untoward outcome. Physical therapy should be started immediately post operatively to ensure rapid healing of bone reconstruction, but it is important to note that chemotherapy post-surgery may interfere with healing1.
Steroids
Corticosteroids are widely used for treatment for many childhood malignancies either to prevent side-effects such as nausea and vomiting or to potentiate other anticancer treatments. Their impact on bone health depends on the cumulative dose and length of exposure. Steroids cause decreased osteoblast activity, increased bone resorption, decrease in muscle strength and can cause a disturbance of calcium balance. Steroids are commonly used as primary cytotoxic treatment for hematologic malignancies. In solid tumors, steroids are used for symptomatic management such as swelling and nausea. For those treatment protocols where the cumulative doses of more than 9000mg/m2 of prednisone equivalent are indicated, children and adolescents are predisposed to low bone mineral density which sometimes doesn’t recover after treatment ends10. Glucocorticoids may promote calcium loss through the kidneys and gut which can lead to increased bone remodeling and osteoclastic activity due to secondary hyperparathyroidism.
Effect of treatment on hormone function
Gonadal dysfunction can occur from cancer treatment, especially high dose alkylating agents frequently used prior to hemopoietic stem cell transplantation. The risk of hypogonadism is related to cumulative dose, age, and sex. The higher the dose, the higher the risk. When cancer treatment occurs during puberty, hormone regulation may be altered with a delay or arrest in pubertal development. Estrogen is the major regulator of bone metabolism in both women and men. If there is a delay in estrogen production, bone accrual is affected, and bone health can be negatively impacted. Estrogen has a role for attaining peak bone mineral density in both males and females as evidenced by lower bone mineral density (BMD) in females who achieve menarche late and in males who have mutations in the estrogen receptor gene and aromatase gene1. Estrogen prevents bone resorption and stimulates growth factor necessary for growth and estrogen deficiency can lead to an excessive increase in bone resorption accompanied by inadequate bone formation. Androgens have a key role in increasing the diameter of bones by the addition of bone tissue on the surface of the bones.1 Direct radiation to the gonads or to the hypothalamic gonadal access can increase the risk for gonadal dysfunction which decreases hormonal production.
Treatment with gonadotropin releasing hormone (GnRH) agonists can be used for short term use in patients with estrogen dependent tumors and for hypothalamic gonadal suppression in patients who develop central precocious puberty. GnRH agonists can also be used to preserve fertility prior to receiving cancer treatment in adolescents and young adults. Treatment with GnRH agonist of six months or more can cause significant bone loss in both the trabecular and cortical bones, with recovery achieved slowly or not at all1.
Further Sequela to Bone
Osteopenia
The prevalence of osteopenia and osteoporosis in childhood cancers is not well documented. All childhood cancers, whether liquid or solid tumors may lead to bone mineral density deficits and increased fracture risk that continue for a lifetime. Treatment related osteopenia is an under-recognized problem in children and young adults receiving chemotherapy11. Methotrexate (MTX), a frontline chemotherapy for osteosarcoma, has direct cytotoxic effect on osteoblasts and is associated with reduced bone volume and bone formation. Higher cumulative doses of MTX are associated with greater risk of osteopenia1. Frequently chemotherapy regimens combine cisplatin and MTX leading to nephrotoxicity and skeletal abnormalities. Ifosfamide, another common agent, can damage renal tubular function and may induce Fanconi syndrome, which can result in electrolyte wasting and reduction in serum calcium required for bone building1.
Avascular Necrosis
Growth arrest can occur because of surgery or from radiation and chemotherapy, increasing the risk of fracture due cell destruction and necrosis. Childhood cancer patients are at risk for a potentially devastating side effect of avascular necrosis (AVN), where the temporary or permanent interruption of blood supply to bone leads to tissue death, AVN occurs frequently after hemopoietic stem cell transplant yet can be seen with chemotherapy, radiation therapy or steroid use1. (AVN is associated with decreased bone stability and pain, requiring surgical repair in severe cases, though there no clear practice guidelines on the best management of AVN.
Research is needed to improve early diagnosis of AVN. Serial total body magnetic resonance imaging has been suggested yet may not be realistic in routine practice1. Children with cancer have a significantly increased risk of AVN as compared to with the healthy childhood population1. Further investigation is needed to determine genetic risk, monitoring, and treatment.
Assessment, Management, and Monitoring of Bone Health During Treatment and Survivorship
Assessment
Prior to initiating treatment of childhood cancers, it is necessary to assess lifestyle, nutritional status, and baseline bone health. Evaluation of bone health should include laboratory studies to assess bone metabolism and renal function as well as other factors that could induce secondary osteoporosis. Obtaining baseline anthropometric evaluations including height, weight and body mass index are needed to evaluate growth disorders or other issues that could interfere with bone health1. Bone fragility is a silent condition that increases bone fracture risk and may be present in pediatric patients at the time of cancer diagnosis. Aristizabal et al, performed a rare study investigating associated sociodemographic factors and vitamin D status in the newly diagnosed pediatric cancer patient12. Their findings showed that vitamin D deficiency and insufficiency affect approximately two-thirds of the pediatric cancer patients in their institutions. More studies like this are needed and baseline assessment of all pediatric cancer patients is required as the biggest risk factor for fragility fracture is low bone mass. Fragility fractures are broken bones related to osteoporosis. Assessment for BMD is done for patients at risk for osteoporosis in the post treatment phase. Bone mineral density is based on age and pubertal development. Low bone mineral density is when the z score is <−2 or T score <−2.5. Bone mineral density can sometimes improve after childhood cancer treatment ends, but for some patients followed into adulthood, they frequently have decreased bone mineral density and therefore an increased risk for fracture. The Children’s Oncology Group Long Term Follow Up guidelines13 recommends obtaining bone mineral density by dual-energy X-ray absorptiometry (DEXA) scan for those children with history of growth hormone deficiency, hypogonadism, delayed puberty, hyperthyroidism, or having received treatment with methotrexate, corticosteroids or hemopoietic stem cell transplantation. DEXA scan is obtained at the spine and femur and frequency of re-evaluations is determined based on risk of fracture and results of baseline evaluations. Bone mass is a key determinant of osteoporosis and fragility fractures. Epidemiologic studies have shown that a 10% increase in peak bone mass reduces the risk of fracture later in life by 50%14.
Management
Treatment of low bone mineral density in children who have received pediatric cancer directed therapies that impact bone is aimed at correcting the underlying cause1. Growth hormone deficiency (GHD) can develop in those children whose treatment impacts the hypothalamic pituitary axis and GHD is associated with decreased bone mineral density with an associated increased fracture risk. Children whose treatment causes them to be growth hormone deficient and are treated with growth hormone during their growing years usually stop treatment at epiphyseal fusion. Not all children with growth hormone deficiency go on to receive growth hormone as adults. Patients who received gonadotoxic agents including high dose alkylating agents, myeloablative treatments and gonad directed radiation causing testosterone and estrogen deficiencies should be referred to endocrinology to evaluate if treatment with testosterone and estrogen respectively is indicated1.
Bone-modifying Agents
There is limited evidence with the use of bisphosphonates in children. They are utilized in bone reformation post-surgical interventions and as an adjuvant to chemotherapy based on evidence found in adult literature. Meyers et al preformed a single arm study utilizing pamidronate 2 mg/kg/dose (max dose 90 mg) for 12 doses to first line therapy in treating osteosarcoma11. Their experience suggested that pamidronate could be safely incorporated with chemotherapy and suggests that bisphosphonates may improve the durability of surgical reconstructions. The single arm study showed hypocalcemia was common requiring supplementation, thus monitoring calcium levels and oral intake is recommended11.
Piperno-Neumann et al. conducted a randomized, multicenter, open-label, phase 3 trial comparing chemotherapy alone compared with chemotherapy plus 10 cycles of Zoledronate inn patients between 5 through 50 years of age with newly diagnosed high grade osteosarcoma15. Zoledronate was chosen for study based on preclinical data suggesting that it exerts pleiotropic antitumor effects in vitro animal studies and demonstrates beneficial effects on improving bone density in adults. Side effects of jaw necrosis and gastrointestinal issues are seen during adult administration and need to be considered and further studied in children. Results showed no significant difference with orthopedic complications were noted with the addition of Zoledronate15. Again, more research is needed.
Denosumab is an inhibitor of receptor of nuclear factor kappa-B ligand (RANKL). Denosumab is a human monoclonal antibody of the IgG2 immunoglobulin isotype, and binds specifically, thereby creating the same effect as endogenous osteoprotegrin, which inhibits osteoclast activity, resulting in rapid suppression of bone resorption16. As opposed to bisphosphonates, denosumab does not incorporate into bone matrix and bone turnover is not suppressed after its cessation. After drug discontinuation, its effects on bone turnover are rapidly reversable. Rebound increased bone turnover with stopping denosumab has led to severe hypercalcemia and phosphoremia in several pediatric patients16. Boyce examined the use of denosumab in children to combat pediatric bone disorders and concluded that though denosumab is promising for anti-resorption for targeted intervention in children with RANKL pathway disorders, its use should be limited to clinical trials and for compassionate use to enhance those with significantly impaired quality of life16. At this point, bisphosphonates or denosumab for general bone building in children cannot be recommended outside of further clinical trials1.
Calcium and Vitamin D
Patient education should focus on obtaining adequate calcium and vitamin D intake through natural food sources and through supplementation if necessary. Calcium intake and supplementation should be recommended according to national guidelines: one to three years old need 700 mg of calcium per day, four to eight years old need 1,000 mg per day, and nine to 18-year-olds need 1,300 mg per day17. When considering the loss of calcium with childhood cancer treatments, it becomes a necessity to supplement pending serial blood work.
Aside from chemical supplements, foods provide calcium. Milk, yogurt, cheese, and other dairy products provide an excellent source of calcium. Vegan sources of calcium include seeds, beans, lentils and green leavy vegetables. Calcium may also be obtained by “calcium enriched or fortified” drinks and cereals1. In general, our bodies can only absorb 500 gm of elemental calcium at a time; thus, supplementation timing should be taken into consideration18.
Optimal vitamin D is essential for absorption of calcium into the bone and required for bone health. The needed use of preventative sunscreen to protect against skin cancer decreases absorption of vitamin D19. Recommendations for dietary vitamin D intake need to keep within the standard national guidelines with periodic 25OHD monitoring to detect levels <20 ng/ml. Supplements are recommended in children with low levels, to maintain levels greater than or equal to 20 ng/ml year long19. Subsequent 25OHD levels should be measured every 6 months while on treatment. Vitamin D and calcium may be found in foods, for example, yogurt and milk. As children on treatment frequently have difficulty taking in an appropriate diet recommendation for 400 IU Vitamin D daily.
Physical Activity
Physical activity should be encouraged throughout treatment and survivorship to optimize bone health since radiation, surgery and chemotherapy cycles can result in increased sedentary time.
Weight bearing exercise is essential for bone mineral density development in children and maintenance through their adult years. This is important pre, during and post cancer treatment. Post limb salvage, physical activity is key for bone reconstruction, and children with bone tumors should be working with physical therapists from the time of diagnosis to achieve the best outcomes post their needed surgical interventions.
After treatment ends, guidelines and recommendations that focus on attaining and maintaining bone health should be encouraged. Children need encouragement in optimizing their weight bearing activities, such as walking and lifting low weights. Swimming would not be an optimal choice for bone building as it does not allow the individual to bear weight. Choice of recommendations depends on the child’s individual health, age, body weight, residence, and dietary and cultural habits1. Limb salvage and or history of treatment with cardiotoxic medications, will possibly prevent an individual from participating in exercise that will put stress on the compromised body part.
Implications for practice and conclusion
Pediatric cancer disease and treatment leads to increased fragility of bone health that extends through adulthood. Bone health should be monitored pre, during and post treatment to achieve wellness with cure. As with many pediatric driven needs, more evidence is needed to support optimal bone health, as much is extrapolated from the adult literature. Children are not small adults; children are still in development and not just maintenance. Much of the information for which childhood recommendations are made is built from the knowledge obtained through adult studies. The need for large observational and randomized trials are needed in healthy children as well as children diagnosed with cancer to develop more definitive recommendations for optimal bone health in children. Professional collaboration including nurses, surgeons, radiation oncologists, medical oncologists, and primary care providers is required to achieve optimal outcomes for bone health. Further interdisciplinary research is needed to better understand bone health needs and management.
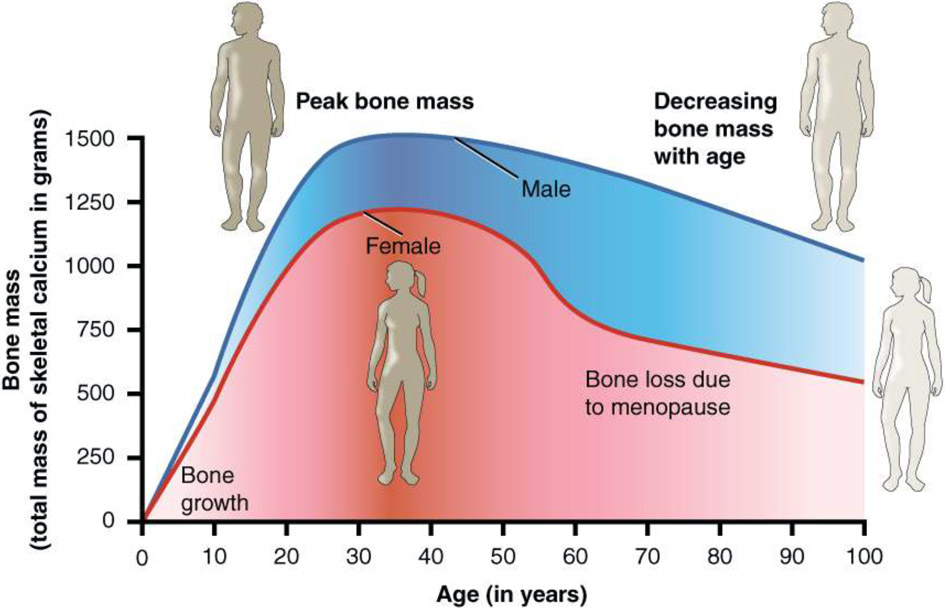
Figure 1.
Graph Showing Relationship Between Age and Bone Mass Bone density peaks at about 30 years of age5. (Section 6.6 Exercise, Nutrition, Hormones, and Bone Tissue, Reprinted from OpenStax, CC 4.0)
Funding:
This work was funded, in part, through a grant (P30CA008748) from the National Institutes of Health, National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marcucci G, Beltrami G, Tamburini A, et al. Bone health in childhood cancer: review of the literature and recommendations for the management of bone health in childhood cancer survivors. Ann Oncol. 2019;30(6):908–920. [DOI] [PubMed] [Google Scholar]
- 2.Kids and Their Bones: A Guide for Parents ∣ NIH Osteoporosis and Related Bone Diseases National Resource Center. Published 2018. Accessed February 28, 2022. https://www.bones.nih.gov/health-info/bone/bone-health/juvenile [Google Scholar]
- 3.Wolff J The Law of Bone Remodelling. Springer; 1986. doi: 10.1007/978-3-642-71031-5 [DOI] [Google Scholar]
- 4.Clarke B Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3 Suppl 3(Suppl 3):S131–9. doi: 10.2215/CJN.04151206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betts JG, Young KA, Wise JA, et al. 6.6 Exercise, Nutrition, Hormones, and Bone Tissue. In: Anatomy and Physiology. OpenStax; 2013. https://openstax.org/books/anatomy-and-physiology/pages/preface [Google Scholar]
- 6.Lee V Bone tumours in childhood and adolescence. Paediatr Child Health (Oxford). 2018;28(4):164–168. doi: 10.1016/J.PAED.2018.01.004 [DOI] [Google Scholar]
- 7.Jussila M-P, Remes T, Anttonen J, et al. Late vertebral side effects in long-term survivors of irradiated childhood brain tumor. PLoS One. 2018;13(12):e0209193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao SS, El Abiad JM, Puvanesarajah V, Levin AS, Jones LC, Morris CD. Osteonecrosis in pediatric cancer survivors: Epidemiology, risk factors, and treatment. Surg Oncol. 2019;28:214–221. doi: 10.1016/j.suronc.2019.02.001 [DOI] [PubMed] [Google Scholar]
- 9.Hwang CL, Yu CJ, Shih JY, Yang PC, Wu YT. Effects of exercise training on exercise capacity in patients with non-small cell lung cancer receiving targeted therapy. Support Care Cancer. 2012;20(12):3169–3177. doi: 10.1007/S00520-012-1452-5 [DOI] [PubMed] [Google Scholar]
- 10.Wasilewski-Masker K, Kaste SC, Hudson MM, Esiashvili N, Mattano LA, Meacham LR. Bone mineral density deficits in survivors of childhood cancer: long-term follow-up guidelines and review of the literature. Pediatrics. 2008;121(3):e705–13. doi: 10.1542/peds.2007-1396 [DOI] [PubMed] [Google Scholar]
- 11.Meyers PA, Healey JH, Chou AJ, et al. Addition of pamidronate to chemotherapy for the treatment of osteosarcoma. Cancer. 2011;117(8):1736–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aristizabal P, Sherer M, Perdomo BP, et al. Sociodemographic and clinical characteristics associated with vitamin D status in newly diagnosed pediatric cancer patients. Pediatr Hematol Oncol. 2020;37(4):314–325. doi: 10.1080/08880018.2020.1721629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Children’s Oncology Group. Children’s Oncology Group. 2018. Long-Term Follow-Up Guidelines, version 5.. Published 2018. Accessed March 7, 2022. www.survivorshipguidelines.org [Google Scholar]
- 14.Zhu W, Cheng Z, Howard VJ, et al. Is adiposity associated with objectively measured physical activity and sedentary behaviors in older adults? BMC Geriatr. 2020;20(1). doi: 10.1186/s12877-020-01664-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piperno-Neumann S, Le Deley M-C, Rédini F, et al. Zoledronate in combination with chemotherapy and surgery to treat osteosarcoma (OS2006): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17(8):1070–1080. [DOI] [PubMed] [Google Scholar]
- 16.Boyce AM. Denosumab: an emerging therapy in pediatric bone disorders. Curr Osteoporos Rep. 2017;15(4):283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu X, Zheng H. Factors influencing peak bone mass gain. Front Med. 2021;15:53–69. [DOI] [PubMed] [Google Scholar]
- 18.Cha S, Perlman SL. Calcium Requirements in Children. Accessed January 28, 2022. https://www.hss.edu/conditions_calcium-requirements-children.asp [Google Scholar]
- 19.van Atteveld JE, Verhagen IE, van den Heuvel-Eibrink MM, et al. Vitamin D supplementation for children with cancer: A systematic review and consensus recommendations. Cancer Med. Published online 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]