Abstract
Background
Isolated systolic hypertension (ISH), is the most common form of hypertension in older adults. However, the ISH prevalence is not well known in many developing countries such as Iran. This study was conducted to determine the ISH prevalence and its related risk factors in an Iranian population.
Methods
Data were obtained from the second phase of the Shahroud eye cohort study (ShECS) in 2014. ShECS is a longitudinal population-based study which the first phase had been conducted in 2009 using the stratified multistage cluster sampling design on 5190 people aged 40 to 70 years. The ISH prevalence was determined based on the eighth Joint National Commission guidelines for different demographic variables. The associated risk factors were estimated by multiple logistic regression and a two-tailed p-value less than 0.05 was considered significant.
Results
The ISH prevalence was 15.89% (95% CI: 14.88–16.96). It was 15.68% (14.12–17.39) and 15.87% (14.54–17.29) for men and women, respectively. The prevalence of ISH increased significantly with increasing age. The 65–70 compared to 45–69 year age group (OR = 4.21), body mass index (OR = 1.03), diabetes (OR = 1.64), retirement, compared to practitioner job (OR = 1.53), and waist to hip ratio (WHR) (OR = 9.81) were significantly associated with ISH prevalence.
Conclusions
ISH is highly prevalent among the older adult population in Iran. Given the risk of cardiovascular disease associated with ISH, it is recommended to conduct education and public health interventions to improve the detection, prevention, and treatment of ISH.
Keywords: Isolated systolic hypertension, Older adult, Risk factor, Prevalence, Iran
Introduction
Hypertension is a serious medical condition and a major cause of premature death especially in low- and middle-income countries [1]. The disease is estimated cause to 10.7 million deaths (about 33.2% of deaths attributed to all risk factors) and 212 million Disability Adjusted Life Years (DALYs) (about 20.9% of DALYs attributed to all risk factors) worldwide [2]. According to the result of the global burden of disease study, hypertension is directly responsible for about 45% of deaths due to heart disease and 51% of deaths due to stroke [3]. Given the increase in the world's elderly population, the prevalence of this disease is expected to increase to 31.9% by 2025 [4, 5]. Hypertension has many different forms, including systolic-diastolic hypertension (SDH), isolated systolic hypertension (ISH), and isolated diastolic hypertension (IDH) [6, 7]. The ISH, which is defined as systolic blood pressure ≥ 140 mmHg and diastolic blood pressure < 90 mmHg [8], is the most common form of hypertension in older adults and affects approximately 50% of people over 60 years of age [9]. However, in recent decades the ISH prevalence has also increased in young adults due to the epidemic of overweight and obesity [10, 11]. The prevalence of ISH has been reported different from 6.51 to 35%, which depend on the definition of ISH and distribution of age, sex, race and other variables in the studied populations [9, 12–15]. It has shown that systolic blood pressure plays a greater role in cardiovascular disease than diastolic blood pressure [16]. Also, other epidemiological and interventional studies have shown that ISH considerably increased the risk of cardiovascular disease, stroke, chronic kidney disease, and dementia [17–22]. However, the prevalence and incidence of ISH in many developing countries, such as Iran, are not well known and this form of hypertension has received less attention as a strong risk factor. Therefore, the present study aimed at determining the prevalence of ISH and its related risk factors in the Iranian population.
Methods
The present study was conducted based on the data obtained from the second phase of the Shahroud eye cohort study (ShECS) in 2014. Shahroud eye cohort study is a longitudinal population-based study. The first phase of this study was conducted in 2009 in people aged 40–64 years old in Shahroud, northeast Iran. In ShECS, using the stratified multistage clustered sampling design, a total of 6311 people were randomly selected. For this purpose, each health care center (Shahroud has 9 comprehensive health service centers) was used as a stratum in Shahroud, and in each stratum were determined the number of clusters based on the population covered. The number of 300 clusters, and at least 20 individuals aged 40 to 64 years were selected from each cluster to participate in the study [23]. Of the 6311 individuals selected for this study, 5190 people (82.23%) participated in the study. After obtaining informed consent from the participants, in addition to demographic information including sex, age, economic status, education years, employment status, marital status, and smoking, participants underwent clinical examination, anthropometric examinations, blood pressure measurements, optometry and ophthalmology examinations, and tests. Of all 5190 people in the first phase, 453 people (8.7%) did not participate in the second phase of the study and 103 people had missing data. Finally, 4634 people were analyzed in this study. The study protocol was reviewed and approved by the Ethics Committee of Shahroud University of Medical Sciences. The details of the methodology of this study have already been published [23].
Blood pressure was measured by trained nursing staff twice for each participant using an Omron M3 Intellisense (HEM-7051-E, Omron Corp, Tokyo, Japan) automated electronic oscillometric blood pressure monitor, with medium/large cuffs at the right arm in the sitting position. The final blood pressure was the mean of the two measurements.
In the present study, consistent with the International Society of Hypertension guidelines [8], SDH was defined as systolic BP ≥ 140 mmHg and diastolic BP ≥ 90 mmHg, and ISH was defined as systolic BP ≥ 140 mmHg and diastolic BP < 90 mmHg. The prevalence of ISH was estimated in different age groups, sex, marital status, job status, diabetes status, abdominal obesity (waist circumference ≥ 102 cm in men and ≥ 88 cm in women), education years, and economic status with 95% confidence intervals. In ShECS, the economic status has been determined through principal component analysis on home assets [24].
The association between the categorical variables was assessed using the chi-square test. For quantitative variables, means were compared using the independent t-test. Variables that had a P-value of less than 0.2 in univariate analysis were included in multivariate analyses. The associated risk factors were estimated by multiple logistic regression analysis with the presence of ISH as the dependent variable. A two-tailed P-value less than 0.05 was considered significant. The effects of cluster sampling were considered in calculating the standard errors and confidence intervals.
Results
Among the 4737 participants in the second phase of the ShECS, the data required for this study were available for 4634 (41.13% were male) and analyzed for this report. The range and mean age of participants were 45–69 and 55.85 ± 6.21 years respectively. Most of the participants were married (89.73%) and in a low economic class (56.44%).
The mean education years, fasting blood sugar (FBS), and BMI of participants were 7.29 ± 4.66 years, 107.16 ± 40.52 mg/dl, and 28.87 ± 4.99 kg/m2, respectively. Other baseline characteristics of participants are provided in Table 1 according to isolated systolic hypertension.
Table 1.
Baseline characteristics of participants with and without Isolated Systolic Hypertension
| Variables | With ISH | Without ISH | Total | P-value |
|---|---|---|---|---|
| N (%), | N (%), | N (%), | ||
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Age Groups (Year) | ||||
| 45–49 | 58 (6.78) | 797 (93.22) | 855 (18.45) | < 0.001 |
| 50–54 | 149 (11.85) | 1108 (88.15) | 1257 (27.15) | |
| 55–59 | 184 (15.85) | 977 (84.15) | 1161 (25.05) | |
| 60–64 | 183 (21.73) | 659 (78.27) | 842 (18.17) | |
| 65–69 | 158 (30.51) | 361 (69.56) | 519 (11.18) | |
| Gender | ||||
| Men | 299 (15.69) | 1607 (84.31) | 1906 (41.13) | 0.862 |
| Women | 433 (15.87) | 2295 (84.13) | 2728 (58.87) | |
| Educational level | ||||
| Illiterate | 117 (22.81) | 396 (77.19) | 513 (11.08) | < 0.001 |
| Primary | 241 (16.45) | 1224 (83.55) | 1465 (31.63) | |
| Guidance | 113 (16.24) | 583 (83.76) | 696 (15.03) | |
| Highschool | 195 (13.58) | 1241 (86.42) | 1436 (31.01) | |
| College | 66 (12.67) | 455 (87.33) | 512 (11.25) | |
| Economic Status | ||||
| Low | 287 (18.76) | 1273 (81.03) | 1555 (33.56) | < 0.001 |
| Moderate | 238 (15.36) | 1331 (84.67) | 1549 (33.43) | |
| High | 207 (13.31) | 1360 (86.51) | 1530 (33.02) | |
| Marital status | ||||
| No | 82 (17.23) | 394 (82.77) | 476 (10.27) | 0.361 |
| Yes | 650 (15.63) | 3508 (84.37) | 4158 (89.73) | |
| Job | ||||
| Practitioner | 90 (9.83) | 826 (90.17) | 916 (19.77) | < 0.001 |
| Retired | 249 (19.78) | 1010 (80.22) | 1259 (27.17) | |
| Housekeeper | 385 (16.11) | 2005 (83.39) | 2390 (51.58) | |
| Others | 8 (11.59) | 61 (88.41) | 69 (1.49) | |
| Current smoking | ||||
| No | 639 (16.09) | 3332 (83.91) | 3971 (85.69) | 0.174 |
| Yes | 93 (14.03) | 570 (85.97) | 663 (14.31) | |
| Yes | 16 (26.23) | 45 (73.77) | 61 (1.32) | |
| Diabetes | ||||
| Nondiabetic | 466 (13.31) | 3033 (86.68) | 3499 (76.15) | < 0.001 |
| Diabetic | 259 (23.63) | 837 (76.37) | 1096 (23.85) | |
| Abdominal obesity | ||||
| No | 187 (13.36) | 1213 (86.64) | 1400 (30.25) | < 0.003 |
| Yes | 544 (16.85) | 2684 (83.15) | 3228 (69.75) | |
| Education (Year) | 6.41 ± 4.73 | 7.47 ± 4.63 | 7.31 ± 4.66 | < 0.001 |
| Body Mass Index (kg/m2) | 29.67 ± 4.92 | 28.73 ± 4.99 | 28.88 ± 4.99 | < 0.001 |
| Fasting Blood Sugar (mg/dL) | 116.91 ± 47.30 | 105.34 ± 38.86 | 107.16 ± 40.52 | < 0.001 |
| Triglyceride (mg/dL) | 192.11 ± 106.77 | 173.72 ± 97.08 | 176.62 ± 98.88 | < 0.001 |
| Total cholesterol (mg/dL) | 198.52 ± 43.22 | 197.77 ± 42.26 | 197.92 ± 41.57 | 0.563 |
| Glycated haemoglobin (%) | 5.81 ± 1.52 | 5.33 ± 1.41 | 5.41 ± 1.44 | < 0.001 |
| Height (cm) | 158.87 ± 8.86 | 159.71 ± 8.66 | 159.57 ± 8.68 | < 0.01 |
| Weight (kg) | 74.73 ± 12.56 | 73.09 ± 12.71 | 73.35 ± 12.69 | < 0.001 |
| Waist circumference (cm) | 104.51 ± 10.58 | 101.06 ± 10.83 | 101.61 ± 10.86 | < 0.001 |
| Wrist circumference (cm) | 17.22 ± 1.36 | 17.07 ± 1.33 | 17.09 ± 1.34 | < 0.005 |
| Hip circumference (cm) | 106.05 ± 9.74 | 104.96 ± 9.26 | 105.13 ± 9.34 | < 0.004 |
| Waist to Hip Ratio | 0.99 ± 0.07 | 0.96 ± 0.08 | 0.96 ± 0.7 | < 0.001 |
ISH isolated systolic hypertension, SD Standard Deviation
The overall prevalence of ISH was 15.89% (95% CI: 14.88–16.96%). The prevalence of ISH increased significantly with increasing age and it was 30.51% (26.68–34.60%) in the 65–70 years age group (Table 2). In all age groups, the ISH prevalence was higher in women than men, except in the age group of 65 to 70 years (Fig. 1), although this difference was not statistically significant.
Table 2.
Prevalence of isolated systolic hypertension by sociodemographic variables in the study population
| Variables | Prevalence of ISH | 95% Confidence interval | P-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age groups (year) | ||||
| 45–49 | 6.78 | 5.27 | 8.67 | < 0.001 |
| 50–54 | 11.85 | 10.17 | 13.75 | |
| 55–59 | 15.85 | 13.85 | 18.06 | |
| 60–64 | 21.73 | 19.07 | 24.65 | |
| 65–69 | 30.51 | 26.68 | 34.60 | |
| Gender | 0.861 | |||
| Men | 15.68 | 14.12 | 17.39 | |
| Women | 15.87 | 14.54 | 17.29 | |
| Education level | < 0.001 | |||
| Illiterate | 22.81 | 19.37 | 26.64 | |
| Primary | 16.45 | 14.63 | 18.43 | |
| Guidance | 16.23 | 13.67 | 19.16 | |
| Highschool | 13.57 | 11.91 | 15.45 | |
| College | 12.66 | 10.07 | 15.81 | |
| Economic status | < 0.001 | |||
| Low | 18.75 | 16.87 | 20.79 | |
| Middle | 15.36 | 13.65 | 17.24 | |
| High | 13.31 | 11.71 | 15.09 | |
| Marital status | 0.362 | |||
| Yes | 15.63 | 14.55 | 16.76 | |
| No | 17.22 | 14.08 | 20.89 | |
| Job | < 0.001 | |||
| Practitioner | 9.82 | 8.05 | 11.93 | |
| Retired | 19.77 | 17.66 | 22.07 | |
| Housekeeper | 16.11 | 14.68 | 17.63 | |
| Others | 11.59 | 5.87 | 21.61 | |
| Current smoking | 0.174 | |||
| Yes | 14.02 | 11.58 | 16.88 | |
| No | 16.09 | 14.98 | 17.26 | |
| Diabetes | < 0.001 | |||
| Diabetic | 23.63 | 21.21 | 26.23 | |
| Nondiabetic | 13.31 | 12.23 | 14.48 | |
| Body Mass Index | < 0.001 | |||
| < 18.5 | 4.34 | 1.07 | 16.01 | |
| 18.5–24.9 | 12.84 | 10.82 | 15.17 | |
| 25–29.9 | 15.43 | 13.89 | 17.11 | |
| ≥ 30 | 18.05 | 16.31 | 19.93 | |
| Abdominal obesity | < 0.003 | |||
| Yes | 16.85 | 15.59 | 18.18 | |
| No | 13.35 | 11.67 | 15.24 | |
ISH Isolated Systolic Hypertension, CI Confidence Interval
Fig. 1.
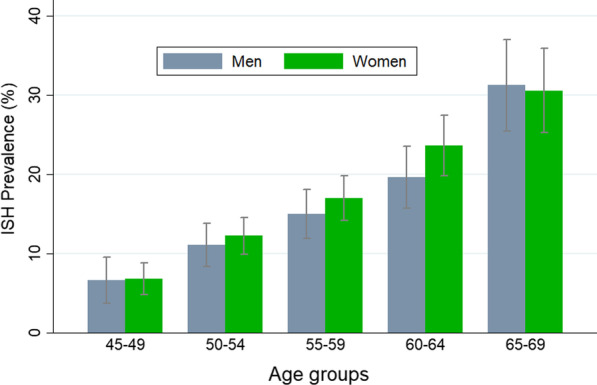
The prevalence of isolated systolic hypertension (ISH) in the study population by age and gender. Error bars indicate 95% confidence intervals for prevalence
The prevalence of ISH was higher in the low economic class 18.96% (95% CI: 17.10–20.98%). The prevalence of ISH significantly decreased with increasing education level and it was 22.81 (95% CI: 19.37–26.64) and 12.66 (95% CI: 10.07–15.81) in illiterate and college-educated respectively (Table 2).
On forward stepwise multiple logistic regression analysis, 65–70 years age group [OR = 4.21 (3.01–5.88)], BMI [OR = 1.03 (1.01–1.05)], diabetes [OR = 1.64 (1.36–1.98)], retirement [OR = 1.53 (1.17–2.01)], and waist to hip ratio (WHR) [OR = 9.81 (3.14–30.61)] were significantly associated with higher odds of ISH (Table 3).
Table 3.
Simple and multiple logistic regression models for the association of various risk factors with isolated systolic hypertension
| Independent variables | Crude Odds Ratio | P-value | Adjusted Odds Ratio | P-value |
|---|---|---|---|---|
| (95% CI) | (95% CI) | |||
| Age groups (year) | ||||
| 45–49 | Reference | – | Reference | – |
| 50–54 | 1.84 (1.35–2.51) | < 0.001 | 1.68 (1.23–2.51) | < 0.001 |
| 55–59 | 2.58 (1.85–3.61) | < 0.001 | 2.12 (1.51–2.98) | < 0.001 |
| 60–69 | 3.81 (2.79–5.21) | < 0.001 | 2.91 (2.08–4.06) | < 0.001 |
| 70–74 | 6.03 (4.41–8.23) | < 0.001 | 4.21 (3.01–5.88) | < 0.001 |
| Education (year) | 0.95 (0.93–0.96) | < 0.001 | 0.98 (0.96–1.00) | 0.113 |
| Economic status | ||||
| High | Reference | – | Reference | – |
| Middle | 1.51 (1.24–1.82) | < 0.001 | 1.05 (0.84–1.31) | 0.632 |
| Low | 1.18 (0.96–1.44) | 0.112 | 0.99 (0.81–1.22) | 0.991 |
| Job type | ||||
| Practitioner | Reference | – | Reference | – |
| Retried | 2.26 (1.77–2.87) | < 0.001 | 1.53 (1.17–2.01) | 0.002 |
| Housekeeper | 1.76 (1.40–2.21) | < 0.001 | 1.21 (0.93–1.57) | 0.146 |
| Others | 1.20 (0.57–2.51) | 0.63 | 0.97 (0.48–1.96) | 0.948 |
| Diabetes | 2.01 (1.69–2.41) | < 0.001 | 1.64 (1.36–1.98) | < 0.001 |
| Current smoking | 0.85 (0.67–1.08) | 0.170 | 0.91 (0.69–1.19) | 0.374 |
| Body Mass Index | 1.04 (1.02–1.05) | < 0.001 | 1.03 (1.01–1.05) | 0.002 |
| Waist to Hip Ratio | 47.32 (16.79–133.36) | < 0.001 | 9.81 (3.14–30.61) | 0.001 |
CI Confidence Intervals
Discussion
In this study, ISH prevalence for the overall population, men, and women was 15.89%, 15.68%, and 15.87% respectively. To the best of our knowledge, no reliable study has previously investigated the ISH prevalence in Iran. Therefore, a valid comparison is not possible. Only in one study, the ISH incidence was reported to be 5.7 / 1000 people person-years in Iran [25]. The results of previous studies of ISH prevalence in different parts of the world are also sparse. However, the estimated prevalence of ISH in the current study appears to be slightly higher than the reported ISH prevalence in other countries. For example, in Taiwan, the prevalence of ISH has been reported as 12.6% in the overall population, 12.3% in males, and 12.9% in females [14]. The prevalence of ISH has been reported 7.6% in China and 10.1% in South Korea and 35% in Portugal [9, 13, 26] and 10.2% among the Mongolian population [15]. In western countries, the prevalence of ISH has been reported at 9.4% in the USA, and 8.1% in Canada [11, 27].
One of the most important factors that may cause the differences in the results of various studies is the age distribution of the study population [5, 27, 28]. The findings of the present study showed that the ISH prevalence increased with age increasing. So that the ISH prevalence in the 65 to 70 years age group was about 4.5-fold higher than in the 45–49.9 years age group. These findings are in line with the results of Framingham study. In the Framingham heart study prevalence of ISH has been reported 35% to 40% in the age group of 50–59 years and 65% to 70% in the age group of over 60 years [29]. In the study conducted in the USA, the ISH prevalence has been reported at 6% in the age group of 40–59 years, and 29.6% in the age group of over 60 years [11]. In Korean adults, also, the prevalence of ISH has been reported 20.2% in the age group of 40—49 and 63.2%, in the age group over 70 years [9].The studies conducted in the USA [30], South Korea [31], and China [26] also have been shown that the ISH prevalence increased steadily with increasing age [28]. Previous studies have shown that with increasing age, arteries tend to lose their elasticity with reduced compliance to the stroke volume [31–33]. Therefore, the increases in ISH prevalence with age may be in relationship to arteriosclerosis and an age-dependent decrease in compliance of the aorta and large capacitance arteries.
Another factor that may cause differences in the results of various studies is the study population sex distribution. So that in the Korean study, the increase in ISH prevalence after the age of 60 years was reported to be higher in women than men [9]. In the Canadian study, also, the mean of ISH values at a younger age was lower for women than for men but higher after 60 years of age [34]. Another study in Taiwan has shown that, at all ages, the prevalence of ISH in women is higher than in men and increases with advancing age in both sexes [14].
In the present study, in line with the above-mentioned studies, the ISH prevalence was approximately higher in women in all age groups. However, Given that the above-mentioned studies [9, 14, 34] have not reported a P-value or confidence interval for the ISH prevalence in both genders, the comparison is partly difficult.
The results of the current study indicated that obesity increases the odds of ISH by 3.73 times. This is in line with the results of previous studies in South Korea [31], China [35], Taiwan [36], and rural India [7, 28]. For example, in a national survey in Korea, the odds ratio of developing ISH in obese people was 3.16 times more than those with a normal weight [31]. Also, another study in India has shown that obesity increases the odds of ISH by 2.21 [7]. According to the available evidence, in Asian people, obesity has a greater effect on hypertension than in Western people. So that in Asian people, the effect of BMI of 25 kg/m2 on blood pressure is similar to the effect of BMI of 30 kg/m2 in Western people [28]. It should also be noted that, as shown in the present study, obesity and overweight (BMI = 28.87 kg/m2) are highly prevalent in Iranian adults and it is a neglected priority [37, 38].
According to the results of this study, other factors significantly associated with the prevalence of ISH were diabetes and WHR which is consistent with the previous studies [31, 39, 40]. The prevalence of hypertension in diabetes patients is most common in comparison to the general population. So that in a population-based study, the prevalence of hypertension has reported 24% in type 1 diabetic and 60.2% in type 2 diabetes patients [41–43]. The association of diabetes and WHR with ISH might be related to the sympathetic nervous system, as the clinical and experimental evidence has shown a significant dependency between obesity, hypertension, hyperinsulinemia, and diabetes through the sympathetic nervous system [39, 41, 44–46]. This dependency may be due to the activation of the renin–angiotensin–aldosterone (RAA) system in type 1 diabetes and insulin resistance or hyperinsulinemia in type 2 diabetes patients [41, 47, 48].
The present study had several limitations. First, this study had a cross-sectional design, therefore, the observed association between ISH and its risk factors cannot be considered as a causal association and needs further investigation in prospective studies. Second, some of the previous studies have reported a significant association between alcohol intake, salt intake, and physical activity with the prevalence of ISH [35]. However, in this study, we were not collecting these variables. Third, must keep in mind that some ISH cases may not be included in the definition of ISH due to receiving antihypertensive medications. This may lead to an underestimation of the ISH prevalence. Four, In the present study, blood pressure was measured in one arm and blood pressure diagnosis was based on a single office visit. While usually 2–3 office visits at 1–4-week intervals and blood pressure measurements in both arms are recommended to confirm the diagnosis of hypertension.
Conclusions
The present study showed that isolated systolic hypertension was highly prevalent among the middle-aged and older adult population in Iran. Several risk factors including age, BMI, WHR, and diabetes were associated with ISH prevalence. Given the risk of cardiovascular disease associated with ISH, these results emphasize the importance of education and the need for public health interventions to improve the detection, prevention, and treatment of ISH.
Acknowledgements
The Shahroud eye cohort study was supported by the Noor Ophthalmology Research Center and Shahroud University of Medical Sciences (Grant No. 9329).
Abbreviations
- ISH
Isolated Systolic Hypertension
- ShECS
Shahroud Eye Cohort Study
- WHR
Waist to Hip Ratio
- OR
Odds Ratio
- DALYs
Disability Adjusted Life Years
- BP
Blood Pressure
- IDH
Isolated Diastolic Hypertension
- SDH
Systolic Diastolic Hypertension
- FBS
Fasting Blood Surge
- BMI
Body Mass Index
- RAA
Renin Angiotensin Aldosterone
- SD
Standard Deviation
- CI
Confidence Interval
Author contributions
AH drafted the manuscript and conducted all statistical analyses and was the primary author of the article. HE and AKH contributed to the conceptualization of the paper and the statistical analyses and critically revised the manuscript. MHE, HH, and AF conceived and designed the study and contributed to the preparation of the study protocol, contributed to the conceptualization of the paper and the statistical analyses, and critically revised the manuscript. All authors read and approved the final manuscript.
Funding
Shahroud Eye Cohort Study is supported by Noor Eye Hospital and Shahroud University of Medical Sciences (project number: 9826).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Helsinki Declaration. All procedures involving participants were approved by the Ethics Committee of Shahroud University of Medical Sciences, Shahroud, Iran (930/09). We obtained written informed consent from all participants.
Consent for publication
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Hypertension, key facts. Avaiable at: https://www.who.int/news-room/fact-sheets/detail/hypertension. Accessed 22 Agu 2022
- 2.Mensah GA. Epidemiology and global burden of hypertension. In: Camm AJ, Lüscher TF, Maurer G, Serruys PW, editors. The ESC Textbook of Cardiovascular Medicine: Oxford University Press; 2018. p. 291–97.
- 3.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jingi A, Dzudie A, Noubiap J, Menanga A, Aminde L, Fesuh B, et al. PT075 trend in the prevalence, awareness, and control of hypertension in Cameroon: a systematic review and projections for 2025 and 2035. Glob Heart. 2016;11(2):e138–e139. [Google Scholar]
- 5.Bavishi C, Goel S, Messerli FH. Isolated systolic hypertension: an update after SPRINT. Am J Med. 2016;129(12):1251–1258. doi: 10.1016/j.amjmed.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 6.McEniery CM, Franklin SS, Cockcroft JR, Wilkinson IB. Isolated systolic hypertension in young people is not spurious and should be treated: pro side of the argument. Hypertension. 2016;68(2):269–275. doi: 10.1161/HYPERTENSIONAHA.116.06547. [DOI] [PubMed] [Google Scholar]
- 7.Midha T, Lalchandani A, Nath B, Kumari R, Pandey U. Prevalence of isolated diastolic hypertension and associated risk factors among adults in Kanpur, India. Indian Heart J. 2012;64(4):374–379. doi: 10.1016/j.ihj.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026. [DOI] [PubMed] [Google Scholar]
- 9.Kim NR, Kim HC. Prevalence and trends of isolated systolic hypertension among Korean adults: the Korea National Health and nutrition examination survey, 1998–2012. Korean Circ J. 2015;45(6):492–499. doi: 10.4070/kcj.2015.45.6.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grebla RC, Rodriguez CJ, Borrell LN, Pickering TG. Prevalence and determinants of isolated systolic hypertension among young adults: the 1999–2004 US National Health and Nutrition Examination Survey. J Hypertens. 2010;28(1):15. doi: 10.1097/HJH.0b013e328331b7ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Rodriguez CJ, Wang K. Prevalence and trends of isolated systolic hypertension among untreated adults in the United States. J Am Soc Hypertens. 2015;9(3):197–205. doi: 10.1016/j.jash.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chuang SY, Chang HY, Tsai TY, Cheng HM, Pan WH, Chen CH. Isolated systolic hypertension and central blood pressure: implications from the national nutrition and health survey in Taiwan. J Clin Hypertens. 2021;23(3):656–664. doi: 10.1111/jch.14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clara JG, De Macedo ME, Pego M. Prevalence of isolated systolic hypertension in the population over 55 years old. Results from a national study. Revista portuguesa de cardiologia: orgao oficial da Sociedade Portuguesa de Cardiologia Portuguese J Cardiol Off J Portuguese Soc Cardiol. 2007;26(1):11–8. [PubMed] [Google Scholar]
- 14.Chuang S, Cheng H, Chou P, Chen C. Prevalence of isolated systolic hypertension and the awareness, treatment, and control rate of hypertension in Kinmen. Acta Cardiologica Sinica. 2006;22(2):83. [Google Scholar]
- 15.Li J, Xu C, Sun Z, Zheng L, Li J, Zhang D, et al. Prevalence and risk factors for isolated untreated systolic hypertension in rural Mongolian and Han populations. Acta Cardiol. 2008;63(3):389–393. doi: 10.2143/AC.63.3.1020317. [DOI] [PubMed] [Google Scholar]
- 16.Flint AC, Conell C, Ren X, Banki NM, Chan SL, Rao VA, et al. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med. 2019;381(3):243–251. doi: 10.1056/NEJMoa1803180. [DOI] [PubMed] [Google Scholar]
- 17.Lee H, Yano Y, Cho SMJ, Park JH, Park S, Lloyd-Jones DM, et al. Cardiovascular risk of isolated systolic or diastolic hypertension in young adults. Circulation. 2020;141(22):1778–1786. doi: 10.1161/CIRCULATIONAHA.119.044838. [DOI] [PubMed] [Google Scholar]
- 18.Kjeldsen SE. Hypertension and cardiovascular risk: general aspects. Pharmacol Res. 2018;129:95–99. doi: 10.1016/j.phrs.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Barri YM. Hypertension and kidney disease: a deadly connection. Curr Hypertens Rep. 2008;10(1):39–45. doi: 10.1007/s11906-008-0009-y. [DOI] [PubMed] [Google Scholar]
- 20.Nagai M, Hoshide S, Kario K. Hypertension and dementia. Am J Hypertens. 2010;23(2):116–124. doi: 10.1038/ajh.2009.212. [DOI] [PubMed] [Google Scholar]
- 21.Ekundayo OJ, Allman RM, Sanders PW, Aban I, Love TE, Arnett D, et al. Isolated systolic hypertension and incident heart failure in older adults: a propensity-matched study. Hypertension. 2009;53(3):458–465. doi: 10.1161/HYPERTENSIONAHA.108.119792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharda M. Isolated systolic hypertension in very elderly. J Indian Acad Geriatr. 2022;18(1):32. [Google Scholar]
- 23.Fotouhi A, Hashemi H, Shariati M, Emamian MH, Yazdani K, Jafarzadehpur E, et al. Cohort profile: Shahroud eye cohort study. Int J Epidemiol. 2013;42(5):1300–1308. doi: 10.1093/ije/dys161. [DOI] [PubMed] [Google Scholar]
- 24.Emamian MH, Zeraati H, Majdzadeh R, Shariati M, Hashemi H, Fotouhi A. The gap of visual impairment between economic groups in Shahroud, Iran: a Blinder-Oaxaca decomposition. Am J Epidemiol. 2011;173(12):1463–1467. doi: 10.1093/aje/kwr050. [DOI] [PubMed] [Google Scholar]
- 25.Asgari S, Khalili D, Mehrabi Y, Kazempour-Ardebili S, Azizi F, Hadaegh F. Incidence and risk factors of isolated systolic and diastolic hypertension: a 10 year follow-up of the Tehran Lipids and Glucose Study. Blood Press. 2016;25(3):177–183. doi: 10.3109/08037051.2015.1116221. [DOI] [PubMed] [Google Scholar]
- 26.Huang J, Wildman RP, Gu D, Muntner P, Su S, He J. Prevalence of isolated systolic and isolated diastolic hypertension subtypes in China. Am J Hypertens. 2004;17(10):955–962. doi: 10.1016/j.amjhyper.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Joffres MR, Hamet P, MacLean DR, L’italien GJ, Fodor G. Distribution of blood pressure and hypertension in Canada and the United States. Am J Hypertens. 2001;14(11):1099–105. doi: 10.1016/s0895-7061(01)02211-7. [DOI] [PubMed] [Google Scholar]
- 28.Tsai TY, Cheng HM, Chuang SY, Chia YC, Soenarta AA, Minh HV, et al. Isolated systolic hypertension in Asia. J Clin Hypertens. 2021;23(3):467–474. doi: 10.1111/jch.14111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kannel WB. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA. 1996;275(20):1571–1576. [PubMed] [Google Scholar]
- 30.Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37(3):869–874. doi: 10.1161/01.hyp.37.3.869. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Kim S, Choi Y, Yoon D, Lee J, Park H, et al. The prevalence and risk factors associated with isolated untreated systolic hypertension in Korea: the Korean National Health and Nutrition Survey 2001. J Hum Hypertens. 2007;21(2):107–113. doi: 10.1038/sj.jhh.1002119. [DOI] [PubMed] [Google Scholar]
- 32.Tomiyama H, Shiina K, Nakano H, Iwasaki Y, Matsumoto C, Fujii M, et al. Arterial stiffness and pressure wave reflection in the development of isolated diastolic hypertension. J Hypertens. 2020;38(10):2000–2007. doi: 10.1097/HJH.0000000000002519. [DOI] [PubMed] [Google Scholar]
- 33.Beltran A, McVeigh G, Morgan D, Glasser SP, Neutel JM, Weber M, et al. Arterial compliance abnormalities in isolated systolic hypertension. Am J Hypertens. 2001;14(10):1007–1011. doi: 10.1016/s0895-7061(01)02160-4. [DOI] [PubMed] [Google Scholar]
- 34.Wilkins K, Campbell NR, Joffres MR, McAlister FA, Nichol M, Quach S, et al. Blood pressure in Canadian adults. Health Rep. 2010;21(1):37. [PubMed] [Google Scholar]
- 35.Xu C, Sun Z, Zheng L, Zhang D, Li J, Zhang X, et al. Prevalence of and risk factors for isolated systolic hypertension in the rural adult population of Liaoning Province, China. J Int Med Res. 2008;36(2):353–356. doi: 10.1177/147323000803600219. [DOI] [PubMed] [Google Scholar]
- 36.Yeh C-J, Pan W-H, Jong Y-S, Kuo Y-Y, Lo C-H. Incidence and predictors of isolated systolic hypertension and isolated diastolic hypertension in Taiwan. J Formos Med Assoc. 2001;100(10):668–675. [PubMed] [Google Scholar]
- 37.Khabazkhoob M, Emamian MH, Hashemi H, Shariati M, Fotouhi A. Prevalence of overweight and obesity in the middle-age population: a priority for the health system. Iran J Public Health. 2017;46(6):827–834. [PMC free article] [PubMed] [Google Scholar]
- 38.Rahmani A, Sayehmiri K, Asadollahi K, Sarokhani D, Islami F, Sarokhani M. Investigation of the prevalence of obesity in Iran: a systematic review and meta-analysis study. Acta Med Iran. 2015;53(10):596–607. [PubMed] [Google Scholar]
- 39.Ko GT, Cockram CS, Chow C-C, Chan W-B, So W-Y, Ma R, et al. Effects of body mass index, plasma glucose and cholesterol levels on isolated systolic hypertension. Int J Cardiol. 2005;101(3):429–433. doi: 10.1016/j.ijcard.2004.03.059. [DOI] [PubMed] [Google Scholar]
- 40.Qian JD, Li XM, Chen DS, Zhu JQ, Liu XZ. Comparative analysis of the association between traditional and lipid-related obesity indicators and isolated systolic hypertension. BMC Cardiovasc Disord. 2022;22(1):1–8. doi: 10.1186/s12872-022-02564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perin PC, Maule S, Quadri R. Sympathetic nervous system, diabetes, and hypertension. Clin Exp Hypertens. 2001;23(1–2):45–55. doi: 10.1081/ceh-100001196. [DOI] [PubMed] [Google Scholar]
- 42.Colosia AD, Palencia R, Khan S. Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: a systematic literature review. Diabetes Metabol Syndrome Obesity Targets Therapy. 2013;6:327. doi: 10.2147/DMSO.S51325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kabakov E, Norymberg C, Osher E, Koffler M, Tordjman K, Greenman Y, et al. Prevalence of hypertension in type 2 diabetes mellitus: impact of the tightening definition of high blood pressure and association with confounding risk factors. J Cardiometab Syndr. 2006;1(2):95–101. doi: 10.1111/j.1559-4564.2006.05513.x. [DOI] [PubMed] [Google Scholar]
- 44.Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res. 2015;116(6):976–990. doi: 10.1161/CIRCRESAHA.116.303604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sorof JM, Poffenbarger T, Franco K, Bernard L, Portman RJ. Isolated systolic hypertension, obesity, and hyperkinetic hemodynamic states in children. J Pediatr. 2002;140(6):660–666. doi: 10.1067/mpd.2002.125228. [DOI] [PubMed] [Google Scholar]
- 46.Khosravi A, Emamian MH, Hashemi H, Fotouhi A. Pre-hypertension and the risk of diabetes mellitus incidence using a marginal structural model in an Iranian prospective cohort study. Epidemiol Health. 2018;2:40. doi: 10.4178/epih.e2018026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skyler JS, Marks JB, Schneiderman N. Hypertension in patients with diabetes mellitus. Am J Hypertens. 1995;8(12):100S–S105. doi: 10.1016/0895-7061(95)00307-x. [DOI] [PubMed] [Google Scholar]
- 48.Chillarón JJ, Sales MP, Flores-Le-Roux JA, Murillo J, Benaiges D, Castells I, et al. Insulin resistance and hypertension in patients with type 1 diabetes. J Diabetes Complicat. 2011;25(4):232–236. doi: 10.1016/j.jdiacomp.2011.03.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


