Abstract
The expression of denitrification by a facultatively anaerobic bacterium requires as exogenous signals a low oxygen tension concomitant with an N oxide. We have studied the role of nitric oxide (NO), nitrous oxide (N2O), and nitrite as signal molecules for the expression of the denitrification apparatus of Pseudomonas stutzeri. Transcriptional kinetics of structural genes were monitored by Northern blot analysis in a 60-min time frame after cells were exposed to an N oxide signal. To differentiate the inducer role of NO from that of nitrite, mRNA kinetics were monitored under anoxic conditions in a nirF strain, where NO generation from nitrite is prevented because of a defect in heme D1 biosynthesis. NO-triggered responses were monitored from the nirSTB operon (encoding cytochrome cd1 nitrite reductase), the norCB operon (encoding NO reductase), nosZ (encoding nitrous oxide reductase), and nosR (encoding a putative regulator). Transcription of nirSTB and norCB was activated by 5 to 50 nM NO, whereas the nosZ promoter required about 250 nM. Nitrite at 5 to 50 nM elicited no response. At a threshold concentration of 650 nM N2O, we observed in the anoxic cell the transient appearance of nosZ and nosR transcripts. Constant levels of transcripts of both genes were observed in an anoxic cell sparged with N2O. NO at 250 nM stimulated in this cell type the expression of nos genes severalfold. The transcription factor DnrD, a member of the FNR-CRP family, was found to be part of the NO-triggered signal transduction pathway. However, overexpression of dnrD in an engineered strain did not result in NirS synthesis, indicating a need for activation of DnrD. NO modified the transcriptional pattern of the dnrD operon by inducing the transcription of dnrN and dnrO, located upstream of dnrD. Insertional mutagenesis of dnrN altered the kinetic response of the nirSTB operon towards nitrite. Our data establish NO and DnrD as key elements in the regulatory network of denitrification in P. stutzeri. The NO response adds to the previously identified nitrate-nitrite response mediated by the NarXL two-component system for the expression of respiratory nitrate reductase encoded by the narGHJI operon.
Nitric oxide (NO) is generated and reduced by bacterial denitrification. The NO generator in the denitrifying cell is respiratory nitrite reductase, which is either the tetraheme cytochrome cd1 nitrite reductase, encoded by the nirS gene, or the Cu-containing nitrite reductase, encoded by the nirK gene (for a review, see reference 54). Although both nitrite reductases exhibit some oxygen reductase activity, there is no evidence that this property would attribute to them a dual function in anaerobic and aerobic respiratory metabolism. The concept of NO as a bacterial signal molecule has its roots in observations of nitrite reductase mutants, which exhibit low levels of NO reduction (18, 38, 52). During genetic studies of heme D1 biosynthesis, we found that mutagenesis of nir genes other than nirS, irrespective of their encoded functions, strongly reduced the expression of the norCB operon, which codes for the NO reductase complex. The key observation to explain this effect came from interspecies exchange of nirK. In spite of the different biochemical natures of the nirK and nirS gene products, it is possible to express nirK in active form in a NirS− background (24). Expression of active nirK was used in a rescue strategy to relieve the low expression of norCB in a nirS mutant. Since NirK and NirS proteins both generate NO, we proposed NO as an inducer of its own reductase and the existence of an NO-signaling mechanism (38, 55). Studies of the nirK gene of Rhodobacter sphaeroides (35, 45) and the nirS gene of Paracoccus denitrificans (46) have subsequently shown that NO-releasing compounds activate gene expression.
Here we have investigated the roles of NO, N2O, and nitrite as signal molecules in the expression of denitrification genes and the interlacing of their regulons with the dnrD operon. The denitrification regulator DnrD, a member of the DNR branch of the FNR-CRP family, is necessary for the expression of the nirSTB and norCB operons in Pseudomonas stutzeri (47). A dnrD mutant possesses neither nitrite reductase nor NO reductase. We had found a complex transcriptional pattern of the dnrD region in response to denitrifying conditions. However, both the cause of the transcriptional pattern and the organization of the underlying operon remained unclear. We show here by direct transcriptional analysis that NO and DnrD fulfill key roles in expressing the nitrite-denitrifying system of P. stutzeri. Further, we show that N2O is required for activation of genes for nitrous oxide respiration, nosZ and nosR, whose expression is strongly enhanced by an NO signal.
MATERIALS AND METHODS
Strains and plasmids.
P. stutzeri strains used in this work were derivatives of MK21 (56), a spontaneously streptomycin-resistant mutant of strain ATCC 14405. The generation of strains MK220 (nirF::Gmr) (38), MK418 (nosR::Kmr) (16), and MRD235 (dnrD::Kmr) (47) by insertional mutagenesis was described previously. The Escherichia coli strains used for propagation of plasmids were DH10B (Gibco-BRL) and JM110 (51). Vectors used for cloning and sequencing were pBluescript II SK (Stratagene), pUCP22 (49), and pBSL15 (2), with the neomycinphosphotransferase II (nptII) gene conferring resistance to kanamycin.
Media, antibiotics, and growth conditions.
Strains of P. stutzeri were grown on a synthetic, asparagine-citrate-containing (AC) medium at 30°C (12). Unless stated otherwise, aerobic and denitrifying cultures were established as previously described (17). For studying mRNA kinetics in response to the addition of an N oxide, the following protocol was used. Aerobically grown cells (gyratory shaker speed set at 240 rpm) were shifted first to a low-oxygen supply (shaker speed reduced to 120 rpm) and incubated for 3 h. Anoxic conditions were then established by transferring the cells into a sealed serum flask under an argon atmosphere for about 30 min before mRNA kinetics were monitored. For anoxic N2O cells, a culture was grown first aerobically to an optical density at 660 nm (OD660) of ≈0.6. Cells were harvested by centrifugation, suspended with fresh AC medium in a 100-ml flask, and sparged for 3 h with a slow stream of N2O before being challenged with the NO signal. Solute concentrations of NO and N2O were calculated from published values (48). NO was synthesized from acidified nitrite in the presence of Fe(II). In a 100-ml argon-filled and then evacuated gas storage vessel, 5 ml of 1 M KNO2 was added slowly from a syringe to 4.5 ml of 1 M FeSO4 in 1 M H2SO4. The vessel was equipped with a rubber septum as the gas sampling port. Sodium nitroprusside (SNP) was purchased from Merck (Darmstadt, Germany); S-nitrosoglutathione (GSNO) was synthesized according to a published method (27). Its purity was estimated from the UV-visible light absorption spectrum. Stock solutions (50 mM) of SNP and GSNO in 50 mM MOPS (morpholinepropanesulfonic acid) were prepared anaerobically under an oxygen-free argon atmosphere immediately before use.
E. coli was cultured in Luria-Bertani medium at 37°C. The following antibiotics were used at the indicated concentrations (in micrograms per milliliter): ampicillin, 100; kanamycin, 50; streptomycin, 200; and gentamicin, 30.
Recombinant DNA techniques.
Plasmid DNA was prepared by a modified alkaline cell lysis method (22). Spin column purification through a silica membrane (Qiagen) was used to purify plasmid DNA and for preparative isolation of DNA fragments from agarose slabs. The identity of products from PCR or cloning procedures was verified by sequence analysis. Genomic DNA was extracted by the adaptation of a published method (9). Electroporation was used for the transformation of plasmids into E. coli (19) and P. stutzeri (21). For DNA manipulations, standard protocols (42) or the instructions of manufacturers of commercial products were followed. Restriction endonucleases and other enzymes were purchased from MBI Fermentas (Vilnius, Lithuania), New England Biolabs (Beverly, Mass.), or Roche Diagnostics (Mannheim, Germany).
Cloning and DNA sequence determination.
A 2.4-kb BamHI-KpnI restriction fragment carrying dnrD and the upstream and downstream flanking regions was cloned into pBluescript II SK to yield p146BK (47). For complementation analysis, the 2.4-kb BamHI-KpnI fragment was ligated into the broad-host-range vector pUCP22 to result in plasmid pUCP146BK. Plasmid p146E carries a 2.0-kb Eco47III fragment comprising 523 bp of the 5′ sequence of dnrN and upstream sequences. An ALF sequencer (Amersham Pharmacia Biotech) was used for automatic fluorescence-based sequence analysis of plasmids after cycle sequencing with thermosequenase. Data banks were searched with FASTA3 (39) via the internet server of the European Bioinformatics Institute.
Construction of a dnrN strain.
The dnrN locus was mutagenized by replacing an internal 38-bp SalI-NdeI fragment of plasmid p146BK with a Kmr gene cartridge. A strain was selected with the nptII gene in opposite orientation to dnrN. The resulting construct, p239SN::Kmr, was transformed into P. stutzeri cells by electroporation. Without an origin of replication for episomal propagation in P. stutzeri, p239SN::Kmr represents a suicide vector enabling the selection of the integration of the kanamycin resistance cassette as the result of homologous recombination. The insertion in dnrN of mutant MRD236 was confirmed by PCR and Southern hybridization.
RNA analysis.
Cell samples for RNA preparation (10 ml; OD660, ≈0.6) were harvested by syringe, centrifuged, and shock frozen in liquid nitrogen to be analyzed later by Northern blotting. The first sample was drawn immediately before addition of an inducer to give the basal transcript level. NO, N2O, and deoxygenated solutions of sodium nitrate, sodium nitrite, hemoglobin, and synthetic NO donors were added in the concentrations indicated in the figures using a gas-tight syringe. For Northern blot analysis, total cellular RNA was obtained from about 8 × 109 cells of P. stutzeri by extraction with hot phenol (1). A frozen cell pellet was suspended in 200 μl of 20 mM Tris-HCl, pH 8.0, and subjected to cell lysis and inactivation of RNase by the addition of 3 ml of lysis buffer (20 mM sodium acetate [pH 5.3], 0.5% sodium dodecyl sulfate, 1 mM EDTA) and 3 ml of phenol heated to 60°C, which had previously been equilibrated with 20 mM sodium acetate, pH 5.3. A 5-min incubation of the sample at 60°C was followed by centrifugation (10 min, 15,000 × g, 15°C) to separate phases. The upper phase containing the total cellular RNA was extracted first with phenol-chloroform-isoamyl alcohol (25:24:1, vol/vol/vol) and subsequently with chloroform-isoamyl alcohol (24:1, vol/vol). After addition of 9 ml of absolute ethanol and overnight precipitation at −20°C, the RNA was pelleted by centrifugation (30 min, 15,000 × g, 4°C) using a swing-out rotor. The pellet was washed with 80% ethanol and suspended in 400 μl of lysis buffer. RNA was precipitated again by adding 1 ml of absolute ethanol; the pellet was washed once more with 80% ethanol and suspended in 100 μl of 10 mM Tris-HCl, pH 8.0. The yield of the preparation was determined by measuring the absorption at 260 nm.
An appropriate volume to give 10 μg of RNA was precipitated with ethanol, washed, dried, and suspended in 10 μl of formamide. After addition of 3.5 μl of formaldehyde, 4.5 μl of quartz-distilled water, and 2 μl of 5× MOPS buffer (1× MOPS buffer is 20 mM MOPS [pH 7.0], 8 mM sodium acetate, and 1 mM EDTA), denaturation was done by a 15-min incubation at 65°C. The denatured RNA solutions were chilled on ice before they were mixed with gel loading buffer containing 50% glycerol, 1 mM EDTA (pH 8.0), 0.25% bromophenol blue, and 50 μg of ethidium bromide per ml. Unless stated otherwise, RNA samples (10 μg) were electrophoretically separated in 1.2% agarose gels with 0.41 M formaldehyde as the denaturing reagent (6). The running buffer for electrophoresis was 1× MOPS. Gels were run for 5 h at 75 V in the cold. Equal sample loadings were ascertained by densitometer analysis of the UV-induced fluorescence of the 23S and 16S rRNA bands after staining with ethidium bromide. The fdxA gene, encoding a P. stutzeri ferredoxin (41), was used as an internal standard since its level of mRNA was constant under the experimental conditions.
Immediately after the electrophoresis, transfer of RNA to positively charged nylon membranes (Roche Diagnostics) was done by downward capillary blotting with 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) transfer buffer (10). Hybridization and detection of digoxigenin-labeled probes were carried out using a published method (20) or according to the instructions of the EasyHyb system (Roche Diagnostics).
The gene probes for Northern blot analysis were amplified from plasmid p146BK with the following primers: for dnrN, 5′-GCATCGACGCCTCGGCATTG-3′ and 5′-CGCCAGGGTTTTCCCAGTCACGAC-3′; for dnrO, 5′-CCGAGGCGTCGATGCCGGTA-3′ and 5′-TTCCGGGAGTTGGCAATTGGC-3′; for dnrD, 5′-TAGCGATCTGGCGACCTTCCTG-3′ and 5′-ATGGTGCTTCACCGCGTACA-3′; for dnrP, 5′-AGCGGATAACAATTTCACAGGA-3′ and 5′-ATCGACGAAGCCATCATCAC-3′; and for orf102, 5′-AAGTGGCTCCGCATGACG-3′ and 5′-CCGCAATGCCTGGAAGGT-3′. Gene probes for nosZ and nosR were amplified from cosmid cDEN1 using the primer pairs 5′-GTTGCTGCCACGGCTCTC-3′ and 5′-GTCGGCGTCGGTGTTGTC-3′ and 5′-TTCGAGATGGCGATCTTCACTG-3′ and 5′-TTCACTGTCGACTCAGGGTTCCACCACTTG-3′, respectively. The degenerate primer sequences for fdxA were reverse translated from the amino acid sequences 5′-ACCTCGAGATGACCTTCGT(G/C)GT(G/C)ACCGAC-3′ and 5′-TCGAATTCTCAGCG(C/T)TCCAGGTACTGCAGCTTG-3′ (41).
PCR-derived probes specific for the genes nirS and norB were prepared by the incorporation of digoxigenin-dUTP as described elsewhere (28). The dioxetane derivative CDP-Star (Roche Diagnostics) was used as a chemiluminescent substrate for membrane-based detection of alkaline phosphatase conjugates. Signal intensities on Northern blots were quantified densitometrically with Gel-Pro analyzer software (Media Cybernetics, Silver Spring, Md.).
Cell extract, enzyme assays, and immunochemical methods.
For the preparation of crude extract, cells were harvested in the cold by centrifugation for 20 min at 10,000 × g, washed in 50 mM MgCl2–25 mM Tris-HCl (pH 7.5), and suspended in 25 mM Tris-HCl (pH 7.5) to an OD660 of ≈50. Cell extract was prepared by pulsed sonication of a cell suspension for 5 min with a microtip and subsequent removal of cell debris by centrifugation (10 min, 32,000 × g). Protein concentration was determined by the Lowry method. Activities of nitrite reductase and NO reductase were determined as described previously (55). Cell extract was separated by discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis (36). For immunoblotting, we used the conditions described for cytochrome cd1 (33) and the cytochrome b subunit of NO reductase (55).
Nucleotide sequence accession number.
The nucleotide sequence data reported here have been deposited in the EMBL nucleotide sequence data bank under the accession number AJ298925.
RESULTS
Experimental rationale.
Because several N oxides are generated as intermediates by the denitrification process, it is problematic to assert which one is a signal molecule. Nitrite and NO have been equally considered as inducers of the activation of the nirS gene in Pseudomonas aeruginosa (5, 30). If reduction is not impeded, added nitrite will simultaneously represent an NO source. On the other hand, added NO is oxidized to nitrite when oxygen infiltrates the test system and hence represents under these conditions again both putative inducer species. To circumvent these problems, we used the nirF mutant MK220 (nirF::Gmr), which is unable to generate NO from nitrite. Transcription of the nirSTB operon in MK220 is not impaired, because the mutational defect is in heme D1 biosynthesis and an enzymatically inactive cytochrome cd1 nitrite reductase is still synthesized (38). Our experiments were done under anoxic conditions to prevent NO oxidation. With this setup, effects caused by NO were clearly differentiated from those caused by nitrite.
NO is a signal molecule for the expression of the nirSTB and norCB operons.
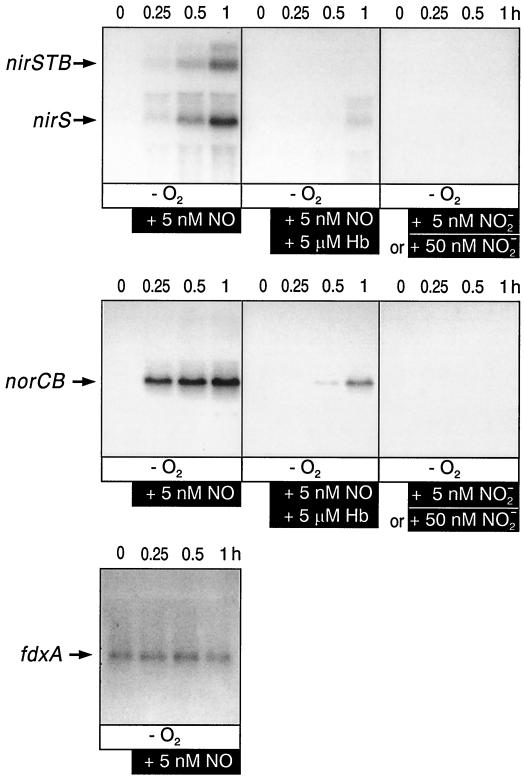
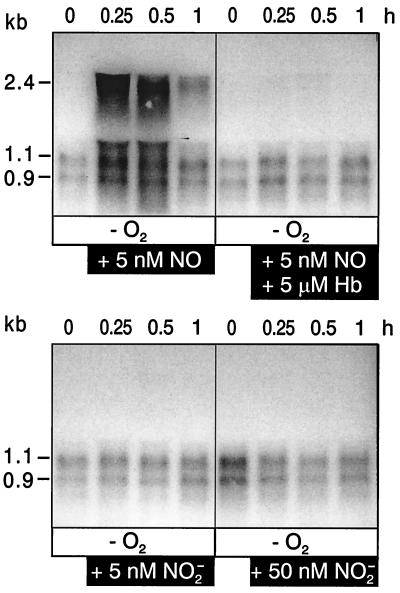
First, we addressed the question of whether nitrite or its reduction product NO is the inducer for nirSTB (nitrite reductase) and norCB (NO reductase) operon expression. We applied a single pulse of NO in gaseous form and monitored mRNA kinetics by Northern blot analysis during 60 min. This procedure had been found to be optimal with respect to induction kinetics of nirSTB, norCB and nosZ (N2O reductase) transcripts and mRNA stability (29). Kinetic experiments were important, since in a temporally variable response pattern, conclusions drawn from single points may not refer to comparable conditions. We found that exogenous NO activated the nirSTB and norCB operons and that the inducing effect observed with NO was counteracted by the NO scavenger hemoglobin (Fig. 1). The inducing effect of NO could not be elicited by nitrite. Nitrite, at a concentration that was the same as or 10-fold higher than that of NO, did not activate transcription at all. As a control for mRNA isolation, integrity, transfer, and hybridization, we used the non-NO-responsive fdxA (ferredoxin) gene of P. stutzeri (Fig. 1).
FIG. 1.
NO is required for the transcription of the nirSTB and norCB operons. Northern blot analysis was used to monitor anaerobic cells (labeled −O2) of mutant MK220 (nirF::Gmr) for the appearance of transcripts from both operons in response to the addition of NO or nitrite. NO gas or deoxygenated solutions of nitrite were added to cells under an argon atmosphere to make the cell suspension 5 or 50 nM in the respective N oxide. The specificity of the NO response was asserted by the addition of deoxygenated hemoglobin (+Hb; 5 μM). The kinetics of nirSTB and norCB transcription were monitored during 1 h. The fdxA signal is shown as a control to show equal gel loadings and mRNA integrity by monitoring a constitutive mRNA species.
Nanomolar concentrations of NO activate the nirSTB and norCB operons.
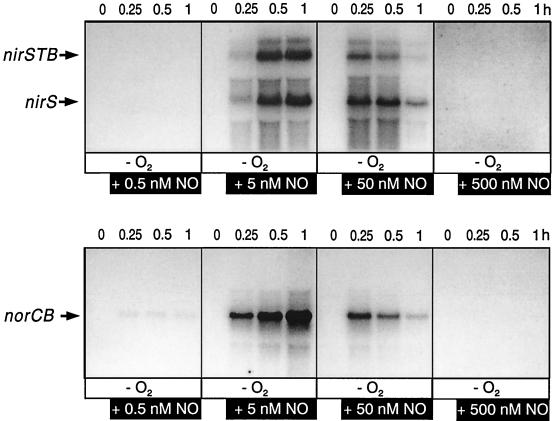
We investigated next the sensitivity of P. stutzeri toward NO and determined the approximate optimal concentration at which NO was effective as a signal molecule. Our data showed a clear concentration dependency of nirSTB and norCB transcription on NO and that NO was effective in a relatively narrow window (Fig. 2). NO promoted transcription of the target genes at a concentration of 5 nM already with highest efficiency. Transcript levels reached their maximum 1 h after induction. A 10-fold increase of NO to 50 nM led to a high transcript level at 15 min but was followed by a continuous decrease. At the 60-min sampling point, nirSTB and norCB transcripts were barely detectable anymore. At 500 nM NO, no transcripts were found. Thus, NO at an approximately 5 nM concentration provided a positive signal whereas NO at or above 50 nM inhibited nirSTB and norCB expression or destabilized the respective transcripts. Our results provide the first evidence of concentration dependency for NO as a signal molecule initiating transcription of the nirSTB and norCB operons and show that this signal must be perceived by a highly sensitive sensory system.
FIG. 2.
NO as a signal molecule for transcription is effective in the low nanomolar range. (Top panels) Concentration dependency on NO of transcripts from the nirSTB operon; (bottom panels) the same for transcripts from the norCB operon. Anaerobically incubated cells of MK220 (labeled −O2) were analyzed during 1 h for mRNA transcripts from the nirSTB and norCB operons by Northern blotting in response to increasing amounts of NO. NO gas was added to cell suspensions to obtain concentrations from 0.5 to 500 nM in solution. The shift to denitrifying conditions consisted of 3 h of incubation of aerobic cells at a shaking speed of 120 rpm (thereby generating O2-limited conditions), followed by 30 min under an Ar atmosphere. The zero time point was taken to be at the end of this period.
Synthetic NO donors induce the expression of the nirSTB and norCB operons.
The effect of NO gas was compared to the effects of the synthetic NO donors SNP (releasing NO+) and GSNO (releasing both NO and NO+). The use of NO donors instead of NO gas was expected to result in similar expression patterns. However, P. stutzeri was found to be less sensitive to SNP. We observed that this compound triggered the response of the nirSTB and norCB operons around 1 mM but that at and above 5 mM no transcripts were found. In contrast, the addition of GSNO in concentrations of 50 μM to 5 mM resulted in a uniform response and the inhibitory effect at the high concentration end was not observed (data not shown). Unlike SNP, the nitrosothiol GSNO is likely to be metabolized. Uptake and/or intracellular transformation of this compound may be rate limiting for NO release, and the solute concentration of GSNO will not necessarily have to translate into the same concentration as that of the inducer molecule. Overall, our results with P. stutzeri show that adding NO gas is the more effective induction mode and is preferable to the use of synthetic NO donors because of the problematic quantitation of NO set free in the latter case and the higher concentration required.
NO functions as a coinducer for N2O respiration involving DnrD.
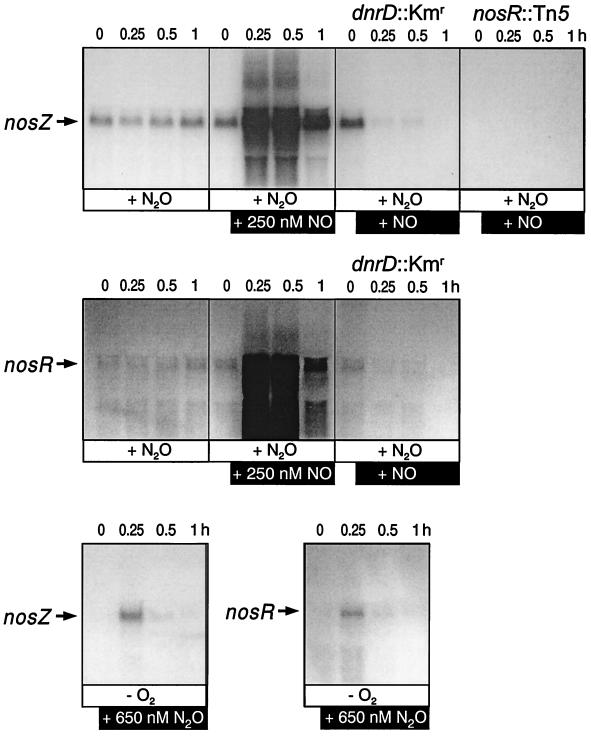
To test a possible regulatory linkage between NO signaling and N2O respiration, we pulsed anoxic cells of MK220 under an N2O atmosphere with NO and monitored the kinetics of mRNA synthesis of nosZ and nosR. The nosZ promoter remained silent in cells exposed to 5 or 50 nM NO, concentrations which were effective in initiating transcription from the nirS and norC promoters (data not shown). However, at 250 nM, NO exhibited a strong activating effect on the transcription of nosZ and nosR (Fig. 3 top and middle panels). As estimated from the signal intensities, the amount of transcript in the N2O-respiring cell was raised several orders of magnitude in response to NO. Anoxic N2O cells of strain MRD235, lacking DnrD, did not show the NO effect on nosZ and nosR transcription. Addition of NO to the dnrD mutant even lowered the levels of nosZ mRNA found at the saturating concentration of N2O. However, other than having an essential role in the expression of the nirSTB and norCB operons, DnrD functions only as a modulator for the nos genes, because an overnight nitrate-induced culture of MRD235 exhibits NosZ protein (47).
FIG. 3.
Both NO and N2O are required as signal molecules for high-level transcription of nosZ. (Top panels) The NO response of nosZ is dependent on DnrD and NosR. Cells were placed in AC medium in an N2O atmosphere and saturated for 3 h with the gas by sparging (+N2O) (see Materials and Methods). Transcripts were monitored at the indicated time intervals after the addition of 250 nM NO. (Middle panels) Expression of nosR is under NO-dependent control of DnrD. Growth conditions were as described for the top panels. (Bottom panels) A pulse of N2O elicits the transient transcription of nosZ and nosR. Cells were grown under O2-limited conditions in AC medium (−O2), pulsed with 650 nM N2O, and probed by Northern blot analysis. RNA for Northern hybridization was prepared from strains MK220 (nirF::Kmr) in panels without further specifications, MRD235 (dnrD::Kmr), and MK418 (nosR::Tn5).
Requirements for the expression of nosZ.
Since P. stutzeri grows with N2O as the sole respiratory substrate, we were interested in the approximate threshold concentration of N2O necessary for activating nosZ when cells were shifted to anoxic conditions. In comparing sensitivities to the respective inducers, we found the N2O signaling system of P. stutzeri to be less sensitive than NO-triggered signal transduction. Addition of at least 650 nM N2O was necessary for the transient appearance of nosZ and nosR transcripts in anaerobic cells (Fig. 3, bottom panels). NosR is necessary for the expression of N2O reductase by exerting direct or indirect transcriptional control on nosZ (16). In line with this observation was the absence of nosZ mRNA in a nosR strain (Fig. 3, top panels). Cells sparged with N2O exhibited constant levels of nosZ and nosR transcripts. In the same cells no transcripts of nirSTB and norCB were detectable. Hence, it seems that there is no regulatory linkage where N2O signaling affects the transcription of the enzymes leading to the production of N2O from nitrite. This result agrees with those of a previous chemostat study of N2O-grown cells which did not exhibit immunochemically detectable nitrite reductase (34). In the same study we noted, however, that an N2O-grown cell has a substantial amount of respiratory nitrate reductase. It remains to be explored how this regulatory cross talk between nos and nar genes is accomplished mechanistically.
Transcriptional organization of the dnrD operon.
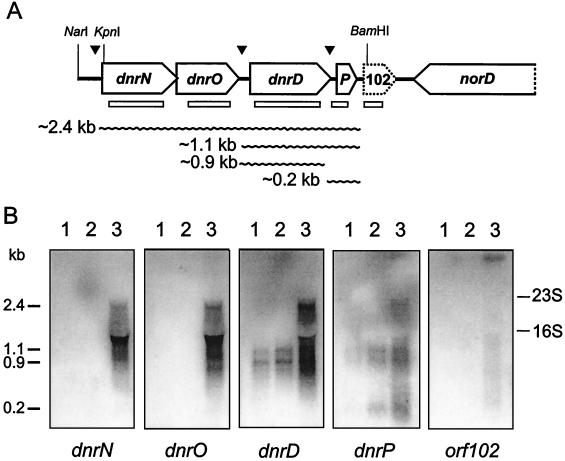
DnrD is a transcriptional activator of the FNR family, which is necessary for nitrite and NO reductase expression. Under denitrifying conditions, the dnrD gene is transcribed as part of an operon together with flanking genes (47). Upstream of dnrD we have identified two open reading frames, dnrN and dnrO, with coding capacities for proteins of 239 (Mr, 27,151) and 194 (Mr, 18,690) amino acids, respectively (Fig. 4A). Downstream of dnrD we found an open reading frame encoding a small protein of 63 amino acids (Mr, 7,379). The dnrD region is located opposite the norD gene, which was sequenced partially as ORF3 when the norCB operon was first identified (55). DnrN is homologous to NorA of Ralstonia eutropha, which is of unknown function and encoded upstream of norB (accession number O30367), to the Staphylococcus aureus ScdA protein (accession number P72360), to the hypothetical proteins YtfE of E. coli (accession number P39313) and Haemophilus influenzae (accession number P45312), and to a somewhat smaller protein (molecular mass, ≈18 kDa) of Neisseria meningitidis (accession numbers AAF41739 and CAB84804). The existence of homologs in different bacteria attributes a broader significance to DnrN; also, a dnrP homolog is present in the nor gene cluster of P. aeruginosa (3).
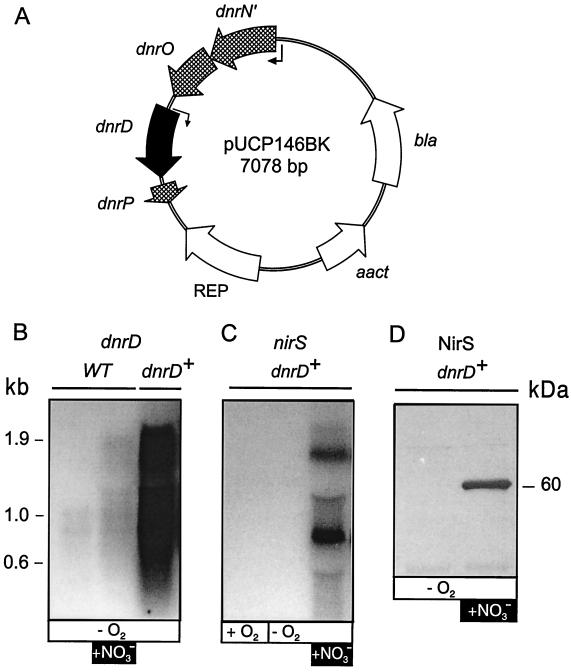
FIG. 4.
Multicistronic dnrD operon of P. stutzeri. (A) Physical map and transcriptional organization of the dnrD operon. Wavy lines indicate the observed transcripts; open bars represent sizes and locations of probes used in Northern hybridization. Arrowheads point to putative FNR boxes. P, dnrP gene. (B) Transcripts as detected by Northern hybridization with the gene probes indicated in panel A. Total RNA was prepared from aerobically cultivated MK21 without nitrate (lane 1), from cells shifted to O2-limited growth conditions for 15 min (shaking speed, 120 rpm) (lane 2), and from O2-limited cells incubated for 15 min with 0.1% sodium nitrate (denitrifying conditions) (lane 3). The dnrP probe overlapped a few nucleotides with dnrD, hence the presence of a 0.9-kb signal upon hybridization.
We used individual probes for each of the five open reading frames of the dnrD region to deduce the mRNA species discussed below and the transcriptional organization of a multicistronic operon (Fig. 4B). Cells grown aerobically or under O2 limitation exhibited two signals, with the dnrD probe representing a monocistronic transcript and a bicistronic mRNA species originating from cotranscription of dnrD with dnrP. dnrP was also found to have a monocistronic transcript of approximately 200 bp. Putative FNR boxes in the promoter regions of dnrD, TTGAc-N4-ATCAA (a lowercase letter indicates deviation from the FNR consensus), and dnrP, TTGAT-N4-GTCAA, are suggestive of transcriptional control by an FNR factor, which may be DnrD, and thus for dnrD involve an element of autoregulation. The presence of an FNR recognition sequence suggests that dnrP is, at least in part, controlled by its own promoter and makes the small transcript less likely to result from mRNA processing. A 2.4-kb transcript, hybridizing with the probes for dnrN, dnrO, dnrD, and dnrP, was detectable only under denitrifying conditions. The 3′ end of dnrN and the start of dnrO overlap by 4 nucleotides, indicative of their being translationally coupled. The transcript size of 2.4 kb of the dnrD operon agrees well with the physical distance between dnrN and dnrP obtained from sequencing. orf102 is not considered to be part of the dnrD operon and may not encode a protein. Its codon bias differs from that of other P. stutzeri genes. The probe for orf102 hybridized with none of the dnrD transcripts but did hybridize with an mRNA species which, considering its size, may originate only from the norCB operon and norD by read-through on the complementary strand. This strand, however, also exhibits no candidate open reading frame with the expected codon usage. The signal is weak and is usually not detected together with norCB transcripts, which require only short times of exposure for detection.
NO selectively activates the promoter of dnrN.
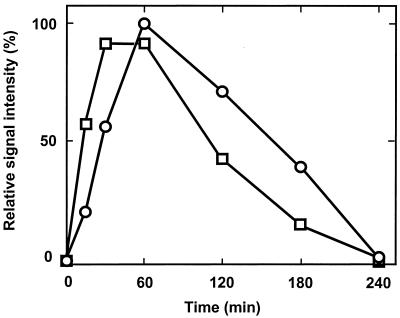
The 2.4-kb transcript hybridizing with the probe for dnrD was detected as a unique response to the same NO concentrations, 5 to 50 nM, effective for transcription of nirSTB and norCB (Fig. 5). Nitrite at these concentrations was without response. The large transcript was not observed when NO was scavenged with hemoglobin. Thus, transcription of the dnrD operon concurred with the expression of its target genes at the same signal strength. A dnrN mutant strain, MRD236, was constructed by insertional mutagenesis of the parent strain MK21 (see Materials and Methods). The dnrN mutant is affected in the NO-dependent formation of the multicistronic transcript because of the polar nature of the mutation, whereas transcription of dnrD and dnrP from their own promoters should proceed. MRD236, possessing nitrite reductase (NirS), was challenged with nitrite to mimick the action of NO. Nitrate was not used to avoid potential superpositioned regulatory effects of this substrate (29). The growth rate of the mutant and the synthesis and activity of nitrite and NO reductases did not deviate significantly from that of the wild type. As a phenotype, we observed a slower induction of the nirSTB transcript (which shifted the maximum transcript level by 15 to 30 min) and an increased transcript stability (Fig. 6), whereas the kinetic pattern of norCB transcription remained unaltered. The kinetics of the monocistronic nirS transcript (not shown) followed the pattern of nirSTB. The promoter of dnrN carries a degenerate FNR box, gTGAT-N4-AcCAg. Assuming a standard distance of 41.5 nucleotides from the center of this regulatory motif, the start of transcription of dnrN would be at G, 54 nucleotides upstream of the start codon.
FIG. 5.
Expression of dnrD is amplified in response to NO, but not nitrite, by activating the promoter of dnrN. The appearance of a 2.4-kb transcript on addition of NO is indicative of the operon structure comprising dnrN, dnrO, dnrD, and dnrP. Anaerobically incubated cells (labeled −O2) were monitored for dnrD transcripts in response to NO or nitrite by Northern hybridization. NO trapping by hemoglobin (Hb) and use of mutant MK220 (nirF::Gmr) ensured the specificity of the N oxide signal. Growth conditions were identical to those described in the legend to Fig. 2.
FIG. 6.
Disruption of dnrN alters the transcriptional response of the nirSTB operon. Cells were grown for 3 h aerobically (240 rpm) in AC medium and then shifted to nitrite-denitrifying conditions by adding sodium nitrite to a 0.05% final concentration and decreasing simultaneously the shaker speed to 120 rpm. RNA was prepared from MK21, representing wild-type traits (□), and MRD236 (dnrN::Kmr) (○). The zero time point represents nitrite addition. Signals from Northern blot analysis detecting nirSTB mRNA were quantified densitometrically.
Overexpression of dnrD does not result in the activation of DnrD target genes.
Since activation of the dnrN promoter by the inducer NO comprises the transcriptionally coupled dnrD gene, the NO response probably involves an increase in DnrD protein. The level of DnrD generated and maintained in the cell may critically influence nirSTB and norCB transcription. To investigate whether the observed responses depended on the level of the DnrD protein alone or whether DnrD requires prior activation, we uncoupled the transcription of dnrD from the requirement for NO by constructing plasmid pUCP146BK (Fig. 7A). A translational fusion of dnrN with the 3′-end-truncated lacZ gene encoding only the first nine amino acids subjected the dnrD operon to the control of the lacZ promoter. Plasmid pUCP146BK complemented the dnrD mutation of strain MRD235. Under conditions of oxygen limitation, even in the absence of an N oxide, transcription of the dnrD operon from the complementing plasmid was enhanced by several orders of magnitude compared with that of the wild type (Fig. 7B). Although we currently have no means to detect DnrD protein in the cell, we infer from parallel data on nirS and norCB gene expression that transcripts observed up to 60 min are translated into immunochemically detectable protein. However, in the dnrD-overexpressing strain, no nitrite reductase protein (NirS) was found. Addition of nitrate was required to detect nirS mRNA in MRD235c (Fig. 7C) or the nitrite reductase protein (Fig. 7D). Nitrate was added in this case to provide the sum of regulatory signals required for the induction of denitrification. Figure 7B shows the 15-min sample in Northern blotting, and a comparable amount of transcripts was found in the 60-min sample.
FIG. 7.
Overexpression of dnrD is insufficient for NirS synthesis. (A) Expression vector for dnrD derived from pUCP20 (49). REP, replication locus. (B) Northern blot analysis for dnrD expression. O2-limited cells of MK21 (wild type [WT]) and of the dnrD mutant MRD235c (dnrD+) complemented with the vector pUCP146BK were analyzed (10 μg of total RNA). The sample for RNA extraction was drawn after 15 min. For the cell sample in the second lane, the medium was made 0.1% in sodium nitrate to generate denitrifying conditions. (C) Northern blot analysis for nirSTB expression. Aerobically grown cells (first lane) where shifted to O2 limitation (second lane) or nitrate-denitrifying conditions (third lane). (D) Western blot analysis for cytochrome cd1 nitrite reductase by a polyclonal anti-NirS antiserum. Cultures were incubated overnight under conditions of O2 limitation (120 rpm) in the absence (first lane) or presence (second lane) of 0.1% sodium nitrate. Equal amounts of cell extract (15 μg) were separated by electrophoresis (36).
DISCUSSION
The differential effects exerted by NO and nitrite in the nirF mutant and the use of hemoglobin as an NO scavenger (25) clearly assigned to NO the role of a signal molecule. The cellular origin of NO acting as an inducer for nitrite denitrification when nitrate or nitrite is added is an open issue. As determined by membrane-inlet mass spectrometry, nitrate and nitrite concentrations of 15 and 5 mM, respectively, are optimal in P. stutzeri for the complete denitrification of these substrates to dinitrogen (23). About the same concentrations were found to be active for induction of nirSTB, norCB, and nosZ in narXL strains. narXL encodes a two-component, nitrate-responsive (to a lesser extent also nitrite-responsive) regulatory system consisting of the nitrate sensor NarX and the response regulator NarL, which activate the narGHJI operon for respiratory nitrate reductase (29). Acidic conditions in the periplasm might result in the nonenzymatic generation of NO due to nitrite accumulation from nitrate respiration (nar system) or periplasmic nitrate reduction (nap system). Nitrate respiration itself has been reported to yield NO (31, 32), observations that might merit consideration in light of the signal role of NO. In addition to the NO-responsive pathway, there may still be a system responsive to high concentrations of nitrate and nitrite. In narX and narL strains, millimolar concentrations of nitrate or nitrite induced the transcription of nirSTB, norCB, and nosZ (29). Other than the nirF strain used here, the narXL strains have an intact background of nitrite denitrification. It is also noted that, in a narXL background, the periplasmic system of nitrate reduction is expected to be active and generates nitrite as the precursor of NO.
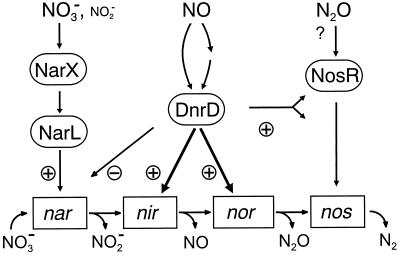
In a previous study we have shown that DnrD is required for the expression of the nirSTB and norCB operons (47). Similar roles are played by other FNR factors of the DNR branch such as DNR of P. aeruginosa (4) and NNR of Paracoccus denitrificans (43). We show here that DnrD also has a role in the NO-dependent response of nosZ and nosR genes required for the respiration of N2O. This respiration constitutes an independent way of energy conservation (54), but it is clear from our study that, when N2O reduction forms part of a complete denitrification process, nos genes are partly under the control of an NO-triggered signal transduction pathway. Considering the elevated levels of respiratory nitrate reductase found in the dnrD mutant (47), which goes in parallel with increased activity (data not shown), it seems that DnrD also has a modulating role in the expression of the narGHJI operon. Our data show that DnrD is a central regulator in P. stutzeri by interlacing to various extents the regulons of denitrification, represented by the four substrates reduced (Fig. 8). As we have shown, the anoxic cell responds also to an N2O signal although with less sensitivity than that to NO. NosR is an essential component for N2O respiration. It has a conspicuous domain structure extending to both sides of the cytoplasmic membrane (15, 54), which makes it predestined for transmembrane signaling; however, it is unknown whether this protein processes an N2O signal.
FIG. 8.
N oxide signaling, components, and hierarchies in the regulation of denitrification in P. stutzeri. Boxed nar, nir, nor, and nos designations represent as regulatory targets the narGHJI operon (nitrate reductase), the nirSTB operon (nitrite reductase), the norCB operon (NO reductase), and nosZ (N2O reductase), respectively. The substrates and products of each N oxide-reducing system are shown. The way NO interacts with DnrD is an open issue. The modulating role of DnrD on the expression of nosR and/or nosZ is shown by a double-headed arrow. Whether N2O acts via NosR is unknown. For further discussion, see the text.
The effective concentration of NO as an inducer was in the low nanomolar range, close to the steady-state concentrations of free NO during denitrification. Concentrations of free NO of 20 to 30 and 50 nM were determined by direct measurement (26) and by gas stripping (53), respectively. The apparent Km value for NO consumption by denitrifying bacteria and soil samples falls in a range from around 1 to about 70 nM (for a review, see reference 11). Synthetic NO donors were active as inducers; however, the signaling system of P. stutzeri responded to them with a lower sensitivity. NO donors were shown by reporter gene fusions to be active in R. sphaeroides 2.4.3, which required NO concentrations near 1 μM, supplied as SNP, for maximal expression of nirK and norB (35). Comparable data obtained with Paracoccus denitrificans, expressing lacZ reporter gene fusions with the promoters of nirS and norC, showed that induction with 0.1 mM SNP was necessary to mimic the nitrate (3 mM) response (46).
Cytotoxic effects of NO on cell division and viability were assumed to occur around 1 mM or above, and protective measures of a denitrifying cell against NO are seen as a necessary element of denitrification (54). In the present study, 0.5 mM NO fully inhibited transcription of the nirSTB and norCB operons. Recently, flavohemoglobin was shown to be involved in NO detoxification (14). Its synthesis in E. coli is maximally induced by 0.2 mM SNP (37). Flavohemoglobin is thought to eliminate NO either aerobically by oxidation to nitrate or anaerobically by reduction to N2O (for a review, see reference 40). It might have a similar protective function in denitrifiers, and its NO-scavenging activity may contribute to the necessity of having a highly sensitive NO signaling system. Flavohemoglobins are widely distributed in bacteria, but there is no systematic study of the occurrence of this protein in denitrifiers. It is present in R. eutropha (13), and we also found a copy of the hmp gene by Southern hybridization in the P. stutzeri genome (unpublished data).
The N2O-respiring cell allows one to deduce a further aspect of NO action. Increasing the concentration of NO to 50 nM caused a negative effect on the level of nirSTB and norCB transcripts in the anoxic cell, with a strong decline in the amount of transcripts at 60 min after induction. On the other hand, 250 nM NO was clearly stimulatory for nosZ transcription in the N2O cell. Thus, the effect observed at the 50 nM NO concentration with nir and nor mRNAs cannot be conceived as an unspecific, entirely toxic effect on the bacterial cell, since otherwise we would have had to find the same response for the nosZ transcripts. To what extent NO at a critical concentration exerts differential transcriptional control over certain target genes and affects stability of distinct mRNA species requires further studies.
We have previously found a complex structure of the nosZ promoter with multiple transcription initiation sites. Promoter P3 was active predominantly under denitrifying conditions (15). A putative FNR box, TTGAT-N4-GTgcA, at a distance of −52.5 from the transcript initiation site may indicate a direct participation of DnrD in the transcription control of nosZ. The spacing of the recognition site, however, is unusual when compared to that of E. coli. Only FNR-binding sites centered at 41, 61, 71, 82, and 92 bp upstream from the transcript start site were shown to meet the spacing requirements for transcriptional activation by FNR (50). Currently, we cannot differentiate whether DnrD exerts its control on nosZ via the synthesis of NosR or whether it is required to bind to the nosZ promoter. NosR was shown to be necessary for the transcription of nosZ. The dnrD mutant MKD235 lacked nosR transcripts. Considering the structure of the nosR promoter, FNR boxes for putative binding of DNR are located at −137.5 (aaGAT-N4-ATCAA), +67.5 (TTGtT-N4-GTCAt), and +127.5 (TTGAT-N4-ATCAA) (16). Again, the positions of these regulatory elements, if at all active, are outside the established locations for activation by an FNR-like factor. Thus, models for FNR-dependent gene activation cannot be applied without modification to DnrD-dependent gene regulation.
Overexpression of dnrD was insufficient to activate transcription of nirSTB. Only after addition of nitrate, nirS mRNA was found and nitrite reductase was detected immunochemically. This result indicates that cells overexpressing dnrD require the activation of DnrD before transcribing its target genes and that the activity of DnrD is unlikely to be exerted mainly by NO-induced autoregulation. We assume that DnrD-dependent transcription requires the reversible modification of the DnrD protein to enhance the affinity to binding sites in its target promoters. Interaction with NO may be of a direct nature at a metal site or an —SH or —OH group, or it may be indirect via a signal transduction pathway involving one or several ancillary components, perhaps even including participation of an organic cofactor. DnrD has been expressed in E. coli as a hybrid with the maltose-binding protein and found to carry heme B (U. Honisch and W. G. Zumft, unpublished data), which is reminiscent of the CO-responsive transcription factor CooA, a hemoprotein of the FNR family (44). However, attribution of any specific role to the heme group was not possible. One possibility of directly activating DnrD by NO is via nitration of a tyrosine residue by peroxynitrite (7, 8). The fact that NO exerted its inducer role under anaerobic conditions and was active in the N2O-grown cell allows us to conclude that oxygen in the form of superoxide leading to peroxynitrite formation from NO is not part of the NO signal transduction pathway. A mechanism of DnrD activation other than by tyrosine nitration will have to be found.
ACKNOWLEDGMENTS
This work was supported by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Aiba H, Adhya S, de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli. J Biol Chem. 1981;256:11905–11910. [PubMed] [Google Scholar]
- 2.Alexeyev M F. Three kanamycin resistance gene cassettes with different polylinkers. BioTechniques. 1995;18:52–55. [PubMed] [Google Scholar]
- 3.Arai H, Igarashi Y, Kodama T. The structural genes for nitric oxide reductase from Pseudomonas aeruginosa. Biochim Biophys Acta. 1995;1261:279–284. doi: 10.1016/0167-4781(95)00018-c. [DOI] [PubMed] [Google Scholar]
- 4.Arai H, Kodama T, Igarashi Y. Cascade regulation of the two CRP/FNR-related transcriptional regulators (ANR and DNR) and the denitrification enzymes in Pseudomonas aeruginosa. Mol Microbiol. 1997;25:1141–1148. doi: 10.1046/j.1365-2958.1997.5431906.x. [DOI] [PubMed] [Google Scholar]
- 5.Arai H, Kodama T, Igarashi Y. Effect of nitrogen oxides on expression of the nir and nor genes for denitrification in Pseudomonas aeruginosa. FEMS Microbiol Lett. 1999;170:19–24. doi: 10.1111/j.1574-6968.1999.tb13350.x. [DOI] [PubMed] [Google Scholar]
- 6.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1995. [Google Scholar]
- 7.Beckman J S. Oxidative damage and tyrosine nitration from peroxynitrite. Chem Res Toxicol. 1996;9:836–844. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- 8.Berlett B S, Friguet B, Yim M B, Chock P B, Stadtman E R. Peroxynitrite-mediated nitration of tyrosine residues in Escherichia coliglutamine synthetase mimics adenylylation: relevance to signal transduction. Proc Natl Acad Sci USA. 1996;93:1776–1780. doi: 10.1073/pnas.93.5.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, Kuo T. A simple and rapid method for the preparation of gram-negative bacterial genomic DNA. Nucleic Acids Res. 1993;21:2260. doi: 10.1093/nar/21.9.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chomczynski P, Mackey K. One-hour downward capillary blotting of RNA at neutral pH. Anal Biochem. 1994;221:303–305. doi: 10.1006/abio.1994.1416. [DOI] [PubMed] [Google Scholar]
- 11.Conrad R. Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO) Microbiol Rev. 1996;60:609–640. doi: 10.1128/mr.60.4.609-640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coyle C L, Zumft W G, Kroneck P M H, Körner H, Jakob W. Nitrous oxide reductase from denitrifying Pseudomonas perfectomarina, purification and properties of a novel multicopper enzyme. Eur J Biochem. 1985;153:459–467. doi: 10.1111/j.1432-1033.1985.tb09324.x. [DOI] [PubMed] [Google Scholar]
- 13.Cramm R, Siddiqui R A, Friedrich B. Primary sequence and evidence for a physiological function of the flavohemoprotein of Alcaligenes eutrophus. J Biol Chem. 1994;269:7349–7354. [PubMed] [Google Scholar]
- 14.Crawford M J, Goldberg D E. Role for the Salmonellaflavohemoglobin in protection from nitric oxide. J Biol Chem. 1998;273:12543–12547. doi: 10.1074/jbc.273.20.12543. [DOI] [PubMed] [Google Scholar]
- 15.Cuypers H, Berghöfer J, Zumft W G. Multiple nosZ promoters and anaerobic expression of nos genes necessary for Pseudomonas stutzerinitrous oxide reductase and assembly of its copper centers. Biochim Biophys Acta. 1995;1264:183–190. doi: 10.1016/0167-4781(95)00128-4. [DOI] [PubMed] [Google Scholar]
- 16.Cuypers H, Viebrock-Sambale A, Zumft W G. NosR, a membrane-bound regulatory component necessary for expression of nitrous oxide reductase in denitrifying Pseudomonas stutzeri. J Bacteriol. 1992;174:5332–5339. doi: 10.1128/jb.174.16.5332-5339.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuypers H, Zumft W G. Anaerobic control of denitrification in Pseudomonas stutzeri escapes mutagenesis of an fnr-like gene. J Bacteriol. 1993;175:7236–7246. doi: 10.1128/jb.175.22.7236-7246.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Boer A P N, Reijnders W N M, Kuenen J G, Stouthamer A H, van Spanning R J M. Isolation, sequencing and mutational analysis of a gene cluster involved in nitrite reduction in Paracoccus denitrificans. Antonie Leeuwenhoek. 1994;66:111–127. doi: 10.1007/BF00871635. [DOI] [PubMed] [Google Scholar]
- 19.Dower W J, Miller J F, Ragsdale C W. High efficiency transformation of E. coliby high voltage electroporation. Nucleic Acids Res. 1988;16:6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engler-Blum G, Meier M, Frank J, Müller G A. Reduction of background problems in nonradioactive Northern and Southern blot analyses enables higher sensitivity than 32P-based hybridizations. Anal Biochem. 1993;210:235–244. doi: 10.1006/abio.1993.1189. [DOI] [PubMed] [Google Scholar]
- 21.Farinha M A, Kropinski A M. High efficiency electroporation of Pseudomonas aeruginosausing frozen cell suspensions. FEMS Microbiol Lett. 1990;70:221–225. doi: 10.1111/j.1574-6968.1990.tb13982.x. [DOI] [PubMed] [Google Scholar]
- 22.Feliciello I, Chinali G. A modified alkaline lysis method for the preparation of highly purified plasmid DNA from Escherichia coli. Anal Biochem. 1993;212:394–401. doi: 10.1006/abio.1993.1346. [DOI] [PubMed] [Google Scholar]
- 23.Firth J R, Edwards C. Effects of cultural conditions on denitrification by Pseudomonas stutzerimeasured by membrane inlet mass spectrometry. J Appl Microbiol. 1999;87:353–358. doi: 10.1046/j.1365-2672.1999.00820.x. [DOI] [PubMed] [Google Scholar]
- 24.Glockner A B, Jüngst A, Zumft W G. Copper-containing nitrite reductase from Pseudomonas aureofaciens is functional in a mutationally cytochrome cd1-free background (NirS−) of Pseudomonas stutzeri. Arch Microbiol. 1993;160:18–26. doi: 10.1007/BF00258141. [DOI] [PubMed] [Google Scholar]
- 25.Goretski J, Hollocher T C. Trapping of nitric oxide produced during denitrification by extracellular hemoglobin. J Biol Chem. 1988;263:2316–2323. [PubMed] [Google Scholar]
- 26.Goretski J, Zafiriou O C, Hollocher T C. Steady-state nitric oxide concentrations during denitrification. J Biol Chem. 1990;265:11535–11538. [PubMed] [Google Scholar]
- 27.Hart T W. Some observations concerning the S-nitroso and S-phenylsulphonyl derivatives of l-cysteine and glutathione. Tetrahedron Lett. 1995;26:2013–2016. [Google Scholar]
- 28.Härtig E, Schiek U, Vollack K-U, Zumft W G. Nitrate and nitrite control of respiratory nitrate reduction in denitrifying Pseudomonas stutzeri by a two-component regulatory system homologous to NarXL of Escherichia coli. J Bacteriol. 1999;181:3658–3665. doi: 10.1128/jb.181.12.3658-3665.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Härtig E, Zumft W G. Kinetics of nirS expression (cytochrome cd1 nitrite reductase) in Pseudomonas stutzeriduring the transition from aerobic respiration to denitrification: evidence for a denitrification-specific nitrate- and nitrite-responsive regulatory system. J Bacteriol. 1999;181:161–166. doi: 10.1128/jb.181.1.161-166.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasegawa N, Arai H, Igarashi Y. Activation of a consensus FNR-dependent promoter by DNR of Pseudomonas aeruginosain response to nitrite. FEMS Microbiol Lett. 1998;166:213–217. doi: 10.1111/j.1574-6968.1998.tb13892.x. [DOI] [PubMed] [Google Scholar]
- 31.Ji X-B, Hollocher T C. Nitrate reductase of Escherichia colias a NO-producing nitrite reductase. Biochem Arch. 1989;5:61–66. [Google Scholar]
- 32.Kalkowski I, Conrad R. Metabolism of nitric oxide in denitrifying Pseudomonas aeruginosa and nitrate-respiring Bacillus cereus. FEMS Microbiol Lett. 1991;82:107–111. doi: 10.1016/0378-1097(91)90429-e. [DOI] [PubMed] [Google Scholar]
- 33.Körner H, Frunzke K, Döhler K, Zumft W G. Immunochemical patterns of distribution of nitrous oxide reductase and nitrite reductase (cytochrome cd1) among denitrifying pseudomonads. Arch Microbiol. 1987;148:20–24. doi: 10.1007/BF00429641. [DOI] [PubMed] [Google Scholar]
- 34.Körner H, Zumft W G. Expression of denitrification enzymes in response to the dissolved oxygen level and respiratory substrate in continuous culture of Pseudomonas stutzeri. Appl Environ Microbiol. 1989;55:1670–1676. doi: 10.1128/aem.55.7.1670-1676.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwiatkowski A V, Shapleigh J P. Requirement of nitric oxide for induction of genes whose products are involved in nitric oxide metabolism in Rhodobacter sphaeroides2.4.3. J Biol Chem. 1996;271:24382–24388. doi: 10.1074/jbc.271.40.24382. [DOI] [PubMed] [Google Scholar]
- 36.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 37.Membrillo-Hernández J, Coopamah M D, Anjum M F, Stevanin T M, Kelly A, Hughes M N, Poole R K. The flavohemoglobin of Escherichia coliconfers resistance to a nitrosating agent, a “nitric oxide releaser,” and paraquat and is essential for transcriptional responses to oxidatve stress. J Biol Chem. 1999;274:748–754. doi: 10.1074/jbc.274.2.748. [DOI] [PubMed] [Google Scholar]
- 38.Palmedo G, Seither P, Körner H, Matthews J C, Burkhalter R S, Timkovich R, Zumft W G. Resolution of the nirD locus for heme d1 synthesis of cytochrome cd1 (respiratory nitrite reductase) from Pseudomonas stutzeri. Eur J Biochem. 1995;232:737–746. [PubMed] [Google Scholar]
- 39.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poole R K, Hughes M N. New functions for the ancient globin family: bacterial responses to nitric oxide and nitrosative stress. Mol Microbiol. 2000;36:775–783. doi: 10.1046/j.1365-2958.2000.01889.x. [DOI] [PubMed] [Google Scholar]
- 41.Saeki K, Wakabayashi S, Zumft W G, Matsubara H. Pseudomonas stutzeri ferredoxin: close similarity to Azotobacter vinelandii and Pseudomonas ovalisferredoxins. J Biochem. 1988;104:242–246. doi: 10.1093/oxfordjournals.jbchem.a122450. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 43.Saunders N F W, Houben E N G, Koefoed S, de Weert S, Reijnders W N M, Westerhoff H V, de Boer A P N, van Spanning R J M. Transcription regulation of the nir gene cluster encoding nitrite reductase of Paracoccus denitrificansinvolves NNR and NirI, a novel type of membrane protein. Mol Microbiol. 1999;34:24–36. doi: 10.1046/j.1365-2958.1999.01563.x. [DOI] [PubMed] [Google Scholar]
- 44.Shelver D, Kerby R L, He Y, Roberts G P. CooA, a CO-sensing transcription factor from Rhodospirillum rubrum, is a CO-binding heme protein. Proc Natl Acad Sci USA. 1997;94:11216–11220. doi: 10.1073/pnas.94.21.11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tosques I E, Kwiatkowski A V, Shi J, Shapleigh J P. Characterization and regulation of the gene encoding nitrite reductase in Rhodobacter sphaeroides2.4.3. J Bacteriol. 1996;179:1090–1095. doi: 10.1128/jb.179.4.1090-1095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Spanning R J M, Houben E, Reijnders W N M, Spiro S, Westerhoff H V, Saunders N. Nitric oxide is a signal for NNR-mediated transcription activation in Paracoccus denitrificans. J Bacteriol. 1999;181:4129–4132. doi: 10.1128/jb.181.13.4129-4132.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vollack K-U, Härtig E, Körner H, Zumft W G. Multiple transcription factors of the FNR family in denitrifying Pseudomonas stutzeri: characterization of four fnr-like genes, regulatory responses and cognate metabolic processes. Mol Microbiol. 1999;31:1681–1694. doi: 10.1046/j.1365-2958.1999.01302.x. [DOI] [PubMed] [Google Scholar]
- 48.Weast R C, Astle M J, Beyer W H, editors. CRC handbook of chemistry and physics. 64th ed. 1983. pp. B–117. . CRC Press Inc., Boca Raton, Fla. [Google Scholar]
- 49.West S E H, Schweizer H P, Dall C, Sample A K, Runyen-Janecky L J. Construction of improved Escherichia-Pseudomonas shuttle vectors derived from pUC18/19 and sequence of the region required for their replication in Pseudomonas aeruginosa. Gene. 1994;128:81–86. doi: 10.1016/0378-1119(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 50.Wing H J, Williams S M, Busby S J W. Spacing requirements for transcription activation by Escherichia coliFNR protein. J Bacteriol. 1995;177:6704–6710. doi: 10.1128/jb.177.23.6704-6710.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 52.Ye R W, Arunakumari A, Averill B A, Tiedje J M. Mutants of Pseudomonas fluorescensdeficient in dissimilatory nitrite reduction are also altered in nitric oxide reduction. J Bacteriol. 1992;174:2560–2564. doi: 10.1128/jb.174.8.2560-2564.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zafiriou O C, Hanley Q S, Snyder G. Nitric oxide and nitrous oxide production and cycling during dissimilatory nitrite reduction by Pseudomonas perfectomarina. J Biol Chem. 1989;264:5694–5699. [PubMed] [Google Scholar]
- 54.Zumft W G. Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev. 1997;61:533–616. doi: 10.1128/mmbr.61.4.533-616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zumft W G, Braun C, Cuypers H. Nitric oxide reductase from Pseudomonas stutzeri: primary structure and gene organization of a novel bacterial cytochrome bccomplex. Eur J Biochem. 1994;219:481–490. doi: 10.1111/j.1432-1033.1994.tb19962.x. [DOI] [PubMed] [Google Scholar]
- 56.Zumft W G, Döhler K, Körner H. Isolation and characterization of transposon Tn5-induced mutants of Pseudomonas perfectomarinadefective in nitrous oxide respiration. J Bacteriol. 1985;163:918–924. doi: 10.1128/jb.163.3.918-924.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]