Abstract
Background
Identifying pregnancies at risk for preterm birth, one of the leading causes of worldwide infant mortality, has the potential to improve prenatal care. However, we lack broadly applicable methods to accurately predict preterm birth risk. The dense longitudinal information present in electronic health records (EHRs) is enabling scalable and cost-efficient risk modeling of many diseases, but EHR resources have been largely untapped in the study of pregnancy.
Methods
Here, we apply machine learning to diverse data from EHRs with 35,282 deliveries to predict singleton preterm birth.
Results
We find that machine learning models based on billing codes alone can predict preterm birth risk at various gestational ages (e.g., ROC-AUC = 0.75, PR-AUC = 0.40 at 28 weeks of gestation) and outperform comparable models trained using known risk factors (e.g., ROC-AUC = 0.65, PR-AUC = 0.25 at 28 weeks). Examining the patterns learned by the model reveals it stratifies deliveries into interpretable groups, including high-risk preterm birth subtypes enriched for distinct comorbidities. Our machine learning approach also predicts preterm birth subtypes (spontaneous vs. indicated), mode of delivery, and recurrent preterm birth. Finally, we demonstrate the portability of our approach by showing that the prediction models maintain their accuracy on a large, independent cohort (5978 deliveries) from a different healthcare system.
Conclusions
By leveraging rich phenotypic and genetic features derived from EHRs, we suggest that machine learning algorithms have great potential to improve medical care during pregnancy. However, further work is needed before these models can be applied in clinical settings.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-022-02522-x.
Keywords: Preterm birth, Machine learning, Electronic health records, Artificial intelligence
Background
Preterm birth, occurring before 37 weeks of completed gestation, affects approximately 10% of pregnancies globally [1–3] and is the leading cause of infant mortality worldwide [4, 5]. The causes of preterm birth are multifactorial since different biological pathways and environmental exposures can trigger premature labor [6]. Large epidemiological studies have identified many risk factors, including multiple gestations [1], cervical anatomic abnormalities [7], and maternal age [8]. Notably, even though a history of preterm birth [9] is one of the strongest risk factors, the recurrence rate remains low at < 30% [10, 11]. Additionally, the maternal race is associated with risk for preterm birth; Black women have twice the prevalence compared to White women [1, 12]. Preterm births have a heterogenous clinical presentation and cluster based on maternal, fetal, or placental conditions [3]. These obstetric and systemic comorbidities (e.g., pre-existing diabetes, cardiovascular disease) can also increase the risk of preterm birth [13, 14].
Despite our understanding of numerous risk factors, there are no accurate methods to predict preterm birth. Some biomarkers associate with preterm birth, but their best performance is limited to a subset of all cases [15, 16]. Recently, analysis of maternal cell-free RNA and integrated -omic models have emerged as promising approaches [17–19], but initial results were based on a small pregnancy cohort and require further validation. In silico classifiers based on demographic and clinical risk factors have the advantage of not requiring serology or invasive testing. However, even in large cohorts (> 1 million individuals), demographic- and risk factor-based models report limited discrimination (AUC = 0.63–0.74) [20–24]. To date, we lack effective screening tools and preventative strategies for prematurity [25].
EHRs are scalable, readily available, and cost-efficient for disease-risk modeling [26]. EHRs capture longitudinal data across a broad set of phenotypes with detailed temporal resolution. EHR data can be combined with socio-demographic factors and family medical history to comprehensively model disease risk [27–29]. EHRs are also increasingly being augmented by linking patient records to molecular data, such as DNA and laboratory test results [30]. Since preterm birth has a substantial heritable risk [31], combining rich phenotypes with genetic risk may lead to better prediction.
Machine learning models have shown promise for accurate risk stratification across a variety of clinical domains [32–34]. However, despite the rapid adoption of machine learning in translational research, a review of 107 risk prediction studies reported that most models used only few variables, did not consider longitudinal data, and rarely evaluated the model performance across multiple sites [35]. Studies using machine learning to predict preterm birth have relied on small cohorts and subsets of preterm birth and are rarely replicated in external datasets [22, 36–38]. Pregnancy research is especially well poised to benefit from machine learning approaches [27]. Per standard of care during pregnancy, women are carefully monitored with frequent prenatal visits, medical imaging, and clinical laboratory tests. Compared to other clinical contexts, pregnancy and the corresponding clinical surveillance occur in a defined time frame based on gestational length. Thus, EHRs are well-suited for modeling pregnancy complications, especially when combined with the well-documented outcomes at the end of pregnancy.
In this study, we combine multiple sources of data from EHRs to predict preterm birth using machine learning. From Vanderbilt’s EHR database (> 3.2 million records) and linked genetic biobank (> 100,000 individuals), we identified a large cohort of women (n = 35,282) with documented deliveries. We trained models (gradient-boosted decision trees) that combine demographic factors, clinical history, laboratory tests, and genetic risk with billing codes to predict preterm birth. We find models trained on only billing codes show potential for predicting preterm birth and outperform a similar model using only known clinical risk factors. By investigating the patterns learned by our models, we identify clusters with distinct preterm birth risk and comorbidity profiles. Finally, we demonstrate the generalizability of billing code-based models trained at Vanderbilt on an external, independent cohort from the University of California, San Francisco (UCSF, n = 5978). Our findings provide a proof of concept that machine learning on rich phenotypes in EHRs shows promise for portable, accurate, and non-invasive prediction of preterm birth. The strong predictive performance across clinical context and preterm birth subtypes argues that machine learning models have the potential to add value during the management of pregnancy; however, further work is needed before these models can be applied in clinical settings.
Results
Assembling pregnancy cohort and ascertaining delivery type from Vanderbilt EHRs
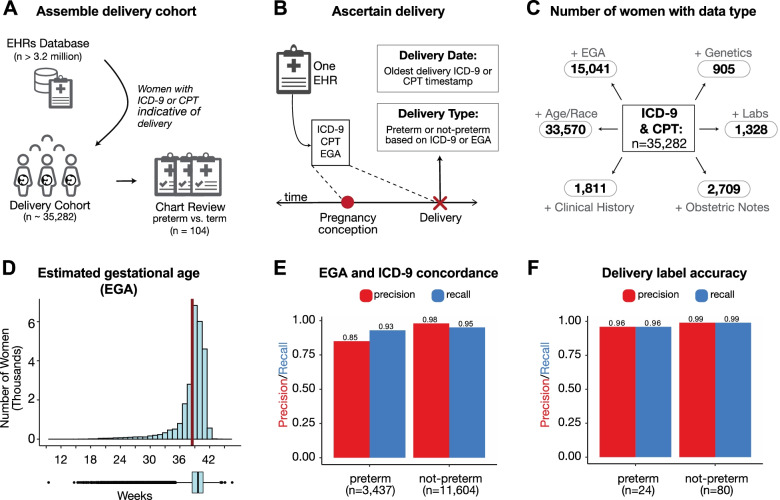
From the Vanderbilt EHR database (> 3.2 million patients), we identified a “delivery cohort” of 35,282 women with at least one delivery in the Vanderbilt hospital system (Fig. 1A). In addition to ICD and CPT billing codes, we extracted demographic data, past medical histories, obstetric notes, clinical labs, and genome-wide genetic data for the delivery cohort. Because billing codes were the most prevalent data in this cohort (n = 35,282), we quantified the pairwise overlap between billing codes and each other data type. The largest subset included women with billing codes paired with demographic data (n = 33,570). The smallest subset was women with billing codes paired with genetic data (n = 905; Fig. 1C). The mean maternal age at the first delivery in the delivery cohort was 27.3 years (23.0–31.0 years, 25th and 75th percentiles, Additional file 1: Fig. S1A). The majority of women in the cohort self- or third-party-reported as White (n = 21,343), Black (n = 6178), or Hispanic (n = 3979; Additional file 1: Fig. S1B). The estimated gestational age (EGA) distribution had a mean of 38.5 weeks (38.0 to 40.3 weeks, 25th to 75th percentile; Fig. 1D). The rate of multiple gestations (e.g., twins, triplets) was 7.6% (n = 1353). Since multiple gestation pregnancies are more likely to deliver preterm, we developed prediction models using singleton pregnancies unless otherwise stated.
Fig. 1.
Definition and attributes of Vanderbilt delivery cohort. A Schematic overview of the assembly of the delivery cohort from electronic health records (EHRs). Using billing codes, women with at least one delivery were extracted from the EHR database (n = 35,282). B Delivery date and type were ascertained using ICD-9, CPT, and/or estimated gestational age (EGA) from each woman’s EHR (the “Methods” section). From this cohort, 104 randomly selected EHRs were chart reviewed to validate the preterm birth label for the first recorded delivery. C Number of women in the billing code cohort with estimated gestational age (+EGA), demographics (+Age, self- or third-party-reported race), clinical labs (+Labs), clinical obstetric notes (+Obstetric notes), patient clinical history (+Clinical History), and genetic data (+Genetics). D The EGA distribution at delivery (mean 38.5 weeks (red line); 38.0–40.3 weeks, 25th and 75th percentiles). Less than 0.015% (n = 49) deliveries have EGA below 20 weeks. E The concordance between estimated gestational age (EGA) within 3 days of delivery and ICD-9-based delivery type for the 15,041 women with sufficient data for both. Precision and recall values were > 93% across labels except for preterm precision (85%). F Accuracy of delivery type phenotyping. The phenotyping algorithm was evaluated by chart review of 104 randomly selected women. The approach has high precision and recall for binary classification of “preterm” or “not preterm”
To determine the delivery date and type (preterm vs. not preterm) at scale across our large cohort, we developed a phenotyping algorithm using delivery-specific billing codes and estimated gestational age at delivery. For women with multiple pregnancies, we only considered the earliest delivery. We find that labeling preterm births using delivery-specific billing codes has high concordance (PPV ≥ 0.85, recall ≥ 0.95) with EGA-based delivery labels (Fig. 1E). Our final algorithm combined billing codes and EGA when available (n = 15,041, Fig. 1C). To evaluate the accuracy of the ascertained delivery labels, a domain expert blinded to the delivery type reviewed clinical notes from 104 EHRs selected at random from the delivery cohort. The algorithm had high accuracy: precision (positive predictive value) of 96% and recall (sensitivity) of 96% using the chart-reviewed label as the gold standard (Fig. 1F).
Boosted decision trees using billing codes to identify preterm deliveries
Using this richly phenotyped delivery cohort, we evaluated how well the entire clinical phenome, defined as billing codes (ICD-9 and CPT) before and after delivery, could identify preterm births. With counts of each billing code (excluding those used to ascertain delivery type), we trained gradient-boosted decision trees [39] to classify each mother’s first delivery as preterm or not preterm. Boosted decision trees are well-suited for EHR data because they require a minimal transformation of the raw data, are robust to correlated features, and capture non-linear relationships [40]. Moreover, boosted decision trees have been successfully applied on a variety of clinical tasks [28, 41, 42].
In all evaluations, we held out 20% of the cohort as a test set and used the remaining 80% for training and validation (Fig. 2A). Boosted decision tree models trained on ICD-9 and CPT codes accurately identified preterm births (singletons and multiple gestations) with PR-AUC = 0.86 (chance = 0.22) and ROC-AUC = 0.95 (Additional file 1: Fig. S2A, B). While the combined ICD-9- and CPT-based model achieved the best performance, models trained on either ICD-9 or CPT individually also performed well (PR-AUC ≥ 0.82; chance = 0.22, ROC-AUC ≥ 0.93). All three models demonstrated good calibration with low Brier scores (≤ 0.092; Additional file 1: Fig. S2C). Thus, billing codes across an EHR show potential as a discriminatory feature for predicting preterm birth.
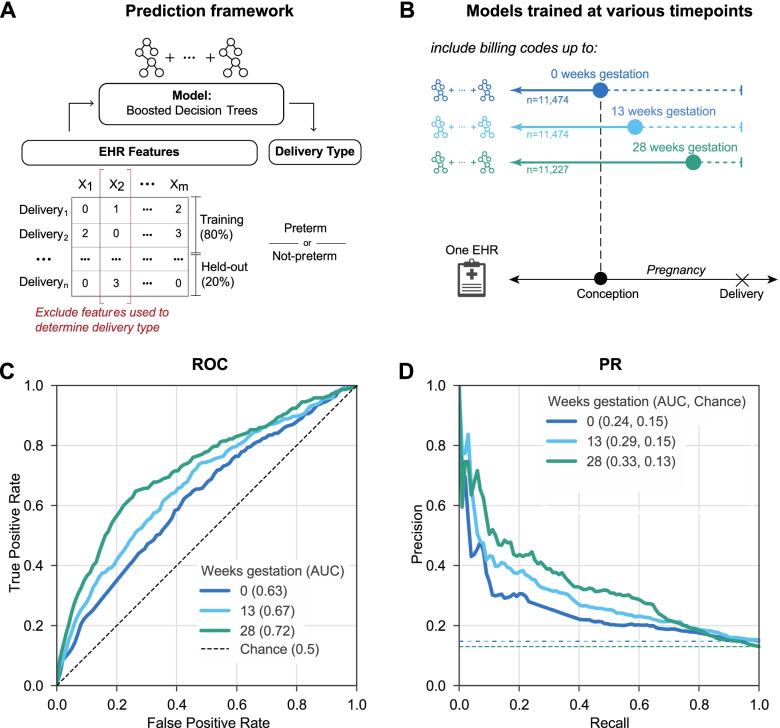
Fig. 2.
Machine learning accurately predicts preterm birth using billing codes present before 28 weeks of gestation. A Machine learning framework for training and evaluating all models. We train models (boosted decision trees) on 80% of each cohort to predict the delivery as preterm or not preterm. EHR features used to ascertain delivery type are excluded from the training. Performance is reported on the held-out cohort consisting of 20% of deliveries using the area under the ROC and precision-recall curves (ROC-AUC, PR-AUC). B We trained models using billing codes (ICD-9 and CPT) present before each of the following time points during pregnancy: 0, 13, and 28 weeks of gestation. These time points were selected to approximate gestational trimesters. Women who already delivered were excluded at each time point. To facilitate comparison across time points, we downsampled the cohorts available so that the models were trained on a cohort with similar numbers of women (n = 11,227 to 11,474). C The ROC-AUC increased from conception at 0 weeks (0.63, dark blue line) to 28 weeks of gestation (0.72, green line) compared to a chance (black dashed line) AUC of 0.5. D The model at 28 weeks of gestation achieved the highest PR-AUC (0.33). This is an underestimate of the possible performance; the performance improves further when all women with data available at 28 weeks are considered (ROC-AUC 0.75 and PR-AUC 0.40). Chance (dashed lines) represents the preterm birth prevalence in each cohort
Accurate prediction of preterm birth at 28 weeks of gestation
To evaluate preterm birth prediction in a clinical context, we trained a boosted decision tree model (Fig. 2A) on billing codes present before each of the following time points: 0, 13, and 28 weeks of gestation (Fig. 2B). These time points were selected to approximately reflect pregnancy trimesters. We downsampled to achieve a comparable number of singleton deliveries across each time point (n = 11,227 to 11,474) to mitigate sample size as a potential confounder while comparing the performance. We only considered active pregnancies at each time point; for example, delivery at 27 weeks would not be included in the 28-week model, since the outcome would already be known. The ROC-AUC increased from conception (0 weeks; 0.63) to the highest performance at 28 weeks (0.72; Fig. 2C). The PR-AUC (Fig. 2D), which accounts for preterm birth prevalence, is highest at 28 weeks (0.33, chance = 0.13). However, as we show in the next section, this is an underestimate of the ability to predict preterm delivery at 28 weeks due to the down-sampling of the number of training examples. As expected, when we included multiple gestations, the model performed even better (PR-AUC = 0.42 at 28 weeks, chance = 0.14; Additional file 1: Fig. S3). The results were similar when models were trained using billing codes available before different time points from the date of delivery (Additional file 1: Fig. S4).
To test whether differences in contact with the health system between cases and controls were driving performance, we trained a classifier based on the total number of codes in an individual’s EHR before delivery to predict preterm birth. This simple classifier failed to discriminate between delivery types with PR-AUC and ROC-AUC only slightly higher than chance (PR-AUC = 0.19, chance = 0.19; ROC-AUC = 0.56, chance = 0.5, Additional file 1: Fig. S5). Therefore, cumulative disease burden or the number of contacts alone is not informative for predicting preterm birth.
Integrating other EHR features does not improve model performance
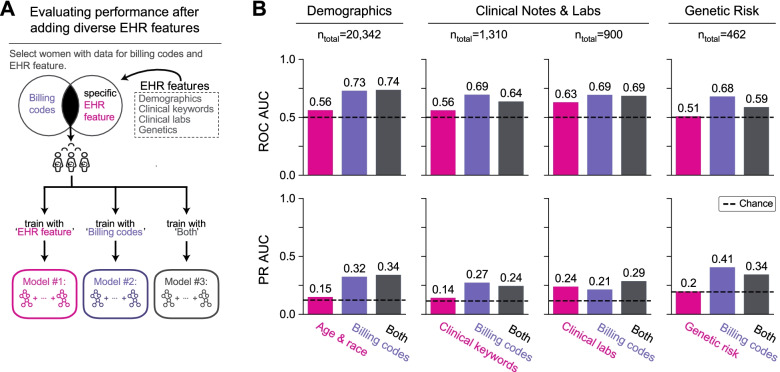
In addition to billing codes, EHRs capture aspects of an individual’s health through different types of structured and unstructured data. We tested whether incorporating additional features from EHRs can improve preterm birth prediction. Models were evaluated using data available at 28 weeks of gestation; we selected this time point as a tradeoff between being sufficiently early for some potential interventions and late enough for sufficient data to be present to enable accurate predictions using billing codes. From the EHRs, we extracted sets of features including demographic variables (age, race), clinical keywords from obstetric notes, clinical lab tests ran during the pregnancy, and predicted genetic risk (polygenic risk score for preterm birth). To measure the performance gain for each feature set, we compared the models trained using the feature set only, billing codes only, and billing codes combined with the feature set (Fig. 3A). Within each feature set, the same pregnancies comprised the training and held-out sets for the three models. However, the number of deliveries (training + held-out sets) varied widely across feature sets (n = 462 to 20,342) due to the differing availability of each feature type.
Fig. 3.
Demographic, clinical, and genetic features do not improve preterm birth prediction compared to billing codes. A Framework for evaluating change in preterm birth prediction performance after incorporating diverse types of EHR features with billing codes (ICD-9 and CPT codes). We used only features and billing codes occurring before 28 weeks of gestation. EHR features are grouped by sets of demographic factors (age and race), clinical keywords (UMLS concept unique identifiers from obstetric notes), clinical labs, and genetic risk (polygenic risk score for preterm birth). We compared three models for each feature set: (1) using only the feature set being evaluated (pink), (2) using only billing codes (“Billing codes,” purple), and (3) using the feature set combined with billing codes (“Both,” gray). For each feature set, we considered the subset of women who had at least one recorded value for the EHR feature and billing codes. All three models for a given EHR feature set considered the same pregnancies, but there are differences in the cohorts considered across the feature set due to the differences in data availability; ntotal is the number of women (training and held-out) for each feature set. B Each of the three models (x-axis) and their ROC-AUC and PR-AUC (y-axis) are shown. Each of the additional EHR features performed worse than the billing codes-only model and did not substantially improve performance when combined with the billing codes. Dotted lines represent a chance of 0.5 for ROC-AUC and the preterm birth prevalence for PR-AUC. Even when including EHR features before and after delivery in this framework revealed the same pattern with no substantial improvement in predictive performance compared to the billing codes-only model (Additional file 1: Fig. S6)
Models using only demographic factors, clinical keywords, and genetic risk had ROC-AUC and PR-AUC similar to chance (Fig. 3B). Clinical labs had moderate predictive power with ROC-AUC of 0.63 and PR-AUC of 0.24 (Fig. 3B). Compared to models using only billing codes, adding additional feature sets did not substantially improve performance (Fig. 3B). We note that some feature sets, such as clinical labs and genetic risk, were evaluated on held-out sets with small numbers of deliveries (180 and 92, respectively). However, even after increasing the sample size by including women who may have features either before or after delivery, we did not observe a consistent gain in performance compared to models trained using only billing codes (Additional file 1: Fig. S6).
Models using billing codes outperform predictions from risk factors
Although there are well-known risk factors for preterm birth, few validated risk calculators exist and even fewer are routinely implemented in clinical practice [43]. We evaluated how a prediction model incorporating only common risk factors associated with moderate to high risk for preterm birth compared to a model using billing codes, which captured a broad range of comorbidities, at 28 weeks of gestation (Fig. 4A). We included maternal and fetal risk factors that occurred before and during the pregnancy and across many organ systems [3, 13, 23, 44], race [20], age at delivery [45–47], pre-gestational and gestational diabetes [48], sickle cell disease [49], fetal abnormalities [13], pre-pregnancy hypertension, gestational hypertension (including pre-eclampsia or eclampsia) [1, 50], and cervical abnormalities [51] (the “Methods” section). However, we note that we did not include some known lifestyle risk factors, like smoking, alcohol consumption, or physical activity, due to the difficulty of accurately extracting them from the EHR.
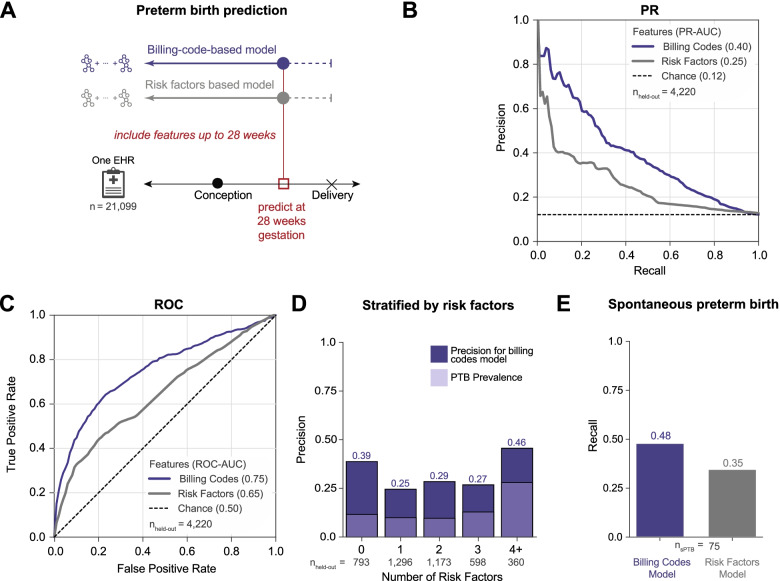
Fig. 4.
Billing code-based model outperforms a model based on clinical risk factors. A We compared the performance of boosted decision trees trained using either billing codes (ICD-9 and CPT) present before 28 weeks of gestation (purple) or known clinical risk factors (gray) to predict preterm delivery. Clinical risk factors (the “Methods” section) included self- or third-party-reported race (Black, Asian, or Hispanic), age at delivery (> 34 or < 18 years old), non-gestational diabetes, gestational diabetes, sickle cell disease, presence of fetal abnormalities, pre-pregnancy BMI > 35, pre-pregnancy hypertension (> 120/80), gestational hypertension, preeclampsia, eclampsia, and cervical abnormalities. Both models were trained and evaluated on the same cohort of women (n = 21,099). B Precision-recall and C ROC curves for the model using billing codes (purple line) or clinical risk factors (gray line). Preterm births are predicted more accurately by models using billing codes at 28 weeks of gestation (PR-AUC = 0.40, ROC-AUC = 0.75) than using clinical risk factors as features (PR-AUC = 0.25, ROC-AUC = 0.65). For the precision-recall curves, chance performance is determined by the preterm birth prevalence (dashed black line). D Billing code-based prediction model performance stratified by the number of risk factors for an individual. The billing code-based model detects more preterm cases and has higher precision (dark purple) across all numbers of risk factors compared to preterm (PTB) prevalence (light purple). E The model using billing codes also performs well at predicting the subset of spontaneous preterm births in the held-out set (recall = 0.48) compared to risk factors (recall = 0.35)
The billing code-based model significantly outperformed a model trained with clinical risk factors at predicting preterm birth at 28 weeks of gestation (PR-AUC = 0.40 vs. 0.25, ROC-AUC = 0.75 vs. 0.65; Fig. 4B, C). The stronger performance of the billing code-based classifier was true for women across the spectrum of comorbidity burden; it had higher precision across individuals with different numbers of risk factors. Performance peaked for individuals with 0 (precision = 0.39) and 4+ (precision = 0.46) risk factors, but we did not observe a trend between model performance and increasing number of clinical risk factors (Fig. 4D). This suggests that machine learning approaches incorporating a comprehensive clinical phenome can add value to predicting preterm birth.
Machine learning models can predict spontaneous preterm births
The multifactorial etiologies of preterm birth lead to clinical presentations with different comorbidities and trajectories. Medically indicated and idiopathic spontaneous preterm births are distinct in etiologies and outcomes. Identifying pregnancies that ultimately result in spontaneous preterm deliveries is particularly valuable, and we anticipated that spontaneous preterm birth would be more challenging to predict than preterm birth overall. To test this, we identified spontaneous preterm births in the held-out set at 28 weeks of gestation by excluding women with medically induced labor, a cesarean section delivery, or PPROM (the “Methods” section). We intentionally used a conservative phenotyping strategy that aimed to minimize false-positive spontaneous preterm births to evaluate the model’s ability to predict spontaneous preterm births. The prediction model trained using billing codes up to 28 weeks of gestation classified 48% (recall) of all spontaneous preterm births (n = 75) as preterm; this is significantly higher than the risk factor only model (recall = 35%; Fig. 4E).
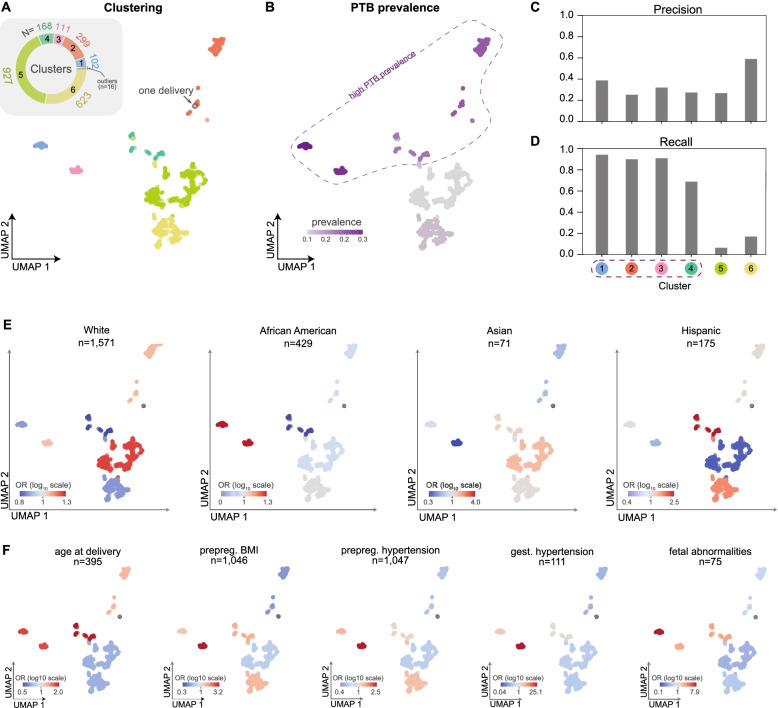
Preterm birth prediction algorithm stratifies deliveries into clusters with different preterm birth risks and distinct comorbidity signatures
Understanding the statistical patterns identified by machine learning models is crucial for their adoption into clinical practice. Unlike deep learning approaches, decision tree-based models are easier to interpret. We calculated the feature importance as measured by the SHapley Additive exPlanation (SHAP) scores [52, 53] for each delivery and feature pair in the held-out cohort for the model using billing codes at 28 weeks of gestation (“Billing code-based model,” Fig. 4A). SHAP scores quantify the marginal additive contribution of each feature to the model predictions for each individual. Next, we performed a density-based clustering on the patient by feature importance matrix and visualized clusters using UMAP (Fig. 5A, the “Methods” section). This approach focuses the clustering on the features for each individual prioritized by the algorithm. We identified six clusters with 927 to 102 women. PTB prevalence (Fig. 5B) was the highest in clusters 1 to 4 (blue, pink, green, orange, Fig. 5A) indicating a greater risk for preterm birth for women in these clusters. Performance varied across the clusters; the yellow cluster with low PTB prevalence had the highest PPV while clusters with higher PTB prevalence had a higher recall (Fig. 5C, D).
Fig. 5.
Machine learning-based clustering of deliveries identifies subgroups with distinct PTB prevalence, clinical features, and prediction accuracy. A For the model predicting preterm birth at 28 weeks of gestation using billing codes (ICD-9 and CPT, Fig. 4A), we assigned deliveries from the held-out test set (n = 2246) to one of six clusters (colors) using density-based clustering (HDBSCAN) on the SHAP feature importance matrix. For visualization of the clusters, we used UMAP to embed the deliveries into a low-dimensional space based on the matrix of feature importance values. The inset pie chart displays the count of individuals in each cluster. B The preterm birth prevalence (color bar) in each cluster. The algorithm discovered four clusters with high PTB prevalence (enclosed by a dashed line). C Precision and D recall for preterm birth classification within each cluster. E The enrichment (odds ratios, color bar in log10 scale) of race as derived from EHRs for each cluster (Additional file 1: Table S1). F The enrichment (log10 odds ratio) of relevant clinical risk factors in each cluster (Additional file 1: Table S2). Risk factors include age at delivery (> 34 or < 18 years old), pre-pregnancy BMI (prepreg BMI), pre-pregnancy hypertension (prepreg hypertension), gestational hypertension (gest hypertension), and fetal abnormalities. We report the total number of women in the delivery cohort at high risk for each clinical risk factor (n). Enrichments for additional risk factors are given in Additional file 1: Fig. S7
To evaluate whether clusters had distinct phenotype profiles, we calculated the enrichment of demographic and clinical risk factor traits within each cluster as the odds ratio from Fisher’s exact test (the “Methods” section). Enrichment (or depletion) of a particular demographic or clinical risk factor in a given cluster denotes higher (or lower) odds of that factor being present in women within the given cluster compared to other factors. These traits were extracted from structured fields in EHRs or ascertained using combinations of billing codes. Although these billing codes are used to train the model, the combination of codes used to ascertain risk factor traits is not encoded in the training data. White women are significantly enriched in cluster 5 (odds ratio, OR = 1.2, p-value = 0.02, Fisher’s exact test, Fig. 5E), which means women in this cluster are more likely to be White than not White. Hispanic women also had significant positive enrichment in cluster 4 (OR = 2.5, p-value = 0.0002) and cluster 6 (OR = 1.6, p-value = 0.008) and were depleted (negative enrichment) in cluster 5 (OR = 0.5, p-value = 4.42E−6, Fig. 5D). African American and Asian women also exhibit modest enrichment in different clusters (Additional file 1: Table S1).
We also tested for enrichment of clinical risk factors of preterm birth in the clusters. We observed distinct patterns of enrichment and depletion for each clinical risk factor (Fig. 5F, Additional file 1: Fig. S7). Gestational hypertension had strong enrichment in cluster 3 (OR = 26.4, p-value = 9.0E−39). Fetal abnormalities demonstrated a similar pattern with strong enrichment in cluster 1 (OR = 8.5, p-value = 2.07E−10). Extreme age at delivery (> 34 or < 18 years old) was enriched, though weakly (OR = 1.2 to 2.2) for all clusters except clusters 5 and 6. Pre-pregnancy BMI, pre-pregnancy hypertension, and gestational hypertension had similar patterns with the strongest enrichment in cluster 3. The remaining clinical risk factors show similar patterns and are provided in Additional file 1: Fig. S7 and Additional file 1: Table S2.
By analyzing the feature importance values through UMAP embeddings, we identify interpretable clusters of individuals discovered by the machine learning model that reflect the complex and multi-faceted paths to preterm birth. Overall, the learned rules highlight the relationships between clinical factors and preterm birth prevalence. For example, some risk factors, such as age at delivery, are enriched in all clusters with high preterm birth prevalence. Other factors, such as pre-pregnancy BMI and hypertension, are strongly enriched only in specific clusters with high preterm birth prevalence. Thus, this approach enables us to interpret phenotypic patterns of risk and identify subgroups among cases learned from complex EHR features by the prediction model.
Performance varies based on clinical context and delivery history
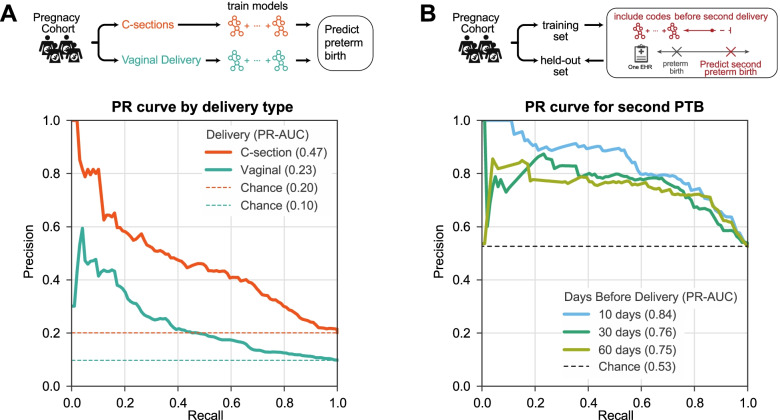
To further explore the sensitivity of the performance of our approach to clinical context and patient history, we evaluated how delivery type (vaginal vs. cesarean section) and a previous preterm birth influence preterm birth prediction. We trained two classifiers using billing codes (ICD-9 and CPT) occurring before 28 weeks of gestation: one on a cohort of cesarean section (n = 5475) singleton deliveries and one on vaginal deliveries (n = 15,487). Preterm birth prediction accuracy was higher in the cesarean section cohort (PR-AUC = 0.47, chance = 0.20) compared to the vaginal delivery cohort (PR-AUC = 0.23, chance = 0.10; Fig. 6A). Cesarean sections also had higher ROC-AUC compared to vaginal deliveries (0.75 vs. 0.68, Additional file 1: Fig. S8). As expected, the preterm birth prevalence was higher in the cesarean section cohort.
Fig. 6.
Preterm birth prediction accuracy is influenced by clinical context. A Preterm birth prediction models trained and evaluated only on cesarean section (C-section) deliveries perform better (PR-AUC = 0.47) than those trained only on vaginal delivery (PR-AUC = 0.23). ROC-AUC patterns were similar (Additional file 1: Fig. S8). Billing codes (ICD-9 and CPT) present before 28 weeks of gestation were used to train a model to distinguish preterm from non-preterm birth for either C-sections (n = 5475) or vaginal deliveries (n=15,487). B Recurrent preterm birth can be accurately predicted from billing codes. We trained the models to predict preterm birth for the second delivery in a cohort of 1416 high-risk women with a prior preterm birth documented in their EHR. Three models were trained using data available 10 days, 30 days, and 60 days before the date of the second delivery. Models accurately predict the birth type in this cohort of women with a history of preterm birth (PR-AUC ≥ 0.75). ROC-AUC varied from 0.82 at 10 days to 0.77 at 60 days before the second delivery (Additional file 1: Fig. S9). Expected performance by chance is the preterm birth prevalence in each cohort (dashed lines)
Women with a history of preterm birth are at significantly higher risk for a subsequent preterm birth than women without a previous history. Therefore, it is particularly important to understand the drivers of risk in this cohort. We tested if models trained on EHR data of women with a history of preterm birth could accurately predict the status of their next birth. We assembled 1416 women with preterm birth and a subsequent delivery in the cohort and split them into a training set (80%) and a held-out test set (20%) to evaluate the model performance (the “Methods” section). For these women, 53% of the second deliveries were preterm. Due to limited availability of estimated gestational age data for recurrent preterm births, which is necessary to approximate the date of conception, we trained models using billing codes (ICD-9 and CPT) present before each of the following time points: 10, 30, and 60 days before the delivery. These models were all able to discriminate term from preterm deliveries better than chance (Fig. 6B; PR-AUCs ≥ 0.75). The model predicting a second preterm birth as early as 60 days before delivery achieved a high performance with PR-AUC = 0.75 (Fig. 6B, chance = 0.53) and ROC-AUC = 0.77 (Additional file 1: Fig. S9).
Models trained at Vanderbilt accurately predict preterm birth in an independent cohort at UCSF
To evaluate whether preterm birth prediction models trained on the Vanderbilt cohort performed well on EHR data from other databases, we compared their performance on the held-out Vanderbilt cohort (n = 4215) and an independent cohort from UCSF (n = 5978). The UCSF cohort was ascertained using similar rules as the Vanderbilt cohort (the “Methods” section); age and distribution of race are provided in Table 1. However, we note that the UCSF cohort has a lower preterm birth prevalence (6%) compared to the Vanderbilt cohort (13%).
Table 1.
Demographic distribution of UCSF and Vanderbilt cohorts. We identified women with preterm and not preterm deliveries at UCSF and Vanderbilt using similar ascertainment (the “Methods” section). For each woman, we predicted the earliest delivery in their EHR. We report age at delivery (patient age) as mean with standard deviation (SD) in parenthesis and self- or third-party-reported race for both cohorts as the count and the column-wise proportion in parenthesis. The T-tests and chi-squared tests of independence were used to compare distributions stratified by delivery label
| UCSF | Vanderbilt | |||||
|---|---|---|---|---|---|---|
| Not preterm | Preterm | p-value | Not preterm | Preterm | p-value | |
| n | 5615 | 363 | 18,498 | 2651 | ||
| Patient age (mean (SD)) | 36.65 (5.08) | 36.54 (5.96) | 0.691 | 27.71 (5.75) | 27.73 (6.38) | 0.876 |
| Patient race (%) | < 0.001 | < 0.001 | ||||
| American Indian or Alaska Native | 26 (0.5) | 3 (0.8) | 47 (0.2) | 4 (0.01) | ||
| Asian | 1336 (23.8) | 51 (14.0) | 1051 (5.8) | 100 (3.8) | ||
| Black or African American | 336 (6.0) | 31 (8.5) | 2962 (16.5) | 486 (18.8) | ||
| Declined | 72 (1.3) | 5 (1.4) | NA | NA | ||
| Hispanic | NA | NA | 2379 | 322 | ||
| Native Hawaiian/Pacific Islander | 86 (1.5) | 3 (0.8) | NA | NA | ||
| Others | 866 (15.4) | 77 (21.2) | 162 (0.9) | 12 (0.04) | ||
| Unknown | 200 (3.6) | 32 (8.8) | 619 (3.3) | 69 (2.6) | ||
| White or Caucasian | 2693 (48.0) | 161 (44.4) | 11,278 (63.0) | 1658 (64.2) | ||
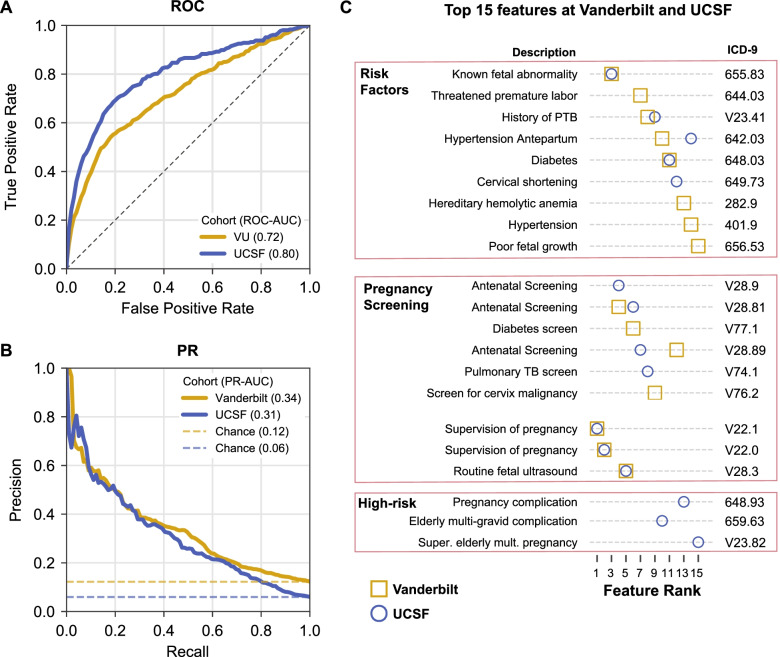
To facilitate the comparison, we trained models to predict preterm birth in the Vanderbilt cohort using only ICD-9 codes present before 28 weeks of gestation. We will refer to this as the “Vanderbilt-28wk model.” We did not consider CPT codes in this analysis due to the differences in the available billing code data between Vanderbilt and UCSF. As expected from the previous results, the Vanderbilt-28wk model accurately predicted preterm birth in the held-out set from Vanderbilt (PR-AUC of 0.34, chance = 0.12), but the performance was slightly lower than using both ICD and CPT codes (Fig. 4B). The Vanderbilt-28wk model also achieved strong performance in the UCSF cohort. The Vanderbilt-28wk model had a higher ROC-AUC (0.80) in the UCSF cohort compared to the Vanderbilt cohort (0.72; Fig. 7A) and PR-AUC of 0.31 vs. 0.34 at Vanderbilt (Fig. 7B). The higher ROC is due to the lower prevalence of preterm birth in the UCSF cohort and the sensitivity of ROC-AUC to class imbalance [54]. Overall, the Vanderbilt-28wk model shows striking reproducibility across two independent cohorts.
Fig. 7.
Preterm birth prediction models accurately generalize to an independent cohort. Performance of preterm birth prediction models trained at Vanderbilt applied to UCSF cohort. Models were trained using ICD-9 codes present before 28 weeks of gestation at Vanderbilt on 16,857 women and evaluated on a held-out set at Vanderbilt (n = 4215, gold) and UCSF cohort (n = 5978, blue). A Models accurately predicted preterm birth at Vanderbilt (ROC-AUC = 0.72) and at UCSF (ROC-AUC = 0.80). The higher ROC-AUC at UCSF is driven by the lower prevalence of preterm birth in this cohort. B Models performed better than baseline prevalence (chance) based on the precision-recall curve at Vanderbilt (PR-AUC = 0.34) and at UCSF (PR-AUC = 0.31). Note that in contrast to the models presented previously, this one was trained only on ICD-9 codes, due to the lack of CPT codes in the UCSF cohort. C The top 15 features with the highest mean absolute SHAP score in the Vanderbilt cohort (gold square) or UCSF cohort (blue circle). The majority of the features were shared across cohorts and captured known risk factors (fetal abnormalities, history of preterm birth, etc.), pregnancy screening visits, and supervision of high-risk pregnancies. Feature importance estimates were strongly correlated between the two cohorts (Additional file 1: Fig. S10). Cohort demographics are given in Table 1
Similar features are predictive across the independent cohorts
The architecture of boosted decision trees enables straightforward identification of features (ICD-9 codes) with the largest influence on the model predictions. We used SHAP [52, 55] scores to quantify the marginal additive contribution of each feature to the model predictions for each individual. For each feature in the Vanderbilt-28wk model based on ICD-9 codes, we calculated the mean absolute SHAP values across all women in the held-out set. The mean absolute SHAP value for each feature was highly correlated (Spearman R = 0.93, p-value < 2.2E−308) between the held-out Vanderbilt set and the UCSF cohort (Additional file 1: Fig. S10). The top 15 features ranked based on the mean absolute SHAP value captured known risk factors (fetal abnormalities, history of preterm birth, etc.), pregnancy screening, and supervision of high-risk pregnancies (Fig. 7C). Ten of the top 15 features were shared across both cohorts. The full list of SHAP values across all features is provided in Additional file 1: Table S3. This suggests that the model’s discovered combination of phenotypes, including expected risk factors, and the corresponding weights assigned by the machine learning model are generalizable across cohorts.
Logistic regression using top features does not outperform gradient-boosted trees
We evaluated how a simpler model that may be easier to implement in routine clinical practice would compare to the full gradient-boosted decision tree model. Using the only top 15 features identified in the Vanderbilt-28wk model (Fig. 7C), we evaluated the performance of a logistic regression model to predict preterm birth. The logistic regression model was trained and evaluated on the Vanderbilt cohort using the same training and held-out set as the Vanderbilt-28wk model. It performed well, but worse (ROC-AUC = 0.70, PR-AUC = 0.30, Additional file 1: Fig. S11) than the Vanderbilt-28wk model (ROC-AUC = 0.72, PR-AUC = 0.34, Fig. 7A, B).
Model performance is similar for Black and White women but lower for Hispanic and Asian women
Given systemic biases in healthcare, it is critical to evaluate the accuracy of prediction algorithms, especially those based on EHR or genetic data, on individuals of different races and ancestries. Furthermore, preterm birth prevalence varies by race with Black women at twice the risk compared to White women [1]. We evaluated the performance of the Vanderbilt-28wk model on the held-out set after stratifying individuals by race. The model performance was very similar for Black women compared to White women (ROC-AUC 0.72 vs. 0.73; PR-AUC = 0.37 vs. 0.36). Our cohort included a substantial number of Black women, and preterm birth prevalence in the cohort was only slightly higher in Black (14%) compared to White (12%) women. (This is likely driven by Vanderbilt serving more high-risk pregnancies compared to a national baseline.) However, when evaluating the performance of Hispanic and Asian women, accuracy was substantially lower for both ROC-AUC (0.68 and 0.64, Additional file 1: Fig. S12) and PR-AUC (0.29 and 0.14, Additional file 1: Fig. S12). We suspect that the lower performance of Hispanic and Asian women may result from the smaller size and lower preterm birth prevalence in Asians.
Discussion
Preterm birth is a major health challenge that affects 5–20% of pregnancies [1, 2, 12] and leads to significant morbidity and mortality [56, 57]. Predicting preterm birth risk could inform clinical management, but no accurate classification strategies are routinely implemented [25]. Here, we take a step toward addressing this need by demonstrating the potential for machine learning on dense phenotyping from EHRs to predict preterm birth in challenging clinical contexts (e.g., spontaneous and recurrent preterm births). However, we emphasize that more work is needed before these approaches are ready for the clinic. Compared to other data types in the EHRs, models using billing codes alone had the highest prediction accuracy and outperformed those using clinical risk factors. Demonstrating the potential broad applicability of our approach, the model accuracy was similar in an external independent cohort. Combinations of many known risk factors and patterns of care drove prediction; this suggests that the algorithm builds on existing knowledge. Thus, we conclude that machine learning based on EHR data has the potential to predict preterm birth accurately across multiple healthcare systems.
Decision tree-based models are robust to correlated features can identify complex non-linear combinations and remain transparent for interpretation after training. In addition to these advantages, decision tree-based models have demonstrated strong performance in various clinical prediction tasks [58–60]. Pregnancy is a clinical context with close monitoring and well-defined endpoints that may similarly benefit from machine learning approaches, yet few studies have applied decision tree-based machine learning models to large pregnancy cohorts with rich clinical data [61].
Our approach has several distinct advantages compared to published preterm birth prediction models. First, our models have robust performance. Previous models using risk factors (diabetes, hypertension, sickle cell disease, history of preterm birth) to predict preterm birth, despite having cohorts up to two million women [23], have reported ROC-AUCs between 0.69 and 0.74 [20–22, 24]. Our models obtain a ROC-AUC of 0.75 and PR-AUC of 0.40 using data available at 28 weeks of gestation even after excluding multiple gestations. Furthermore, given the unbalanced classification problem (preterm births are less common than non-preterm), we report high PR-AUCs in addition to high ROC-AUCs. The improvement in our models is likely driven by richer longitudinal phenotypes accessible from EHRs and complex models capable of identifying non-linear patterns. These factors also likely contributed to the decision tree-based models outperforming the logistic regression model (Additional file 1: Fig. S11). A recent deep learning model trained using word embeddings from EHRs achieved a high performance (ROC-AUC = 0.83 [61]). This model was evaluated over a stratified high-risk cohort consisting of birth before 28 weeks of gestation. We did not stratify preterm births by severity since more than 85% of preterm births occur after 32 weeks of gestation [62]; however, this is an important topic for future work. Our models achieve comparable performance with the benefit of easier interpretability, which is an advantage over deep learning approaches, and we discuss this further below.
Second, our models use readily available data throughout pregnancy that do not require invasive sampling. While some studies have also obtained high ROC-AUCs (e.g., 0.81–0.88), they used serum biomarkers across small cohorts [17] or acute obstetric changes within days of delivery [16]. The potential to enable cost-effective and broad application is illustrated by our evaluation of the classifiers on EHR data from UCSF; however, substantial further work is needed to move from this proof-of-concept analysis to clinic-ready models. Furthermore, the rich characterization of the phenome provided by EHRs leveraged by our approach could also complement more invasive biochemical assays.
Third, the gradient-boosted decision trees we implement are easier to interpret than “black box” deep learning models that cannot easily identify features driving predictions. Transparency is an important, if not necessary, characteristic of machine/artificial learning models deployed in clinical practice [63, 64], and it can facilitate the discovery of insights and hypotheses to motivate future work. We reveal the patterns learned by our model by clustering deliveries using feature importance profiles. The enrichment for known risk factors (e.g., gestational hypertension, fetal abnormalities, and pre-pregnancy BMI) in clusters with high preterm birth prevalence establishes confidence in our machine learning-based prediction models. In addition, we can quantify the strength of enrichment and combination of risk factors across clusters with distinct comorbid patterns. Since preterm birth is a heterogenous phenotype [6], and stratifying pregnancies based on clinical features may be critical to uncovering the biological basis of labor [3, 65, 66], the learned rules from our model offer a possible method for subphenotyping.
Finally, our approach generalizes across hospital systems. We demonstrate that billing code-based models trained at Vanderbilt achieve similar accuracy in an independent cohort from UCSF. The generalizability of machine learning models can be constrained by the sampling of the training data. Thus, the accurate prediction in an independent dataset from an external institution points to several inherent strengths of the approach. First, successful replication indicates the models’ ability to learn predictive signals despite regional variation in assigning billing codes to an EHR. Even with different demographic distribution between the two cohorts (e.g., a greater proportion of African American and Asian women in the Vanderbilt and UCSF cohorts, respectively, Table 1), the overall model performance is very similar. Second, the large cohorts used to train and evaluate models at Vanderbilt and USCF guard against the potential weakness of EHRs, such as miscoding or omission of key data points. These errors are unavoidable in EHRs [67], but the large cohort used to train our models mitigates these errors and enables the high accuracy in the UCSF dataset, even with its different demographics. Additionally, idiosyncratic patterns of patient care at the institution used to develop the algorithm, which would be present in the Vanderbilt training and held-out sets, are unlikely to be present in the external UCSF cohort and inflate the out-of-sample accuracy. Third, the top features driving model performance are shared across institutions and reflect combinations of known risk factors and patterns of care. This aids interpretability of the underlying algorithm and likely reflects underlying pathophysiology that is innate to preterm birth.
We see several avenues for further improving our algorithm. First, some of the top features reflected routine obstetric care for high-risk pregnancies. Thus, factors that are already known to the physician or that arise from a different clinical pathway initiated by a clinician based on their assessment that a pregnancy is a high risk contribute to our prediction. This is not unique to preterm birth prediction and is a concern in any study based on longitudinal EHRs. To mitigate this effect, the learning problem could be engineered to force the algorithm to discover new unappreciated risk factors. However, we also note that prediction based on a combination of known and novel risk factors is still valuable. Second, we were surprised that the addition of features beyond billing codes, such as lab values, concepts extracted from clinical notes, and genetic information did not significantly improve performance. In some cases, any redundant information already captured by the billing codes would not improve the model’s accuracy; this is likely true for clinical notes. However, other sources, like currently available genetic data and polygenic risk scores, may not effectively capture underlying etiologies of preterm birth. Thus, these sources may not add more discriminatory power due to limitations in the current data. Indeed, the largest published genome-wide study for preterm birth only explains a very small fraction of the heritability [31], and a polygenic risk score derived from it was not predictive in our cohort. The relatively small sample size of individuals with genetic data may also limit its predictive utility in a broadly defined delivery cohort. For example, genetic risk prediction may have greater utility in certain subtypes of preterm birth (e.g., individuals with a strong family history of preterm birth). We also note that many lifestyle factors, such as smoking, alcohol consumption, diet, and physical activity, have been implicated for increasing preterm birth risk [1, 68]. Many of these data are recorded in unstructured fields in EHRs, and there are active efforts to develop accurate algorithms to extract these data from EHR [69, 70]. As these approaches become robust, including lifestyle factors may further improve preterm birth prediction. Further subphenotyping of preterm birth will not only aid in the prediction, but also understanding its multifactorial etiology and developing personalized treatment strategies. Subphenotyping by gestational age to predict preterm birth earlier during gestation, especially before 22 weeks, would provide physicians more time for therapeutic interventions. Finally, while we evaluated the ability of our classifiers to discriminate preterm births, further studies evaluating the calibration of these models are necessary to better risk stratify pregnancies.
The strong predictive performance of our models suggests that they have the potential to be clinically useful. Compared to a machine learning model trained using only known risk factors, the billing code-based classifier incorporated a broad set of clinical features and predicted preterm birth with higher accuracy. Furthermore, the superior performance was not driven by the number of risk factors or the total burden of billing codes. These results indicate the algorithm is not simply identifying less healthy individuals or those with greater healthcare usage. The models also accurately predicted many preterm births in challenging and important clinical contexts such as spontaneous and recurrent preterm birth. Spontaneous preterm births are common [1, 12, 71], and unlike iatrogenic deliveries, they are more difficult to predict because they are driven by unknown multifactorial etiologies [12, 25]. Similarly, since a prior history of preterm birth is one of the strongest risk factors [72], distinguishing pregnancies most at risk for recurrent preterm birth has the potential to provide clinical value.
However, we emphasize that additional work is needed before this approach is ready for clinical application. Though it has strong performance, a more comprehensive evaluation of the algorithm against the current clinical practice is needed to determine how early and how much improvement in the standard of care this approach could provide [73]. Furthermore, while our model performed similarly on White and Black women, the two most represented groups in the training set, the lower performance on Hispanic and Asian women highlights that future approaches must be evaluated to ensure that they do not introduce or amplify biases against specific groups or types of preterm birth [74]. In addition, as noted above, we anticipate further gains in the clinical value of this approach as more modalities of data become incorporated in the EHR [75], and more data from diverse populations become available. Addressing these questions and taking other necessary steps toward clinical utility will require the close collaboration of diverse experts from basic, clinical, social, and implementation sciences.
Conclusions
Our results provide a proof of concept that machine learning algorithms can use the dense phenotype information collected during pregnancy in EHRs to predict preterm birth. The prediction accuracy across clinical contexts and compared to existing risk factors suggests such modeling strategies can be clinically useful. We are optimistic that with the increasingly widespread adoption of EHRs, improvement in tools for extracting meaningful data from them, and integration of complementary molecular data, machine learning approaches can improve the clinical management of preterm birth.
Methods
Ascertaining delivery type and date for the Vanderbilt cohort
We identified women with at least one delivery (n = 35,282, “delivery-cohort”) at Vanderbilt Hospital based on the presence of delivery-specific billing codes, which included the International Classification of Diseases ninth and tenth editions (ICD-9, ICD-10) and Current Procedural Terminology (CPT) or estimated gestational age (EGA) documented in the EHR. Combining delivery-specific ICD-9/10 (“delivery-ICDs”), CPT (“delivery-CPTs”), and EGA data, we developed an algorithm to label each delivery as preterm or not preterm. Women with multiple gestations (e.g., twins, triplets) were identified using ICD and CPT codes and excluded for singleton-based analyses. See Additional file 1: Supplementary Materials and Methods for the exact codes considered.
We demarcate multiple deliveries by grouping delivery-ICDs in intervals of 37 weeks starting with the most recent delivery-ICD. This step is repeated until all delivery-ICDs in a patient’s EHR are assigned to a pregnancy. We chose 37-week intervals to maximally discriminate between pregnancies.
For each delivery, we assign labels (preterm, term, or postterm) ascertained using the delivery-ICDs. EGA values, extracted from structured fields across clinical notes, were mapped to multiple pregnancies using the same procedure. For women with multiple EGA recorded in their EHR, the most recent EGA value determined the time interval to group preceding EGA values. Based on the most recent EGA value for each pregnancy, we assigned labels to each delivery (EGA < 37 weeks: preterm; ≥ 37 and < 42 weeks: term, ≥ 42 weeks: postterm). After pooling the delivery labels based on delivery-ICDs and EGA, we assigned a consensus delivery label by selecting the oldest gestational age-based classification (i.e., postterm > term > preterm). By incorporating both billing code- and EGA-based delivery labels and selecting the oldest gestational classification, we expect this to increase the accuracy of this algorithm, which we evaluate by chart review (described in detail below).
Since CPT codes do not encode delivery type, we combined the delivery-CPTs with timestamps of delivery-ICDs and EGAs to approximate the date of delivery. Delivery-CPTs were grouped into multiple pregnancies as described above. The most recent timestamp from delivery-CPTs, delivery-ICDs, and EGA values was used as the approximate delivery date for a given pregnancy.
Validating delivery type based on chart review
To validate the delivery type ascertained from billing codes and EGA, we used chart-reviewed labels as the gold standard. For 104 randomly selected EHRs from the delivery cohort, we extracted the date and gestational age at delivery from clinical notes. For the earliest delivery recorded in the EHR, we assigned a chart review-based label according to the gestational age at delivery (< 37 weeks: preterm; 37 and 42 weeks: term, ≥ 42 weeks: postterm). The precision/positive predictive value (PPV) for the ascertained delivery type as a binary variable (“preterm” or “not preterm”) was calculated using the chart reviewed label as the gold standard. To compare the ascertainment strategy to a simpler phenotyping algorithm, we compared the concordance of the label derived from delivery-ICDs to one based on the gestational age within 3 days of delivery. This simpler phenotyping approach resulted in a lower positive predictive value (85%) and recall (93%; Additional file 1: Fig. S1B) compared to the billing code-based ascertainment strategy.
Training and evaluating gradient-boosted decision trees to predict preterm birth
All models for predicting preterm birth used boosted decision trees as implemented in XGBoost v0.82 [39]. Unless stated otherwise, we trained models to predict the earliest delivery in a woman’s EHR as preterm or not preterm. The delivery cohort was randomly split into training (80%) and held-out (20%) sets with an equal proportion of preterm cases. For prediction tasks, we used only ICD-9 and excluded ICD-10 codes to avoid potential confounding effects. The total count of billing codes within a specified time frame was used as features to train our models; if a woman never had a billing code in her EHR, we encoded these as “0.” For all models, we excluded ICD-9, CPT codes, and EGA used to ascertain delivery type and date. On the training set, we use the tree of Parzen estimators as implemented in hyperopt v0.1.1 [76] to optimize hyperparameters by maximizing the mean average precision. The best set of hyperparameters was selected after 1000 trials using 3-fold cross-validation over the training set (80:20 split with an equal proportion of preterm cases). We evaluated the performance of all models on the held-out set using Scikit-learn v0.20.2 [77]. All performance metrics reported are on the held-out set. For precision-recall curves, we define the baseline chance performance for each model as the prevalence of preterm cases. To ensure no data leaks were present in our training protocol, we trained and evaluated a model using a randomly generated dataset (n = 1000 samples) with a 22% preterm prevalence. As expected, this model did not do better than chance (ROC-AUC = 0.50, PR-AUC = 0.22, data not shown). All trained models with their optimized hyperparameters are provided at https://github.com/abraham-abin13/ptb_predict_ml.
Predicting preterm birth at different weeks of gestation
As the first step, we evaluated whether billing codes could discriminate between delivery types. Models were trained to predict preterm birth using the total counts of each ICD-9, CPT, or ICD-9 and CPT code across a woman’s EHR. We excluded any codes used to ascertain the delivery type or date. All three models were trained and evaluated on the same cohort of women who had at least one ICD-9 and CPT code (Additional file 1: Fig. S2).
Next, we evaluated the machine learning models at 0, 13, 28, and 35 weeks of gestation by training using only features present before each time point. For the subset of women in our delivery cohort with EGA, we calculated the date of conception by subtracting EGA (recorded within 3 days of delivery) from the date of delivery. Next, we trained the models using ICD-9 and CPT codes time-stamped before different gestational time points with only singleton (Fig. 2B) or including multiple gestations (Additional file 1: Fig. S3). The same cohort of women was used to train and evaluate across models. The sample size varied slightly (n = 11,843 to 10,799) since women who already delivered were excluded at each time point.
In addition to evaluating the models based on the date of conception, we trained the models at different time points before the date of delivery (Additional file 1: Fig. S4) using the same cohort of women by requiring every individual in this cohort to have at least one ICD-9 or CPT code before each time point. Evaluating the models before the date of delivery increased the sample size (n = 15,481) compared to a prospective conception-based design (n = 12,410) and yielded similar results.
Evaluating the predictive potential of demographic, clinical, and genetic features from EHRs
In addition to billing codes, we extracted the structured and unstructured features from the EHRs (Fig. 3A). We evaluated the models using features present before 28 weeks of gestation (Fig. 3) and features present before or after delivery (Additional file 1: Fig. S6). Structured data included self or third-party reported race (Fig. 1E), age at delivery, past medical and family history (92 features, see Additional file 1: Supplementary Materials and Methods), and clinical labs. For training models, we only included clinical labs obtained during the first pregnancy and excluded values greater than four standard deviations from the mean. To capture the trajectory of each clinical lab’s values across pregnancy (307 clinical labs, see Additional file 1: Supplementary Materials and Methods), we trained the models using the mean, median, minimum, and maximum lab measurements. For unstructured clinical text in obstetric and nursing clinical notes, we applied CLAMP [78] to extract Unified Medical Language System (UMLS) concept unique identifiers (CUIs) and included those with positive assertions with > 0.5% frequency across all EHRs. When training preterm birth prediction models, we one-hot encoded the categorical features. No transformations were applied to the continuous features.
A subset of women (n = 905) was genotyped on the Illumina MEGAEX platform. We applied standard genome-wide association study (GWAS) quality control steps [79] using PLINK v1.90b4s [80]. We calculated a polygenic risk score for each White woman with genotype data based on the largest available preterm birth GWAS [31] using PRSice-2 [81, 82]. We assumed an additive model and summed the number of risk alleles at single nucleotide polymorphisms (SNPs) weighted by their strength of association with preterm birth (effect size). PRSice determined the optimum number of SNPs by testing the polygenic risk score for association with preterm birth in our delivery-cohort at different GWAS p-value thresholds. We included the date of birth and five genetic principal components to control for ancestry. Our final polygenic risk score used 356 preterm birth-associated SNPs (GWAS p-value < 0.00025).
Using the structured and unstructured data derived from the EHR, we evaluated whether adding EHR features to billing codes could improve preterm birth prediction. Since the number of women varied across EHR feature, we created subsets of the delivery cohort for each EHR feature. Each subset included women with at least one recorded value for the EHR feature and billing codes. Then, we trained three models as described above for each subset: (1) using only the EHR feature being evaluated, (2) using ICD-9 and CPT codes, and (3) using the EHR feature with ICD-9 and CPT codes. Thus, all three models for a given EHR feature were trained and evaluated on the same cohort of deliveries (Fig. 3A).
Predicting preterm birth using billing codes and clinical risk factors at 28 weeks of gestation
We compared the performance of a model trained using billing codes (ICD-9 and CPT) present before 28 weeks of gestation with a model trained using clinical risk factors to predict preterm delivery (Fig. 4). Both models were trained and evaluated on the same cohort of women (n = 21,099). We selected well-established obstetric risk factors that included maternal and fetal factors across organ systems, occurred before and during pregnancy, and had moderate to high risk for preterm birth [3, 13, 23, 44]. For each individual, risk factors were encoded as high-risk or low-risk binary values. Risk factors such as non-gestational diabetes status [48], gestational diabetes [48], gestational hypertension, pre-eclampsia or eclampsia [1, 50], fetal abnormalities [13], cervical abnormalities [51], and sickle cell disease [49] status were defined based on at least one corresponding ICD-9 code occurring before the date of delivery (Additional file 1: Supplementary Materials and Methods). The remaining factors, such as race (Black, Asian, or Hispanic was encoded as higher risk) [20], age at delivery (> 34 or < 18 years old) [45–47], pre-pregnancy BMI ≥ 35, and pre-pregnancy hypertension (> 120/80) [1, 50], were extracted from structured fields in EHR. Pre-pregnancy value was defined as the most recent measurement occurring before 9 months of the delivery date.
Density-based clustering on feature importance values
To better understand the decision-making process of our machine learning models, we calculated the feature importance value for the model predicting preterm birth at 28 weeks of gestation. We used SHapley Additive exPlanation values (SHAP) [52, 53, 55] to determine the marginal additive contribution of each feature for each individual. First, we calculated a matrix of SHAP values of features by individuals from the held-out cohort. Since the shape of this matrix was too large to perform the density-based clustering, we created an embedding using 30 Uniform Manifold Approximation and Projection (UMAP) components with default parameters as implemented in UMAPv0.3.8 [83]. Next, we performed a density-based hierarchical clustering using HDBSCANv0.8.26 [84]. We used default parameters (metric=Euclidean) and tried a range of values for two hyperparameters: minimum number of individuals in each cluster (“min_clust_size”) and threshold for determining outlier individuals who do not belong to a cluster (“min_samples”). After tuning these two hyperparameters, we selected the clustering model with the highest density-based cluster validity score [84], which measures the within- and between-cluster density connectedness. We find a min_clust_size = 110 and min_samples = 10 had the highest density-based cluster validity (DBCV) score with 6 distinct clusters with one cluster for outliers (Additional file 1: Fig. S13). A minority of women (n = 16) were not assigned to a cluster (“outliers”). To visualize the cluster assignments, we performed UMAP on the feature importance matrix with default settings and two UMAP components and colored each individual by their cluster membership. Finally, we calculated the preterm birth prevalence and accuracy within each cluster.
Comorbidity enrichment within clusters
We tested for enrichment of clinical risk factors within each cluster by using Fisher’s exact test as implemented in Scipy [85]. For each risk factor, we constructed a contingency table based on a given cluster membership and being high risk for the risk factor. We report enrichment as the odds ratio with the color bar showing the log10 scale of the odds ratio. For sickle cell disease, one cluster did not have any cases of sickle cell disease.
Evaluating model performance on spontaneous preterm births, by delivery type and recurrent preterm birth
We compared how models trained used billing codes (ICD-9 and CPT) performed in different clinical contexts. First, we evaluated the accuracy of predicting spontaneous preterm birth using models trained to predict all types of preterm births. From all preterm cases in the held-out set, we excluded women who met any of the following criteria to create a cohort of spontaneous preterm births: medically induced labor, delivery by cesarean section, or preterm premature rupture of membranes. The ICD-9 and CPT codes used to identify the exclusion criteria are provided in Additional file 1: Supplementary Materials and Methods. We calculated recall/sensitivity as the number of predicted spontaneous preterm births out of all spontaneous preterm births in the held-out set. We used the same approach to quantify the performance of models trained using clinical risk factors (Fig. 4E).
We trained the models to predict preterm birth among cesarean sections and vaginal deliveries separately using billing codes (ICD-9 and CPT) as features. Deliveries were labeled as cesarean sections or vaginal deliveries if they had at least one relevant billing code (ICD-9 or CPT) occurring within 10 days of the date of first delivery in the EHR. Billing codes used to determine the delivery type are provided in Additional file 1: Supplementary Materials and Methods. Deliveries with billing codes for both cesarean and vaginal deliveries were excluded. We trained separate models to predict cesarean and vaginal deliveries (Fig. 6A and Additional file 1: Fig. S8).
We evaluated how well models using billing codes could predict recurrent preterm birth. From our delivery cohort, we retained women whose first delivery in the EHR was preterm and a second delivery for which we ascertained the type (preterm vs. not preterm) as described above for the first delivery. We trained models using billing codes (ICD-9 and CPT) at time points before the date of delivery because the majority of this cohort did not have reliable EGA at the second delivery. As described earlier, separate models were trained using billing codes timestamped before the time point being evaluated (Fig. 6B, Additional file 1: Fig. S9).
Preterm birth prediction in independent UCSF cohort
We evaluated how well models trained at Vanderbilt using billing codes perform in an external cohort assembled at UCSF. Only the first delivery in the EHR was used for prediction. Women with twins or multiple gestations, identified using billing codes (Additional file 1: Supplementary Materials and Methods), were excluded. Delivery type (preterm vs. not preterm) was assigned based on the presence of ICD-10 codes. Term (or not preterm) deliveries were determined by the presence of an ICD-10 code beginning with the character “O80,” specifying an encounter for full-term delivery. Preterm deliveries were determined by both the absence of ICD-10 codes beginning with “O80” and the presence of codes beginning with “O60.1,” the family of codes for preterm labor with preterm delivery. We trained models using ICD-9 codes present before 28 weeks of gestation on the Vanderbilt cohort to predict preterm birth. We refer to this model as the “Vanderbilt-28wk model” throughout the manuscript. CPT codes were not used since they were not available from the UCSF EHR system. The Vanderbilt-28wk model was evaluated on the Vanderbilt held-out set and the independent UCSF cohort.
Feature interpretation from boosted decision tree models
To determine the feature importance, we used SHAP values [52, 53, 55] to determine the marginal additive contribution of each feature for the Vanderbilt-28wk model. For the held-out Vanderbilt cohort and the UCSF cohort, a SHAP value was calculated for each feature per individual. Feature importance was summarized by taking the mean of the absolute value of SHAP scores across individuals, and the top fifteen features based on the mean absolute SHAP value in either the Vanderbilt or UCSF cohorts are reported. To compare how feature importance differed between Vanderbilt and UCSF, we computed the Pearson correlation of the mean absolute SHAP values.
Training and evaluating a logistic regression model using only top features
Using only the top 15 features obtained from our Vanderbilt-28wk model as predictors, we trained a logistic regression using Scikit-learn v0.20.2 [67] with the following parameters: random_state=0, max_iter=10000,solver=‘liblinear’, class_weight=‘balanced’. The model was trained using the same training set from the Vanderbilt cohort (i.e., ICD-9 codes present before 28 weeks) used for comparing to the UCSF dataset. Performance was evaluated also on the same held-out set from the Vanderbilt cohort using ROC-AUC and PR-AUC.
Comparing model performance after stratifying by race
For the Vanderbilt-28wk model, we evaluated model performance on the Vanderbilt held-out set stratified by race. We excluded individuals (n = 284) from this analysis if their race was annotated as “other” or had multiple categories because their subset counts were low (n < 143), therefore more likely to have sampling variability. Stratifying the held-out set by race resulted in four categories (White, Black, Hispanic, Asian). Next, we evaluated the model performance on each subset and report the ROC-AUC and PR-AUC with the preterm birth prevalence within each subset.
Supplementary Information
Additional file 1. Supplementary figures, tables, and methods.
Acknowledgements
We thank the members of the Capra Lab, members of the Vanderbilt MSTP, and the March of Dimes Prematurity Research Center Ohio Collaborative for the thoughtful discussion on this project.
Abbreviations
- AUC
Area under the curve
- CPT
Current Procedural Terminology
- CUI
Concepts unique identifier
- EGA
Estimated gestational age
- EHR
Electronic health record
- GWAS
Genome-wide association study
- ICD-10
International Classification of Diseases tenth edition
- ICD-9
International Classification of Diseases ninth edition
- PPV
Positive predictive value
- PR-AUC
Precision-recall-area under the curve
- PTB
Preterm birth
- ROC-AUC
Receiver operation curve-area under the curve
- SHAP
SHapley Additive exPlanation
- SNP
Single nucleotide polymorphisms
- UCSF
University of California, San Francisco
- UMAP
Uniform Manifold Approximation and Projection
- UMLS
Unified Medical Language System
Authors’ contributions
Conceptualization and methodology: A.A. and J.A.C. conceived and designed the study. J.M.N provided clinical interpretation and aided in the feature selection. Data curation: A.A. and C.A.B. extracted the billing codes and clinical notes and performed the concept extraction on the Vanderbilt cohort. A.A., P.S., and L.K.D. extracted, cleaned, and provided clinical laboratory data during pregnancy on the Vanderbilt cohort. Resources: D.R.V. provided obstetric and nursing notes on the Vanderbilt cohort. B.L., I.K., and M.S. extracted the delivery cohort from UCSF. Formal analysis and investigation: A.A. performed all the analyses on the Vanderbilt cohort under supervision from J.A.C. B.L. and I.K. evaluated the models on UCSF cohorts under supervision from M.S. Funding acquisition: J.A.C. Writing: A.A. wrote the manuscript with guidance from J.A.C., J.M.N., M.S., L.M., and A.R. The authors read and approved the final manuscript.
Funding
AA was supported by the American Heart Association fellowship 20PRE35080073, National Institutes of Health (NIH, T32GM007347), the March of Dimes, and the Burroughs Wellcome Fund. MS, IK, and BL were supported by the March of Dimes and NIH (NLM K01LM012381). JAC was supported by the NIH (R35GM127087), NIH (1R01HD101669), March of Dimes, and the Burroughs Wellcome Fund. This work was conducted in part using the resources of the Advanced Computing Center for Research and Education at Vanderbilt University. The datasets used for the analyses described were obtained from Vanderbilt University Medical Center’s BioVU which is supported by numerous sources: institutional funding, private agencies, and federal grants. These include the NIH-funded Shared Instrumentation Grant S10RR025141 and CTSA grants UL1TR002243, UL1TR000445, and UL1RR024975. Genomic data are also supported by investigator-led projects that include U01HG004798, R01NS032830, RC2GM092618, P50GM115305, U01HG006378, U19HL065962, and R01HD074711 and additional funding sources listed at https://victr.vumc.org/biovu-funding/. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the March of Dimes, or the Burroughs Wellcome Fund.
Availability of data and materials
The machine learning models and analysis code supporting the conclusions of this article are available in the https://github.com/abraham-abin13/ptb_predict_ml repository. Individual-level data is not provided due to privacy concerns and can be applied for through Vanderbilt University’s BioVU program. No public datasets were used in this manuscript.
Declarations
Ethics approval and consent to participate
This study exclusively utilized the information extracted from the medical records in the Vanderbilt University Medical Center (VUMC) “Synthetic Derivative” database (SD). The SD is a de-identified copy of the main hospital medical record databases created for research purposes. The de-identification of SD records was achieved primarily through the application of a commercial electronic program, which was applied and assessed for acceptable effectiveness in scrubbing identifiers. For instance, if the name “John Smith” appeared in the original medical record, its corresponding record in the SD does not contain “John Smith.” Instead, it is permanently replaced with a tag [NAMEAAA, BBB] to maintain the semantic integrity of the text. Similarly, dates, such as “January 1, 2004,” have been replaced with a randomly generated date, such as “February 3, 2003.”
The SD database (which contains over 3 million electronic records, with no defined exclusions) was accessed through database queries. Searches are logged and audited annually. As no HIPAA identifiers are available in the SD database, and this work does not plan to re-identify these records using the identified VUMC database, this study meets the criteria for non-human subjects research. Nonetheless, to ensure confidentiality and appropriate use of the SD, all relevant key personnel for this study entered a data use agreement, which prohibits any use of the data not described in this application, including the re-identification of the SD records.
Consent for publication
Not applicable.
Competing interests
LJM is a consultant for Mirvie, Inc. All other authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet Lond Engl. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller A-B, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet Lond Engl. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 3.Barros FC, Papageorghiou AT, Victora CG, Noble JA, Pang R, Iams J, et al. The distribution of clinical phenotypes of preterm birth syndrome. JAMA Pediatr. 2015;169:220–210. doi: 10.1001/jamapediatrics.2014.3040. [DOI] [PubMed] [Google Scholar]
- 4.Callaghan WM, MacDorman MF, Rasmussen SA, Qin C, Lackritz EM. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics. 2006;118:1566–1573. doi: 10.1542/peds.2006-0860. [DOI] [PubMed] [Google Scholar]
- 5.Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388:3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iams J, Goldenberg R, Meis P, Mercer B, Moawad A, Das A, et al. The length of the cervix and the risk of spontaneous premature delivery. New Engl J Med. 1996;334:567–573. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs F, Monet B, Ducruet T, Chaillet N, Audibert F. Effect of maternal age on the risk of preterm birth: a large cohort study. PLoS One. 2018;13:e0191002. doi: 10.1371/journal.pone.0191002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mercer BM, Goldenberg RL, Moawad AH, Meis PJ, Iams JD, Das AF, et al. The preterm prediction study: effect of gestational age and cause of preterm birth on subsequent obstetric outcome. Am J Obstet Gynecol. 1999;181:1216–1221. doi: 10.1016/S0002-9378(99)70111-0. [DOI] [PubMed] [Google Scholar]
- 10.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Pineles BL, Gotsch F, et al. Recurrent preterm birth. Semin Perinatol. 2007;31:142–158. doi: 10.1053/j.semperi.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ananth CV, Kirby RS, Vintzileos AM. Recurrence of preterm birth in twin pregnancies in the presence of a prior singleton preterm birth. J Maternal Fetal Neonatal Med. 2008;21:289–295. doi: 10.1080/14767050802010206. [DOI] [PubMed] [Google Scholar]
- 12.Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010;362:529–535. doi: 10.1056/NEJMra0904308. [DOI] [PubMed] [Google Scholar]
- 13.Auger N, Le TUN, Park AL, Luo Z-C. Association between maternal comorbidity and preterm birth by severity and clinical subtype: retrospective cohort study. BMC Pregnancy Childbirth. 2011;11:75. doi: 10.1186/1471-2393-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter M, Fowler S, Holden A, Xenakis E, Dudley D. The late preterm birth rate and its association with comorbidities in a population-based study. Am J Perinatol. 2011;28:703–708. doi: 10.1055/s-0031-1280592. [DOI] [PubMed] [Google Scholar]
- 15.Francesca L, Laura M, Giuseppe R, Francesco DA, Ersilia B, Leonardo P, et al. Biomarkers for predicting spontaneous preterm birth: an umbrella systematic review. J Matern Fetal Neonatal Med. 2019;0:726–734. doi: 10.1080/14767058.2017.1297404. [DOI] [PubMed] [Google Scholar]
- 16.Dabi Y, Nedellec S, Bonneau C, Trouchard B, Rouzier R, Benachi A. Clinical validation of a model predicting the risk of preterm delivery. PLoS One. 2017;12:e0171801. doi: 10.1371/journal.pone.0171801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ngo TTM, Moufarrej MN, Rasmussen M-LH, Camunas-Soler J, Pan W, Okamoto J, et al. Noninvasive blood tests for fetal development predict gestational age and preterm delivery. Science. 2018;360:1133–1136. doi: 10.1126/science.aar3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarca AL, Pataki BÁ, Romero R, Sirota M, Guan Y, Kutum R, et al. Crowdsourcing assessment of maternal blood multi-omics for predicting gestational age and preterm birth. Cell Rep Med. 2021;2:100323. doi: 10.1016/j.xcrm.2021.100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stelzer IA, Ghaemi MS, Han X, Ando K, Hédou JJ, Feyaerts D, et al. Integrated trajectories of the maternal metabolome, proteome, and immunome predict labor onset. Sci Transl Med. 2021;13:eabd9898. doi: 10.1126/scitranslmed.abd9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaaf JM, Ravelli ACJ, Mol BWJ, Abu-Hanna A. Development of a prognostic model for predicting spontaneous singleton preterm birth. Eur J Obstet Gynecol Reprod Biol. 2012;164:150–155. doi: 10.1016/j.ejogrb.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Morken NH, Källen K, Jacobsson B. Predicting risk of spontaneous preterm delivery in women with a singleton pregnancy. Paediatr Perinat Epidemiol. 2014;28:11–22. doi: 10.1111/ppe.12087. [DOI] [PubMed] [Google Scholar]
- 22.Weber A, Darmstadt GL, Gruber S, Foeller ME, Carmichael SL, Stevenson DK, et al. Application of machine-learning to predict early spontaneous preterm birth among nulliparous non-Hispanic black and white women. Ann Epidemiol. 2018;28:783–789.e1. doi: 10.1016/j.annepidem.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Baer RJ, McLemore MR, Adler N, Oltman SP, Chambers BD, Kuppermann M, et al. Pre-pregnancy or first-trimester risk scoring to identify women at high risk of preterm birth. Eur J Obstet Gynecol. 2018;231:235–240. doi: 10.1016/j.ejogrb.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tucker CM, Berrien K, Menard MK, Herring AH, Daniels J, Rowley DL, et al. Predicting preterm birth among women screened by North Carolina’s pregnancy medical home program. Matern Child Health J. 2015;19:2438–2452. doi: 10.1007/s10995-015-1763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suff N, Story L, Shennan A. The prediction of preterm delivery: what is new? Semin Fetal Neonat M. 2018;24:27–32. doi: 10.1016/j.siny.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Abul-Husn NS, Kenny EE. Personalized medicine and the power of electronic health records. Cell. 2019;177:58–69. doi: 10.1016/j.cell.2019.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paquette AG, Hood L, Price ND, Sadovsky Y. Deep phenotyping during pregnancy for predictive and preventive medicine. Sci Transl Med. 2020;12:eaay1059. doi: 10.1126/scitranslmed.aay1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Artzi NS, Shilo S, Hadar E, Rossman H, Barbash-Hazan S, Ben-Haroush A, et al. Prediction of gestational diabetes based on nationwide electronic health records. Nat Med. 2020;26:71–76. doi: 10.1038/s41591-019-0724-8. [DOI] [PubMed] [Google Scholar]
- 29.Ravizza S, Huschto T, Adamov A, Böhm L, Büsser A, Flöther FF, et al. Predicting the early risk of chronic kidney disease in patients with diabetes using real-world data. Nat Med. 2019;25:57–59. doi: 10.1038/s41591-018-0239-8. [DOI] [PubMed] [Google Scholar]
- 30.Li R, Chen Y, Ritchie MD, Moore JH. Electronic health records and polygenic risk scores for predicting disease risk. Nat Publ Group. 2020;31:1–10. doi: 10.1038/s41576-020-0224-1. [DOI] [PubMed] [Google Scholar]
- 31.Zhang G, Feenstra B, Bacelis J, Liu X, Muglia LM, Juodakis J, et al. Genetic associations with gestational duration and spontaneous preterm birth. New Engl J Med. 2017;377:1156–1167. doi: 10.1056/NEJMoa1612665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomašev N, Glorot X, Rae JW, Zielinski M, Askham H, Saraiva A, et al. A clinically applicable approach to continuous prediction of future acute kidney injury. Nature. 2019;572:116–119. doi: 10.1038/s41586-019-1390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao C, Choi E, Sun J. Opportunities and challenges in developing deep learning models using electronic health records data: a systematic review. J Am Med Inform Assn. 2018;25:1419–1428. doi: 10.1093/jamia/ocy068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao J, Feng Q, Wu P, Lupu RA, Wilke RA, Wells QS, et al. Learning from longitudinal data in electronic health record and genetic data to improve cardiovascular event prediction. Sci Rep. 2019;9:1–10. doi: 10.1038/s41598-018-37186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldstein BA, Navar AM, Pencina MJ, Ioannidis JPA. Opportunities and challenges in developing risk prediction models with electronic health records data: a systematic review. J Am Med Inform Assoc. 2017;24:198–208. doi: 10.1093/jamia/ocw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aung MT, Yu Y, Ferguson KK, Cantonwine DE, Zeng L, McElrath TF, et al. Prediction and associations of preterm birth and its subtypes with eicosanoid enzymatic pathways and inflammatory markers. Sci Rep. 2019;9:17049. doi: 10.1038/s41598-019-53448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rittenhouse KJ, Vwalika B, Keil A, Winston J, Stoner M, Price JT, et al. Improving preterm newborn identification in low-resource settings with machine learning. PLoS One. 2019;14:e0198919. doi: 10.1371/journal.pone.0198919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fergus P, Cheung P, Hussain A, Al-Jumeily D, Dobbins C, Iram S. Prediction of preterm deliveries from EHG signals using machine learning. PLoS One. 2013;8:e77154. doi: 10.1371/journal.pone.0077154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen T, Guestrin C. Xgboost: A scalable tree boosting system. In: Proceedings of the 22nd ACM SIGKDD International conference on knowledge discovery and data mining. 2016. p. 785–94.
- 40.Hastie T, Tibshirani R, Friedman J. The elements of statistical learning, data mining, inference, and prediction. 2009. [Google Scholar]
- 41.Corey KM, Kashyap S, Lorenzi E, Lagoo-Deenadayalan SA, Heller K, Whalen K, et al. Development and validation of machine learning models to identify high-risk surgical patients using automatically curated electronic health record data (Pythia): a retrospective, single-site study. PLoS Med. 2018;15:e1002701. doi: 10.1371/journal.pmed.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jing L, Cerna AEU, Good CW, Sauers NM, Schneider G, Hartzel DN, et al. A machine learning approach to management of heart failure populations. Jacc Hear Fail. 2020;8:578–587. doi: 10.1016/j.jchf.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 43.Carter J, Seed PT, Watson HA, David AL, Sandall J, Shennan AH, et al. Development and validation of predictive models for QUiPP App v.2: tool for predicting preterm birth in women with symptoms of threatened preterm labor. Ultrasound Obstet Gynecol. 2020;55:357–367. doi: 10.1002/uog.20422. [DOI] [PubMed] [Google Scholar]
- 44.Vogel JP, Chawanpaiboon S, Moller A-B, Watananirun K, Bonet M, Lumbiganon P. The global epidemiology of preterm birth. Best Pract Res Cl Ob. 2018;52:3–12. doi: 10.1016/j.bpobgyn.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Smith GCS, Pell JP. Teenage pregnancy and risk of adverse perinatal outcomes associated with first and second births: population based retrospective cohort study. Obstet Gynecol Surv. 2002;57:136–137. doi: 10.1097/00006254-200203000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waldenström U, Aasheim V, Nilsen ABV, Rasmussen S, Pettersson HJ, Schytt E, et al. Adverse pregnancy outcomes related to advanced maternal age compared with smoking and being overweight. Obstet Gynecol. 2014;123:104–112. doi: 10.1097/AOG.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 47.Carolan M. Maternal age ≥45 years and maternal and perinatal outcomes: a review of the evidence. Midwifery. 2013;29:479–489. doi: 10.1016/j.midw.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Ray JG, Vermeulen MJ, Shapiro JL, Kenshole AB. Maternal and neonatal outcomes in pregestational and gestational diabetes mellitus, and the influence of maternal obesity and weight gain: the DEPOSIT study. Qjm Int J Med. 2001;94:347–356. doi: 10.1093/qjmed/94.7.347. [DOI] [PubMed] [Google Scholar]
- 49.Whiteman V, Salinas A, Weldeselasse HE, August EM, Mbah AK, Aliyu MH, et al. Impact of sickle cell disease and thalassemias in infants on birth outcomes. Eur J Obstet Gyn R B. 2013;170:324–328. doi: 10.1016/j.ejogrb.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 50.Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertens Res. 2017;40:213–220. doi: 10.1038/hr.2016.126. [DOI] [PubMed] [Google Scholar]
- 51.Koullali B, Oudijk MA, Nijman TAJ, Mol BWJ, Pajkrt E. Risk assessment and management to prevent preterm birth. Semin Fetal Neonatal Med. 2016;21:80–88. doi: 10.1016/j.siny.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Lundberg SM, Lee S-I, et al. A unified approach to interpreting model predictions. In: Guyon I, Luxburg UV, Bengio S, Wallach H, Fergus R, Vishwanathan S, et al., editors. Advances in Neural Information Processing Systems 30. Curran Associates, Inc.; 2017. pp. 4765–4774. [Google Scholar]
- 53.Lundberg SM, Erion G, Chen H, DeGrave A, Prutkin JM, Nair B, et al. From local explanations to global understanding with explainable AI for trees. Nat Mach Intell. 2020;2:56–67. doi: 10.1038/s42256-019-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davis J, Goadrich M. The relationship between Precision-Recall and ROC curves. In: Proceedings of the 23rd International Conference on Machine learning. 2006. p. 233–24 .
- 55.Lundberg SM, Nair B, Vavilala MS, Horibe M, Eisses MJ, Adams T, et al. Explainable machine-learning predictions for the prevention of hypoxaemia during surgery. Nat Biomed Eng. 2018;2:749–760. doi: 10.1038/s41551-018-0304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006–2010. Obstet Gynecol. 2015;125:5–12. doi: 10.1097/AOG.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 57.Hirshberg A, Srinivas SK. Epidemiology of maternal morbidity and mortality. Semin Perinatol. 2017;41:332–337. doi: 10.1053/j.semperi.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 58.Kopitar L, Kocbek P, Cilar L, Sheikh A, Stiglic G. Early detection of type 2 diabetes mellitus using machine learning-based prediction models. Sci Rep-Uk. 2020;10:11981. doi: 10.1038/s41598-020-68771-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan L, Zhang H-T, Goncalves J, Xiao Y, Wang M, Guo Y, et al. An interpretable mortality prediction model for COVID-19 patients. Nat Mach Intell. 2020;2:283–288. doi: 10.1038/s42256-020-0180-7. [DOI] [Google Scholar]
- 60.Couronné R, Probst P, Boulesteix A-L. Random forest versus logistic regression: a large-scale benchmark experiment. Bmc Bioinformatics. 2018;19:270. doi: 10.1186/s12859-018-2264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao C, Osmundson S, Edwards DRV, Jackson GP, Malin BA, Chen Y. Deep learning predicts extreme preterm birth from electronic health records. J Biomed Inform. 2019;100:103334. doi: 10.1016/j.jbi.2019.103334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Torchin H, Ancel P-Y. Epidemiology and risk factors of preterm birth. J De Gynecol Obstetrique Et Biologie De La Reprod. 2016;45:1213–1230. doi: 10.1016/j.jgyn.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 63.Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25:44–56. doi: 10.1038/s41591-018-0300-7. [DOI] [PubMed] [Google Scholar]
- 64.He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med. 2019;25:30–36. doi: 10.1038/s41591-018-0307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Esplin MS. The importance of clinical phenotype in understanding and preventing spontaneous preterm birth. Am J Perinatol. 2016;33:236–244. doi: 10.1055/s-0035-1571146. [DOI] [PubMed] [Google Scholar]
- 66.Manuck TA, Esplin MS, Biggio J, Bukowski R, Parry S, Zhang H, et al. The phenotype of spontaneous preterm birth: application of a clinical phenotyping tool. Am J Obstet Gynecol. 2015;212:487.e1–487.e11. doi: 10.1016/j.ajog.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Phelan M, Bhavsar NA, Goldstein BA. Illustrating informed presence bias in electronic health records data: how patient interactions with a health system can impact inference. Egems Wash Dc. 2017;5:22. doi: 10.5334/egems.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Outcomes I of M (US) C on UPB and AH, Behrman RE, Butler AS. Preterm birth: causes, consequences, and prevention. 2007. 10.17226/11622. [PubMed]
- 69.Kukhareva PV, Caverly TJ, Li H, Katki HA, Cheung LC, Reese TJ, et al. Inaccuracies in electronic health records smoking data and a potential approach to address resulting underestimation in determining lung cancer screening eligibility. J Am Med Inform Assoc. 2022. 10.1093/jamia/ocac020. [DOI] [PMC free article] [PubMed]
- 70.Garies S, Cummings M, Quan H, McBrien K, Drummond N, Manca D, et al. Methods to improve the quality of smoking records in a primary care EMR database: exploring multiple imputation and pattern-matching algorithms. Bmc Med Inform Decis. 2020;20:56. doi: 10.1186/s12911-020-1068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moutquin J-M. Classification and heterogeneity of preterm birth. BJOG. 2003;110:30–33. doi: 10.1016/S1470-0328(03)00021-1. [DOI] [PubMed] [Google Scholar]
- 72.Phillips C, Velji Z, Hanly C, Metcalfe A. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ Open. 2017;7:e015402. doi: 10.1136/bmjopen-2016-015402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shah NH, Milstein A, Bagley SC. Making machine learning models clinically useful. JAMA. 2019;322:1351–1352. doi: 10.1001/jama.2019.10306. [DOI] [PubMed] [Google Scholar]
- 74.Gianfrancesco MA, Tamang S, Yazdany J, Schmajuk G. Potential biases in machine learning algorithms using electronic health record data. JAMA Intern Med. 2018;178:1544. doi: 10.1001/jamainternmed.2018.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weng C, Shah N, Hripcsak G. Deep phenotyping: embracing complexity and temporality—towards scalability, portability, and interoperability. J Biomed Inform. 2020;105:103433. doi: 10.1016/j.jbi.2020.103433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bergstra J, Yamins D, Cox D. International conference on machine learning. 2013. Making a science of model search: hyperparameter optimization in hundreds of dimensions for vision architectures; pp. 115–123. [Google Scholar]
- 77.Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al. Scikit-learn: machine learning in Python. J Mach Learn Res. 2011;12:2825–2830. [Google Scholar]
- 78.Soysal E, Wang J, Jiang M, Wu Y, Pakhomov S, Liu H, et al. CLAMP – a toolkit for efficiently building customized clinical natural language processing pipelines. J Am Med Inform Assn. 2017;25:331–336. doi: 10.1093/jamia/ocx132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marees AT, de Kluiver H, Stringer S, Vorspan F, Curis E, Marie‐Claire C, et al. A tutorial on conducting genome‐wide association studies: Quality control and statistical analysis. Int J Methods Psychiatr Res. 2018;27(2):e1608. [DOI] [PMC free article] [PubMed]
- 80.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Euesden J, Lewis CM, O’Reilly PF. PRSice: polygenic risk score software. Bioinformatics. 2015;31:1466–1468. doi: 10.1093/bioinformatics/btu848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Choi SW, O’Reilly PF. PRSice-2: polygenic risk score software for biobank-scale data. Gigascience. 2019:giz082. [DOI] [PMC free article] [PubMed]
- 83.McInnes L, Healy J, Melville J. UMAP: Uniform manifold approximation and projection for dimension reduction. arXiv preprint. 2018;arXiv:1802.03426.
- 84.McInnes L, Healy J, Astels S. hdbscan: Hierarchical density based clustering. J Open Source Softw. 2017;2(11):205.
- 85.Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods. 2020;17:261–272. doi: 10.1038/s41592-019-0686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary figures, tables, and methods.
Data Availability Statement
The machine learning models and analysis code supporting the conclusions of this article are available in the https://github.com/abraham-abin13/ptb_predict_ml repository. Individual-level data is not provided due to privacy concerns and can be applied for through Vanderbilt University’s BioVU program. No public datasets were used in this manuscript.