Abstract
Background
There are limited data on noninvasive methods to identify hepatic steatosis in coexisting hepatitis B virus (HBV) infection.
Aims
To evaluate the diagnostic performance of noninvasive serum-based scores to detect steatosis using two distinct chronic HBV cohorts with liver histology evaluation.
Methods
Chronic HBV cohorts with untreated HBV mono-infection (N = 302) and with treated HBV–HIV (N = 92) were included. Liver histology was scored centrally. Four serum-based scores were calculated: hepatic steatosis index (HSI), non-alcoholic fatty liver disease Liver Fat Score (NAFLD-LFS), visceral adiposity index (VAI), and triglyceride glucose (TyG) index. Optimal cutoffs (highest sensitivity + specificity) to detect ≥ 5% HS, stratified by cohort, were evaluated.
Results
HBV–HIV (vs. HBV mono-infected) patients were older (median 50 vs. 43 years), and a higher proportion were male (92% vs. 60%), were black (51% vs. 8%), had the metabolic syndrome (41% vs. 25%), and suppressed HBV DNA (< 1000 IU/mL; 82% vs. 9%). Applying optimal cutoffs, the area under the receiver operator curve for detecting ≥ 5% steatosis in HBV-only and HBV–HIV, respectively, was 0.69 and 0.61 for HSI, 0.70 and 0.76 for NAFLD-LFS, 0.68 and 0.64 for TyG, and 0.68 and 0.69 for VAI. The accuracy of optimal cutoffs ranged from 61% (NAFLD-LFS) to 67% (TyG) among HBV-only and 56% (HSI) to 76% (NAFLD-LFS) among HBV–HIV. Negative predictive values were higher than positive predictive values for all scores in both groups.
Conclusion
The relative utility of scores to identify steatosis in chronic HBV differs by co-infection/anti-HBV medication status. However, even with population-specific cutoffs, several common serum-based scores have only moderate utility. ClinicalTrials.gov NCT01924455.
Keywords: Steatosis, Noninvasive, HBV, HBV–HIV
Introduction
Nonalcoholic fatty liver disease (NAFLD) is one of the most common causes of chronic liver disease in the USA [1] and worldwide [2]. In addition to independently increasing the risk of hepatic decompensation and hepatocellular carcinoma (HCC) [3-5], NAFLD contributes to the overall morbidity and mortality associated with other liver diseases, such as chronic hepatitis B virus (HBV) [6]. NAFLD and its hallmark, hepatic steatosis, which affects over one-fourth of adults with chronic HBV in North America [7, 8], are associated with elevated alanine aminotransferase (ALT) levels in adults with chronic HBV [7-10] independent of HBV infection. In addition, hepatic steatosis has been associated with systemic inflammation [11, 12], cardiovascular disease [13-16], and atherosclerosis [17]. Therefore, identification of hepatic steatosis in those with chronic HBV, i.e., over 2 million children and adults in North America [18] and over 250 million worldwide [19], is important for informing appropriate management of this population. For example, anti-HBV treatment would not be indicated for high ALT with low HBV viral replication if the elevated ALT is caused by hepatic steatosis and not HBV-related hepatocellular damage.
Although assessment of liver histology is optimal for evaluation of coexisting hepatic steatosis in the context of HBV, due to the invasiveness of liver biopsy, noninvasive tests (i.e., imaging and serum-based algorithms) to identify steatosis have received increased attention over the last decade. Imaging-based tests for steatosis include B-mode ultrasound (US), continuous attenuated parameter (CAP) as part of vibration controlled transient elastography (VCTE), and magnetic resonance (MR) imaging and spectroscopy techniques. B-mode US, used as part of hepatocellular carcinoma (HCC) surveillance, is unable to accurately detect mild–moderate hepatic steatosis (< 20–30%) [20, 21]. CAP and MRI have better performance across the spectrum of hepatic steatosis severity. However, they are not widely available and increase the cost of care [22-24]. In addition, other imaging-based methods of elastography, such as shear wave elastography (SWE), do not provide steatosis assessment. Serum-based algorithms, which may include demographic and clinical variables, such as sex, body mass index (BMI), waist circumference (WC), diabetes and metabolic syndrome, and laboratory variables, such as aspartate aminotransferase (AST), ALT, glucose, insulin, triglycerides, and high-density lipoprotein (HDL) cholesterol [16, 25-31], have the advantage of broader applicability and can be incorporated into clinical practice in a variety of settings, including primary care settings, with minimal expense.
Popular serum-based algorithms for hepatic steatosis include the hepatic steatosis index (HSI) [17, 27, 32-34], the nonalcoholic fatty liver disease Liver Fat Score (NAFLD-LFS) [34-36], visceral adiposity index (VAI), and the triglyceride glucose index (TyG index) [34, 37-41]. It is important to note, however, that of these four scores, only the HSI has been tested in HBV [32] and some of these scores have only been evaluated in relation to histology-determined hepatic steatosis in a sample in which 95% of participants had at least mild hepatic steatosis and suspected NAFLD [34]. Thus, their applicability in the context of HBV is largely unknown. In addition, it is unknown whether HIV co-infection or use of antivirals to suppress HBV as part of anti-HIV therapy might impact their utility.
To address this gap in knowledge, our aims were to 1) determine the diagnostic performance of four popular serum-based scores (LFS, HSI, VAI, and TyG index), including their ability to quantitatively predict histology-determined ≥ 5% hepatic steatosis in two distinct HBV cohorts of adults with chronic HBV, and 2) evaluate whether the performance of these tests differs between those with an untreated HBV mono-infection versus HBV–HIV co-infection on anti-HBV therapy.
Participants and Methods
All participants were enrolled in the Hepatitis B Research Network (HBRN) adult cohort study or the HBV–HIV cohort ancillary study of the HBRN, as previously described [42-44]. In brief, all participants were HBV surface antigen (HBsAg) positive, age 18 years or older, and did not have a history of hepatic decompensation, HCC, or solid organ or bone marrow transplant. While the HBRN adult cohort excluded those with HIV co-infection, the presence of anti-HIV was required in the HBV–HIV cohort. Liver biopsy was performed as part of the baseline research assessment of the HBV–HIV study. In contrast, liver biopsy was only assessed in HBRN adult cohort participants if performed for clinical care (such as for elevated liver enzymes) or as a requirement for study entry into the HBRN Immune Active treatment trial (NCT01369212). Informed consent was obtained from all individual participants included in the study.
Of adults enrolled in either cohort, the following exclusion criteria were employed: 1) no liver biopsy or inadequate liver tissue to determine hepatic steatosis, 2) acute HBV, 3) history of treatment for alcohol-related liver disease or at-risk alcohol intake (defined as ≥ 5 drinks on ≥ 1 day in past month in men/women, or > 4 drinks/day or > 14 drinks/week in men, or > 3 drinks/day or 7 drinks/week in women [45]) in the year prior to the biopsy, and 4) insufficient clinical or laboratory data obtained within 24 weeks of biopsy to calculate at least one of the four serum-based hepatic steatosis scores. Among the non-HIV cohort only, participants were also excluded from this report if they had chronic hepatitis C or delta co-infection (thus, the group is referred to as HBV-only hereafter), or were on HBV treatment within 4 weeks prior to the liver biopsy or collection of clinical data (whereas participants of the HBV–HIV cohort were generally on anti-HBV therapy as part of their combined anti-retroviral regimen (cART)).
Assessment of Liver Histology
Unstained slides of liver biopsies were submitted for hematoxylin and eosin (H&E) and Masson trichrome staining by a central laboratory. Histologic findings were scored blindly with respect to clinical and laboratory data, by the HBRN Pathology Committee (DEK, chair) composed of pathologists from participating HBRN clinical centers. Biopsies determined as inadequate (i.e., not representative) by the HBRN Pathology committee (≈1%) were excluded. Hepatic steatosis was graded based on the proportion of steatotic hepatocytes assessed at low magnification: none (0%), minimal (> 0– < 5%), mild (5–33%), moderate (34–66%), and severe (≥ 67%) [46].
Assessment of Clinical and Laboratory Data and Data Definitions
Self-reported race and ethnicity were categorized as non-Hispanic black, non-Hispanic white, Asian, and other, due to the low frequency of other groups. Past-year alcohol consumption was graded as none or minimal (< 1 drink per month) or low risk (more than none or minimal but ≤ 4 drinks/day or 14 drinks/week in men, ≤ 3 drinks/day or 7 drinks/week in women) [45]. Current cART was determined from self-report and medical records.
Laboratory testing by local site included plasma glucose, insulin, HDL cholesterol, triglycerides, AST, ALT, platelet counts, hemoglobin, qualitative HBV e-antigen (HBeAg) and surface-antigen (HBsAg), and HBV DNA. HBV DNA and quantitative HBsAg and HBeAg testing was done centrally, as previously described, at a HBRN-funded virology laboratory (University of Washington, Seattle, WA) [44]. Additionally, among the HBV–HIV cohort only, the following tests were performed centrally in a commercial laboratory (TrueHealth Diagnostic Laboratories, Inc., Richmond, VA): plasma glucose, insulin, total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides. Triglycerides, glucose, and insulin from samples taken after fewer than 8 h of fasting were excluded.
BMI was calculated from measured weight in kilograms (kg) divided by height in meters (m) squared. Overweight was defined as BMI of 23– < 27.5 kg/m2 if Asian and 25– < 30 kg/m2 for all other racial groups, and obesity was defined as BMI ≥ 27.5 kg/m2 if Asian and ≥ 30 kg/m2 for all other racial groups [47]. WC was measured following a standard protocol. High-risk WC was defined as ≥ 80 cm for Asian women and ≥ 88 cm for women of all other races, ≥ 90 cm for Asian men, and ≥ 102 cm for men of all other races [48]. Anti-diabetic and lipid medications were self-reported and obtained through medical records. Diabetes (type 1 or type 2) was defined as self-report of the condition, current use of anti-diabetic medications or fasting glucose ≥ 126 mg/dL. Metabolic syndrome was defined as the presence of at least 3 of 5 standard criteria: 1) abnormal glucose metabolism (fasting glucose ≥ 100 mg/dL or diabetes), 2) elevated blood pressure (systolic pressure ≥ 130 and/or diastolic pressure ≥ 85 mmHg) or use of antihypertensive medications, 3) high-risk WC, 4) elevated triglycerides (≥ 150 mg/dL) or current use of lipid-lowering agent, and 5) low HDL cholesterol level (HDL < 50 mg/dL in females and < 40 mg/dL in males) [49]. Hyperlipidemia was defined as self-report of the condition, current use of lipid lowering medication or low-density lipoprotein (LDL) ≥ 160 mg/dL. Insulin sensitivity was assessed with HOMA-IR: fasting insulin (mU/L) x fasting glucose (mmol/L)/22.5. The upper limit of normal (ULN) for ALT was 30 U/L for males and 19 U/L for females [50].
The closest clinical and laboratory data obtained near the time of biopsy were used to calculate the following published steatosis scores:
HSI: 8 * ALT/AST + BMI (+ 2 if diabetes; + 2 if female) [27].
NAFLD-LFS: −2.89 + 1.18 * metabolic syndrome (yes = 1/no = 0) + 0.45 * diabetes (yes = 2/no = 0) + 0.15 * insulin (mU/L) + 0.04 * AST(U/L)—0.94 * AST/ALT [35].
TyG index: ln[(triglycerides)(mg/dL) * 8 glucose (mg/dL)/2] [41].
VAI: [WC/39.68 + (1.88*BMI)] * (triglycerides/1.03) * (1.31/HDL), for males; [WC/36.58 + (1.89 * BMI)] * (triglycerides/0.81) * (1.52/HDL), for females [38].
Statistical Analysis
Because the HBV–HIV cohort was on anti-HBV therapy as part of cART and the HBV alone was not on anti-HBV therapy at the time of biopsy, our aim was not to compare the two cohorts, but rather to compare the performance of non-invasive models for steatosis in each distinct cohort. Analyses were conducted using SAS, version 9.4 (SAS Institute Inc., Cary, NC, 2000). Descriptive statistics summarized demographic, clinical, virologic, and histologic characteristics. The Pearson χ2 test or Fisher exact test for categorical variables, the Kruskal–Wallis test for continuous variables, and the Cochran–Armitage trend test for ordinal variables were used to compare characteristics of the HBV-only versus HBV–HIV groups. All further analyses were stratified by co-infection status.
The distribution of each serum-based hepatic steatosis-related score by hepatic steatosis grade is presented graphically using boxplots. Correlations between each score and hepatic steatosis grade were assessed with Kendall’s tau-b. Given the low frequency of moderate and severe hepatic steatosis (i.e., ≥ 33%) in both groups, hepatic steatosis of at least mild severity (i.e., ≥ 5%) was evaluated in the remainder of the analyses.
The discriminatory capacity of each hepatic steatosis-related score (continuous) for the diagnosis of ≥ 5% hepatic steatosis was calculated using receiver operating characteristics (ROC) curves. DeLong’s test was used to evaluate whether the areas under the ROC (AUROCs) differed by score. The AUROCs, sensitivity (SE), specificity (SP), SE + SP, positive predictive value (PPV), negative predictive value (NPV), and the diagnosis accuracy (i.e., sum of true positives and true negatives over the number of participants) were calculated for the optimal cutoff of each ROC curve, defined by the highest SE + SP, as well as for previously established cutoffs (> 41 for HSI, > 0.16 for NAFLD-LFS, > 8.38 for TyG, > 1.25 for VAI) [34].
Multivariable logistic regression was used to determine whether population-specific predictive models for differentiating ≥ 5% hepatic steatosis versus < 5% hepatic steatosis performed better than the four established models that we tested. The following established risk factors were considered: age, sex, black race, alcohol risk, WC, BMI, metabolic syndrome, glucose, diabetes, hypertension, hyperlipidemia, HDL cholesterol, triglycerides, ALT, AST, ALT/AST, and hemoglobin [27, 32, 35, 38, 41]. Serum uric acid and gamma glutamyl transferase (GGT) [25, 32] were not available for consideration. Using backward elimination, variables with p < 0.10 were retained. Models were first built excluding variables missing in over 10% of participants and then built with consideration of all variables.
Results
Study Participants
Of the 1996 adult HBRN cohort participants, 302 met inclusion criteria for this report, and of the 135 HBV–HIV participants, 92 met inclusion criteria for this report (Supplemental Fig. 1). The vast majority (260 of 302 [86.1%] and 90 of 92 [97.8%] in the HBV-only and HBV–HIV analysis samples, respectively) had clinical data (i.e., score components) collected within 12 weeks of biopsy. The sample size differed by score, reflecting differences in data completeness by score components. For example, among HBV-only participants, 296 had the HSI, which does not include a data component requiring a fasting blood draw, versus 115, 101, and 71 participants for the TyG index, VAI, and NAFLD-LFS, respectively.
Participant Sociodemographic, Clinical, and Virologic Characteristics
Among the HBV–HIV participants, 6.5% (n = 6) were anti-HCV positive and 4.4% (n = 4) were anti-HDV positive. All but 3 (96.7%) were on cART treatment at the time of biopsy (most common was Tenofovir, alone or in combination). Specifically, 95.7% (n = 88) were on a nucleoside reverse transcriptase inhibitor (NRTI), 33.7% (n = 31) were on a non-nucleoside reverse transcriptase inhibitor (NNRTI), and 45.7% (n = 42) were on a protease inhibitor (PI). The majority had < 20 copies/mL of HIV RNA (77.9%; n = 67) and were HIV stage 1 (CD4 ≥ 500 cells/mm3) (58.6%; n = 51). The HBV–HIV versus HBV-only group differed with respect to most characteristics (Table 1). For example, participants with HBV–HIV versus HBV-only were older (median age 50 vs 43 years), and a higher percentage were male (92.4% vs 59.6%), were black (50.6% vs 7.6%), were North American born (100% vs 13.7%), and reported low-risk (versus no/minimal risk) alcohol consumption; a smaller percentage were Asian (5.7% vs 81.4%) (p for all < 0.001). Their BMI and WC were also higher, they had lower HDL cholesterol, and a higher percentage had hyperlipidemia and metabolic syndrome versus HBV-only (p for all < 0.05). However, diabetes prevalence was similar (p = 0.65). Although a higher percentage with HBV–HIV were HBeAg positive, a higher percentage had suppressed HBV DNA (81.5% vs 9.0%) and their median ALT, AST, and total bilirubin were lower versus the untreated HBV-only group (p for all < 0.001).
Table 1.
Demographics, virologic characteristics, and cardiometabolic health among North American adults with chronic HBV, by co-infection status
| Variable | HBV-only n = 302 |
HBV–HIV n = 92 |
P value |
|---|---|---|---|
| Age at time of biopsy (years) | < 0.001 | ||
| Median(25th:75th) | 43 (35: 53) | 50 (44: 56) | |
| Sex, n (%) | < 0.001 | ||
| Male | 180 (59.6%) | 85 (92.4%) | |
| Race, n (%) | n = 301 | n = 87 | < 0.001 |
| White | 27 (9.0%) | 35 (40.2%) | |
| Black | 23 (7.6%) | 44 (50.6%) | |
| Asian | 245 (81.4%) | 5 (5.7%) | |
| Other | 6 (2.0%) | 3 (3.4%) | |
| US/Canada birth, n (%) | n = 285 | n = 78 | < 0.001 |
| Yes | 39 (13.7%) | 78 (100.0%) | |
| Alcohol risk, n (%) | n = 296 | n = 92 | 0.014 |
| None or minimal | 225 (76.0%) | 58 (63.0%) | |
| Low risk | 71 (24.0%) | 34 (37.0%) | |
| HBeAg status, n (%) | n = 296 | 0.001 | |
| Positive | 111 (37.5%) | 53 (57.6%) | |
| HBV DNA (IU/mL) | n = 301 | < 0.001* | |
| < 1000 | 27 (9.0%) | 75 (81.5%) | |
| 1000– < 20,000 | 59 (19.6%) | 8 (8.7%) | |
| ≥ 20,000 | 215 (71.4%) | 9 (9.8%) | |
| HIV RNA (copies/mL) | NA | n = 81 | |
| < 20 | 62 (76.5%) | ||
| 20–< 400 | 12 (14.8%) | ||
| 400–< 10,000 | 4 (4.9%) | ||
| ≥ 10,000 | 3 (3.7%) | ||
| CD4 (cells/mm3) | NA | n = 87 | |
| Median (25th:75th) | 572 (374: 718) | ||
| CD4% | NA | n = 87 | |
| Median (25th:75th) | 25.4 (18.0: 36.0) | ||
| ALT (U/L) | n = 91 | < 0.001 | |
| Median(25th:75th) | 58 (37: 94) | 27 (19: 39) | |
| High ALT (> 1 ULN), n (%)a | n = 91 | < 0.001c | |
| Yes | 275 (91.1%) | 39 (42.9%) | |
| AST (U/L) | n = 299 | n = 91 | < 0.001 |
| Median(25th:75th) | 40 (28: 59) | 27 (22: 38) | |
| High AST (> 1 ULN), n (%)b | n = 299 | n = 91 | < 0.001* |
| Yes | 149 (49.8%) | 19 (20.9%) | |
| AST/ALT ratio | n = 299 | n = 91 | < 0.001 |
| Median(25th:75th) | 0.7 (0.6: 0.9) | 1.0 (0.8: 1.3) | |
| Hemoglobin (g/dL) | n = 248 | n = 91 | < 0.001 |
| Median(25th:75th) | 14.8 (13.5: 15.7) | 14.9 (13.6: 15.6) | |
| Platelets (× 103/mm3) | n = 279 | 0.29 | |
| Median (25th:75th) | 195 (170: 234) | 202 (173: 233) | |
| Body mass index (kg/m2) | n = 299 | n = 89 | 0.001 |
| Median (25th:75th) | 24.4 (21.9: 27.3) | 26.7 (22.7: 30.4) | |
| Weight status (race specific), n (%) | n = 299 | n = 89 | 0.20c |
| Underweight/normal | 115 (38.5%) | 29 (32.6%) | |
| Overweight | 121 (40.5%) | 36 (40.4%) | |
| Obese | 63 (21.1%) | 24 (27.0%) | |
| Waist circumference (cm) in females | n = 86 | n = 5 | 0.001 |
| Median (25th:75th) | 81.1 (74.9: 86.4) | 102.0 (97.0: 105.4) | |
| Waist circumference (cm) in males | n = 136 | n = 73 | 0.001 |
| Median (25th:75th) | 87.8 (81.7: 95.7) | 94.0 (86.0: 102.0) | |
| Hyperlipidemia, n (%) | n = 301 | 0.004 | |
| Yes | 56 (18.6%) | 30 (32.6%) | |
| HDL (mg/dL) in females | n = 68 | n = 7 | 0.024 |
| Median (25th:75th) | 61 (50: 73) | 49 (41: 55) | |
| HDL (mg/dL) in males | n = 88 | n = 85 | 0.05 |
| Median (25th:75th) | 46 (39: 55) | 41 (36: 50) | |
| Triglycerides (mg/dL) | n = 124 | n = 74 | 0.002 |
| Median (25th:75th) | 87 (67: 115) | 114 (76: 171) | |
| Hypertension, n (%) | < 0.001 | ||
| Yes | 83 (27.5%) | 46 (50.0%) | |
| Glucose (mg/dL) | n = 159 | n = 78 | 0.02 |
| Median (25th:75th) | 87 (80: 96) | 90 (84: 101) | |
| Diabetes, n (%) | 0.65 | ||
| Yes | 28 (9.3%) | 10 (10.9%) | |
| Metabolic syndrome, n (%) | n = 152 | n = 81 | 0.013 |
| Yes | 38 (25.0%) | 33 (40.7%) | |
| Insulin (mcU/mL) | n = 75 | n = 65 | 0.002 |
| Median (25th:75th) | 8.1 (4.5: 12.2) | 11.0 (8.0: 19.0) | |
| HOMA-IR | n = 71 | n = 65 | < 0.001 |
| Median (25th:75th) | 1.7 (0.9: 2.4) | 2.5 (1.7: 4.9) |
ALT Alanine aminotransferase, AST aspartate aminotransferase, DNA deoxyribonucleic acid. HBeAg, quantitative hepatitis B e-antigen, HBV hepatitis B virus, HDL high-density lipoproteins HIV human immunodeficiency virus, HOMA-IR homeostatic model assessment of insulin resistance, LDL low-density lipoproteins
Upper limit of normal (ULN) ALT ≤ 30 IU/L in men, ALT ≤ 19 U/L in women
Upper limit of normal (ULN) AST by each local laboratory
From the Cochran–Armitage trend test
Assessment of Liver Histology
There was not a significant difference in prevalence of ≥ 5% hepatic steatosis (27.2% HBV–HIV versus 30.5% HBV-only; p = 0.55). However, there was indication of a possible trend (p = 0.05) toward higher grade levels of hepatic steatosis among HBV-only participants (e.g., 11.3% vs 4.3% ≥ 33% hepatic steatosis; Table 2).
Table 2.
Hepatic steatosis severity and noninvasive serum-based scores among North American adults with chronic HBV, by co-infection status
| Variable | HBV-only n = 302 |
HBV–HIV n = 92 |
p value |
|---|---|---|---|
| Steatosis percentage of hepatocytes, n (%) | n = 302 | n = 92 | 0.054 |
| None | 53 (17.5%) | 24 (26.1%) | |
| > None- < 5% | 157 (52.0%) | 43 (46.7%) | |
| 5–33% | 58 (19.2%) | 21 (22.8%) | |
| 34–66% | 28 (9.3%) | 4 (4.3%) | |
| ≥ 67% | 6 (2.0%) | 0 (0.0%) | |
| Scores | |||
| HSI | n = 296 | n = 89 | 0.006 |
| Median(25th:75th) | 37.2 (33.2: 41.8) | 35.0 (31.1: 40.6) | |
| NAFLD-LFS | n = 71 | n = 62 | 0.60 |
| Median(25th:75th) | − 0.2 (− 1.2: 1.4) | − 0.1 (− 1.5: 1.8) | |
| TyG index | n = 115 | n = 73 | 0.001 |
| Median(25th:75th) | 4.5 (4.3: 4.6) | 4.6 (4.4: 4.9) | |
| VAI | n = 101 | n = 59 | 0.007 |
| Median(25th:75th) | 2.4 (1.7: 3.9) | 3.1 (2.1: 5.3) |
HBV, hepatitis B virus, HIV human immunodeficiency virus, HSI hepatic steatosis index, NAFLD-LFS nonalcoholic fatty liver disease Liver Fat Score, TyG triglyceride glucose, VAI visceral adiposity index
Serum-Based Scores
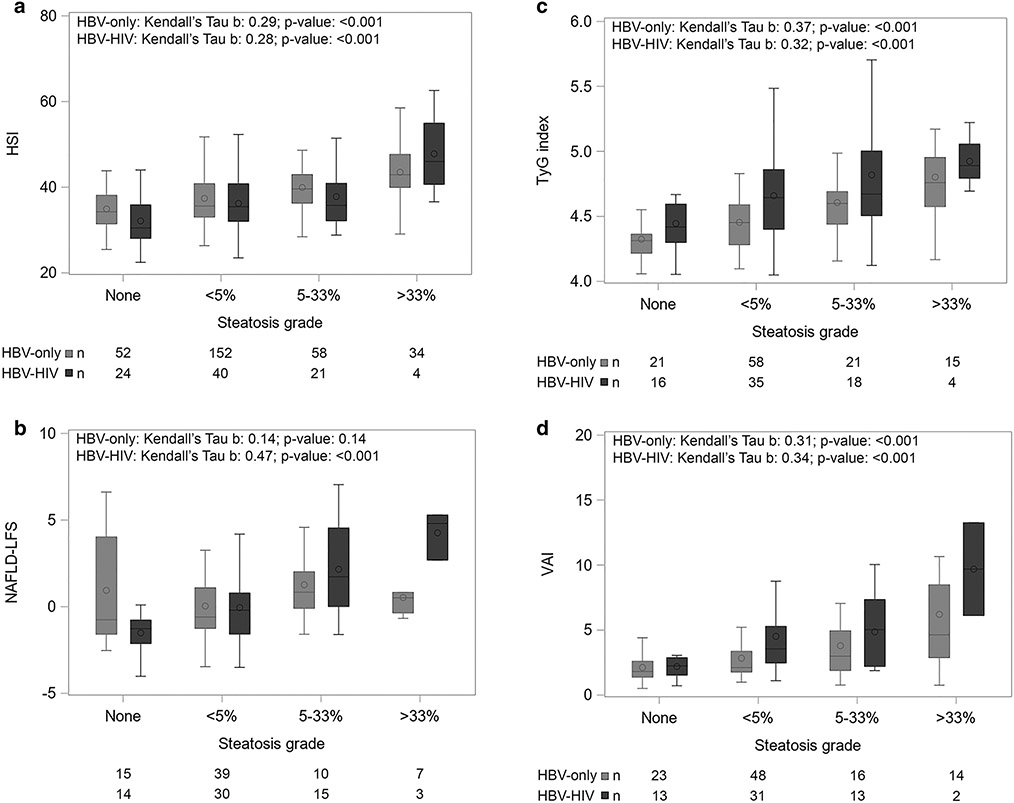
HBV–HIV versus HBV-only participants had a lower median HSI, but a higher median TyG index and VAI (p for all < 0.01), while there was not a significant difference in median NAFLD-FLS (p = 0.60; Table 2). The distribution of each noninvasive serum score by severity of hepatic steatosis is shown in Fig. 1. Correlations were fairly weak (Kendall’s tau-b (τ): range 0.28–0.37; p ≤ 0.01) for HSI, TyG index, and VAI among both groups (Fig. 1). The correlation with NAFLD-LFS was slightly stronger (τ = 0.47; p < 0.0001) among the HBV–HIV group, but not significant (τ = 0.14; p = 0.14) among HBV-only.
Fig. 1.
Boxplots of steatosis grade by serum-based steatosis-related scores among North American adults with chronic HBV, by co-infection status. a. HSI; b. NAFLD-LFS; c. TyG index; d. VAI. HBV, hepatitis B virus; HIV human immunodeficiency virus, HSI hepatic steatosis index, NAFLD-LFS nonalcoholic fatty liver disease Liver Fat Score, TyG triglyceride glucose, VAI visceral adiposity index
Discriminatory Capacity of Serum-Based Scores
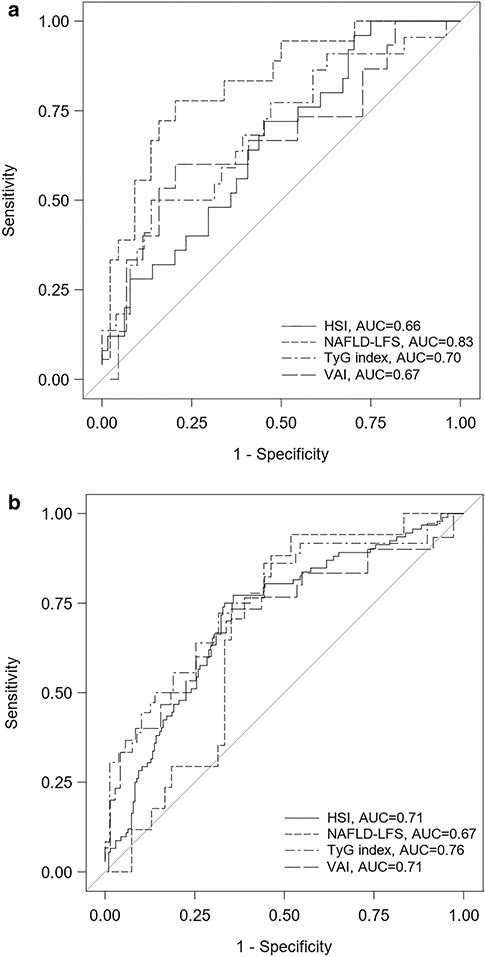
The ROC for differentiation of ≥ 5% hepatic steatosis by each noninvasive serum score is shown in Fig. 2. Within the HBV-only sample, there was not a significant difference in AUROCs between scores (DeLong’s p-value range for all comparisons: 0.19–0.95). Within the HBV–HIV sample, NAFLD-LFS had a higher AUROC (0.83) than HSI (0.66; p < 0.01) or VAI (0.67; p = 0.04); other pairwise comparisons were not significant (DeLong’s p value range for comparisons: 0.17–0.65). With the exception of the NAFLD-LFS, AUROC point estimates were higher in the HBV-only vs HBV–HIV group: 0.71 (95%CI, 0.65–0.77) vs 0.66 (95%CI, 0.54–0.78) for HSI, 0.67 (95%CI, 0.54–0.80) vs 0.83 (95%CI, 0.72–0.94) for NAFLD-LFS, 0.76 (95%CI, 0.66–0.86) vs 0.70 (95%CI, 0.56–0.83) for TyG index, and 0.71 (95%CI, 0.59–0.83) vs 0.67 (95%CI, 0.50–0.84) for VAI. However, the 95% CIs overlapped.
Fig. 2.
The diagnostic accuracy of established noninvasive serum scores in identifying ≥ 5% steatosis among North American adults with chronic HBV, by co-infection status. a. HBV-only; b. HBV–HIV. HBV, Hepatitis B virus; HSI Human immunodeficiency virus, HSI Hepatic Steatosis index, NAFLD-LFS nonalcoholic fatty liver disease Liver Fat Score, TyG triglyceride glucose, VAI visceral adiposity index
Optimal and Established Cutoffs
The percentage of HBV-only participants meeting scores’ optimal thresholds for ≥ 5% ranged from 47.0% to 57.7%; for established thresholds, the range was 0–90.1%. The percentage of HBV–HIV participants meeting score’s optimal thresholds for ≥ 5% ranged from 30.1% to 56.2%; for established thresholds, the range was 0–93.2%. For all scores but the HSI, a lower percentage of HBV–HIV versus HBV-only participants met the optimal threshold indicative of ≥ 5% hepatic steatosis (p for all < 0.05). In contrast, the percentage of participants meeting the established threshold for each score indicative of ≥ 5% hepatic steatosis did not significantly differ by group (p for all > 0.40).
The diagnostic accuracy of optimal cutoffs (i.e., producing the highest SE + SP) and established cutoffs of serum-based scores in identifying ≥ 5% hepatic steatosis is reported in Table 3. Among HBV-only participants, SE + SP of each score was similar when the optimal cutoff (HSI > 37; NAFLD-LFS > −0.41; TyG index > 4.50; VAI > 2.48) was applied (SE + SP range: 135.3–140.4). The TyG index had the highest PPV (48.2%), followed by the HSI (46.4%), while the NAFLD-LFS had the highest NPV (93.3%), followed by the HSI (85.3%).
Table 3.
The diagnostic accuracy of optimal and established cutoffsa for serum-based scores in identifying ≥ 5% hepatic steatosis among North American adults with chronic HBV, by co-infection status
| Score cutoff | n (%) identified | AUROC (95% CI) | SE, % | SP, % | SE + SP | PPV, % | NPV, % | AC, % |
|---|---|---|---|---|---|---|---|---|
| HBV-only | ||||||||
| HSI > 37 | 153 (51.7) | 0.69 (0.63, 0.74) | 77.2 | 59.8 | 137.0 | 46.4 | 85.3 | 65.2 |
| Established > 41 | 75 (25.3) | 0.63 (0.55, 0.71) | 46.7 | 79.9 | 126.6 | 51.2 | 76.9 | 69.6 |
| NAFLD-LFS > −0.41 | 41 (57.7) | 0.70 (0.54, 0.86) | 88.2 | 51.9 | 140.1 | 36.6 | 93.3 | 60.6 |
| Established > 0.16 | 30 (42.3) | 0.65 (0.49, 0.80) | 64.7 | 64.8 | 129.5 | 36.7 | 85.4 | 64.8 |
| TyG index > 4.50 | 54 (47.0) | 0.68 (0.57, 0.80) | 72.2 | 64.6 | 136.8 | 48.2 | 83.6 | 67.0 |
| Established > 8.38 | 0 | |||||||
| VAI > 2.48 | 49 (48.5) | 0.68 (0.56, 0.79) | 73.3 | 62.0 | 135.3 | 44.9 | 84.6 | 65.4 |
| Established > 1.25 | 91 (90.1) | 0.50 (0.38, 0.62) | 90.0 | 9.9 | 99.9 | 29.7 | 70.0 | 33.7 |
| HBV–HIV | ||||||||
| HSI > 34 | 50 (56.2) | 0.61 (0.47, 0.75) | 72.0 | 50.0 | 122.0 | 36.0 | 82.1 | 56.2 |
| Established > 41 | 19 (21.3) | 0.56 (0.42, 0.70) | 32.0 | 79.7 | 111.7 | 38.1 | 75.0 | 66.3 |
| NAFLD-LFS > 0.32 | 25 (40.3) | 0.76 (0.63, 0.90) | 77.8 | 75.0 | 152.8 | 56.0 | 89.2 | 75.8 |
| Established > 0.16 | 28 (45.2) | 0.73 (0.57, 0.89) | 77.8 | 68.2 | 146.0 | 50.0 | 88.2 | 71.0 |
| TyG index > 4.84 | 22 (30.1) | 0.64 (0.50, 0.78) | 50.0 | 78.4 | 128.4 | 50.0 | 78.4 | 69.9 |
| Established > 8.38 | 0 | NA | ||||||
| VAI > 4.76 | 19 (32.2) | 0.69 (0.53, 0.84) | 60.0 | 77.3 | 137.3 | 47.4 | 85.0 | 72.9 |
| Established > 1.25 | 55 (93.2) | 0.55 (0.37, 0.72) | 100.0 | 9.1 | 109.1 | 27.3 | 100.0 | 32.2 |
AC accuracy, AUROC area under the receiver operating characteristic curve, HBV hepatitis B virus, HIV human immunodeficiency virus, HSI hepatic steatosis index, NAFLD-LFS nonalcoholic fatty liver disease Liver Fat Score, NPV negative predictive value, TyG triglyceride glucose, PPV positive predictive value, SE sensitivity, SP specificity, VAI visceral adiposity index
The cutoff with the highest SE + SP; the higher number, the higher the diagnostic accuracy
There was more variation in the diagnostic accuracy of scores among HBV–HIV participants. The optimal NAFLD-LFS cutoff (> 0.32) yielded the highest SE + SP (152.8), as well as the highest PPV (56.0%) and highest NPR (89.2%), compared to the optimal cutoffs for VAI (> 4.76), TyG index (> 4.84), and HSI (> 34) (Table 3).
Among both groups, the established cutoff for TyG index identified no participants, and the established cutoff for VAI identified over 90% of participants, resulting in a very low PPV (27.3% in HBV-only, 29.7% in HBV–HIV). However, the established cutoffs for HSI and NAFLD-LFS did not perform much worse than the group-specific optimal cutoffs (Table 3).
Population-Specific Predictive Models
Among potential risk factors collected in each cohort (age, sex, black race, alcohol risk, WC, BMI, metabolic syndrome, glucose, diabetes, hypertension, hyperlipidemia, HDL cholesterol, triglycerides, ALT, AST, ALT/AST, and hemoglobin), those associated with steatosis at p < 0.10 are shown in Table 4. Among the HBV-only group, older age, non-black race, moderate (versus none/minimal) alcohol intake, higher BMI, and having hypertension were associated with higher odds of ≥ 5% hepatic steatosis. HBV DNA or HBeAg status was not associated with steatosis. Non-black race and having hypertension were also associated with higher odds of ≥ 5% hepatic steatosis in the HBV–HIV group, as was higher ALT; however, moderate (versus none/minimal) alcohol intake was associated with lower odds of ≥ 5% hepatic steatosis (Table 4). Despite creating population-specific predictive models from a wide array of established risk factors, for the most part, they did not perform better than existing models. For example, among the HBV-only group, the new score had an AUC of 0.78 (95%CI, 0.73–0.83) versus 0.76, (95%CI, 0.66–0.86) for the TyG index. Likewise, among the HBV–HIV group, the new score had an AUC of 0.81 (95%CI, 0.72–0.90) versus 0.83 (95%CI, 0.72–0.94) for the NAFLD-LFS. However, if triglyceride level, which was missing in the majority of participants, was added to the HBV-only group model, the AUC increased to 0.84 (95%CI, 0.77, 0.91). Adding variables with over 10% missing to the HBV–HIV model did not improve its predictive ability.
Table 4.
Associations between demographic and clinical characteristics and steatosis ≥ 5% among North American adults with chronic HBV, by co-infection status
| n | HBV-only C = 0.780a Adjusted OR |
p-value | n | HBV–HIV C = 0.811 Adjusted OR |
p-value | |
|---|---|---|---|---|---|---|
| Age, per 10 years | 293 | 1.26 (0.97, 1.65) | 0.08 | NA | ||
| Race (ref = black) | 22 | 44 | 0.047 | |||
| Non-black | 271 | 9.87 (2.58, 37.75) | < 0.001 | 47 | 3.17 (1.02, 9.86) | |
| Alcohol risk (ref = none/minimal) | 211 | 57 | 0.08 | |||
| Low | 82 | 1.90 (1.01, 3.59) | 0.048 | 34 | 0.34 (0.10, 1.15) | |
| Body Mass Index, per 5 kg/m2 | 293 | 2.36 (1.61, 3.45) | < .0001 | NA | ||
| Hypertension (ref = No) | 223 | 46 | 0.022 | |||
| Yes | 70 | 2.52 (1.27, 4.99) | 0.008 | 45 | 3.84 (1.22, 12.12) | |
| ALT (log2 U/L) | NA | 91 | 2.99 (1.56, 5.74) | 0.001 |
ALT alanine aminotransferase, HBV hepatitis B virus, NA not applicable
C = .840 if triglyceride level added to model (N = 120 due to missing triglyceride level)
Discussion
In this analysis, using four published scores to predict ≥ 5% hepatic steatosis, we found that even when applying optimal (i.e., population-specific) cutoffs, these scores only have moderate utility in HBV mono-infected and HBV–HIV co-infected groups, respectively. While our aim was not to compare liver histology or predictors of steatosis between these distinct cohorts, we do show that in the HBV–HIV group, the NAFLD-LFS had the highest correlation with degree of hepatic steatosis, and it best differentiated at least 5% hepatic steatosis versus the HSI, TyG index, and VAI. While the NAFLD-LFS did not correlate strongly with hepatic steatosis severity and did not perform as well in the HBV-only group (perhaps due to low data ascertainment of insulin), it was also the best performing of the four scores among those with HBV-only when an optimal cutoff was applied. In general, the optimal cutoffs for each score performed markedly better than the established cutoffs, indicating that validity studies in non-HBV samples may not be applicable.
Because the HBV and HBV–HIV cohorts differed by indication for biopsy and cART use for HBV suppression, the groups were not combined. The common use of cART containing tenofovir among the HBV–HIV group only explains the higher percentage of HBV DNA suppression and lower ALT and AST levels. Still, the HBV–HIV group appeared to have more health-related risk factors for hepatic steatosis; for example, a much higher percentage of the sample was black race, which was related to a lower risk of hepatic steatosis in both groups. There was also a large difference in sex distributions between groups. However, while sex is a component of some of the serum-based scores, sex was not related to hepatic steatosis in either group. Because the majority of those with HIV had an undetectable HIV RNA and HBV DNA, they were not associated with steatosis. Despite differences in demographics, health status, virologic parameters, and indications for biopsy, the proportion of those with ≥ 5% hepatic steatosis in our two cohorts was similar at 30% in the HBV mono-infection group and 27% in those with HBV–HIV co-infection. This allowed for comparison of the relative performance of the noninvasive makers to detect hepatic steatosis in these two distinct cohorts, independent of steatosis prevalence. While differences in the performance of scores between cohorts may be due to differences in hepatic steatosis risk factors, difference in sample size may have also played a role, as better performance is expected with a larger sample. Additionally, the NAFLD-LFS could only be calculated among one-third of the HBV-only group due to unavailability of fasting insulin levels in that cohort.
There are limited data on noninvasive serum testing for hepatic steatosis in chronic HBV [32, 47, 48] and none in HBV–HIV. Previous studies have demonstrated that the performance for each serum-based model for hepatic steatosis varies depending on the population’s risk for hepatic steatosis, the severity of hepatic steatosis to be identified and score cutoff [30, 31]. The performance of the HSI is perhaps the most variable of the four scores we evaluated [17, 27, 32-34], perhaps due to its inclusion of BMI as a high BMI, especially if over 40 kg/m2, leads to a high HSI, regardless of other risk factors [32, 47]. In comparison, the performance of the NAFLD-LFS and TyG index, neither of which include BMI, has been less variable across studies. Still, among three studies the reported sensitivity, specificity, and AUROC for NAFLD-LFS ranges from 65 to 86%, 71 to 87%, and 0.68 to 0.87, respectively [33-35], and among two studies, the TyG index ranges from 70 to 94%, 60 to 92%, and 0.68 to 0.90, respectively [34, 37]. We only identified one prior report that has evaluated the performance of the VAI, which also includes BMI, to identify hepatic steatosis; reported sensitivity was 79%, specificity was 92%, and AUROC was 0.92 [34].
A novel model for predicting hepatic steatosis in HBV was recently developed among 364 chronic HBV patients from China, of whom 118 (32%) had at least 5% hepatic steatosis. They identified five risk factors (age, hemoglobin, uric acid, BMI, triglycerides) [32]. Their model (steatosis in HBV [SIHBV]) performed better than several published models including HSI (AUROC 0.85 vs. 0.62). However, NAFLD-LFS, TyG index, and VAI were not evaluated. We were unable to test SIHBV in the current study because uric acid was not measured. However, because the models we tested performed poorly, we also explored whether a new model would work better. Unfortunately, neither population-specific model performed better than the best-performing established model for each cohort. Still, it is interesting to note that non-black race and hypertension were identified as risk factors in both the HBV-only and HBV–HIV groups, as neither race nor hypertension is components of other hepatic steatosis scores.
Strengths of this study are the geographic diversity of the sample, the assessment in both untreated chronic HBV and treated HIV-HBV cohorts, the central reading of liver biopsies by the HBRN pathology committee, and the evaluation of four competing serum-based scores for hepatic steatosis. The main limitations of this study were exclusion of variables of interest, the use of local laboratories for the steatosis indices calculations, and incomplete data attainment. Although the SIHBV [32] or the fatty liver index (FLI) [17, 49] was of interest, we were unable to evaluate them because required components (e.g., serum uric acid for SIHBV; gamma glutamyl transpeptidase for FLI) were not collected in either HBRN cohort. All four scores selected for study required at least one fasting laboratory measure (e.g., fasting glucose or HbA1c to determine diabetes status for the HSI; fasting triglycerides for the TyG index and VAI). However, the NAFLD-FLS required fasting glucose or HbA1c for diabetes, fasting triglycerides to determine metabolic syndrome, and fasting insulin. Thus, it was missing most frequently, especially among the HBV-only cohort, which may have impacted its performance. Because fasting insulin is not done routinely in clinical practice, NAFLD-LFS may have limited applicability. Additional limitations of our study include the self-selection of patients who were willing to undergo liver biopsy in the HBV–HIV cohort and lack of systematic biopsy for the HBV-only cohort, which might affect the representativeness of the samples. Although several older cART medications, such as PIs and NRTIs, have been linked to steatosis, we did not have accurate records of their past use in HBV–HIV patients. In addition, we could not account for duration of infections or impact or duration of anti-HBV therapy alone or as part of prior cART in HBV–HIV subjects due to poor participant recall. As with any liver biopsy study, there may have been sampling error. Furthermore, not all components of each score were measured on all participants on the same day as the liver biopsy, but by ensuring close proximity (the majority < 12 weeks) of the liver biopsy and noninvasive serum-based score measurements, the potential impact on diagnostic accuracy of noninvasive markers was reduced. Because our sample sizes were modest, we were not able to perform sensitivity analysis among participants with metabolic risk factors (e.g., obesity, diabetes, hypertension) to determine whether the noninvasive tests perform better in such a subgroup. Additionally, we were unable to split our cohort into derivation and validation subsets; thus, the cutoffs identified in this study require validation in other cohorts. However, given the poor PPV, future work should focus on determining a better score for use in HBV. Steatohepatitis and higher thresholds for steatosis (> 33%) were not specifically evaluated in our study due to a combination of the sample size and their low frequency (16.4% and < 10%, respectively). However, our outcome (i.e., ≥ 5% hepatic steatosis) included almost all cases of steatohepatitis [7]. We also did not differentiate type 1 vs. type 2 diabetes nor were we able to control for compliance with anti-hypertensive, anti-diabetes, or anti-lipid medications. Notwithstanding these, our study is one of very few studies on noninvasive assessment compared to liver histology to identify hepatic steatosis in these understudied populations.
Hepatic steatosis is important to identify as it contributes to the overall morbidity and mortality of other liver diseases, including chronic HBV [6], and is associated with increased liver enzymes independent of HBV [7-10] and cardiovascular disease [13-16]. Although US is recommended for HCC surveillance, it is insensitive to detect mild degrees of steatosis (< 20%) that may cause increased liver enzymes. Because noninvasive assessments of fibrosis and steatosis are increasingly utilized, most patients do not undergo liver biopsy. While serum-based algorithms can be performed at the point of care and in both primary care and specialty care and have the potential to enhance identification of those with hepatic steatosis, our results indicate that even with population-specific cutoffs, several common serum-based scores have only moderate utility and are better to rule out (high NPV) than diagnosis mild–severe hepatic steatosis in chronic HBV. The relative utility of each score was different in the untreated HBV mono-infected cohort than the cART-treated HBV–HIV cohort. Additional studies to validate other serum-based scores and imaging techniques (CAP, MR) alone or in combination in those with chronic HBV alone or in those with HBV–HIV are needed.
Supplementary Material
Funding
This study was funded by NIDDK (R01-DK94818) as an ancillary study (NCT01924455) of the Hepatitis B Research Network to Dr. Richard K. Sterling. Dr. Sulkowski was partially supported by K24DA034621. Dr. Khalili was partially supported by K24AA022523
Abbreviations
- HBV
Hepatitis B virus
- HIV
Human immunodeficiency virus
- cART
Combination antiretroviral therapy
- AIDS
Acquired immunodeficiency syndrome
- TDF
Tenofovir disoproxil fumarate
- FTC
Emtricitabine
- 3TC
Lamivudine
- HBRN
Hepatitis B Research Network
- NIH
National Institutes of Health
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- HBsAg
Hepatitis B surface antigen
- HBsAb
Anti-hepatitis B surface antibody
- HBeAg
Hepatitis B envelop antigen
- HBeAb
Anti-hepatitis B envelop antibody
- DNA
Deoxyribonucleic acid
- HDV
Hepatitis delta virus
- HCV
Hepatitis C virus
- RNA
Ribonucleic acid
- BMI
Body mass index
- HAI
Histologic activity index
- AST
Aspartate aminotransferase
- ALT
Alanine aminotransferase
- ALP
Alkaline phosphatase
- NASH
Nonalcoholic steatohepatitis
- IQR
Interquartile range
- NAFLD-LFS
Nonalcoholic fatty liver disease Liver Fat Score
- VAI
Visceral adiposity index
- TyG
Triglyceride glucose index
- HDL
High-density lipoprotein
- LDL
Low-density lipoprotein
- CAP
Continuous attenuated parameter
- VCTE
Vibration-controlled transient elastography
- NRTI
Nucleoside reverse transcriptase inhibitor
- PI
Protease inhibitor
Footnotes
Supplementary information The online version of this article (doi:https://doi.org/10.1007/s10620-021-06860-3) contains supplementary material, which is available to authorized users.
Conflict of interest Richard Sterling has received research grant from Abbott, AbbVie, Gilead, and Roche and serves on the data safety and monitoring board for Pfizer and Baxter. Mandana Khalili is a recipient of research grant (to her institution) from Gilead Sciences Inc. and Intercept Pharmaceuticals, and she has served as consultant for Gilead Sciences Inc. Raymond Chung has research grants (to institution) from Gilead, AbbVie, BMS, Merck, Boehringer, Roche, and Janssen. Mauricio Lisker-Melman serves on the speaker bureau for AbbVie, Gilead Sciences Inc., and SimplySpeaking. Mamta K. Jain has received research funding from Gilead Sciences, Janssen Pharmaceuticals, Merck, and GlaxoSmithKline. She has served on the scientific advisory board for Gilead Sciences. David Wong, Wendy King, Marc Ghany, and David Kleiner do not have any disclosures relevant to this project.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Human and animal rights The studies have been approved by the appropriate institutional and/or national research ethics committee and have been performed in accordance with the ethical standards as laid down in the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
References
- 1.Younossi ZM, Stepanova M, Younossi Y et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 2020;69:564–568. 10.1136/gutjnl-2019-318813. Epub 2019 Jul 31 [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z, Anstee QM, Marietti M et al. Global burden of NAHEPATIC STEATOSIS and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20 [DOI] [PubMed] [Google Scholar]
- 3.Unalp-Arida A, Ruhl CE. Noninvasive fatty liver markers predict liver disease mortality in the U.S. population. Hepatology. 2016;63:1170–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee YB, Ha Y, Chon YE et al. Association between hepatic steatosis and the development of hepatocellular carcinoma in patients with chronic hepatitis B. Clin Mol Hepatol. 2019;25:52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan AW, Wong GL, Chan HY et al. Concurrent fatty liver increases risk of hepatocellular carcinoma among patients with chronic hepatitis B. J Gastroenterol Hepatol. 2017;32:667–676 [DOI] [PubMed] [Google Scholar]
- 6.Suliman I, Abdelgelil N, Kassamali F, Hassanein TI. The effects of hepatic steatosis on the natural history of HBV infection. Clin Liver Dis. 2019;23:433–450 [DOI] [PubMed] [Google Scholar]
- 7.Khalili M, King W, Kleiner D, et al. HBV-HIV Cohort Study of the Hepatitis B Research Network. Fatty Liver Disease in a Prospective North American Cohort of Adults with HIV and Hepatitis B Coinfection. Clin Infect Dis. 2020. Sep 1:ciaa1303. 10.1093/cid/ciaa1303. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khalili M, King W, Kleiner D. Nonalcoholic Fatty Liver Disease (NAFLD) and its Clinical and Metabolic Correlates in a Prospective North American Cohort with HIV-HBV Coinfection on Combined Anti-Retroviral Therapy (cART). Hepatology. 2019;70:1362A–1363A [Google Scholar]
- 9.Shi FY, Jing L, Wenjun C et al. Fatty liver disease index: a simple screening tool to facilitate diagnosis of nonalcoholic fatty liver disease in the Chinese population. Dig Dis Sci. 2013;58:3326–3334. 10.1007/s10620-013-2774-y. [DOI] [PubMed] [Google Scholar]
- 10.Demir K, Akyuz F, Ozdil S et al. What is the reason of elevated alanine aminotransferase level in HBeAg negative patients with low viremia: NAFLD or chronic hepatitis? Ann Hepatol. 2007;6:92–96 [PubMed] [Google Scholar]
- 11.Fricker ZP, Pedley A, Massaro JM. Liver fat is associated with markers of inflammation and oxidative stress in analysis of data from the Framingham heart study. Clin Gastroenterol Hepatol. 2019;17:1157–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Liu L, Wang B, Wang J, Chen D. Simple steatosis is a more relevant source of serum inflammatory markers than omental adipose tissue. Clin Res Hepatol Gastroenterol. 2014;38:46–54 [DOI] [PubMed] [Google Scholar]
- 13.Otgonsuren M, Estep MJ, Hossain N et al. Single non-invasive model to diagnose non-alcoholic fatty liver disease (NAHE-PATIC STEATOSIS) and non-alcoholic steatohepatitis (NASH). J Gastroenterol Hepatol. 2014;29:2006–13 [DOI] [PubMed] [Google Scholar]
- 14.Sviklāne L, Olmane E, Dzērve Z et al. Fatty liver index and hepatic steatosis index for prediction of non-alcoholic fatty liver disease in type 1 diabetes. J Gastroenterol Hepatol. 2018;33:270–276 [DOI] [PubMed] [Google Scholar]
- 15.Gastaldelli A, Kozakova M, Højlund K et al. RISC Investigators. Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology. 2009;49:1537–44. [DOI] [PubMed] [Google Scholar]
- 16.Paris R, Giral P, Khan JF et al. LIDO Study Group. Fatty liver is an independent predictor of early carotid atherosclerosis. J Hepatol. 2016;65:95–102. [DOI] [PubMed] [Google Scholar]
- 17.Kozakova M, Palombo C, Eng MP, et al. RISC Investigators. Fatty liver index, gamma-glutamyltransferase, and early carotid plaques. Hepatology. 2012;55:1406–15. [DOI] [PubMed] [Google Scholar]
- 18.Kowdley KV, Wang CC, Welch S, Roberts H, Brosgart CL. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology. 2012;56:422–433 [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization Hepatitis B Fact Sheet. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. Accessed November, 2020.
- 20.Schwenzer NF, Springer F, Schraml C et al. Noninvasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51:433–445 [DOI] [PubMed] [Google Scholar]
- 21.Bril F, Ortiz-Lopez C, Lomonaco R et al. Clinical value of liver ultrasound for the diagnosis of nonalcoholic fatty liver disease in overweight and obese patients. Liver Int. 2015;35:2139–46. [DOI] [PubMed] [Google Scholar]
- 22.Paige JS, Bernstein GS, Heba E, et al. A Pilot Comparative Study of Quantitative Ultrasound, Conventional Ultrasound, and MRI for Predicting Histology-Determined Steatosis Grade in Adult Nonalcoholic Fatty Liver Disease. AJR Am J Roentgenol. 2017;208:W168–W177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siddiqui MS, Vuppalanchi R, Van Natta ML et al. NASH Clinical Research Network. Vibration-Controlled Transient Elastography to Assess Fibrosis and Steatosis in Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2019;17:156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlas T, Petroff D, Garnov N et al. Non-invasive assessment of hepatic steatosis in patients with NAFLD using controlled attenuation parameter and 1H-MR spectroscopy. PLoS One. 2014;9:e91987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bedogni G, Bellentani S, Miglioli L et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;2:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calori G, Lattuada G, Ragogna F et al. Fatty liver index and mortality: the Cremona study in the 15th year of follow-up. Hepatology. 2011;54:145–152 [DOI] [PubMed] [Google Scholar]
- 27.Lee JH, Kim D, Kim HJ. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42:503–508 [DOI] [PubMed] [Google Scholar]
- 28.Bedogni G, Kahn, Bellentani S, Tiribelli C, A simple index of lipid over accumulation is a good marker of liver steatosis. BMC Gastroenterol. 2010;25;10:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuthbertson DJ, Weickert MO, Lythgoe D et al. External validation of the fatty liver index and lipid accumulation product indices, using 1H-magnetic resonance spectroscopy, to identify hepatic steatosis in healthy controls and obese, insulin-resistant individuals. Eur J Endocrinol. 2014;171:561–569 [DOI] [PubMed] [Google Scholar]
- 30.Stern C, Castera L. Non-invasive diagnosis of hepatic steatosis. Hepatol Int. 2017;11:70–78 [DOI] [PubMed] [Google Scholar]
- 31.Festi D, Schiumerini R, Marzi L et al. Review article: the diagnosis of non-alcoholic fatty liver disease—availability and accuracy of non-invasive methods. Aliment Pharmacol Ther. 2013;37:392–400 [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, Wang G, Kang K, Wu G, Wang P. Diagnostic accuracy and clinical utility of a new noninvasive index for hepatic steatosis in patients with hepatitis B virus infection. Sci Rep. 2016;6:32875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ooi GJ, Earnest A, Kemp WW, Burton PR, Laurie C, Majeed A, Johnson N, McLean C, Roberts SK, Brown WA. Evaluating feasibility and accuracy of non-invasive tests for nonalcoholic fatty liver disease in severe and morbid obesity. Int J Obes (Lond). 2018;42:1900–1911 [DOI] [PubMed] [Google Scholar]
- 34.Fedchuk L, Nascimbeni F, Pais R et al. LIDO Study Group. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2014;40:1209–22. [DOI] [PubMed] [Google Scholar]
- 35.Kotronen A, Peltonen M, Hakkarainen A et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137:865–872 [DOI] [PubMed] [Google Scholar]
- 36.Wlazlo N, van Greevenbroek MM, Ferreira I, Bravenboer B, Stehouwer CD. The diagnosis of non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2012;35:204–5; author reply205–6.) [DOI] [PubMed] [Google Scholar]
- 37.Simental-Mendía LE, Simental-Mendía E, Rodríguez-Hernández H et al. The product of triglycerides and glucose as biomarker for screening simple steatosis and NASH in asymptomatic women. Ann Hepatol. 2016;15:715–20. [DOI] [PubMed] [Google Scholar]
- 38.Petta S, Amato M, Cabibi D et al. Visceral adiposity index is associated with histological findings and high viral load in patients with chronic hepatitis C due to genotype 1. Hepatology. 2010;52:1543–1552 [DOI] [PubMed] [Google Scholar]
- 39.Musso G, Cassader M, Gambino R. Diagnostic accuracy of adipose insulin resistance index and visceral adiposity index for progressive liver histology and cardiovascular risk in nonalcoholic fatty liver disease. Hepatology. 2012;56:788–789 [DOI] [PubMed] [Google Scholar]
- 40.Amato MC, Giordano C, Galia M et al. ; for the AlkaMeSy Study Group. Visceral adiposity index (VAI): a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33:920–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic hyperin-sulinemic clamp. J Clin Endocrinol Metab. 2010;95:3347–51. [DOI] [PubMed] [Google Scholar]
- 42.Sterling RK, Wahed AS, King WC, et al. and the HBV-HIV Cohort Study of the Hepatitis B Research Network. Spectrum of Liver Disease in Hepatitis B Virus (HBV) Patients Co-infected with Human Immunodeficiency Virus (HIV): Results of the HBV-HIV Cohort Study. Am J Gastroenterol. 2019;114:746–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghany MG, Perrillo R, Li R et al. Hepatitis B Research Network; Hepatitis B Research Network. Characteristics of adults in the hepatitis B research network in North America reflect their country of origin and hepatitis B virus genotype. Clin Gastroenterol Hepatol. 2015;13:183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Bisceglie AM, Lombardero M, Teckman et al. Research Network HBRN. Determination of hepatitis B phenotype using biochemical and serological markers. J Viral Hepat. 2017;24:320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.NIAAA. What is "low-risk" drinking? National Institute on Alcohol Abuse and Alcoholism. http://rethinkingdrinking.niaaa.nih.gov/IsYourDrinkingPatternRisky/WhatsLowRiskDrinking.asp, Accessed July 15, 2016 [Google Scholar]
- 46.Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis. 2012;32:3–13 [DOI] [PubMed] [Google Scholar]
- 47.Chon YE, Jung KS, Kim SU, Park JY, Park YN, Kim DY, Ahn SH, Chon CY, Lee HW, Park Y, Han KH. Controlled attenuation parameter (CAP) for detection of hepatic steatosis in patients with chronic liver diseases: a prospective study of a native Korean population. Liver Int. 2014;34:102–109 [DOI] [PubMed] [Google Scholar]
- 48.Xu L, Lu W, Li P et al. A comparison of hepatic steatosis index, controlled attenuation parameter and ultrasound as noninvasive diagnostic tools for steatosis in chronic hepatitis B. Dig Liver Dis. 2017;49:910–917 [DOI] [PubMed] [Google Scholar]
- 49.Borman MA, Ladak F, Crotty P et al. The Fatty Liver Index has limited utility for the detection and quantification of hepatic steatosis in obese patients. Hepatol Int. 2013;7:592–599 [DOI] [PubMed] [Google Scholar]
- 50.Prati D, Taioli E, Zanella A et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.