Abstract
SARS-CoV-2 variants have continuously emerged even as highly effective vaccines have been widely deployed. Reduced neutralization observed against variants of concern (VOC) raises the question as to whether other antiviral antibody activities are similarly compromised, or if they might compensate for lost neutralization activity. In this study, the breadth and potency of antibody recognition and effector function was surveyed in both healthy individuals as well as immunologically vulnerable subjects following either natural infection or receipt of an mRNA vaccine. Considering pregnant women as a model cohort with higher risk of severe illness and death, we observed similar binding and functional breadth for healthy and immunologically vulnerable populations. In contrast, considerably greater functional antibody breadth and potency across VOC was associated with vaccination than prior infection. However, greater antibody functional activity targeting the endemic coronavirus OC43 was noted among convalescent individuals, illustrating a dichotomy in recognition between close and distant human coronavirus strains that was associated with exposure history. Probing the full-length spike and receptor binding domain (RBD) revealed that antibody-mediated Fc effector functions were better maintained against full-length spike as compared to RBD. This analysis of antibody functions in healthy and vulnerable populations across a panel of SARS-CoV-2 VOC and extending through endemic alphacoronavirus strains suggests the differential potential for antibody effector functions to contribute to protecting vaccinated and convalescent subjects as the pandemic progresses and novel variants continue to evolve.
One Sentence Summary
As compared to natural infection with SARS-CoV-2, vaccination drives superior functional antibody breadth raising hopes for candidate universal CoV vaccines.
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the virus causing the COVID-19 pandemic, has continued to evolve despite the widespread use of multiple highly effective vaccines1–3. Notable SARS-CoV-2 variants have arisen and expanded across the globe, including Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and most recently Omicron (B.1.1.529). While vaccination is a critical intervention in combatting the pandemic, many studies of both infection and vaccination have shown reduced neutralizing potency toward variants of concern (VOC)4–14.
Fortunately, studies have also shown that both infection and vaccination lead to measurable levels of antibodies and T cell responses against VOC15–23. Because binding antibodies can elicit potent antibody Fc domain-dependent effector functions that contribute to protection even in the absence of neutralization24–27, they may help to compensate for compromised neutralization potency. These activities, including antibody dependent cellular phagocytosis (ADCP), cellular cytotoxicity (ADCC), and complement deposition (ADCD), rely on interactions with soluble and cell-expressed antibody Fc receptors on diverse innate immune cells that can drive direct lysis of virions or infected cells, as well as trigger inflammatory cascades to amplify host defense13,28–31. Fc effector functions have correlated with protection in animal models of SARS-CoV-2 and in humans in other disease settings27,32–35. The potential clinical relevance of antibody-mediated Fc effector functions is suggested by observations that vaccines appear to remain highly effective in preventing serious disease despite reduced levels of neutralizing antibodies.
Importantly, studies have shown that Fc effector activities are elicited following vaccination and infection25,28,36 and antibodies with the capacity to elicit multiple effector functions appear to recognize diverse epitopes on the spike (S) protein25,37. SARS-CoV-2 variants generally accrue most mutations in the receptor binding domain (RBD) and the N terminal domain (NTD), where the most common epitopes for neutralizing Abs are found38–40. The emergence of the Omicron variant, with far more mutations than other VOC, including in other regions of the spike protein, has led to reductions in neutralization potency against this variant8,41,42. These observations have raised interest in determining whether and which other antibody activities may help confer protection from severe disease from sequence distant strains, and how well these responses are induced by infection or vaccination.
To this end, immunity resulting from vaccination and natural infection are known to exhibit a number of distinctions with respect to antibody responses15,43,44. Within groups, individuals also show considerable variability in response magnitudes and characteristics45–47. Lastly, populations at increased risk for severe disease, such as pregnant women48, may also exhibit further distinctions in humoral response attributes49. How these distinct axes of variability associate with the magnitude and breadth of antibody effector functions across VOC is not known, but has important implications for continued protection of diverse individuals and in the face of further viral diversification. The breadth of Fc effector functions across VOC in vulnerable and healthy subjects, following natural infection or vaccination, can provide new insight into the potential contributions of mechanisms beyond neutralization to protection from SARS-CoV-2 disease.
Results
Distinct Ig isotype responses induced by infection and vaccination across SARS-CoV-2 VOC, independent of immunological vulnerability
To study antibody Fc mediated effector functions across SARS-CoV-2 VOC, serum samples from individuals who either received two doses of an approved mRNA vaccine (n=87), were previously infected when the Wuhan strain was predominant (n=57), or were SARS-CoV-2 naïve (n=38) (Table 1) were first evaluated for the magnitude and characteristics of antibody responses to various CoV strains and subdomains (Supplemental Table 1). As a model of a uniquely vulnerable population, a subset of samples from vaccinated (n=50) and convalescent (n=38) individuals were collected from pregnant women, who are at greater risk of hospitalization for covid-1948. To define similarities and differences in antibody profiles among seropositive individuals, dimensionality reduction was performed on biophysical antibody features using Uniform Manifold Approximation and Projection (UMAP)50. Subjects were distributed across the profile landscape into three distinct clusters, which were almost perfectly segregated by whether their responses were elicited by vaccination or infection (Figure 1A). In contrast, major differences between pregnant and non-pregnant individuals were not observed, motivating combined analysis of subjects at different risk levels for subsequent analysis.
Table 1. Cohort characteristics.
NA indicates not applicable or available, and IQR indicates interquartile range. Adapted from Crowley, et. Al, 2021.
| Characteristic | Convalescent n=19 |
Pregnant Convalescent n=38 |
Vaccinated n=37 |
Pregnant Vaccinated n=50 |
Naïve controls n=38 |
|---|---|---|---|---|---|
| Median age (IQR), years | 52 (45–62) | 31 (27–35) | NA | 32 (29–35) | 39 (28–50) |
| Age range (n, %), years | - | - | 21–30 (14, 38%) 31–40 (8, 22%) 41–50 (10, 27%) 51–60 (5, 14%) |
- | - |
| Sex (n, %) | |||||
| Female | 10 (53%) | 38 (100%) | 17 (46%) | 50 (100%) | 22 (58%) |
| Male | 9 (47%) | 0 (0%) | 20 (54%) | 0 (0%) | 16 (42%) |
| Median days since PCR+ or symptom onset (IQR) | 37 (31–43) | 49 (22–78) | NA | NA | NA |
| Median days since second vaccine dose (IQR) | NA | NA | 8 (7–11) | 20 (12–29) | NA |
| Location | US | Belgium | US | Israel | US |
| IRB | DHMC | CHU St. Pierre | JHMI | Hadassah Medical Center | BiolVT clinical sites |
| Collection period | March 2020 – April 2020 | June 2020 – December 2020 | December 2020 – February 2021 | February 2021 | October 2020 |
| Symptoms or positive test | April 2020 – June 2020 | March 2020 – November 2020 | NA | NA | NA |
| Predominant strain | Wuhan | Wuhan | NA | NA | NA |
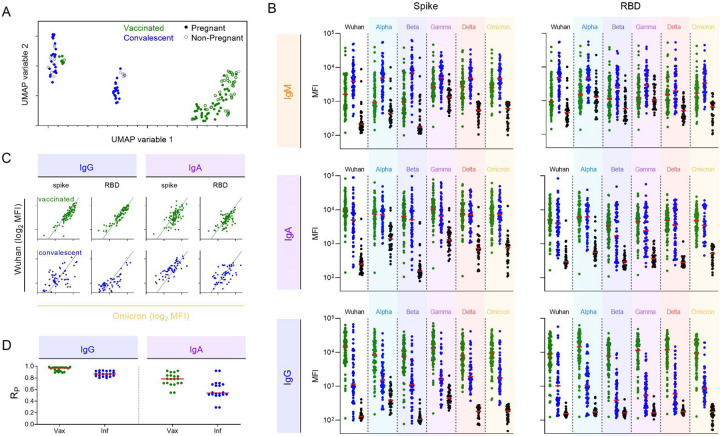
Figure 1. IgM, IgA, and IgG Antibody responses to VOCs following mRNA vaccination or natural infection.
A. Coronavirus-specific antibody response features after dimensional reduction in pregnant (filled) and non-pregnant (open) individuals who were previously infected (blue) or vaccinated (green). B. Median Fluorescent Intensity (MFI) of IgM (top), IgA (center), and IgG (bottom) responses to spike (left) and RBD (right) of SARS-CoV-2 VOCs as defined by multiplex assay. Responses among SARS-CoV-2 naïve subjects are shown in black. Bar indicates median response. C. Representative scatterplots between IgG (left) and IgA (right) responses specific for Wuhan (y-axis) and Omicron (x-axis) in subjects following vaccination (top) or infection (bottom). Diagonal line indicates x=y. D. Pearson correlation coefficient (RP) for IgG (left) and IgA (right) responses across all pairs of variants in vaccinated (green) and infected (blue) subjects.
IgM, IgA, and IgG responses to both the complete spike extracellular domain or the receptor binding domain (RBD) from the ancestral Wuhan strain as well as Alpha, Beta, Gamma, Delta, and Omicron differed among convalescent and vaccinated subjects and naïve controls (Figure 1B). Despite collection at a somewhat later timepoint following antigen exposure, IgM responses were elevated among individuals in the natural infection cohort. In contrast, vaccinated subjects had higher levels of IgG antibodies to both spike and RBD across diverse strains. Medians and ranges in IgA response magnitudes were generally similar between groups, though responses to RBD from some strains appeared bimodal among convalescent individuals with a dichotomy of high and low responders. Distinctions between the two clusters of convalescent subjects in the UMAP analysis can be explained by the bimodal distribution.
To begin to support generalization of observations about relative responses across diverse VOC, correlations were calculated between Wuhan and other VOC for each antibody isotype in each subject group. As can be seen for Omicron, the most sequence distinct variant tested (Figure 1C), responses were generally more strongly correlated for IgG than IgA, for RBD than spike, and for vaccinated subjects than convalescent subjects (Figure 1D). For IgG responses, strong correlations were observed between variants, providing evidence that subjects with high levels of antibody binding to WT are also likely to have high levels of antibodies that bind to future variants. Likewise, these results suggest that individuals who generate lower levels of antibody to contemporaneously circulating strains will have lower levels of antibodies that cross-react to future variants.
Breadth of Antibody responses varies by antigen exposure history and isotype
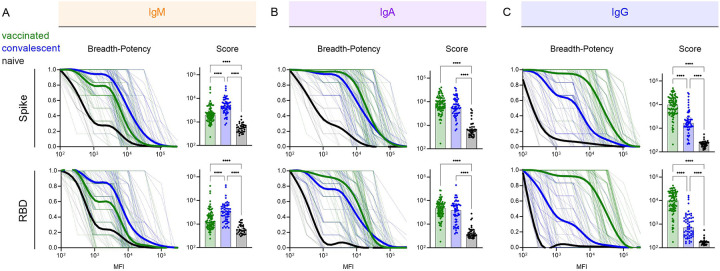
Because the breadth of the antibody binding responses across VOC may relate to infection risk for future variants, antibody binding breadth was assessed for each subject. Breadth-potency curves and breadth scores, defined as the geometric mean response across variants, were calculated for IgM, IgA, and IgG (Figure 2A–C) for the panel of VOC across the full spike extracellular domain or only the RBD. Each isotype showed a distinct breadth profile. IgM breadth was greater in convalescent than vaccinated subjects (Figure 2A). IgA breadth was similar (Figure 2B), and IgG breadth was considerably greater among vaccinated individuals (Figure 2C). Differences in breadth were somewhat more pronounced for RBD than for spike.
Figure 2. Variable Ab response isotype breadth across VOC.
A-C. (Left) Breadth-potency curves representing the fraction of subjects with a response exceeding a given level for IgM (A), IgA (B), and IgG (C) antibody responses. Population mean for naïve (black), vaccinated (green), and convalescent (blue) subjects is shown with a thick line and individual subjects are illustrated in thin lines. (Right) Breadth scores for each subject. Bar indicates mean. Statistical significance was defined by ANOVA Kruskal-Wallis test with Dunn’s correction (****p<0.0001). Response to spike are shown at top, and to RBD at bottom.
Vaccination induces breadth in IgG subclasses and FcγR binding-propensity across SARS-CoV-2 VOC
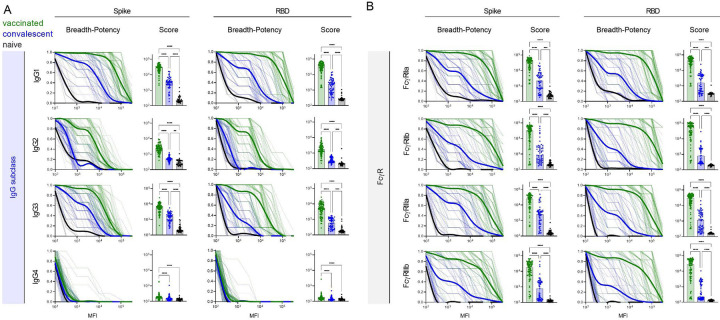
The robust breadth of the overall IgG response led us to further explore potential differences in subclass and FcγR -binding capacity among antibodies to VOC, as both are known to mediate differences in antibody effector functions35,51,52. Breadth-potency curves and breadth scores for each IgG subclass against the spike and RBD antigens were calculated (Figure 3A). Each IgG subclass response was broader and more potent across VOC in seropositive subjects than in naïve controls, and breadth and potency was also universally greater in vaccinated than convalescent subjects. However, the relative magnitude of these differences varied among subclasses. Differences between seropositive subject groups were most pronounced for IgG1, IgG2, and IgG3, whereas IgG4 responses, though distinct from controls, were low in both groups of seropositive subjects. Compared to IgG1 and IgG3, IgG2 responses in convalescent subjects were more similar to naïve subjects than to those observed in vaccinated individuals. Collectively, these profiles show the greatest magnitude in antibody breadth for the most cytotoxic IgG subclasses (IgG1 and IgG3), intermediate breadth for moderately cytotoxic IgG2, and low breadth and potency for the relatively inert IgG4 subclass. Again, differences in the breadth among subclasses between vaccinated and convalescent subjects tended to be greater in RBD than whole spike.
Figure 3. Vaccinated subjects exhibit potentiated IgG subclass and FcγR-binding responses across VOC.
A. (Left) Breadth-potency curves representing the fraction of subjects with a response exceeding a given level for IgG1, IgG2, IgG3, and IgG4 antibody responses. Population mean for naïve (black), vaccinated (green), and convalescent (blue) subjects is shown with a thick line and individual subjects are illustrated in thin lines. (Right) IgG subclass breadth scores for each subject. Bar indicates mean. B. (Left) Breadth-potency curves representing the fraction of subjects with a response exceeding a given level for FcγRIIa, FcγRIIb, FcγRIIIa, and FcγRIIIb-binding antibody responses. Population mean for naïve (black), vaccinated (green), and infected (blue) subjects is shown with a thick line and individual subjects are illustrated in thin lines. (Right) IgG subclass breadth scores for each subject. Bar indicates mean. Statistical significance was defined by ANOVA Kruskal-Wallis test with Dunn’s correction (**p<0.01, ***p<0.001****p<0.0001). Response to spike are shown at left, and to RBD at right.
To further explore potential differences in the antiviral activity of SARS-CoV-2-specifc antibody responses, their ability to bind to recombinant FcγR tetramers was assessed. Again breadth-potency curves, and breadth scores were calculated (Figure 3B), and were universally elevated among seropositive subjects relative to naïve controls, and in vaccinated subjects relative to convalescent subjects. In general, the magnitude of differences in breadth and potency between vaccinated and convalescent subjects were greater in FcγR binding than in measures of individual IgG subclasses, or total IgG. These differences were again somewhat greater for RBD than whole spike, and exhibited differences of up to almost two orders of magnitude in median breadth score, suggesting that antibodies elicited by vaccination may be highly functional against SARS-CoV-2 variants.
Greater breadth of antibody effector functions across SARS-CoV-2 VOCs
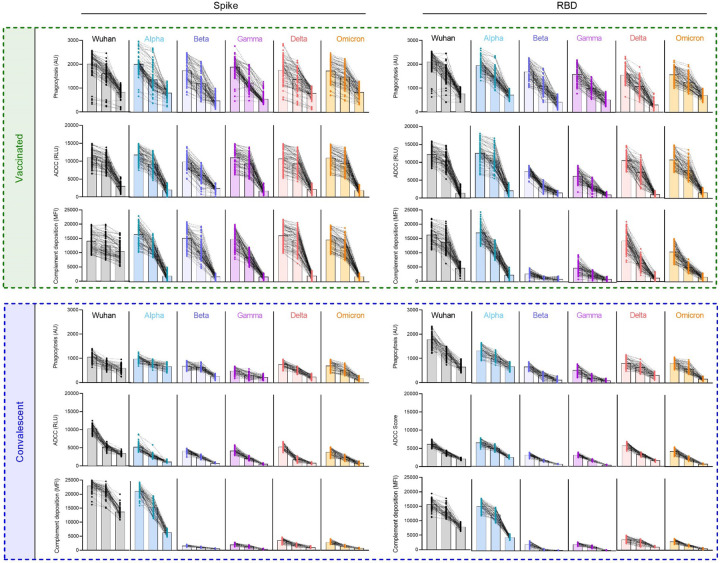
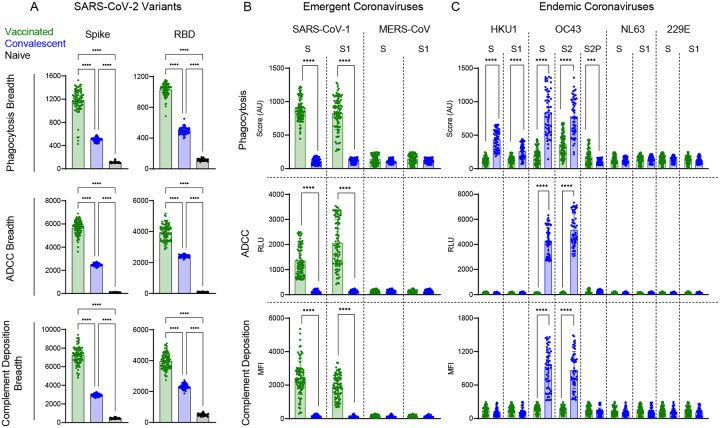
While the neutralization potency of antibodies raised from immunization or infection with ancestral strains is known to be reduced toward new VOC53,54, whether similar losses in the extent of antibody effector functions are observed is less well studied11,36,55. For each subject, phagocytosis, ADCC, and complement deposition activities were assessed using full length spike and RBD antigens for a panel of VOC across three different serum concentrations (Figure 4). With the exception of complement deposition against Wuhan strain whole spike protein, functional antibody responses were equal or greater in vaccinated than convalescent subjects for both Wuhan and diverse VOC. Among effector functions tested, complement deposition was the activity most strongly impacted by strain differences, for example, showing high activity in convalescent subjects only for Wuhan and alpha strains. It was also the most sensitive to serum concentration. Whereas phagocytosis and ADCC activity across VOC were reasonably well conserved at high and intermediate serum concentrations, and often remained detectable at the lowest serum concentration in vaccinated subjects, decreases in activity were at intermediate concentrations were more pronounced in complement deposition activity. Functional responses to RBD were somewhat more sensitive to decreasing serum concentration than were those to spike. This difference was most apparent in ADCC and complement deposition activities observed against beta and gamma strain RBD in vaccinated subjects. Overall, breadth scores for each effector function showed the dramatically improved breath of functional antibody responses across SARS-CoV-19 variants among vaccinated as compared to convalescent subjects toward both spike and RBD (Figure 5A), and suggest that viral variation has a greater effect on neutralization than on Fc-dependent effector functions.
Figure 4. mRNA vaccination results in superior breadth of SARS-CoV-2-specific Ab effector function.
Ab effector function activities directed to spike (left) and RBD (right) in vaccinated (top) and convalescent (bottom) subjects. Phagocytosis, ADCC, and Complement deposition activities were assessed at each of three serum dilutions (1:50, 1:100, 1:250) for each indicated SARS-CoV-2 variant. Individual traces for each subject across dilutions are displayed. Bar indicated the median activity.
Figure 5. Functional breadth across hCoV is imprinted by vaccination or infection history.
A. Functional breadth in vaccinated (green), convalescent (blue), and naïve (black) subjects in phagocytosis (top), ADCC (center), and complement deposition (bottom) as defined by the geometric mean of each activity directed to spike (left) and RBD (right) across variants. Bar indicates mean. Statistical significance was defined by ANOVA Kruskal-Wallis test with Dunn’s correction (****p<0.0001). B-C. Phagocytosis (top), ADCC (center), and complement deposition (bottom) activities observed in vaccinated (green) and convalescent (blue) subjects across other emergent (B) and endemic (C) CoV spike antigens consisting of unstabilized (S), stabilized (S2P) and S1 or S2 subdomains of spike from indicated hCoV strains. Statistical differences were measured by Mann Whitney test (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Functional dichotomy observed across more distant Coronaviruses
While breadth of effector function across SARS-CoV-2 variants that have arisen during the pandemic is important as new VOC arise, we also wanted to explore whether Fc effector functions may be elicited towards more distant coronaviruses, including pathogenic coronaviruses, such as SARS-CoV-1 and MERS, which share 80% and 40% sequence identity to SARS-CoV-256,57, as well as against endemic coronaviruses including beta CoV OC43 and HKU1, and alpha CoV NL63, and 229E that historically account for 10–15% of respiratory infections in children and adults each year58. We observed a striking dichotomy between vaccinated and convalescent subjects in terms of the phagocytosis, ADCC, and complement deposition activities across coronavirus strains. While robust SARS-CoV-1-specific effector function was observed for whole S and the S1 domain in vaccinated subjects, cross-reactive functional antibodies to these targets were not observed among convalescent subjects (Figure 5B). In contrast, robust activity toward whole S and the S2 domain of the endemic coronavirus OC43 was detected among convalescent subjects (Figure 5C). Some phagocytic activity was seen against HKU1 in convalescent subjects, although to a lesser extent than OC43. Vaccinated individuals showed some phagocytic activity toward these targets, but no evidence of ADCC or complement deposition. Intriguingly, despite their lower S and S2-specific phagocytic activity, vaccinated subjects exhibited greater phagocytic activity directed to stabilized OC43 spike (S2P), suggesting that not only sequence but conformational state are key factors in defining antibody cross-reactivity profiles among even distantly related coronaviruses. Neither subject group exhibited functional responses to MERS S or its S1 domain, or to other alphacoronaviruses 229E and NL63. In sum, both natural infection and vaccination appear to induce antibodies with better maintained effector function breadth than neutralization breadth. However, the dichotomy in functional breadth between VOC, emergent, and endemic CoV observed between vaccinated and convalescent subjects, coupled with distinctions in antibody isotypes and preferences in recognition of different conformational states and subdomains point to distinctions in responses that may relate to site and persistence of antigen exposure, conformation of antigen, or other factors that could be used to improve functional breadth in pursuit of “universal” CoV vaccines.
Discussion
The continued emergence of SARS-CoV-2 variants has raised concern that vaccines based on the Wuhan strain will exhibit reduced efficacy over time in the face of viral evolution14. This fear is further exacerbated in vulnerable populations among which vaccine effectiveness is already reduced59,60. Whereas many studies have shown that vaccination results in reduced neutralization of variants as compared to the Wuhan strain30,54, vaccines remain protective from severe disease61, suggesting that either a threshold effect exists or that contributions from other mechanisms may be at play. This latter possibility is well-supported by studies providing evidence that Fc effector functions are more resistant to changes from VOC pointing to a potential component of protection from severe disease18,55.
The continually evolving viral landscape of SARS-CoV-2 strains shows the importance of needing to further understand how effector functions may provide protection from severe disease when vaccine neutralization is reduced. To this end, and relative to the more considerably reduced neutralization potencies expected, we report well-maintained antibody effector functions across diverse VOC. Broad effector function was observed for both vaccinated and convalescent subjects, and for both whole spike and RBD, although the former was somewhat broader than the latter. This data suggests that for VOC against which neutralization activity may be insufficient, Fc-mediated effector functions have the potential to compensate for decreased neutralization and contribute to protection.
This study shows that the breadth of antibody recognition across SARS-CoV-2 VOC varies by isotype and by antigen exposure history. Whereas vaccinated subjects showed considerably greater FcγR-binding IgG antibodies of diverse subclasses, convalescent subjects exhibited greater IgM breadth. IgA breadth was comparable between populations. The distinctions among isotypes may reflect differences in exposure route, duration, and costimulatory factors. As mucosal vaccine continue to advance toward the clinic, these possibilities can be tested. Our data also suggests that the differences observed in the breadth of recognition of VOC and emergent CoV as compared to endemic CoV between vaccination and infection likely at least partially relate to distinctions in antigenic conformations between native and proline-stabilized forms of spike. Additionally, binding antibody breadth does not appear to simply scale with greater IgG response magnitude, as for each antigen tested, at least some convalescent subjects exhibited responses of similar magnitude to vaccinated subjects, yet no convalescent subject exhibited a similar breadth score. Instead of response magnitude, these differences in breadth across VOC may relate to differences at the level of B cell responses between vaccinees and convalescent individuals6,62. Considerable antibody breadth and potency across VOC was similarly observed for diverse antibody effector functions, including phagocytosis mediated by monocytes, a surrogate measure of ADCC, and complement deposition. Among these functions, complement deposition was generally more sensitive to both antigenic variation and to serum concentration than the other activities tested.
While the observation of greater breadth and potency induced by vaccination than natural infection could be extended to SARS-CoV-1, neither exposure induced functional antibodies to MERS, and depending on spike conformation, the opposite pattern was observed for the endemic CoV OC43. These observations establish an intriguing dichotomy: breadth across CoV-2 strains was greater among vaccine recipients, but breadth across more distant beta and alpha coronaviruses was greater among convalescent subjects. While the origin of this dichotomy cannot be defined from this study, the observation that functional breadth toward stabilized and unstabilized spike differed between populations suggests that it may be related to the conformational state of the spike antigen to which the immune system was exposed. This data suggests differential antigenicity and immunogenicity of the stabilized spike protein used in mRNA vaccines, and has important implications for efforts to develop pan-CoV vaccines.
We initially set out to characterize functional breadth in a vulnerable population, but found only limited differences in the binding and functional profiles of antibodies in pregnant women as compared to healthy controls. While this observation has important implications for vulnerable populations, it is important to note that even in the context of similarly functional antibodies, the effector capacity of susceptible individuals may be compromised due to alterations in the cellular effectors and availability or regulation of complement cascade factors. While the assays used herein have correlated with improved protection in vivo in various settings63–67, only three activities were evaluated. Similarly, only five major VOC were evaluated and subjects were infected or vaccinated with the Wuhan strain. The breadth of effector functions induced by strains other than Wuhan, or against future viral variants may exhibit different or similar degrees of conservation as observed here. Other limitations include the use of surrogate measure of effector function reliant on recombinant antigen and cell lines as opposed to infected cells or virions and primary effectors.
Nonetheless, these observations of well-maintained functional antibody breadth following vaccination and infection, even among vulnerable individuals, have important implications. They suggest that antibodies elicited by either prior infection or vaccination with a given strain have the potential to restrict replication of and disease caused by future variants. Similarly, given evidence that these activities contribute to the efficacy of convalescent plasma68 and monoclonal antibody therapy24,27,69,70, our observations support the potential value of these interventions even in the face of continually diversifying virus. Our observations of enhanced breadth of IgG dependent Fc effector functions provides additional evidence of beneficial aspects of vaccine-induced immunity as compared to natural infection. Future studies could define the impact hybrid immunity resulting from combinations of infection and vaccination, or after combination of distinct vaccine regimens, and variant specific boosters may have on functional breadth in healthy and immune vulnerable populations. Overall, this work provides insights into how vaccines and prior natural infection may provide protection by antibody functional mechanisms. These observations promote vaccination as able to drive superior functional antibody breadth, and set expectations for cross-variant antibody effector function and may serve as a useful comparator in studies of candidate vaccines aimed at improving breadth of protection and in evaluating population susceptibility as exposure histories and infecting virus continue to evolve.
Methods
Human Subjects
Vaccinated subjects (n=87) received two doses of either mRNA-1273 (n=2) or BNT162b2 (n=85) vaccines. Vaccinated subjects were pregnant women in Israel (n=50), who were screened for lack of anti- N SARS-CoV-2 antibody respones or non-pregnant subjects from the United States (n=37), among which four subjects had prior history of SARS-CoV-2 infection. Convalescent subjects were pregnant women from Belgium (n=38) or non-pregnant subjects from Dartmouth Hitchcock Medical Center in the United States (n=19) with infection status defined by RT-PCR. Collection of these samples occurred when Wuhan was the dominant strain in circulation, but viruses were not typed. Naïve serum was obtained from a commercial source prior to approval of vaccines and was screened for anti-N SARS-CoV-2 antibody responses to exclude donors with previous infection. Characteristics for each cohort are described in Table 1. Subjects provided informed written consent and studies were reviewed and approved by IRBs at individual collection sites and Dartmouth.
Antigen and Fc Receptor Expression
Antigens were purchased from commercial sources or transiently expressed in Expi293 or HEK293 cells and purified via affinity chromatography following manufacturers protocols (Supplemental Table 1). Fc receptors were expressed and purified as described previously71.
Fc Array
Antigen-specific antibodies were characterized using the Fc array assay as described previously72. Briefly, antigens were covalently coupled to MagPlex microspheres (Luminex Corporation) using two-step carbodiimide chemistry. Experimental controls included pooled human polyclonal serum IgG (IVIG), S309 an antibody from a SARS-CoV patient that cross-reacts SARS-CoV and SARS-CoV-2, and VRC01, an HIV specific antibody73,74. Serum dilutions used in experiments were based on experience from previous work and a small pilot experiment of test concentrations. Final dilutions used in assays varied from 1:250 to 1:5000 depending on detection reagent. Antigen-specific antibodies were detected by R-phycoerythrin-conjugated secondary reagents specific to human immunoglobulin isotypes and subclasses and by Fc receptor tetramers as described previously75,76. Median fluorescent intensity data was acquired on a FlexMap 3D array reader (Luminex Corporation). Samples were run in technical duplicates.
Phagocytosis
Characterization of the phagocytic activity of serum antibodies was performed as described previously77. Briefly, 1 uM yellow-green fluorescent beads (Thermo Fisher, F8813) were covalently conjugated to spike or RBD antigens. Beads were then incubated with serum samples for 4 hr with THP-1 cells (ATCC TIB-202). Cells were fixed and analyzed by flow cytometry using a MACSQuant Analyzer (Miltenyi Biotec). Scores were calculated as the percentage of cells that phagocytosed one or more fluorescent beads multiplied by the MFI of this population. S309 and VRC01 antibodies were included as positive and negative controls, respectively. Additional control wells with no added antibody were used to determine the level of antibody-independent phagocytosis. Serum samples were assayed at three different dilutions, which were determined by an initial pilot experiment to determine the optimal dilution series for measuring signal compared to background. All samples were run in three biological replicates.
Antibody Dependent Cellular Cytotoxicity (ADCC)
A surrogate for antibody-mediated cellular cytotoxicity was measured using a CD16 reporter assay system used previously (78). Jurkat Lucia NFAT (Invivogen, jktl-nfat-cd16) cells were cultured according to manufacturer’s instructions. Cultured cells express CD16 (FcγRIIIa) which when engaged on the cell surface leads to luciferase secretion from the cell. First, high binding 96-well plates were coated overnight at 4°C with 1 μg/mL of spike or RBD antigen. Following incubation, plates were washed (PBS + 0.1% Tween20) and blocked (PBS + 2.5% BSA) at room temperature for 1 hr. Following plate washing, 100,000 cells per well and dilute serum samples were added to each well in cell culture media lacking antibiotics in a 200 μl volume. Following 24 hr incubation, 25 μL of supernatant from each well was transferred into a white 96 well plate which 75 μL of quantiluc substrate was immediately added. Following 10 min incubation, plates were read on a SpectraMax plate reader (Molecular Devices). VRC01 was used as a negative control; cell stimulation cocktail (Therm, 00-4970-93) and ionomycin and S309 served as positive controls. All samples were run in three biological replicates.
Antibody Dependent Complement Deposition (ADCD)
Antibody-dependent complement deposition (ADCD) experiments were performed essentially as previously described79. Serum samples were first heat inactivated at 56°C for 30 min. Samples were then incubated for 2 hr at room temperature with assay microspheres. The optimal dilutions for serum was determined from a small pilot experiment testing a range of dilutions. Human complement serum (Sigma, S1764) was diluted 1:100 in gel veronal buffer (Sigma-Aldrich, GVB++, G6514) and mixed with samples at RT with shaking for 1 hr. After washing, samples were incubated with murine anti-C3b (Cedarlane #CL7636AP) at RT for 1 hr followed by staining with anti-mouse IgG1-PE secondary Ab (Southern Biotech #1070–09) at RT for 30 min. A final wash was performed and samples were resuspended into Luminex sheath fluid and MFI acquired on a FlexMap 3D reader. Assay controls with no antibody, an irrelevant VRC01 antibody, and with heat-inactivated complement were used a negative controls. S309 was used as a positive control. All samples were run in three biological replicates.
Data Analysis and Statistical Quantification
Statistical analysis was performed in GraphPad Prism (version 9.7). Breadth-potency curves were defined as the proportion of antigen-specificities exhibiting a signal above a given intensity. Curves were generated using the LOWESS curve fit method in Prism for each respective subject group. Breadth scores were calculated by taking the geometric mean across antigen specificities for each subject. The sample size for each figure includes all subjects from their respective groups.
Supplementary Material
Acknowledgements
We would like to thank all participants who enrolled in this study and the study and laboratory staff who helped collect and process the samples. A number of antigen expression constructs were provided by Dr. Jason McLellan (UT Austin), and the positive control mAb S309 was provided by Dr. Jiwon Lee (Dartmouth). The following reagent was produced under HHSN272201400008C and obtained through BEI Resources, NIAID, NIH: Spike Glycoprotein Receptor Binding Domain (RBD) from SARS-Related Coronavirus 2, Wuhan-Hu-1 with C-Terminal Histidine Tag, Recombinant from Baculovirus, NR-52307. The following reagent was deposited by the Centers for Disease Control and Prevention and obtained through BEI Resources, NIAID, NIH: SARS-Related Coronavirus 2, Isolate USA-WA1/2020, NR-52281.
Funding
This work was supported in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, as well as extramural support from the National Institute of Allergy and Infectious Diseases (R01AI120938, R01AI120938S1 and R01AI128779 to A.A.R.T), National Heart Lung and Blood Institute (K23HL151826 to E.M.B), National Cancer Institute (2 P30 CA 023108-41 to M.E.A.), National Institute of General Medical Sciences (P20-GM113132 BioMT Molecular Tools Core to M.E.A.). A.M. is Research Director at the F.R.S., FNRS, Belgium.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
References:
- 1.Baden L. R. et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med 384, 403–416, doi: 10.1056/NEJMoa2035389 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson L. A. et al. An mRNA Vaccine against SARS-CoV-2 - Preliminary Report. N Engl J Med 383, 1920–1931, doi: 10.1056/NEJMoa2022483 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack F. P. et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 383, 2603–2615, doi: 10.1056/NEJMoa2034577 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pegu A. et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science 373, 1372–1377, doi: 10.1126/science.abj4176 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sievers B. L. et al. Antibodies elicited by SARS-CoV-2 infection or mRNA vaccines have reduced neutralizing activity against Beta and Omicron pseudoviruses. Sci Transl Med 14, eabn7842, doi: 10.1126/scitranslmed.abn7842 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z. et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature 592, 616–622, doi: 10.1038/s41586-021-03324-6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rees-Spear C. et al. The effect of spike mutations on SARS-CoV-2 neutralization. Cell Rep 34, 108890, doi: 10.1016/j.celrep.2021.108890 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt F. et al. Plasma Neutralization of the SARS-CoV-2 Omicron Variant. N Engl J Med 386, 599–601, doi: 10.1056/NEJMc2119641 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang P. et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 593, 130–135, doi: 10.1038/s41586-021-03398-2 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Zhou D. et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell 184, 2348–2361.e2346, doi: 10.1016/j.cell.2021.02.037 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zohar T. et al. Compromised Humoral Functional Evolution Tracks with SARS-CoV-2 Mortality. Cell 183, 1508–1519.e1512, doi: 10.1016/j.cell.2020.10.052 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng S. et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med 27, 2032–2040, doi: 10.1038/s41591-021-01540-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen R. E. et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nature Medicine 27, 717–726, doi: 10.1038/s41591-021-01294-w (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L. et al. Differential neutralization and inhibition of SARS-CoV-2 variants by antibodies elicited by COVID-19 mRNA vaccines. Nature Communications 13, 4350, doi: 10.1038/s41467-022-31929-6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amanat F. et al. SARS-CoV-2 mRNA vaccination induces functionally diverse antibodies to NTD, RBD, and S2. Cell 184, 3936–3948.e3910, doi: 10.1016/j.cell.2021.06.005 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarke A. et al. Impact of SARS-CoV-2 variants on the total CD4(+) and CD8(+) T cell reactivity in infected or vaccinated individuals. Cell Rep Med 2, 100355, doi: 10.1016/j.xcrm.2021.100355 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arunachalam P. S. et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature 596, 410–416, doi: 10.1038/s41586-021-03791-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geers D. et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci Immunol 6, doi: 10.1126/sciimmunol.abj1750 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarke A. et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 185, 847–859.e811, doi: 10.1016/j.cell.2022.01.015 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dan J. M. et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 371, eabf4063, doi:doi: 10.1126/science.abf4063 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Windsor I. W. et al. Antibodies induced by an ancestral SARS-CoV-2 strain that cross-neutralize variants from Alpha to Omicron BA.1. Science Immunology 7, eabo3425, doi:doi: 10.1126/sciimmunol.abo3425 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redd A. D. et al. Minimal Crossover between Mutations Associated with Omicron Variant of SARS-CoV-2 and CD8(+) T-Cell Epitopes Identified in COVID-19 Convalescent Individuals. mBio 13, e0361721, doi: 10.1128/mbio.03617-21 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redd A. D. et al. CD8+ T-Cell Responses in COVID-19 Convalescent Individuals Target Conserved Epitopes From Multiple Prominent SARS-CoV-2 Circulating Variants. Open Forum Infect Dis 8, ofab143, doi: 10.1093/ofid/ofab143 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beaudoin-Bussières G. et al. A Fc-enhanced NTD-binding non-neutralizing antibody delays virus spread and synergizes with a nAb to protect mice from lethal SARS-CoV-2 infection. Cell Rep 38, 110368, doi: 10.1016/j.celrep.2022.110368 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adeniji O. S. et al. COVID-19 Severity Is Associated with Differential Antibody Fc-Mediated Innate Immune Functions. mBio 12, doi: 10.1128/mBio.00281-21 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson S. I. et al. SARS-CoV-2 Omicron triggers cross-reactive neutralization and Fc effector functions in previously vaccinated, but not unvaccinated, individuals. Cell Host Microbe 30, 880–886.e884, doi: 10.1016/j.chom.2022.03.029 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winkler E. S. et al. Human neutralizing antibodies against SARS-CoV-2 require intact Fc effector functions for optimal therapeutic protection. Cell 184, 1804–1820.e1816, doi: 10.1016/j.cell.2021.02.026 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tauzin A. et al. A single dose of the SARS-CoV-2 vaccine BNT162b2 elicits Fc-mediated antibody effector functions and T cell responses. Cell Host Microbe 29, 1137–1150.e1136, doi: 10.1016/j.chom.2021.06.001 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Beltran W. F. et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 184, 2372–2383.e2379, doi: 10.1016/j.cell.2021.03.013 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas C. et al. Impact of circulating SARS-CoV-2 variants on mRNA vaccine-induced immunity. Nature 600, 523–529, doi: 10.1038/s41586-021-04085-y (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z. et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature 592, 616–622, doi: 10.1038/s41586-021-03324-6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dias A. G. Jr. et al. Antibody Fc characteristics and effector functions correlate with protection from symptomatic dengue virus type 3 infection. Sci Transl Med 14, eabm3151, doi: 10.1126/scitranslmed.abm3151 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gunn B. M. et al. A Fc engineering approach to define functional humoral correlates of immunity against Ebola virus. Immunity 54, 815–828.e815, doi: 10.1016/j.immuni.2021.03.009 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zohar T. et al. Upper and lower respiratory tract correlates of protection against respiratory syncytial virus following vaccination of nonhuman primates. Cell Host Microbe 30, 41–52.e45, doi: 10.1016/j.chom.2021.11.006 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Chung A. W. et al. Polyfunctional Fc-Effector Profiles Mediated by IgG Subclass Selection Distinguish RV144 and VAX003 Vaccines. Science Translational Medicine 6, 228ra238–228ra238, doi:doi: 10.1126/scitranslmed.3007736 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Kaplonek P. et al. mRNA-1273 vaccine-induced antibodies maintain Fc effector functions across SARS-CoV-2 variants of concern. Immunity 55, 355–365.e354, doi: 10.1016/j.immuni.2022.01.001 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ullah I. et al. Live imaging of SARS-CoV-2 infection in mice reveals that neutralizing antibodies require Fc function for optimal efficacy. Immunity 54, 2143–2158.e2115, doi: 10.1016/j.immuni.2021.08.015 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li D., Sempowski G. D., Saunders K. O., Acharya P. & Haynes B. F. SARS-CoV-2 Neutralizing Antibodies for COVID-19 Prevention and Treatment. Annual Review of Medicine 73, 1–16, doi: 10.1146/annurev-med-042420-113838 (2022). [DOI] [PubMed] [Google Scholar]
- 39.Suryadevara N. et al. Neutralizing and protective human monoclonal antibodies recognizing the N-terminal domain of the SARS-CoV-2 spike protein. Cell 184, 2316–2331.e2315, doi: 10.1016/j.cell.2021.03.029 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnes C. O. et al. Structures of Human Antibodies Bound to SARS-CoV-2 Spike Reveal Common Epitopes and Recurrent Features of Antibodies. Cell 182, 828–842.e816, doi: 10.1016/j.cell.2020.06.025 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cui Z. et al. Structural and functional characterizations of infectivity and immune evasion of SARS-CoV-2 Omicron. Cell 185, 860–871.e813, doi: 10.1016/j.cell.2022.01.019 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dejnirattisai W. et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 185, 467–484.e415, doi: 10.1016/j.cell.2021.12.046 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z. et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 595, 426–431, doi: 10.1038/s41586-021-03696-9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bates T. A. et al. Neutralization of SARS-CoV-2 variants by convalescent and BNT162b2 vaccinated serum. Nature Communications 12, 5135, doi: 10.1038/s41467-021-25479-6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Long Q.-X. et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nature Medicine 26, 845–848, doi: 10.1038/s41591-020-0897-1 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Röltgen K. et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Science Immunology 5, eabe0240, doi:doi: 10.1126/sciimmunol.abe0240 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robbiani D. F. et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 584, 437–442, doi: 10.1038/s41586-020-2456-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ellington S. et al. Characteristics of Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status - United States, January 22-June 7, 2020. MMWR Morb Mortal Wkly Rep 69, 769–775, doi: 10.15585/mmwr.mm6925a1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atyeo C. et al. COVID-19 mRNA vaccines drive differential antibody Fc-functional profiles in pregnant, lactating, and nonpregnant women. Sci Transl Med 13, eabi8631, doi: 10.1126/scitranslmed.abi8631 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McInnes L., Healy J. & Melville J. UMAP: Uniform Manifold Approximation and Projection for Dimension Reduction. arXiv 1802.03426 (2020). [Google Scholar]
- 51.Nimmerjahn F. & Ravetch J. V. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science 310, 1510–1512, doi: 10.1126/science.1118948 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Vidarsson G., Dekkers G. & Rispens T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Frontiers in Immunology 5, doi: 10.3389/fimmu.2014.00520 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pajon R. et al. SARS-CoV-2 Omicron Variant Neutralization after mRNA-1273 Booster Vaccination. New England Journal of Medicine 386, 1088–1091, doi: 10.1056/NEJMc2119912 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muik A. et al. Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine–elicited human sera. Science 375, 678–680, doi:doi: 10.1126/science.abn7591 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaplonek P. et al. mRNA-1273 and BNT162b2 COVID-19 vaccines elicit antibodies with differences in Fc-mediated effector functions. Science Translational Medicine 14, eabm2311, doi:doi: 10.1126/scitranslmed.abm2311 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou H. et al. A Review of SARS-CoV2: Compared With SARS-CoV and MERS-CoV. Frontiers in Medicine 8, doi: 10.3389/fmed.2021.628370 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walls A. C. et al. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 181, 281–292.e286, doi: 10.1016/j.cell.2020.02.058 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang C. et al. Antigenic structure of the human coronavirus OC43 spike reveals exposed and occluded neutralizing epitopes. Nature Communications 13, 2921, doi: 10.1038/s41467-022-30658-0 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee A. R. Y. B. et al. Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ 376, e068632, doi: 10.1136/bmj-2021-068632 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zauche L. H. et al. Receipt of mRNA Covid-19 Vaccines and Risk of Spontaneous Abortion. New England Journal of Medicine 385, 1533–1535, doi: 10.1056/NEJMc2113891 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tseng H. F. et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nature Medicine 28, 1063–1071, doi: 10.1038/s41591-022-01753-y (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reynolds C. J. et al. Immune boosting by B.1.1.529 (Omicron) depends on previous SARS-CoV-2 exposure. Science 377, eabq1841, doi:doi: 10.1126/science.abq1841 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ackerman M. E. et al. Route of immunization defines multiple mechanisms of vaccine-mediated protection against SIV. Nat Med 24, 1590–1598, doi: 10.1038/s41591-018-0161-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Felber B. K. et al. Co-immunization of DNA and Protein in the Same Anatomical Sites Induces Superior Protective Immune Responses against SHIV Challenge. Cell Rep 31, 107624, doi: 10.1016/j.celrep.2020.107624 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller-Novak L. K. et al. Analysis of Complement-Mediated Lysis of Simian Immunodeficiency Virus (SIV) and SIV-Infected Cells Reveals Sex Differences in Vaccine-Induced Immune Responses in Rhesus Macaques. J Virol 92, doi: 10.1128/jvi.00721-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Om K. et al. Adjuvanted HIV-1 vaccine promotes antibody-dependent phagocytic responses and protects against heterologous SHIV challenge. PLoS Pathog 16, e1008764, doi: 10.1371/journal.ppat.1008764 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suscovich T. J. et al. Mapping functional humoral correlates of protection against malaria challenge following RTS,S/AS01 vaccination. Sci Transl Med 12, doi: 10.1126/scitranslmed.abb4757 (2020). [DOI] [PubMed] [Google Scholar]
- 68.Bégin P. et al. Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial. Nature Medicine 27, 2012–2024, doi: 10.1038/s41591-021-01488-2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chan C. E. Z. et al. The Fc-mediated effector functions of a potent SARS-CoV-2 neutralizing antibody, SC31, isolated from an early convalescent COVID-19 patient, are essential for the optimal therapeutic efficacy of the antibody. PLOS ONE 16, e0253487, doi: 10.1371/journal.pone.0253487 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamin R. et al. Fc-engineered antibody therapeutics with improved anti-SARS-CoV-2 efficacy. Nature 599, 465–470, doi: 10.1038/s41586-021-04017-w (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boesch A. W. et al. Highly parallel characterization of IgG Fc binding interactions. MAbs 6, 915–927, doi: 10.4161/mabs.28808 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown E. P. et al. High-throughput, multiplexed IgG subclassing of antigen-specific antibodies from clinical samples. J Immunol Methods 386, 117–123, doi: 10.1016/j.jim.2012.09.007 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pinto D. et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 583, 290–295, doi: 10.1038/s41586-020-2349-y (2020). [DOI] [PubMed] [Google Scholar]
- 74.Wu X. et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329, 856–861, doi: 10.1126/science.1187659 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brown E. P. et al. Multiplexed Fc array for evaluation of antigen-specific antibody effector profiles. J Immunol Methods 443, 33–44, doi: 10.1016/j.jim.2017.01.010 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brown E. P. et al. Optimization and qualification of an Fc Array assay for assessments of antibodies against HIV-1/SIV. J Immunol Methods 455, 24–33, doi: 10.1016/j.jim.2018.01.013 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ackerman M. E. et al. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J Immunol Methods 366, 8–19, doi: 10.1016/j.jim.2010.12.016 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Butler S. E. et al. Distinct Features and Functions of Systemic and Mucosal Humoral Immunity Among SARS-CoV-2 Convalescent Individuals. Frontiers in Immunology 11, doi: 10.3389/fimmu.2020.618685 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fischinger S. et al. A high-throughput, bead-based, antigen-specific assay to assess the ability of antibodies to induce complement activation. J Immunol Methods 473, 112630, doi: 10.1016/j.jim.2019.07.002 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.