Abstract
Objectives
Metaplastic breast cancer (MBC) is a rare neoplasm accounting for <1% of all breast cancer. We evaluated the clinical characteristics and survival outcomes of MBC.
Methods
Patients diagnosed with pathologically proven MBC were reviewed from the institutional breast cancer database from 2000 to 2017.
Results
A total of 136 patients diagnosed with MBC were included in the study. The median age of the diagnosis was 60 years, and 60% of patients were stage II at diagnosis, and 22% were stage III. About two-thirds of the patients were triple-negative; 93% had nuclear grade III, and 25% had a lymphovascular invasion. Squamous differentiation (29%) was the most common histologic subtype, followed by the spindle subtype (21%). The most common distant metastases were lung (22%), followed by bone (13%). Moreover, 60% had a mastectomy, 19% had endocrine therapy, 58% had radiation, 51% received anthracycline-based chemotherapy, 26% had non-anthracycline chemotherapy, and 22% received no chemotherapy. In the entire cohort, the two-year overall survival (OS) and five-year OS were 79% and 69%, respectively, and the two-year progression-free survival (PFS) and five-year PFS were 72% and 61%, respectively. On multivariable analysis, the stage of MBC (stage III: hazard ratio (HR), 5.065 (95% confidence interval (CI), 1.02-25.27) (p=0.048)), poor functional status (Eastern Cooperative Oncology Group (ECOG) score, 2; HR, 24.736 (95% CI, 1.92-318.73) (p=0.014)), and distant metastasis to the brain (HR, 8.453 (95% CI, 1.88-38.04) (p=0.005)) and lung (HR, 42.102 (95% CI, 7.20-246.36) (p<0.001)) were significant predictors of decreased OS.
Conclusions
MBC demonstrated early disease progression and poor overall survival. The stage of MBC, decreased performance status, and metastasis to the lung and brain were independent poor prognostic factors.
Keywords: triple negative, prognosis, hormone receptors, radiation, mastectomy, chemotherapy, histology, ecog, metastasis, metaplastic breast cancer
Introduction
Metaplastic breast cancer (MBC) is a rare neoplasm that accounts for less than 1% of all breast cancers, and it is characterized by histologic and molecular heterogeneity. MBC is histologically characterized by the differentiation of the neoplastic epithelium into squamous cells and/or mesenchymal-looking elements [1]. Evidence also suggests that MBC has an aggressive nature and tends to have a worse prognosis of MBC as compared to other breast cancers. This is possibly due to its rarity, tumor heterogeneity, and lack of targeted treatment [2,3]. Various clinical and immunohistochemical factors along with genetic markers have been described in the literature; however, no validated prognostic markers have been identified. To date, MBC remains a clinical challenge for physicians regarding pathogenesis, clinicopathological features, and its management [2]. Most MBCs are triple-negative; hence, they are managed in a similar way to triple-negative breast cancer (TNBC) with anthracycline, taxane, and platinum-based therapy. There is no standard therapeutic approach available for this breast cancer subtype.
In this study, we evaluated the clinical, histopathologic, and molecular characteristics across all locations of our health system. We also sought to identify potential factors attributing to the progression of the disease and survival.
Materials and methods
Patients
The complete list of the study cohort from January 2000 to December 2017 was identified from a report generated from the institutional database. The study was approved by the Cleveland Clinic Institutional Review Board (IRB# 17-404). The database for pathologically proven MBC cases was maintained in the breast tumor registry from three centers in the Cleveland Clinic health system. The inclusion criteria for the study include age > 18 years, female gender, and all pathologically diagnosed and proven cases of MBC from the database. Patients aged <18 years and males were excluded. A total of 136 patients fulfilled the inclusion criteria and were included in the study for analysis.
The retrospective chart review was done using an electronic medical record for data abstraction. Study variables for data collection include age at the diagnosis, demographics, Eastern Cooperative Oncology Group (ECOG) performance status, histopathology, staging, hormone receptor status, lymphovascular invasion, molecular markers, treatment received (surgery, radiation therapy, chemotherapy, and hormonal therapy), date of local and distant progression, and date of death or last follow-up. The stage of MBC was documented based on the breast cancer staging system by the American Joint Committee on Cancer (AJCC).
Pathology
The histopathology of breast tissue was evaluated by an expert pathologist in breast cancer and documented in the electronic medical record. MBC was defined based on the World Health Organization (WHO) classification for breast tumors [1]. It is a unique group of invasive ductal carcinoma, which is characterized by the differentiation of tumor cells into purely epithelial or mixed epithelial and mesenchymal components. The epithelial group includes squamous, adenocarcinoma with spindle cell differentiation, and adenocarcinoma, including mucoepidermoid. Mixed epithelial and mesenchymal components are comprised of carcinoma with chondroid metaplasia, carcinoma with osseous metaplasia, and carcinosarcoma. We broadly categorized histology into four groups for analysis as follows: squamous subtype, spindle cell subtype, mixed epithelial plus mesenchymal differentiation, and other types of metaplastic carcinoma with no special type. Other histology of MBC with no special type includes ductal carcinoma in situ micropapillary pattern, heterologous type with matrix production, high-grade metaplastic carcinoma, large cells with intermingling mature plasma cells, matrix-producing carcinoma, and sarcomatoid features, and metaplastic carcinoma.
Using immunohistochemical stains at our institution, p53, estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) status were assessed. Variables were collected for histologic subtypes; nuclear grades; lymphovascular invasion; PR, ER, and HER2 status; and p63 and BRCA mutation.
Statistical analysis
Categorical data are summarized as frequencies and percentages, whereas continuous data as medians and ranges. Overall survival (OS) and progression-free survival (PFS) were the primary outcomes. OS was calculated from the date of the diagnosis of metaplastic breast cancer to the date of death or last follow-up. PFS was calculated from the date of the diagnosis of MBC to the date of distant progression or local progression of disease or death or follow-up, whichever comes first. Time to event variable was summarized using the Kaplan-Meier method; the log-rank test and univariate cox regression model were used to estimate the association between outcomes and patient clinical and pathological characteristics, including tumor-node-metastasis (TNM) stage (I-IV), distance metastasis, hormone receptor status, histologic subtypes, type of therapy, and performance status. The variables that were selected through stepwise selection were included in the multivariable Cox model. All statistical analyses were performed using R version 3.5.0. p-value < 0.05 was considered statistically significant.
Results
Patient characteristics
The median age at diagnosis was 60 years (27-92 years), with a median follow-up of 51.5 months. Most of the patients were white (n=101 (74%)), followed by black (n=31 (23%)). ECOG performance status was classified as 0, 1, 2, and 3 in 77 (57%), 38 (28%), 12 (9%), and nine (6%) patients, respectively. Lymphovascular invasion was demonstrated in 27 (25.7%) patients; 114 (93.4%) had nuclear grade III, five (4%) had nuclear grade II, and three (2.4%) had nuclear grade I. Eighty-two (60.3%) patients were diagnosed at stage II, 28 (20.6%) at stage I, 19 (14%) at stage III, and seven (5.1%) at stage IV. A total of 101 (74.3%) patients were triple-negative. Estrogen receptor (ER), progesterone receptor (PR), and HER2/neu expression were positive in 22 (16.2%), 12 (8.8%), and 14 (10.3%) patients, respectively; only one patient was triple-positive. On further breakdown for hormone receptor positivity, nine patients had HER2/neu expression, 10 had ER, four had both HER2 and ER, seven had both ER and PR, four had PR only, and one had all three receptors positive.
On histologic evaluation, spindle cell tumor was observed in 29 (21%) patients, and 40 (29%) patients showed squamous subtype. Fifty-five (40%) patients had mixed epithelial plus mesenchymal carcinoma, and 12 (9%) were found to be other types of metaplastic carcinoma with no specific type. Breast pathologists documented the diagnosis of MBC in the chart. Histology documentation in the pathology report was utilized to divide MBCs into different subtypes. Seven patients had BRCA mutations (five had BRCA1 and two had BRCA2). Forty-three (31.6%) patients expressed p63. The most common distant metastases were observed in the lungs (n=30 (22%)), followed by the bone (n=18 (13%)) and brain (n=9 (7%)). The adrenal and spleen were rare sites of metastasis, each occurring in one patient. Fifty (40%) patients had a lumpectomy, 76 (60%) had a mastectomy, 25 (20%) received hormonal therapy, 76 (58%) received radiation, 51% received anthracycline-based chemotherapy, 26% had non-anthracycline chemotherapy, and 22% received no chemotherapy. Unfortunately, not all clinical and pathological data were available for patients included in the study during the chart review. Complete demographics, clinical, and pathological variables are outlined in Table 1.
Table 1. Patient characteristics.
AJCC, American Joint Committee on Cancer; p63, tumor protein p63; MBC, metaplastic breast cancer; ECOG, Eastern Cooperative Oncology Group; NA, data not available from the chart review; HER2, human epidermal growth factor receptor 2
#Other histology in our study includes ductal carcinoma in situ micropapillary pattern, heterologous type with matrix production, high-grade metaplastic carcinoma, large cells with intermingling mature plasma cells, matrix-producing carcinoma, and sarcomatoid features, and metaplastic carcinoma.
| Variables | Level | Overall |
| Age at diagnosis (median (range)) | 60 (27, 92) | |
| Race (%) | Black | 31 (23.5) |
| White | 101 (76.5) | |
| Others | 4 (2.9) | |
| ECOG performance status (%) | 0 | 77 (56.6) |
| 1 | 38 (27.9) | |
| 2 | 12 (8.8) | |
| 3 | 9 (6.6) | |
| Lymphovascular invasion (%) | No | 78 (57.3) |
| Yes | 27 (19.8) | |
| NA | 31 (22.8) | |
| p63 (%) | Negative | 93 (68.4) |
| Positive | 43 (31.6) | |
| BRCA status (%) | BRCA1 positive | 5 (3.7) |
| BRCA2 positive | 2 (1.5) | |
| NA | 129 (94.8) | |
| Nuclear grade (%) | I | 3 (2.4) |
| II | 5 (4) | |
| III | 114 (93.4) | |
| NA | 14 | |
| AJCC stage (%) | I | 28 (20.6) |
| II | 82 (60.3) | |
| III | 19 (14) | |
| IV | 7 (5.1) | |
| Hormonal therapy (%) | No | 103 (80.5) |
| Yes | 25 (19.5) | |
| NA | 8 | |
| Radiation therapy (%) | No | 55 (42) |
| Yes | 76 (58) | |
| NA | 5 | |
| Chemotherapy type (%) | Anthracycline | 70 (51.5) |
| Non-anthracycline | 36 (26.5) | |
| None | 30 (22) | |
| Surgery (%) | Mastectomy | 76 (60.3) |
| Lumpectomy | 50 (39.7) | |
| Histologic subtypes (%) | Spindle cell | 29 (21.3) |
| Squamous | 40 (29.4) | |
| Mixed MBC/mesenchymal | 55 (40.4) | |
| #Others | 12 (8.8) | |
| Triple-negative status (%) | No | 35 (25.7) |
| Yes | 101 (74.3) | |
| Estrogen receptor positivity (%) | No | 114 (83.8) |
| Yes | 22 (16.2) | |
| Progesterone receptor positivity (%) | No | 124 (91.2) |
| Yes | 12 (8.8) | |
| HER2 expression (%) | No | 122 (89.7) |
| Yes | 14 (10.3) | |
| Metastasis to the lung (%) | No | 106 (77.9) |
| Yes | 30 (22.1) | |
| Metastasis to the liver (%) | No | 130 (95.6) |
| Yes | 6 (4.4) | |
| Metastasis to the bone (%) | No | 118 (86.8) |
| Yes | 18 (13.2) | |
| Metastasis to the adrenal (%) | No | 135 (99.3) |
| Yes | 1 (0.7) | |
| Metastasis to the spleen (%) | No | 135 (99.3) |
| Yes | 1 (0.7) | |
| Metastasis to the brain (%) | No | 127 (93.4) |
| Yes | 9 (6.6) |
Survival analysis
At the time of analysis, a total of 44 patients had died, and 36 patients were found to have progression (local or distant). Four patients were excluded from survival analysis due to missing information. Two patients had missing dates of diagnosis, and two patients had missing dates of the last follow-up or date of death. The two-year and five-year OS rates in the entire cohort were 79% and 69%, respectively. We observed a two-year PFS rate of 72% and a five-year PFS rate of 61%.
Progression-free survival
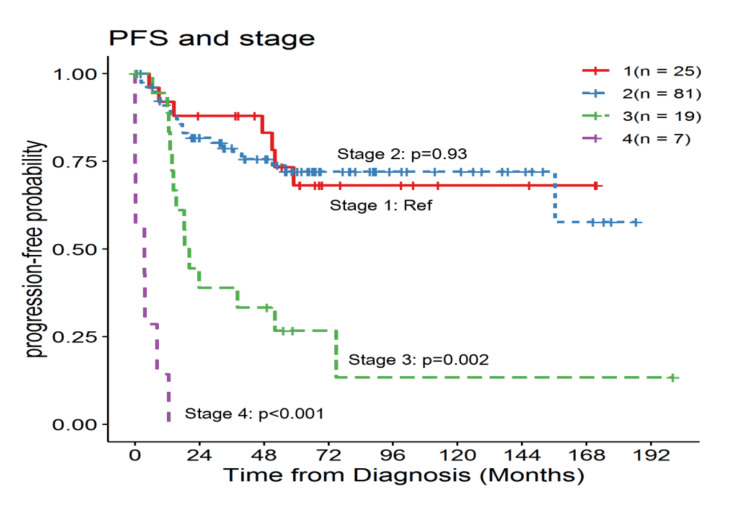
Univariable analysis for PFS was statistically significant for the stage of the MBC and higher functional status (ECOG 0 and 1 ). Lumpectomy and mastectomy were the types of surgery included as a variable for analysis. The five-year PFS rates for stages I, II, and III were 68%, 72%, and 27%, respectively (Figure 1). Stage IV had worse survival with only a one-year PFS rate of 14%. Metastases to the lung, brain, bone, and liver were found to have worse PFS. The complete univariable analysis is summarized in Table 2.
Table 2. Univariable analysis for progression-free survival.
AJCC, American Joint Committee on Cancer; MBC, metaplastic breast cancer; N, number of patients; HR, hazard ratio; CI, confidence interval; NA, not applicable; Ref, reference; ECOG, Eastern Cooperative Oncology Group; HER2, human epidermal growth factor receptor 2
^Four patients were excluded from survival analysis due to missing information such as the date of diagnosis, last follow-up, or date of death.
*Progression was treated as an event and death as a competing risk event.
#Other histology in our study includes ductal carcinoma in situ micropapillary pattern, heterologous type with matrix production, high-grade metaplastic carcinoma, large cells with intermingling mature plasma cells, matrix-producing carcinoma, and sarcomatoid features, and metaplastic carcinoma.
| Variable | Variable level | N^ | Number of events* | One-year rate (% (range)) | Two-year rate (% (range)) | Five-year rate (% (range)) | HR (95% CI) | p-value |
| Race | Black | 31 | 13 | 94% (77%-98%) | 71% (52%-84%) | 61% (41%-75%) | Ref | |
| White | 97 | 35 | 86% (77%-92%) | 73% (62%-81%) | 60% (48%-70%) | 0.93 (0.49-1.76) | 0.83 | |
| Lymphovascular invasion | No | 77 | 23 | 91% (81%-95%) | 81% (70%-88%) | 65% (51%-75%) | Ref | |
| Yes | 27 | 13 | 89% (69%-96%) | 64% (43%-80%) | 56% (34%-73%) | 1.62 (0.81-3.24) | 0.17 | |
| ECOG performance status | 0 | 74 | 18 | 94% (86%-98%) | 85% (75%-92%) | Ref | ||
| 1 | 37 | 12 | 89% (74%-96%) | 78% (61%-88%) | 1.40 (0.67-2.91) | 0.37 | ||
| 2 | 12 | 12 | 58% (27%-80%) | 17% (3%-41%) | 12.19 (5.44-27.30) | <0.001 | ||
| 3 | 9 | 7 | 67% (28%-88%) | 22% (3%-51%) | 8.46 (3.31-21.60) | <0.001 | ||
| p63 | Negative | 89 | 35 | 87% (78%-93%) | 70% (59%-78%) | 59% (48%-69%) | Ref | |
| Positive | 43 | 14 | 88% (73%-95%) | 78% (62%-88%) | 64% (45%-78%) | 0.92 (0.49-1.71) | 0.78 | |
| AJCC stage | I | 25 | 7 | 92% (72%-98%) | 88% (76%-100%) | 68% (51%-91%) | Ref | |
| II | 81 | 21 | 91% (82%-96%) | 82% (73%-91%) | 72% (62%-83%) | 1.05 (0.45-2.48) | 0.90 | |
| III | 19 | 14 | 94% (67%-99%) | 39% (22%-69%) | 27% (12%-59%) | 4.17 (1.67-10.40) | 0.002 | |
| IV | 7 | 7 | 14% (1%-46%) | 44.64 (13.40-148.71) | <0.001 | |||
| Hormonal therapy | No | 100 | 36 | 88% (79%-93%) | 74% (64%-82%) | 62% (51%-72%) | Ref | |
| Yes | 24 | 9 | 91% (70%-98%) | 74% (51%-87%) | 59% (35%-76%) | 1.04 (0.50-2.17) | 0.91 | |
| Radiation therapy | No | 53 | 19 | 81% (67%-89%) | 71% (57%-82%) | 63% (47%-75%) | Ref | |
| Yes | 74 | 28 | 93% (84%-97%) | 73% (61%-82%) | 60% (47%-70%) | 0.99 (0.55-1.77) | 0.97 | |
| Chemotherapy type | Anthracycline | 68 | 21 | 93% (83%-97%) | 80% (69%-88%) | 70% (56%-80%) | Ref | |
| Non-anthracycline | 36 | 13 | 85% (68%-94%) | 73% (55%-85%) | 56% (35%-72%) | 1.48 (0.74-2.98) | 0.27 | |
| Surgery | Mastectomy | 74 | 28 | 89% (79%-94%) | 74% (62%-82%) | 61% (48%-72%) | Ref | |
| Lumpectomy | 48 | 13 | 96% (84%-99%) | 82% (68%-91%) | 69% (52%-81%) | 0.66 (0.34-1.28) | 0.22 | |
| Histologic subtypes | Spindle cell | 27 | 11 | 92% (73%-98%) | 73% (51%-86%) | 56% (33%-74%) | Ref | |
| Squamous | 40 | 19 | 82% (67%-91%) | 65% (48%-78%) | 54% (37%-68%) | 1.15 (0.55-2.42) | 0.71 | |
| Mixed MBC/mesenchymal | 54 | 16 | 88% (76%-95%) | 76% (62%-86%) | 66% (50%-78%) | 0.69 (0.32-1.49) | 0.34 | |
| #Other | 11 | 3 | 91% (51%-99%) | 82% (45%-95%) | 72% (35%-90%) | 0.54 (0.15-1.95) | 0.35 | |
| Estrogen receptor | No | 111 | 41 | 88% (80%-93%) | 73% (63%-80%) | 61% (50%-70%) | Ref | |
| Yes | 21 | 8 | 86% (62%-95%) | 71% (47%-86%) | 61% (36%-78%) | 1.00 (0.47, 2.14) | > 0.99 | |
| Progesterone receptor | No | 121 | 46 | 86% (79%-91%) | 71% (62%-78%) | 60% (50%-69%) | Ref | |
| Yes | 11 | 3 | 100% (NA) | 90% (47%-99%) | 64% (24%-87%) | 0.66 (0.20-2.11) | 0.48 | |
| HER2 expression | No | 119 | 45 | 87% (79%-92%) | 73% (63%-80%) | 59% (49%-68%) | Ref | |
| Yes | 13 | 4 | 92% (57%-99%) | 69% (37%-87%) | 69% (37%-87%) | 0.76 (0.27-2.12) | 0.60 | |
| Metastasis to the lung | No | 102 | 20 | 95% (88%-98%) | 84% (75%-90%) | Ref | ||
| Yes | 30 | 29 | 63% (44%-78%) | 33% (18%-50%) | 11.41 (6.15-21.18) | <0.001 | ||
| Metastasis to the bone | No | 114 | 31 | 90% (83%-94%) | 79% (70%-85%) | Ref | ||
| Yes | 18 | 18 | 72% (46%-87%) | 33% (14%-55%) | 6.65 (3.60-12.28) | <0.001 | ||
| Metastasis to the brain | No | 123 | 40 | 87% (80%-92%) | 76% (67%-83%) | Ref | ||
| Yes | 9 | 9 | 89% (43%-98%) | 22% (3%-51%) | 5.00 (2.38-10.50) | <0.001 | ||
| Metastasis to the liver | No | 126 | 43 | 89% (81%-93%) | 74% (65%-81%) | Ref | ||
| Yes | 6 | 6 | 67% (19%-90%) | 33% (5%-68%) | 5.16 (2.15-12.36) | <0.001 |
Figure 1. Stage-by-stage progression-free survival probability curve.
PFS: progression-free survival; Ref: reference
In multivariable analysis, the stage of MBC (stage II: HR, 5.15 (95% CI, 1.00-26.52) (p=0.05); stage III: HR, 35.90 (95% CI, 5.64-228.59) (p<0.001)), metastasis to the lung (HR, 67.01 (95% CI, 16.65-269.63) (p<0.001)), and metastasis to the brain (HR, 12.28 (95% CI, 3.29-45.84) (p<0.001)) were independent predictors of progression (Table 3). Patients with liver metastasis were observed to have less tendency for progression with an HR of 0.14 (95% CI, 0.03-0.70) (p=0.016).
Table 3. Multivariable analysis for progression-free survival.
HR, hazard ratio; CI, confidence interval; Ref, reference
*Analysis was done using a reference level of no hormonal therapy and no lung, liver, and brain metastases.
| Variable | HR (95% CI) | p-value |
| Stage II (ref = stage I) | 5.15 (1.00-26.52) | 0.05 |
| Stage III (ref = stage I) | 35.90 (5.64-228.59) | <0.001 |
| Stage IV (ref = stage I) | 4,149.63 (139.71-123,249.44) | <0.001 |
| *Hormonal therapy (ref = no) | 0.27 (0.07-1.02) | 0.054 |
| *Lung metastasis (ref = no) | 67.01 (16.65-269.63) | <0.001 |
| *Liver metastasis (ref = no) | 0.14 (0.03-0.70) | 0.016 |
| *Brain metastasis (ref = no) | 12.28 (3.29-45.84) | <0.001 |
Overall survival
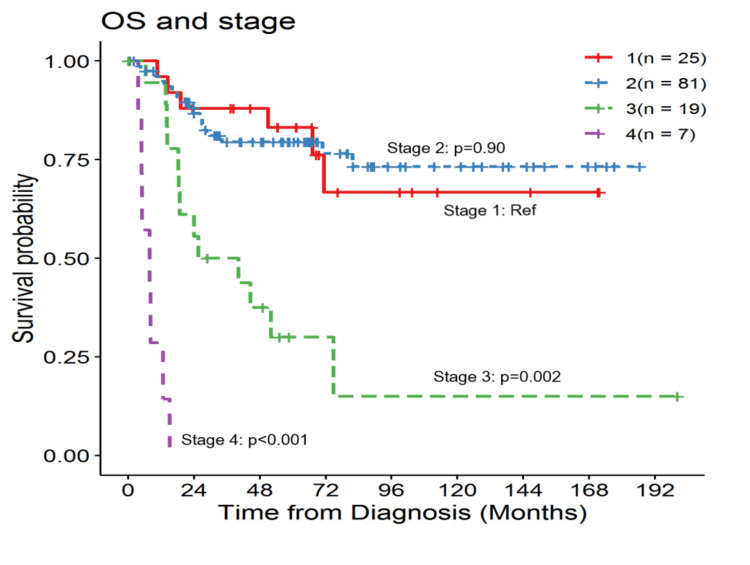
The five-year OS rate at stage I was 83%. There was no difference in OS between stage I and stage II. On univariable analysis with stage I as the reference, the five-year OS rate at stage II was 79% with an HR of 0.96 (95% CI, 0.38-2.44) (p=0.93). The five-year OS at stage III was 30% with an HR of 4.53 (95% CI, 1.71-12.01) (p=0.002). For stage IV, only 29% survived at the end of one year with an HR of 43.26 (95% CI, 12.34-151.64) (p=0.001) (Figure 2). Better functional or performance status (ECOG 0 and 1) demonstrated better five-year OS, and poor functional or performance status (ECOG 3 and 4) was correlated with increased mortality. Lymphovascular invasion, p63 positivity, hormonal therapy, radiation therapy, and histologic subtype did not show better outcomes.
Figure 2. Stage-by-stage overall survival probability curve.
OS, overall survival; Ref: reference
Metastasis to the lung and brain at any time during follow-up was associated with poor survival. In patients with lung metastasis, the two-year and five-year OS rates were 53% and 27%, respectively, with an HR of 7.85 (95% CI, 4.18-14.72) (p<0.001). The complete analysis is summarized in Table 4.
Table 4. Univariable analysis for overall survival.
AJCC, American Joint Committee on Cancer; MBC, metaplastic breast cancer; N, number of patients; HR, hazard ratio; CI, confidence interval; NA, not applicable; Ref, reference; ECOG, Eastern Cooperative Oncology Group; HER2, human epidermal growth factor receptor 2
^Four patients were excluded from survival analysis due to missing information such as the date of diagnosis, last follow-up, or date of death.
*Progression was treated as an event and death as a competing risk event.
#Other histology in our study includes ductal carcinoma in situ micropapillary pattern, heterologous type with matrix production, high-grade metaplastic carcinoma, large cells with intermingling mature plasma cells, matrix-producing carcinoma, and sarcomatoid features, and metaplastic carcinoma.
| Variable | Variable level | N^ | Number of events* | One-year rate (% (range)) | Two-year rate (% (range)) | Five-year rate (% (range)) | HR (95% CI) | p-value |
| Race | Black | 31 | 12 | 94% (77%-98%) | 81% (62%-91%) | 63% (43%-78%) | Ref | |
| White | 97 | 30 | 91% (83%-96%) | 78% (68%-85%) | 70% (60%-79%) | 0.88 (0.45-1.71) | 0.70 | |
| Lymphovascular invasion | No | 77 | 20 | 95% (86%-98%) | 86% (76%-92%) | 75% (63%-84%) | Ref | |
| Yes | 27 | 10 | 96% (76%-99%) | 72% (50%-86%) | 63% (40%-79%) | 1.47 (0.68-3.14) | 0.32 | |
| ECOG performance status | 0 | 74 | 13 | 99% (90%-100%) | 88% (78%-94%) | Ref | ||
| 1 | 37 | 12 | 95% (80%-99%) | 86% (71%-94%) | 1.90 (0.87-4.17) | 0.11 | ||
| 2 | 12 | 12 | 58% (27%-80%) | 25% (6%-50%) | 17.81 (7.55-41.98) | <0.001 | ||
| 3 | 9 | 6 | 78% (36%-94%) | 56% (20%-80%) | 9.31 (3.33-26.09) | <0.001 | ||
| p63 | Negative | 89 | 32 | 93% (85%-97%) | 77% (66%-84%) | 67% (55%-76%) | Ref | |
| Positive | 43 | 11 | 90% (76%-96%) | 85% (70%-93%) | 74% (57%-85%) | 0.80 (0.40-1.58) | 0.51 | |
| AJCC stage | I | 25 | 6 | 96% (75%-99%) | 88% (76%-100%) | 83% (69%-100%) | Ref | |
| II | 81 | 17 | 96% (88%-99%) | 88% (81%-96%) | 79% (71%-89%) | 0.96 (0.38-2.44) | 0.93 | |
| III | 19 | 13 | 94% (67%-99%) | 61% (42%-88%) | 30% (14%-64%) | 4.53 (1.71-12.01) | 0.002 | |
| IV | 7 | 7 | 29% (4%-61%) | 43.26 (12.34-151.64) | <0.001 | |||
| Hormonal therapy | No | 100 | 32 | 92% (84%-96%) | 78% (68%-85%) | 68% (57%-77%) | Ref | |
| Yes | 24 | 7 | 100% (NA) | 96% (73%-99%) | 77% (54%-90%) | 0.86 (0.38-1.96) | 0.72 | |
| Radiation therapy | No | 53 | 16 | 87% (74%-93%) | 77% (63%-86%) | 70% (55%-81%) | Ref | |
| Yes | 74 | 25 | 97% (89%-99%) | 81% (70%-89%) | 69% (56%-78%) | 1.01 (0.54-1.90) | 0.97 | |
| Chemotherapy type | Anthracycline | 68 | 18 | 97% (89%-99%) | 85% (74%-92%) | 77% (64%-85%) | Ref | |
| Non-anthracycline | 36 | 12 | 85% (67%-93%) | 79% (61%-89%) | 67% (46%-81%) | 1.52 (0.73-3.15) | 0.26 | |
| Surgery | Mastectomy | 74 | 24 | 94% (86%-98%) | 82% (71%-89%) | 69% (56%-79%) | Ref | |
| Lumpectomy | 48 | 11 | 98% (85%-100%) | 89% (75%-95%) | 79% (63%-89%) | 0.66 (0.32-1.34) | 0.25 | |
| Histologic subtypes | Spindle cell | 27 | 10 | 92% (73%-98%) | 77% (56%-89%) | 67% (44%-82%) | Ref | |
| Squamous | 40 | 15 | 90% (76%-96%) | 77% (61%-88%) | 64% (46%-77%) | 0.97 (0.43-2.15) | 0.94 | |
| Mixed MBC/mesenchymal | 54 | 15 | 94% (83%-98%) | 80% (66%-89%) | 73% (58%-83%) | 0.71 (0.32-1.58) | 0.40 | |
| #Others | 11 | 3 | 91% (51%-99%) | 91% (51%-99%) | 71% (34%-90%) | 0.56 (0.15-2.05) | 0.38 | |
| Estrogen receptor | No | 111 | 36 | 92% (84%-9%) | 77% (68%-84%) | 67% (57%-75%) | Ref | |
| Yes | 21 | 7 | 95% (71%-99%) | 90% (67%-98%) | 75% (51%-89%) | 0.95 (0.42-2.13) | 0.89 | |
| Progesterone receptor | No | 121 | 40 | 91% (85%-95%) | 78% (69%-84%) | 67% (57%-75%) | Ref | |
| Yes | 11 | 3 | 100% (NA) | 100% (NA) | 89% (43%-98%) | 0.70 (0.22-2.26) | 0.55 | |
| HER2-neu expression | No | 119 | 39 | 92% (85%-96%) | 80% (71%-86%) | 68% (59%-76%) | Ref | |
| Yes | 13 | 4 | 92% (57%-99%) | 77% (44%-92%) | 69% (37%-87%) | 0.87 (0.3-2.43) | 0.79 | |
| Metastasis to the lung | No | 102 | 18 | 96% (89%-98%) | 88% (79%-93%) | 81% (72%-88%) | Ref | |
| Yes | 30 | 25 | 80% (61%-90%) | 53% (34%-69%) | 27% (11%-44%) | 7.85 (4.18-14.72) | <0.001 | |
| Metastasis to the bone | No | 114 | 28 | 95% (88%-97%) | 82% (74%-88%) | 76% (67%-83%) | Ref | |
| Yes | 18 | 15 | 78% (51%-91%) | 61% (35%-79%) | 24% (7%-46%) | 4.90 (2.57-9.34) | <0.001 | |
| Metastasis to the brain | No | 123 | 34 | 92% (85%-95%) | 83% (75%-89%) | 72% (63%-80%) | Ref | |
| Yes | 9 | 9 | 100% (100%-100%) | 33% (8%-62%) | 22% (3%-51%) | 5.53 (2.61-11.72) | <0.001 | |
| Metastasis to the liver | No | 126 | 39 | 93% (86%-96%) | 81% (73%-87%) | Ref | ||
| Yes | 6 | 4 | 83% (27%-97%) | 50% (11%-80%) | 3.06 (1.08-8.67) | 0.036 |
The potential predictors of outcome in univariable analysis were utilized for multivariable analysis. No difference in survival was observed in the early stages of MBC (stages I and II). Performance status proved to be predictive of OS (ECOG score 1: HR, 3.141 (95% CI, 1.00-9.88) (p=0.05); ECOG score 2: HR, 24.736 (95% CI, 1.92-318.73) (p=0.014)). Distant metastases to the lung (HR, 42.102 (95% CI 7.20-246.36) (p<0.001)) and brain (HR, 8.453 (95% CI, 1.88-38.04) (p= 0.005)) were statistically significant for worse OS in multivariable analysis (Table 5). Interestingly, patients with bone metastasis showed a tendency for a lower risk of death with an HR of 0.052 (95% CI, 0.01-0.48) (p=0.009). Other variables such as hormonal therapy, radiation therapy, and histologic subtype did not show any statistical significance for OS.
Table 5. Multivariable analysis for overall survival.
ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; CI, confidence interval; Ref, reference
*Analysis was done using a reference level of no lung, bone, and brain metastasis.
| Variable | HR (95% CI) | p-value |
| Stage II (ref = stage I) | 0.671 (0.15-3.07) | 0.61 |
| Stage III (ref = stage I) | 5.065 (1.02-25.27) | 0.048 |
| Stage IV (ref = stage I) | 5.968 (0.55-64.47) | 0.14 |
| Hormonal therapy (ref = no) | 0.142 (0.02-1.17) | 0.07 |
| Radiation therapy (ref = no) | 3.626 (0.71-18.60) | 0.12 |
| ECOG 1 (ref = ECOG 0) | 3.141 (1.00-9.88) | 0.05 |
| ECOG 2 (ref = ECOG 0) | 24.736 (1.92-318.73) | 0.014 |
| ECOG 3 (ref = ECOG 0) | 1.704 (0.18-16.48) | 0.65 |
| *Lung metastasis (ref = no) | 42.102 (7.20-246.36) | <0.001 |
| *Bone metastasis (ref = no) | 0.052 (0.01-0.48) | 0.009 |
| *Brain metastasis (ref = no) | 8.453 (1.88-38.04) | 0.005 |
Discussion
The identification of MBC has evolved over the past two decades since the official recognition of this subtype by the WHO [3]. Clinically, most MBC patients present with a well-circumscribed palpable mass, and the presentation resembles an invasive ductal carcinoma. However, previous studies reported larger tumor sizes, greater than 5 cm, which is associated with worse outcomes [4-6]. A large national database study by Pezzi et al. reported an increased proportion of MBC in older patients with a mean age of 61.1 years and a higher prevalence in African-American and Hispanic patients [6]. In our study cohort, the median age of diagnosis was 60 years (27-92 years), and most tumors were diagnosed at AJCC stage II, followed by stage I. The white population (76%) represented the dominant cohort of the study, which could possibly be due to the large referral center with better access to care and regional demographics of the MBC population.
Currently, MBC is classified into purely epithelial and mixed epithelial and mesenchymal components based on the updated fourth edition of the WHO classification for breast tumors [7]. It is a heterogeneous tumor with diverse histologic subtypes. Previous studies demonstrated variation in the prevalence of histologic subtypes in different ethnic populations. The spindle cell subtype resembles a low-grade sarcoma, which is the most common histologic subtype found in western patients and is reported to be associated with poor prognosis [8,9]. The squamous subtype was found to be more common in the Asian population [4,8]. However, Zhang et al. found the most common histology subtype of spindle cell carcinoma (34%) followed by the squamous subtype (31%) in the Chinese population [10]. In our study cohort, we identified the squamous subtype (29%) more commonly in our study population, followed by the spindle subtype (21%).
Tumor protein p53 is a gene that functions as a tumor suppressor. The p53 mutation has been commonly reported in MBC with a high frequency ranging from 53% to 64% [11,12]. Tumor protein p63, which is a member of the p53 family, is expressed in MBC tumor cells and is also a myoepithelial marker [13]. Evidence suggests that p63 can be utilized as a diagnostic marker for MBC but with no prognostic value [13,14]. We found 43 (31%) patients positive for p63 expression in the entire cohort. No difference in outcome was found between patients positive for p63 versus negative for p63 expression.
MBC is known for its rapid tumor growth and an increased tendency for recurrence [3,15]. Typically, tumors are of higher nuclear grade and characterized by the absence of ER, PR, and HER2/neu expression [7,16,17]. Moreover, the prognosis of metaplastic triple-negative breast cancer is worse when compared to non-metaplastic triple-negative breast cancer [18]. In our study cohort, approximately two-thirds of the patients had metaplastic triple-negative breast cancer. The cohort with any hormone receptor positivity and treatment with hormonal therapy did not show any improvement in survival when compared with the metaplastic triple-negative cohort. We also observed the heightened potential of tumor metastasis to the lung (22%), followed by the bone (13%), brain (6%), and liver (4%). In addition, there was evidence of rare metastasis to the spleen and adrenal gland in the study cohort. Evidence suggests the metastatic spread of tumors to the lungs, bone, and brain via vasculature instead of lymphatics [19]. Patients with metastasis to the lung and brain in our study cohort demonstrated significantly poor survival outcomes.
Rakha et al. reported histologic subtype as an independent prognostic factor in MBC; the spindle subtype tumor had aggressive behavior with a worse prognosis as compared to the matrix-producing tumor and squamous subtype [8]. However, in other studies, the histologic subtype did not show any statistical significance as a prognostic factor [10,20]. In our study cohort, histologic subtypes did not prove to be a predictor of outcomes (Tables 6, 7). Nevertheless, young age (<40 years), skin invasion, and squamous carcinoma subtype with nodal involvement have been identified as independent predictors of outcome in MBC patients [15].
Table 6. Progression-free survival based on histology.
HR, hazard ratio; CI, confidence interval; Ref: reference; NA, not available; N, number of patients; MBC, metaplastic breast cancer
#Other histology in our study includes ductal carcinoma in situ micropapillary pattern, heterologous type with matrix production, high-grade metaplastic carcinoma, large cells with intermingling mature plasma cells, matrix-producing carcinoma, and sarcomatoid features, and metaplastic carcinoma.
| Variable | Variable level | N | Number of events | Estimated median (month) | One-year rate | Two-year rate | Five-year rate | HR (95% CI) | p-value |
| Histology | Spindle cell | 27 | 11 | 74.8 | 92% (73%-98%) | 73% (51%-86%) | 56% (33%-74%) | Ref | |
| Squamous | 40 | 19 | 156.3 | 82% (67%-91%) | 65% (48%-78%) | 54% (37%-68%) | 1.15 (0.55-2.42) | 0.71 | |
| Mixed MBC/mesenchymal | 54 | 16 | NA | 88% (76%-95%) | 76% (62%-86%) | 66% (50%-78%) | 0.69 (0.32-1.49) | 0.34 | |
| #Other | 11 | 3 | NA | 91% (51%-99%) | 82% (45%-95%) | 72% (35%-90%) | 0.54 (0.15-1.95) | 0.35 |
Table 7. Overall survival based on histology.
HR, hazard ratio; CI, confidence interval; Ref: reference; NA, not available; N, number of patients; MBC, metaplastic breast cancer
#Other histology in our study includes ductal carcinoma in situ micropapillary pattern, heterologous type with matrix production, high-grade metaplastic carcinoma, large cells with intermingling mature plasma cells, matrix-producing carcinoma, and sarcomatoid features, and metaplastic carcinoma.
| Variable | Variable level | N | Number of events | Estimated median (month) | One-year rate | Two-year rate | Five-year rate | HR (95%CI) | p-value |
| Histology | Spindle cell | 27 | 10 | 74.8 | 92% (73%-98%) | 77% (56%-89%) | 67% (44%-82%) | Ref | |
| Squamous | 40 | 15 | NA | 90% (76%-96%) | 77% (61%-88%) | 64% (46%-77%) | 0.97 (0.43-2.15) | 0.94 | |
| Mixed MBC/mesenchymal | 54 | 15 | NA | 94% (83%-98%) | 80% (66%-89%) | 73% (58%-83%) | 0.71 (0.32-1.58) | 0.40 | |
| #Other | 11 | 3 | NA | 91% (51%-99%) | 91% (51%-99%) | 71% (34%-90%) | 0.56 (0.15-2.05) | 0.38 |
In one of the large US population-based studies, 1,011 MBC patients were compared with 253,818 infiltrating ductal carcinoma (IDC) [21]. The study noted significantly worse five-year survival in MBC patients than in IDC patients (78% versus 93% (p<0.0001)). Besides, MBC was associated with higher tumor grades and larger tumor sizes. Similar findings were demonstrated in another study; authors reported decreased five-year OS when compared with invasive ductal carcinoma and triple-negative invasive ductal carcinoma (54.5% versus 85.1% versus 73.3% (p<0.001)) [5]. El Zein et al. also observed a worse prognosis in MBC than in triple-negative breast cancer (TNBC) [22]. The study documented that MBC patients had almost double the risk of local recurrence than TNBC patients (95% CI, 1.01-3.83 (p=0.05)). The authors also noted worse disease-free survival (DFS) and OS in MBC patients when compared with matched TNBC patients (p<0.001, p=0.033). Further review of the literature revealed a five-year OS ranging from 50% to 83% in various retrospective studies [10,17,19,23-29]. Most of the studies demonstrated a five-year DFS of 41%-65% [10,19,23,26-30], except for one study that demonstrated a five-year DFS of 84% [24].
In our study, we observed a five-year OS of 69% and PFS of 61%, which correlates with previous studies. On multivariable analysis, the stage of the disease, performance status (ECOG score), and distant metastasis to the lung, bone, brain, and liver proved to be significant predictors of outcomes in the MBC patient population. More importantly, on analysis for prognostication of MBC, we observed that patients with bone metastasis have a lower risk of death. On the other hand, a lower risk of progression was identified in the cohort of patients with liver metastasis. However, larger study samples would be needed to identify if these factors are truly associated with positive outcomes.
Our study is limited by its retrospective design with the possibility of selection bias and a small sample size. Few patients in the study cohort had missing variables; therefore, the total number of patients included for analysis for some of the specified variables was less than 136. We intended to do a descriptive analysis because of the above reasons. We utilized the pathology report documented by breast pathologists for MBC in our retrospective cohort study and did not review slides. Patients were not divided based on the type of presentation, such as primary first-time diagnosed MBC and concurrent malignancy in the contralateral breast. Another weakness of the study is that progression included both local and distant progression; no analysis was explicitly conducted for local recurrence. As far as treatment is concerned, we did not describe whether the patient received neoadjuvant or adjuvant chemotherapy, the combination of surgery with radiation therapy or hormonal therapy, or chemotherapy.
Despite the above limitations, the strength of the study is a sizable cohort of MBC patients over a long period of time depicting clinical and pathological characteristics.
Conclusions
Our study demonstrated the aggressive nature of MBC with early progression and overall poor prognosis. Histologically, tumors were heterogeneous with no difference in outcome based on histologic subtypes. However, the stage of metaplastic breast cancer, poor functional status (ECOG score), and metastasis to the lung and brain are identified as independent predictors of poor survival outcomes in the entire cohort. Our experience with MBC with diverse clinicopathological findings and relevant prognostic factors is another addition to the literature. Tumor pathogenesis, diverse histology, and molecular heterogeneity pose a significant challenge in the diagnosis and management of MBC. The lack of a more specific therapeutic approach for this rare subtype of breast cancer is an unmet need that warrants further research and randomized clinical trials.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. The Cleveland Clinic Institutional Review Board (IRB) issued approval 17-404. The Cleveland Clinic IRB approved this retrospective study on metaplastic breast cancer.
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.A brief overview of the WHO classification of breast tumors, 4th edition, focusing on issues and updates from the 3rd edition. Sinn HP, Kreipe H. Breast Care (Basel) 2013;8:149–154. doi: 10.1159/000350774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Management and outcomes in metaplastic breast cancer. Tzanninis IG, Kotteas EA, Ntanasis-Stathopoulos I, Kontogianni P, Fotopoulos G. Clin Breast Cancer. 2016;16:437–443. doi: 10.1016/j.clbc.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Metaplastic breast cancer: clinical overview and molecular aberrations for potential targeted therapy. Abouharb S, Moulder S. Curr Oncol Rep. 2015;17:431. doi: 10.1007/s11912-014-0431-z. [DOI] [PubMed] [Google Scholar]
- 4.The prognostic significance of metaplastic carcinoma of the breast (MCB)--a case controlled comparison study with infiltrating ductal carcinoma. Lai HW, Tseng LM, Chang TW, et al. Breast. 2013;22:968–973. doi: 10.1016/j.breast.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Unique clinicopathological features of metaplastic breast carcinoma compared with invasive ductal carcinoma and poor prognostic indicators. Song Y, Liu X, Zhang G, et al. World J Surg Oncol. 2013;11:129. doi: 10.1186/1477-7819-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Characteristics and treatment of metaplastic breast cancer: analysis of 892 cases from the National Cancer Data Base. Pezzi CM, Patel-Parekh L, Cole K, Franko J, Klimberg VS, Bland K. Ann Surg Oncol. 2007;14:166–173. doi: 10.1245/s10434-006-9124-7. [DOI] [PubMed] [Google Scholar]
- 7.Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. Lyon, France: World Health Organization; 2012. WHO classification of tumours of the breast who classification of tumours, 4th edition, volume 4. [Google Scholar]
- 8.Prognostic factors in metaplastic carcinoma of the breast: a multi-institutional study. Rakha EA, Tan PH, Varga Z, et al. Br J Cancer. 2015;112:283–289. doi: 10.1038/bjc.2014.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metaplastic breast cancer: histologic characteristics, prognostic factors and systemic treatment strategies. Schwartz TL, Mogal H, Papageorgiou C, Veerapong J, Hsueh EC. Exp Hematol Oncol. 2013;2:31. doi: 10.1186/2162-3619-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinicopathological features and prognosis of metaplastic breast carcinoma: experience of a major Chinese Cancer Center. Zhang Y, Lv F, Yang Y, et al. PLoS One. 2015;10:0. doi: 10.1371/journal.pone.0131409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genomic profiling of metaplastic breast carcinomas reveals genetic heterogeneity and relationship to ductal carcinoma. Krings G, Chen YY. Mod Pathol. 2018;31:1661–1674. doi: 10.1038/s41379-018-0081-z. [DOI] [PubMed] [Google Scholar]
- 12.Genomic and transcriptomic heterogeneity in metaplastic carcinomas of the breast. Piscuoglio S, Ng CK, Geyer FC, et al. NPJ Breast Cancer. 2017;3:48. doi: 10.1038/s41523-017-0048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.p63 is useful in the diagnosis of mammary metaplastic carcinomas. Tse GM, Tan PH, Chaiwun B, et al. Pathology. 2006;38:16–20. doi: 10.1080/00313020500444625. [DOI] [PubMed] [Google Scholar]
- 14.Diagnostic utility of snail in metaplastic breast carcinoma. Nassar A, Sookhan N, Santisteban M, Bryant SC, Boughey JC, Giorgadze T, Degnim A. Diagn Pathol. 2010;5:76. doi: 10.1186/1746-1596-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metaplastic carcinoma of the breast. McKinnon E, Xiao P. Arch Pathol Lab Med. 2015;139:819–822. doi: 10.5858/arpa.2013-0358-RS. [DOI] [PubMed] [Google Scholar]
- 16.Metaplastic carcinoma of the breast: a clinicopathological review. Tse GM, Tan PH, Putti TC, Lui PC, Chaiwun B, Law BK. J Clin Pathol. 2006;59:1079–1083. doi: 10.1136/jcp.2005.030536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Predictive factors on outcomes in metaplastic breast cancer. Leyrer CM, Berriochoa CA, Agrawal S, et al. Breast Cancer Res Treat. 2017;165:499–504. doi: 10.1007/s10549-017-4367-5. [DOI] [PubMed] [Google Scholar]
- 18.The prognoses of metaplastic breast cancer patients compared to those of triple-negative breast cancer patients. Bae SY, Lee SK, Koo MY, et al. Breast Cancer Res Treat. 2011;126:471–478. doi: 10.1007/s10549-011-1359-8. [DOI] [PubMed] [Google Scholar]
- 19.Metaplastic carcinoma of the breast, an unusual disease with worse prognosis: the experience of the European Institute of Oncology and review of the literature. Luini A, Aguilar M, Gatti G, et al. Breast Cancer Res Treat. 2007;101:349–353. doi: 10.1007/s10549-006-9301-1. [DOI] [PubMed] [Google Scholar]
- 20.Metaplastic breast cancer: a comparison between the most common histologies with poor immunohistochemistry factors. Barquet-Muñoz SA, Villarreal-Colin SP, Herrera-Montalvo LA, et al. BMC Cancer. 2015;15:75. doi: 10.1186/s12885-015-1079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Survival outcomes of metaplastic breast cancer patients: results from a US population-based analysis. Nelson RA, Guye ML, Luu T, Lai LL. Ann Surg Oncol. 2015;22:24–31. doi: 10.1245/s10434-014-3890-4. [DOI] [PubMed] [Google Scholar]
- 22.Metaplastic carcinoma of the breast is more aggressive than triple-negative breast cancer: a study from a single institution and review of literature. El Zein D, Hughes M, Kumar S, Peng X, Oyasiji T, Jabbour H, Khoury T. Clin Breast Cancer. 2017;17:382–391. doi: 10.1016/j.clbc.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biphasic metaplastic sarcomatoid carcinoma of the breast. Hennessy BT, Giordano S, Broglio K, et al. Ann Oncol. 2006;17:605–613. doi: 10.1093/annonc/mdl006. [DOI] [PubMed] [Google Scholar]
- 24.Metaplastic breast cancer: clinical significance. Beatty JD, Atwood M, Tickman R, Reiner M. Am J Surg. 2006;191:657–664. doi: 10.1016/j.amjsurg.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 25.Metaplastic carcinomas of the breast. I. Matrix-producing carcinoma. Wargotz ES, Norris HJ. Hum Pathol. 1989;20:628–635. doi: 10.1016/0046-8177(89)90149-4. [DOI] [PubMed] [Google Scholar]
- 26.Worse prognosis of metaplastic breast cancer patients than other patients with triple-negative breast cancer. Jung SY, Kim HY, Nam BH, et al. Breast Cancer Res Treat. 2010;120:627–637. doi: 10.1007/s10549-010-0780-8. [DOI] [PubMed] [Google Scholar]
- 27.Metaplastic breast cancer: clinicopathological features and its prognosis. Lee H, Jung SY, Ro JY, et al. J Clin Pathol. 2012;65:441–446. doi: 10.1136/jclinpath-2011-200586. [DOI] [PubMed] [Google Scholar]
- 28.Metaplastic breast carcinoma: a clinical analysis of 26 cases. Jin X, Yu D, Guo C, Gong X, Li N, Zhao Y. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6958196/ Int J Clin Exp Pathol. 2018;11:2112–2117. [PMC free article] [PubMed] [Google Scholar]
- 29.Metaplastic breast carcinoma: analysis of 31 cases from a single institute. Fayaz S, Demian GA, Eissa HE, Amanguno H, Abuzalouf S. J Egypt Natl Canc Inst. 2017;29:141–145. doi: 10.1016/j.jnci.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Metaplastic sarcomatoid carcinoma of the breast appears more aggressive than other triple receptor-negative breast cancers. Lester TR, Hunt KK, Nayeemuddin KM, et al. Breast Cancer Res Treat. 2012;131:41–48. doi: 10.1007/s10549-011-1393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]