Abstract
The filamentous cyanobacterium Anabaena sp. strain PCC 7120 forms a developmental pattern of single heterocysts separated by approximately 10 vegetative cells. Heterocysts differentiate from vegetative cells and are specialized for nitrogen fixation. The patS gene, which encodes a small peptide that inhibits heterocyst differentiation, is expressed in proheterocysts and plays a critical role in establishing the heterocyst pattern. Here we present further analysis of patS expression and heterocyst pattern formation. A patS-gfp reporter strain revealed clusters of patS-expressing cells during the early stage of heterocyst differentiation. PatS signaling is likely to be involved in the resolution of these clusters. Differentiating cells were inhibited by PatS during the time period 6 to 12 h after heterocyst induction, when groups of differentiating cells were being resolved to a single proheterocyst. Increased transcription of patS during development coincided with expression from a new transcription start site. In vegetative cells grown on nitrate, the 5′ end of a transcript for patS was localized 314 bases upstream from the first translation initiation codon. After heterocyst induction, a new transcript with a 5′ end at −39 bases replaced the vegetative cell transcript. A patS mutant grown for several days under nitrogen-fixing conditions showed partial restoration of the normal heterocyst pattern, presumably because of a gradient of nitrogen compounds supplied by the heterocysts. The patS mutant formed heterocysts when grown in the presence of nitrate but showed no nitrogenase activity and no obvious heterocyst pattern. We conclude that PatS and products of nitrogen fixation are the main signals determining the heterocyst pattern.
How a developmental pattern of differentiated cells arises out of a group of apparently equivalent cells is a fundamental question in biology. In prokaryotes, cyanobacterial heterocyst development serves as a simple model of developmental pattern formation. The filamentous cyanobacterium Anabaena sp. strain PCC 7120 grows as vegetative cells in media containing combined nitrogen. Vegetative cells carry out oxygenic photosynthesis similar to that of algae and higher plants (10). In the absence of combined nitrogen, 1 out of every 8 to 15 vegetative cells differentiates into a nitrogen-fixing heterocyst at semiregular intervals along the filament. The differentiation of a vegetative cell into a heterocyst takes approximately 24 h and involves a variety of structural, biochemical, and genetic changes (27). Heterocysts are terminally differentiated and highly specialized cells that house oxygen-sensitive nitrogenase, which converts atmospheric nitrogen to ammonia. The thick envelope and active respiration of the heterocyst protect nitrogenase from oxygen (6, 23). Heterocyst development allows the spatial separation of two incompatible biochemical processes: photosynthesis and nitrogen fixation.
Under nitrogen-fixing conditions, heterocystous cyanobacteria grow as simple multicellular organisms consisting of two interdependent cell types that cooperate by exchanging metabolites. Heterocysts provide nitrogen fixation products to neighboring vegetative cells and in turn receive carbohydrate products of photosynthesis (27). Heterocyst spacing and frequency are apparently regulated to optimize the distribution of fixed nitrogen within a long filament to allow optimal growth. It is assumed that multiple internal and external signals must be integrated by a regulatory network that controls cell differentiation and pattern formation (25, 27).
We have previously found that patS, which is predicted to encode a 17- or 13-amino-acid peptide, is crucial for the formation and maintenance of the normal heterocyst pattern (28). The overexpression of patS completely blocked heterocyst development. The exogenous addition of a pentapeptide corresponding to the last five COOH-terminal residues of PatS also inhibited heterocyst differentiation, indicating that a processed form of PatS may be a diffusible inhibitory signal regulating development. A patS deletion mutant produced chains of contiguous heterocysts and abnormally short intervals of vegetative cells between the heterocysts. This striking phenotype is consistent with a defect in lateral inhibition by an intercellular signal originating from the differentiating cells. The multiple-contiguous-heterocyst phenotype of the patS mutant was complemented by the expression of patS from an early proheterocyst-specific promoter. This result indicates that patS functions nonautonomously by producing a signal from proheterocysts that inhibits neighboring cells from differentiating. We also showed that both transcription and translation of patS increased early after the onset of differentiation by Northern RNA analysis and patS-lacZ fusion analysis, respectively. The increased patS expression was localized to differentiating cells by analyzing the activity of a patS-gfp fusion. At later times during development, patS-gfp expression was detected exclusively in proheterocysts. The patS product appears to be an inhibitory peptide produced by differentiating cells and functions in cell-cell signaling to regulate the pattern by lateral inhibition.
In this report, we further examine patS expression and the characteristics of the patS mutant. We show that clusters of adjacent cells initially expressed patS and then resolved to a single proheterocyst approximately 12 h after induction. Inhibitory PatS signaling may be involved in the resolution of the clusters because cells were not committed to complete heterocyst differentiation until 9 to 14 h after heterocyst induction. The patS gene appears to be transcribed from at least two promoters, one of which is induced after nitrogen step-down. Analysis of pattern maintenance in the wild type and the patS mutant suggests that a gradient of nitrogen fixation products contributes to pattern formation. Heterocyst formation by the patS null mutant grown in the presence of nitrate as a uniform external source of nitrogen failed to show a normal pattern. These results suggest that PatS and fixed nitrogen produced by heterocysts are the major diffusible signals regulating the frequency and spacing of heterocysts.
MATERIALS AND METHODS
Strains and culture conditions.
Anabaena sp. strain PCC 7120 and its derivatives were grown in 100 ml of BG-11 medium or BG-110 medium (which lacks sodium nitrate) at 30°C as previously described (8). For medium containing ammonium, BG-110 was supplemented with 2 mM ammonium chloride and 5 mM MOPS [3-(N-morpholino)propanesulfonic acid] (pH 8.0). For time course RNA extractions, 8-liter large-scale cultures were grown in the liquid medium of Allen and Arnon diluted eightfold, with some modifications (24). Cultures induced for heterocyst formation were grown in flasks with 100 ml of BG-110 medium or in 24-well tissue culture plates (Falcon) with 2 ml of BG-110 medium. For the timing of commitment experiments, PatS-5 pentapeptide (RGSGR; Genosys Biotechnologies) (28) or NaNO3 was added to cultures of the wild-type strain at various times after transfer to 200 μl of BG-110 medium in 96-well plates. For strains containing the patS-gfp reporter plasmid pAM1951, neomycin at 12.5 and 25 μg/ml was added to liquid and solid media, respectively. The patS mutant strain AMC451 (28) was grown in media supplemented with spectinomycin and streptomycin at 1 μg/ml each.
Escherichia coli strains were maintained in Luria-Bertani liquid or agar medium. For plasmid preparation, strains were grown in 0.5× TB liquid medium as described previously (8). Media were supplemented with appropriate antibiotics according to standard protocols (2). E. coli strain DH10B was used for plasmid maintenance, except that JM107 was used for derivatives of pKEN2-GFPmut2 (4, 5).
Plasmid constructions.
Conjugal plasmid pAM1951 contains a patS-gfp transcriptional fusion. It was made by digesting pAM1035 (28) with BamHI and ThaI to isolate a 762-bp fragment containing the patS open reading frame and 724 bp of upstream sequence. This fragment was ligated into a plasmid containing the gfp gene, pKEN2-GFPmut2 (4, 5); this plasmid had been digested with XbaI, filled in by the Klenow enzyme, and digested with BamHI. The resulting plasmid, pAM1877, was digested with HindIII, and the ends were blunted with the Klenow enzyme; the plasmid was digested with SacI to release patS-gfp on a fragment that was then ligated into shuttle vector pAM505 (28) digested with SacI and SmaI. The resulting construct, pAM1951, was confirmed by restriction digestion and DNA sequencing through the junction of patS and gfp.
Fluorescence microscopy.
Fluorescence micrographs were taken on a Zeiss Axioplan II microscope with a ×40 objective using fluorescein isothiocyanate-specific illumination (484 ± 8 nm) and green fluorescent protein (GFP)-specific emission (518 ± 13 nm) filter sets. The images were captured with a Hamamatsu 3 charge-coupled device (CCD) camera (C5810) attached to the microscope via an HR coupler (Diagnostic Instruments, Inc.). The images were processed with Adobe Photoshop version 4.0.
Primer extension assays.
RNA was extracted by an acidic hot phenol method (14) from Anabaena sp. strain PCC 7120 vegetative cells grown for 6 days (optical density at 750 nm [OD750], approximately 0.5) in 100 ml of BG-11 medium. RNA was extracted from differentiating wild-type filaments and purified by centrifugation through 5.7 M CsCl (24). The filaments were synchronously induced to form heterocysts by nitrogen step-down as previously described (24). Primer extension was performed with 30 μg of total RNA and primer A or B and with 15 or 100 μg of total RNA and primer C (see Fig. 4A). The [γ-32P]ATP-end-labeled primers were annealed to RNA and extended with avian myeloblastosis virus reverse transcriptase at 42°C or with displayTHERMO-RT (Display Systems Biotech) at 50°C. DNA sequencing was performed by using the dideoxynucleotide chain termination sequencing method (SequiTherm cycle sequencing kit; Epicentre Technologies) and the same primers as those used for reverse transcription. The cDNA and sequencing products were analyzed on a 6% polyacrylamide sequencing gel.
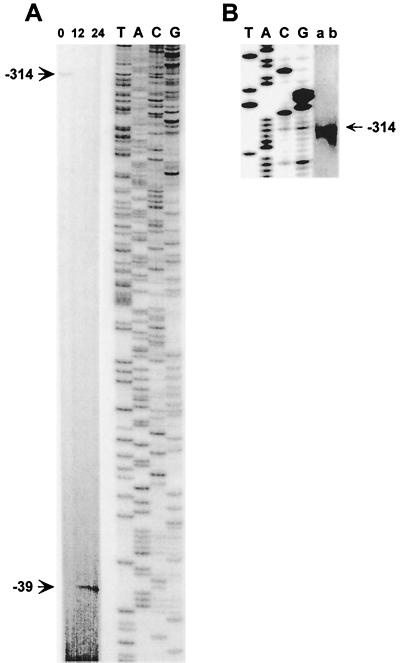
FIG. 4.
Identification of the 5′ ends of patS transcripts by primer extension. Total RNA from filaments grown with 17.6 mM NaNO3 (lane 0) or from filaments collected at 12 and 24 h after nitrogen step-down was hybridized to 32P-end-labeled primer A (A) or C (B), extended with reverse transcriptase, and analyzed on a 6% polyacrylamide gel. Samples of 30 μg of total RNA (A) and 15 or 100 μg of total RNA (B, lane a or b, respectively) were used. The fragments are labeled to indicate the position of the 5′ end with respect to the patS translation initiation codon shown in Fig. 3A. Lanes T, A, C, and G are dideoxynucleotide sequencing ladders of pAM1035 produced with the corresponding primers A and C.
Heterocyst pattern.
For heterocyst induction, early-exponential-growth-phase (OD750 = 0.1 to 0.2) vegetative cell filaments from 100-ml shaken cultures of BG-11 medium or ammonium-containing medium were collected by centrifugation, washed with water, and resuspended in 0.1 to 0.05 the original volume of BG-110 medium or BG-11 medium (for AMC451). Samples (2 ml) were transferred to 24-well plates and incubated without shaking under standard conditions. For each time point, samples were carefully pipetted to glass slides to avoid breaking filaments. The percentage of heterocysts and their spacing in filaments were scored visually by bright-field or Hoffman modulation contrast microscopy (×400 magnification). Heterocysts were distinguished by their thick cell envelope, changes in the granularity of the cytoplasm, and cyanophycin granule formation at the cell poles. Dividing vegetative cells were counted as two cells only if an obvious septum had formed at the site of constriction. Only lengths of vegetative cells between heterocysts along filaments were reported as an interval. Terminal sequences of vegetative cells were recorded but were not scored as an interval between heterocysts. A relatively small number of detached heterocysts were observed and could have represented a slight undercounting of multiple contiguous heterocysts. Over 1,000 total vegetative cells and heterocysts were counted at each time point for some of the experiments (see Fig. 5 and 6); for others (see Fig. 2), over 500 total cells were counted at each time point.
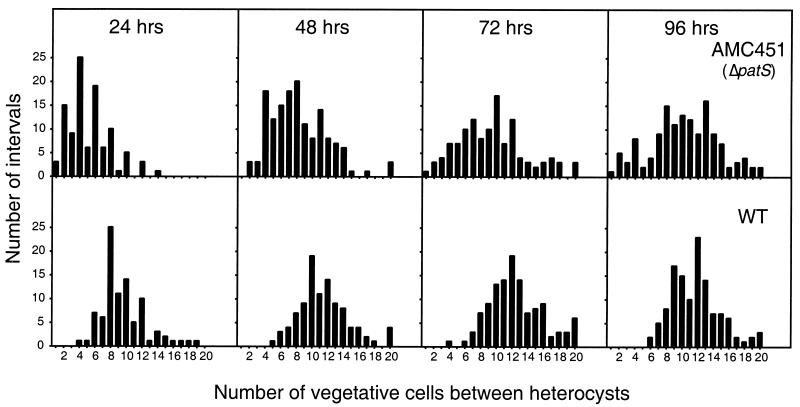
FIG. 5.
Heterocyst pattern maintenance of the wild type and the patS deletion strain AMC451 during prolonged culturing. The two strains were grown in liquid BG-11 medium to an OD750 of 0.2 and then induced by transfer to BG-110 medium. The number of vegetative cells between heterocysts was determined microscopically every 24 h after heterocyst induction. Interval lengths of greater than or equal to 20 are shown as 20. One representative experiment of two independent experiments is shown.
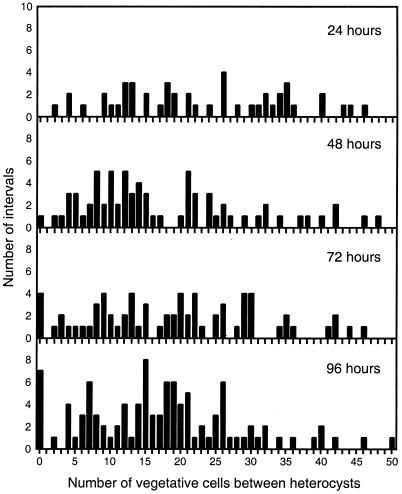
FIG. 6.
The patS deletion strain AMC451 does not show a normal heterocyst pattern in the presence of NaNO3. Strain AMC451 was grown in BG-110 medium supplemented with 2 mM NH4Cl to completely suppress heterocyst development. Mid-log-phase cells were transferred to BG-11 medium containing 17.6 mM NaNO3. The spacing between heterocysts was determined every 24 h for 4 days. One representative experiment of two independent experiments is shown.
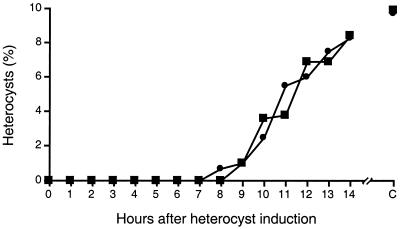
FIG. 2.
Timing of commitment. Wild-type Anabaena sp. strain PCC 7120 was grown in liquid BG-11 medium to early log phase and then induced in nitrogen-free BG-110 medium. At the times indicated, PatS-5 pentapeptide (1 μM final concentration [squares]) or NaNO3 (17.6 mM final concentration [circles]) was added to a sample of the culture. The percentage of heterocysts was determined 24 h after induction by counting over 500 total vegetative cells and heterocysts for each sample. C, control sample, with no addition of PatS-5 or NaNO3. One representative experiment of three independent experiments (two independent experiments for NaNO3) is shown.
Acetylene reduction assays.
Wild-type and AMC451 cultures were grown in BG-110 medium supplemented with 2 mM ammonium chloride to early exponential growth phase (OD750 = approximately 0.2), induced by transfer to BG-110 (wild type and AMC451) or BG-11 (AMC451) medium, and incubated under standard conditions for approximately 52 h. Induced filaments were concentrated to an OD750 of 2.0, and 1-ml samples were transferred to tubes (100 by 16 mm) fitted with serum stoppers. The tubes were injected with 1 ml of acetylene gas and incubated horizontally at approximately 25°C with a photosynthetically active irradiance of 120 to 150 μmol of photons m−2 s−1. Gas samples (0.2 ml) were removed at intervals over a 2-h period for analysis of ethylene production by gas chromatography with a 6-ft Porapak N column at 50°C.
RESULTS
Spatial pattern of patS expression during heterocyst development.
Under our standard growth conditions, proheterocysts are distinguishable 15 to 18 h after nitrogen step-down and mature heterocysts containing polar cyanophycin granules are visible by 18 to 24 h. In our previous studies, the GFP fluorescence of a patS-gfp reporter strain was examined at 12 and 18 h after nitrogen step-down (28). At 12 h after induction, strong GFP fluorescence was localized to mostly individual cells in a pattern that resembled that of mature heterocysts. However, fainter GFP fluorescence was also present in cells neighboring the bright cells. By 18 h, strong GFP fluorescence was found only in developing proheterocysts (28). In this report, we further examined the pattern of patS-gfp expression from 0 to 14 h after heterocyst induction.
A reporter gene encoding an optimized version of GFP (GFPmut2) (4) was used to analyze the spatial pattern of patS expression (28). The patS-gfp transcriptional fusion on plasmid pAM1951 was transferred by conjugation to wild-type Anabaena sp. strain PCC 7120. Plasmid pAM1951 is based on a low-copy-number shuttle vector containing a pDU1 origin of replication, which has a copy number of approximately one per chromosome (13). After nitrogen step-down, patS-gfp expression was often observed initially in clusters of several adjacent cells that then resolved to single bright cells separated from one another by approximately 10 dim vegetative cells (Fig. 1).
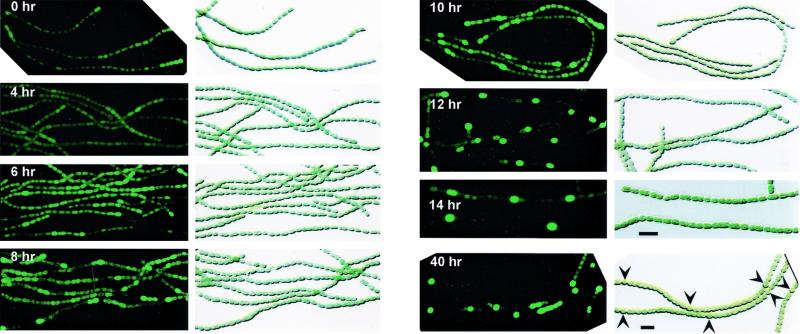
FIG. 1.
Temporal and spatial expression of patS-gfp reporter transcriptional fusion in Anabaena sp. strain PCC 7120 filaments subjected to nitrogen step-down. (Left panels) Fluorescence micrographs. (Right panels) Corresponding differential interference contrast photomicrographs. The strain was grown in nitrate-containing BG-11 medium (0 h) and then transferred to BG-110 medium to induce heterocyst development for the times indicated. Arrowheads show mature heterocysts. Scale bars, 10 μm. All photomicrographs except for those at 14 h were taken at the same magnification. Images were photographed and processed with the same settings to allow qualitative comparisons of fluorescence intensities.
Low-level expression of the patS-gfp fusion was observed in nitrate-grown cultures (Fig. 1). This GFP fluorescence varied somewhat along the filaments without any obvious pattern. Filaments were transferred to BG-110 medium containing no combined nitrogen to induce heterocyst development. At 4 h after heterocyst induction, patS-gfp expression was relatively unchanged (Fig. 1). Variable weak GFP fluorescence was observed from vegetative cells along the filaments. At 6 h, many individual cells and small groups of cells showed increased GFP fluorescence. By 8 h, many individual cells showed strong GFP fluorescence in a pattern similar to that of mature heterocysts. However, lower levels of GFP fluorescence were also observed in pairs of cells, groups of cells, and cells separated by only short stretches (three to six cells) of nonfluorescing cells. At 10 h, most clusters had resolved to single bright cells or contained one cell that was brighter than the rest. By 12 h, the contrast between fluorescent cells and nonfluorescent cells had increased and the pattern of single fluorescent cells had become more obvious. Although morphological differentiation of proheterocysts was not obvious at 14 h, a clear pattern of fluorescent cells similar to that of mature heterocysts was displayed. However, the pattern was not perfect; some short intervals between bright cells and pairs of fluorescent cells were seen. As in our previous studies (28), strong GFP fluorescence was found exclusively within morphologically distinguishable proheterocysts 18 h after induction (data not shown).
GFP fluorescence was still bright in mature heterocysts at 24 h but became dimmer in heterocysts at later times. We have not determined if this finding is due to decreased patS-gfp expression or to the reducing environment in heterocysts (4, 27). Once mature heterocysts start supplying fixed nitrogen to neighboring vegetative cells, the filaments resume growth and vegetative cells between heterocysts begin dividing. Between 24 and 40 h after induction, the number of vegetative cells between two heterocysts had increased and GFP fluorescence had begun to appear in cells midway between two heterocysts. Single fluorescing cells as well as two adjacent cells or short chains of fluorescing cells were observed. The fluorescence gradually resolved to single cells as the second round of heterocyst formation progressed. At 40 h, about one-third of the intervals that contained fluorescing cells had resolved to single cells at positions predicted for the formation of new heterocysts (Fig. 1). Morphologically distinguishable proheterocysts between two preexisting heterocysts showed the brightest fluorescence.
Anabaena sp. strain PCC 7120 containing a control plasmid (pAM1954) in which a vegetative cell-specific promoter (PrbcL (16)) was fused to gfp showed high levels of fluorescence only in vegetative cells (28). Anabaena sp. strain PCC 7120 containing a control plasmid (pAM1956) with a promoterless gfp gene showed no detectable GFP fluorescence.
Timing of commitment to complete heterocyst differentiation.
The resolution of clusters of differentiating cells to a single heterocyst requires that all but one cell respond to inhibitory signals. Heterocyst differentiation passes through an intermediate stage that can regress back to the vegetative state (1). At some point, however, proheterocysts are committed to complete the process of differentiation. If PatS inhibitory signaling is responsible for the resolution of groups of differentiating cells, then the cells that regress must be inhibited by PatS during the time period 6 to 12 h after induction, in which the resolution of clusters occurs. If differentiating cells are no longer sensitive to PatS inhibition at this time, then other factors must be involved in resolving clusters.
We tested the time of commitment to heterocyst formation by the ability to respond to inhibition by the PatS-5 pentapeptide (28) or NaNO3. The peptide was added to cultures at a final concentration of 1 μM every hour for 14 h after induction in BG-110 nitrogen-free medium. A heterocyst-inhibiting concentration of 17.6 mM NaNO3 was also tested in separate experiments as a comparison. Heterocyst formation was completely blocked by the addition of PatS-5 pentapeptide for up to 8 h after induction (Fig. 2). Between 9 and 14 h after induction, an increasing proportion of cells became committed to complete heterocyst differentiation and were no longer sensitive to inhibition by the PatS-5 pentapeptide. Similar results were obtained when NaNO3 was added to the induced culture (Fig. 2). These results show that differentiating cells are sensitive to inhibition by PatS or nitrate during the time period when groups of cells are being resolved. Heterocyst maturation and nitrogen fixation begin 18 to 24 h after nitrogen step-down under our growth conditions. Therefore, heterocysts cannot be supplying fixed nitrogen to the filaments before cells become committed to differentiate. A previous study showed that a patS mutant formed multiple contiguous heterocysts and short vegetative cell intervals between heterocysts (28). This phenotype appears to represent a failure of the mutant to resolve clusters of differentiating cells. These data are consistent with a model in which PatS inhibitory signaling resolves clusters of differentiating cells to a single heterocyst.
Regulation of patS transcription during heterocyst development.
Our patS-gfp transcriptional reporter studies showed low levels of expression in vegetative cells grown in nitrate-containing medium and strong expression after nitrogen step-down in differentiating cells. To further study the transcriptional regulation of patS during heterocyst development, we examined patS mRNA from vegetative cells and induced filaments by primer extension.
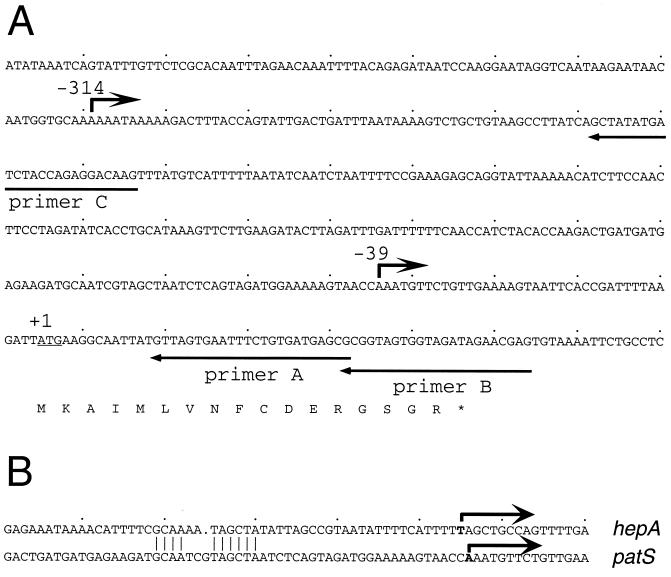
Primer extension analysis of patS mRNA showed the presence of two transcripts that differed in abundance depending on nitrogen availability. Total RNA was isolated from filaments grown in nitrate-containing medium and at 12 and 24 h after nitrogen step-down. Primer extension of RNA from nitrate-grown filaments using a primer internal to the patS gene (primer A; Fig. 3A) revealed a vegetative transcript with a 5′ end at −314 bases upstream of the translational initiation codon (Fig. 4A). Another primer, C (Fig. 3A), was used to confirm the 5′ end of this transcript at −314 bases (Fig. 4B). Primer extension of RNA from cultures induced for 12 and 24 h lacked the transcript at −314 bases but instead produced a distinct band at −39 bases from the patS translational initiation codon. The induced transcript 5′ end at −39 bases was confirmed by primer extension with a second primer (primer B; Fig. 3A) (data not shown). The primer extension data, together with the gfp reporter data, indicate that patS is transcribed from a weak promoter in vegetative cells and from a strong developmentally regulated promoter induced in differentiating cells.
FIG. 3.
Nucleotide sequence of the patS upstream region. (A) The primers used for primer extension assays and the putative transcription start sites at −39 and −314 are labeled. The first ATG translation initiation codon is underlined and indicated by +1. (B) Nucleotide sequence comparison of the putative promoter regions of the two early heterocyst-specific genes, patS and hepA. Similar nucleotide sequences centered at about −31 bases upstream of the developmentally regulated transcription start sites are marked by vertical lines.
The 5′ ends of patS transcripts mapped by primer extension identify putative transcription start sites. Sequences centered at about −31 bases upstream of the patS developmentally induced transcription start site are similar to sequences at the same position of the hepA promoter region (29) (Fig. 3B). HepA is required for the synthesis of the heterocyst envelope polysaccharide layer. The hepA gene is inactive in vegetative cells and is induced in proheterocysts at between 4.5 and 7 h after nitrogen step-down (3, 26, 29). The similar regulation of these two transcripts suggests that shared sequence motifs could be involved in their developmental regulation and may warrant further study.
PatS is required for both establishment and maintenance of a normal pattern.
The crucial role of patS in the initial establishment of the heterocyst pattern was demonstrated by the short-vegetative-cell-interval and multiple-contiguous-heterocyst phenotype of the patS deletion strain AMC451 at 24 h after nitrogen step-down (28). AMC451 heterocysts appear morphologically normal and are functional in supporting long-term diazotrophic growth. AMC451 filaments induced for 52 h in BG-110 medium showed nitrogenase activity, as measured by acetylene reduction, that was approximately one-third that of the wild type. By examining the heterocyst pattern of AMC451 for several days after induction, we showed that patS is also required for pattern maintenance, as new heterocysts were formed (Fig. 5). In addition, as suggested in earlier models (27), our data also support the idea that once heterocysts start fixing nitrogen and growth is resumed, the products of nitrogen fixation contribute to the maintenance of the heterocyst pattern. However, our data cannot exclude the possibility that another signal molecule from heterocysts is used to reflect nitrogenase activity.
The number of vegetative cells between heterocysts in the wild-type strain and in strain AMC451 during growth under nitrogen-fixing conditions was scored (Fig. 5). The wild-type culture showed a slight lengthening of the intervals between heterocysts as incubation continued and the filaments began to grow. There was a gradual shift in the mean interval length of vegetative cells from 9.6 cells at 24 h to 12.5 and 11.7 cells at 72 and 96 h, respectively (Fig. 5). The patS deletion strain AMC451 also displayed an increase in the interval length between heterocysts as growth continued under nitrogen-fixing conditions. The heterocyst pattern of the patS mutant at 72 and 96 h began to resemble that of the wild type. The mean interval length increased from 5.2 cells at 24 h to 10.3 cells at 96 h. The multiple-contiguous-heterocyst phenotype of AMC451 was noticeably reduced at 72 and 96 h (data not shown), presumably because of the accumulation of single mature heterocysts that suppressed adjacent cells from differentiating. However, even after growth for several days, AMC451 continued to produce double heterocysts (data not shown) and short intervals between heterocysts (Fig. 5, 72 and 96 h).
These results demonstrate two important points. First, signals other than PatS, presumably products of nitrogen fixation supplied by heterocysts (see below), contribute significantly to maintaining the overall spacing of heterocysts along a filament. Second, PatS signaling continues to play a role in pattern maintenance during diazotrophic growth by resolving clusters to ensure the formation of a single new heterocyst in the interval between two preexisting heterocysts.
Abnormal heterocyst spacing is produced by a patS mutant in nitrogen-replete medium.
The results shown in Fig. 5 are consistent with the longstanding hypothesis that a gradient of nitrogen fixation products produced by mature heterocysts suppresses the differentiation of neighboring vegetative cells (25, 27). This hypothesis has been difficult to prove because nitrogen fixation mutants cannot continue growth under diazotrophic conditions. We took advantage of the fact that the patS null mutant strain AMC451 formed heterocysts in BG-11 medium, which contains 17.6 mM sodium nitrate, but showed no other indication of nitrogen starvation. Wild-type Anabaena sp. strain PCC 7120 did not form heterocysts in liquid BG-11 medium under our growth conditions. The heterocysts formed by AMC451 in the presence of nitrate were morphologically normal but showed no nitrogenase activity, as measured by an acetylene reduction assay. It has been previously reported that glutamine can reduce nitrogenase activity in heterocysts of Anabaena variabilis (19). Even if these heterocysts were supplying the filament with some nitrogen from their reserves, we would expect that this supply would be masked by the abundant supply of nitrate from the medium. If PatS and nitrogen fixation products are the major diffusible signals controlling heterocyst pattern, then filaments of AMC451 grown in BG-11 medium should lack gradients of both signals and fail to show a normal pattern. In contrast, if these heterocysts produced an additional pattern formation signal other than fixed nitrogen, we would expect to see its influence on the heterocyst pattern under these conditions. Although it is possible that the production of or response to an additional heterocyst inhibition signal is inhibited in the presence of nitrate, this possibility would seem to be at odds with the fact that wild-type filaments normally suppress heterocysts under these conditions.
AMC451 was grown in a medium supplemented with 2 mM ammonium chloride, which completely inhibits heterocyst formation by this strain. The culture was then transferred to BG-11 medium, in which AMC451 forms heterocysts even though the cells are uniformly supplied with sodium nitrate. The pattern of heterocysts was examined every 24 h for 4 days (Fig. 6). The percentage of heterocysts reached 4.4% by 96 h, a percentage about half that of the wild type in medium lacking a source of reduced nitrogen. Compared with the pattern shown by AMC451 in Fig. 5, no obvious heterocyst pattern was evident at the later time points in the presence of nitrate. The 48-h data set showed some resemblance to the normal pattern. It is conceivable that a temporary gradient of nitrogen produced from stored compounds in heterocysts exerted some influence on spacing during this time period after induction. It is particularly important to note that, as expected for the patS mutant, adjacent heterocysts (indicated as an interval of zero) as well as abnormally short intervals were present in all samples. If heterocyst spacing were due to a signal molecule other than PatS and nitrogen fixation products, we would have expected a more normal pattern, similar to that shown in Fig. 5.
We conclude that the spacing of 8 to 15 vegetative cells between heterocysts in strain AMC451 growing diazotrophically (Fig. 5, 96 h) is due to the supply of nitrogen fixation products from heterocysts. The wild-type pattern (Fig. 5, 96 h) results from the additional influence of PatS, which suppresses multiple contiguous heterocysts and short intervals. Based on previous models (25, 27), gradients of both diffusible signals will be created along the filaments as the molecules are consumed by vegetative cells. Our results indicate that gradients of PatS and nitrogen fixation products originating from heterocysts are the two main diffusible signals that regulate the heterocyst pattern.
DISCUSSION
Pattern formation and cell fate determination have been studied intensively in a number of eukaryotic organisms (7, 9, 11, 12, 15). Competition and lateral inhibition among equivalent cell groups are common themes underlying the mechanisms of pattern formation and organogenesis (18). Anabaena heterocyst development provides an opportunity to study simple one-dimensional developmental pattern formation in a multicellular organism consisting of only two cell types. Only a relatively small fraction of cells (about 8 to 10%) at semiregular intervals along a filament undergo heterocyst differentiation. Strict control of heterocyst frequency and spacing is expected to be an important selective advantage because heterocyst differentiation represents a considerable energy investment and loss of reproductive capacity. A mechanism to establish appropriate spacing of heterocysts is required to ensure the efficient distribution of both fixed carbon and fixed nitrogen along a filament.
We had previously identified a gene, patS, which is expressed in differentiating cells and encodes a diffusible inhibitor of heterocyst development that controls development by lateral inhibition (28). We hypothesized that a processed PatS peptide produced by the cells that first begin to differentiate would diffuse along the filament within the periplasmic space to inhibit their neighbors.
Our further examination of PatS signaling provides additional insights into the regulation of heterocyst development. PatS is likely to be involved in the resolution of groups of differentiating cells and the suppression of short vegetative cell intervals between heterocysts. PatS signaling is important for both the initial pattern formed by vegetative cell filaments and the maintenance of the pattern as filaments continue to grow diazotrophically and produce new heterocysts. In addition, products of nitrogen fixation appear to provide an important second diffusible inhibitor of heterocyst differentiation that maintains the spacing pattern between heterocysts. Primer extension and patS-gfp reporter fusion studies indicated that patS is expressed from two promoters: one that is active in vegetative cells and one that is developmentally regulated and shows similarity to the hepA promoter.
The normal heterocyst pattern requires the resolution of clusters of differentiating cells. Six hours after nitrogen step-down, a patS-gfp reporter produced GFP fluorescence in many pairs or small groups of cells (Fig. 1). By monitoring a time course of development, we showed that the brightest of these cells eventually differentiated into heterocysts and that the less fluorescent cells eventually lost their fluorescence and remained vegetative cells. PatS inhibitory signaling is likely to be involved in the resolution of these clusters of differentiating cells. This suggestion is supported by the phenotype of a patS null mutant, which fails to resolve clusters and forms multiple contiguous heterocysts (28). During the resolutions of clusters, the cells that continue to complete differentiation into a heterocyst must be less sensitive to the PatS inhibitory signal (by a mechanism that is still not known). The coincident appearance of single bright patS-gfp cells (Fig. 1) and cells committed to complete differentiation (Fig. 2) suggests a possible linkage between these events. The timing of increased patS expression in developing cells at 6 and 8 h occurred well before cells became committed. The resolution of clusters to single bright cells was mostly complete by 12 h, slightly before the time period in which most cells have become committed to differentiate. Although a few cells were committed to form heterocysts by 9 h after induction, some cells were still not committed at 13 h after induction. We predict that at 9 to 13 h after induction, the cells in unresolved clusters are still sensitive to inhibition by externally added PatS-5 pentapeptide and therefore are also sensitive to the inhibitory PatS signal that is responsible for resolving the clusters.
In an effort to understand the regulation of patS expression during heterocyst development, the 5′ ends of patS transcripts were mapped by primer extension of RNA samples obtained before and after the onset of heterocyst development (Fig. 4). Transcript 5′ ends at −314 or −39 bases were detected in RNA from nitrate-grown filaments or differentiating filaments, respectively. Together with the results of the patS-gfp expression studies, these results indicate that patS is expressed from two promoters: one expressed in vegetative cells and the other induced upon nitrogen step-down in differentiating cells. The Anabaena sp. strain PCC 7120 glnA gene also contains multiple promoters that allow its expression in both vegetative cells and heterocysts (22), and primer extension analysis of the Anabaena sp. strain PCC 7120 ntcA gene identified multiple transcripts that vary in abundance in response to nitrogen availability (17).
Little is known about early heterocyst-specific promoters, but interestingly, sequences upstream of the induced patS transcript at −39 bases show some similarity to the promoter region of another early heterocyst-specific gene, hepA (29). The expression of both hepA and patS is induced relatively early after nitrogen step-down: at 4.5 to 7 h for hepA (3) and at approximately 6 h for patS. It is possible that these genes share a regulatory factor(s) that controls their developmental regulation. Complementation of the patS null strain with patS expressed from the hepA promoter (28) also suggests that the two genes are similar in terms of temporal regulation. Examination of DNA sequences upstream of the vegetative transcript at −314 bases failed to reveal an obvious similarity to the E. coli sigma-70 consensus promoter or to promoters of other Anabaena sp. strain PCC 7120 genes.
It has been assumed that nitrogen fixation products contribute to the heterocyst pattern by satisfying the nitrogen requirements of cells adjacent to heterocysts (27). However, an additional signal, now known to be PatS, was required to explain how the heterocyst pattern was initially established before heterocysts had matured and how nitrogen fixation mutants could initially establish a nearly normal pattern after nitrogen step-down. The availability of a patS null mutant allowed us to address the contribution made to the pattern by other factors. Our examination of a patS null mutant showed that after mature heterocysts had been formed, heterocyst spacing became more similar to the wild-type pattern (Fig. 5). The simplest explanation is that the gradient of nitrogen fixation products produced by heterocysts suppresses the development of neighboring vegetative cells to produce normal spacing. This notion also explains the shift of the wild-type strain to slightly longer intervals after several days of diazotrophic growth.
The role of heterocyst nitrogen fixation products in pattern formation cannot be directly addressed with nitrogen fixation mutants because they cannot continue to grow in the absence of an external source of reduced nitrogen. However, we have observed that nitrogen fixation mutants produce shorter vegetative cell intervals than does the wild type (unpublished observations). To determine if heterocyst spacing is due to the supply of nitrogen fixation products or to a specialized signal molecule, we made use of the patS null mutant, AMC451, which forms heterocysts when grown in the presence of nitrate. AMC451 is not known to have any deficiency in nitrate metabolism. Our expectation was that when grown in medium containing nitrate, the patS mutant would fail to display the normal pattern if spacing were due to the supply of fixed nitrogen from heterocysts. If another signal molecule controlled spacing, then its influence might be revealed by the formation of a partially normal pattern. Our results did not show evidence of a normal pattern and therefore argue against additional diffusible heterocyst-specific factors, other than PatS and nitrogen fixation products, controlling the pattern.
It is thought that only a subset of vegetative cells is capable of immediately initiating heterocyst differentiation after filaments are induced by nitrogen step-down. It has been speculated that this effect could be due to either stochastic or regulated differences in vegetative cells along a filament based on their position in the cell cycle, a predisposition based on cell lineage or specific signals that create a cryptic pattern, or differential stores of nitrogen reserves. Although our experiments do not address which mechanism might be correct, it is clear that only a subset of cells showed increased patS-gfp expression 4 to 8 h after induction.
A recent report suggests that the nitrogen sufficiency of individual cells may not control heterocyst induction or pattern (21). A. variabilis contains two Mo-dependent nitrogenases: one, Nif1, is expressed in heterocysts, and the second, Nif2, is expressed under anoxic conditions in vegetative cells (20). Under the anoxic growth conditions that allow the expression of Nif2, which include a dinitrogen atmosphere, fructose as a carbon source, and a herbicide to inhibit oxygen production by photosystem II, filaments form heterocysts even though the Nif2 system provides enough fixed nitrogen to support the long-term growth of a nif1 mutant (21). The intriguing possibility is raised that filaments respond specifically to the availability of external fixed nitrogen to control heterocyst development by producing differentiation signals that affect the entire filament (21). The molecular form of fixed nitrogen appears to be a factor because an earlier study showed that externally supplied glutamine does not inhibit heterocyst development in A. variabilis (19). We believe that these results can be interpreted to indicate that heterocyst development is controlled by a threshold of nitrogen sufficiency that is relatively high compared to levels that would be so low as to slow the growth rate. They also indicate that different nitrogen compounds are perceived differently by the organism in terms of the regulation of heterocyst development, even though they support an apparently normal growth rate. It should be adaptive for Anabaena to form heterocysts before the external nitrogen supply reaches a level too low to support normal growth. Similarly, the control of heterocyst spacing in a filament should allow the differentiation of new heterocysts at a level of nitrogen sufficiency that is higher than that which would adversely affect the growth rate. Filaments containing a fraction of cells that had their growth limited by nitrogen starvation would be at a selective disadvantage.
We believe that the heterocyst pattern in Anabaena sp. strain PCC 7120 is influenced by several factors: (i) a predisposition of some cells to begin development sooner than other cells; (ii) PatS inhibition of adjacent heterocysts and short vegetative cell intervals; and (iii) the supply of nitrogen fixation products from mature heterocysts, which suppress the development of nearby cells. An additional related factor is that filaments in stationary growth phase appear to have a substantially decreased ability to differentiate heterocysts (unpublished observations). PatS appears to be important for the creation of the initial pattern and then for pattern maintenance by resolving pairs or small groups of cells to a single heterocyst. We suspect that the role of PatS signaling is most important for pattern maintenance in rapidly growing cultures, in which cells are more likely to begin to differentiate simultaneously. Under these conditions, the products of nitrogen fixation from mature heterocysts would be produced too late to suppress the differentiation of adjacent and nearby cells that had also begun to differentiate. An important aspect of PatS signaling remains unresolved. How does the signaling mechanism allow one cell in a pair or cluster of differentiating cells to avoid self-inhibition by PatS and at the same time allow inhibition of nearby cells? We expect that identification and analysis of the components of the PatS signaling pathway will be required to answer this intriguing question.
ACKNOWLEDGMENTS
We thank members of our laboratory, especially Ivan Khudyakov and Duan Liu, for helpful discussions and Marty Lee and Scott Holliday for critically reading the manuscript. We are grateful to Wu Xiaoqiang and David Zuberer and members of his laboratory for help with acetylene reduction assays.
This work was supported by National Institutes of Health grant GM36890.
REFERENCES
- 1.Adams D G, Carr N G. Control of heterocyst development in the cyanobacterium Anabaena cylindrica. J Gen Microbiol. 1989;135:839–849. [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley-Interscience; 1994. [Google Scholar]
- 3.Cai Y, Wolk C P. Anabaena sp. strain PCC 7120 responds to nitrogen deprivation with a cascade-like sequence of transcriptional activations. J Bacteriol. 1997;179:267–271. doi: 10.1128/jb.179.1.267-271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cormack B P, Valdivia R H, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 5.Ezaz-Nikpay K, Uchino K, Lerner R E, Verdine G L. Construction of an overproduction vector containing the novel srp (sterically repressed) promoter. Protein Sci. 1994;3:132–138. doi: 10.1002/pro.5560030117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fay P. Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol Rev. 1992;56:340–373. doi: 10.1128/mr.56.2.340-373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman M. Cell determination strategies in the Drosophila eye. Development. 1997;124:261–270. doi: 10.1242/dev.124.2.261. [DOI] [PubMed] [Google Scholar]
- 8.Golden J W, Whorff L L, Wiest D R. Independent regulation of nifHDK operon transcription and DNA rearrangement during heterocyst differentiation in the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1991;173:7098–7105. doi: 10.1128/jb.173.22.7098-7105.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez F, Swales L, Bejsovec A, Skaer H, Martinez Arias A. Secretion and movement of wingless protein in the epidermis of the Drosophila embryo. Mech Dev. 1991;35:43–54. doi: 10.1016/0925-4773(91)90040-d. [DOI] [PubMed] [Google Scholar]
- 10.Ho K K, Krogmann D W. Photosynthesis. In: Carr N G, Whitton B A, editors. The biology of cyanobacteria. Oxford, England: Blackwell Scientific Publications Ltd.; 1982. pp. 191–214. [Google Scholar]
- 11.Kessler D S, Melton D A. Vertebrate embryonic induction: mesodermal and neural patterning. Science. 1994;266:596–604. doi: 10.1126/science.7939714. [DOI] [PubMed] [Google Scholar]
- 12.Kimble J, Simpson P. The LIN-12/Notch signaling pathway and its regulation. Annu Rev Cell Dev Biol. 1997;13:333–361. doi: 10.1146/annurev.cellbio.13.1.333. [DOI] [PubMed] [Google Scholar]
- 13.Lang J D, Haselkorn R. A vector for analysis of promoters in the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1991;173:2729–2731. doi: 10.1128/jb.173.8.2729-2731.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohamed A, Jansson C. Influence of light on accumulation of photosynthesis-specific transcripts in the cyanobacterium Synechocystis 6803. Plant Mol Biol. 1989;13:693–700. doi: 10.1007/BF00016024. [DOI] [PubMed] [Google Scholar]
- 15.Poznanski A, Keller R. The role of planar and early vertical signaling in patterning the expression of Hoxb-1 in Xenopus. Dev Biol. 1997;184:351–366. doi: 10.1006/dbio.1996.8500. [DOI] [PubMed] [Google Scholar]
- 16.Ramasubramanian T S, Wei T-F, Golden J W. Two Anabaena sp. strain PCC 7120 DNA-binding factors interact with vegetative cell- and heterocyst-specific genes. J Bacteriol. 1994;176:1214–1223. doi: 10.1128/jb.176.5.1214-1223.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramasubramanian T S, Wei T-F, Oldham A K, Golden J W. Transcription of the Anabaena sp. strain PCC 7120 ntcA gene: multiple transcripts and NtcA binding. J Bacteriol. 1996;178:922–926. doi: 10.1128/jb.178.3.922-926.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simpson P. Lateral inhibition and the development of the sensory bristles of the adult peripheral nervous system of Drosophila. Development. 1990;109:509–519. doi: 10.1242/dev.109.3.509. [DOI] [PubMed] [Google Scholar]
- 19.Thiel T, Leone M. Effect of glutamine on growth and heterocyst differentiation in the cyanobacterium Anabaena variabilis. J Bacteriol. 1986;168:769–774. doi: 10.1128/jb.168.2.769-774.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thiel T, Lyons E M, Erker J C, Ernst A. A second nitrogenase in vegetative cells of a heterocyst-forming cyanobacterium. Proc Natl Acad Sci USA. 1995;92:9358–9362. doi: 10.1073/pnas.92.20.9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thiel T, Pratte B. Effect on heterocyst differentiation of nitrogen fixation in vegetative cells of the cyanobacterium Anabaena variabilis ATCC 29413. J Bacteriol. 2001;183:280–286. doi: 10.1128/JB.183.1.280-286.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tumer N E, Robinson S J, Haselkorn R. Different promoters for the Anabaena glutamine synthetase gene during growth using molecular or fixed nitrogen. Nature (London) 1983;306:337–342. [Google Scholar]
- 23.Walsby A E. The permeability of heterocysts to the gases nitrogen and oxygen. Proc R Soc Lond Ser B. 1985;226:345–366. [Google Scholar]
- 24.Wei T-F, Ramasubramanian T S, Golden J W. Anabaena sp. strain PCC 7120 ntcA gene required for growth on nitrate and heterocyst development. J Bacteriol. 1994;176:4473–4482. doi: 10.1128/jb.176.15.4473-4482.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolk C P. Alternative models for the development of the pattern of spaced heterocysts in Anabaena (Cyanophyta) Plant Syst Evol. 1989;164:27–31. [Google Scholar]
- 26.Wolk C P, Elhai J, Kuritz T, Holland D. Amplified expression of a transcriptional pattern formed during development of Anabaena. Mol Microbiol. 1993;7:441–445. doi: 10.1111/j.1365-2958.1993.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 27.Wolk C P, Ernst A, Elhai J. Heterocyst metabolism and development. In: Bryant D A, editor. The molecular biology of cyanobacteria. Vol. 1. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 769–823. [Google Scholar]
- 28.Yoon H S, Golden J W. Heterocyst pattern formation controlled by a diffusible peptide. Science. 1998;282:935–938. doi: 10.1126/science.282.5390.935. [DOI] [PubMed] [Google Scholar]
- 29.Zhu J, Kong R, Wolk C P. Regulation of hepA of Anabaena sp. strain PCC 7120 by elements 5′ from the gene and by hepK. J Bacteriol. 1998;180:4233–4242. doi: 10.1128/jb.180.16.4233-4242.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]