Abstract
Replication factor C (RFC) and proliferating cell nuclear antigen (PCNA) are accessory proteins essential for processive DNA synthesis in the domain Eucarya. The function of RFC is to load PCNA, a processivity factor of eukaryotic DNA polymerases δ and ɛ, onto primed DNA templates. RFC-like genes, arranged in tandem in the Pyrococcus furiosus genome, were cloned and expressed individually in Escherichia coli cells to determine their roles in DNA synthesis. The P. furiosus RFC (PfuRFC) consists of a small subunit (RFCS) and a large subunit (RFCL). Highly purified RFCS possesses an ATPase activity, which was stimulated up to twofold in the presence of both single-stranded DNA (ssDNA) and P. furiosus PCNA (PfuPCNA). The ATPase activity of PfuRFC itself was as strong as that of RFCS. However, in the presence of PfuPCNA and ssDNA, PfuRFC exhibited a 10-fold increase in ATPase activity under the same conditions. RFCL formed very large complexes by itself and had an extremely weak ATPase activity, which was not stimulated by PfuPCNA and DNA. The PfuRFC stimulated PfuPCNA-dependent DNA synthesis by both polymerase I and polymerase II from P. furiosus. We propose that PfuRFC is required for efficient loading of PfuPCNA and that the role of RFC in processive DNA synthesis is conserved in Archaea and Eucarya.
In the eukaryotic DNA replication system, replication factor C (RFC) and proliferating cell nuclear antigen (PCNA) are DNA polymerase auxiliary proteins implicated in replicative and repair DNA synthesis (reviewed in reference 42). The replicative DNA polymerases (polδ and polɛ) in Eucarya are highly processive, i.e., they can polymerize long stretches of DNA without dissociating from the template. This property is conferred upon both DNA polymerases by PCNA, a ring-shaped homotrimeric protein capable of encircling and sliding along duplex DNA. PCNA works as an elongation factor for DNA polymerases by tethering the polymerases to the DNA template. For the loading of PCNA onto DNA, a clamp loader consisting of four distinct small subunits and one large subunit is required. The clamp loader, commonly known as RFC, performs this function in an ATP-dependent manner by (i) recognizing the primer terminus, (ii) binding to and opening the donut-shaped PCNA, and (iii) linking the opened PCNA topologically to the DNA. In the bacteria and bacteriophage systems, the replicative DNA polymerases also require the clamp molecule for their processive DNA synthesis. The molecular mechanisms of the clamp-loading process have been basically conserved, although the amino acid sequences of each molecule are distinctly different from those of eukaryotic proteins. Escherichia coli DNA polymerase III (Pol III) γ-subunit and T4 gp44/gp62 are well known as the clamp loaders for their sliding clamps, Pol III β-subunit and T4 gp45, respectively (20, 44).
Since the discovery of Archaea, the third domain of life, the molecular mechanisms of their DNA transactions have become a very interesting subject. However, the current knowledge of the archaeal DNA replication mechanism is still rudimentary. Moreover, an understanding of how the hyperthermophilic Archaea maintain their genetic information systems in cells growing under conditions unfavorable to the stability of DNA is of particular interest to biologists. Several genes encoding eukaryotic-like DNA replication proteins are present in archaeal genomes (4, 7, 12, 24). This has led to the proposal that the archaeal DNA replication mechanism is basically similar to that of Eucarya. Except for the euryarchaeotic heterodimeric DNA polymerase (3, 11, 13, 40), all archaeal DNA polymerases described to date are single subunit proteins with sequences similar to those of the family B (α-like) DNA polymerases, which include the chromosomal DNA replicases of Eucarya (4, 12, 32). The archaeal family B DNA polymerases have low processivity in vitro, and their ability to replicate the genome has been questioned (29). Our recent results, however, show that the rates of DNA synthesis by Pyrococcus furiosus DNA polymerase I (Pol BI) and DNA polymerase II (Pol D) are enhanced by the addition of P. furiosus PCNA (PfuPCNA) (5). Surprisingly, we found that PfuPCNA can self-assemble onto circular DNA without the assistance of RFC in vitro, even though the genomes of Archaea, including the pyrococci, contain genes encoding RFC-like proteins (4). Recent reports have shown that the two-subunit RFCs from Methanobacterium thermoautotrophicum and Sulfolobus solfataricus function to load the PCNA homologs in these organisms onto the DNA strand (21, 33).
To determine the functions of the two RFC-like proteins in P. furiosus, the corresponding genes located in tandem in the genome were cloned separately and expressed in E. coli, and their products were biochemically characterized in this study. The results of our analyses provide evidence for the basic conservation of the role of RFC in DNA synthesis in both Archaea and Eucarya.
MATERIALS AND METHODS
Cloning of P. furiosus genes encoding the RFC small and large subunits.
The genes encoding RFC-like proteins in Pyrococcus horikoshii (18) were used to search for their homologs in an incomplete genome sequence of P. furiosus (http://comb5-156.umbi.umd.edu/bags.html). Two primers, RFCSF (5′-ATGAGCGAAGAGATTAGAGAAGTTT-3′) and RFCSR (5′-ATCACTTCTTCCCAATTAGGGTGAAC-3′), were designed for PCR amplification of a 2.5-kb fragment from the genomic DNA of P. furiosus. The PCR product was cloned into a TA-cloning vector (pT7 Blue; Novagen), and the nucleotide sequences were determined from the several independent clones by using a capillary sequencer (ABI Prism 310 Genetic Analyzer; Applied Biosystems). Similar to its homolog in P. horikoshii, the gene encoding the putative RFC small subunit (RFCS) in P. furiosus contained an intervening sequence (an intein coded by 1,575 nucleotides [∼60 kDa]). Therefore, four primers were designed to fuse the two exteins via PCR to obtain the entire rfcS gene (see Fig. 1). The primers utilized were RFCSF1 (5′-TCATATGAGCGAAGAGATTAGAGAAGTTAAG-3′, NdeI tagged), RFCSF2 (5′-GCAGGCCCCCCTGGTGTCGGAAAGACTACAGCGGCTTTGGCCCTTG-3′), RFCSR2 (5′-CAAGGGCCAAAGCCGCTGTAGTCTTTCCGACACCAGGGGGGCCTG-3′), and RFCSR1 (5′-AGGTCGACCATCACTTCTTCCCAATTAGGGTGAAC-3′, SalI tagged). The DNA encoding the N-terminal (180 nucleotides) and C-terminal (804 nucleotides) exteins were amplified by the combinations RFCSF1-RFCSR2 and RFCSF2-RFCSR1, respectively. To fuse the two exteins together, portions of each PCR product served as templates in a second PCR with RFCSF1 and RFCSR1 as the primers. The PCR product (984 nucleotides), which contained an NdeI and a SalI site at the N and C termini, respectively, was cloned into pT7 Blue, and the integrity of the nucleotide sequence was confirmed as described above. The PCR-amplified rfcS gene fragment was digested with NdeI and SalI and was cloned into the corresponding site of the pET21a vector (Novagen). This construct was designated pTRFS. The putative RFC large subunit (RFCL) of P. furiosus was also amplified by PCR by the use of two primers, namely, RFCLF1 (5′-AGCCATATGCCAGAGCTTCCCTGGGTAGAA-3′, NdeI tagged) and RFCLR1 (5′-AGGTCGACTCACTTTTTAAGAAAGTCAAAGAGAG-3′, SalI tagged). The nucleotide sequence of the PCR product was determined as described above, and the rfcL gene was cloned into NdeI/SalI-digested pET28a′ (the kanamycin resistance marker of pET28a [Novagen] was substituted with that for ampicillin resistance) to fuse the N terminus of the gene product with the His tag sequence encoded by this vector. This construct was designated pTRFLhis.
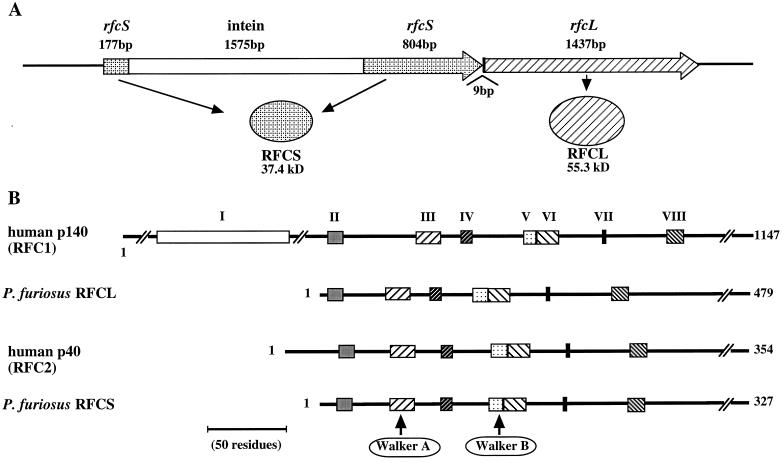
FIG. 1.
Gene organization and amino acid sequence comparison of PfuRFC with human RFC. (A) The genes for RFCS and RFCL are arranged in tandem on the P. furiosus genome. Open reading frames are indicated by the large arrows with each encoded product. An intein gene is inserted into the site for Walker motif A of RFCS. (B) Amino acid sequences of RFCS and RFCL are compared with those of the human RFC subunits. The conserved RFC boxes are indicated by closed boxes and are numbered at the top. The amino acid lengths of each subunit are indicated on the right, Walker A and B motifs characteristic for NTP binding proteins are involved in boxes III and V, respectively.
Production of recombinant RFCS.
E. coli BL21(DE3) cells containing pTRFS were grown in 1 liter of Luria-Bertani (LB) medium with ampicillin (100 μg/ml) at 30°C for 16 h without induction by isopropyl-β-d-thiogalactopyranoside (IPTG). After being harvested by centrifugation, the cell pellet was suspended in 38 ml of buffer A (50 mM Tris-HCl, pH 8.5; 10% glycerol; 2 mM β-mercaptoethanol; 0.1 M NaCl) and was lysed by using a French pressure cell (Aminco). The cell debris was removed by centrifugation at 30,000 × g for 10 min, and the supernatant was heated at 80°C for 20 min, followed by recentrifugation to partially remove the denatured E. coli proteins. Polyethyleneimine (Sigma) was added to a concentration of 0.15%, and the mixture was stirred on ice for 30 min. After centrifugation, ammonium sulfate was added to the supernatant to 80% saturation. The precipitates were collected by centrifugation (30,000 × g for 20 min), suspended in 6 ml of buffer B (10 mM potassium phosphate, pH 6.8; 7 mM β-mercaptoethanol; 0.05 mM CaCl2; 10% glycerol), and dialyzed overnight against 1 liter of the same buffer. The dialyzed material was applied to a buffer B-equilibrated hydroxyapatite column (Econo-Pac CHT-II, 5 ml; Bio-Rad), and the column was washed with one column volume of buffer B. The chromatography was then developed with a 40-ml linear gradient (0 to 60%) of buffer C (1 M potassium phosphate, pH 6.8). The RFCS protein eluted at a concentration of 0.35 M potassium phosphate.
Production of recombinant hisRFCL.
Epicurian coli BL21-CodonPlus (DE3)-RIL cells (Stratagene) harboring pTRFLhis were grown at 37°C in LB medium with ampicillin (100 μg/ml) and chloramphenicol (20 μg/ml) to an optical density at 600 nm of 0.4. The cells in the culture were then induced by the addition of IPTG to a final concentration of 0.25 mM. After a further 24 h of cultivation at 20°C, the cells were harvested by centrifugation. The cells were suspended in buffer D (20 mM Tris-HCl, pH 8.0; 300 mM NaCl) and were disrupted by a single passage through a French pressure cell. The cell debris was removed by centrifugation at 30,000 × g for 15 min, and the supernatant was collected. After the metal affinity column (TALON-NX Metal Resin; Clontech) was equilibrated with buffer D, it was loaded with the supernatant and washed with 10 column volumes of the washing buffer (buffer D plus 5 mM imidazole). The bound protein was eluted with elution buffer (buffer D plus 100 mM imidazole). For further purification, the fractions were pooled and applied to a gel filtration column (G3000SWXL, Tosoh Co., Tokyo, Japan) equilibrated with a buffer containing 0.1 M sodium phosphate (pH 6.7) and 0.1 M sodium chloride. The chromatography was performed with the same buffer at a flow rate of 0.5 ml/min.
Production of recombinant RFC complex.
Purified RFCS (a hydroxyapatite column fraction) and hisRFCL (a metal affinity column fraction) were mixed in a ratio of 20:1 based on the absorbance at 280 nm. The mixture was dialyzed against buffer E (50 mM potassium phosphate, pH 6.8; 7 mM β-mercaptoethanol; 0.1 M NaCl; 10% glycerol). The dialysate was applied to a buffer E-equilibrated anion-exchange column (HiTrap Q, 5 ml; Amersham Pharmacia Biotech), and the column was developed with a 50-ml linear gradient of 0.1 to 1.0 M NaCl in buffer E. The excess RFCS did not bind to the column, and P. furiosus RFC (PfuRFC) was eluted at a 0.3 M NaCl concentration. The PfuRFC was further diluted 1 to 3 in volume with buffer E without NaCl and was applied to an affinity column (HiTrap Heparin, 5 ml; Amersham Pharmacia Biotech). The column was developed with a 50-ml linear gradient of 0.1 to 1.0 M NaCl in buffer E, and PfuRFC was eluted at a 0.45 M NaCl concentration.
Protein concentrations.
The protein concentrations of each purified sample were calculated by measuring the absorbance at 280 nm after denaturation by 6 M guanidine chloride. Theoretical molar absorption coefficients for each molecule were calculated based on the number of tryptophans and tyrosines they contain, as described earlier (16). The molar extinction coefficients are 19,060 and 66,000/mol-cm for RFCS and RFCL, respectively.
Analytical gel filtration.
Purified RFCS, RFCL, and PfuRFC were subjected to a gel filtration analysis using the SMART system (Amersham Pharmacia) to estimate the molecular mass of each molecule in the solution. Molecular standard markers containing thyroglobulin (670 kDa), bovine gamma globulin (158 kDa), chicken ovalbumin (44 kDa), equine myoglobin (17 kDa), and vitamin B12 (1.35 kDa) (Bio-Rad) were used in a different run under the same conditions.
N-terminal amino acid sequencing.
A sample containing full length of hisRFCL was fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 7.5% gel, electroblotted onto a polyvinylidene difluoride (PVDF) membrane (Sequi-blot, 0.2 μm; Bio-Rad), stained with Coomassie brilliant blue R250, and destained with 5% methanol. The protein bands were excised and subjected to automated Edman degradation in an Applied Biosystems Procise model 492 protein sequencer. RFCS in solution was sequenced directly by using a commercial kit (ProSorb; Perkin-Elmer) according to the manufacturer's instructions.
Western blot analysis.
Rabbit polyclonal antibodies were raised against homogeneous RFCS and RFCL prepared by the procedure as described above. Protein samples separated by SDS-PAGE were electroblotted onto a PVDF membrane and analyzed with the enhanced chemiluminescence system (Amersham Pharmacia) according to the supplier's protocol.
ATPase activity.
Reaction mixtures (20 μl) contained 25 mM Tris-HCl (pH 7.5), 6 mM MgCl2, 0.1 mM dithiothreitol, 0.05% bovine serum albumin, 50 μM nonradioactive ATP, 16 nM [γ-32P]rATP or [α-32P]dATP, and the protein (300 ng/20 μl) under investigation. DNA, when added, was in the form of M13mp18 single-stranded DNA (ssDNA) or singly primed ssDNA (pri-DNA) or else M13mp18 double-stranded DNA (dsDNA) at a concentration of 10 ng/μl. Reactions were initiated at 65°C by the addition of ATP. Aliquots (3 μl) were removed from each reaction mixture at the indicated times after the initiation of the reaction and were dispensed into Eppendorf tubes containing 3 μl of cold 50 mM EDTA to terminate the reaction. Each sample was evaluated for ATP hydrolysis by spotting them onto a polyethyleneimine-cellulose thin-layer plate (Merck, Darmstadt, Germany), which was developed in 1 M LiCl and 0.5 M formic acid. A laser-excited image analyzer (BAS-5000; Fuji Film, Tokyo, Japan) was used to quantify the amounts of ATP hydrolyzed.
Thermostability.
Protein samples were heated on a heating block, at 80, 90, and 100°C for 20 min in buffer A with or without NaCl (200 mM). Each preparation was then tested for ATPase activity at 65°C in the presence of M13mp18 ssDNA and PfuPCNA.
Band mobility shift assay.
An ssDNA (5′-AGCTACCATGCCTGCACGAATTAAGCAATTCGTAATCATGGTCATAGCT-3′) was end labeled by [γ-32P]ATP and T4 polynucleotide kinase (Toyobo, Osaka, Japan). The product was used as the ssDNA substrate. A dsDNA was created by annealing a complementary DNA strand to the ssDNA in TE buffer (10 mM Tris-HCl, pH 8.0; 1 mM EDTA). pri-DNA was created by annealing the d17-mer (5′-AGCTATGACCATGATTA-3′). After the addition of increasing amounts of either RFCS or the PfuRFC complex to respective tubes containing 0.5 pmol of DNA, the binding reactions (10 μl) were incubated for 5 min at 60°C in the buffer containing 20 mM Tris-acetate (pH 8.0) and 0.5 mM magnesium acetate. The reactions were terminated by adding 3 μl of loading buffer (20 mM Tris-acetate, pH 8.0; 10% glycerol; 0.1% bromophenol blue), and 9 μl of each product was resolved by 1% agarose gel in 0.1× TAE buffer. The signals were then detected by autoradiography.
DNA polymerase assay.
Pol I and Pol II were prepared as described earlier (14, 22). The primer extension abilities of P. furiosus Pol I and Pol II in the absence or presence of PfuPCNA and PfuRFC were detected directly by using alkali agarose gel electrophoresis. As the template-primer substrate for one reaction, two pmol of 32P-labeled primer were annealed to the M13mp18 circular ssDNA (0.2 μg) by heating the mixture in a DNA polymerase reaction buffer containing 20 mM Tris-HCl (pH 8.8), 100 mM NaCl, 5 mM MgCl2, and 2 mM β-mercaptoethanol at 95°C for 3 min, followed by cooling of the mixture gradually to room temperature. The DNA polymerase (0.25 U) under investigation was then added to the reaction mixture containing the template primer and the respective accessory factors (PfuPCNA and PfuRFC). The reaction was initiated by the addition of deoxynucleotide triphosphates (dNTP) to a concentration of 250 μM. The DNA polymerization reaction was carried out at 70°C for 4 min, and 6 μl of stop solution (98% deionized formamide, 1 mM EDTA, 0.1% xylene cyanol, 0.1% bromophenol blue) was added. A 12-μl portion of each reaction was analyzed on a 1% alkali agarose gel in 50 mM sodium hydroxide and 1 mM EDTA.
Phylogenetic analysis.
Eighty-eight amino acid sequences of RFCs and relatives were collected from sequence databases. The sequence data of RuvB and the Pol III δ and τ subunits available in databases were too numerous to be included in the phylogenetic analysis. Therefore, some sequences were selected from the RuvB and Pol III subgroups based on the E-value of less than 0.04 by the PSI-BLAST analysis using the sequence of RFCL as the query. In addition, to avoid being redundant, only one sequence among those from the highly closed organisms was used in these two subgroups. A multiple sequence alignment was constructed with the alignment software, CLUSTAL W 1.7 (36). The obtained alignment was modified by visual inspection to adjust the gap positions to exclude the gaps in the secondary structure elements as much as possible. For the molecular phylogenetic analysis, the sites including gaps were excluded from the multiple alignment. To construct a phylogenetic tree, the genetic distance between every pair of aligned sequences was calculated as a maximum likelihood (ML) estimate (9), using the Jones, Taylor, and Thornton model (16) for the amino acid substitutions. Based on the distance, a tree was constructed by the neighbor-joining (NJ) method (35). The statistical significance of the NJ tree topology was evaluated by a bootstrap analysis (8) with 1,000 iterative constructions of the NJ tree. For the molecular phylogenetic analyses, two software packages, PHYLIP 3.5c (J. Felsenstein, Department of Genetics, University of Washington, Seattle [1993]) and MOLPHY 2.3b3 (J. Adachi and M. Hasegawa, Institute of Statistical Mathematics, Tokyo, Japan [1996]) were used. The trees thus obtained were drawn by TREEVIEW (30).
Nucleotide sequence and accession numbers.
The nucleotide sequences of the genes for extein 1 and extein 2 in P. furiosus rfcS appear in the DDBJ/EMBL/GenBank nucleotide sequence databases under the accession numbers AB037374 and AB037375, respectively. The sequence of the rfcL gene also appears in the databases under the accession number AB034755.
RESULTS
Cloning of the genes encoding putative RFC homologs of P. furiosus.
In the P. furiosus genome, the two genes encoding homologous sequences to the eukaryotic RFC are arranged in tandem, and a region possibly encoding an intein was found in the first gene (Fig. 1A). The insertion position is at the Walker A motif for the NTP binding, which is consistent with the fact that intein genes disrupt the motifs important for the activity of the host proteins. This intervening sequence in the gene was removed, and the two exteins were fused together by a PCR method as described in the Materials and Methods. The downstream gene was also amplified by a PCR method and was cloned. These two gene products consisted of 327 and 479 amino acids, and their molecular weights were calculated to be 37,400 and 55,285, respectively. From this observation, we named these proteins RFCS and RFCL for small and large subunit, respectively. Within the N-terminal half of both RFCS and RFCL, there were highly conserved motifs, designated RFC boxes II to VIII in eukaryotic RFC (Fig. 1B). Box I, also known as the ligase motif, is found only in the large subunit of eukaryotic RFC. This motif was absent in the archaeal RFCL. The amino acid sequence of RFCS shared 58, 58, 59, and 69% identities with its archaeal homologs in Aeropyrum pernix, Methanobacterium thermoautotrophicum, Archaeoglobus fulgidus, and Methanococcus jannaschii, respectively. Among the RFC small subunits in the Eucarya, the Saccharomyces cerevisiae RFC4 and RFC2 shared identities of 42 and 39%, respectively, with RFCS. The human RFC40 exhibited the highest identity of 41%, followed by the RFC37 (40%), to RFCS. The sequence identities of RFCL between P. furiosus and other archaeal organisms were 29% (M. thermoautotrophicum) to 37% (M. jannaschii). All of the archaeal RFCLs lack the N-terminal region of the eukaryotic RFC large subunit, including box I, as described above. The RFCL was, therefore, compared with its eukaryotic counterparts from the amino acid sequence beginning from RFC box II. RFCL showed 10% identity to the RFC large subunit of S. cerevisiae, while the homologs in Drosophila melanogaster, Homo sapiens, and Caenorhabditis elegans were 18, 18, and 19%, respectively.
Expression and purification of RFCS, hisRFCL, and PfuRFC.
The genes for RFCS and RFCL were cloned and expressed individually in E. coli cells. As shown in Fig. 2, recombinant RFCS was purified to near homogeneity through heat treatment to denature the majority of host (E. coli) proteins, polyethyleneimine treatment to remove DNA, ammonium sulfate precipitation, and hydroxyapatite column chromatography. The N-terminal amino acid sequence of the purified RFCS was determined. The first eight amino acids were SEEIREVK, which matched the amino acids from positions 2 to 9 of the predicted RFCS. The initiation methionine was removed in E. coli cells. On the other hand, due to the difficulties encountered in producing the RFCL in E. coli with its native sequence, the protein was produced as a fusion protein containing an N-terminal His6 tag (hisRFCL) and was purified by Co-chelate affinity chromatography and a subsequent gel filtration column chromatography (Fig. 2). The hisRFCL was unstable under salt-free conditions and tended to aggregate in buffers without salt. However, dialysis against buffer A containing 300 mM NaCl reversed the protein aggregation. From 1 liter of E. coli culture, about 10 mg of RFCS and 5 mg of hisRFCL were purified. A complex of RFCS and hisRFCL was made by mixing the two purified proteins, anion-exchange chromatography, and heparin affinity chromatography. The two proteins eluted in the same fraction throughout the column chromatographies (Fig. 2), and the complex was designated PfuRFC. To determine the stoichiometric ratio of RFCS and RFCL in the PfuRFC, the Coomassie brilliant blue-stained gel was scanned with a densitometer, and each band was quantitated from the calibration curve obtained by using purified RFCS and hisRFCL, respectively, which were quantified from the absorbance values at 280 nm after denaturation by 6 M guanidine chloride (Materials and Methods). This densitometric analysis indicated a RFCS/RFCL ratio of 4:1 in the purified PfuRFC. For further analysis, the purified proteins were subjected to analytical gel filtration. RFCS and PfuRFC eluted at the positions corresponding to molecular masses of about 140 to 145 and 240 to 260 kDa, respectively. These results suggest that the PfuRFC complex consists of three to four RFCS and one to two RFCL proteins. When hisRFCL was subjected to gel filtration, it eluted at the void volume of the column. The RFCL may inappropriately aggregate by itself, and it is possible that RFCS is necessary for it to exist in the proper form of the RFC complex. Further biochemical and structural analyses are necessary to determine the active form of the PfuRFC complex.
FIG. 2.
Purification of recombinant RFCS, RFCL, and PfuRFC from E. coli cells. Recombinant proteins purified as described in the text were loaded onto a 12% polyacrylamide gel, which was stained with Coomassie brilliant blue. Lane M indicates the molecular size marker (New England Biolabs). The gel was scanned with a PDI 420oe Densitometer, and each band on the gel was quantitated using the Quantity One software.
Detection of RFCS and RFCL in a P. furiosus cell extract.
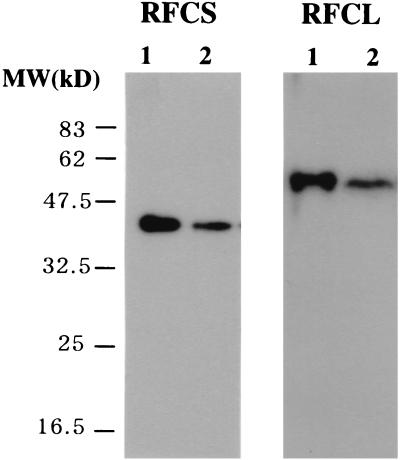
To ascertain whether recombinant PfuRFC corresponded to the proteins produced in P. furiosus cells, polyclonal antibodies were prepared by using the highly purified recombinant RFCS and hisRFCL, respectively. As shown in Fig. 3, proteins with sizes corresponding to the recombinant RFCS and hisRCFL were detected in the crude cell extract of P. furiosus by Western blot analysis. This result indicates that the genes for RFC-like open reading frames are actually expressed in P. furiosus. The size of the native RFCS protein (37.4 kDa) shows that the predicted intein in the RFCS precursor (93.7 kDa) was actually spliced out in P. furiosus cells.
FIG. 3.
Identification of RFCS and RFCL in P. furiosus cells. P. furiosus cell extracts (2.5 μg of cells for RFCS and 300 μg of cells for RFCL) and purified RFCS (0.25 ng) or RFCL (15 ng) were separated by SDS–12% PAGE. These gels were subjected to Western blot analyses with anti-RFCS and anti-RFCL antisera, respectively. Lanes: 1, recombinant RFCS or RFCL; 2, P. furiosus cell extract.
ATPase activity of the RFCS, RFCL, and PfuRFC complex.
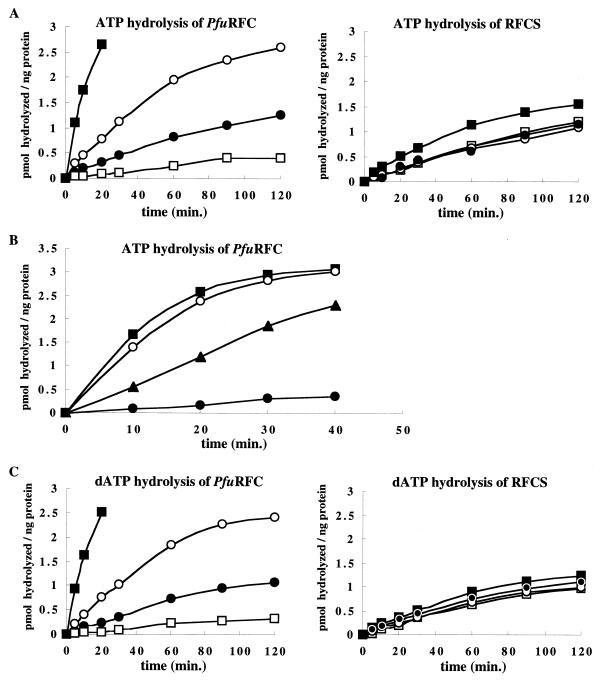
ATP hydrolysis is required by eukaryotic RFC for the loading of PCNA onto circular DNA. Therefore, the RFC-like proteins were examined for their ability to hydrolyze ATP. We initially tested the ATP hydrolysis of RFCS, RFCL, and PfuRFC separately. The results showed that RFCS possesses an ATPase activity equal to that of PfuRFC (0.01 pmol/ng/min). RFCL contained an extremely weak ATPase activity (<0.003 pmol/ng/min), even though it may aggregate, as described above. As shown in Fig. 4A, the addition of PfuPCNA to the reaction mixture tended to suppress the ATPase activities of both RFCS and PfuRFC. In the presence of ssDNA, the ATPase activity of PfuRFC, but not RFCS, was stimulated threefold. Furthermore, the ATPase activities of both RFCS and PfuRFC were enhanced 2- and 10-fold, respectively, in the presence of both ssDNA and PfuPCNA (Fig. 4A). At the concentration of RFC utilized, an ssDNA concentration of more than 0.05 pmol/μl failed to further stimulate both RFCS and PfuRFC (data not shown). With regard to PfuPCNA, beyond a concentration of 2 ng/μl, RFCS failed to show a further response, and moreover, a negative effect was observed with the higher concentration of PfuPCNA in the case of PfuRFC (data not shown). To test the dependency of the stimulation on the DNA structure, the ATPase activity of PfuRFC was measured in the presence of three types of DNA. Eukaryotic RFC is known to recognize the primer terminus (23, 38), and therefore, the partial double-stranded oligonucleotide (pri-DNA: d30-mer was annealed to the M13 ssDNA) was used to mimic the template-primer DNA. The experiment revealed that the effects of ssDNA and pri-DNA on the ATPase activity of PfuRFC were more pronounced than that with dsDNA (Fig. 4B). Under these experimental conditions, a preference for pri-DNA compared with ssDNA was not observed. The ability of RFCS and PfuRFC to hydrolyze dATP was also investigated, since dATP could be the source of energy for loading the clamp onto the DNA. PfuRFC hydrolyzed dATP with the same efficiency as that for ATP (Fig. 4C). This activity was slightly inhibited by PfuPCNA, as observed for the hydrolysis of ATP. Likewise, dATP hydrolysis by PfuRFC was enhanced in the presence of ssDNA and PfuPCNA, as observed when ATP was the substrate. Hydrolysis of [α-32P]dATP in the presence of an excess of unlabeled ATP was higher than that in the presence of an excess of unlabeled dATP, which suggests a slight preference for dATP by PfuRFC. The thermostabilities of the PfuRFC proteins were studied by heating the proteins for 20 min at various temperatures and assaying the proteins for residual ATPase activity in the presence of ssDNA and PfuPCNA. The presence of salt in the enzyme solution seems to confer further thermostability to the protein. Both RFCS and PfuRFC were highly thermostable, and heating at 100°C for 20 min only slightly affected the activity in the absence of salt (data not shown).
FIG. 4.
ATPase activity of RFCS and PfuRFC. The PfuRFC or RFCS protein (each at 0.3 μg), [α-32P]ATP or [α-32P]dATP (0.05 μCi/μl), and 50 mM ATP or dATP were incubated with or without DNA and PfuPCNA at 65°C for the indicated times. Aliquots of the reactions were analyzed by thin-layer chromatography, and the amounts of hydrolyzed ATP or dATP were quantified from the autoradiogram using a laser excited image analyzer. The graphs show the values after subtraction of the background. (A) Effects of DNA and PfuPCNA on the activity. Symbols: ●, RFC protein only; ○, with ssDNA; □, with PfuPCNA; ■, with ssDNA and PfuPCNA. (B) Dependency of the stimulation on the DNA structure. Symbols: ●, PfuRFC only; ▴, with dsDNA; ○, with ssDNA; ■, with priDNA. (C) dATPase activity of RFCS and PfuRFC. Symbols are as described for panel A.
DNA binding.
Since the ATPase activities of RFCS and PfuRFC were stimulated by DNA, as described above, the abilities of RFCS and PfuRFC to bind to DNA were investigated by using a gel retardation assay. As shown in Fig. 5A, PfuRFC had a stable binding activity to the ssDNA (d49-mer). RFCS also had the activity, with the same affinity as that of PfuRFC. However, the high-molecular-weight RFCL had little activity with this DNA. To analyze the dependency of the binding ability of PfuRFC on the DNA structure, ssDNA, pri-DNA, and dsDNA were subjected to the assay. PfuRFC bound to both ssDNA and pri-DNA with the same affinity (Fig. 5B). These binding abilities of PfuRFC were not affected by ATP (data not shown). The oligonucleotides used for the gel retardation assay may be too short to serve as suitable DNA substrates and, therefore, a filter binding assay was performed using poly(dA)280 with or without annealing to oligo(dT)25–30. At equal concentrations of DNA, no distinct difference of the binding affinity to PfuRFC was observed between the two DNA forms (Fig. 5C). These results are consistent with the equal enhancement of the ATPase activity by the ssDNA and pri-DNA, as described above. This lack of a PfuRFC preference for the DNA form is in contrast to other reports including archaeal RFCs (21, 33) but is consistent with the recent electron microscopic observation of human RFC-DNA complexes (19).
FIG. 5.
DNA binding activities of RFCS, RFCL, and PfuRFC. (A and B) Various concentrations of proteins were incubated with a 32P-labeled DNA (d49-mer as ssDNA or d45-d17-mer as pri-DNA) at 60°C for 5 min. The reaction products were analyzed by 1% agarose gel electrophoresis followed by autoradiography. (C) Alternatively, the reaction mixtures using 32P-labeled poly(dA)200 or poly(dA)200-oligo(dT)25–30 were subjected to a nitrocellulose filter binding assay. The protein-bound DNA trapped on the filter was quantified by scintillation counting. Symbols: ●, poly(dA)200 without ATP; ○, poly(dA)200 with ATP; ■, poly(dA)200-oligo(dT)25–30 without ATP; □, poly(dA)200-oligo(dT)25–30 with ATP.
Effect of PfuRFC on the PCNA-dependent DNA synthesis of Pol I and Pol II.
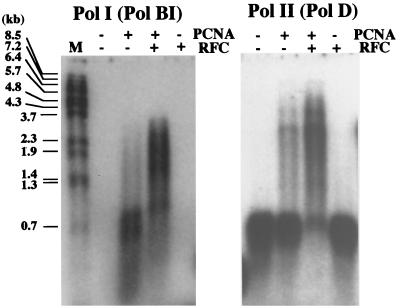
To investigate the clamp loading activity of PfuRFC, an in vitro primer extension assay was performed using Pol I and Pol II from P. furiosus. As shown in our previous study (5), the primer extension activities of Pol I and Pol II were enhanced by PfuPCNA. When PfuRFC was added to the reactions in this study, these PCNA-dependent syntheses were further enhanced. PfuPCNA clearly enhanced the extension ability of Pol I and Pol II, but a pause at a specific site in the template (M13 ssDNA), which caused the accumulation of 0.7- to 0.8-kbp products, was observed. This pause was clearly relieved upon the addition of PfuRFC, and higher yields of longer products were synthesized in both cases of the Pol I and Pol II reactions (Fig. 6). The results suggest that, in addition to the loading ability, PfuRFC possesses the ability to unload PfuPCNA from the DNA at the pause site, which results in the longer synthesis by the reassembly of the Pol-PCNA complex on the DNA strand. It is well known that the eukaryotic RFC absolutely requires ATP for the loading of PCNA onto DNA. Unexpectedly, we found that the addition of PfuRFC to the reaction mixture, in the absence of ATP, resulted in increased PfuPCNA-dependent DNA synthesis.
FIG. 6.
Effect of PfuRFC on the PfuPCNA-dependent DNA synthesis of P. furiosus Pol I and Pol II. The primer extension abilities of Pol I and Pol II were compared with M13 single-stranded circular DNA as the template in the presence or absence of PfuPCNA and PfuRFC. The reaction mixtures were analyzed by 1% alkali agarose gel electrophoresis, and the products were visualized by autoradiography. The sizes indicated on the left were from BstPI-digested λ phage DNA labeled by 32P at each 5′ end.
DISCUSSION
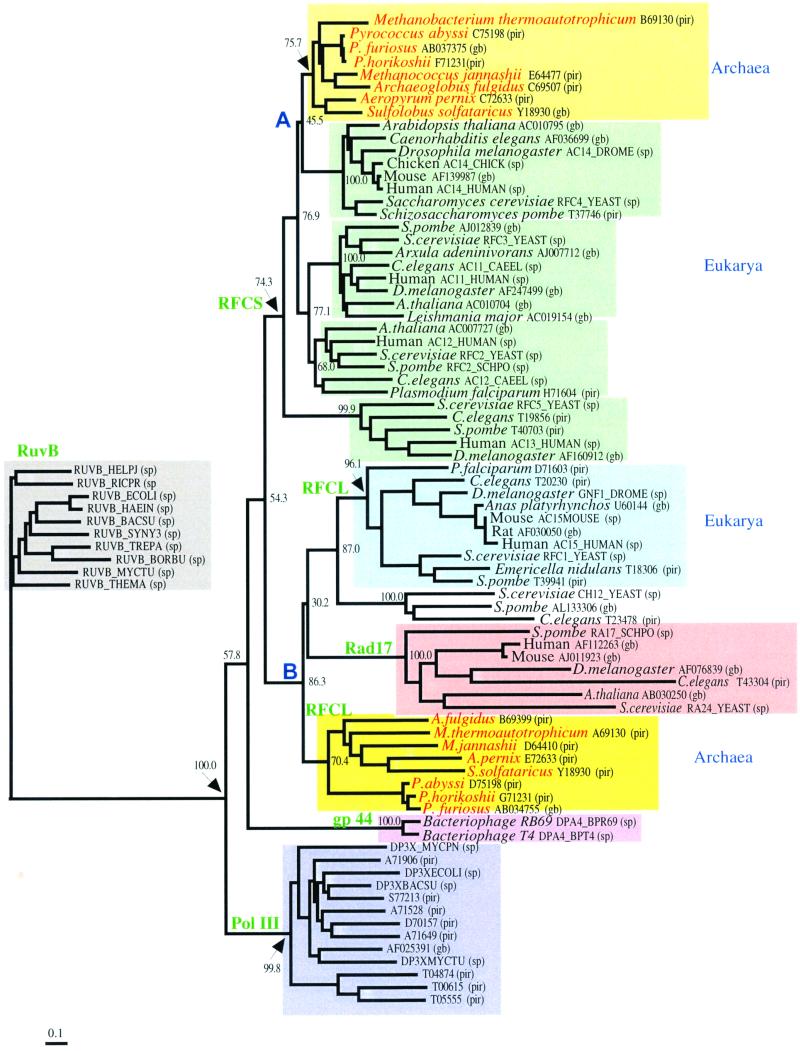
We have described that the products of two genes encoding RFC-like proteins in P. furiosus possess biological properties similar to those of known clamp loaders. PfuRFC stimulated PCNA-dependent DNA synthesis with both Pol I and Pol II in P. furiosus. This result indicates that the basic DNA replication mechanism found in the other biological domains is conserved in Archaea, and both Pol I and Pol II are the replicative enzymes in P. furiosus cells. The eukaryotic RFC consists of four small subunits and a large subunit. The small subunits share considerable amino acid sequence identity at the N-terminal half, and a similar sequence is also found in the large subunit. Despite this redundancy, all of the RFC subunits are required for the viability of yeast cells (6, 10, 25, 26, 28). The archaeal RFC may consist of only two different proteins. The absence of the N-terminal region containing the ligase motif (box I) from the archaeal large subunit suggests that a gene fusion or a loss of this motif might have occurred after the archaeal/eukaryotic divergence. Using the sequences in the public databases, we constructed an unrooted phylogenetic tree of the RFCs and their relatives (Fig. 7). Cdc6 and Orc1 were reported to be relatives of the RFCs (31). When we included them in the multiple alignment for the phylogenetic analysis, the number of alignment sites available for the estimation of genetic distance was reduced, due to the high sequence divergence. Therefore, these two groups were removed from the analysis. In order to simplify the figure, only the bootstrap probabilities for the clustering used for our evolutionary discussion are shown. The tree constructed by the ML method was similar in topology to the NJ tree, although many of the nodes showed low bootstrap probabilities (data not shown). Here, we set the significance level of the topology to be 70.0%. The tree consisted of 11 clusters. One of them was composed of the bacterial RuvBs, which were introduced into the molecular phylogenetic analysis as an outgroup for the remainders. The Pol III γ and δ′ subunits, which are known to work as parts of the clamp loader in E. coli DNA replication, form a cluster. The bacteriophage T4 gp44, which is also known to form a clamp loader complex together with the gp62 protein, and a relative derived from bacteriophages also form a different cluster. Among these clamp loader molecules, the RFCs are relatively close to each other, and they are roughly classified into two groups. One of them composes the RFC large subunits and Rad17. This group was further divided into three clusters: the RFC large subunits in Eucarya, the RFC large subunit in Archaea, and Rad17 in Eucarya. Due to the low bootstrap probabilities, it was difficult to determine the order of the evolutionary divergence of these clusters. The other group consisted of the RFC small subunits, which were further classified into five clusters. One of them consisted of the archaeal RFC small subunit, and each of the remaining four clusters corresponded to the isoforms of the eukaryotic RFC small subunits. As with the RFC large subunits, it was quite difficult to determine the order of divergence from the sequence data. The tree suggests that the divergence of the eukaryotic isoforms preceded the divergence between Archaea and Eucarya. Nodes A and B in Fig. 7 are considered to correspond to the divergence of Eucarya and Archaea. The branch lengths from node A to the terminal nodes were shorter than those from node B to the terminal nodes. This observation suggests that the RFC small subunits have been subjected to stronger functional constraints than the RFC large subunits and the Rad17s. At this stage, we do not know the details of the functional divergence of the eukaryotic RFC isoforms. Further experimental and theoretical studies on the function of the RFC complex will reveal the meaning of the differences in the branch lengths.
FIG. 7.
Phylogenetic analysis of RFC superfamily proteins. Only the bootstrap probabilities for the clustering used for the evolutionary discussion in this study are shown. The length of the bar indicates 0.1 amino acid substitutions per site. Database accession numbers are shown for each protein (pir, Protein Information Resource; gb, GenBank; sp, Swissprot). Proteins in the groups of bacterial Pol III and RuvB are shown only by their accession numbers. Nodes A and B are considered to correspond to the divergence of Eucarya and Archaea.
The primary role of RFC in DNA synthesis is to load PCNA onto DNA templates for processive DNA synthesis by the eukaryotic replicative DNA polymerases. The mode of interaction between PCNA and RFC is, however, not clearly understood. PCNA also interacts with several proteins involved in DNA transactions in the cell, including the flap endonuclease (Fen1), the DNA mismatch repair proteins MSH2 and MLH1, the nucleotide excision repair endonuclease (XP-G), a cyclin kinase inhibitor (p21), human DNA-(cytosine-5) methyltransferase (MCMT), and DNA ligase I (reviewed in reference 37). The majority of these proteins share a common motif, referred to as the PCNA interacting protein (PIP)-box (43). In the Archaea, we have shown that the two DNA polymerases from P. furiosus that interact with PfuPCNA also contain PIP-boxes (5). Interestingly, an obvious PIP-box is not found in the eukaryotic RFC (17). In each of the known archaeal RFCLs, however, a putative PIP-box is highly conserved at the extreme C terminus (5), and RFCL has been shown to interact with PfuPCNA (5). It will be interesting to analyze the similarities and the differences of the interactions between the archaeal and eukaryotic mechanisms.
The strengths of the ATPase activities from PfuRFC and RFCS were similar, but in the presence of DNA and PfuPCNA the activity of PfuRFC was much more stimulated than that of RFCS. Due to the aggregation of purified RFCL, we could not assess the strength of the intrinsic ATPase activity of RFCL. However, it is presumed that the ATPase activity of PfuRFC is inherent to the small subunit, from the analogy with eukaryotic RFC, where a subcomplex of the three small subunits (p40, p36, and p37) was shown to contain a basal DNA-dependent ATPase activity (1). The eukaryotic subcomplex also requires the interaction with p140 for maximal stimulation by DNA and PCNA (2, 34). Both PfuRFC and RFCS were demonstrated to bind to ssDNA and dsDNA. None of the four small subunits of human RFC (hRFC) binds to DNA (41) and, therefore, the subunit complex may be required for DNA binding. It is interesting that in the absence of PfuPCNA, ssDNA stimulated the ATPase activity of PfuRFC but not that of RFCS. Unlike the hRFC subcomplex, the archaeal RFCS was able to support PCNA-dependent DNA replication in a concentration-dependent manner, although with much less efficiency than with PfuRFC (S. Ishino and Y Ishino, unpublished data).
One remarkable observation is that ATP was not required in the PCNA-loading reactions of PfuRFC, in contrast to reports on eukaryotic RFC, which requires ATP for efficient PCNA-dependent DNA synthesis by Pol δ (23, 39). In the case of eukaryotic RFC, dATP, dGTP, or dCTP can substitute for ATP in supporting DNA synthesis by Pol δ; however, their efficiencies are clearly lower than that with ATP. We never observed higher efficiency of DNA synthesis by either Pol I or Pol II with the addition of ATP to the reactions. Since PfuRFC hydrolyzes dATP as efficiently as ATP, dATP in the dNTP mixture added as a DNA polymerase substrate may also serve as a cofactor for the loading of PfuPCNA. The direct detection of PCNA loading by PfuRFC in the absence and presence of ATP is required to test this hypothesis. Further investigations on this subject will undoubtedly yield very interesting insights into archaeal DNA replication.
Finally, PfuRFC is extremely stable and, therefore, it is a suitable experimental material for detailed structure-function analyses. Furthermore, this archaeal clamp loader, consisting of only two different proteins, may yield critical clues toward understanding the molecular recognition mechanisms among the DNA replication proteins common to Archaea and Eucarya.
ACKNOWLEDGMENTS
We thank A. Sugino, T. Tsurimoto, and H. Shinagawa for helpful discussions. We are grateful to Y. Shimura, the director of Biomolecular Engineering Research Institute, for continuous encouragement.
REFERENCES
- 1.Cai J, Gibbs E, Uhlmann F, Phillips B, Yao N, O'Donnell M, Hurwitz J. A complex consisting of human replication factor C p40, p37, and p36 subunits is a DNA-dependent ATPase and an intermediate in the assembly of the holoenzyme. J Biol Chem. 1997;272:18974–18981. doi: 10.1074/jbc.272.30.18974. [DOI] [PubMed] [Google Scholar]
- 2.Cai J, Yao N, Gibbs E, Finkelstein J, Phillips B, O'Donnell M, Hurwitz J. ATP hydrolysis catalyzed by human replication factor C requires participation of multiple subunits. Proc Natl Acad Sci USA. 1998;95:11607–11612. doi: 10.1073/pnas.95.20.11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cann I K O, Komori K, Toh H, Kanai S, Ishino Y. A heterodimeric DNA polymerase: evidence that members of euryarchaeota possess a distinct DNA polymerase. Proc Natl Acad Sci USA. 1998;95:14250–14255. doi: 10.1073/pnas.95.24.14250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cann I K O, Ishino Y. Archaeal DNA replication: identifying the pieces to solve a puzzle. Genetics. 1999;152:1249–1267. doi: 10.1093/genetics/152.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cann I K O, Ishino S, Hayashi I, Komori K, Toh H, Morikawa K, Ishino Y. Functional interactions of a homolog of proliferating cell nuclear antigen with DNA polymerases in Archaea. J Bacteriol. 1999;181:6591–6599. doi: 10.1128/jb.181.21.6591-6599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cullmann G, Fien K, Kobayashi R, Stillman B. Characterization of the five replication factor C genes of Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:4661–4671. doi: 10.1128/mcb.15.9.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edgell D R, Doolittle W F. Archaea and the origin(s) of DNA replication proteins. Cell. 1997;89:995–998. doi: 10.1016/s0092-8674(00)80285-8. [DOI] [PubMed] [Google Scholar]
- 8.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 9.Felsenstein J. Inferring phylogenies from protein sequences by parsimony, distance, and likelihood methods. Methods Enzymol. 1996;266:418–427. doi: 10.1016/s0076-6879(96)66026-1. [DOI] [PubMed] [Google Scholar]
- 10.Gary S L, Burgers M J. Identification of the fifth subunit of Saccharomyces cerevisiae replication factor C. Nucleic Acids Res. 1995;23:4986–4991. doi: 10.1093/nar/23.24.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imamura M, Uemori T, Kato I, Ishino Y. A non-α-like DNA polymerase from the hyperthermophilic archaeon Pyrococcus furiosus. Biol Pharmacol Bull. 1995;18:1647–1652. doi: 10.1248/bpb.18.1647. [DOI] [PubMed] [Google Scholar]
- 12.Ishino Y, Cann I K O. The euryarchaeotes, a subdomain of Archaea, survive on a single DNA polymerase: fact or farce? Genes Genet Syst. 1998;73:323–336. doi: 10.1266/ggs.73.323. [DOI] [PubMed] [Google Scholar]
- 13.Ishino Y, Komori K, Cann I K O, Koga Y. A novel DNA polymerase family found in Archaea. J Bacteriol. 1998;180:2232–2236. doi: 10.1128/jb.180.8.2232-2236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishino Y, Ishino S. DNA polymerases from Euryarchaeota. Methods Enzymol. 2001;334:249–260. doi: 10.1016/s0076-6879(01)34473-7. [DOI] [PubMed] [Google Scholar]
- 15.Jarvis T C, Paul L S, von Hippel P H. Structure and enzymatic studies of the T4 DNA replication system. J Biol Chem. 1989;264:12709–12716. [PubMed] [Google Scholar]
- 16.Jones D T, Taylor W R, Thornton J M. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- 17.Jonsson Z O, Hindges R, Hübscher U. Regulation of DNA replication and repair proteins through interaction with the front side of proliferating cell nuclear antigen. EMBO J. 1998;17:2412–2425. doi: 10.1093/emboj/17.8.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawarabayasi Y, Sawada M, Horikawa H, Haikawa H, Hino Y, Yamamoto S, Sekine M, Baba S, Kosugi H, Hosoyama A, Nagai Y, Sakai M, Ogura K, Otsuka R, Nakazawa H, Takamiya M, Ohfuku Y, Funahashi T, Tanaka T, Kudoh Y, Yamazaki J, Kushida N, Oguchi A, Aoki K, Yoshizawa T, Nakamura Y, Robb F T, Horikoshi K, Masuchi Y, Shizuya H, Kikuchi H. Complete sequence and gene organization of the genome of a hyper-thermophilic archaebacterium, Pyrococcus horikoshii OT3. DNA Res. 1998;5:55–76. doi: 10.1093/dnares/5.2.55. [DOI] [PubMed] [Google Scholar]
- 19.Keller R C, Mossi R, Maga G, Wellinger R E, Hübscher U, Sogo J M. Electron microscopic analysis reveals that replication factor C is sequestered by single-stranded DNA. Nucleic Acids Res. 1999;27:3433–3437. doi: 10.1093/nar/27.17.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelman Z, O'Donnel M. DNA polymerase III holoenzyme: structure and function of a chromosomal replicating machine. Annu Rev Biochem. 1995;64:171–200. doi: 10.1146/annurev.bi.64.070195.001131. [DOI] [PubMed] [Google Scholar]
- 21.Kelman Z, Hurwitz J. A unique organization of the protein subunits of the DNA polymerase clamp loader in the archaeon Methanobacterium thermoautotrophicum ΔH. J Biol Chem. 2000;275:7327–7336. doi: 10.1074/jbc.275.10.7327. [DOI] [PubMed] [Google Scholar]
- 22.Komori K, Ishino Y. Functional interdependence of DNA polymerizing and 3′→5′ exonucleolytic activities in Pyrococcus furiosus DNA polymerase I. Protein Eng. 2000;13:41–47. doi: 10.1093/protein/13.1.41. [DOI] [PubMed] [Google Scholar]
- 23.Lee S-H, Kwong A D, Pan Z-Q, Hurwitz J. Studies on the activator 1 protein complex, an accessory factor for proliferating cell nuclear antigen-dependent DNA polymerase δ. J Biol Chem. 1991;266:594–602. [PubMed] [Google Scholar]
- 24.Leipe D D, Aravind L, Koonin E V. Did DNA replication evolve twice independently? Nucleic Acids Res. 1999;27:3389–3401. doi: 10.1093/nar/27.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Burgers P M. Cloning and characterization of the essential Saccharomyces cerevisiae RFC4 gene encoding the 37 kDa subunit of replication factor C. J Biol Chem. 1994;269:21880–21884. [PubMed] [Google Scholar]
- 26.Li X, Burgers P M. Molecular cloning and expression of the Saccharomyces cerevisiae RFC3 gene, an essential component of replication factor C. Proc Natl Acad Sci USA. 1994;91:868–872. doi: 10.1073/pnas.91.3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mossi R, Hübscher U. Clamping down on clamps and clamp loaders-the eukaryotic replication factor C. Eur J Biochem. 1998;254:209–216. [PubMed] [Google Scholar]
- 28.Noskov V, Maki S, Kawasaki Y, Leem S-H, Ono B-I, Araki H, Pavlov Y, Sugino A. The RFC2 gene encoding a subunit of replication factor C of Saccharomyces cerevisiae. Nucleic Acids Res. 1994;22:1527–1535. doi: 10.1093/nar/22.9.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsen G J, Woese C R. Archaeal genomics: an overview. Cell. 1997;89:991–994. doi: 10.1016/s0092-8674(00)80284-6. [DOI] [PubMed] [Google Scholar]
- 30.Page R D. TreeView: an application to display phylogenetic trees on personal computers. Comp Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 31.Perkins G, Diffley J F. Nucleotide-dependent prereplicative complex assembly by Cdc6p, a homolog of eukaryotic and prokaryotic clamp-loaders. Mol Cell. 1998;2:23–32. doi: 10.1016/s1097-2765(00)80110-0. [DOI] [PubMed] [Google Scholar]
- 32.Perler F B, Kumar S, Kong H. Thermostable DNA polymerases. Adv Protein Chem. 1996;48:377–435. doi: 10.1016/s0065-3233(08)60367-8. [DOI] [PubMed] [Google Scholar]
- 33.Pisani F M, De Felice M, Carpentieri F, Rossi M. Biochemical characterization of a clamp-loader complex homologous to eukaryotic replication factor C from the hyperthermophilic archaeon Sulfolobus solfataricus. J Mol Biol. 2000;301:61–73. doi: 10.1006/jmbi.2000.3964. [DOI] [PubMed] [Google Scholar]
- 34.Podust V N, Tiwari N, Ott R, Fanning E. Functional interactions among the subunits of replication factor C potentiate and modulate its ATPase activity. J Biol Chem. 1998;273:12935–12942. doi: 10.1074/jbc.273.21.12935. [DOI] [PubMed] [Google Scholar]
- 35.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 36.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsurimoto T. PCNA, a multifunctional ring on DNA. Biochim Biophys Acta. 1998;1443:23–39. doi: 10.1016/s0167-4781(98)00204-8. [DOI] [PubMed] [Google Scholar]
- 38.Tsurimoto T, Stillman B. Purification of a cellular replication factor, RF-C, that is required for coordinated synthesis of leading and lagging strands during simian virus 40 DNA replication in vitro. Mol Cell Biol. 1989;9:609–619. doi: 10.1128/mcb.9.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsurimoto T, Stillman B. Replication factors required for SV40 DNA replication in vitro. I. DNA structure-specific recognition of a primer-template junction by eukaryotic DNA polymerases and their accessory proteins. J Biol Chem. 1991;266:1950–1960. [PubMed] [Google Scholar]
- 40.Uemori T, Sato Y, Kato I, Doi H, Ishino Y. A novel DNA polymerase in the hyperthermophilic archaeon, Pyrococcus furiosus: gene cloning, expression, and characterization. Genes Cells. 1997;2:499–512. doi: 10.1046/j.1365-2443.1997.1380336.x. [DOI] [PubMed] [Google Scholar]
- 41.Uhlmann F, Cai J, Gibbs E, O'Donnell M, Hurwitz J. Deletion analysis of the large subunit p140 in human replication factor C reveals regions required for complex formation and replication activities. J Biol Chem. 1997;272:10058–10064. doi: 10.1074/jbc.272.15.10058. [DOI] [PubMed] [Google Scholar]
- 42.Waga S, Stillman B. The DNA replication fork in eukaryotic cells. Annu Rev Biochem. 1998;67:721–751. doi: 10.1146/annurev.biochem.67.1.721. [DOI] [PubMed] [Google Scholar]
- 43.Warbrick E. PCNA binding through a conserved motif. Bioessays. 1998;20:195–199. doi: 10.1002/(SICI)1521-1878(199803)20:3<195::AID-BIES2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 44.Young M C, Reddy M K, von Hippel P H. Structure and function of the bacteriophage T4 DNA polymerase holoenzyme. Biochemistry. 1992;31:8675–8690. doi: 10.1021/bi00152a001. [DOI] [PubMed] [Google Scholar]