Abstract
The treatment in acute lymphoblastic Leukemia (ALL) has evolved and improved dramatically over the past four decades. We assessed the outcome of ALL overall, and the two major subsets of Philadelphia chromosome (Ph)-positive and Ph-negative ALL by age, time periods, ethnicity, median household income, and geographic county area. A total of 12 788 patients diagnosed with ALL from 1980 to 2017 were included. We performed an analysis to better evaluate the outcome evolution in ALL according to time period and patient’s demographic factors. The overall 5-year survival rates have improved significantly over time, from 51% before 1990 to 72% since 2010. The survival rates for children (age 0 to 14 years) and adolescents (age 15 to 19 years) have improved from 73% and 55% before 1990 to 93% and 74% since 2010, respectively. Similarly, the rates had improved from 33% to 59% for adults 20 to 29 years old, 24% to 59% for 30 to 39 years old, and 14% to 43% for 40 to 59 years old between the two time periods. The rates remained under 30% in older patients (60+ years). Since 2010, patients with Ph-negative ALL had 5-year survival rate of 73% and those with Ph-positive ALL 50%. African Americans, Hispanic ethnicity, and lower household income were associated with inferior survival. The outcome of patients with ALL showed continued improvement across all age groups in the US. The recent introduction of targeted therapies, together with optimized supportive care, will continue to improve outcomes, particularly in older patients.
1 |. INTRODUCTION
Acute lymphoblastic leukemia (ALL) accounts for about 10% of leukemias in the United States. About 6000 new cases of ALL are diagnosed each year.1 The conventional treatment of ALL consists of multiple chemotherapeutic agents used in sequences of induction, consolidation, and maintenance over 2.5 to 3 years, with central nervous system (CNS) prophylaxis, and the use of allogeneic stem cell transplantation (ASCT) in first or later remissions.2 Recently, such intensive chemotherapy regimens, patterned after the original curative pediatric regimens, have resulted in cure rates of 80+% in childhood ALL, 40–50% in adult ALL, and less than 15% in older ALL.3–5
Since 2000, the treatment of adult and older ALL (age 60+ years) has undergone rapid positive changes. These have included: (1) the addition of imatinib, and, later, more potent BCR-ABL1 tyrosine kinase inhibitors, to chemotherapy in Philadelphia chromosome (Ph)-positive and certain subtypes of Ph-like ALL3,6–10; (2) the incorporation of CD20-directed antibodies (rituximab, ofatumumab) into the treatment of Burkitt disease and CD20-positive B-ALL11–14; (3) the development of bispecific antibody constructs, antibody drug conjugates, and chimeric-antigen receptor (CAR) T-cell therapies targeting CD19 and CD2215-18; and (4) the risk stratification by measurable residual disease.19–23
Older patients with ALL have not benefited as much as younger patients from the intensive chemotherapy-based regimens due to the inherently more resistant disease features, comorbidities, and the poor tolerance of intensive chemotherapy (treatment interruptions, mortality in complete remission), as well as less transition to ASCT.24–27 In these patients, the historical cure rates are 10% or less.
Previous studies analyzed SEER data in specific subsets of patients with ALL.27–29 In this study, we analyzed the SEER data from 1980 until 2017 across all patient populations in order to quantify the improvement in ALL outcomes over the past four decades. We also evaluated the outcome evolution separately in Ph-positive and Ph-negative ALL, coded into the SEER data since 2010. Finally, we explored whether the outcome was associated with financial status, geography, or ethnicity.
2 |. PATIENTS AND METHODS
2.1 |. Patient database
The data from the SEER registries covering about 35% of the US population were used to identify patients diagnosed with ALL from 1980 until 2017. The SEER data originally included the states of Connecticut, Iowa, New Mexico, Utah, and Hawaii, as well as the metropolitan areas of Detroit and San Francisco-Oakland. This later expanded to include Alaska, Arizona, California (San Jose-Monterey, Greater California), Georgia, Idaho, Kentucky, Louisiana, Massachusetts, New Jersey, New York, Washington (Seattle-Puget Sound), and Wisconsin.
We used the third edition of the International Classification of Disease for Oncology histology codes 9811/3, 9812/3, 9813/3, 9814/3, 9815/3, 9816/3, 9817/3, 9818/3, 9826/3, 9835/3, and 9836/3 to identify all patients with diagnostic codes: “B lymphoblastic leukemia/lymphoma, NOS,” “B lymphoblastic leukemia/lymphoma with t(9;22)(q34;q11.2); BCR-ABL1,” “B lymphoblastic leukemia/lymphoma with t(v;11q23); MLL rearranged,” “B lymphoblastic leukemia/lymphoma with t(12;21)(p13;q22);TEL-AML1,” “B lymphoblastic leukemia/lymphoma with hyperdiploidy,” “B lymphoblastic leukemia/lymphoma with hypodiploidy,” “B lymphoblastic leukemia/lymphoma with t(5;14)(q31;q32); IL3-IGH,” “B lymphoblastic leukemia/lymphoma with t(1;19)(q23;p13.3); E2A PBX1,” “Burkitt cell leukemia,” “Precursor cell lymphoblastic leukemia, NOS,” and “Precursor B-cell lymphoblastic leukemia,” respectively.
A total of 14 165 patients were identified in the SEER database.30 We excluded 202 patients with adult T-cell leukemia/lymphoma, 489 patients who were reported by autopsy or death certificates without follow-up, and 686 patients with ALL and prior history of malignancy. The remaining 12 788 patients with de novo ALL were included in this analysis: 9727 before 2010, and 3061 since 2010. Ph-positive ALL was coded (code 9812/3) separately into the SEER data since 2010 (Supplemental Figure S1). A total of 549 coded patients with Ph-positive ALL were identified.
Since the Ph-positive code was available only since 2010, we analyzed the outcome of patients by time period and age group overall from 1980 until 2017. We then analyzed separately the outcomes of Ph-positive and Ph-negative ALL in the period of 2010–2017.
2.2 |. Data analysis
The pre-treatment characteristics of patients were summarized using descriptive statistics. Categorical variables were compared using the chi-square test. To evaluate the outcome by age at diagnosis, patients were grouped by age 0–14, 15–19, 20–29, 30–39, 40–59, 60–69, and 70+ years. The age group cutoffs were selected by clinical relevance including pediatric, adolescence, young adult, middle age, older patients with potential candidates for intensive therapy and allogeneic stem cell transplant, and older patients who were not typically suitable for intensive therapy. We summarized the ethnicity of Asian or Pacific Islanders, American Indian/Alaska Native, and Unknown as Others. To evaluate the outcome by annual median household income at the county levels, patients were grouped by annual household income levels <$50 000, $50 000–$75 000, and >$75 000.31,32 The overall survival was calculated from the time of diagnosis to the time of death from any cause or last follow-up, whichever comes first.33 We analyzed the overall survival using the Kaplan–Meier method with the log-rank test.34,35 We performed subgroup analysis by the presence of Ph status. We assessed 4- and 8-week mortality in each age group. To evaluate the number and percentage of cause of death at 3-month periods, histograms and stacked proportional histograms were assessed between 2000 and 2017 overall and by every 5-year period. Multiple imputations were performed for missing covariates using age at diagnosis, gender, ethnicity, diagnostic year, and geographic county areas. Poor financial status was defined as median annual household income less than $50 000/year. The geographic county areas were divided by the 2013 Rural–Urban Continuum Codes, which distinguished metropolitan counties by the population size of their metro area, and nonmetropolitan counties by degree of urbanization and adjacency to a metro area. Univariate and multivariate Cox proportional regression analyses were performed to identify prognostic factors for survival. In the univariate and multivariate models, covariates included age at diagnosis, gender, diagnostic year, type of ALL, median household income, ethnicity, and geographic areas. SEER*Stat 8.3.6.1 statistical software, SPSS 24.0 software (IBM), and R 3.6.3 software (R Development Core Team) were used for data analyses.
3 |. RESULTS
3.1 |. Patients characteristics
Among the 12 788 patients evaluated, 5591 (44%) were females (Table 1). The median age was 11 years (range 0 to 85 + years); 13% of patients were aged 60 years or older. In patients with Ph-positive ALL in the 2010–2017 cohort, 33% were 60 years or older. Overall, 81% of patients were white and 65% were non-Hispanic white. The annual median household income was available in 77% of patients, including 878 (8%), 4792 (54%), and 4240 (38%) with an annual median income of <$50 000, $50 000–75 000, >$75 000, respectively (Supplemental Table S1). Forty-four percent of patients lived in metropolitan areas with a population of more than one million people.
TABLE 1.
Patient characteristics
| ALL subgroups |
||||
|---|---|---|---|---|
| Variables | All N = 12 788 | ALL N = 12 591 | Ph-positive ALL N = 197 | p |
| Age, No. (%) | ||||
| 0–14 years | 7247 (57) | 7231 (57) | 16(8) | <.001 |
| 15–19 years | 857 (7) | 849 (7) | 8 (4) | |
| 20–29 years | 912 (7) | 898 (7) | 14 (7) | |
| 30–39 years | 736 (6) | 701 (6) | 35 (18) | |
| 40–59 years | 1423 (11) | 1364 (11) | 59 (30) | |
| 60–69 years | 723 (6) | 679 (5) | 44 (22) | |
| 70–85+ years | 890 (7) | 869 (7) | 21 (11) | |
| Sex, No. (%) | ||||
| Female | 5591 (44) | 5502 (44) | 89 (45) | .678 |
| Ethnicity, No. (%) | ||||
| Non-Hispanic white | 8369 (65) | 8249 (66) | 120 (61) | .105 |
| Hispanic white | 1976 (16) | 1950 (16) | 26 (13) | |
| Black | 935 (7) | 916 (7) | 19 (10) | |
| Other | 1508 (12) | 1476 (12) | 32 (16) | |
| Diagnostic year, No. (%) | ||||
| 1980–1989 | 2861 (23) | 2861 (23) | 0 | <.001 |
| 1990–1999 | 3280 (26) | 3280 (26) | 0 | |
| 2000–2009 | 3586 (28) | 3586 (29) | 0 | |
| 2010–2017 | 3061 (24) | 2864 (23) | 197 (100)a | |
Abbreviations: ALL, acute lymphoblastic leukemia; Ph, Philadelphia chromosome.
The histologic code of Philadelphia chromosome-positive acute lymphoblastic leukemia were available since 2010.
3.2 |. Overall outcomes
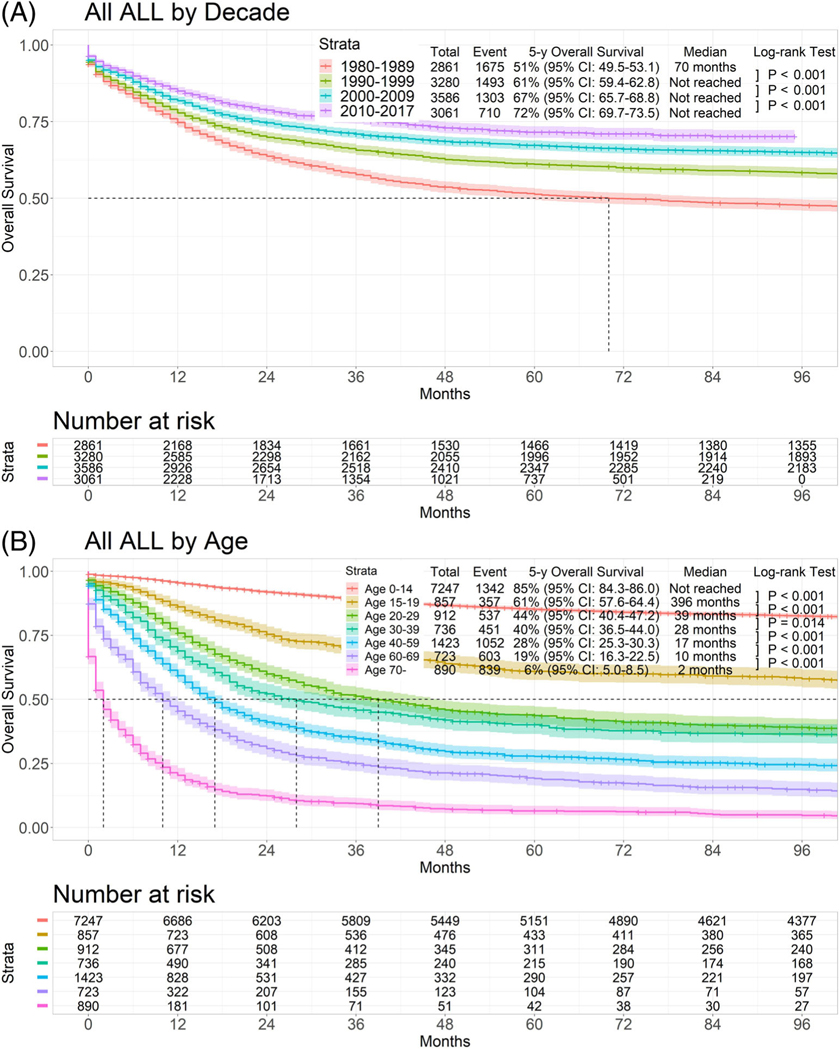
The 5-year overall survival rate for the total population by treatment decade was 51% in 1980–1989, 61% in 1990–1999, 67% in 2000–2009, and 72% in 2010–2017 (p < .001) (Figure 1(A)). Figure 1(B) shows the estimated 5-year survival rates in the seven different age groups. The estimated 5-year survival rate was 85% in patients 0–14 years old, 61% in patients 15–19 years old, 44% in patients 20–29 years old, 40% in patients 30–39 years old, 28% in patients 40–59 years old, 19% in patients 60–69 years old, and 6% in patients 70 years and older (p < .001).
FIGURE 1.
Survival of all types of acute lymphoblastic leukemia by age and decade
3.3 |. Outcomes by time period and age
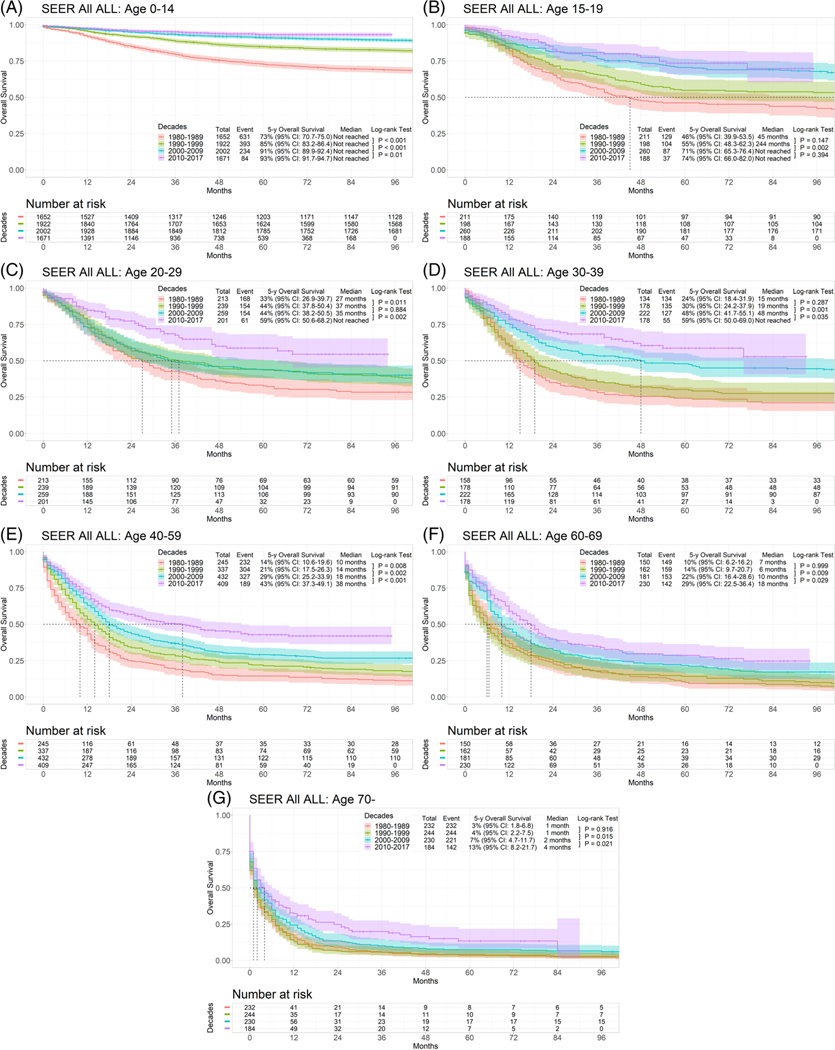
Next we analyzed the outcomes by time period and age (Table 2; Figures 2(A)–(G)). Across all age groups, the estimated 5-year survival rates continued to improve, highlighting the continued beneficial progress in ALL therapy across all age groups. By contrast, the 5-year survival rates for patients 70 years and older have made no progress, with the possible exception of the recent decade (2010–2017) when they appeared to have a small gain in survival.
TABLE 2.
Summary of 5-year and 10-year survival in patients with acute lymphoblastic leukemia by age and time period
| Estimated 5-year survival rate (%) |
||||||
|---|---|---|---|---|---|---|
| Variables | N | Overall | 1980–1989 | 1990–1999 | 2000–2019 | 2010–2017 |
| Age 0–14 years | 7247 | 85 | 73 | 85 | 91 | 93 |
| Age 15–19 years | 857 | 61 | 46 | 55 | 71 | 74 |
| Age 20–29 years | 912 | 44 | 33 | 44 | 44 | 59 |
| Age 30–39 years | 736 | 40 | 24 | 30 | 48 | 59 |
| Age 40–59 years | 1423 | 28 | 14 | 21 | 29 | 43 |
| Age 60–69 years | 723 | 19 | 10 | 14 | 22 | 29 |
| Age 70+ years | 890 | 6 | 3 | 4 | 7 | 13 |
| Estimated 10-year survival rate (%) |
||||||
| Variables | N | Overall | 1980–1989 | 1990–1999 | 2000–2019 | 2010–2017 |
| Age 0–14 years | 7247 | 82 | 67 | 82 | 88 | NA |
| Age 15–19 years | 857 | 57 | 42 | 53 | 66 | NA |
| Age 20–29 years | 912 | 38 | 28 | 38 | 40 | NA |
| Age 30–39 years | 736 | 35 | 21 | 27 | 42 | NA |
| Age 40–59 years | 1423 | 23 | 11 | 17 | 25 | NA |
| Age 60–69 years | 723 | 13 | 7 | 9 | 15 | NA |
| Age 70+ years | 890 | 4 | 1 | 3 | 5 | NA |
Abbreviation: NA, not available.
FIGURE 2.
Survival of acute lymphoblastic leukemia by decade in each age group
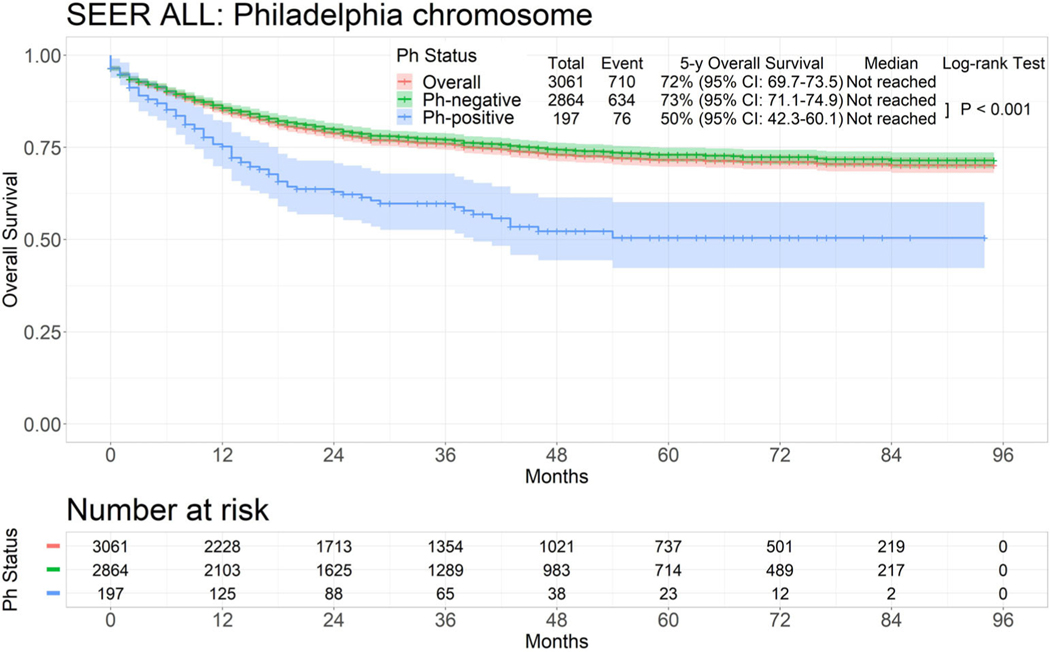
With the introduction of tyrosine kinase inhibitors (TKIs), Ph-positive ALL was considered a separate ALL entity as of 2010. We then assessed the outcomes in Ph-positive and Ph-negative ALL in the time period of 2010–2017. The 5-year overall survival rates were 72% overall, 73% for the 2864 patients with Ph-negative ALL, and 50% for the 197 patients with Ph-positive ALL (p < .001; Figure 3). The estimated 5-year survival rates were 94% (N = 16), 83% (N = 8), 38% (N = 14), 58% (N = 35), 44% (N = 59), 32% (N = 44), and not available in patients with Ph-positive ALL between 0 and 14 years old, 15 and 19 years old, 20 and 29 years old, 30 and 39 years old, 40 and 59 years old, 60 and 69 years old, and 70 years and older, respectively (p < .001; Supplemental Table S2 and Supplemental Figure S2). In patients with Ph-negative ALL, the 5-year survival rates were 93%, 73%, 59%, 59%, 42%, and 28%, respectively (p < .001; Supplemental Figure S3). The 5-year survival rate for patients 70 years and older was 11%. Since the incidence of Ph-positive ALL increases with age, by performing age-matched comparison older patients with Ph-positive ALL had better outcome due to the availability of TKIs (Supplemental Figure S2F and G).
FIGURE 3.
Survival of acute lymphoblastic leukemia by Philadelphia chromosome status
3.4 |. Four-week and eight-week mortality
The 4- and 8-week mortality rates continued to improve over the four time periods. The 4-week mortality decreased from 10% in 1980–1989 to 5% in 2010–2017 (Supplemental Tables S3–S5). The 4- and 8-week mortality rates increased with age. Overall, these rates were 5% and 7% (5% and 9% in Ph-positive ALL) between 2010 and 2017. Of note, among patients 60 to 69 years old, the 4-week mortality was 27% in 1980–1989 and decreased to 13% in 2010–2017. Among patients 70 years and older, the 4-week mortality was 51% in 1980–1989, decreased, but remained at 37%, in 2010–2017.
3.5 |. Cause of death
We then assessed the cause of death in patients with ALL between 2000 and 2017 overall and by every 5-year period. As expected, the most common cause of death was due to leukemia (77%, Supplemental Figure S4). The rate of death from ALL decreased with every 5-year period from 81% during the first 5-year period to 26% during the third 5-year period. In contrast, the proportion of death from second malignancies and cardiovascular diseases increased from 8% and 2% during the first 5-year period to 25% and 12% during the third 5-year period, respectively (p < .001; Supplemental Figure S4). Thus, among patients who survived for 10 years since the diagnosis, second malignancies and late relapse were the most common causes of death (26% and 25%, respectively), followed by cardiovascular diseases (12%).
3.6 |. Socioeconomic evaluations
We evaluated the effect of financial status, ethnicity, and population density, but only since 2000, to highlight their effects in the recent (and more relevant) time period 2000–2017 and to minimize the effect of inflation over decades. The associations between financial status and race with outcomes are shown in Supplemental Figures S4A and B. Poorer financial status (median household income less than $50 000/year) correlated with worse outcome overall (Supplemental Figure S5A). Outcome was also different by ethnicity, which is interrelated with financial status (Supplemental Figure S5B). Hispanic whites were significantly underrepresented in the older age group. Among non-Hispanic whites, 6% were 70 years or older, versus 2% among Hispanics (p < .001) (average life span 81.8 years among Hispanics and 78.6 years among non-Hispanic whites). Among ethnicity groups, Hispanic whites and Blacks had a tendency of worse survival (Supplemental Figure S5B). Blacks and Hispanic whites remained independent adverse prognostic factors in multivariate analysis (Supplemental Table S6). Finally, patients living in a small metropolitan area (population density < 250 000) had a tendency of worse outcome, with a 5-year survival rate of 62% compared to 72% in the metropolitan areas above 1 million (p = .013 by univariate Cox regression; p = .088 by log-rank) (Supplemental Figure S5C); by multivariate analysis, lower population density was adverse independent prognostic factor for survival (Supplemental Table S6).
The multivariate analysis for survival confirmed that the early time period, older age at diagnosis, Hispanic or Black, lower median annual income, lower population density, and male gender were independent adverse prognostic factors for survival (Supplemental Table S6). In patients aged 15 years and older, gender was not a prognostic factor for survival by univariate analysis (p = .993; HR, 1.000; 95% CI, 0.938–1.067).
4 |. DISCUSSION
The SEER data in ALL from 1980 to 2017 highlights multiple important points.
The most important finding is that survival has improved significantly over time, especially for adults since 2000. The estimated 5-year survival rate improved from 51% before 1990 to 72% since 2010 (Figure 1(A)). Still, age is highly correlated with outcome regardless of the time period. The estimated survival rates for children (age 0 to 14 years) and adolescents (15 to 19 years) had improved from 73% and 55% before 1990 to 93% and 74% since 2010, respectively. The rates had also improved from 33% to 59% for adults 20 to 29 years old, 24% to 59% for adults 30 to 39 years old, and 14% to 43% for those 14% to 43% between the same time periods. Although the 5-year survival rates remained under 30% in older ALL (age 60+ years), a small gain had occurred since 2010, from 22% to 29% for patients between 60 and 69 years old and 7% to 13% for those aged 70+. Recently, the incorporation of inotuzumab and blinatumomab to lower-intensity chemotherapy over a 12–18-month treatment period resulted in a high rate of complete response (90 + %) and an estimated 5-year survival rate of 50 + % for adults with ALL.36,37 Among 727 older patients (aged >65 years) with ALL treated through the Medicare health insurance program, Li and colleagues14 reported a median overall survival of 10 months (95% CI, 8.3–12.7).26 Given most older patients with ALL historically did not receive any therapy, the combination of targeted therapy with low-intensity therapy offers the opportunity for more tolerable and effective therapy in older patients.26,38
A second important observation is that modifications of the dose-schedules of chemotherapy and in supportive care measures in both children and adults continued to improve outcomes in each age group over the four time periods. Since 2000, the outcomes improved in adult ALL, with a shift from regimens that mimicked adult AML regimens (shorter maintenance, reliance on ASCT) to regimens that mimicked pediatric-inspired regimens (intensification of the non-myelosuppressive drugs like steroids and vincristine in pediatric-inspired ALL regimens that incorporate asparaginase, Hyper-CVAD regimen and its derivatives).11,39–41
A third important observation is the remarkable improved outcome for adults since 2010, the time when Ph-positive ALL was separated from Ph-negative ALL. The introduction of BCR-ABL1 tyrosine kinase inhibitors to chemotherapy in Ph-positive ALL regimens started in 2000.6–9 The estimated 5-year survival rate in adult ALL of 50% in Ph-positive disease reflects the addition of first and second-generation TKIs.6,7 This improvement was markedly observed in older patients 60+ years (5-year OS rates of 20% and 33% in Ph-negative and in Ph-positive ALL, respectively) (p = .015). The addition of CD20 antibodies (rituximab, ofatumumab) to chemotherapy started in 2000, but was established as a standard of care in adult patients with ALL since 2017.11–14,42–44 The use of inotuzumab and blinatumomab in frontline chemotherapy regimen is too recent to influence the SEER data results.36,37,45 The estimated survival of 73% in Ph-negative ALL could soon be significantly improved with the addition of CD20, CD19, and CD22 antibodies to the chemotherapy in adult (age 30–46 years)45 and older ALL (age 60 + years).36,37
In our analysis, we confirmed the independent associations of several factors affecting survival, including ethnicity (African American and Hispanic ethnicity associated with worse outcome). The poorer outcome among African Americans may be due to several factors, including a higher incidence of co-morbidities (hypertension, diabetes, cardiovascular problems, and obesity) and financial status. Hispanic patients have a higher prevalence of Ph-like ALL phenotype,46 which is associated with poor outcome.29,46–48
There are several limitations in our study. First, the SEER database does not include treatment data. Park and colleagues used the US National Cancer Institute’s SEER database to assess survival among 1675 older adults (aged ≥60 years) with ALL in the USA between 1980 and 2011.27 Median overall survival was 4 months (95% CI 3–5) and 3-year overall survival was 12.8% (95% CI 11.2–14.5). These data included patients who did not receive any chemotherapeutic agent. Therefore, although these outcomes reflect real-life practice, they are substantially influenced by patients who did not receive any anti-leukemic treatment. The recent approval of novel generation TKIs, antibody drug conjugates, and bispecific antibody constructs may not be fully reflected in the survival improvement in this study. It is possible that novel agents may further improve ALL outcomes with longer follow-up. Second, the assessment of the socioeconomic factors is limited to an evaluation closely interrelated with racial disparity. The broader inclusion of socioeconomic variables such as education levels, individual median income or household income with the adjustment of inflation over decades, and insurance status might better reflect the reasons for differences in outcome independent of ethnic status. The impact of ethnic disparities by multivariate analysis (other than Hispanic ethnicity, associated with Ph-like ALL) needs to be interpreted cautiously. Third, the coding data of Ph + status was not available before 2010. It is possible that patients with ALL received treatment with or without TKI therapy. However, we confirmed the survival improvement consistently in the total cohort over decades. Given the small number of patients with Ph-positive ALL, the results of outcome needs cautious interpretation.
In summary, the SEER data shows a continued improvement in patient outcomes over the four calendar periods and across all age categories, even in older ALL (age 70+ years) in the past decade. Ongoing trials incorporating the more potent BCR-ABL1 TKIs (ponatinib) into the Ph-positive ALL therapy, and more potent antibodies (inotuzumab, blinatumomab) into intensive and lower-intensity chemotherapy in Ph-negative ALL during induction consolidation and maintenance, will improve the ALL outcomes further across all age categories in the next decade in the real world.
Supplementary Material
ACKNOWLEDGMENTS
Supported in part by National Cancer Institute Grant No. CA021765 and the American Lebanese Syrian Associated Charities. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. National Institutes of Health. This work was supported by the National Institutes of Health/National Cancer Institute Cancer Center Support Grant CA016672 from the MD Anderson Cancer Center.
Funding information
American Lebanese Syrian Associated Charities; National Cancer Institute Grant, Grant/Award Number: CA021765
CONFLICT OF INTEREST
KS received research funding from Novartis, honoraria from Otsuka, and consultation fees from Daiichi-Sankyo, Novartis, and Pfizer. EJ received research grants and consulting fees from AbbVie, Adaptive Biotechnologies, Amgen, Astellas, Bristol-Myers Squibb, Daiichi Sankyo, Novartis, Pfizer, and Takeda. NJS received research grants from Takeda and Astellas and consulting fees from Takeda, AstraZeneca, and Amgen. NJ received research grants and consulting fees from Servier, Pharmacyclics, AstraZeneca, Genentech, Verastem, Pfizer, AbbVie, ADC Therapeutics, Precision Biosciences, and Adaptive Biotechnologies; research grants from Bristol-Myers Squibb, Celgene, Seattle Genetics, and Incyte; and consulting fees from Janssen. FR received honoraria from Sunesis and Jazz, and Orsenix; research funding from Bristol-Myers Squibb, Abbvie, Seattle Genetics, and Xencor; consultancy and honoraria from Astellas Pharmaceuticals; honoraria and research funding from Macrogenix; research funding, honoraria and speaker bureau from Amgen; honoraria and consultancy from Astellas Pharmaceuticals. HK received research grants and honoraria from AbbVie, Agios, Amgen, Immunogen, and Pfizer; research grants from Ariad, Astex, Bristol-Myers Squibb, Cyclacel, Daiichi-Sankyo, Jazz Pharma, and Novartis; and honoraria from Takeda; and attended an advisory board for Actinium. All other authors declare no competing interests.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The SEER Data is available after the request and subsequent approval at the SEER data repository.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Jabbour E, O’Brien S, Konopleva M, Kantarjian H. New insights into the pathophysiology and therapy of adult acute lymphoblastic leukemia. Cancer. 2015;121:2517–2528. [DOI] [PubMed] [Google Scholar]
- 3.Jabbour E, Pui CH, Kantarjian H. Progress and innovations in the management of adult acute lymphoblastic leukemia. JAMA Oncol. 2018;4: 1413–1420. [DOI] [PubMed] [Google Scholar]
- 4.Pui CH, Sandlund JT, Pei D, et al. Improved outcome for children with acute lymphoblastic leukemia: results of total therapy study XIIIB at St Jude Children’s research hospital. Blood. 2004;104:2690–2696. [DOI] [PubMed] [Google Scholar]
- 5.Teachey DT, Pui CH. Comparative features and outcomes between paediatric T-cell and B-cell acute lymphoblastic leukaemia. Lancet Oncol. 2019;20:e142–e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103:4396–4407. [DOI] [PubMed] [Google Scholar]
- 7.Ravandi F, Othus M, O’Brien SM, et al. US intergroup study of chemotherapy plus dasatinib and allogeneic stem cell transplant in Philadelphia chromosome positive ALL. Blood Adv. 2016;1:250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jabbour E, Kantarjian H, Ravandi F, et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: a single-Centre, phase 2 study. Lancet Oncol. 2015;16:1547–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jabbour E, Short NJ, Ravandi F, et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: long-term follow-up of a single-Centre, phase 2 study. Lancet Haematol. 2018;5: e618–e627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slayton WB, Schultz KR, Kairalla JA, et al. Dasatinib plus intensive chemotherapy in children, adolescents, and young adults with Philadelphia chromosome-positive acute lymphoblastic leukemia: results of Children’s oncology group trial AALL0622. J Clin Oncol. 2018;36:2306–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jabbour E, Richard-Carpentier G, Sasaki Y, et al. Hyper-CVAD regimen in combination with ofatumumab as frontline therapy for adults with Philadelphia chromosome-negative B-cell acute lymphoblastic leukaemia: a single-arm, phase 2 trial. Lancet Haematol. 2020;7:e523–e533. [DOI] [PubMed] [Google Scholar]
- 12.Thomas DA, O’Brien S, Faderl S, et al. Chemoimmunotherapy with a modified hyper-CVAD and rituximab regimen improves outcome in de novo Philadelphia chromosome-negative precursor B-lineage acute lymphoblastic leukemia. J Clin Oncol. 2010;28:3880–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoelzer D, Gökbuget N. Chemoimmunotherapy in acute lymphoblastic leukemia. Blood Rev. 2012;26:25–32. [DOI] [PubMed] [Google Scholar]
- 14.Maury S, Chevret S, Thomas X, et al. Rituximab in B-lineage adult acute lymphoblastic leukemia. N Engl J Med. 2016;375:1044–1053. [DOI] [PubMed] [Google Scholar]
- 15.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab Ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375:740–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer. 2019;125:2474–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376:836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yilmaz M, Kantarjian H, Wang X, et al. The early achievement of measurable residual disease negativity in the treatment of adults with Philadelphia-negative B-cell acute lymphoblastic leukemia is a strong predictor for survival. Am J Hematol. 2020;95:144–150. [DOI] [PubMed] [Google Scholar]
- 20.Issa GC, Kantarjian HM, Yin CC, et al. Prognostic impact of pre-treatment cytogenetics in adult Philadelphia chromosome-negative acute lymphoblastic leukemia in the era of minimal residual disease. Cancer. 2017;123:459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Short NJ, Kantarjian HM, Sasaki K, et al. Prognostic significance of day 14 bone marrow evaluation in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia. Cancer. 2016; 122:3812–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Short NJ, Jabbour E, Sasaki K, et al. Impact of complete molecular response on survival in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2016;128:504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borowitz MJ, Devidas M, Hunger SP, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s oncology group study. Blood. 2008;111:5477–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Brien S, Thomas DA, Ravandi F, Faderl S, Pierce S, Kantarjian H. Results of the hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone regimen in elderly patients with acute lymphocytic leukemia. Cancer. 2008;113:2097–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goekbuget N, Beck J, Brueggemann M, et al. Moderate intensive chemotherapy including CNS-prophylaxis with liposomal Cytarabine is feasible and effective in older patients with Ph-negative acute lymphoblastic leukemia (ALL): results of a prospective trial from the German multicenter study Group for Adult ALL (GMALL). Blood. 2012; 120:1493–1493. [Google Scholar]
- 26.Li S, Molony JT, Chia V, Katz AJ. Patient characteristics and treatment patterns in elderly patients newly diagnosed with acute lymphoblastic leukemia (ALL) using 100% medicare ALL data. Blood. 2016;128:3981–3981. [Google Scholar]
- 27.Geyer MB, Hsu M, Devlin SM, Tallman MS, Douer D, Park JH. Overall survival among older US adults with ALL remains low despite modest improvement since 1980: SEER analysis. Blood. 2017;129:1878–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pulte D, Gondos A, Brenner H. Improvement in survival in younger patients with acute lymphoblastic leukemia from the 1980s to the early 21st century. Blood. 2009;113:1408–1411. [DOI] [PubMed] [Google Scholar]
- 29.Shah N, Rockwell B, Kazemi M, et al. Ethnic disparities in survival of adult B-cell acute lymphoblastic leukemia in modern era - a SEER analysis. Leuk Lymphoma. 2020;61:3503–3506. [DOI] [PubMed] [Google Scholar]
- 30.Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat Database: Incidence - SEER Research Data, 9 Registries, Nov 2019 Sub (1975–2017) - Linked To County Attributes - Time Dependent (1990–2017) Income/Rurality, 1969–2018 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2020, based on the November 2019 submission.
- 31.Fintel AE, Jamy O, Martin MG. Influence of insurance and marital status on outcomes of adolescents and young adults with acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk. 2015;15:364–367. [DOI] [PubMed] [Google Scholar]
- 32.Kahn JM, Keegan TH, Tao L, et al. Racial disparities in the survival of American children, adolescents, and young adults with acute lymphoblastic leukemia, acute myelogenous leukemia, and Hodgkin lymphoma. Cancer. 2016;122:2723–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathoulin-Pelissier S, Gourgou-Bourgade S, Bonnetain F, Kramar A. Survival end point reporting in randomized cancer clinical trials: a review of major journals. J Clin Oncol. 2008;26:3721–3726. [DOI] [PubMed] [Google Scholar]
- 34.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 35.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 36.Kantarjian H, Ravandi F, Short NJ, et al. Inotuzumab ozogamicin in combination with low-intensity chemotherapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2018;19:240–248. [DOI] [PubMed] [Google Scholar]
- 37.Jabbour EJ, Sasaki K, Ravandi F, et al. Inotuzumab ozogamicin in combination with low-intensity chemotherapy (mini-HCVD) with or without blinatumomab versus standard intensive chemotherapy (HCVAD) as frontline therapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukemia: a propensity score analysis. Cancer. 2019;125:2579–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Short NJ, Kantarjian H, Jabbour E, Ravandi F. Novel therapies for older adults with acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2018;13:91–99. [DOI] [PubMed] [Google Scholar]
- 39.Stock W, Luger SM, Advani AS, et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood. 2019;133:1548–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rytting ME, Jabbour EJ, Jorgensen JL, et al. Final results of a single institution experience with a pediatric-based regimen, the augmented Berlin-Frankfurt-Münster, in adolescents and young adults with acute lymphoblastic leukemia, and comparison to the hyper-CVAD regimen. Am J Hematol. 2016;91:819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jabbour E, Kantarjian H. The hyper-CVAD regimen is an optimal pediatric-inspired regimen for adolescents and adults with acute lymphoblastic leukemia. Clin Lymph Myelom Leukem. 2020. 10.1016/j.clml.2020.09.001. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 42.Ceppi F, Weitzman S, Woessmann W, et al. Safety and efficacy of intrathecal rituximab in children with B cell lymphoid CD20+ malignancies: an international retrospective study. Am J Hematol. 2016;91:486–491. [DOI] [PubMed] [Google Scholar]
- 43.Sasaki K, Kantarjian H, Wierda W, et al. Phase 2 study of hyper-CMAD with liposomal vincristine for patients with newly diagnosed acute lymphoblastic leukemia. Am J Hematol. 2020;95:734–739. [DOI] [PubMed] [Google Scholar]
- 44.Cassaday RD, Stevenson PA, Wood BL, et al. Description and prognostic significance of the kinetics of minimal residual disease status in adults with acute lymphoblastic leukemia treated with HyperCVAD. Am J Hematol. 2018;93:546–552. [DOI] [PubMed] [Google Scholar]
- 45.Short NJ, Kantarjian HM, Ravandi F, et al. Hyper-CVAD and sequential Blinatumomab in adults with newly diagnosed Philadelphia chromosome-negative B-cell acute lymphoblastic leukemia: results from a phase II study. Blood. 2020;136:9–11. [Google Scholar]
- 46.Perez-Andreu V, Roberts KG, Harvey RC, et al. Inherited GATA3 variants are associated with Ph-like childhood acute lymphoblastic leukemia and risk of relapse. Nat Genet. 2013;45:1494–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts KG, Li Y, Payne-Turner D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014; 371:1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jain N, Roberts KG, Jabbour E, et al. Ph-like acute lymphoblastic leukemia: a high-risk subtype in adults. Blood. 2017;129:572–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The SEER Data is available after the request and subsequent approval at the SEER data repository.