Abstract
Friedreich's ataxia (FRDA), a neurodegenerative disease characterized by ataxia and other neurological features, affects 1 in 50,000–100,000 individuals in the USA. However, FRDA also includes cardiac, orthopedic and endocrine dysfunction, giving rise to many secondary disease characteristics. The multifaceted approach for clinical care has necessitated the development of disease-specific clinical care guidelines. New developments in FRDA include the advancement of clinical drug trials targeting the NRF2 pathway and frataxin restoration. Additionally, a novel understanding of gene silencing in FRDA, reflecting a variegated silencing pattern, will have applications to current and future therapeutic interventions. Finally, new perspectives on the neuroanatomy of FRDA and its developmental features will refine the time course and anatomical targeting of novel approaches.
Keywords: : antioxidant, clinical care guideline, clinical trial, Frataxin, multisystem, NRF2
Plain language summary
Friedreich's ataxia (FRDA), mainly referred to as a disorder of balance, is characterized by loss of coordination (ataxia) in the arms and legs and other neurological features, affecting about 1 in 50,000 people in the USA. FRDA also includes serious heart disease, aggressive scoliosis, diabetes and many other disease characteristics. Due to various clinical care needs, disease-specific clinical care guidelines have been created. New developments in FRDA include the advancement of clinical drug trials targeting cell signaling pathways and restoration of the deficient protein found in individuals with FRDA. Additionally, a new understanding of the role of the various genetic factors that contribute to the development of FRDA could affect current and future therapies. Finally, new perspectives on the early developmental features of FRDA will help refine the time course and accelerate new treatments.
Practice points.
-
Clinical features
-
○
Friedreich's ataxia (FRDA) is a progressive neurodegenerative disease with features outside the nervous system;
-
○
Cardiac disease is the most common cause of death in FRDA;
-
○
Bone health including osteopenia is a new area of clinical care in FRDA.
-
○
-
Clinical care requires the integration of care across many pathophysiological systems.
-
○
Clinical guidelines exist for essentially all aspects of medical care in FRDA;
-
○
Such guidelines generally do not reveal FRDA-specific treatments, but instead reinforce the need for high-quality standard medical care in FRDA.
-
○
-
Clinical trials are investigating agents that ameliorate mitochondrial dysfunction in FRDA or raise frataxin levels.
-
○
Two new areas of treatment include the enhancement of the NRF2 pathway, which mediates the mitochondrial reaction to oxidant damage;
-
○
and restoration of deficient frataxin, which potentially reverses the primary cause of FRDA.
-
○
-
New scientific advances continue to influence clinical understanding of FRDA.
-
○
A new explanation of epigenetic silencing in FRDA stresses a variegated silencing mechanism, which explains many clinical phenomena in FRDA such as the overlap in frataxin levels between carriers and patients;
-
○
Assessment of the neuroanatomic features of FRDA clarifies the targets of new therapies and helps understand the new perspectives on the developmental aspects of the disease.
-
○
Background
Friedreich's ataxia (FRDA), a progressive neurodegenerative disease, affects 1 in every 50,000-100,000 individuals in the USA, making FRDA the most commonly inherited autosomal recessive ataxia [1–5]. Typically, onset of FRDA occurs between the ages of 5 and 20 years of age but it can present outside this range with some patients presenting as late as their 60s [6,7]. FRDA most commonly results from homozygous inheritance of FXN alleles that contain a trinucleotide GAA repeat expansion in intron 1 [1–5]. Normal alleles have fewer than 33 GAA repeats; 96% of patients with FRDA are homozygous for abnormal GAA expansions of 66–1500 repeats. The remaining 4% of patients carry a point mutation or deletion in one FXN allele and an expanded GAA repeat on the other [1–5]. The trinucleotide expansion disrupts transcription of FXN leading to deficiency of the protein frataxin in those with FRDA. The clinical severity of FRDA correlates with the length of the GAA expansion (particularly on the shorter of the two alleles) as assessed by age of onset, rate of progression, or amount of residual frataxin [1–5,8–10].
Frataxin, a nuclear-encoded mitochondrial protein, plays a crucial role in mitochondrial iron metabolism and iron homeostasis [1–5], particularly in the maintenance of iron-sulfur clusters for enzymes involved in oxidative phosphorylation, the Krebs Cycle, and other cellular events [11–13]. Frataxin deficient cells exhibit oxidative stress in vitro, mitochondrial iron accumulation, decreased ATP production, and cellular dysfunction [14]. While frataxin deficiency is ubiquitous across tissues in FRDA, cells most affected clinically in FRDA include large sensory neurons of dorsal root ganglia (DRG), the dentate nucleus of the cerebellum, upper motor neurons giving rise to the corticospinal tract, cardiomyocytes, pancreatic islet cells and other selected cells of the brain and retina [15]. In the present review, we briefly summarize the clinical features of the disorder (including novel aspects such as bone health) and the availability and rationale behind clinical care guidelines. We subsequently update the status of clinical trials and discuss the details of the present approaches as well as novel basic science understanding that shapes future investigation of FRDA.
Clinical features & care
Multisystem nature of FRDA
Traditionally classified as a neurodegenerative disease with ataxia as the most common presentation, modern viewpoints approach FRDA as a multisystem disorder with diverse manifestations that require a variety of clinical therapies and comprehensive care distinct from other neurological disorders [5]. In addition to ataxia and neurological dysfunction, patients with FRDA often develop cardiomyopathy, diabetes, scoliosis and hearing and vision loss [1–5,10,16] (Table 1)
Table 1. . Comparison of the frequency of clinical features of Friedreich's ataxia between two major natural history studies, the Friedreich Ataxia Clinical Outcome Measure Study and the European Friedreich Ataxia Consortium for Translational Studies.
| Feature | FACOMS | EFACTS |
|---|---|---|
| Neurologic dysfunction | 100 | 100 |
| Cardiomyopathy | 51 | 40 |
| Scoliosis | 76 | 74 |
| Diabetes | 5-8 | 7.1 |
| Visual loss | 50 | 37 |
| Hearing loss | 16 | NA |
| Depression | NA | 14 |
| Age at onset (years) | 13.1 | 15.7 |
| Age at baseline (years) | 25.1 | 33.9 |
EFACTS: European Friedreich Ataxia Consortium for Translational Studies; FACOMS: Friedreich Ataxia Clinical Outcome Measure Study; NA: Not applicable.
Neurological
Limb and gait ataxia is the most common presentation in FRDA patients [10,16]. Other neurological features include dysarthria, absent reflexes, and loss of vibration and proprioception sensation [17]. The three major neuroanatomical systems affected by FRDA include the proprioceptive system, the dentate nucleus of the cerebellum and the corticospinal tracts [15]. Degeneration of these systems accounts for limb ataxia, spasticity, dysarthria, lower extremity weakness and loss of proprioceptive sensation and deep tendon reflexes [15]. There is no symptomatic pharmacological treatment for the ataxia.
Visual, auditory and nonproprioceptive sensory systems are also affected in FRDA, giving rise to other neurological characteristics. These include vision loss (caused largely by optic atrophy and reduced retinal nerve fiber layer thickness related to retinal ganglion cell death), hearing loss (without loss of primary tone, reflecting auditory neuropathy), urinary urgency and neuropathic pain (reflecting sensory nerve loss). Neuropathy and spasticity can be treated symptomatically with agents for neuropathic pain (gabapentin, duloxetine, etc.) or spasticity (baclofen, tizanidine), though dosing changes over the course of FRDA as the disease evolves.
Vision
While most FRDA patients have some neuroophthalmological findings, significant afferent visual impairment is rare until later in the disease [18,19]. Early findings are diverse in FRDA including efferent abnormalities such as saccadic movements and fixation instability, abnormal neurophysiological findings in visual evoked potential tests, progressively decreasing low contrast visual acuity, impaired spatial perception, visual field abnormalities and reduced retinal nerve fiber layer (RNFL) on optical coherence tomography (OCT) scans [18–26]. Severity in all of these findings ranges from minimally abnormal to severe and typically correlates with GAA repeat length and age.
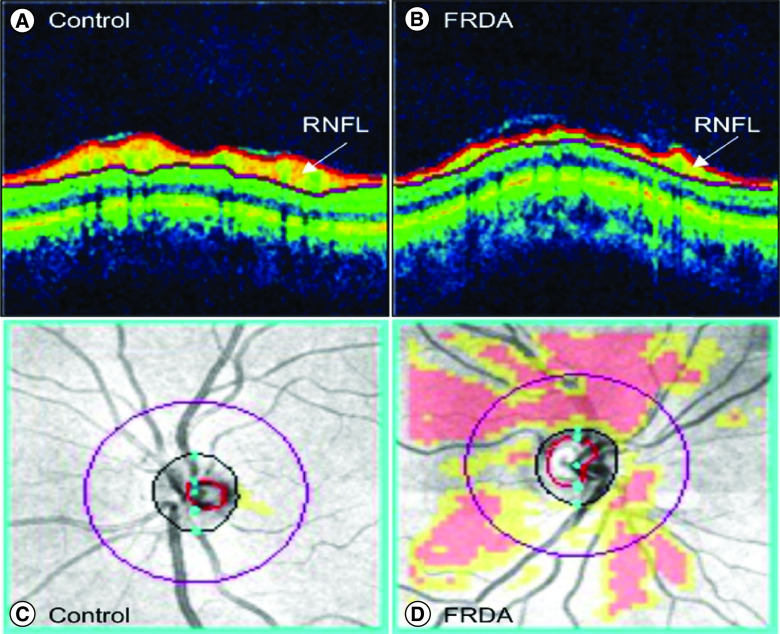
The most common cause of afferent visual dysfunction in FRDA is progressive optic neuropathy, beginning early in disease and continuing through the course of FRDA. This can either present as a slowly progressive event or as a rapid decline in vision similar to what is seen in Leber's hereditary optic neuropathy [20]. Optic neuropathy is most severe in patients with a presentation before age 10, with long GAA repeats or with point mutations including G130V [25]. The optic neuropathy of FRDA is characterized by loss of peripheral fields with later loss of central fields. Contrast letter acuity is also lost early. In the most severely affected patients vision progresses to levels worse than 20/800, a highly disabling situation. The optic neuropathy can be assessed using optical coherence tomography (OCT) which reveals moderate to severe thinning of the retinal nerve fiber layer RNFL (Figure 1) [18]. Surprisingly, when compared with MS patients with similar visual acuity, FRDA patients have thinner RNFLs, suggesting a slower progression with greater compensatory abilities of the visual system in FRDA [18].
Figure 1. . Retinal nerve fiber layer pathology in Friedreich's ataxia.
Optical coherence tomography scans of the retina in (A) a healthy control subject (age and sex matched) and (B) a subject with FRDA. The RNFL (white arrows) is outlined in red. Overlay of RNFL thickness deviation map on fundus images of the optic disk in (C) the same healthy control and (D) the same subject with FRDA. Red and yellow regions reveal areas of the retina that deviate in thickness compared with the normal distribution. Yellow: <5th percentile, red: <1st percentile.
FRDA: Friedreich's ataxia; RNFL: Retinal nerve fiber layer.
Hearing
There is a wide phenotypic spectrum of auditory sensitivity and processing impairment among individuals with FRDA. The severity of auditory impairment shows a correlation, with GAA1 repeat length >500 increasing the likelihood of auditory and spatial processing difficulty in an individual [27,28]. Only 8–13% of individuals with FRDA develop with sensorineural hearing loss based on pure tone audiometry [27]. However, 90% manifest other types of hearing impairment associated with difficulty understanding speech when background noise is present and impairments in selective attention on a singular voice based on location. This type of hearing loss has been termed ‘auditory neuropathy’, showing preserved cochlear structures with axonopathy of the eighth nerve and auditory brainstem [28,29]. Abnormal brainstem auditory evoked responses (BAERs) have been identified in FRDA, associated with significant hearing loss [30]. Speech perception is greatly affected in FRDA, particularly when presented with a competing signal. Typically, FRDA individuals are only able to access about 50% of information available compared with their peers [31,32 ]. Because pure tones are affected to a minor degree, typical hearing aids are of little value. More sophisticated directional aids can help, though they are usually expensive [32].
Cardiac
Cardiac dysfunction is the leading cause of death for individuals with FRDA, accounting for about 60% of mortality [33]. Most people with FRDA show some form of cardiac abnormality on ECG or echocardiograms, including T-wave repolarization abnormalities seen in about 85% of individuals with FRDA [34–37]. FRDA patients also develop concentric left ventricular hypertrophy, with later appearance of fibrosis [38–40]. Whether hypertrophy and later fibrosis are obligately connected is unclear. Arrhythmias are also common in patients with FRDA, particularly atrial arrhythmias. Though atrial arrhythmias are not usually fatal in isolation, they can contribute to overall mortality in combination with other cardiac issues [38]. About 30% of FRDA patients die from heart failure, the most common cause of death for FRDA patients [34,38].
The direct clinical implications of structural heart disease are difficult to evaluate in individuals with FRDA. Due to progressive ataxia, individuals are often limited in their physical activity, making the assessment of cardiac dysfunction difficult to quantify [38,39]. However, clinically significant cardiac dysfunction can appear late in the disease as well as earlier, particularly during times of physiological stress. Diastolic dysfunction becomes discernable during times of volume overload or volume depletion, such as in the context of excess administration of i.v. fluids or during gastrointestinal illnesses associated with diarrhea or vomiting, respectively [39]. Monitoring FRDA cardiomyopathy is also complicated. Echocardiograms change slowly over time, and most parameters identified on echo have little prognostic significance. Assessment of cardiac strain has been useful in cross-sectional studies, but longitudinal analysis has not been useful [40–43]. Few FRDA-specific biomarkers exist, and assessment of classical cardiac biomarkers can be paradoxical. For example, serum troponin I values, usually a marker of cardiac damage in the setting of myocardial infarction, are elevated in individuals with FRDA even in the absence of symptomatology or structural heart disease [43,44]. While this may track with hypertrophy to some degree, the correlation is insufficient to connect troponin levels with cardiac changes in acute situations, and cardiac troponin I levels in FRDA individuals always require comparison to a baseline value for any interpretation.
Orthopedic – scoliosis & pes cavus
Scoliosis occurs in most patients with FRDA. Ninety percent of individuals with early onset of FRDA symptoms develop intermediate to severe scoliosis, while those who present later (>14 years old) are more likely to develop mild scoliosis or no scoliosis at all [45]. In FRDA, scoliosis is not always progressive. The main predictors of progression are symptom onset before the age of 10 years and presence of scoliosis before the age of 15 years [46–49]. In most cases, bracing appears to provide some slowing of progression, but curvature angles greater than approximately 45 degrees normally require surgery (spinal fusion).
Pes cavus is also common in individuals with FRDA. Occurring in 55–75% of FRDA patients, pes cavus results in an abnormally high foot arch that does not flatten with pressure [14]. Although pes cavus does not commonly contribute to morbidity, it may play a role in decreased functionality of the foot [1–5].
Endocrine – diabetes mellitus I (type 1) & II (type 2)
Around 10% of FRDA patients develop diabetes. Diabetes has a wide range of features among individuals with FRDA with the greatest determinant of prevalence and pathogenic mechanism being age [50–54]. Diabetes in FRDA can present as nonimmune ‘type 1’ (insulin-requiring) or insulin-resistant ‘type 2’, but more commonly presents as an intermediate between the two types [55,56]. Those FRDA patients who present with diabetes at an early age more closely resemble type 1 diabetes requiring insulin to prevent diabetic ketoacidosis [56], while individuals with later-onset FRDA can develop type 2 diabetes, with age and increasing adiposity being cofounding factors in the disease [54]. Early-onset diabetes is also associated with a greater genetic severity, and presence of diabetes is an independent risk factor for more affected neurological function in FRDA.
Other features of FRDA
Sleep apnea
Obstructive sleep apnea (OSS) has been documented in FRDA, though it remains understudied [57]. Posture, duration of disease and reduced strength in respiratory muscles have all been linked to OSS in FRDA, and the prevalence correlates with disease duration [57]. Scoliosis and deficits in chest wall mechanics are linked to the impaired airways that cause OSS, perhaps providing an explanation for its occurrence in FRDA [58]. In later disease, atrophy can appear in the brainstem in FRDA where the major respiratory neurons are localized, suggesting a neuroanatomical basis for OSS [58]. When severe, sleep apnea is generally treated through continuous positive airway pressure to prevent further cardiopulmonary and neurologic complications [5].
Bone density
Bone density in individuals with FRDA can become compromised as a result of multiple risk factors. Firstly, bone density in adults with FRDA is below the expected range, with 20% of adults with FRDA presenting with low areal bone mineral density (BMD) by dual-energy x-ray absorptiometry scanning [59]. Additionally, early onset of FRDA can diminish peak bone mass in children leading to later development of adult osteoporosis [60]. While there is no significant difference between the blood calcium levels of FRDA individuals and healthy controls, other pathophysiological reasonings for overall decreased BMD have been proposed. Early difficulty with ambulation and mobility may play a role in bone health and density, with less ambulatory individuals bearing little to no weight on their bones. Weight bearing exercises promote an increase and maintenance of BMD in children to accelerate bone growth. The use of wheelchairs at a young age creates effects on the BMD of children and young adults [61]. Additionally, FRDA may have implications in developmental or acquired deficits in skeletal muscle leading to a decreased BMD [62,63]. Despite these increased risks of decreased bone health, recommended dietary allowance values for bone health important nutrients are currently being met by less than 50% of adult individuals with FRDA [61]. However, 60.5% of individuals reported supplementation of either calcium, vitamin D3 or a multivitamin for bone health purposes [61].
Urination
A large number of people with FRDA report urinary dysfunction, usually a sense of urgency rather than true incontinence. This presumptively reflects abnormalities related to neuronal innervation of the bladder and other components of the parasympathetic nervous system, anticholinergic therapy can minimize symptomatology but is not curative.
Depression & mental illness
Another distinct under-recognized and understudied feature of FRDA is depression [64–70], with 92% of FRDA patients reporting symptoms of depression in some series. Depression uniformly affects quality-of-life, whether such depression is an endogenous disease-related feature or a reaction to disease-specific events [5]. The exact pattern of symptomatology can also vary between case series. In either case, no singular disease-specific therapy has been identified for treatment of depression in FRDA, though pharmacological interventions including selective serotonin uptake inhibitors are generally useful [5].
Care guidelines
The field of clinical FRDA research has seen a proliferation in studies aimed at exploring such factors as frataxin levels, disease progression, and disease-modifying agents, all of which potentially impact clinical care [71]. Unfortunately, tangible improvements in clinical care in recent years are less well defined. There is at present no approved therapy that significantly alters disease symptomatology or progression; thus, providing care in FRDA remains challenging [71]. The multisystem nature of FRDA creates the necessity for both disease-specific guidelines for clinician care and a multidisciplinary approach to care treatment. In addition, the complexity and variation of FRDA symptoms onset create clinical challenges that are specific to FRDA [5,71]. In particular, the broad age differential (ages 5–90) of FRDA patients requires clinical approaches over the lifetime of patients.
The creation of clinical management guidelines has aided in resolving such challenges in several ways. Such guidelines provide a consistent framework to aid in healthcare decisions between patient and practitioner [71], particularly in situations in which the clinician is not well versed in FRDA. Clinical care guidelines simultaneously highlight the breaches in the evidence while providing a roadmap for the ongoing research that will one day underscore future revisions, which are expected in the upcoming year [71].
The current guidelines on clinical care management in patients with FRDA were developed by thirty-nine expert clinicians in the disease field from across the globe, with representation from the USA, Australia, Europe and Canada [71]. Each recommendation was assigned a grading (A–D) to specify the strength of evidence underlying the recommendation and to discern whether improved health outcomes were likely to arise through its application (consensus clin care guidelines). Those recommendations designated grade A were defined by evidence that could reliably guide practice [71], while assignments of grade D signified weak evidence backing the recommendation and that caution should be taken in its application [71]. In cases where no definitive grading level evidence was obtainable, good practice points were appointed, each formulated upon clinical experience and expert opinion [71].
Unfortunately, few controlled studies have at present identified guidelines or treatments with grade A or B evidence, highlighting the inherent gaps and difficulty in identifying effective interventions for FRDA patients. Consequently, most approaches to treatment of FRDA rely on good practice or expert observations [71]; thus, the major impetus of present guidelines is not directing clinicians toward novel therapies, but instead overcoming the hesitancy in prescribing standard of care symptomatic therapies to patients with FRDA. Essentially all pharmacological guidelines in FRDA reflect interventions based on symptoms or structural abnormalities (i.e., heart failure or hypertrophy) rather than disease-specific pathophysiology (such as frataxin deficiency mediated cardiac biology). Thus, all of the medications discussed in the present guidelines are derived from approaches to analogous situations in other diseases, including medications for neuropathic pain, spasticity or heart failure. Thus, as there are many manners to treat syndromes such as these, the guidelines put forward are truly guidelines, to be interpreted in the context of comorbidities, therapeutic urgency, patient preferences and abilities, and practicalities of healthcare systems including accessibility to treatments and health insurance. Judgement and medical art remain important aspects of the treatment of FRDA.
Interestingly, there are no identified medicines that are relatively contraindicated (even in chemotherapy) in a manner specific to FRDA, contrasting with disorders like type I Charcot Marie tooth [72–74]. One could argue that varenicline should be contraindicated based on its clinical trial being stopped due to progression of ataxia in some subjects, but the absence of discoveries of available medications specifically altering FRDA in a negative manner remains surprising. One possibility is that such discoveries would most likely be made during the presymptomatic period, as after diagnosis individuals become more reluctant to try novel approaches. Even new agents like SGLT2 inhibitors for low ejection frataxin heart failure have acquired little data in FRDA. These deficiencies illustrate that further studies of existing drugs through studies like large case series, and systematic trials of new agents (either through formal clinical trials or off-label use in justifiable situations) are needed to advance treatment, even as other research investigates the disease-specific pathophysiology of FRDA and gives rise to clinical trials of novel approaches.
Clinical trials
While FRDA currently has no approved treatment, widespread use of non-prescription antioxidants such as Vitamin E and coenzyme Q10 remains common among FRDA patients (Supplementary Table 1). While there may be suggested benefits in cardiac and skeletal energy metabolism from these supplements [75,76], they have not succeeded in blinded clinical trials for efficacy [75,76]. In those situations where supplements or antioxidants have been tested and shown modest benefit in open label studies (such as idebenone), they have failed to reach a registration level end points [77–85]. The recent failure of RT001 even suggests that reactive oxygen species (ROS) production, which provides the rationale for antioxidant use, may not be a large part of the pathophysiology of FRDA in vivo [86,87]. A variety of repurposed agents that raise frataxin levels to some degree (such as erythropoietin and gamma interferon) have also shown no clear benefit in systematic trials [88–103]. Finally, with the exception of omaveloxolone, downstream modulators have not reached end points in registration level trials, though some open-label or biomarker-based studies have suggested the potential for benefit. Further work is needed on these agents such as deferiprone, PTC743, steroid therapy, letiriglitozone and related compounds [104–116].
New developments in FRDA
Omaveloxolone: NRF2 as a downstream target
FRDA can be treated in two ways: reversal of frataxin deficiency (gene therapy, protein replacement, epigenetic therapy, reversal of FXN silencing) or amelioration of pathogenic downstream events. While initial approaches were directed at downstream ROS production, such ROS production may be less important in FRDA than other downstream events. In particular, the lack of antioxidant reserve associated with abnormalities of activation of the transcription factor NRF2 appears to play a more important role than direct ROS production in vivo [116–118]. NRF2 binds to antioxidant response elements in DNA to activate genes protecting cells from oxidative damage. NRF2 is tonically degraded by the ubiquitin ligase Keap1. Cells and tissue from FRDA patients show deficiencies in NRF2 activation in response to oxidants, with low basal levels of enzymes (particularly heme oxygenase-1 [HO-1], NAD(P)H quinone oxidoreductase 1 [NQO1], Cu/Zn and Mn-superoxide dismutases) induced in response to oxidant stress. Specifically, in FRDA, NRF2 appears mislocalized and fails to enter the nucleus in FRDA cells; consequently, little activation of these enzymes occurs even in the presence of oxidant stress. Elevated levels of KEAP1 have also been found in FRDA models, which would shut off NRF2 [116–123]. Augmentation of NRF2 levels and activation should reverse these events. Restoration of NRF2 with EPI743 or sulforaphane in FRDA cells models has also been shown to block ferroptosis, an iron-based cell death mechanism in FRDA [124].
NRF2 activation thus offers a target for downstream pharmacological intervention in FRDA. Several agents such as omaveloxolone and dimethyl fumarate can increase levels of NRF2 by blocking its binding to Keap1 and thus preventing its degradation [125–128]. DMF and omaveloxolone appear beneficial in cell models, while omaveloxolone has proceeded to clinical trials. A phase II dose-finding study in FRDA reveals a benefit on neurological exam scores in FRDA as well as benefit in correcting abnormal lipid metabolism; it also reverses intrinsic biomarkers of FRDA such as decreased ferritin levels [125]. In a pivotal study at 160 mg per day, omaveloxolone led to a clinically significant improvement in neurological exam scores and a tendency toward improvement in activities of daily living and a variety of secondary outcome measures [127]. Benefit was maintained over 2–3 years as revealed by analysis of open-label extension data showing that the rate of progression over 2–3 years dropped substantially from natural history controls. Adverse events were relatively limited, suggesting that omaveloxolone may provide a safe, efficacious therapy for FRDA.
Frataxin restoration
A second major therapeutic advance in FRDA may arise from a set of interventions referred to as frataxin restoration. The primary cause of FRDA is congenital deficiency of active frataxin leading to mitochondrial dysfunction and other downstream events [1–5]. In theory, sustained restoration of frataxin, if done to a sufficient degree and at an early enough time and in sufficient numbers of cells, should provide an ideal treatment if it is free of adverse events. Experimental data supports this as removal of the GAA repeat or transfection of frataxin cDNA in cell culture restores cellular properties [129]. One way to restore frataxin is via epigenetic approaches to reactivate the gene. In FRDA mouse models, epigenetic activation of FXN with HDAC inhibitors increases frataxin levels and reverses behavioral deficits [130–134]. A separate approach is to restore frataxin directly with protein or gene replacement therapy. Protein replacement therapy restores cellular function in vitro and reverses cardiomyopathy in FRDA mouse models [135–137]. Gene therapy ameliorates disease in virtually all tissues it reaches in FRDA mouse models [138–141]. At a scientific level, the concept that frataxin restoration may be highly efficacious certainly holds; frataxin replacement appears to be ideal for treatment of FRDA subject to the constraints of finding the correct dosage, determining when to intervene, maintaining a critical duration of efficacy and providing ability to penetrate affected tissues.
The difficulties in frataxin replacement via protein replacement or gene therapy are mainly pragmatic issues. For protein replacement specifically, the large size and need for broad distribution of frataxin make it difficult for administration of cell-permeable forms of frataxin to reach all relevant cells. In a phase I study, CT1601(tat frataxin), increased functional frataxin in skin, buccal cells and blood [142]. However, its ability to alter downstream targets and disease related tissues is unknown, and its potential to readily cross the blood–brain barrier may be incomplete. At present, CT1601 is on hold reflecting preclinical issues in primates.
Frataxin gene therapy in humans also may be limited by distribution issues. Most gene therapy paradigms at present utilize different serotypes of AAV vector to deliver the FXN gene, each targeting different cells, and few readily target brain without intraparenchymal administration [143,144]. This leaves cardiac tissue as an achievable target with little penetration into brain. Another challenge is that the development of immunity to AAVs associated with initial treatment likely prevents repeat administration [145]. The need to transduce large numbers of cells leads to a different problem: overexpression of frataxin in some cells, leading to toxicity [146]. The interplay between transduction of insufficient numbers of cells and excessive levels of frataxin can be difficult to navigate. Conceptually, gene therapy should couple a vector and delivery mechanism that transduces a large number of cells but produces only the minimum necessary amount of frataxin once introduced. This can be difficult to achieve and may require more optimization. Although use of gene editing methods may alleviate the toxicity from overexpression, the difficulty in delivery of these agents is more pronounced. Although scientifically sound, gene therapy and gene editing as treatments for FRDA may await improved delivery approaches.
Attempts to reactivate FXN expression have shown some promise in trials, but also have encountered obstacles. HDAC inhibition with RPG-2833 reached clinical trials and showed evidence of reactivation in short phase I study, but the potential for toxic metabolites appeared [134]. In addition, tissue selectivity in penetration and/or efficacy confound this therapeutic approach. Novel agents such as GeneTac may overcome many of these issues [147]. GeneTac, (DT216) designed as a selective agent to overcome FXN silencing by binding to the GAA repeat and recruiting transcription factors, shows wide tissue distributed in animals, making it an excellent candidate if no unexpected adverse events appear.
Still, the approaches of frataxin restoration so far have not truly had to address some of the limits of this type of therapy, in particular the questions of when must frataxin be restored, where must it be restored and how much is the minimum amount of restoration needed for efficacy. Knowing these answers may be needed to optimize therapy. New understanding of the mechanism of FXN silencing and of the exact neuroanatomical locations of neurodegeneration and its exact timing are now beginning to answer such questions.
Advancements in the understanding of FRDA: application to therapeutic interventions
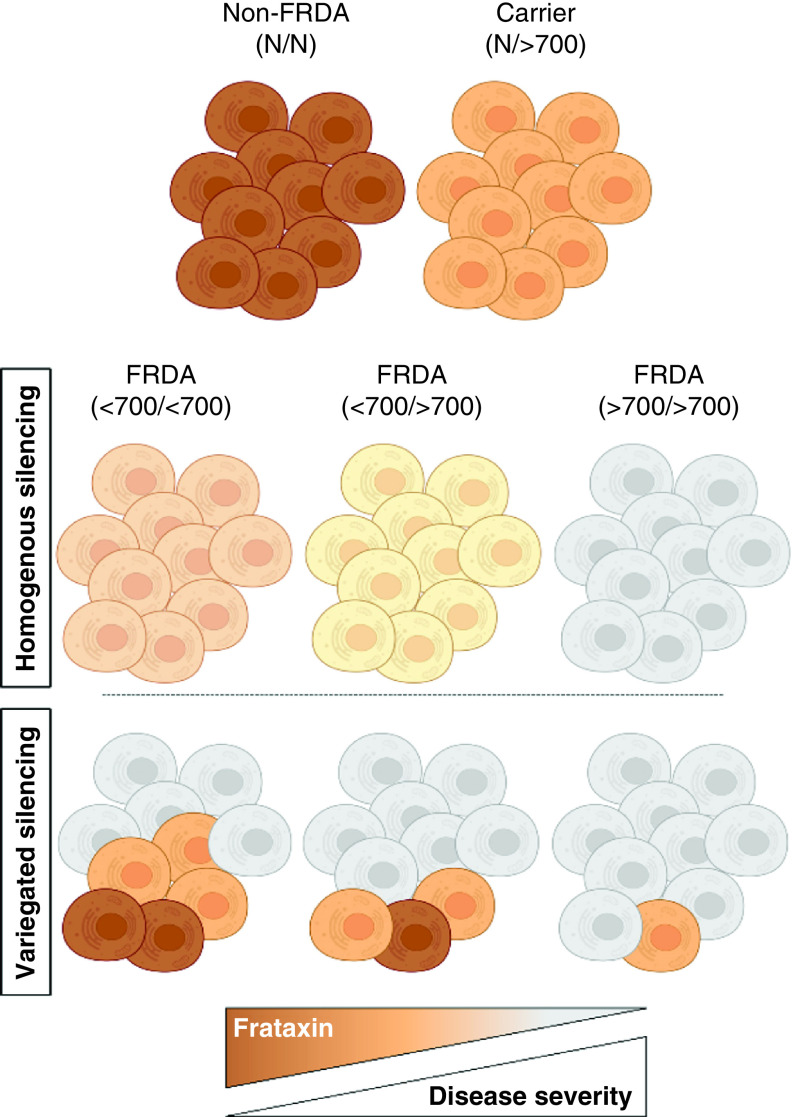
It is widely agreed upon that the GAA repeat expansion leads to epigenetic silencing of the FXN gene in FRDA [148–159]. Because the GAA repeat expansion is present in every cell in FRDA, it has been historically presumed that the FXN gene is silenced to the same degree in every cell, resulting in all cells equally expressing 5–30% of non-FRDA frataxin levels based on GAA repeat expansion length. The first indication that an alternative model of gene silencing in FRDA should be considered emerged from studying an FRDA cell model that contained a transgene with ∼200 GAA triplets upstream of a fluorescent reporter [151]. The GAA expansion acted as a source of spreading repressive chromatin that resulted in epigenetic silencing of the transgene, but not in every cell. The resulting phenotype was a stochastic pattern of gene silencing within a population of cells where some cells, although they too contained the GAA repeat expansion, escaped silencing. This variegated pattern of expression is analogous to position effect variegation (PEV), a phenomenon that was originally described in Drosophila [160]. In PEV, a gene is located abnormally close to a source of repressive chromatin, perhaps due to an inversion, resulting in epigenetic silencing of the gene, but only in a proportion of cells. The subsequent phenotype in Drosophila is a mosaic compound eye. Two recent studies, one in patient-derived cells and the other in a large clinical dataset, further support that the mechanism of FXN silencing in FRDA is analogous to PEV [148,161]. These studies showed that patients with shorter GAA repeat expansions retain a population of cells that escapes FXN silencing even while containing a GAA repeat expansion. The proportion of escaped cells reaches a minimum when GAA repeat length approaches 500–700 triplets, producing a ceiling effect on everything downstream (including clinical severity; Figure 2)
Figure 2. . A model of variegated silencing in Friedreich's ataxia.
Each cluster of 10 cells represents a theoretical person who does not have FRDA (non-FRDA), is a carrier of one GAA repeat expansion (carrier) or is one of three people who have FRDA with various GAA repeat lengths. Parentheses indicate the GAA repeat lengths: N = normal length GAA; >700 = more than 700 triplets; <700 = fewer than 700 triplets. The relative color saturation of cells represents the relative amount of frataxin (darker color = more frataxin). Homogenous silencing (top set of FRDA subjects) shows the same amount of frataxin in every cell, and less frataxin in each cell as the GAA repeat length increases. Variegated silencing (bottom set of FRDA subjects) shows how most cells in people with FRDA have very little frataxin (gray/no color), and few cells have escaped silencing, a proportion that increases with decreasing GAA repeat length. Protein/RNA assays would give the same result in either silencing model for a given repeat length i.e. shorter repeat patients would have ∼30% residual frataxin. Recent studies however support the variegated silencing model in FRDA.
FRDA: Friedreich's ataxia.
Shifting the view of FXN gene silencing to the PEV model has revealed a subpopulation of patients, those with >700 GAA triplets, that is relatively homogenous in their disease progression. This has important implications for pre-clinical and clinical data analysis and reveals that the severity (or presence) of disease features in FRDA is not dictated by relative frataxin deficiency alone, but rather the relative proportion of cells/FXN genes that escape GAA repeat-mediated silencing. Refinement of this model will allow better understanding of the selection and stratification of subjects for clinical trials as well as interpretation of the results of such trials.
Perhaps more importantly, the concept of variegated silencing leads to a reevaluation of the quantification of frataxin levels and their restoration in disease. Disease severity reflects the number of cells that are maximally silenced, not the absolute frataxin level. This explains why carriers who have the lowest levels manifest no symptoms: they still have one functioning FXN allele in almost all cells. This also suggests why the carriers with lower levels of frataxin differ from the patients with a short, expanded allele. They have similar frataxin levels, but the mechanism by which they got such levels differs. Thus, the benchmark of restoring levels of frataxin to carrier levels is flawed. In terms of restoration of frataxin level, variegated silencing emphasizes the importance of reaching many cells (particularly affected ones) more than raising the level astronomically in a few cells. In other recessive diseases, the amount of protein needed to prevent the appearance of a phenotype is frequently less than 10% of normal. New studies will need to determine the amount of frataxin needed at an individual cell level in order to better inform future approaches.
The developmental component & neuroanatomy of FRDA
Although classically viewed as a neurodegenerative disorder, many clues suggest a major developmental component to FRDA, particularly in the loss of peripheral nerve components [162]. Deep tendon reflexes are lost before clinical presentation in most individuals, and many patients recall never having reflexes even from young age. Intrinsic hand muscle atrophy and pes cavus also develop before presentation, suggesting long-standing loss. In addition, spine size is small in FRDA and MRI scans reveal dorsal column atrophy at the earliest scans [163].
In recent years, systematic scientific examination reveals the loss of proprioceptive input and dorsal column function may be near-complete by presentation. First, autopsy studies suggest that many of the dorsal roots fail to enter the spinal cord, consistent with a longstanding developmental injury [163]. In addition, somatosensory evoked potentials and sensory nerve action potentials are absent at presentation and, if retained, fail to progress over time [164–166]. Using MEG, a more sensitive manner to detect such potentials, somatosensory response can be identified at presentation but are of extremely low amplitude and are delayed in reaching the cortex. In addition, the size of such potentials correlates with genetic severity (GAA repeat length) but not disease duration [167–170]. All of these suggest an early origin to somatosensory dysfunction, with significant CNS remodeling before clinical presentation. Such dysfunction depends on early genetic events rather than disease duration and changes the ‘textbook’ anatomy of FRDA, suggesting that while proprioceptive loss provides an early mechanism in disease causation, it does not contribute to progressive disease and may not represent a target for restorative therapies.
Where are the appropriate sites of active neurodegeneration during the progression of FRDA? Most likely the dentate nucleus of the cerebellum and the motor cortex provide the major, specific sites of degeneration, with other areas such as the retina playing roles in specific disease features [3]. In addition, sites such as the Purkinje cells may not die but appear to remodel or repair over the course of time, suggesting pathological involvement [171]. Understanding this issue is crucial to the advancement in new therapies that require specific neuroanatomic targeting, such as gene therapy. Even still, many of the neurophysiological features of FRDA may reflect age of onset more than disease duration [66–164,172], emphasizing the degree of abnormality that precedes symptomatic presentation.
The importance of developmental influences also provides a rationale for questioning the detailed relevance of animal models in FRDA. Models in which frataxin levels are knocked down after birth or later may not provide evidence of developmental events. Consequently, results from such models may not readily extrapolate to predictions on drug response in human diseases. Thus, interpretation of the role of animals and their utility is important for consideration in therapeutic development programs. This will require understanding of the specific ways that animal models match neuroanatomically selective biomarkers in human FRDA.
Conclusion
FRDA is a progressive, neurodegenerative disease that affects both children and adults. Disease specific clinical care guidelines have been adapted to urge physicians to treat each FRDA symptom individually using standard of care therapies. There have been advances in areas of drug development such as NRF2 activation and frataxin restoration, which could have implications on novel, effective therapies in the future. Likewise, new perspectives on variegated gene silencing in FRDA could have implications on how future therapies approach the application of frataxin restoration, as well as lead to better stratification of subjects in clinical trials and improve pre-clinical/clinical trial data analysis. Lastly, evidence suggested a developmental component of FRDA could play a large role in determining target areas for novel therapies.
Future perspective
The past 25 years have seen enormous growth in research on FRDA leading to a better understanding of the disease at all levels. This includes refinement of the clinical phenotype, improved clinical care through collaboration and systematic observation, and Basic and clinical research leading to clinical trials delivering at least one agent to the doorstep of approval. Still, further work is needed to translate the present findings to optimal clinical response in patients, particularly in regard to crucial questions:
How long can the benefits of downstream agents like omaveloxolone last? While present data does not show any wearing off, homeostatic mechanisms at some point will likely blunt the effect of drug acting downstream from frataxin deficiency. At that point new agents will certainly be necessary acting either on other components of the pathophysiology or on frataxin deficiency itself;
How much do deficiencies in understating of the detail of frataxin restoration prevent success in clinical trials? New information from concepts like variegated silencing and new findings on the developmental pathology and anatomy in FRDA can refine approaches to restoration of frataxin. That may be enough for success, but developments outside the FRDA field such as development of new delivery systems and understanding of general toxicities of gene therapy and protein restoration may also prove important.
Overall, such questions lead to a situation in which further knowledge is still needed for optimization of therapy in FRDA.
Supplementary Material
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/nmt-2022-0011
Author contributions
M Keita, K McIntyre, LN Rodden and DR Lynch wrote the first draft. M Keita, K McIntyre, LN Rodden, K Schadt and DR Lynch provided critical revisions.
Financial & competing interests disclosure
DR Lynch receives grant money from the NIH, FDA, FARA, Reata, Retrotope, Design and PTC. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Koeppen AH. Friedreich's ataxia: pathology, pathogenesis and molecular genetics. J. Neurol. Sci. 303(1-2), 1–12 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandolfo M. Friedreich ataxia: new pathways. J. Child Neurol. 27(9), 1204–1211 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandolfo M. Friedreich ataxia. Handb. Clin. Neurol. 103, 275–294 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Pandolfo M. Friedreich ataxia: the clinical picture. J. Neurol. 256(Suppl. 1), 3–8 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Lynch DR, Schadt K, Kichula E, McCormack S, Lin KY. Friedreich Ataxia: Multidisciplinary Clinical Care. J. Multidiscip. Healthcare. 14, 1645–1658 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDaniel DO, Keats B, Vedanarayanan VV, Subramony SH. Sequence variation in GAA repeat expansions may cause differential phenotype display in Friedreich's ataxia. Mov. Disord. 16(6), 1153–1158 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Stolle CA, Frackelton EC, McCallum J et al. Novel, complex interruptions of the GAA repeat in small, expanded alleles of two affected siblings with late-onset Friedreich ataxia. Mov. Disord. 23(9), 1303–6 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Lazaropoulos M, Dong Y, Clark E et al. Frataxin levels in peripheral tissue in Friedreich ataxia. Ann. Clin. Transl. Neurol. 2(8), 831–842 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dürr A, Cossee M, Agid Y et al. Clinical and genetic abnormalities in patients with Friedreich's ataxia. N. Engl. J. Med. 335(16), 1169–1175 (1996). [DOI] [PubMed] [Google Scholar]; • The original article linking clinical phenomena and GAA repeat length in Friedreich ataxia (FRDA).
- 10.Patel M, Isaacs CJ, Seyer L et al. Progression of Friedreich ataxia: quantitative characterization over 5 years. Ann. Clin. Transl. Neurol. 3(9), 684–694 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martelli A, Puccio H. Dysregulation of cellular iron metabolism in Friedreich ataxia: from primary iron-sulfur cluster deficit to mitochondrial iron accumulation. Front. Pharmacol. 5, 130 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rötig A, de Lonlay P, Chretien D et al. Aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia. Nat. Genet. 17(2), 215–217 (1997). [DOI] [PubMed] [Google Scholar]
- 13.Musco G, Stier G, Kolmerer B et al. Towards a structural understanding of Friedreich's ataxia: the solution structure of frataxin. Structure 8(7), 695–707 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Delatycki MB, Camakaris J, Brooks H et al. Direct evidence that mitochondrial iron accumulation occurs in Friedreich ataxia. Ann. Neurol. 45(5), 673–675 (1999). [PubMed] [Google Scholar]
- 15.Harding IH, Lynch DR, Koeppen AH, Pandolfo M. Central nervous system therapeutic targets in Friedreich ataxia. Hum. Gene Ther. 31(23-24), 1226–1236 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Collaborative expert review identifying present thoughts on neuroanatomy of FRDA.
- 16.Reetz K, Dogan I, Costa AS et al. Biological and clinical characteristics of the European Friedreich's Ataxia Consortium for Translational Studies (EFACTS) cohort: a cross-sectional analysis of baseline data. Lancet Neurol. 14(2), 174–182 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Corben LA, Tai G, Wilson C, Collins V, Churchyard AJ, Delatycki MB. A comparison of three measures of upper limb function in Friedreich ataxia. J. Neurol. 257(4), 518–523 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Seyer LA, Galetta K, Wilson J et al. Analysis of the visual system in Friedreich ataxia. J. Neurol. 260(9), 2362–2369 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Fortuna F, Barboni P, Liguori R et al. Visual system involvement in patients with Friedreich's ataxia. Brain 132(Pt 1), 116–123 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Givre SJ, Wall M, Kardon RH. Visual loss and recovery in a patient with Friedreich ataxia. J. Neuroophthalmol. 20(4), 229–233 (2000). [PubMed] [Google Scholar]
- 21.Pinto F, Amantini A, de Scisciolo G, Scaioli V, Guidi L, Frosini R. Visual involvement in Friedreich's ataxia: PERG and VEP study. Eur. Neurol. 28(5), 246–251 (1988). [DOI] [PubMed] [Google Scholar]
- 22.Carroll WM, Kriss A, Baraitser M, Barrett G, Halliday AM. The incidence and nature of visual pathway involvement in Friedreich's ataxia. A clinical and visual evoked potential study of 22 patients. Brain 103(2), 413–434 (1980). [DOI] [PubMed] [Google Scholar]
- 23.Bogdanova-Mihaylova P, Plapp HM, Chen H et al. Longitudinal assessment using optical coherence tomography in patients with Friedreich's ataxia. Tomography. 7(4), 915–931 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noval S, Contreras I, Sanz-Gallego I, Manrique RK, Arpa J. Ophthalmic features of Friedreich ataxia. Eye (Lond.). 26(2), 315–320 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamedani AG, Hauser LA, Perlman S et al. Longitudinal analysis of contrast acuity in Friedreich ataxia. Neurol. Genet. 4(4), e250 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afsharian P, Nolan-Kenney R, Lynch AE, Balcer LJ, Lynch DR. Correlation of visual quality of life with clinical and visual status in Friedreich ataxia. J. Neuroophthalmol. 40(2), 213–217 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Rance G, Corben L, Barker E et al. Auditory perception in individuals with Friedreich's ataxia. Audiol Neurootol. 15(4), 229–40 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Koohi N, Thomas-Black G, Giunti P, Bamiou DE. Auditory phenotypic variability in Friedreich's ataxia patients. Cerebellum 20, 497–508 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rance G, Starr A. Pathophysiological mechanisms and functional hearing consequences of auditory neuropathy. Brain 138(11), 3141–3158 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain (Pt 3), 741–53 (1996). [DOI] [PubMed] [Google Scholar]
- 31.Knezevic W, Stewart-Wynne EG. Brainstem auditory evoked responses in hereditary spinocerebellar ataxias. Clin. Exp. Neurol. 21, 149–155 (1985). [PubMed] [Google Scholar]
- 32.Rance G, Corben LA, Du Bourg E, King A, Delatycki MB. Successful treatment of auditory perceptual disorder in individuals with Friedreich ataxia. Neuroscience. 171(2), 552 –5 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Tsou AY, Paulsen EK, Lagedrost SJ et al. Cross-sectional analysis of electrocardiograms in a large heterogeneous cohort of Friedreich ataxia subjects. J. Child Neurol. 27(9), 1187–1192 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Legrand L, Weinsaft JW, Pousset F et al. Characterizing cardiac phenotype in Friedreich's ataxia: The CARFA study. Arch. Cardiovasc. Dis. 115(1), 17–28 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Pousset F, Legrand L, Monin ML et al. A 22-year follow-up study of long-term cardiac outcome and predictors of survival in Friedreich ataxia. JAMA Neurol. 72(11), 1334–1341 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Hanson E, Sheldon M, Pacheco B, Alkubeysi M, Raizada V. Heart disease in Friedreich's ataxia. World J. Cardiol. 11(1), 1–12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Payne RM. The heart in Friedreich's ataxia: basic findings and clinical implications. Prog Pediatr Cardiol. 31(2), 103–109 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lynch DR, Regner SR, Schadt KA, Friedman LS, Lin KY, St John Sutton MG. Management and therapy for cardiomyopathy in Friedreich's ataxia. Expert Rev. Cardiovasc. Ther. 10(6), 767–777 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Koeppen AH, Ramirez RL, Becker AB et al. The pathogenesis of cardiomyopathy in Friedreich ataxia. PLoS ONE 10(3), e0116396 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dedobbeleer C, Rai M, Donal E, Pandolfo M, Unger P. Normal left ventricular ejection fraction and mass but subclinical myocardial dysfunction in patients with Friedreich's ataxia. Eur Heart J Cardiovasc. Imaging. 13(4), 346–352 (2012). [DOI] [PubMed] [Google Scholar]
- 41.St John Sutton M, Ky B, Regner SR et al. Longitudinal strain in Friedreich ataxia: a potential marker for early left ventricular dysfunction. Echocardiography 31(1), 50–57 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Legrand L, Heuze C, Diallo A et al. Prognostic value of longitudinal strain and ejection fraction in Friedreich's ataxia. Int. J. Cardiol. 330, 259–265 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Friedman LS, Schadt KA, Regner SR et al. Elevation of serum cardiac troponin I in a cross-sectional cohort of asymptomatic subjects with Friedreich ataxia. Int. J. Cardiol. 167(4), 1622–1624 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Legrand L, Maupain C, Monin ML et al. Significance of NT-proBNP and high-sensitivity troponin in Friedreich ataxia. J. Clin. Med. 9(6), 1630 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rummey C, Flynn JM, Corben LA et al. Scoliosis in Friedreich's ataxia: longitudinal characterization in a large heterogeneous cohort. Ann. Clin. Transl. Neurol. 8(6), 1239–1250 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsirikos AI, Smith G. Scoliosis in patients with Friedreich's ataxia. J. Bone Joint Surg. Br. 94(5), 684–689 (2012). [DOI] [PubMed] [Google Scholar]
- 47.Simon AL, Meyblum J, Roche B et al. Scoliosis in patients with friedreich ataxia: results of a consecutive prospective series. Spine Deform. 7(5), 812–821 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Reetz K, Dogan I, Hohenfeld C et al. Nonataxia symptoms in Friedreich Ataxia: report from the Registry of the European Friedreich's Ataxia Consortium for Translational Studies (EFACTS). Neurology 91(10), e917–e930 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Milbrandt TA, Kunes JR, Karol LA. Friedreich's ataxia and scoliosis: the experience at two institutions. J. Pediatr. Orthop. 28(2), 234–238 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Tamaroff J, DeDio A, Wade K et al. Friedreich's Ataxia related Diabetes: epidemiology and management practices. Diabetes Res. Clin. Pract. 186, 109828 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Azzi AS, Cosentino C, Kibanda J, Féry F, Cnop M. OGTT is recommended for glucose homeostasis assessments in Friedreich ataxia. Ann. Clin. Transl. Neurol. 6(1), 161–166 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCormick A, Farmer J, Perlman S et al. Impact of diabetes in the Friedreich ataxia clinical outcome measures study. Ann. Clin. Transl. Neurol. 4(9), 622–631 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Isaacs CJ, Brigatti KW, Kucheruk O et al. Effects of genetic severity on glucose homeostasis in Friedreich ataxia. Muscle Nerve 54(5), 887–894 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chakraborty PP, Ray S, Bhattacharjee R et al. First presentation of diabetes as diabetic ketoacidosis in a case of Friedreich's ataxia. Clin. Diabetes. 33(2), 84–86 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greeley NR, Regner S, Willi S, Lynch DR. Cross-sectional analysis of glucose metabolism in Friedreich ataxia. J. Neurol. Sci. 342(1-2), 29–35 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Pappa A, Häusler MG, Veigel A et al. Diabetes mellitus in Friedreich Ataxia: a case series of 19 patients from the German-Austrian diabetes mellitus registry. Diabetes Res. Clin. Pract. 141, 229–236 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Corben LA, Ho M, Copland J, Tai G, Delatycki MB. Increased prevalence of sleep-disordered breathing in Friedreich ataxia. Neurology 81(1), 46–51- (2013). [DOI] [PubMed] [Google Scholar]
- 58.Reddy PL, Grewal RP. Friedreich's ataxia: a clinical and genetic analysis. Clin. Neurol. Neurosurg. 109(2), 200–202 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Eigentler A, Nachbauer W, Donnemiller E, Poewe W, Gasser RW, Boesch S. Low bone mineral density in Friedreich ataxia. Cerebellum. 13(5), 549–557 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Weber DR. Bone health in childhood chronic disease. Endocrinol. Metab. Clin. North Am. 49(4), 637–650 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dunn J, Tamaroff J, DeDio A et al. Bone mineral density and current bone health screening practices in Friedreich's ataxia. Front. Neurosci. 16, 818750 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han EY, Choi JH, Kim SH, Im SH. The effect of weight bearing on bone mineral density and bone growth in children with cerebral palsy: a randomized controlled preliminary trial. Medicine 96(10), e5896 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ward LM, Hadjiyannakis S, McMillan HJ, Noritz G, Weber DR. Bone health and osteoporosis management of the patient with duchenne muscular dystrophy. Pediatrics 142, S34–S42 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Musegante AF, Almeida PN, Monteiro RT, Barroso U Jr. Urinary symptoms and urodynamics findings in patients with Friedreich's ataxia. Int. Braz. J. Urol. 39(6), 867–874 (2013). [DOI] [PubMed] [Google Scholar]
- 65.Nieto A, Hernández-Torres A, Pérez-Flores J, Montón F. Depressive symptoms in Friedreich ataxia. Int. J. Clin. Health Psychol. 18(1), 18–26 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pérez-Flores J, Hernández-Torres A, Montón F, Nieto A. Health-related quality of life and depressive symptoms in Friedreich ataxia. Qual. Life Res. 29(2), 413–420 (2020). [DOI] [PubMed] [Google Scholar]
- 67.Costabile T, Capretti V, Abate F et al. Emotion recognition and psychological comorbidity in Friedreich's ataxia. Cerebellum. 17(3), 336–345 (2018). [DOI] [PubMed] [Google Scholar]
- 68.Sayah S, Rotgé JY, Francisque H et al. Personality and neuropsychological profiles in Friedreich ataxia. Cerebellum. 17(2), 204–212 (2018). [DOI] [PubMed] [Google Scholar]
- 69.Flood MK, Perlman SL. The mental status of patients with Friedreich's ataxia. J. Neurosci. Nurs. 19(5), 251–255 (1987). [DOI] [PubMed] [Google Scholar]
- 70.Xiong E, Lynch AE, Corben LA et al. Health related quality of life in Friedreich Ataxia in a large heterogeneous cohort. J. Neurol. Sci. 410, 116642 (2020). [DOI] [PubMed] [Google Scholar]
- 71.Corben LA, Lynch D, Pandolfo M, Schulz JB, Delatycki MB. Clinical Management Guidelines Writing Group. Consensus clinical management guidelines for Friedreich ataxia. Orphanet. J. Rare Dis. 9, 184 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deutsch EC, Seyer LA, Perlman SL, Yu J, Lynch DR. Clinical monitoring in a patient with Friedreich ataxia and osteogenic sarcoma. J. Child Neurol. 27(9), 1159–1163 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Griffiths JD, Stark RJ, Ding JC, Cooper IA. Vincristine neurotoxicity in Charcot-Marie-Tooth syndrome. Med. J. Aust. 143(7), 305–306 (1985). [DOI] [PubMed] [Google Scholar]
- 74.Graf WD, Chance PF, Lensch MW, Eng LJ, Lipe HP, Bird TD. Severe vincristine neuropathy in Charcot-Marie-Tooth disease type 1A. Cancer 77(7), 1356–1362 (1996). [DOI] [PubMed] [Google Scholar]
- 75.Kearney M, Orrell RW, Fahey M, Pandolfo M. Antioxidants and other pharmacological treatments for Friedreich ataxia. Cochrane Database Syst. Rev. 4, CD007791 (2012). [DOI] [PubMed] [Google Scholar]
- 76.Myers L, Farmer JM, Wilson RB et al. Antioxidant use in Friedreich ataxia. J. Neurol. Sci. 267(1-2), 174–176 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meier T, Perlman SL, Rummey C, Coppard NJ, Lynch DR. Assessment of neurological efficacy of idebenone in pediatric patients with Friedreich's ataxia: data from a 6-month controlled study followed by a 12-month open-label extension study. J. Neurol. 259(2), 284–291 (2012). [DOI] [PubMed] [Google Scholar]
- 78.Lagedrost SJ, Sutton MS, Cohen MS et al. Idebenone in Friedreich ataxia cardiomyopathy-results from a 6-month phase III study (IONIA). Am. Heart J. 161(3), 639–645.e1 (2011). [DOI] [PubMed] [Google Scholar]
- 79.Lynch DR, Perlman SL, Meier T. A Phase III, double-blind, placebo-controlled trial of idebenone in friedreich ataxia. Arch. Neurol. 67(8), 941–947 (2010). [DOI] [PubMed] [Google Scholar]
- 80.Rinaldi C, Tucci T, Maione S, Giunta A, De Michele G, Filla A. Low-dose idebenone treatment in Friedreich's ataxia with and without cardiac hypertrophy. J. Neurol. 256(9), 1434–1437 (2009). [DOI] [PubMed] [Google Scholar]
- 81.Schulz JB, Di Prospero NA, Fischbeck K. Clinical experience with high dose idebenone in Friedreich ataxia. J. Neurol. 256(Suppl. 1), 42–5 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meier T, Buyse G. Idebenone: an emerging therapy for Friedreich ataxia. J. Neurol. 256(Suppl. 1), 25–30 (2009). [DOI] [PubMed] [Google Scholar]
- 83.Pineda M, Arpa J, Montero R et al. Idebenone treatment in paediatric and adult patients with Friedreich ataxia: long-term follow-up. Eur. J. Paediatr. Neurol. 12(6), 470–475 (2008). [DOI] [PubMed] [Google Scholar]
- 84.Di Prospero NA, Baker A, Jeffries N, Fischbeck KH. Neurological effects of high-dose idebenone in patients with Friedreich's ataxia: a randomised, placebo-controlled trial. Lancet Neurol. 6(10), 878–886 (2007). [DOI] [PubMed] [Google Scholar]
- 85.Di Prospero NA, Sumner CJ, Penzak SR, Ravina B, Fischbeck KH, Taylor JP. Safety, tolerability, and pharmacokinetics of high dose idebenone in patients with Friedreich ataxia. Arch. Neurol. 64(6), 803–808 (2007). [DOI] [PubMed] [Google Scholar]
- 86.Zesiewicz T, Heerinckx F, De Jager R et al. Randomized, clinical trial of RT001: early signals of efficacy in Friedreich's ataxia. Mov. Disord. 33(6), 1000–1005 (2018). [DOI] [PubMed] [Google Scholar]
- 87.Brenna JT, James G, Midei M et al. J. Pharm. Sci. 109(11), 3496–3503 (2020). [DOI] [PubMed] [Google Scholar]
- 88.Mariotti C, Fancellu R, Caldarazzo S et al. Erythropoietin in Friedreich ataxia: no effect on frataxin in a randomized controlled trial. Mov. Disord. 27(3), 446–449 (2012). [DOI] [PubMed] [Google Scholar]
- 89.Saccà F, Puorro G, Marsili A et al. Long-term effect of epoetin alfa on clinical and biochemical markers in friedreich ataxia. Mov. Disord. 31(5), 734–741 (2016). [DOI] [PubMed] [Google Scholar]
- 90.Boesch S, Nachbauer W, Mariotti C et al. Safety and tolerability of carbamylated erythropoietin in Friedreich's ataxia. Mov. Disord. 29(7), 935–939 (2014). [DOI] [PubMed] [Google Scholar]
- 91.Nachbauer W, Boesch S, Schneider R et al. Bioenergetics of the calf muscle in Friedreich ataxia patients measured by 31P-MRS before and after treatment with recombinant human erythropoietin. PLoS ONE 8(7), e69229 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nachbauer W, Hering S, Seifert M et al. Effects of erythropoietin on frataxin levels and mitochondrial function in Friedreich ataxia–a dose-response trial. Cerebellum. 10(4), 763–769 (2011). [DOI] [PubMed] [Google Scholar]
- 93.Saccà F, Piro R, De Michele G et al. Epoetin alfa increases frataxin production in Friedreich's ataxia without affecting hematocrit. Mov. Disord. 26(4), 739–742 (2011). [DOI] [PubMed] [Google Scholar]
- 94.Boesch S, Sturm B, Hering S et al. Neurological effects of recombinant human erythropoietin in Friedreich's ataxia: a clinical pilot trial. Mov. Disord. 23(13), 1940–1944 (2008). [DOI] [PubMed] [Google Scholar]
- 95.Boesch S, Sturm B, Hering S, Goldenberg H, Poewe W, Scheiber-Mojdehkar B. Friedreich's ataxia: clinical pilot trial with recombinant human erythropoietin. Ann. Neurol. 62(5), 521–524 (2007). [DOI] [PubMed] [Google Scholar]
- 96.Britti E, Delaspre F, Sanz-Alcázar A et al. Calcitriol increases frataxin levels and restores mitochondrial function in cell models of Friedreich ataxia. Biochem. J. 478(1), 1–20 (2021). [DOI] [PubMed] [Google Scholar]
- 97.Alfedi G, Luffarelli R, Condò I et al. Drug repositioning screening identifies etravirine as a potential therapeutic for friedreich's ataxia. Mov. Disord. 34(3), 323–334 (2019). [DOI] [PubMed] [Google Scholar]
- 98.Vavla M, Arrigoni F, Toschi N et al. Sensitivity of neuroimaging indicators in monitoring the effects of interferon gamma treatment in Friedreich's ataxia. Front. Neurosci. 14, 872 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vavla M, D'Angelo MG, Arrigoni F. Safety and efficacy of interferon γ in friedreich's ataxia. Mov. Disord. 35(2), 370–371 (2020). [DOI] [PubMed] [Google Scholar]
- 100.Lynch DR, Hauser L, McCormick A et al. Randomized, double-blind, placebo-controlled study of interferon-γ 1b in Friedreich ataxia. Ann. Clin. Transl. Neurol. 6(3), 546–553 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wyller VB, Jacobsen K, Dahl MB et al. Interferon gamma may improve cardiac function in Friedreich's ataxia cardiomyopathy. Int. J. Cardiol. 221, 376–378 (2016). [DOI] [PubMed] [Google Scholar]
- 102.Marcotulli C, Fortuni S, Arcuri G et al. GIFT-1, a phase IIa clinical trial to test the safety and efficacy of IFNγ administration in FRDA patients. Neurol. Sci. 37(3), 361–364 (2016). [DOI] [PubMed] [Google Scholar]
- 103.Seyer L, Greeley N, Foerster D et al. Open-label pilot study of interferon gamma-1b in Friedreich ataxia. Acta Neurol. Scand. 132(1), 7–15 (2015). [DOI] [PubMed] [Google Scholar]
- 104.Pandolfo M, Arpa J, Delatycki MB et al. Deferiprone in Friedreich ataxia: a 6-month randomized controlled trial. Ann. Neurol. 76(4), 509–521 (2014). [DOI] [PubMed] [Google Scholar]
- 105.Arpa J, Sanz-Gallego I, Rodríguez-de-Rivera FJ et al. Triple therapy with deferiprone, idebenone and riboflavin in Friedreich's ataxia - open-label trial. Acta Neurol. Scand. 129(1), 32–40 (2014). [DOI] [PubMed] [Google Scholar]
- 106.Arpa J, Sanz-Gallego I, Rodríguez-de-Rivera FJ et al. Triple therapy with darbepoetin alfa, idebenone, and riboflavin in Friedreich's ataxia: an open-label trial. Cerebellum. 12(5), 713–720 (2013). [DOI] [PubMed] [Google Scholar]
- 107.Patel M, Schadt K, McCormick A, Isaacs C, Dong YN, Lynch DR. Open-label pilot study of oral methylprednisolone for the treatment of patients with friedreich ataxia. Muscle Nerve 60(5), 571–575 (2019). [DOI] [PubMed] [Google Scholar]
- 108.Qureshi MY, Patterson MC, Clark V et al. Safety and efficacy of (+)-epicatechin in subjects with Friedreich's ataxia: a phase II, open-label, prospective study. J. Inherit. Metab. Dis. 44(2), 502–514 (2021). [DOI] [PubMed] [Google Scholar]
- 109.Yiu EM, Tai G, Peverill RE et al. An open-label trial in Friedreich ataxia suggests clinical benefit with high-dose resveratrol, without effect on frataxin levels. J. Neurol. 262(5), 1344–1353 (2015). [DOI] [PubMed] [Google Scholar]
- 110.Lynch DR, Willi SM, Wilson RB et al. A0001 in Friedreich ataxia: biochemical characterization and effects in a clinical trial. Mov. Disord. 27(8), 1026–1033 (2012). [DOI] [PubMed] [Google Scholar]
- 111.Zesiewicz T, Salemi JL, Perlman S et al. Double-blind, randomized, and controlled trial of EPI-743 in Friedreich's ataxia. Neurodegener. Dis. Manag. 8(4), 233–242 (2018). [DOI] [PubMed] [Google Scholar]
- 112.Enns GM, Kinsman SL, Perlman SL et al. Initial experience in the treatment of inherited mitochondrial disease with EPI-743. Mol. Genet. Metab. 105(1), 91–102 (2012). [DOI] [PubMed] [Google Scholar]
- 113.Rodríguez-Pascau L, Britti E, Calap-Quintana P et al. PPAR gamma agonist leriglitazone improves frataxin-loss impairments in cellular and animal models of Friedreich Ataxia. Neurobiol. Dis. 148, 105162 (2021). [DOI] [PubMed] [Google Scholar]
- 114.García-Giménez JL, Sanchis-Gomar F, Pallardó FV. Could thiazolidinediones increase the risk of heart failure in Friedreich's ataxia patients? Mov. Disord. 26(5), 769–771 (2011). [DOI] [PubMed] [Google Scholar]
- 115.Marmolino D, Acquaviva F, Pinelli M et al. PPAR-gamma agonist Azelaoyl PAF increases frataxin protein and mRNA expression: new implications for the Friedreich's ataxia therapy. Cerebellum. 8(2), 98–103 (2009). [DOI] [PubMed] [Google Scholar]
- 116.D'Oria V, Petrini S, Travaglini L et al. Frataxin deficiency leads to reduced expression and impaired translocation of NF-E2-related factor (Nrf2) in cultured motor neurons. Int. J. Mol. Sci. 14(4), 7853–7865 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shan Y, Schoenfeld RA, Hayashi G et al. Frataxin deficiency leads to defects in expression of antioxidants and Nrf2 expression in dorsal root ganglia of the Friedreich's ataxia YG8R mouse model. Antioxid. Redox Signal. 19(13), 1481–1493 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Paupe V, Dassa EP, Goncalves S et al. Impaired nuclear Nrf2 translocation undermines the oxidative stress response in Friedreich ataxia. PLoS ONE 4(1), e4253 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.La Rosa P, Russo M, D'Amico J et al. Nrf2 induction re-establishes a proper neuronal differentiation program in Friedreich's ataxia neural stem cells. Front. Cell. Neurosci. 13, 356 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Abeti R, Baccaro A, Esteras N, Giunti P. Novel Nrf2-inducer prevents mitochondrial defects and oxidative stress in Friedreich's ataxia models. Front. Cell. Neurosci. 12, 188 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Petrillo S, Piermarini E, Pastore A et al. Nrf2-inducers counteract neurodegeneration in frataxin-silenced motor neurons: disclosing new therapeutic targets for Friedreich's ataxia. Int. J. Mol. Sci. 18(10), 2173 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Anzovino A, Chiang S, Brown BE, Hawkins CL, Richardson DR, Huang ML. Molecular alterations in a mouse cardiac model of Friedreich ataxia: an impaired Nrf2 response mediated via upregulation of Keap1 and activation of the Gsk3β Axis. Am. J. Pathol. 187(12), 2858–2875 (2017). [DOI] [PubMed] [Google Scholar]
- 123.Abeti R, Uzun E, Renganathan I, Honda T, Pook MA, Giunti P. Targeting lipid peroxidation and mitochondrial imbalance in Friedreich's ataxia. Pharmacol. Res. 99, 344–350 (2015). [DOI] [PubMed] [Google Scholar]
- 124.La Rosa P, Petrillo S, Turchi R et al. The Nrf2 induction prevents ferroptosis in Friedreich's ataxia. Redox Biol. 38, 101791 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lynch DR, Chin MP, Delatycki MB et al. Safety and efficacy of omaveloxolone in Friedreich ataxia (MOXIe Study). Ann. Neurol. 89(2), 212–225 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Along with reference 127, one of two double blind trials showing benefits of omaveloxolone.
- 126.Petrillo S, D'Amico J, La Rosa P, Bertini ES, Piemonte F. Targeting NRF2 for the treatment of Friedreich's ataxia: a comparison among drugs. Int. J. Mol. Sci. 20(20), 5211 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lynch DR, Farmer J, Hauser L et al. Safety, pharmacodynamics, and potential benefit of omaveloxolone in Friedreich ataxia. Ann. Clin. Transl. Neurol. 6(1), 15–26 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Along with reference 125, one of two double blind trials showing benefits of omaveloxolone.
- 128.Sahdeo S, Scott BD, McMackin MZ et al. Dyclonine rescues frataxin deficiency in animal models and buccal cells of patients with Friedreich's ataxia. Hum. Mol. Genet. 23(25), 6848–6862 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li Y, Polak U, Bhalla AD et al. Excision of expanded GAA repeats alleviates the molecular phenotype of Friedreich's ataxia. Mol. Ther. 23(6), 1055–1065 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Codazzi F, Hu A, Rai M et al. Friedreich ataxia-induced pluripotent stem cell-derived neurons show a cellular phenotype that is corrected by a benzamide HDAC inhibitor. Hum. Mol. Genet. 25(22), 4847–4855 (2016). [DOI] [PubMed] [Google Scholar]
- 131.Herman D, Jenssen K, Burnett R, Soragni E, Perlman SL, Gottesfeld JM. Histone deacetylase inhibitors reverse gene silencing in Friedreich's ataxia. Nat. Chem. Biol. 2(10), 551–8 (2006). [DOI] [PubMed] [Google Scholar]
- 132.Rai M, Soragni E, Jenssen K et al. HDAC inhibitors correct frataxin deficiency in a Friedreich ataxia mouse model. PLoS ONE 3(4), e1958 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chutake YK, Lam CC, Costello WN, Anderson MP, Bidichandani SI. Reversal of epigenetic promoter silencing in Friedreich ataxia by a class I histone deacetylase inhibitor. Nucleic Acids Res. 44(11), 5095–5104 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Soragni E, Miao W, Iudicello M et al. Epigenetic therapy for Friedreich ataxia. Ann. Neurol. 76(4), 489–508 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vyas PM, Tomamichel WJ, Pride PM et al. A TAT-frataxin fusion protein increases lifespan and cardiac function in a conditional Friedreich's ataxia mouse model. Hum. Mol. Genet. 21(6), 1230–1247 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Britti E, Delaspre F, Feldman A et al. Frataxin-deficient neurons and mice models of Friedreich ataxia are improved by TAT-MTScs-FXN treatment. J. Cell Mol. Med. 22(2), 834–848 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mincheva-Tasheva S, Obis E, Tamarit J, Ros J. Apoptotic cell death and altered calcium homeostasis caused by frataxin depletion in dorsal root ganglia neurons can be prevented by BH4 domain of Bcl-xL protein. Hum. Mol. Genet. 23(7), 1829–1841 (2014). [DOI] [PubMed] [Google Scholar]
- 138.Perdomini M, Belbellaa B, Monassier L et al. Prevention and reversal of severe mitochondrial cardiomyopathy by gene therapy in a mouse model of Friedreich's ataxia. Nat. Med. 20(5), 542–547 (2014). [DOI] [PubMed] [Google Scholar]
- 139.Piguet F, de Montigny C, Vaucamps N, Reutenauer L, Eisenmann A, Puccio H. Rapid and complete reversal of sensory ataxia by gene therapy in a novel model of Friedreich ataxia. Mol. Ther. 26(8), 1940–1952 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Belbellaa B, Reutenauer L, Monassier L, Puccio H. Correction of half the cardiomyocytes fully rescue Friedreich ataxia mitochondrial cardiomyopathy through cell-autonomous mechanisms. Hum. Mol. Genet. 28(8), 1274–1285 (2019). [DOI] [PubMed] [Google Scholar]
- 141.Cherif K, Gérard C, Rousseau J, Ouellet DL, Chapdelaine P, Tremblay JP. Increased Frataxin Expression Induced in Friedreich Ataxia Cells by Platinum TALE-VP64s or Platinum TALE SunTag. Mol. Ther. Nucleic Acids. 12, 19–32 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.ClinicalTrials.gov. Single Ascending Dose Study of CTI-1601 Versus Placebo in Subjects With Friedreich's Ataxia (2020). https://clinicaltrials.gov/ct2/show/NCT04176991
- 143.Fischell JM, Fishman PS. A multifaceted approach to optimizing AAV delivery to the brain for the treatment of neurodegenerative diseases. Front Neurosci. 15, 747726 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hinderer C, Bell P, Katz N et al. Evaluation of intrathecal routes of administration for adeno-associated viral vectors in large animals. Hum. Gene Ther. 29(1), 15–24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Taymans JM, Vandenberghe LH, Haute CV et al. Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain. Hum. Gene Ther. 18(3), 195–206 (2007). [DOI] [PubMed] [Google Scholar]
- 146.Belbellaa B, Reutenauer L, Messaddeq N, Monassier L, Puccio H. High levels of Frataxin overexpression lead to mitochondrial and cardiac toxicity in mouse models. Mol. Ther. Methods Clin. Dev. 19, 120–138 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Erwin GS, Grieshop MP, Ali A et al. Synthetic transcription elongation factors license transcription across repressive chromatin. Science 358(6370), 1617–1622 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Article showing the elegant approach to the Gene TAC drug design.
- 148.Rodden LN, Chutake YK, Gilliam K et al. Methylated and unmethylated epialleles support variegated epigenetic silencing in Friedreich ataxia. Hum. Mol. Genet. 29(23), 3818–3829 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Initial article on variegated silencing, a mechanism that may revolutionize thought process in FRDA.
- 149.Rodden LN, Gilliam KM, Lam C et al. DNA methylation in Friedreich ataxia silences expression of frataxin isoform E. Sci. Rep. 12(1), 5031 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rodden LN, Gilliam KM, Lam C, Lynch DR, Bidichandani SI. Epigenetic heterogeneity in Friedreich ataxia underlies variable FXN reactivation. Front. Neurosci. 15, 752921 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Saveliev A, Everett C, Sharpe T, Webster Z, Festenstein R. DNA triplet repeats mediate heterochromatin-protein-1-sensitive variegated gene silencing. Nature 422(6934), 909–913 (2003). [DOI] [PubMed] [Google Scholar]
- 152.Herman D, Jenssen K, Burnett R, Soragni E, Perlman SL, Gottesfeld JM. Histone deacetylase inhibitors reverse gene silencing in Friedreich's ataxia. Nat. Chem. Biol. 2(10), 551–558 (2006). [DOI] [PubMed] [Google Scholar]
- 153.Greene E, Mahishi L, Entezam A, Kumari D, Usdin K. Repeat-induced epigenetic changes in intron 1 of the frataxin gene and its consequences in Friedreich ataxia. Nucleic Acids Res. 35(10), 3383–3390 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Soragni E, Herman D, Dent SY, Gottesfeld JM, Wells RD, Napierala M. Long intronic GAA*TTC repeats induce epigenetic changes and reporter gene silencing in a molecular model of Friedreich ataxia. Nucleic Acids Res. 36(19), 6056–6065 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Castaldo I, Pinelli M, Monticelli A et al. DNA methylation in intron 1 of the frataxin gene is related to GAA repeat length and age of onset in Friedreich ataxia patients. J. Med. Genet. 45(12), 808–812 (2008). [DOI] [PubMed] [Google Scholar]
- 156.Al-Mahdawi S, Pinto RM, Ismail O et al. The Friedreich ataxia GAA repeat expansion mutation induces comparable epigenetic changes in human and transgenic mouse brain and heart tissues. Hum. Mol. Genet. 17(5), 735–746 (2008). [DOI] [PubMed] [Google Scholar]
- 157.De Biase I, Chutake YK, Rindler PM, Bidichandani SI. Epigenetic silencing in Friedreich ataxia is associated with depletion of CTCF (CCCTC-binding factor) and antisense transcription. PLoS ONE 4(11), e7914 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Evans-Galea MV, Carrodus N, Rowley SM et al. FXN methylation predicts expression and clinical outcome in Friedreich ataxia. Ann. Neurol. 71(4), 487–497 (2012). [DOI] [PubMed] [Google Scholar]
- 159.Chan PK, Torres R, Yandim C et al. Heterochromatinization induced by GAA-repeat hyperexpansion in Friedreich's ataxia can be reduced upon HDAC inhibition by vitamin B3. Hum. Mol. Genet. 22(13), 2662–2675 (2013). [DOI] [PubMed] [Google Scholar]
- 160.Muller HJ. Types of visible variations induced by x-rays in drosophila. J. Genet. 1930 XXII, 22, 299–333 (1930). [Google Scholar]
- 161.Rodden L, Rummey C, Dong YN, Lynch DR. Clinical evidence for variegated silencing in Friedreich ataxia patients. Neurology Genet. 8(3), e683 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lynch DR, Deutsch EC, Wilson RB, Tennekoon G. answered questions in Friedreich ataxia. J. Child Neurol. 27(9), 1223–1229 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Koeppen AH, Becker AB, Qian J, Gelman BB, Mazurkiewicz JE. Friedreich ataxia: developmental failure of the dorsal root entry zone. J. Neuropathol. Exp. Neurol. 76(11), 969–977 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Santoro L, Perretti A, Lanzillo B et al. Influence of GAA expansion size and disease duration on central nervous system impairment in Friedreich's ataxia: contribution to the understanding of the pathophysiology of the disease. Clin. Neurophysiol. 111(6), 1023–1030 (2000). [DOI] [PubMed] [Google Scholar]
- 165.Coppola G, De Michele G, Cavalcanti F et al. Why do some Friedreich's ataxia patients retain tendon reflexes? A clinical, neurophysiological and molecular study. J. Neurol. 246(5), 353–357 (1999). [DOI] [PubMed] [Google Scholar]
- 166.Santoro L, De Michele G, Perretti A et al. Relation between trinucleotide GAA repeat length and sensory neuropathy in Friedreich's ataxia. J. Neurol. Neurosurg. Psychiatry 66(1), 93–96 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Naeije G, Coquelet N, Wens V, Goldman S, Pandolfo M, De Tiège X. Age of onset modulates resting-state brain network dynamics in Friedreich Ataxia. Hum. Brain. Mapp. 42(16), 5334–5344 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Naeije G, Wens V, Coquelet N et al. Age of onset determines intrinsic functional brain architecture in Friedreich ataxia. Ann. Clin. Transl. Neurol. 7(1), 94–104 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Naeije G, Bourguignon M, Wens V et al. Electrophysiological evidence for limited progression of the proprioceptive impairment in Friedreich ataxia. Clin. Neurophysiol. 131(2), 574–576 (2020). [DOI] [PubMed] [Google Scholar]
- 170.Marty B, Naeije G, Bourguignon M et al. Evidence for genetically determined degeneration of proprioceptive tracts in Friedreich ataxia. Neurology 93(2), e116–e124 (2019). [DOI] [PubMed] [Google Scholar]
- 171.Kemp KC, Cook AJ, Redondo J, Kurian KM, Scolding NJ, Wilkins A. Purkinje cell injury, structural plasticity and fusion in patients with Friedreich's ataxia. Acta Neuropathol. Commun. 4(1), 53 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Quercia N, Somers GR, Halliday W, Kantor PF, Banwell B, Yoon G. Friedreich ataxia presenting as sudden cardiac death in childhood: clinical, genetic and pathological correlation, with implications for genetic testing and counselling. Neuromuscul. Disord. 20(5), 340–342 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.