Abstract
Background
The autonomic nervous system (ANS) is a complex network where sympathetic and parasympathetic domains interact inside and outside of the network. Correlation-based network analysis (NA) is a novel approach enabling the quantification of these interactions. The aim of this study is to assess the applicability of NA to assess relationships between autonomic, sensory, respiratory, cerebrovascular, and inflammatory markers on post-acute sequela of COVID-19 (PASC) and postural tachycardia syndrome (POTS).
Methods
In this retrospective study, datasets from PASC (n = 15), POTS (n = 15), and matched controls (n = 11) were analyzed. Networks were constructed from surveys (autonomic and sensory), autonomic tests (deep breathing, Valsalva maneuver, tilt, and sudomotor test) results using heart rate, blood pressure, cerebral blood flow velocity (CBFv), capnography, skin biopsies for assessment of small fiber neuropathy (SFN), and various inflammatory markers. Networks were characterized by clusters and centrality metrics.
Results
Standard analysis showed widespread abnormalities including reduced orthostatic CBFv in 100%/88% (PASC/POTS), SFN 77%/88%, mild-to-moderate dysautonomia 100%/100%, hypocapnia 87%/100%, and elevated inflammatory markers. NA showed different signatures for both disorders with centrality metrics of vascular and inflammatory variables playing prominent roles in differentiating PASC from POTS.
Conclusions
NA is suitable for a relationship analysis between autonomic and nonautonomic components. Our preliminary analyses indicate that NA can expand the value of autonomic testing and provide new insight into the functioning of the ANS and related systems in complex disease processes such as PASC and POTS.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10072-022-06423-y.
Keywords: Network analysis, Long COVID, PASC, Autonomic testing, Skin biopsy, POTS
Introduction
Commonly used autonomic tests assess cardiovascular parasympathetic functions using the deep breathing test, cardiovascular sympathetic functions using Valsalva maneuver and tilt test, and sudomotor functions. Each test measures one domain. A combination of testing is usually done to capture the complexities of ANS. A common approach is to use the Ewing battery of cardiovascular testing [1] consisting of the deep breathing test, Valsalva maneuver, and response to standing or to the tilt test [1, 2] which can be combined with sudomotor testing [3] or with transcranial Doppler, capnography, and skin biopsies [4]. Nevertheless, even combined testing does not address the main feature of ANS—the interaction of its subsystems at various levels and interactions with other, non-autonomic domains.
Interactions of ANS with inflammatory/autoimmune systems may be of particular importance. Dysautonomia, low-grade inflammation, and related autoimmune dysregulation plays important role in aging [5, 6] and several chronic disorders including hepatitis C [7, 8], HIV [7, 9], and diabetic neuropathy [10, 11]. Many patients with dysautonomia [12], postural tachycardia syndrome (POTS) [13], and idiopathic SFN [14–16] have elevated inflammatory markers and may respond to immunomodulatory therapy but also negative study exists [17]. Obviously, better methods are needed to assess the relationship between inflammation/autoimmunity and dysautonomia.
Recent advances in network analysis (NA) provide the theoretical and practical framework enabling to characterize interaction data [18–21]. ANS is a complex biological network represented as a set of domains that interact with each other and hence it is natural to apply NA to ANS analysis.
In this study, we applied NA on autonomic and related data obtained from patients with post-acute sequela of COVID-19 (PASC) [22, 23] and postural tachycardia syndrome (POTS) [24] in addition to healthy controls. We integrated inflammatory markers to NA since inflammation/autoimmunity plays a role in PASC [23, 25] and in a subset of POTS [12, 13]. The aim was to assess the applicability of NA to autonomic and related data, and to explore its ability to differentiate PASC from POTS, and as such to explore the potential of NA to identify unique pathophysiology features which underly PASC and POTS.
Material and methods
Principles of NA
Networks as defined in bioinformatics, known as graphs in mathematics, represent objects and relationships between them [18, 20, 26]. Networks consist of nodes that are connected by edges. Nodes represent an object and edges represent the relationships between objects. Correlation-based network analysis evaluates correlations of different components to each other. These correlations can be positive or negative. Typically, correlation-based network analysis has been applied to a correlation matrix of studied variables. An underlying assumption when constructing networks is that the property of the whole network is more than a simple summation of properties of individual network objects. Network analysis may provide information that is not revealed by other methods analyzing correlation matrices [27]. Several metrics have been developed that enable to extract network characteristics. Common metrices are clusters and centrality indicators [28]. Clusters or communities denote degree which is equal to a number of connections to a node. Centrality, a measure of importance, indicates how a node is connected [28]. In another words, a node is correlated positively/negatively to another node/other nodes in a quantitative manner and their relationships could be described by the community and three centrality parameters.
The following centrality indicators are typically used:
-
Betweenness: a measure that characterizes nodes serving as bridges between other nodes, and also determines the most influential node.
Betweenness centrality of a node is defined by [29]:
where is the number of shortest paths that pass through and is the number of those paths that pass through , with . Betweenness can be interpreted as the tunnel via the information flows. A larger tunnel diameter allows to flow of more information within it.
-
Closeness: the average distance of internode connections.
Closeness centrality of a node is defined by [30]:
where is the distance between nodes and . Closeness reflects the speed of information flow. A good analogy is to interpret closeness as “how close are you with your friends,” implying closer contact means faster but presumably fewer complex interactions at a time.
Strength or degree centrality refers to number of direct connections a node has with other nodes, it is the simplest measure of node connectivity.
Degree centrality of a node is defined by [31, 32]: with is adjacency matrix where is equal to 1 if nodes and are connected, and is equal to 0 otherwise.
Calculations of NA
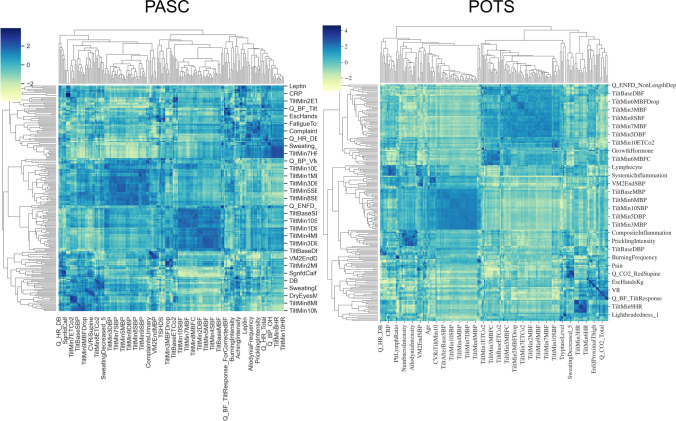
Figure 1 shows a typical flow of calculations. Imputing data is the usual initial step. The preferred approach is to use multiple imputations which reduces the risk of biased estimates of resulting statistics [33]. The next step is a calculation of correlation matrices. Heatmaps with or without hierarchical dendrogram are usually a simple way to visualize correlation matrices. As network metrics, we calculated communities and centrality indicators including betweenness, closeness, and strength. Biological systems form complex structures with the presence of both positive and negative correlations between their components. For easier interpretation of results, we divided correlation matrices into positive and negative correlation matrices and calculated networks for positive and negative correlations separately.
Fig. 1.
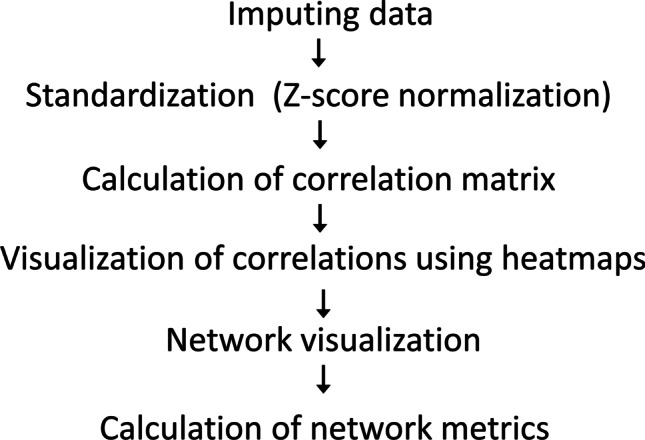
Flow of network calculations
Data collection
We used PASC and POTS patients data published previously [23] except we increased the number of patients in this study. Inclusion and exclusion criteria were described in detail previously. Particular inclusion criteria for PASC were the presence of chronic (> 4 weeks) fatigue (Grade 3 or more on each of the Bristol Rheumatoid Arthritis Fatigue Numerical Rating Scale and brain fog (Grade 3 or more on the Brain Fog scale) which developed within the 6-week interval after acute COVID-19 infection.
POTS was defined as the presence of orthostatic symptoms for > 6 months and an abnormal tilt test with orthostatic tachycardia with heart rate increment ≥ 30 beats per minute without orthostatic hypotension. The POTS criteria for this study also included a clearly defined precipitating event and no systemic disorder that can be associated with POTS. POTS patients were selected randomly from our database using the age and gender matching criteria. Healthy controls were used from our previous study [34].
Surveys, autonomic testing, skin biopsies, and grading
We described testing in detail previously [4, 35]. Shortly, sensory complaints were surveyed by the Neuropathy Total Symptom Score-6 (NTSS) which is a validated instrument for the evaluation of pain features [36]. Autonomic symptoms were obtained from the Survey of Autonomic Symptoms (SAS) which is a validated instrument for the evaluation of autonomic complaints [37]. The Brigham protocol [38] was used for objective and quantitative assessment of autonomic functions and related SFN [4]. Functional cardiovascular autonomic testing included deep breathing (a marker of parasympathetic functions), the Valsalva maneuver and tilt test (both markers of parasympathetic and adrenergic sympathetic functions), and sudomotor evaluation (a marker of postganglionic sudomotor functions). Patients were tilted for 10 min after 10 min of a baseline resting period. The deep breathing test was done for 1 min at 6 breaths per minute. The Valsalva maneuver was performed with expiratory pressure of 40 mmHg for 15 s. The following signals were recorded throughout the testing: electrocardiogram, continuous and intermittent blood pressure, end-tidal CO2, and cerebral blood flow velocity (CBFv) in the middle cerebral artery using transcranial Doppler. Sudomotor testing was done using an electrochemical skin conductance (ESC) device [39]. Epidermal nerve fiber density (ENFD) and sweat gland nerve fiber density (SGNFD) were obtained using established standards [40]. Skin samples were taken from the proximal thigh 20 cm distal to the iliac spine, and from the calf 10 cm above the lateral malleolus using a 3-mm circular punch tool. Skin specimens were immunoperoxidase-stained for the axonal marker PGP 9.5 at Therapath (New York, NY). Test results were graded using the Quantitative Scale for Grading of Cardiovascular Autonomic Reflex Tests and Small Fibers from Skin Biopsies (QASAT) [35]. QASAT is a modular and objective instrument for grading the severity of dysautonomia, small fiber neuropathy, and cerebral blood flow. Hypocapnia, which is frequently seen in PASC and POTS, has profound effect on CBFv. Hypocapnia, e.g., low end-tidal CO2, induces cerebral arteriolar vasoconstriction and reduces CBFv. We calculated how much orthostatic drop in CBFv is due to the effect of hypocapnia versus the drop in CBFv due to cerebral autoregulatory failure assuming the orthostatic hypotension is absent. The parameter OCHOS% gives in % the contribution of both variables. For example, OCHOS% = 30 means that 30% of the drop in CBFv is due to cerebral autoregulatory failure, e.g., arteriolar stiffness, and 70% is due to effect of hypocapnia-induced arteriolar vasoconstriction. We showed detailed calculations of OCHOS% previously [41].
Calculations of inflammatory activity
We calculated indexes of low-grade inflammation including a composite inflammatory activity (CIA) [18] and a systemic inflammation index (SII) [42]. CIA was calculated using interleukin (IL) 6, IL1b, and tumor necrosis α (TNA) inflammatory markers. We also defined expanded CIA (ECIA) by adding other inflammatory markers including high-resolution c-reactive protein (CRP), IL-10, trisulfated heparin disaccharide (TS-HDS) antibody, fibroblast growth factor receptor 3 (FGFR3) antibody [14], growth hormone, adiponectin, leptin, and ganglionic acetylcholine receptor antibody to CIA. CIA and ECIA were obtained by averaging the standardized z scores of the inflammatory markers [18, 43]. Systemic inflammation index (SII) was obtained by using the following formula: SII = platelet * neutrophil/lymphocyte, where all variables are in absolute numbers [42].
Details of NA calculations
For imputation, missing data were substituted using a mean strategy for numerical and the most frequent strategy for categorical data. The correlation matrix has been calculated using Pearson’s method. The network was visualized using the Gephi software [19]. The standardized correlation matrices were imported using the average edges and the undirected graph type with the Force Atlas 2 algorithm. The algorithm detects clusters or communities with similar characteristics and assigns each community a distinct color [44]. A random color is typically set for each community.
Two networks were constructed, a detailed network with both raw and derived variables and a minimal network consisting of QASAT and inflammatory indexes only. The following QASAT indexes were used: parasympathetic cardiovagal (obtained from deep breathing), sympathetic adrenergic (obtained from Valsalva maneuver and tilt test), sudomotor (obtained from ESC), orthostatic tachycardia, cerebral blood flow, end-tidal CO2, and two indexes of small fibers (epidermal and sudomotor) obtained from skin biopsies. Autonomic failure score was obtained by summing parasympathetic cardiovagal, sympathetic adrenergic, and sympathetic sudomotor scores. The reason to construct both networks was to determine if the limited network provides similar information as the detailed network which requires much more demand on computational resources and makes interpretation of results more difficult.
As an additional control, we filled autonomic and inflammatory variables with random numbers and constructed networks using the same method as for real data to compare random data network patterns with presumably deterministic network patterns from patients.
Statistical analysis
A one-way analysis of variance was used to determine the differences between means of all three studied groups for continuous variables. Categorical variables were compared by the χ2 test. The 2-tailed t statistic was used to compare PASC and POTS groups for continuous variables and by χ2 test for categorical variables. Only non-network variables were subjected to statistical analysis. Statistical analysis was obtained using the tableone Python [45] and r package (r-project.org).
Results
Eleven controls, fifteen PASC, and fifteen POTS patients, age, gender, and body mass index matched were evaluated (Table 1). All PASC patients had mild COVID-19 infection, contracted within 2020–2021 years, and were treated with conservative home management with one patient treated with supplemental home oxygen. The typical presentation was the sudden onset of cough, dyspnea, fever, headaches, and generalized malaise. None of the patients with PASC had received the COVID-19 vaccine nor had a history of severe acute respiratory distress syndrome, acute kidney, or liver failure, or reported use of corticosteroids or other immunomodulatory therapy.
Table 1.
Demographics of study subjects
| Variable | Controls | PASC | POTS | P (Controls-PASC-POTS) | P (PASC-POTS) |
|---|---|---|---|---|---|
| N | 11 | 15 | 15 | ||
| Age, years, mean (SD) | 34.9 (8.6) | 35.8 (7.9) | 31.8 (9.8) | 0.749 | 0.16 |
| Gender, f (%) | 10 (90.9) | 12 (92.3) | 14 (93.3) | 0.573 | 0.999 |
| BMI, kg/m2, mean (SD) | 24.6 (6.0) | 26.6(6.7) | 23.6(4.5) | 0.578 | 0.177 |
| Symptom’s duration, years, mean (SD) | 0.0 (0.0) | 0.9 (0.3) | 6.9(4.4) | < 0.001 | 0.001 |
Group analysis showed abnormal QASAT scores in all domains (Table 2). SFN on skin biopsy was detected in 77% (n = 10) of PASC (mixed SFN = 4, sensory SFN = 4, autonomic sudomotor SFN = 2) and 88% (n = 14) of POTS (mixed SFN = 5, sensory SFN = 7, autonomic sudomotor SFN = 2) patients. Autonomic failure was detected in 100% of PASC and 94% (n = 15) of POTS patients. Autonomic failure was mild-to-moderate and no one had orthostatic hypotension. Reduced orthostatic CBFv was detected in 100% of PASC and in 88% (n = 14) POTS patients. Two POTS patients with normal orthostatic CBFv had reduced supine CBFv. Abnormal hypocapnia was detected in 87% (n = 13) of PASC (supine hypocapnia in 67% (n = 10), orthostatic hypocapnia in 40% (n = 6), and in 100% of POTS, supine hypocapnia in 33% (n = 5), orthostatic hypocapnia in 93% (n = 14). POTS patients had higher QASAT scores of cerebral blood flow, end-tidal CO2, and autonomic failure, but lower ENFD and SGNFD scores compared to PASC (Table 2).
Table 2.
Results of testing. Data are expressed as mean (sd)
| QASAT scores | Controls | PASC | POTS | P (Controls-PASC-POTS) | P (PASC-POTS) |
|---|---|---|---|---|---|
| N | 11 | 15 | 15 | ||
| Sympathetic sudomotor (ESC), range 0–6 | 0.0 (0.0) | 1.5 (1.3) | 1.7 (1.8) | 0.397 | 0.776 |
| Parasympathetic cardiovagal (Deep breathing), range 0–3 | 0.0 (0.0) | 0.4 (0.5) | 0.4 (0.7) | 0.113 | 0.904 |
| Increased heart rate response to tilt, range 0–10 | 0.0 (0.0) | 2.3 (3.5) | 8.6 (1.2) | 0.202 | < 0.001 |
| Sympathetic adrenergic (Valsalva maneuver), range 0–3 | 0.0 (0.0) | 1.6 (0.6) | 1.3 (1.0) | < 0.001 | 0.246 |
| Sympathetic adrenergic (Tilt), range 0–10 | 0.0 (0.0) | 0.7 (2.2) | 0.5 (1.2) | 0.436 | 0.782 |
| Cerebral blood flow response to tilt, range 0–10 | 0.0 (0.0) | 6.3 (2.7) | 7.3 (2.5) | < 0.001 | 0.284 |
| Cerebral blood flow, total, range 0–11 | 0.0 (0.0) | 7.1 (2.5) | 8.1 (2.3) | < 0.001 | 0.312 |
| pET-CO2, range 0–10 | 0.0 (0.0) | 1.6 (2.7) | 6.0 (3.5) | 0.026 | 0.001 |
| Autonomic failure, range 0–24 | 0.0 (0.0) | 8.0 (5.1) | 12.9 (3.2) | < 0.001 | 0.005 |
| ENFD, range 0–8 | 0.0 (0.0) | 2.1 (2.4) | 1.5 (2.2) | 0.005 | 0.466 |
| SGNFD, range 0–8 | 0.0 (0.0) | 1.5 (2.4) | 0.8 (1.5) | 0.016 | 0.439 |
| Total, range 0–54 | 0.0 (0.0) | 20.9 (8.8) | 29.5 (5.2) | < 0.001 | 0.005 |
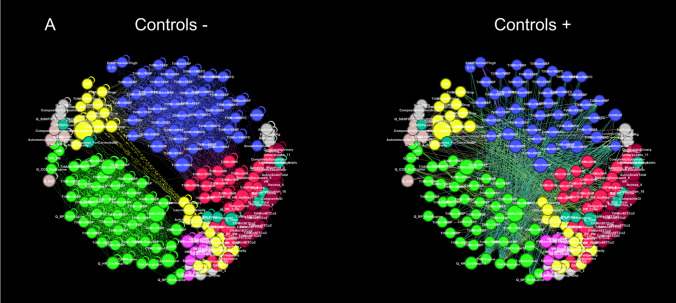
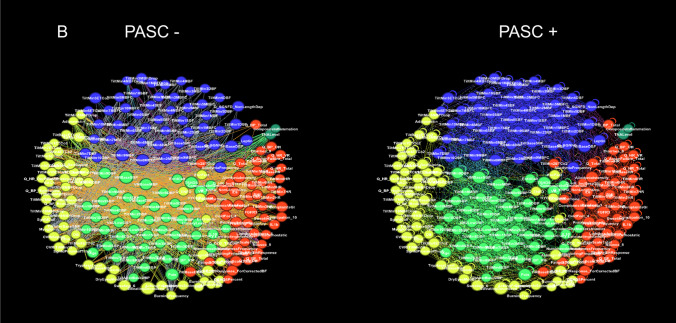
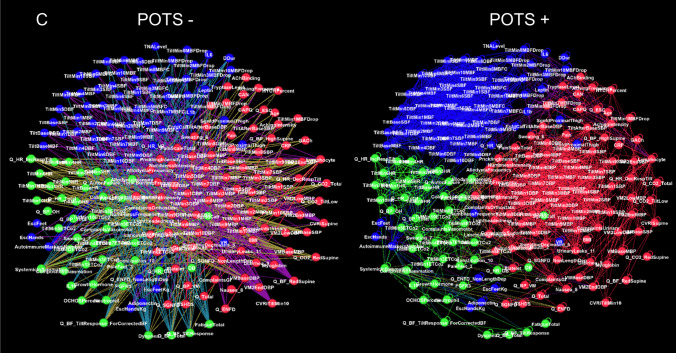
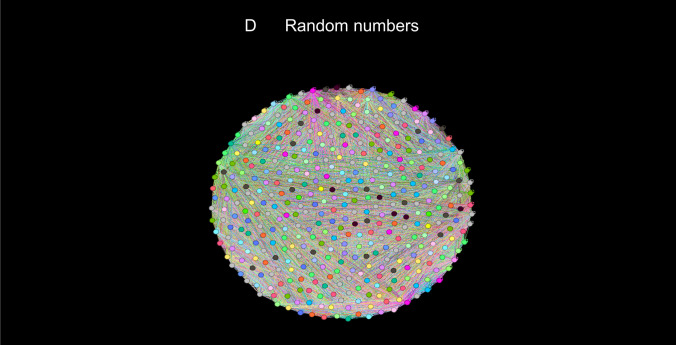
Two networks were constructed, a detailed network consisting of 216 nodes and a limited network consisting of 8 nodes. Visual inspection of heatmaps showed negative and positive correlations concentrated into several dominant clusters (Fig. 2). Largest clusters correspond to CBFv and blood pressure. Network visualization showed the complex structure of underlying networks. Unfiltered networks showed a high level of complexity therefore networks were divided into negative and positive correlation networks. Several dominant clusters emerged which correspond to CBFv, blood pressure, heart rate, and CO2 (Fig. 3). Visual inspection showed qualitative differences between controls, PASC, and POTS. Relatively well-demarcated clusters were seen in controls and POTS. The heart rate cluster was less differentiated in PASC. Negative correlations had a different profile in PASC compared to POTS or controls, and the distinction between various clusters was more blurred. No discernible pattern was found in a network created from random numbers (Fig. 3d). Positive node strength of constipation, sudomotor, and total autonomic scale were higher in PASC while positive node strength of baselined blood pressure, drop of CBFv at the tilt end, systemic inflammation were higher in POTS group (Supplemental material table). Positive node strength of pain and of cold feet was high in both POTS and PASC. Negative betweenness of ESC at hands, CBFv, and composite inflammation were higher at POTS. Negative betweenness of CBFv at the 10th minute of the tilt was higher PASC group. Closeness of sensory and orthostatic symptoms, and baseline blood pressure were higher in POTS (Fig. 4).
Fig. 2.
Heatmaps with hierarchical dendrogram of PASC (left) and POTS (right) patients
Fig. 3.
Detailed networks of controls (A), PASC patients (B), POTS patients (C), and network constructed from random numbers (D). Undirected correlation networks were generated by Force Atlas 2 algorithm. Each community or cluster has a distinct color. Labels: − negative correlations (left panels); + positive correlations (right panels)
Fig. 4.
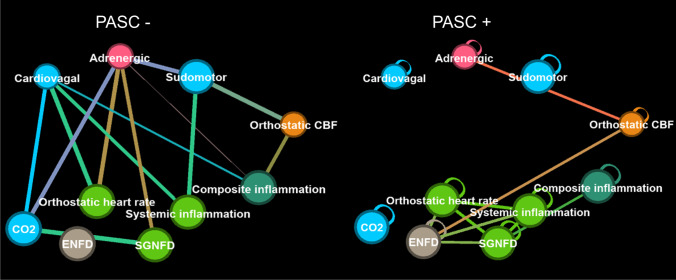
The limited eight-node network of PASC consists of QASAT and inflammatory indexes. Networks of negative correlations are on the left, positive on the right
Limited network showed elevated negative betweenness of QASAT-Cardiovagal in both POTS and PASC (Table 3). All network metrices for QASAT-ENFD for positive correlations were elevated in both POTS and PASC compared to other metrices (Table 3).
Table 3.
Limited network results. The networks were calculated for negative ( −) and positive ( +) correlations
| Node | Closeness | Betweenness | Strength | |||
|---|---|---|---|---|---|---|
| PASC − / + | POTS − / + | PASC − / + | POTS − / + | PASC − / + | POTS − / + | |
| QASAT-Orthostatic tachycardia | 0.6/0.75 | 0.56/0.75 | 1.5/0.65 | 6.6/1.75 | 3/8 | 3/8 |
| QASAT-Cardiovagal | 0.75/0.6 | 0.64/0.64 | 13.1/0.75 | 13.3/0.6 | 6/5 | 5/6 |
| QASAT-Adrenergic | 0.64/0.64 | 0.45/0.75 | 5.6/0.83 | 2/1.6 | 5/6 | 2/8 |
| QASAT-Orthostatic hypoperfusion | 0.47/0.82 | 0.45/0.82 | 0.5/4.45 | 2/2.95 | 2/9 | 2/9 |
| QASAT-CO2 | 0.6/0.69 | 0.64/0.64 | 1.5/0.7 | 6/0.2 | 4/7 | 4/6 |
| QASAT-ENFD | 0.45/0.9 | 0.4/0.9 | 0.0/3.65 | 0/2.63 | 1/10 | 1/10 |
| QASAT-SGNFD | 0.56/0.75 | 0.5/0.82 | 0.5/1.03 | 3/1.9 | 3/8 | 2/9 |
| QASAT-ESC | 0.6/0.69 | 0.5/0.75 | 4.3/2.5 | 3.6/2.15 | 4/7 | 3/8 |
| Composite inflammatory activity | 0.6/0.75 | 0.56/0.75 | 4.2/1.03 | 2.6/0.6 | 3/8 | 3/8 |
| Systemic inflammation index | 0.6/0.75 | 0.56/0.75 | 1.75/1.36 | 2.6/0.6 | 3/8 | 3/8 |
Discussion
This study showed that the application of NA to autonomic testing and related data is straightforward. No alterations of raw data or autonomic indexes were necessary. NA was written in Python software using available packages, which are free and widely used in the scientific community. Network was visualized using Gephi software, which is also free.
NA showed complex interactions within autonomic and between autonomic and nonautonomic domains. Both negative and positive correlations were common. Furthermore, network nodes were forming clusters with distinct connections separating each cluster. No distinct patterns were detected in the network created from random numbers suggesting that the network patterns obtained from patients represent predominantly a deterministic process.
Established analysis
Established analysis using the Brigham protocol confirmed our previous findings [41, 46]. PASC is associated with abnormal orthostatic cerebral blood flow, respiratory dysregulation, and small fiber neuropathy associated with widespread dysautonomia. Our findings are similar to recently reported reduced orthostatic cerebral blood flow [47], respiratory dysregulation with hypocapnia [48], small fiber neuropathy [49, 50], and dysautonomia [51] in PASC. Established analysis showed similar occurrence but more severe dysautonomia in POTS compared to PASC. Cerebral blood flow dysregulation was more common but less severe in PASC compared to POTS. Hypocapnic hyperventilation was more severe in POTS. Although the above described differences between PASC and POTS indicate different etiologic pathways, they cannot clarify the underlying mechanisms.
Differentiation between PASC and POTS using NA
CBFv and blood pressure network nodes formed the largest network clusters, but these relationships were disease dependent. In controls, cerebral blood flow and blood pressure nodes are mainly negatively correlated. In PASC and POTS, positive correlations increased, but negative correlations declined only in POTS. These observations imply different mechanisms underlying vascular dysregulation in these disorders. Image processing with artificial intelligence can be employed in future studies to quantify the observed differences.
Several network metrics differentiated between both disorders. Positive betweenness of OCHOS% node was high in PASC. OCHOS% is a marker of vascular dysfunction. It quantitates in % how much reduction in orthostatic CBFv is due to cerebral arteriolar stiffness associated with cerebral autoregulatory failure versus the effect of hypocapnia-induced cerebral arteriolar vasoconstriction [38]. The first case (OCHOS% = 100) is usually associated with PASC, the second case (OCHOS% = 0) with POTS, although there is a continuous gradient. In contrast, the negative betweenness of the OCHOS% node was high in POTS, implying important but opposite influences of the vascular metrics in both disorders. The higher strength for POTS nodes was observed for the blood pressure baseline before the tilt.
Inflammatory indexes
To detect low-grade inflammation and related autoimmune dysregulation, we evaluated several individual markers as well as composite inflammatory indexes including SII which uses widely available blood tests and CIA which is appears to be more specific for low grade inflammation but it is also more expensive. Several network metrics of inflammatory markers differentiated PASC including TSHDS and FGFR3 antibodies, adiponectin, neutrophil count, IL10, and myoglobin. Betweenness, an information flow marker, of SII for negative correlations was elevated in POTS implying a role of low grade inflammation in POTS. The value of SII as an addendum to autonomic testing should be investigated in feature studies since it is inexpensive and easy-to-obtain inflammatory index.
Comparisons between detailed and limited networks
In this study, two networks have been created—a detailed and a limited, which is much smaller. The detailed network used combination of surveys, raw data, autonomic and other indexes, as well as inflammatory markers. The detailed network showed complex interactions between analyzed variables. A limited network consists of a few nodes and each node is a composite value corresponding to a particular domain or index. In theory, a limited network compresses a large amount of data hopefully without reducing the information content. Also, smaller networks are easier to interpret. Interestingly, the QASAT-Cardiovagal closeness and betweenness were higher in both PASC and POTS compared to other nodes, suggesting that cardiovagal dysregulation plays an important role in both disorders through common effector pathways facilitated by a vagus nerve. Although not explored in this study, the vagus nerve has been shown to mediate and connect neural and immune activity [52]. In addition, all network metrics of ENFD exceeded other nodes in both PASC and POTS implying an important role of peripheral nervous system involvement in both disorders. QASAT-Orthostatic hypoperfusion (negative betweenness was higher in POTS) and CIA (negative betweenness was higher in PASC) appear also to differentiate PASC from POTS.
New information gathered from NA
Network analysis has several unique properties. NA focuses on relationships and not on absolute data values. Hence, no normative data are needed when constructing networks from raw data. NA can be entirely data-driven as no hypotheses are necessary. The main aim of the NA is the detection of the most important nodes in the network which underly the network structure and therefore may be targeted by interventions [21]. Our pilot results point to the interplay between the parasympathetic system and inflammation. Cardiovagal network metrics were elevated in both POTS and PASC patients which is consistent with the role of the vagus nerve in the modulation of the immune system [53]. Another application is to compare networks when underlying structures are not well defined. Such differential networks when applied to gene–gene interactions may lead to new discoveries in gene functions [54]. In our study, a comparison of the PASC to POTS networks showed differences in vascular and inflammatory variables as discussed above. These observations confirm an important role of immune-mediated vascular injury in PASC [55, 56]. Ultimately, the goal of NA is to clarify the etiology and to define optimal treatment.
Limitations
This study has several limitations. A referral bias, a limited number of subjects, women predominance, and restricted age range of our cohort limits the generalization of our results. Also, a one-time assessment does not enable to evaluate the time dynamic of the studied disorders which may of importance, particularly for PASC. Furthermore, we used linear correlations in the network constructs which is suboptimal for revealing nonlinear relationships.
Conclusion
NA is suitable for relationship analysis between autonomic and nonautonomic data. Our preliminary analyses indicate that NA may expand the value of autonomic testing.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Authors thank to Mona Taliaferro/Bay Shore Recycling for funding this study.
Declarations
Ethical approval and informed consent
The study was approved by the BWH Institutional Review Board as a minimal risk study.
Conflict of interest
None.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ewing DJ, Clarke BF. Diagnosis and management of diabetic autonomic neuropathy. Br Med J Clin Res Ed. 1982;285:916–918. doi: 10.1136/bmj.285.6346.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Novak P. Quantitative autonomic testing. J Vis Exp JoVE. 2011 doi: 10.3791/2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Low PA, Tomalia VA, Park K-J. Autonomic function tests: some clinical applications. J Clin Neurol Seoul Korea. 2013;9:1–8. doi: 10.3988/jcn.2013.9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novak P (2019) Autonomic Testing, Oxford University Press
- 5.Calder PC, Bosco N, Bourdet-Sicard R, Capuron L, Delzenne N, Doré J, Franceschi C, Lehtinen MJ, Recker T, Salvioli S, Visioli F. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res Rev. 2017;40:95–119. doi: 10.1016/j.arr.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Masoli JAH, Delgado J. Blood pressure, frailty and dementia. Exp Gerontol. 2021;155:111557. doi: 10.1016/j.exger.2021.111557. [DOI] [PubMed] [Google Scholar]
- 7.Lopez Angel CJ, Pham EA, Du H, Vallania F, Fram BJ, Perez K, Nguyen T, Rosenberg-Hasson Y, Ahmed A, Dekker CL, Grant PM, Khatri P, Maecker HT, Glenn JS, Davis MM, Furman D. Signatures of immune dysfunction in HIV and HCV infection share features with chronic inflammation in aging and persist after viral reduction or elimination. Proc Natl Acad Sci U S A. 2021;118:e2022928118. doi: 10.1073/pnas.2022928118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coutinho BM, Bordalo E, Nascimento OJM. Autonomic evaluation of hepatitis C virus infected patients. Arq Neuropsiquiatr. 2013;71:537–539. doi: 10.1590/0004-282X20130074. [DOI] [PubMed] [Google Scholar]
- 9.Siepmann T, Penzlin AI, Illigens BMW. Autonomic neuropathies. Dtsch Med Wochenschr. 2013;1946(138):1465–1469. doi: 10.1055/s-0033-1343268. [DOI] [PubMed] [Google Scholar]
- 10.Ristikj-Stomnaroska D, Risteska-Nejashmikj V, Papazova M. Role of inflammation in the pathogenesis of diabetic peripheral neuropathy, Open Access Maced. J Med Sci. 2019;7:2267–2270. doi: 10.3889/oamjms.2019.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowdhury M, Nevitt S, Eleftheriadou A, Kanagala P, Esa H, Cuthbertson DJ, Tahrani A, Alam U. Cardiac autonomic neuropathy and risk of cardiovascular disease and mortality in type 1 and type 2 diabetes: a meta-analysis. BMJ Open Diabetes Res Care. 2021;9:e002480. doi: 10.1136/bmjdrc-2021-002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schofield JR, Chemali KR. Intravenous immunoglobulin therapy in refractory autoimmune dysautonomias: a retrospective analysis of 38 patients. Am J Ther. 2019;26:570–582. doi: 10.1097/MJT.0000000000000778. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez B, Hoepner R, Salmen A, Kamber N, Z’Graggen WJ. Immunomodulatory treatment in postural tachycardia syndrome: A case series. Eur J Neurol. 2021;28:1692–1697. doi: 10.1111/ene.14711. [DOI] [PubMed] [Google Scholar]
- 14.Trevino JA, Novak P. TS-HDS and FGFR3 antibodies in small fiber neuropathy and Dysautonomia. Muscle Nerve. 2021;64:70–76. doi: 10.1002/mus.27245. [DOI] [PubMed] [Google Scholar]
- 15.Levine TD, Kafaie J, Zeidman LA, Saperstein DS, Massaquoi R, Bland RJ, Pestronk A. Cryptogenic small-fiber neuropathies: serum autoantibody binding to trisulfated heparan disaccharide and fibroblast growth factor receptor-3. Muscle Nerve. 2019 doi: 10.1002/mus.26748. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Treister R, Lang M, Oaklander AL (2018) IVIg for apparently autoimmune small-fiber polyneuropathy: first analysis of efficacy and safety. Ther Adv Neurol Disord 11. 10.1177/1756285617744484 [DOI] [PMC free article] [PubMed]
- 17.Geerts M, de Greef BTA, Sopacua M, van Kuijk SMJ, Hoeijmakers JGJ, Faber CG, Merkies ISJ. intravenous immunoglobulin therapy in patients with painful idiopathic small fiber Neuropathy. Neurology. 2021;96:e2534–e2545. doi: 10.1212/WNL.0000000000011919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J, Yoon S, Lee S, Hong H, Ha E, Joo Y, Lee EH, Lyoo IK. A double-hit of stress and low-grade inflammation on functional brain network mediates posttraumatic stress symptoms. Nat Commun. 2020;11:1898. doi: 10.1038/s41467-020-15655-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birt HWG, Dennis PG. Inference and analysis of SPIEC-EASI microbiome networks, methods Mol. Biol Clifton NJ. 2021;2232:155–171. doi: 10.1007/978-1-0716-1040-4_14. [DOI] [PubMed] [Google Scholar]
- 20.Jeong H, Mason SP, Barabási AL, Oltvai ZN. Lethality and centrality in protein networks. Nature. 2001;411:41–42. doi: 10.1038/35075138. [DOI] [PubMed] [Google Scholar]
- 21.Hevey D (n.d.) Network analysis: A brief overview and tutorial. Health Psychol Behav Med 6 301–328. 10.1080/21642850.2018.1521283 [DOI] [PMC free article] [PubMed]
- 22.Bisaccia G, Ricci F, Recce V, Serio A, Iannetti G, Chahal AA, Ståhlberg M, Khanji MY, Fedorowski A, Gallina S. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: what do we know? J Cardiovasc Dev Dis. 2021;8:156. doi: 10.3390/jcdd8110156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novak P, Mukerji SS, Alabsi HS, Systrom D, Marciano SP, Felsenstein D, Mullally WJ, Pilgrim DM. Multisystem involvement in post-acute sequelae of coronavirus disease 19. Ann Neurol. 2022;91:367–379. doi: 10.1002/ana.26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garland EM, Celedonio JE, Raj SR. Postural tachycardia syndrome: beyond orthostatic intolerance. Curr Neurol Neurosci Rep. 2015;15:60. doi: 10.1007/s11910-015-0583-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bornstein SR, Voit-Bak K, Donate T, Rodionov RN, Gainetdinov RR, Tselmin S, Kanczkowski W, Müller GM, Achleitner M, Wang J, Licinio J, Bauer M, Young AH, Thuret S, Bechmann N, Straube R (2021) Chronic post-COVID-19 syndrome and chronic fatigue syndrome: Is there a role for extracorporeal apheresis? Mol Psychiatry 1–4. 10.1038/s41380-021-01148-4 [DOI] [PMC free article] [PubMed]
- 26.Mistry D, Wise RP, Dickerson JA. DiffSLC: A graph centrality method to detect essential proteins of a protein-protein interaction network. PLoS One. 2017;12:e0187091. doi: 10.1371/journal.pone.0187091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kojaku S, Masuda N. Constructing networks by filtering correlation matrices: a null model approach. Proc Math Phys Eng Sci. 2019;475:20190578. doi: 10.1098/rspa.2019.0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borgatti S. Centrality and network flow. Soc Netw. 2005;27:55–71. doi: 10.1016/j.socnet.2004.11.008. [DOI] [Google Scholar]
- 29.Freeman L. A set of measures of centrality based on betweeneness. Sociometry. 1977;40:35–41. doi: 10.2307/3033543. [DOI] [Google Scholar]
- 30.Bavelas A. Communication patterns in task-oriented groups. J Acoust Soc Am. 1950;22:725–730. doi: 10.1121/1.1906679. [DOI] [Google Scholar]
- 31.Sabidussi G. The centrality of a graph. Psychometrika. 1966;31:581–603. doi: 10.1007/BF02289527. [DOI] [PubMed] [Google Scholar]
- 32.Nieminen J. On the centrality in a graph. Scand J Psychol. 1974;15:332–336. doi: 10.1111/j.1467-9450.1974.tb00598.x. [DOI] [PubMed] [Google Scholar]
- 33.Austin PC, White IR, Lee DS, van Buuren S (2020) Missing data in clinical research: a tutorial on multiple imputation. Can J Cardiol S0828–282X(20)31111–9. 10.1016/j.cjca.2020.11.010 [DOI] [PMC free article] [PubMed]
- 34.Novak P. Assessment of sympathetic index from the Valsalva maneuver. Neurology. 2011;76:2010–2016. doi: 10.1212/WNL.0b013e31821e5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novak P. Qpack-a Python package for QASAT-quantitative scale for grading cerebral blood flow, autonomic testing, and skin biopsies. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2022 doi: 10.1007/s10072-022-06007-w. [DOI] [PubMed] [Google Scholar]
- 36.Bastyr EJ, Price KL, Bril V. MBBQ Study Group, development and validity testing of the neuropathy total symptom score-6: questionnaire for the study of sensory symptoms of diabetic peripheral neuropathy. Clin Ther. 2005;27:1278–1294. doi: 10.1016/j.clinthera.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Zilliox L, Peltier AC, Wren PA, Anderson A, Smith AG, Singleton JR, Feldman EL, Alexander NB, Russell JW. Assessing autonomic dysfunction in early diabetic neuropathy: the Survey of Autonomic Symptoms. Neurology. 2011;76:1099–1105. doi: 10.1212/WNL.0b013e3182120147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novak P, Mukerji SS, Alabsi HS, Systrom D, Marciano SP, Felsenstein D, Mullally WJ, Pilgrim DM. Multisystem involvement in post-acute sequelae of COVID-19 (PASC) Ann Neurol. 2021 doi: 10.1002/ana.26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novak P. Electrochemical skin conductance: a systematic review. Clin Auton Res Off J Clin Auton Res Soc. 2017 doi: 10.1007/s10286-017-0467-x. [DOI] [PubMed] [Google Scholar]
- 40.Lauria G, Hsieh ST, Johansson O, Kennedy WR, Leger JM, Mellgren SI, Nolano M, Merkies ISJ, Polydefkis M, Smith AG, Sommer C, Valls-Solé J (2010) European Federation of Neurological Societies, Peripheral Nerve Society, European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol 17:903–912, e44–49. 10.1111/j.1468-1331.2010.03023.x [DOI] [PubMed]
- 41.Novak P, Mukerji S, Alabsi H, Systrom D (2022) Multisystem involvement in post-acute sequelae of COVID-19 (PASC). Ann Neurol [DOI] [PMC free article] [PubMed]
- 42.Hu B, Yang X-R, Xu Y, Sun Y-F, Sun C, Guo W, Zhang X, Wang W-M, Qiu S-J, Zhou J, Fan J. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2014;20:6212–6222. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 43.Bonaccio M, Di Castelnuovo A, Pounis G, De Curtis A, Costanzo S, Persichillo M, Cerletti C, Donati MB, de Gaetano G, Iacoviello L. Moli-sani Study Investigators, A score of low-grade inflammation and risk of mortality: prospective findings from the Moli-sani study. Haematologica. 2016;101:1434–1441. doi: 10.3324/haematol.2016.144055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kauffman J, Kittas A, Bennett L, Tsoka S. DyCoNet: a Gephi plugin for community detection in dynamic complex networks. PLoS One. 2014;9:e101357. doi: 10.1371/journal.pone.0101357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pollard TJ, Johnson AEW, Raffa JD, Mark RG. tableone: an open source Python package for producing summary statistics for research papers. JAMIA Open. 2018;1:26–31. doi: 10.1093/jamiaopen/ooy012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Novak P, Giannetti M, Weller E, Hamilton M, Castells M. Mast cell disorders are associated with decreased cerebral blood flow and small fiber neuropathy. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol. 2021;S1081–1206(21):01155–8. doi: 10.1016/j.anai.2021.10.006. [DOI] [PubMed] [Google Scholar]
- 47.van Campen CLMC, Rowe PC, Visser FC. Orthostatic symptoms and reductions in cerebral blood flow in long-haul COVID-19 patients: similarities with myalgic encephalomyelitis/chronic fatigue syndrome. Med Kaunas Lith. 2021;58:28. doi: 10.3390/medicina58010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wood J, Tabacof L, Tosto-Mancuso J, McCarthy D, Kontorovich A, Putrino D (2021) Levels of end-tidal carbon dioxide are low despite normal respiratory rate in individuals with long COVID. J Breath Res 16. 10.1088/1752-7163/ac3c18 [DOI] [PubMed]
- 49.Abrams RMC, Simpson DM, Navis A, Jette N, Zhou L, Shin SC. Small fiber neuropathy associated with SARS-CoV-2 infection. Muscle Nerve. 2021 doi: 10.1002/mus.27458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oaklander AL, Mills AJ, Kelley M, Toran LS, Smith B, Dalakas MC, Nath A. Peripheral neuropathy evaluations of patients with prolonged long COVID. Neurol Neuroimmunol Neuroinflammation. 2022;9:e1146. doi: 10.1212/NXI.0000000000001146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shouman K, Vanichkachorn G, Cheshire WP, Suarez MD, Shelly S, Lamotte GJ, Sandroni P, Benarroch EE, Berini SE, Cutsforth-Gregory JK, Coon EA, Mauermann ML, Low PA, Singer W. Autonomic dysfunction following COVID-19 infection: an early experience. Clin Auton Res Off J Clin Auton Res Soc. 2021;31:385–394. doi: 10.1007/s10286-021-00803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reardon C, Murray K, Lomax AE. Neuroimmune communication in health and disease. Physiol Rev. 2018;98:2287–2316. doi: 10.1152/physrev.00035.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Mahony C, van der Kleij H, Bienenstock J, Shanahan F, O’Mahony L. Loss of vagal anti-inflammatory effect: in vivo visualization and adoptive transfer. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1118–1126. doi: 10.1152/ajpregu.90904.2008. [DOI] [PubMed] [Google Scholar]
- 54.Grimes T, Potter SS, Datta S. Integrating gene regulatory pathways into differential network analysis of gene expression data. Sci Rep. 2019;9:5479. doi: 10.1038/s41598-019-41918-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sollini M, Morbelli S, Ciccarelli M, Cecconi M, Aghemo A, Morelli P, Chiola S, Gelardi F, Chiti A (2021) Long COVID hallmarks on [18F]FDG-PET/CT: a case-control study. Eur J Nucl Med Mol Imaging 1–11. 10.1007/s00259-021-05294-3 [DOI] [PMC free article] [PubMed]
- 56.Christensen RH, Berg RMG (2021)Vascular inflammation as a therapeutic target in COVID-19 “Long Haulers”: HIITing the Spot? Front Cardiovasc Med 8. https://www.frontiersin.org/article/10.3389/fcvm.2021.643626. Accessed 23 Jun 2022 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.