Abstract
Spine fusion is one of the most common orthopedic surgeries, with more than 400,000 cases performed annually. While these procedures correct debilitating pain and deformities, complications occur in up to 45%. As successful fusion rests upon early stability of hardware in bone, patients with structural skeletal deficits may be at particular risk for complications. Few studies have investigated this relationship, and none have used higher order imaging to evaluate microstructural mechanisms for complications. Standard DXA measurements are subject to artifact in patients with spinal disease and therefore provide limited information. The goal of this prospective study was to investigate pre-operative bone quality as a risk factor for early post-operative complications using high resolution peripheral QCT (HR-pQCT) measurements of volumetric BMD (vBMD) and microarchitecture. We hypothesized that patients with low vBMD and abnormal microarchitecture at baseline would have more skeletal complications post-operatively. Conversely, we hypothesized that pre-operative DXA measurements would not be predictive of complications. Fifty-four subjects (mean age 63 years, BMI 27 kg/m2) were enrolled pre-operatively and followed for 6 months after multi-level lumbar spine fusion. Skeletal complications occurred in 14 patients. Patients who developed complications were of similar age and BMI to those who did not. Baseline areal BMD and Trabecular Bone Score by DXA did not differ. In contrast, HR-pQCT revealed that patients who developed complications had lower trabecular vBMD, fewer and thinner trabeculae at both the radius and tibia, and thinner tibial cortices. In summary, abnormalities of both trabecular and cortical microarchitecture were associated the development of complications within the first six months following spine fusion surgery. Our results suggest a mechanism for early skeletal complications after fusion. Given the burgeoning number of fusion surgeries, further studies are necessary to investigate strategies that may improve bone quality and lower the risk of post-operative complications.
Keywords: ANALYSIS QUANTITATION OF BONE, Bone QCT/μCT, DXA, ORTHOPAEDICS, DISEASES OF DISORDERS RELATED TO BONE
Introduction
Spine fusion is among the most common orthopedic procedures, with more than 400,000 cases performed each year in the United States[1–3]. The number of cases is increasing annually, particularly among patients over 65[3]. These complex procedures can correct debilitating spinal deformities, relieve pain and improve neurologic symptoms. However, complications can occur in up to 45% of cases[4–6] and are associated with substantial morbidity and health care costs[7, 8]. Between 2000 and 2010, more than $287 billion dollars were spent on fusion surgeries[9, 10]. Given the invasive nature of spinal fusion, associated costs, and relatively high complication rate, there is a great need to identify modifiable factors associated with these complications. As successful spinal fusion requires early stability of instrumentation in bone, patients with structural skeletal deficits may be at particular risk for complications related to the bone-instrumentation interface. However, few studies have investigated this relationship.
Prior studies that have investigated the relationship between bone health and post-operative complications have utilized dual energy x-ray absorptiometry (DXA) measurements of areal BMD. While DXA is the gold standard for diagnosis of osteoporosis, it is of limited utility in patients presenting for spinal fusion since abnormalities of the spine can falsely elevate DXA readings, masking patients with true osteoporosis[11–13]. Further, DXA cannot assess true volumetric bone density (vBMD) or microarchitecture, important factors that contribute to the overall quality and integrity of bone. Other tools may be necessary to investigate the relationship between poor bone quality and surgical complications. At present, patients with abnormal bone quality are often not identified until the time of surgery when there are unexpected problems with instrumentation or post-operatively when bone related complications, such as adjacent fractures, hardware failure, and proximal junctional kyphosis (PJK), occur. Better identification of high-risk patients prior to surgery could translate to early intervention with anabolic medications, which are currently under investigation and might ultimately minimize these types of complications.
The goal of this prospective study was to investigate pre-operative bone quality as a risk factor for post-operative complications in patients presenting for multi-level lumbar spine fusion. In addition to standard DXA measurements of areal BMD and trabecular bone score (TBS), high resolution peripheral QCT (HR-pQCT) was used to investigate volumetric BMD and microarchitecture at the radius and tibia. We hypothesized that patients with abnormal bone quality would have higher rates of skeletal complications after fusion. Specifically, patients with low volumetric BMD and abnormal microarchitecture by HR-pQCT at baseline would have higher rates of post-operative complications. In contrast, we postulated that spine DXA would be normal in the majority of patients presenting for fusion surgery and would not predict post-operative complications.
Methods
Study Procedures
Patients undergoing posterior spinal fusion deformity surgery were recruited from the Spine Service at the Hospital for Special Surgery (HSS) between December 2017 and December 2019. Patients were included if planned surgery involved the lumbar spine and included fusion of at least two vertebral levels. Both patients having initial surgery and those having revisions were eligible for inclusion. Patients were excluded if they were under the age of 25, pregnant, or had clotting disorders. All subjects provided written informed consent, and the Institutional Review Board of HSS approved this study. A total of 60 patients met inclusion criteria and were enrolled. Six of these patients were withdrawn prior to surgery, either because of an inability to complete pre-operative imaging tests, or because their surgery was cancelled for clinical reasons. Data is presented on the 54 subjects who underwent surgery and completed the baseline study procedures. At the baseline study visit, medical history and medication lists were assessed. Areal bone mineral density (aBMD) was measured by DXA. In addition, HR-pQCT was performed to measure volumetric BMD and microarchitecture. Surgical details were recorded including number of vertebral levels involved, decompression, types of bone graft. Post-operative radiographs were performed at 6 weeks and 6 months to assess for complications in all participants. Re-operation rates were assessed.
Areal BMD
Areal BMD was measured by DXA (Horizon A (S/N 201056) densitometer, Hologic, Inc., Waltham, MA) of the lumbar spine L1–L4 (LS), total hip (TH), femoral neck (FN), 1/3 radius (1/3R). Short term, in vivo precision is 0.70% for the spine, 1.36% for the total hip, and 0.70% for the radius.
Lumbar vertebrae with significant deformity, osteosclerosis, osteophytes, or degenerative disease were excluded from the analysis. Z-scores compared subjects with age matched populations of the same race and sex, as provided by the manufacturer. Spine TBS parameters were extracted from the DXA image using TBS iNsight software (version 2.1; Medimaps Group; Plan-Les-Ouates, Geneva, Switzerland). TBS was calculated as a mean value of the measurement for vertebrae L1–L4 (unitless)[14, 15]. Normal TBS has been recently suggested as TBS value ≥1.31, which is associated with the lowest risk of major osteoporotic fracture[14, 16].
HR-pQCT of the distal radius and tibia
HR-pQCT was performed with an XtremeCT II scanner (Scanco Medical, Brüttisellen, Switzerland) which uses a microfocus X-ray source (68 kVp voltage, 900 μA current, 43 sec integration time) scanning a region 10.2 mm long along the axis of the long bone resulting in VOI of 60.7 μm isotropic voxel size. The non-dominant distal radius and tibia were scanned unless there was a contraindication (prior fracture or metal implant), in which case the contralateral limb was scanned. Region of Interest was defined on a 2-D scout view by placing a reference line at the distal endplate and scans were acquired using a standard fixed offset of 9 mm and 22 mm from the reference line for radius and tibia respectively. A single highly trained operator acquired and analyzed all scans. Scans were scored for motion on a scale of 1–5 and scans with motion score >3 were excluded from analysis. We used the manufacturer’s standard method to filter and binarize the HR-pQCT images. Automated segmentation algorithm was used to segment the cortical and trabecular regions[17]. We assessed standard HR-pQCT morphological microstructure outcomes, including area; density - total, trabecular (Tb) and cortical (Ct) volumetric BMD (vBMD); microstructure - trabecular number (Tb.N), thickness (Tb.Th), and separation (Tb.Sp), cortical thickness (Ct.Th), and cortical porosity (Ct.Po). In vivo short-term reproducibility (CV) for HR-pQCT measures at our center is between 0–5% for all measures except Ct.Po[18].
Radiographic Analysis
Full length scoliosis films or EOS films were obtained on all patients at baseline, 6 weeks and 6 months post-operatively. Radiographs were reviewed for the following complications: rod breakage, screw loosening, pseudoarthrosis, adjacent segment fracture and PJK. PJK was measured from the upper instrumented vertebra (UIV) to the vertebra directly above (UIV+1) and from the UIV to the UIV+2 on both pre and post-operative radiographs. PJK was defined by the two criteria originated by Glattes[19]: (1) a proximal junctional sagittal Cobb angle ≥10° and (2) at least 10° greater than the preoperative measurement.
Statistical Analysis
Descriptive statistics, means and standard deviation, counts and percentages, were calculated and reviewed for completeness and distributions examined. Univariate associations of demographic variables and details of the surgical procedure with the presence or absence of complications were estimated with logistic regression. Mean differences in DXA, TBS and HR-pQCT variables between those with and without complications were compared with independent T-tests using a Satterthwaite’s correction when variances were unequal. SAS 9.4 (SAS Institute, Cary, NC) was used for all analyses, no adjustment for multiple comparisons was applied, and a p-value less than 0.05 was adopted as the criterion for statistical significance.
Results
Study Subjects
This prospective study enrolled patients undergoing multi-level lumbar or thoracolumbar spinal fusion surgery at HSS. Characteristics of the study population are detailed in Table 1. The average age of patients enrolled was 63 ± 11 years. Mean BMI was 27 ± 6 kg/m2; 26% of the cohort were obese (BMI>30 kg/m2). Men comprised 37% of our population, postmenopausal women 56%, and premenopausal women 7%. While most subjects had no tobacco history, 7% were current smokers, and 35% former smokers. Other relevant comorbidities included hypertension (35%), hyperlipidemia (33%), and diabetes (4%). Twelve patients (22%) in our cohort had a history of clinical fragility fracture in adulthood. Of these, six had multiple fractures. Common sites of fracture were forearm (3), humerus (3), rib (3), vertebra (2), and hip (2) in adulthood. At the baseline visit, 39% of subjects reported using calcium and vitamin D supplements, and an additional 35% were taking vitamin D alone. In the cohort as a whole, current teriparatide use was reported in eight patients at baseline, denosumab in three patients, and bisphosphonates in two patients. Among patients with fractures, four were using teriparatide, one denosumab and one alendronate.
Table 1.
Characteristics of the Study Population
| Total | No Complications | Complications | p-value | |
|---|---|---|---|---|
| Sex | ||||
| Male (N, %) | 20, 37% | 19 | 1 | 0.02 |
| Female (N, %) | 34, 63% | 21 | 13 | |
| Premenopausal | 4, 12% | 3 | 1 | |
| Postmenopausal | 30, 88% | 18 | 12 | |
| Age (years) (Mean ± SD) | 63 ± 11 | 63 ± 12 | 65 ± 9 | 0.60 |
| BMI (kg/m2) (Mean ± SD) | 27 ± 6 | 28 ± 6 | 25 ± 6 | 0.27 |
| BMI Classification (N, %) | ||||
| Underweight (BMI<18.5) | 1, 2% | 1 | 0 | 0.69 |
| Normal (18.5<BMI<24.9) | 20, 37% | 13 | 7 | |
| Overweight (25<BMI<29.9) | 19, 35% | 15 | 4 | |
| Obese (BMI>30) | 14, 26% | 11 | 3 | |
| Smoking History | ||||
| Current (N, %) | 4, 7% | 2 | 2 | 0.26 |
| Former Smoker (N, %) | 19, 35% | 16 | 3 | |
| Never Smoker (N, %) | 31, 57% | 22 | 9 | |
| Teriparatide Use (N, %) | 8, 15% | 4 | 4 | 0.27 |
| Vitamin D and Calcium Use (N, %) | 21, 39% | 12 | 9 | 0.06 |
| Vitamin D Use Only (N, %) | 19, 35% | 16 | 3 | 0.46 |
| Revision Surgery (N, %) | 24, 44% | 18 | 6 | 1.00 |
| DXA Z-Scores (Mean ± SD) | ||||
| Lumbar Spine (n:24 10) | 1.7 ± 2.3 | 1.62 ± 2.5 | 1.8 ± 2.2 | 0.87 |
| Right Total Hip (n: 32 14) | 0 ± 1.0 | 0.1 ± 1.0 | −0.3 ± 1.1 | 0.29 |
| Right Femoral Neck (n: 32 14) | 0.1 ± 1.1 | 0.3 ±1.1 | −0.3 ± 1.1 | 0.10 |
| Left Total Hip (n: 33 12) | −0.1 ± 1.0 | −0.0 ± 1.0 | −0.3 ± 1.0 | 0.39 |
| Left Femoral Neck (n: 33 12) | 0 ± 1.2 | 0.1 ± 1.2 | −0.4 ± 1.1 | 0.18 |
| 1/3 Radius (n: 38 14) | 0.2 ± 1.3 | 0.3 ± 1.2 | 0.1 ± 1.5 | 0.66 |
| DXA TBS (Mean ± SD) (n: 23 10) | 1.400 ± 0.226 | 1.380 ± 0.261 | 1.446 ± 0.112 | 0.33 |
Of the 54 patients in our cohort, 30 patients underwent initial surgery, and 24 patients underwent revision of prior spine surgery with instrumentation. The indication for surgery was adult spinal deformity (ASD; scoliosis, kyphosis, flatback syndrome and/or kyphoscoliosis) in the majority of patients (87%). Most patients (91%) had multiple indications for surgery which included, in addition to ASD, spondylolisthesis and spondylosis. For patients undergoing revision of a prior fusion, the majority were performed because of pseudoarthrosis (58%) or fracture/deformity of the vertebrae adjacent to the surgical construct (25%). Most patients (89%) included underwent long fusion (involving ≥ 5 vertebral levels). The mean number of surgical levels was of 8.5 ± 3.3 vertebrae (range: 2–16). The majority of procedures (68%) included decompression for stenosis as well as fusion.
Areal BMD
Pre-operative DXA demonstrated that Z-scores at the LS were well above average, with a mean of +1.7. Measurements at other sites were within the normal range, with mean Z-scores at the hips and radius, falling within 0.5 SD of normal, as shown in Table 1. The mean TBS value was 1.400, falling within the normal range.
Post-Operative Complications
Skeletal complications occurred in 14 patients (26% of the cohort) during the first six months post-operatively. While two patients required wound debridement at 2 and 4 months post-operatively due to infection, neither required revision surgery. No other patient required re-operation during the 6-month follow-up.
Skeletal complications included rod breakage (6%), screw loosening (11%), pseudoarthrosis (17%) and PJK (78%). Three patients had multiple complications. Patients who developed complications were of similar age and BMI to those who did not. Postmenopausal women were more likely to have complications (p<0.02). Only one male patient developed a skeletal complication. Tobacco use did not differ. A higher rate of complications was found among subjects who had a greater number of vertebral levels instrumented (p <0.05). Use of teriparatide and bisphosphonates were not related to risk of complications. We did not find higher rates of complications among patients whose surgery was a revision of a prior fusion.
Development of complications was not related to pre-operative DXA Z-score at any site, the spine, hip or radius. Similarly, low baseline TBS measurements did not predict the development of complications.
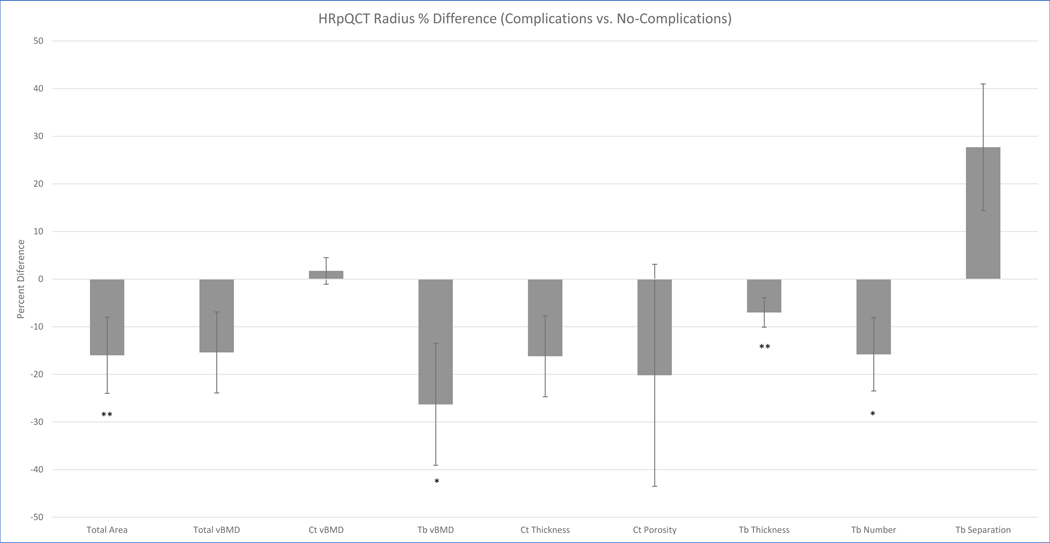
Patients who had pre-operative abnormalities in volumetric BMD and microarchitecture were more likely to develop post-operative skeletal complications (Figures 1 and 2). At the radius, patients with complications had smaller cross-sectional area (−16%, p<0.01). They had lower trabecular vBMD (−26%, p<0.05) and tended to have lower total volumetric BMD (−15%, p-value=0.08). Patients with complications had fewer (−16%, p<0.05) and thinner trabeculae (−7%, p<0.01). Cortical thickness tended to be lower (−16%, p=0.06). Cortical density and porosity did not differ.
Figure 1.
Percent difference in baseline vBMD and microarchitecture at the radius in patients with and without skeletal complications. *p-value<0.05. **p-value<0.01. Trends were observed for lower total vBMD (p=0.08) and lower cortical thickness (p=0.06). Abbreviations: vBMD = volumetric bone mineral density; Ct = cortical; Tb = trabecular.
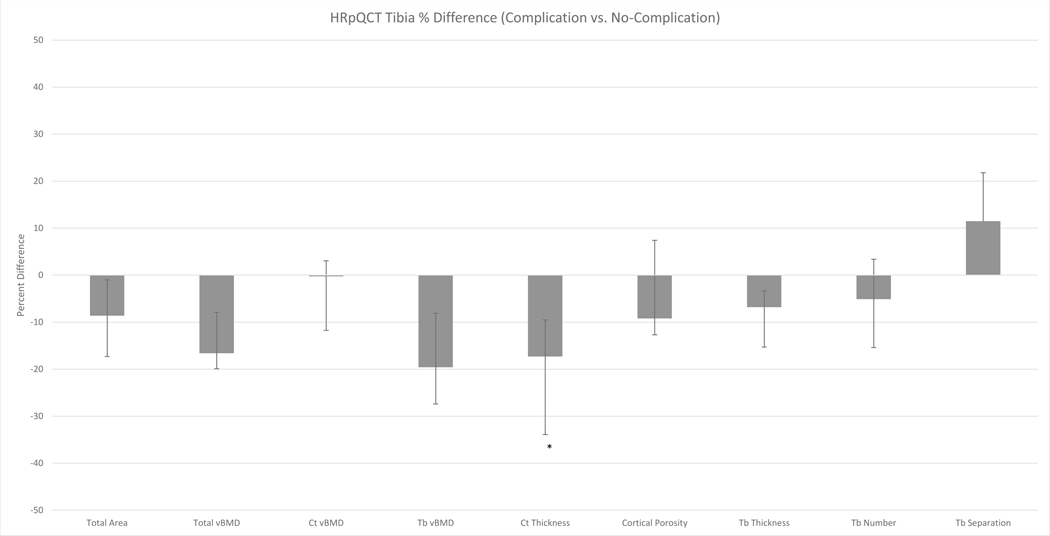
Figure 2.
Percent difference in baseline vBMD and microarchitecture at the tibia in patients with and without skeletal complications. *p-value<0.05. Trends were observed for lower total vBMD (p=0.06), trabecular vBMD (p=0.10) and trabecular thickness (p=0.054). Abbreviations: vBMD = volumetric bone mineral density; Ct = cortical; Tb = trabecular.
At the tibia, bone size was not related to risk of skeletal complications. Patients who developed complications tended to have lower total vBMD (−17%, p=0.06) and trabecular vBMD (−20%, p=0.10). They tended to have thinner trabeculae (−7%, p=0.054). Subjects who developed complications also had thinner cortices (−17%, p<0.05). However, cortical vBMD and porosity did not differ.
Discussion
In this prospective study, we investigated volumetric BMD and microarchitecture as risk factors for early complications following multi-level spinal fusion surgery. We found that patients with lower volumetric BMD and microarchitectural abnormalities by HR-pQCT pre-operatively were more likely to have bone-related complications within the first 6 months following surgery. Our results suggest that adequate bone mass and intact microarchitecture may play a critical role in early hardware stability thereby promoting successful healing and minimizing complications during this acute phase.
To our knowledge, this is the first study using HR-pQCT to assess microarchitecture and relate it to post-operative outcomes in a cohort of spine fusion surgery patients. This technique has the ability to reveal bone quality deficits not detected by DXA and therefore provide novel information about the relationship of bone quality to skeletal complications after fusion surgery. While HR-pQCT is not widely available, its application in this study enabled us to investigate and provide novel data on pre-operative microarchitectural abnormalities as a mechanism for skeletal complications after fusion. We found that patients with low vBMD, fewer, and thinner trabeculae were more likely to have skeletal complications. The fact that we found relationships between vBMD and microarchitecture with complications in the first six months suggests that early skeletal complications may relate to initial stability of hardware in bone. Higher bone density and intact microarchitecture may provide a better anchor for the hardware and prevent micromotion, which enables new bone formation and fusion of the construct.
Although HR-pQCT measurements are performed at peripheral sites, studies by our group and others suggest that patients with peripheral abnormalities in vBMD and microarchitecture have higher rates of vertebral fracture[20, 21]. In prior work, postmenopausal women with vertebral fractures had lower vBMD, more microarchitectural abnormalities and lower stiffness[21]. Women with vertebral fractures also had fewer trabecular plates, fewer axially aligned trabeculae, less connectivity between trabeculae and lower whole bone stiffness[22]. In the current study, we found that patients who developed complications had multiple abnormalities in trabecular bone and thinner cortices. Although vertebral bodies have a only thin cortical rim, prior studies have demonstrated that the cortical shell provides substantial contributions to the biomechanical properties of vertebral bone[23, 24]. Thin cortices at the radius and tibia may therefore be reflective of an inability of the vertebral cortices to provide the necessary support for vertebral bone to withstand the altered distribution of loads post-operatively. While we did not find that higher cortical porosity related to complications, there is a greater degree of variability with this measure compared to the other HR-pQCT parameters. It is conceivable that this variability interfered with our ability to detect a relationship.
Of the prior studies that have investigated the relationship between bone quality and fusion outcomes, the majority were retrospective and utilized only DXA for skeletal assessments. Results of some of these studies suggest that low BMD is a risk factor for PJK[25–28], adjacent fractures[28, 29], screw loosening[28, 30, 31] and hardware subsidence[32]. We did not find that pre-operative DXA measurements were predictive of post-operative complications. Spine measurements in our cohort were well above normal, reflecting the effects of degenerative changes, sclerosis and other artifact. While it was conceivable that TBS measurements would better reflect microarchitecture and be predictive of complications, we did not find that to be the case. This finding may relate to effect of artifact on TBS. Although artifact is less with TBS than areal BMD measurements, some amount still occurs[33].
Our data is consistent with prior literature demonstrating that DXA has limited clinical utility in assessing bone quality in patients with degenerative lumbar scoliosis[34, 35]. While younger patients with scoliosis have been shown to have low BMD[36, 37], in patients that require surgery for adult spinal deformity many spinal segments are degenerated and ultimately sclerotic resulting in normal to high BMD readings on DXA[34]. In contrast, studies utilizing CT measurements have shown that low BMD is common in patients presenting for fusion[25, 26, 28, 38, 39]. In contrast to DXA, central quantitative computed tomography (cQCT) provides a three-dimensional measurement that is less affected by sclerotic changes, vascular calcifications, obesity and other artifacts compared to DXA[40, 41]. A few prior studies have investigated the relationship between spine vBMD and post-operative outcomes, though the majority have utilized measurements obtained from post-operative scans. In a retrospective study of patients who underwent lumbar interbody fusion[42], those with pseudoarthrosis tended to have lower vBMD, on post-operative CT, when compared to patients with successful fusion[42]. In another study, 78% of patients with low BMD by CT had hardware instability, adjacent fractures and other complications[29]. Our group recently demonstrated, in a retrospective study of a different cohort, that patients with low pre-operative spine vBMD at the spine had higher rates of post-operative skeletal complications, and earlier occurrence of complications than those with higher vBMD[39]. Those results are consistent with our findings in this prospective cohort. Both suggest that the quality of bone is integral to early surgical success.
We found that factors other than vBMD and microarchitecture were also related to risk of developing skeletal complications. Postmenopausal women had higher rates of complications, likely related to the combined detrimental skeletal effects of estrogen deficiency and aging. As only one male subject and one premenopausal subject had complications we did not have the power to perform additional analyses compairog risk among these groups Greater number of levels instrumented was associated with increased complications, as has been shown in other cohorts[43, 44]. We did not find that patients having revision surgery had higher rates of complications. While medical complications commonly occur in the first few months after revision surgery[45], few studies have investigated the timing of skeletal complications after revisions. It is conceivable that we would have detected higher rates of skeletal complications in patients after revisions with a longer duration of follow-up. Previous studies have suggested that tobacco use impedes fusion[39, 46, 47]. We did not find a relationship between tobacco use and complications, possibly because very few of our subjects, less than 10%, were current smokers. Several studies have shown less favorable outcomes in obese patients after spine surgery[48–50], including increased rates of revision[39] and other complications. In this cohort we did not find an association between obesity and complication rates. Interestingly, we did not observe a relationship between teriparatide use and complications. We had few patients in our cohort using teriparatide which may have precluded us from finding a relationship. While some studies have demonstrated a beneficial effect of teriparatide treatment on fusion[51–53], these findings are not uniform[54], and most have been small and retrospective[51–53, 55–58]. Further prospective randomized studies are needed to investigate the relationship between anabolic agents and post-operative outcomes.
One of the most common skeletal complications in our cohort was PJK, the development of kyphosis between the transition of fused and unfused spinal segments. In addition to increased deformity, PJK can result in severe pain and neurologic deficits and ultimately necessitate revision surgery. Prior studies have reported an incidence of PJK is between 20% to 40%[19, 59–65]. Female gender, increasing age, and obesity are risk factors associated with the development of post-operative PJK[62, 66, 67]. Low BMD by DXA was related to risk of PJK in some[68], but not all studies[69]. The discrepancy in studies relating aBMD and PJK may relate to the limited ability of DXA to detect skeletal deficits in this population.
There are several important limitations to this work. Our sample size was relatively small. Due to our sample size, and subsequent small number of patients enrolled with different comorbidities, we were unable to investigate whether the presence of specific underlying diseases, such as diabetes, impacted the relationship between microarchitecture and complications. The rate of complications in our cohort may have been higher than those in the general population of patients presenting for spine fusion for several reasons. We enrolled patients having multi-level fusion, in whom complication risks may be higher[43, 44]. Our institution is an international referral center for complex cases. Moreover, we included both patients having initial and revision surgeries. Many patients had an initial surgery elsewhere and then presented to our institution for revision. Therefore, we do not have information about bone health at the time of initial surgery for those patients. While we did not find that there was a difference in complication rates between initial and revision surgery cases, we may have detected differences with a longer duration of follow-up or larger sample size. Others have found that patients who have already failed one surgery are at higher risk for future complications[70]. While our short follow-up duration was intentional as we were specifically interested in investigating bone quality and early complications, further investigation of the relationship of bone quality to long-term complications and fusion rates is needed. Due to our observational design, some patients in the cohort were being treated with osteoporosis medications. As the number of treated patients was small and because treatment decisions were made by the clinical team and not random allocation, we cannot draw conclusions about the impact of these treatments on post-operative outcomes. While our results provide new information that abnormal microarchitectcure is a risk factor for complications after lumbar spine fusion surgery, it is not clear whether these relationships would extend to cervical fusion procedures as well. This is an important question for future studies.
In conclusion, patients with low pre-operative vBMD and abnormal microarchitecture had higher rates of skeletal complications after spine fusion surgery. Abnormalities of both trabecular and cortical microarchitecture were associated the development of complications within the first six months following surgery. These results help to provide a mechanism for early skeletal complications after fusion. They suggest that both trabecular and cortical bone make important contributions to the stability of inserted hardware, as well as the ability to withstand the modified loads of the new surgical construct, so that new bone can be formed. Given the burgeoning number of fusion surgeries performed in older patients, further studies are necessary to investigate the relationship between bone microarchitecture and post-operative complications, as well as optimal strategies for their prevention.
Table 2.
Volumetric Bone Mineral Density and Microarchitecture in Patients with and without Complications (mean ± SD)
| RADIUS | TIBIA | |||||
|---|---|---|---|---|---|---|
| No Complications | Complications | p-value | No Complications | Complications | p-value | |
| Total Area (mm) | 324 ± 84 | 273 ± 35 | 0.004 | 791 ± 190 | 723 ± 107 | 0.263 |
| Total Density (mgHA/cm3) | 293 ± 77 | 248 ± 57 | 0.077 | 269 ± 74 | 224 ± 45 | 0.063 |
| Trabecular Density (mgHA/cm3) | 153 ± 60 | 113 ± 47 | 0.045 | 154 ± 56 | 124 ± 33 | 0.096 |
| Cortical Density (mgHA/cm3) | 865 ± 73 | 880 ± 55 | 0.533 | 838 ± 84 | 836 ± 68 | 0.942 |
| Cortical Thickness (mm) | 1.02 ± 0.27 | 0.85 ± 0.17 | 0.063 | 1.44 ± 0.35 | 1.19 ± 0.25 | 0.031 |
| Cortical Porosity (%) | 1.18 ± 0.83 | 0.95 ± 0.70 | 0.390 | 3.85 ± 1.82 | 3.50 ± 2.01 | 0.584 |
| Trabecular Number (1/mm) | 1.40 ± 0.32 | 1.18 ± 0.31 | 0.046 | 1.27 ± 0.32 | 1.21 ± 0.31 | 0.552 |
| Trabecular Thickness (mm) | 0.24 ± 0.02 | 0.22 ± 0.01 | 0.003 | 0.25 ± 0.03 | 0.23 ± 0.02 | 0.054 |
| Trabecular Separation (mm) | 0.72 ± 0.22 | 0.92 ± 0.44 | 0.173 | 0.79 ± 0.22 | 0.88 ± 0.28 | 0.271 |
Acknowledgements
This research was supported by UL1TR002384 NIH/NCATS awarded by the Weill Cornell CTSC and the Marina Kellen French Foundation
Disclosures
Dr. Kim reports personal fees from Alphatec, personal fees from K2M, personal fees from Zimmerbiomet, outside the submitted work.
Mr. Dash has nothing to disclose.
Dr. Cunningham reports grants and personal fees from K2M, personal fees from Zimmerbiomet, grants from Radius Health, grants from RTI, outside the submitted work.
Dr. Schwab reports grants from ISSGF, personal fees from MSD, personal fees from K2M, personal fees from ZimmerBiomet, personal fees from Globus Medical, Inc., during the conduct of the study; grants and personal fees from MSD, grants and personal fees from K2M, grants and personal fees from ZimmerBiomet, grants and personal fees from NuVasive, grants and personal fees from Medicrea, grants and personal fees from Globus Medical, Inc., grants from ISSGF, grants from DePuy, personal fees from Hospital for Special Surgery, personal fees from Staysis Medical, personal fees from VFT Solutions, outside the submitted work.
Dr. Dowdell has nothing to disclose.
Mr. Harrison has nothing to disclose.
Ms. Zaworski has nothing to disclose.
Ms. Krez has nothing to disclose.
Dr. Lafage reports personal fees from Johnson and Johnson, personal fees from K2M, personal fees from NuVasive, personal fees from Implanet, personal fees from Globus Medical, Inc., personal fees from AO, other from Nemaris Inc., outside the submitted work.
Ms. Agarwal has nothing to disclose.
Dr. Carlson reports personal fees from Globus Medical, Inc., personal fees from Prosidyan, outside the submitted work.
Mr. McMahon reports personal fees from Hospital for Special Surgery, during the conduct of the study; personal fees from Hospital for Special Surgery, outside the submitted work.
Dr. Stein reports grants from Novartis, grants from Radius Health, outside the submitted work.
References
- [1].Hall MJ, DeFrances CJ, Williams SN, Golosinskiy A, Schwartzman A, National Hospital Discharge Survey: 2007 summary, Natl Health Stat Report (29) (2010) 1–20, 24. [PubMed] [Google Scholar]
- [2].Rajaee SS, Bae HW, Kanim LE, Delamarter RB, Spinal fusion in the United States: analysis of trends from 1998 to 2008, Spine (Phila Pa 1976) 37(1) (2012) 67–76. [DOI] [PubMed] [Google Scholar]
- [3].Martin BI, Mirza SK, Spina N, Spiker WR, Lawrence B, Brodke DS, Trends in Lumbar Fusion Procedure Rates and Associated Hospital Costs for Degenerative Spinal Diseases in the United States, 2004 to 2015, Spine (Phila Pa 1976) 44(5) (2019) 369–376. [DOI] [PubMed] [Google Scholar]
- [4].Uribe JS, Deukmedjian AR, Mummaneni PV, Fu KM, Mundis GM Jr., Okonkwo DO, Kanter AS, Eastlack R, Wang MY, Anand N, Fessler RG, La Marca F, Park P, Lafage V, Deviren V, Bess S, Shaffrey CI, International Spine Study G, Complications in adult spinal deformity surgery: an analysis of minimally invasive, hybrid, and open surgical techniques, Neurosurg Focus 36(5) (2014) E15. [DOI] [PubMed] [Google Scholar]
- [5].Dede O, Thuillier D, Pekmezci M, Ames CP, Hu SS, Berven SH, Deviren V, Revision surgery for lumbar pseudarthrosis, Spine J 15(5) (2015) 977–82. [DOI] [PubMed] [Google Scholar]
- [6].Yoon ST, Boden SD, Spine fusion by gene therapy, Gene Ther 11(4) (2004) 360–7. [DOI] [PubMed] [Google Scholar]
- [7].Bess S, Boachie-Adjei O, Burton D, Cunningham M, Shaffrey C, Shelokov A, Hostin R, Schwab F, Wood K, Akbarnia B, International Spine Study G, Pain and disability determine treatment modality for older patients with adult scoliosis, while deformity guides treatment for younger patients, Spine (Phila Pa 1976) 34(20) (2009) 2186–90. [DOI] [PubMed] [Google Scholar]
- [8].Pichelmann MA, Lenke LG, Bridwell KH, Good CR, O’Leary PT, Sides BA, Revision rates following primary adult spinal deformity surgery: six hundred forty-three consecutive patients followed-up to twenty-two years postoperative, Spine (Phila Pa 1976) 35(2) (2010) 219–26. [DOI] [PubMed] [Google Scholar]
- [9].Goz V, Weinreb JH, McCarthy I, Schwab F, Lafage V, Errico TJ, Perioperative complications and mortality after spinal fusions: analysis of trends and risk factors, Spine (Phila Pa 1976) 38(22) (2013) 1970–6. [DOI] [PubMed] [Google Scholar]
- [10].Martin BI, Deyo RA, Mirza SK, Turner JA, Comstock BA, Hollingworth W, Sullivan SD, Expenditures and health status among adults with back and neck problems, JAMA 299(6) (2008) 656–64. [DOI] [PubMed] [Google Scholar]
- [11].Schuit SC, van der Klift M, Weel AE, de Laet CE, Burger H, Seeman E, Hofman A, Uitterlinden AG, van Leeuwen JP, Pols HA, Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study, Bone 34(1) (2004) 195–202. [DOI] [PubMed] [Google Scholar]
- [12].Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Cummings SR, Osteoporotic Fractures Research G, BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures, J Bone Miner Res 18(11) (2003) 1947–54. [DOI] [PubMed] [Google Scholar]
- [13].Blake GM, Fogelman I, The role of DXA bone density scans in the diagnosis and treatment of osteoporosis, Postgrad Med J 83(982) (2007) 509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brunerova L, Ronova P, Veresova J, Beranova P, Potoekova J, Kasalicky P, Rychlik I, Osteoporosis and Impaired Trabecular Bone Score in Hemodialysis Patients, Kidney Blood Press Res 41(3) (2016) 345–54. [DOI] [PubMed] [Google Scholar]
- [15].Hans D, Barthe N, Boutroy S, Pothuaud L, Winzenrieth R, Krieg MA, Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae, J Clin Densitom 14(3) (2011) 302–12. [DOI] [PubMed] [Google Scholar]
- [16].McCloskey EV, Oden A, Harvey NC, Leslie WD, Hans D, Johansson H, Barkmann R, Boutroy S, Brown J, Chapurlat R, Elders PJM, Fujita Y, Gluer CC, Goltzman D, Iki M, Karlsson M, Kindmark A, Kotowicz M, Kurumatani N, Kwok T, Lamy O, Leung J, Lippuner K, Ljunggren O, Lorentzon M, Mellstrom D, Merlijn T, Oei L, Ohlsson C, Pasco JA, Rivadeneira F, Rosengren B, Sornay-Rendu E, Szulc P, Tamaki J, Kanis JA, A Meta-Analysis of Trabecular Bone Score in Fracture Risk Prediction and Its Relationship to FRAX, J Bone Miner Res 31(5) (2016) 940–8. [DOI] [PubMed] [Google Scholar]
- [17].Buie HR, Campbell GM, Klinck RJ, MacNeil JA, Boyd SK, Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis, Bone 41(4) (2007) 505–15. [DOI] [PubMed] [Google Scholar]
- [18].Agarwal S, Rosete F, Zhang C, McMahon DJ, Guo XE, Shane E, Nishiyama KK, In vivo assessment of bone structure and estimated bone strength by first- and second-generation HR-pQCT, Osteoporos Int 27(10) (2016) 2955–66. [DOI] [PubMed] [Google Scholar]
- [19].Glattes RC, Bridwell KH, Lenke LG, Kim YJ, Rinella A, Edwards C 2nd, Proximal junctional kyphosis in adult spinal deformity following long instrumented posterior spinal fusion: incidence, outcomes, and risk factor analysis, Spine (Phila Pa 1976) 30(14) (2005) 1643–9. [DOI] [PubMed] [Google Scholar]
- [20].Sornay-Rendu E, Cabrera-Bravo JL, Boutroy S, Munoz F, Delmas PD, Severity of vertebral fractures is associated with alterations of cortical architecture in postmenopausal women, J Bone Miner Res 24(4) (2009) 737–43. [DOI] [PubMed] [Google Scholar]
- [21].Stein EM, Liu XS, Nickolas TL, Cohen A, McMahon DJ, Zhou B, Zhang C, Kamanda-Kosseh M, Cosman F, Nieves J, Guo XE, Shane E, Microarchitectural abnormalities are more severe in postmenopausal women with vertebral compared to nonvertebral fractures, J Clin Endocrinol Metab 97(10) (2012) E1918–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang J, Stein EM, Zhou B, Nishiyama KK, Yu YE, Shane E, Guo XE, Deterioration of trabecular plate-rod and cortical microarchitecture and reduced bone stiffness at distal radius and tibia in postmenopausal women with vertebral fractures, Bone 88 (2016) 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Roux JP, Wegrzyn J, Arlot ME, Guyen O, Delmas PD, Chapurlat R, Bouxsein ML, Contribution of trabecular and cortical components to biomechanical behavior of human vertebrae: an ex vivo study, J Bone Miner Res 25(2) (2010) 356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Seeman E, Delmas PD, Bone quality--the material and structural basis of bone strength and fragility, The New England journal of medicine 354(21) (2006) 2250–61. [DOI] [PubMed] [Google Scholar]
- [25].Balci A, Kalemci O, Kaya FG, Akyoldas G, Yucesoy K, Ozaksoy D, Early and long-term changes in adjacent vertebral body bone mineral density determined by quantitative computed tomography after posterolateral fusion with transpedicular screw fixation, Clin Neurol Neurosurg 145 (2016) 84–8. [DOI] [PubMed] [Google Scholar]
- [26].Wang H, Ma L, Yang D, Wang T, Yang S, Wang Y, Wang Q, Zhang F, Ding W, Incidence and risk factors for the progression of proximal junctional kyphosis in degenerative lumbar scoliosis following long instrumented posterior spinal fusion, Medicine (Baltimore) 95(32) (2016) e4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu FY, Wang T, Yang SD, Wang H, Yang DL, Ding WY, Incidence and risk factors for proximal junctional kyphosis: a meta-analysis, Eur Spine J 25(8) (2016) 2376–83. [DOI] [PubMed] [Google Scholar]
- [28].Bjerke BT, Zarrabian M, Aleem IS, Fogelson JL, Currier BL, Freedman BA, Bydon M, Nassr A, Incidence of Osteoporosis-Related Complications Following Posterior Lumbar Fusion, Global Spine J 8(6) (2018) 563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Formby PM, Kang DG, Helgeson MD, Wagner SC, Clinical and Radiographic Outcomes of Transforaminal Lumbar Interbody Fusion in Patients with Osteoporosis, Global spine journal 6(7) (2016) 660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bredow J, Boese CK, Werner CM, Siewe J, Lohrer L, Zarghooni K, Eysel P, Scheyerer MJ, Predictive validity of preoperative CT scans and the risk of pedicle screw loosening in spinal surgery, Archives of orthopaedic and trauma surgery 136(8) (2016) 1063–7. [DOI] [PubMed] [Google Scholar]
- [31].Schwaiger BJ, Gersing AS, Baum T, Noel PB, Zimmer C, Bauer JS, Bone mineral density values derived from routine lumbar spine multidetector row CT predict osteoporotic vertebral fractures and screw loosening, AJNR. American journal of neuroradiology 35(8) (2014) 1628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tempel ZJ, Gandhoke GS, Okonkwo DO, Kanter AS, Impaired bone mineral density as a predictor of graft subsidence following minimally invasive transpsoas lateral lumbar interbody fusion, Eur Spine J 24 Suppl 3 (2015) 414–9. [DOI] [PubMed] [Google Scholar]
- [33].Silva BC, Leslie WD, Resch H, Lamy O, Lesnyak O, Binkley N, McCloskey EV, Kanis JA, Bilezikian JP, Trabecular bone score: a noninvasive analytical method based upon the DXA image, J Bone Miner Res 29(3) (2014) 518–30. [DOI] [PubMed] [Google Scholar]
- [34].Pappou IP, Girardi FP, Sandhu HS, Parvataneni HK, Cammisa FP Jr., Schneider R, Frelinghuysen P, Lane JM, Discordantly high spinal bone mineral density values in patients with adult lumbar scoliosis, Spine (Phila Pa 1976) 31(14) (2006) 1614–20. [DOI] [PubMed] [Google Scholar]
- [35].Sarioglu O, Gezer S, Sarioglu FC, Koremezli N, Kara T, Akcali O, Ozaksoy D, Balci A, Evaluation of vertebral bone mineral density in scoliosis by using quantitative computed tomography, Pol J Radiol 84 (2019) e131–e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cheng JC, Guo X, Sher AH, Persistent osteopenia in adolescent idiopathic scoliosis. A longitudinal follow up study, Spine (Phila Pa 1976) 24(12) (1999) 1218–22. [DOI] [PubMed] [Google Scholar]
- [37].Cheng JC, Hung VW, Lee WT, Yeung HY, Lam TP, Ng BK, Guo X, Qin L, Persistent osteopenia in adolescent idiopathic scoliosis--longitudinal monitoring of bone mineral density until skeletal maturity, Stud Health Technol Inform 123 (2006) 47–51. [PubMed] [Google Scholar]
- [38].Burch S, Feldstein M, Hoffmann PF, Keaveny TM, Prevalence of Poor Bone Quality in Women Undergoing Spinal Fusion Using Biomechanical-CT Analysis, Spine (Phila Pa 1976) 41(3) (2016) 246–52. [DOI] [PubMed] [Google Scholar]
- [39].Liu Y, Dash A, Krez A, Kim HJ, Cunningham M, Schwab F, Hughes A, Carlson B, Samuel A, Marty E, Moore H, McMahon DJ, Carrino JA, Bockman RS, Stein EM, Low volumetric bone density is a risk factor for early complications after spine fusion surgery, Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA (2020) 10.1007/s00198-019-05245-7. [DOI] [PubMed] [Google Scholar]
- [40].Farhat GN, Cauley JA, Matthews KA, Newman AB, Johnston J, Mackey R, Edmundowicz D, Sutton-Tyrrell K, Volumetric BMD and vascular calcification in middle-aged women: the Study of Women’s Health Across the Nation, J Bone Miner Res 21(12) (2006) 1839–46. [DOI] [PubMed] [Google Scholar]
- [41].Rehman Q, Lang T, Modin G, Lane NE, Quantitative computed tomography of the lumbar spine, not dual x-ray absorptiometry, is an independent predictor of prevalent vertebral fractures in postmenopausal women with osteopenia receiving long-term glucocorticoid and hormone-replacement therapy, Arthritis Rheum 46(5) (2002) 1292–7. [DOI] [PubMed] [Google Scholar]
- [42].Schreiber JJ, Hughes AP, Taher F, Girardi FP, An association can be found between hounsfield units and success of lumbar spine fusion, HSS journal : the musculoskeletal journal of Hospital for Special Surgery 10(1) (2014) 25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mahesh B, Upendra B, Vijay S, Kumar GA, Reddy S, Complication rate during multilevel lumbar fusion in patients above 60 years, Indian J Orthop 51(2) (2017) 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rollinghoff M, Schluter-Brust K, Groos D, Sobottke R, Michael JW, Eysel P, Delank KS, Mid-range outcomes in 64 consecutive cases of multilevel fusion for degenerative diseases of the lumbar spine, Orthop Rev (Pavia) 2(1) (2010) e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sing DC, Yue JK, Metz LN, Winkler EA, Zhang WR, Burch S, Berven SH, Obesity Is an Independent Risk Factor of Early Complications After Revision Spine Surgery, Spine (Phila Pa 1976) 41(10) (2016) E632–40. [DOI] [PubMed] [Google Scholar]
- [46].How NE, Street JT, Dvorak MF, Fisher CG, Kwon BK, Paquette S, Smith JS, Shaffrey CI, Ailon T, Pseudarthrosis in adult and pediatric spinal deformity surgery: a systematic review of the literature and meta-analysis of incidence, characteristics, and risk factors, Neurosurg Rev 42(2) (2019) 319–336. [DOI] [PubMed] [Google Scholar]
- [47].Phan K, Fadhil M, Chang N, Giang G, Gragnaniello C, Mobbs RJ, Effect of Smoking Status on Successful Arthrodesis, Clinical Outcome, and Complications After Anterior Lumbar Interbody Fusion (ALIF), World Neurosurg 110 (2018) e998–e1003. [DOI] [PubMed] [Google Scholar]
- [48].Jiang J, Teng Y, Fan Z, Khan S, Xia Y, Does obesity affect the surgical outcome and complication rates of spinal surgery? A meta-analysis, Clin Orthop Relat Res 472(3) (2014) 968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Patel N, Bagan B, Vadera S, Maltenfort MG, Deutsch H, Vaccaro AR, Harrop J, Sharan A, Ratliff JK, Obesity and spine surgery: relation to perioperative complications, J Neurosurg Spine 6(4) (2007) 291–7. [DOI] [PubMed] [Google Scholar]
- [50].Yadla S, Malone J, Campbell PG, Maltenfort MG, Harrop JS, Sharan AD, Vaccaro AR, Ratliff JK, Obesity and spine surgery: reassessment based on a prospective evaluation of perioperative complications in elective degenerative thoracolumbar procedures, Spine J 10(7) (2010) 581–7. [DOI] [PubMed] [Google Scholar]
- [51].Ohtori S, Inoue G, Orita S, Yamauchi K, Eguchi Y, Ochiai N, Kishida S, Kuniyoshi K, Aoki Y, Nakamura J, Ishikawa T, Miyagi M, Kamoda H, Suzuki M, Kubota G, Sakuma Y, Oikawa Y, Inage K, Sainoh T, Takaso M, Ozawa T, Takahashi K, Toyone T, Teriparatide accelerates lumbar posterolateral fusion in women with postmenopausal osteoporosis: prospective study, Spine (Phila Pa 1976) 37(23) (2012) E1464–8. [DOI] [PubMed] [Google Scholar]
- [52].Inoue G, Ueno M, Nakazawa T, Imura T, Saito W, Uchida K, Ohtori S, Toyone T, Takahira N, Takaso M, Teriparatide increases the insertional torque of pedicle screws during fusion surgery in patients with postmenopausal osteoporosis, J Neurosurg Spine 21(3) (2014) 425–31. [DOI] [PubMed] [Google Scholar]
- [53].Ohtori S, Inoue G, Orita S, Yamauchi K, Eguchi Y, Ochiai N, Kishida S, Kuniyoshi K, Aoki Y, Nakamura J, Ishikawa T, Miyagi M, Kamoda H, Suzuki M, Kubota G, Sakuma Y, Oikawa Y, Inage K, Sainoh T, Takaso M, Toyone T, Takahashi K, Comparison of teriparatide and bisphosphonate treatment to reduce pedicle screw loosening after lumbar spinal fusion surgery in postmenopausal women with osteoporosis from a bone quality perspective, Spine (Phila Pa 1976) 38(8) (2013) E487–92. [DOI] [PubMed] [Google Scholar]
- [54].Jespersen AB, Andresen ADK, Jacobsen MK, Andersen MO, Carreon LY, Does Systemic Administration of Parathyroid Hormone After Noninstrumented Spinal Fusion Surgery Improve Fusion Rates and Fusion Mass in Elderly Patients Compared to Placebo in Patients With Degenerative Lumbar Spondylolisthesis?, Spine (Phila Pa 1976) 44(3) (2019) 157–162. [DOI] [PubMed] [Google Scholar]
- [55].Kim JW, Park SW, Kim YB, Ko MJ, The Effect of Postoperative Use of Teriparatide Reducing Screw Loosening in Osteoporotic Patients, J Korean Neurosurg Soc 61(4) (2018) 494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ohtori S, Orita S, Yamauchi K, Eguchi Y, Ochiai N, Kuniyoshi K, Aoki Y, Nakamura J, Miyagi M, Suzuki M, Kubota G, Inage K, Sainoh T, Sato J, Shiga Y, Abe K, Fujimoto K, Kanamoto H, Inoue G, Takahashi K, More than 6 Months of Teriparatide Treatment Was More Effective for Bone Union than Shorter Treatment Following Lumbar Posterolateral Fusion Surgery, Asian Spine J 9(4) (2015) 573–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yagi M, Ohne H, Konomi T, Fujiyoshi K, Kaneko S, Komiyama T, Takemitsu M, Yato Y, Machida M, Asazuma T, Teriparatide improves volumetric bone mineral density and fine bone structure in the UIV+1 vertebra, and reduces bone failure type PJK after surgery for adult spinal deformity, Osteoporos Int 27(12) (2016) 3495–3502. [DOI] [PubMed] [Google Scholar]
- [58].Ebata S, Takahashi J, Hasegawa T, Mukaiyama K, Isogai Y, Ohba T, Shibata Y, Ojima T, Yamagata Z, Matsuyama Y, Haro H, Role of Weekly Teriparatide Administration in Osseous Union Enhancement within Six Months After Posterior or Transforaminal Lumbar Interbody Fusion for Osteoporosis-Associated Lumbar Degenerative Disorders: A Multicenter, Prospective Randomized Study, J Bone Joint Surg Am 99(5) (2017) 365–372. [DOI] [PubMed] [Google Scholar]
- [59].Denis F, Sun EC, Winter RB, Incidence and risk factors for proximal and distal junctional kyphosis following surgical treatment for Scheuermann kyphosis: minimum five-year follow-up, Spine (Phila Pa 1976) 34(20) (2009) E729–34. [DOI] [PubMed] [Google Scholar]
- [60].Hassanzadeh H, Gupta S, Jain A, El Dafrawy MH, Skolasky RL, Kebaish KM, Type of Anchor at the Proximal Fusion Level Has a Significant Effect on the Incidence of Proximal Junctional Kyphosis and Outcome in Adults After Long Posterior Spinal Fusion, Spine Deform 1(4) (2013) 299–305. [DOI] [PubMed] [Google Scholar]
- [61].Kim HJ, Bridwell KH, Lenke LG, Park MS, Ahmad A, Song KS, Piyaskulkaew C, Hershman S, Fogelson J, Mesfin A, Proximal junctional kyphosis results in inferior SRS pain subscores in adult deformity patients, Spine (Phila Pa 1976) 38(11) (2013) 896–901. [DOI] [PubMed] [Google Scholar]
- [62].Kim HJ, Lenke LG, Shaffrey CI, Van Alstyne EM, Skelly AC, Proximal junctional kyphosis as a distinct form of adjacent segment pathology after spinal deformity surgery: a systematic review, Spine (Phila Pa 1976) 37(22 Suppl) (2012) S144–64. [DOI] [PubMed] [Google Scholar]
- [63].Kim YJ, Bridwell KH, Lenke LG, Glattes CR, Rhim S, Cheh G, Proximal junctional kyphosis in adult spinal deformity after segmental posterior spinal instrumentation and fusion: minimum five-year follow-up, Spine (Phila Pa 1976) 33(20) (2008) 2179–84. [DOI] [PubMed] [Google Scholar]
- [64].Lee JH, Kim JU, Jang JS, Lee SH, Analysis of the incidence and risk factors for the progression of proximal junctional kyphosis following surgical treatment for lumbar degenerative kyphosis: minimum 2-year follow-up, Br J Neurosurg 28(2) (2014) 252–8. [DOI] [PubMed] [Google Scholar]
- [65].Yagi M, King AB, Boachie-Adjei O, Incidence, risk factors, and natural course of proximal junctional kyphosis: surgical outcomes review of adult idiopathic scoliosis. Minimum 5 years of follow-up, Spine (Phila Pa 1976) 37(17) (2012) 1479–89. [DOI] [PubMed] [Google Scholar]
- [66].Kim HJ, Bridwell KH, Lenke LG, Park MS, Song KS, Piyaskulkaew C, Chuntarapas T, Patients with proximal junctional kyphosis requiring revision surgery have higher postoperative lumbar lordosis and larger sagittal balance corrections, Spine (Phila Pa 1976) 39(9) (2014) E576–80. [DOI] [PubMed] [Google Scholar]
- [67].Kim HJ, Iyer S, Proximal Junctional Kyphosis, J Am Acad Orthop Surg 24(5) (2016) 318–26. [DOI] [PubMed] [Google Scholar]
- [68].Zou L, Liu J, Lu H, Characteristics and risk factors for proximal junctional kyphosis in adult spinal deformity after correction surgery: a systematic review and meta-analysis, Neurosurg Rev 42(3) (2019) 671–682. [DOI] [PubMed] [Google Scholar]
- [69].Yagi M, Akilah KB, Boachie-Adjei O, Incidence, risk factors and classification of proximal junctional kyphosis: surgical outcomes review of adult idiopathic scoliosis, Spine (Phila Pa 1976) 36(1) (2011) E60–8. [DOI] [PubMed] [Google Scholar]
- [70].Rajaee SS, Kanim LE, Bae HW, National trends in revision spinal fusion in the USA: patient characteristics and complications, Bone Joint J 96-B(6) (2014) 807–16. [DOI] [PubMed] [Google Scholar]