Abstract
The AcrAB-TolC efflux pump plays an intrinsic role in resistance to hydrophobic solvents in Escherichia coli. E. coli OST5500 is hypersensitive to solvents due to inactivation of the acrB gene by insertion of IS30. Suppressor mutants showing high solvent resistance were isolated from OST5500. These mutants produced high levels of AcrE and AcrF proteins, which were not produced in OST5500, and in each mutant an insertion sequence (IS1 or IS2) was found integrated upstream of the acrEF operon, coding for the two proteins. The suppressor mutants lost solvent resistance on inactivation of the acrEF operon. The solvent hypersensitivity of OST5500 was suppressed by introduction of the acrEF operon with IS1 or IS2 integrated upstream but not by introduction of the operon lacking the integrated IS. It was concluded that IS integration activated acrEF, resulting in functional complementation of the acrB mutation. The acrB mutation was also complemented by a plasmid containing acrF or acrEF under the control of Plac. The wild-type tolC gene was found to be essential for complementation of the acrB mutation by acrEF. Thus, it is concluded that in these cells a combination of the proteins AcrA, AcrF, and TolC or the proteins AcrE, AcrF, and TolC is functional in solvent efflux instead of the AcrAB-TolC efflux pump.
Hydrophobic organic solvents with log POW values of 2 to 5 can inhibit the growth of microorganisms. When microorganisms are incubated in the presence of a large volume of solvent, the magnitude of growth inhibition is inversely correlated with the log POW of the solvent (2, 9), which is the common logarithm of the partition coefficient (POW) of a solvent, measured in a two-liquid phase system composed of n-octanol and water. It is known that this value is correlated with solvent hydrophobicity (16).
The solvent resistance of a microorganism is determined genetically. In Escherichia coli, the AcrAB-TolC efflux pump plays an intrinsic role in the solvent resistance mechanism (3, 30, 31). In E. coli exposed to a large volume of a particular solvent, the intracellular concentration of the solvent is maintained at a low level (30). This pump consists of three components, AcrA, AcrB, and TolC (8, 18, 19). The inner membrane protein AcrB belongs to the RND (resistance-nodulation-cell division) transporter family and is thought to be a proton antiporter (23, 28, 33). The periplasmic lipoprotein AcrA belongs to the membrane fusion protein family and is a highly asymmetric protein capable of spanning the periplasmic space (6, 32). The outer membrane protein TolC spans the membrane and the periplasm and functions as a channel tunnel (8, 14). Deletion of acrAB or tolC decreases the solvent resistance of E. coli.
The acrEF operon encodes the components of an efflux pump other than the AcrAB efflux pump. AcrE and AcrF are highly homologous to AcrA and AcrB, respectively (12, 18). On the chromosome of E. coli, the acrAB and acrEF loci are located at 10.5 and 73.5 min, respectively (5, 13, 26). The acrEF operon was cloned accidentally as envCD genes because AcrEF functionally complemented the crystal violet sensitivity of the envC mutant (27) of E. coli (11). High expression of the acrEF operon carried on a plasmid suppresses the hypersusceptible phenotype of envC or acrAB mutants to multiple drugs. On the other hand, acrEF null mutants do not show drug sensitivity (10, 20). Therefore, it is not likely that the acrEF operon contributes to the drug resistance of E. coli. The acrEF operon is considered to be expressed only weakly in E. coli under laboratory conditions (20).
In this report, we show that the acrEF operon in E. coli is expressed at a high level on integration of IS1 or IS2 into the chromosome at a site upstream of the operon, resulting in suppression of the solvent-hypersensitive phenotype caused by the acrB mutation. In addition, we show that AcrF also functions with AcrA and that AcrEF requires TolC to improve the solvent resistance of E. coli.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The E. coli strains and plasmids used in this study are listed in Table 1. OST5500 and AX727 were obtained from the National Institute of Genetics (Mishima, Japan) as ME7783 and JE8495, respectively. A number of acrEF null mutants (JA300F, JA300AF, OST5500F, DK3402F, and DK4201F) were constructed by P1 transduction of kanamycin resistance with JZM221A1 (ΔacrEF::Tn5) as the donor strain. Disruption of the acrEF genes was confirmed by PCR analysis using chromosomal DNA prepared from each strain as the template, with the combination of primers 2 and 4 described below. Low-copy-number vectors pMW118, pMW119, and pMW219 (GenBank accession no. AB005475, AB005476, and AB005478, respectively) were purchased from Nippon Gene Co. (Tokyo, Japan). These vectors are derived from pSC101, and the copy numbers are 1 to 5 per cell. pMW118 and pMW119 each contains an ampicillin resistance gene, and pMW219 contains a kanamycin resistance gene. The high-copy-number vector pBluescript KS(+) was purchased from Toyobo Biochemical, Inc. (Osaka, Japan).
TABLE 1.
E. coli strains and plasmids used in this study
| Strain or plasmid | Genotype and solvent resistance | Source or reference |
|---|---|---|
| Strains | ||
| AX727 | F−dnaX str thi lac | 7 |
| DK3402 | Same as OST5500, but resistant to cyclohexane | This study |
| DK3402F | Same as DK3402, but ΔacrEF::Tn5 | This study |
| DK4201 | Same as OST5500, but resistant to diphenyl ether | This study |
| DK4201F | Same as DK4201, but ΔacrEF::Tn5 | This study |
| JA300 | F−thr leuB6 trpC1117 thi rpsL20 hsdS | 3 |
| JA300A | Same as JA300, but ΔacrAB::cat | 30 |
| JA300F | Same as JA300, but ΔacrEF::Tn5 | This study |
| JA300AF | Same as JA300, but ΔacrAB::cat ΔacrEF::Tn5 | This study |
| JA300T | Same as JA300, but ΔtolC | 3 |
| JA300TA | Same as JA300, but ΔtolC and ΔacrAB::cat | 30 |
| OST5500 | HfrC metB resistant to n-nonane and sensitive to n-octane | ME7783a |
| OST5500F | Same as OST5500, but ΔacrEF::Tn5 | This study |
| Plasmids | ||
| pEFrk | SalI-HindIII fragment containing the acrEF operon from OST5500 in pMW219 oriented in the direction opposite that of Plac | This study |
| pEF34 | SalI-SacII fragment containing the acrSE-intergenic region from DK3402 in pEFrk | This study |
| pEF42 | SalI-SacII fragment containing the acrSE-intergenic region from DK4201 in pEFrk | This study |
| pEF55 | SalI-SacII fragment containing the acrSE-intergenic region from OST5500 in pEFrk | This study |
| pHAcrF | XhoI-HindIII fragment containing the acrF gene in pBluescript II oriented in the same direction as Plac | This study |
| pLAcrAB | AvaI-EcoT22I fragment containing the acrAB genes in pMW119 oriented in the same direction as Plac | This study |
| pLAcrA | XhoI-ClaI fragment containing the acrA gene in pMW119 oriented in the same direction as Plac | This study |
| pLKSAcrA | AvaI-EcoRV fragment containing the acrA gene in pMW219 oriented in the same direction as Plac. aad9 is inserted at EcoRV-BamHI sites downstream of acrA | This study |
| pLAcrB | Bpu1102I-EcoT22I fragment containing the acrB gene in pMW119 oriented in the same direction as Plac | This study |
| pLAcrEF | SalI-HindIII fragment containing the acrEF operon in pMW118 oriented in the same direction as Plac | This study |
| pLAcrF | XhoI-HindIII fragment containing the acrF gene in pMW118 oriented in the same direction as Plac | This study |
| pLKTolC | EcoRI-HindIII containing the tolC gene in pMW219 oriented in the same direction as Plac | 3 |
Strain number of the National Institute of Genetics (Mishima, Japan).
Genetic analysis.
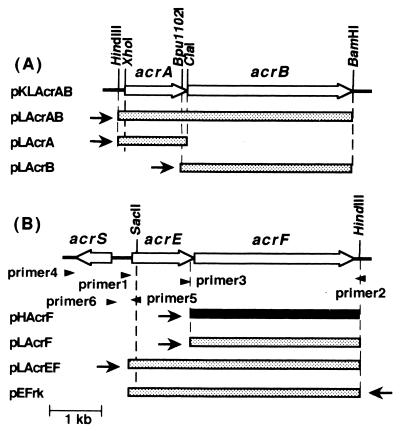
DNA manipulations, including the preparation of E. coli genomic DNA, plasmid preparation, restriction enzyme digestion and ligation, and transformation of E. coli, were carried out by standard methods. The insert DNA containing acrAB was recovered from pLKAcrAB, which was a pMW219 derivative containing acrAB (30), digested with XhoI and BamHI, and inserted in the same sites of vector pMW119 to yield pLAcrAB (Fig. 1). pLAcrAB was digested with ClaI and BamHI. The resulting 5.5-kb ClaI-BamHI DNA fragment containing the acrA region was blunted and ligated to make pLAcrA. pLKAcrAB was digested with EcoRV and BamHI to remove acrB and ligated with a SmaI-BamHI fragment obtained from pFW5 (25) to make pLKSAcrA. The SmaI-BamHI fragment contains aad9, a spectinomycin resistance marker. Thus, the resulting plasmid pLKSAcrA contains two markers, kanamycin resistance and spectinomycin resistance. pLAcrAB was digested with Bpu1102I and HindIII. The resulting 7.4-kb Bpu1102I-HindIII DNA fragment was blunted and ligated to make pLAcrB. In these plasmids, acrA and acrB were under the control of Plac. Also, the acrAB region was amplified using the chromosomal DNA from OST5500 as the template, and the presence of the mutation was confirmed by nucleotide sequencing. The primers used were designed according to the sequence deposited in GenBank (accession no. AE000152) as follows: a forward primer, 5′-AGTTATAAGCTTCATCAATAATCGACGC-3′ (bp 456 to 483 upstream of the initiation codon of acrA), and a reverse primer, 5′-TAAGGATCCACTTTTTCATACTAGCACT-3′ (bp 357 to 384 downstream of the stop codon of acrB).
FIG. 1.
Physical maps and construction of plasmids containing the acrAB (A) and acrEF (B) regions. Open arrows indicate the directions and extents of the acrA, acrB, acrS, acrE, and acrF genes. Arrowheads indicate the positions and directions of extension of the primers. Dotted bars represent the DNA fragments inserted into low-copy-number vectors, and a solid bar represents the DNA fragments inserted into a high-copy-number vector. Arrows indicate the direction of the lac promoter in the vectors.
The acrEF region was amplified by PCR. The primers used, shown in Fig. 1, were designed according to the sequence deposited in GenBank (accession no. AE000405), as follows: primer 1, 5′-CTGCGAGTTAACGCGTCGACTTTTTGGG-3′ (bp 36 to 63 upstream of the initiation codon of acrE); primer 2, 5′-GGGAGCGGGAACTATAGAAAGC-3′ (complementary to the region from bp 93 to 114 downstream of the stop codon of acrF); primer 3, 5′-CTACCGATACCCCTCGAGATAC-3′ (bp 25 to 46 upstream of the initiation codon of acrF); primer 4, 5′-AACGAAGCCGGTTAAGCGAAGTTG-3′ (bp 289 to 312 downstream of the stop codon of acrS); primer 5, 5′-AAGCCGCGGAGATCAGAATAAAGG-3′ (complementary to the region from bp 35 to 57 downstream of the initiation codon of the acrE structural gene); and primer 6, 5′-CAGTTCTTGCCGGGTCGACAGAGC-3′ (complementary to the region from bp 25 to 48 downstream of the initiation codon of acrS). Primer 7 (5′-CACGACGTTGTAAAACGACGGC-3′) was designed based on the sequence of vector pMW118. The underlined sequence is the SalI or XhoI site introduced into each primer. A 4.4-kb SalI-HindIII fragment containing acrEF was amplified using the genomic DNA from strain OST5500 as the template, with the combination of primers 1 and 2, and inserted into the SaI -HindIII sites of pMW118 and pMW219 to make pLAcrEF and pEFrk, respectively. A DNA fragment containing the acrF gene was prepared by PCR from pLAcrEF, using the combination of primers 3 and 7. This fragment was digested with XhoI and HindIII. A resulting 3.2-kb XhoI-HindIII DNA fragment was inserted into the XhoI-HindIII sites of pBluescriptII KS(+) to make pHAcrF, and into the same sites of pMW118 to yield pLAcrF.
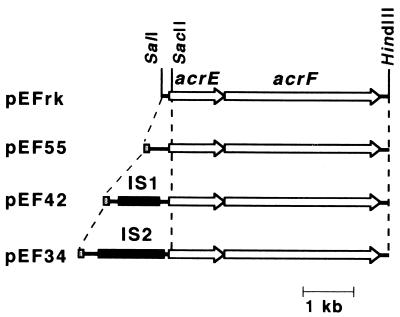
A DNA fragment containing acrS (a putative gene encoding an acrEF repressor, present upstream of acrE) and the intergenic region between acrS and acrE (the acrSE-intergenic region) was amplified from the chromosomal DNA of OST5500, DK4201, and DK3402 using the combination of primers 4 and 5. From the PCR products, the acrSE-intergenic region was amplified using the combination of primers 5 and 6. Each SalI-SacII fragment containing the acrSE-intergenic region was inserted into the SalI-SacII sites of pEFrk to make pEF55, pEF42, and pEF34.
Culture conditions.
The organisms were grown aerobically at 37°C in Luria broth (LB medium; pH 7.0) containing 1% Bacto Tryptone (Difco Laboratories, Detroit, Mich.), 0.5% Bacto Yeast Extract (Difco), and 1% NaCl. This medium supplemented with 0.1% glucose and 10 mM MgSO4 (LBGMg medium) was also used. When necessary, selective antibiotics were added to the medium as follows: ampicillin, 50 μg/ml, kanamycin, 50 μg/ml, or spectinomycin, 100 μg/ml.
Assay of organic solvent resistance.
An overnight culture of E. coli was diluted with 0.9% NaCl (approximately 107 cells/ml). A drop of the cell suspension (5 μl) was spotted on LBGMg agar medium to form a circle with a diameter of 7 to 8 mm. The surface of the agar was overlaid with an appropriate solvent to obtain a solvent depth of 3 mm. Growth was assessed after the plates were incubated at 37°C for 12 h in a sealed vessel. Confluent growth of the cells was considered to be indicative of resistance to the solvent tested. When only a few colonies (fewer than 10) were formed in the circle, the cells were considered to be sensitive to the solvent tested.
Preparation of membrane fractions.
E. coli was grown in LBGMg medium. The cells were harvested during the exponential phase of growth (optical density at 660 nm, 0.6) by centrifugation (5,000 × g for 10 min at 4°C). The cells were suspended in cold 50 mM phosphate buffer (pH 7.0) and broken by sonication in an ice-water bath. Unbroken cells were removed from the lysate by centrifugation in the same manner. The supernatant was centrifuged at 100,000 × g for 45 min at 4°C. The precipitate was washed with the phosphate buffer. The insoluble matter was used as the membrane fraction. The protein content was measured by the method of Lowry et al. (17).
SDS-polyacrylamide gel electrophoresis.
Samples were dissolved in a solubilization buffer containing 1% sodium dodecyl sulfate (SDS), 2.5% (vol/vol) β-mercaptoethanol, 20% (vol/vol) glycerol, and 16 mM Tris-HCl (pH 6.8) and incubated in a boiling-water bath for 5 min or at 37°C for 30 min. The samples were analyzed by electrophoresis on an SDS-polyacrylamide gel mainly by the method described by Laemmli (15).
Recovery and identification of AcrE.
The membranes from DK3402 cells were incubated in 0.5% N-lauroylsarcosine (sarcosyl). Sarcosyl-soluble proteins were recovered by centrifugation (100,000 × g for 30 min at 15°C) and electrophoresed on a 0.1% SDS–10% polyacrylamide gel in Tris-HCl buffer (pH 6.8) (15). Proteins with a molecular mass of approximately 42 kDa were recovered from the gel and electrophoresed on a 0.1% SDS–10% polyacrylamide gel in 30 mM NaH2PO4-NaOH buffer (pH 7.2) (1). The purified 42-kDa protein thereby obtained was dissolved in 70% (vol/vol) formic acid containing 1% CNBr and incubated at room temperature for 4 days. The CNBr digest was electrophoresed on a 0.1% SDS–10% polyacrylamide gel in Tris-Tricine buffer (24). The N-terminal amino acid sequence of an appropriate peptide (ca. 13 kDa) was determined by Edman degradation using a 477A protein sequencer (Applied Biosystems Inc., Foster City, Calif.).
Determination of nucleotide sequences.
DNA nucleotide sequences were determined by the dideoxy chain termination method using a DNA sequencing system (PRISM 377; Perkin-Elmer Applied Biosystems).
Materials.
Plasmid pFW5 containing a spectinomycin resistance cassette was a kind gift from A. Podbielski of the Institute of Medical Microbiology. Antiserum against AcrA and E. coli JZM221A1 were kind gifts from H. Nikaido of the University of California, Berkeley, Calif. The organic solvents used were of the highest purity available and were purchased from Wako Pure Chemical Industries (Osaka, Japan). The log POW values of the solvents were calculated by the addition rule (16) using the log POW calculation software ClogP version 4 (Bio Byte Corp., Claremont, Calif.).
RESULTS
Identification of the acrB mutation as the determinant of the solvent hypersensitivity of OST5500.
Most E. coli strains grow on LBGMg agar medium overlaid with diphenyl ether. However, the solvent resistance assay showed that E. coli OST5500 was resistant to n-nonane and sensitive to n-octane and diphenyl ether (Table 2). Thus, OST5500 is hypersensitive to solvents, compared to most E. coli strains. This solvent resistance is similar to that of ΔacrAB or ΔtolC mutants (30).
TABLE 2.
Solvent resistance of OST5500 and its derivatives
| Strain | Plasmid | Growth on LBGMg agar overlaid witha:
|
||||||
|---|---|---|---|---|---|---|---|---|
| N (5.5) | O (4.9) | CO (4.5) | DE (4.2) | H (3.9) | CH (3.4) | pX (3.1) | ||
| OST5500 | None | + | − | − | − | − | − | − |
| pLAcrAB | + | + | NDb | + | ± | − | − | |
| pLAcrA | + | − | ND | − | − | − | − | |
| pLAcrB | + | + | ND | + | − | − | − | |
| DK4201 | None | + | + | + | + | − | − | − |
| DK3402 | None | + | + | + | + | + | + | − |
A drop of the E. coli cell suspension (5 μl) containing approximately 105 cells was spotted on LBGMg agar medium to form a circle with a diameter of 7 to 8 mm. The surface of the agar was overlaid with the organic solvent indicated. Growth was assessed after the plates were incubated at 37°C for 12 h. Values in parentheses are the log POW values of the organic solvents. Abbreviations: N, n-nonane; O, n-octane; CO, cyclooctane; DE, diphenyl ether; H, n-hexane; CH, cyclohexane; pX, p-xylene; +, confluent growth; ±, poor growth (fewer than 10 small colonies in the circle spotted); −, no growth.
ND, not determined.
We assumed that the solvent hypersensitivity might be due to a defect in the AcrAB-TolC pump. A P1 transduction experiment was done to test this possibility. The acrAB and dnaX loci have been mapped at 10.4 and 10.6 min, respectively (5). E. coli strain AX727 is temperature sensitive because of the dnaX mutation (7) and resistant to diphenyl ether (results not shown). After P1 transduction of AX727 with OST5500 as the donor strain, the transductants were grown at 42°C, the nonpermissive temperature for growth of the recipient. Among 36 temperature-resistant transductants obtained, 24 clones were found to be as sensitive to diphenyl ether as the donor. It was shown that the dnaX gene and the determinant of solvent hypersensitivity were cotransducible. Therefore, it seemed highly possible that acrAB was defective in OST5500.
A plasmid containing the acrAB operon was constructed as described in Materials and Methods. OST5500 acquired n-hexane resistance on transformation of the cells with pLAcrAB (Table 2). The solvent resistance of OST5500 was also improved as a result of transformation of the cells with pLAcrB but not with pLAcrA. These results showed that the solvent hypersensitivity of OST5500 was attributable to some mutation in acrB. A region of the acrAB gene of OST5500 was amplified by PCR, as described in Materials and Methods. The product from OST5500 was about 1 kb longer than that from JA300 (results not shown). Analysis of the nucleotide sequence of this product showed that IS30 had inserted at codon 184 of the acrB gene, accompanied by doubling of the dimer AT.
Isolation of mutants of strain OST5500 showing improved solvent resistance.
From OST5500, we isolated suppressor mutants with high solvent resistance to study the solvent resistance mechanism that was not dependent on AcrAB. OST5500 was grown on LBGMg agar medium overlaid with cyclooctane, diphenyl ether, or n-hexane. These solvents are more toxic than n-nonane under the conditions employed. The mutants that grew on the agar were classified into three groups on the basis of their resistance to solvents, irrespective of the solvents used. The mutants in the first group were resistant to cyclooctane but sensitive to diphenyl ether and n-hexane, those in the second group were resistant to cyclooctane and diphenyl ether but sensitive to n-hexane, and those in the third group were resistant to all of these solvents as well as cyclohexane. Mutants belonging to the first, second, and third groups appeared at frequencies of 1.6 × 10−9, 2.9 × 10−9, and 4.7 × 10−8, respectively. We analyzed the solvent resistance mechanism of two mutants, DK4201 (belonging to the second group) and DK3402 (belonging to the third group). The solvent resistance of DK4201 was similar to that of most E. coli strains, whereas that of DK3402 was greater (Table 2).
Occurrence of the 42-kDa membrane protein AcrE in the mutants.
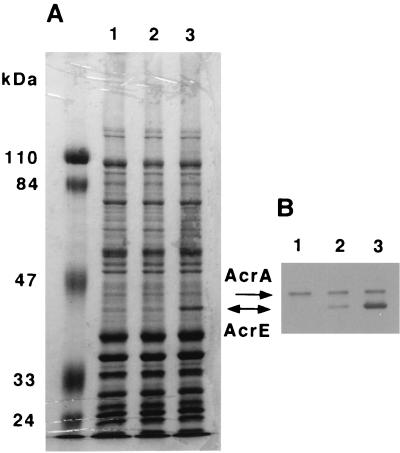
Through a survey examining changes in protein production in the mutants, we found that the mutants had a novel 42-kDa protein in the cell membranes. OST5500, DK4201, and DK3402 were grown in LBGMg. The membrane proteins were electrophoresed on a 0.1% SDS–10% polyacrylamide gel and stained with Coomassie brilliant blue R-250 (Fig. 2A). A 42-kDa protein was clearly evident in DK3402 and faintly detected in DK4201. This 42-kDa protein was not detected in OST5500. The protein was soluble in 0.5% sarcosyl (results not shown), suggesting that it was likely to be a protein associated with the inner membrane.
FIG. 2.
Occurrence of the AcrE protein in the mutants DK4201 and DK3402. Envelope preparations containing 40 μg of protein were electrophoresed on a 0.1% SDS–10% (wt/vol) polyacrylamide gel. Arrows indicate the positions of AcrA and AcrE. Proteins were detected by staining with Coomassie brilliant blue R-250 (A) or by immunostaining with antiserum against AcrA (B). Lanes: 1, OST5500; 2, DK4201; 3, DK3402.
The 42-kDa protein was recovered from the fraction of sarcosyl-soluble membrane proteins of DK3402 and subjected to Edman degradation in an effort to determine the N-terminal amino acid sequence. However, this attempt failed because the N-terminal amino acid of the protein was blocked. A 13-kDa peptide generated from the protein by CNBr treatment showed the amino acid sequence H2N-FVRARIDEG. This sequence is consistent with that of amino acid residues 291 to 299 of the E. coli AcrE protein. Amino acid 290 of AcrE is methionine, susceptible to cleavage by CNBr treatment. It has been reported that AcrE is a periplasmic protein acylated at its N terminus (29). The molecular mass of the mature AcrE polypeptide is calculated to be 38.8 kDa. The molecular mass of AcrE as estimated by SDS-polyacylamide gel electrophoresis was larger than the calculated value, probably due to the N-terminal fatty acid in the acylated protein. On the basis of these findings, the 42-kDa protein found in the mutants was identified as AcrE.
Antiserum against the AcrA protein was found to react with the 42-kDa protein (Fig. 2B). This serological cross-reactivity further supported the identification of the 42-kDa protein produced by DK3402 as AcrE. Our findings concerning the electrophoretic mobility and immunological activity of the 42-kDa protein suggested that AcrE was produced weakly by DK4201, but this protein was not sequenced.
Detection of AcrF in the membranes of the mutants.
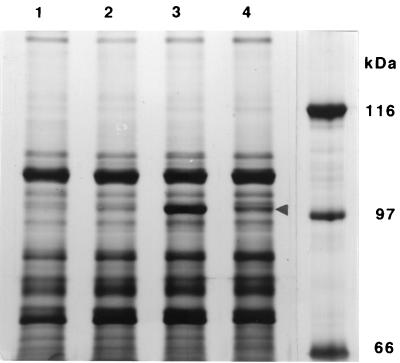
The acrE gene is known to form a transcriptional unit together with acrF. It seemed likely that acrF was expressed in the mutants. It has been reported that AcrF is an integral inner membrane protein with a molecular mass of 111.4 kDa (12). The membrane proteins were solubilized at 37°C for 30 min and electrophoresed on a 0.1% SDS–6% polyacrylamide gel. After silver staining, a 100-kDa protein was clearly evident in DK3402 and weakly detected in DK4201 (Fig. 3). This protein was also produced by OST5500 on introduction of pHAcrF into the cells. Therefore, it was concluded that the 100-kDa protein is AcrF.
FIG. 3.
Occurrence of the AcrF protein in the mutants DK4201 and DK3402. Envelope preparations containing 15 μg of protein were electrophoresed on a 0.1% SDS–6% (wt/vol) polyacrylamide gel. Protein was detected by silver staining. The position of AcrF is indicated by an arrowhead. Lanes: 1, OST5500; 2, DK4201; 3, DK3402; 4, OST5500(pHAcrF).
IS elements integrated upstream of acrEF in the mutants.
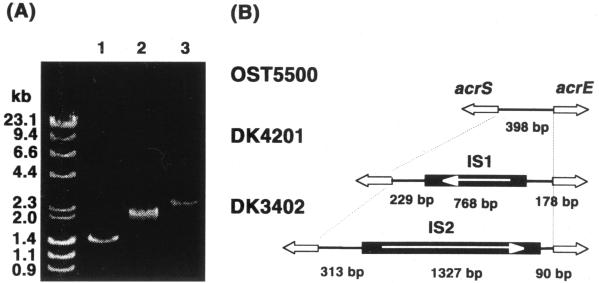
The acrSE intergenic region of the chromoosme was analyzed by PCR for strains OST5500, DK4201, and DK3402 (Fig. 4A). DNA fragments containing the acrS gene and the intergenic region were amplified using the combination of primers 4 and 5 (Fig. 1). It was expected that the length of the product would be 1,431 bp based on the sequences deposited in GenBank. A product with the same length as that expected was amplified from the chromosomal DNA of strain OST5500. The sizes of the products obtained with DK4201 and DK3402 were 2.1 and 2.8 kb, respectively. Therefore, it was evident that an insert of 0.7 or 1.4 kb was present in the intergenic region of strains DK4201 and DK3402, respectively.
FIG. 4.
Structure of the acrSE-intergenic region of OST5500 and its mutants. (A) Length of the acrSE-intergenic region. PCR products containing the intergenic region of OST5500 (lane 1), DK4201 (lane 2), and DK3402 (lane 3), as well as fragment length standards, were electrophoresed on a 0.8% agarose gel. (B) Structure of the acrSE-intergenic region of OST5500, DK4201, and DK3402. Horizontal lines indicate the acrSE intergenic region of the OST5500 chromosomal DNA. Solid bars represent the IS elements. The white arrow shown in the bar indicates the direction of the transposase gene encoded by each IS element. Open arrows indicate the directions of the acrE and acrS genes.
The nucleotide sequence of the intergenic region was determined for each of these PCR products. The length of the intergenic region of OST5500 was 398 bp, and the sequence was the same as that deposited in GenBank (accession no. AE000405). It was shown that IS1 was inserted in the intergenic region in DK4201 and that IS2 was inserted in DK3402. The structure of the intergenic region is shown in Fig. 4B. The IS1 and IS2 sequences were identical to those deposited in GenBank (accession no. U49270 and V00610, respectively). IS1 was inserted 178 bp upstream of the initiation codon of acrE, accompanied by doubling of the nonamer, AACAGTAGA. IS2 was inserted 90 bp upstream of the initiation codon of acrE, accompanied by doubling of the pentamer GTAGG, the same as reported by Klein et al. for IS2 integration in a plasmid containing acrE (11).
Effect of acrEF inactivation on the solvent resistance of E. coli.
The results described above suggested that the IS integration caused high expression of acrEF, resulting in improvement of the solvent resistance of OST5500. We constructed acrEF null mutants of several E. coli strains by P1 transduction of ΔacrEF::Tn5. Inactivation of acrEF did not cause alteration of the solvent resistance of JA300, JA300A, or OST5500 at all. These strains showed different degrees of solvent resistance. Therefore, it was concluded that acrEF did not contribute to maintenance of the high or low solvent resistance displayed by these strains.
On the other hand, inactivation of acrEF caused deterioration of the improved solvent resistance of DK4201 and DK3402. The solvent hypersensitivity of each of these acrEF null mutants was the same as that of OST5500. It was evident that acrEF was involved in the improved solvent resistance of DK4201 and DK3402.
Improvement of the solvent resistance of OST5500 by introduction of acrEF or acrF under the control of Plac into the cells.
In pLAcrEF, acrEF is under the control of the lac promoter of the vector. OST5500(pLAcrEF) was found to be resistant to n-hexane (Table 3). This result indicates that overexpression of the acrEF operon improves the solvent resistance of OST5500. This transformant was resistant to cyclohexane when isopropyl-β-d-thiogalactopyranoside (IPTG) was present in the medium, suggesting that the resistance of this transformant depends on the levels of acrEF expression. The solvent resistance of OST5500 was also improved by introduction of pLAcrF or pHAcrF into the cells. OST5500(pLAcrF) and OST5500(pHAcrF) were resistant to n-octane and diphenyl ether (Table 3), respectively. The improvement of solvent resistance was dependent on the copy number of the plasmids. Also, the solvent resistance of OST5500F was improved by the introduction of pLAcrEF or pHAcrF into the cells, whereas that of JA300AF was not improved by introduction only of pHAcrF; cotransformation of the cells with acrA or acrE was essential to improve the solvent resistance of JA300AF. Therefore, it is concluded that a combination of AcrA and AcrF or AcrE and AcrF was functional in these cells instead of AcrAB.
TABLE 3.
Effect of transformation with a plasmid containing acrF or acrEF on the organic solvent resistance of E. coli
| Strain | Chromosomal genotype
|
Plasmid | IPTG (1 mM) | Growth on LBGMg agar overlaid witha:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| acrA | acrB | acrE | acrF | tolC | D (6.0) | N (5.5) | O (4.9) | DE (4.2) | H (3.9) | CH (3.4) | pX (3.1) | |||
| OST5500 | + | − | + | + | + | None | − | + | + | − | − | − | − | − |
| pLAcrEF | − | + | + | + | + | ± | − | − | ||||||
| pLAcrEF | + | + | + | + | + | + | + | − | ||||||
| pLAcrF | − | + | + | ± | − | − | − | − | ||||||
| pHAcrF | − | + | + | + | + | − | − | − | ||||||
| OST5500F | + | − | − | − | + | None | − | + | + | − | − | − | − | − |
| pLAcrEF | − | + | + | + | + | ± | − | − | ||||||
| pLAcrEF | + | + | + | + | + | + | + | − | ||||||
| pLAcrEF | − | + | + | + | + | − | − | − | ||||||
| JA300 | + | + | + | + | + | None | − | + | + | + | + | + | − | − |
| pLAcrEF | − | + | + | + | + | + | − | − | ||||||
| JA300A | − | − | + | + | + | None | − | + | + | − | − | − | − | − |
| pLAcrEF | − | + | + | + | + | ± | − | − | ||||||
| pHAcrF | − | + | + | − | − | − | − | − | ||||||
| JA300AF | − | − | − | − | + | None | − | + | + | − | − | − | − | − |
| pHAcrF | − | + | + | − | − | − | − | − | ||||||
| pHAcrF + pLKSAcrA | − | + | + | + | + | − | − | − | ||||||
| JA300T | + | + | + | + | − | None | − | + | − | − | − | − | − | − |
| pLAcrEF | − | + | − | − | − | − | − | − | ||||||
| pLKTolC | − | + | + | + | + | + | − | − | ||||||
| JA300TA | − | − | + | + | − | None | − | + | − | − | − | − | − | − |
| pLAcrEF | − | + | − | − | − | − | − | − | ||||||
| pLAcrEF | + | + | − | − | − | − | − | − | ||||||
| pLKTolC | − | + | + | − | − | − | − | − | ||||||
| pLAcrEF + pLKTolC | − | + | + | + | + | ± | − | − | ||||||
| pLAcrEF + pLKTolC | + | + | + | + | + | + | + | − | ||||||
Growth was assessed in the same manner as described in the footnote of Table 2. Abbreviations: D, n-decane; N, n-nonane; O, n-octane; DE, diphenyl ether; H, n-hexane; CH, cyclohexane; pX, p-xylene; +, confluent growth; ±, poor growth; −, no growth.
Solvent resistance of E. coli is greatly diminished by deletion of acrAB or tolC. The solvent resistance of JA300A was restored by transformation of the cells with pLAcrEF but not with pHAcrF (Table 3). The solvent resistance of JA300T or JA300TA was not improved by transformation of the cells with pLAcrEF. The resistance of JA300TA was restored only when both pLAcrEF and pLKTolC were introduced into the cells. The resistance of JA300TA containing only pLAcrEF was not improved. Therefore, it is concluded that AcrE and AcrF contribute to the solvent resistance of E. coli in a manner dependent on TolC.
Evidence of the contribution of IS integration upstream of acrEF to improvement of the solvent resistance of OST5500.
A series of plasmids were constructed containing the acrSE-intergenic region of OST5500, DK4201 or DK3402 in addition to the acrEF of OST5500 (Fig. 5). The solvent resistance of OST5500 transformants carrying each of the plasmids was examined (Table 4). OST5500(pEF55) was as sensitive to solvents as was OST5500. The solvent resistance of OST5500 was improved by transformation of the cells with pEF42 or pEF34. OST5500(pEF42) and OST5500(pEF34) were as resistant to solvents as were DK4201 and DK3402, respectively. These results show that acrEF in the OST5500 chromosome has no effect on solvent resistance. When either IS1 or IS2 was integrated upstream of acrEF, the solvent resistance of OST5500 was improved through functional complementation of acrAB.
FIG. 5.
Insertion of the acrSE intergenic region upstream of acrEF cloned in pEFrk. The acrSE intergenic region was amplified by PCR from chromosomal DNA of OST5500, DK4201, and DK3402. The amplified products were inserted upstream of acrE in pEFrk. The acrE gene was oriented in the direction opposite that of the lac promoter in the vector. Solid bars show the integrated IS elements. Open arrows indicate the directions and extents of acrE and acrF. Dotted bars show the 5′ part of the acrS open reading frame.
TABLE 4.
Effect of IS1 or IS2 integration upstream of the acrEF operon on the solvent resistance of OST5500
| Plasmid | Growth on LBGMg agar overlaid witha:
|
|||||
|---|---|---|---|---|---|---|
| N (5.5) | O (4.9) | DE (4.2) | H (3.9) | CH (3.4) | pX (3.1) | |
| None | + | − | − | − | − | − |
| pEF55 | + | − | − | − | − | − |
| pEF42 | + | + | + | − | − | − |
| pEF34 | + | + | + | + | + | − |
Growth was assessed in the same manner as described in the footnote of Table 2.
DISCUSSION
E. coli OST5500, a strain maintained in our laboratory, was found to be hypersensitive to solvents due to insertional inactivation of acrB by IS30. The two suppressor mutants characterized, which differed in terms of their solvent resistance, produced different levels of AcrE and AcrF. Ma et al. found that acrEF are dormant or expressed only at very low levels in E. coli (20). Kawamura-Sato et al. reported that disruption of acrEF resulted in decreased efflux of indole generated intracellularly although the null mutant was as resistant as the parent to various extracellular drugs (10). Therefore, acrEF might be expressed at a faint level which is hard to detect. However, we did not find AcrE or AcrF among the membrane proteins of OST5500 or OST5500(pEF55). Inactivation of acrEF did not reduce the solvent resistance of OST5500, JA300, or JA300A. These results indicated that the acrEF operon was not actively expressed and that it was not involved in solvent resistance in these strains, irrespective of the hydrophobicity of the solvent.
Nonetheless, integration of the IS element upstream of acrEF caused enhanced expression of acrEF and consequently suppressed the solvent hypersensitivity of OST5500. Such enhanced expression caused by IS2 integration has been reported for a plasmid containing acrEF (11). E. coli has several efflux pumps belonging to the RND transporter family. In recent years, we found that the solvent hypersensitivity of a ΔacrAB mutant of E. coli was partially suppressed by overexpression of emrAB or yhiUV, encoding an efflux pump belonging to the RND family (30). The degree of solvent resistance restored by overexpression of acrEF was comparable to that restored by the introduction of acrAB.
The levels of the AcrEF proteins were lower in DK4201 than in DK3402. A difference in the AcrEF levels was also observed in the case of OST5500(pEF42) and OST5500(pEF34). These results indicate that the activity of the promoter generated by IS1 integration was weaker than that of the promoter generated by IS2 integration. Thus, the extent of improvement of solvent resistance of the mutants or transformants was correlated with the level of acrEF expression. This conclusion is confirmed by our finding that the solvent resistance of OST5500(pLAcrEF) cells grown in the presence of IPTG was greater than that of the same cells grown in the absence of IPTG.
We have reported that overproduction of each transcriptional activator of the mar-sox regulon genes, MarA, SoxS, or Rob, improves the solvent resistance of E. coli (4, 21, 22). The solvent resistance of DK3402 or OST5500(pEF34) is comparable to that of JA300, in which the mar-sox regulon genes are highly expressed. It has been reported that an E. coli strain in which the acrR gene, encoding a repressor for acrAB, was disrupted showed cyclohexane resistance (31). These observations suggest that E. coli acquires cyclohexane resistance by overproduction of either AcrAB or AcrEF in the presence of TolC. The improvement of solvent resistance by AcrEF was dependent on the tolC+ genotype.
Introduction of pLAcrEF or pHAcrF into OST5500 resulted in improvement of the solvent resistance. These findings indicate that the complex formed by the AcrAB proteins can be replaced functionally by a combination of the AcrEF or AcrAF proteins. This interchangeability was also found in JA300AF on cointroduction of a plasmid containing acrA and a plasmid containing acrF. AcrB has 77% amino acid sequence identity to AcrF (18).
It is likely that expression of the acrEF operon is not essential for E. coli cells, in which the acrAB operon is expressed, to survive in a milieu containing harmful compounds. It is interesting from a phylogenetic point of view that the acrEF structural genes are conserved in E. coli although they appear to be dormant or expressed only faintly. This operon might be conserved as part of a strategy which allows E. coli to survive under harmful conditions in the event that the acrAB genes become inactive.
ACKNOWLEDGMENTS
This work was partially supported by a Grant-in-Aid for Scientific Research (B), no. 10450308, to R. Aono from the Ministry of Science, Education and Culture of Japan.
We thank H. Asako for his technical assistance. We are indebted to A. Podbielski of the Institute of Medical Microbiology for the kind gift of the spectinomycin resistance plasmid pFW5. Also, we are indebted to H. Nikaido of the University of California, Berkeley, Calif., for the kind gifts of antiserum against AcrA and the acrEF null mutant JZM221A1.
REFERENCES
- 1.Aono R. Probable detection of Kil peptide derived from colicin E1 plasmid in the envelope fraction of Escherichia coli HB101 carrying pEAP31. Biochem J. 1988;255:365–368. [PMC free article] [PubMed] [Google Scholar]
- 2.Aono R, Kobayashi H, Joblin K N, Horikoshi K. Effects of organic solvents on growth of Escherichia coli K-12. Biosci Biotechnol Biochem. 1994;58:2009–2014. doi: 10.1271/bbb.58.1231. [DOI] [PubMed] [Google Scholar]
- 3.Aono R, Tsukagoshi N, Yamamoto M. Involvement of outer membrane protein TolC, a possible member of the mar-sox regulon, in maintenance and improvement of organic solvent tolerance of Escherichia coli K-12. J Bacteriol. 1998;180:938–944. doi: 10.1128/jb.180.4.938-944.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asako H, Nakajima H, Kobayashi K, Kobayashi M, Aono R. Organic solvent tolerance and antibiotic resistance increased by overexpression of marA in Escherichia coli. Appl Environ Microbiol. 1997;63:1428–1433. doi: 10.1128/aem.63.4.1428-1433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berlyn M K B. Linkage map of Escherichia coli K-12, edition 10. Microbiol Mol Biol Rev. 1998;62:814–984. doi: 10.1128/mmbr.62.3.814-984.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinh T, Paulsen I T, Saier M H., Jr A family of extracytoplasmic proteins that allow transport of large molecules across the outer membranes of Gram-negative bacteria. J Bacteriol. 1994;176:3825–3831. doi: 10.1128/jb.176.13.3825-3831.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filip C C, Allen J S, Gustafson R G, Allen R G, Walker J R. Bacterial cell division regulation: characterization of the dnaH locus of Escherichia coli. J Bacteriol. 1974;119:443–449. doi: 10.1128/jb.119.2.443-449.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fralick J A. Evidence that TolC is required for functioning of the Mar/AcrAB efflux pump of Escherichia coli. J Bacteriol. 1996;178:5803–5805. doi: 10.1128/jb.178.19.5803-5805.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inoue A, Horikoshi K. A Pseudomonas thrives in high concentrations of toluene. Nature. 1989;338:264–265. [Google Scholar]
- 10.Kawamura-Sato K, Shibayama K, Horii T, Iimura Y, Arakawa Y, Ohta M. Role of multiple efflux pumps in Escherichia coli in indole expulsion. FEMS Microbiol Lett. 1999;179:345–352. doi: 10.1111/j.1574-6968.1999.tb08748.x. [DOI] [PubMed] [Google Scholar]
- 11.Klein J R, Henrich B, Plapp R. Molecular cloning of the envC gene of Escherichia coli. Curr Microbiol. 1990;21:341–347. [Google Scholar]
- 12.Klein J R, Henrich B, Plapp R. Molecular analysis and nucleotide sequence of the envCD operon of Escherichia coli. Mol Gen Genet. 1991;230:230–240. doi: 10.1007/BF00290673. [DOI] [PubMed] [Google Scholar]
- 13.Klein J R, Plapp R. Locations of the envCD genes on the physical map of the Escherichia coli chromosome. J Bacteriol. 1992;174:3828–3829. doi: 10.1128/jb.174.11.3828-3829.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koronakis V, Shaarff A, Koronakis E, Luisi B. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature. 2000;405:914–919. doi: 10.1038/35016007. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Leo A J. Calculating log Poct from structures. Chem Rev. 1993;93:1281–1306. [Google Scholar]
- 17.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 18.Ma D, Cook D N, Alberti M, Pon N G, Nikaido H, Hearst J E. Molecular cloning and characterization of acrA and acrE genes of Escherichia coli. J Bacteriol. 1993;175:6299–6313. doi: 10.1128/jb.175.19.6299-6313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma D, Cook D N, Alberti M, Pon N G, Nikaido H, Hearst J E. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol Microbiol. 1995;16:45–55. doi: 10.1111/j.1365-2958.1995.tb02390.x. [DOI] [PubMed] [Google Scholar]
- 20.Ma D, Cook D N, Hearst J E, Nikaido H. Efflux pumps and drug resistance in Gram-negative bacteria. Trends Microbiol. 1994;2:489–493. doi: 10.1016/0966-842x(94)90654-8. [DOI] [PubMed] [Google Scholar]
- 21.Nakajima H, Kobayashi K, Kobayashi M, Asako H, Aono R. Overexpression of robA gene increases organic solvent tolerance and multiple antibiotic and heavy metal ion resistance in Escherichia coli. Appl Environ Microbiol. 1995;61:2302–2307. doi: 10.1128/aem.61.6.2302-2307.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakajima H, Kobayashi M, Negishi T, Aono R. soxRS gene increased the level of organic solvent tolerance in Escherichia coli. Biosci Biotechnol Biochem. 1995;59:1323–1325. doi: 10.1271/bbb.59.1323. [DOI] [PubMed] [Google Scholar]
- 23.Paulsen I T, Brown M H, Skurray R A. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ploug M, Jensen A L, Barkhoit V. Determination of amino acid compositions and NH2-terminal sequences of peptides electroblotted onto PVDF membranes from Tricine-SDS-PAGE: application to peptide mapping of human complement component C3. Anal Biochem. 1989;181:33–39. doi: 10.1016/0003-2697(89)90390-4. [DOI] [PubMed] [Google Scholar]
- 25.Podbielski A, Spellerberg B, Woischnik M, Pohl B, Lütticken R. Novel series of plasmid vectors for gene inactivation and expression analysis in group A streptococci (GAS) Gene. 1996;177:137. doi: 10.1016/0378-1119(96)84178-3. [DOI] [PubMed] [Google Scholar]
- 26.Rodolakis A, Casse F, Starka J. Morphological mutants of Escherichia coli K-12: mapping of the env C mutation. Mol Gen Genet. 1974;130:177–181. doi: 10.1007/BF00269088. [DOI] [PubMed] [Google Scholar]
- 27.Rodolakis A, Thomas P, Starka J. Morphological mutants of Escherichia coli. Isolation and ultrastructure of a chain-forming envC mutant. J Gen Microbiol. 1973;75:409–416. doi: 10.1099/00221287-75-2-409. [DOI] [PubMed] [Google Scholar]
- 28.Saier M H, Tam R, Reizer A, Reizer J. Two novel families of bacterial membrane proteins concerned with nodulation, cell division and transport. Mol Microbiol. 1994;11:841–847. doi: 10.1111/j.1365-2958.1994.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 29.Seiffer D, Klein J R, Plapp R. EnvC, a new lipoprotein of the cytoplasmic membrane of Escherichia coli. FEMS Microbiol Lett. 1993;107:175–178. doi: 10.1111/j.1574-6968.1993.tb06026.x. [DOI] [PubMed] [Google Scholar]
- 30.Tsukagoshi N, Aono R. Entry and release of solvents in Escherichia coli in an organic-aqueous two-liquid phase system and substrate specificity of the AcrAB-TolC solvent-extruding pump. J Bacteriol. 2000;182:4803–4810. doi: 10.1128/jb.182.17.4803-4810.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White D G, Goldman J D, Demple B, Levy S B. Role of the acrAB locus in organic solvent tolerance mediated by expression of marA, soxS, or robA in Escherichia coli. J Bacteriol. 1997;179:6122–6126. doi: 10.1128/jb.179.19.6122-6126.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zgurskaya H I, Nikaido H. AcrA is a highly asymmetric protein capable of spanning the periplasm. J Mol Biol. 1999;285:409–420. doi: 10.1006/jmbi.1998.2313. [DOI] [PubMed] [Google Scholar]
- 33.Zgurskaya H I, Nikaido H. Bypassing the periplasm: reconstitution of the AcrAB multidrug efflux pump of Escherichia coli. Proc Nat Acad Sci USA. 1999;96:7190–7195. doi: 10.1073/pnas.96.13.7190. [DOI] [PMC free article] [PubMed] [Google Scholar]