Abstract
Background
Studying sex differences in the efficacy of immunotherapy may contribute to the practice of the precision medicine, especially in non-small cell lung cancer (NSCLC), a kind of cancer with sexual bimorphism.
Methods
Published randomized controlled trials (RCTs), published by PubMed, Medline, Embase, and Scopus, before 15 June 2022, testing immunotherapy (CTLA-4 or PD-1/L1 inhibitor alone, combination or with chemotherapy) versus non-immunotherapy (receiving chemotherapy or placebo only) were included to assess different efficacy between males and females. The primary endpoint was overall survival (OS). This meta-analysis was registered with PROSPERO (CRD42022298439).
Results
Sixteen RCTs, involving 10,155 patients with advanced NSCLC, was collected in this meta-analysis. The pooled HR comparing immunotherapy vs non-immunotherapy were 0.76 (95%CI 0.71–0.81) for males and 0.74 (95%CI 0.63–0.87) for females. The pooled HRs comparing immune-checkpoint inhibitors (ICIs) plus chemotherapy versus chemotherapy were 0.79 (95%CI 0.70–0.89) for males and 0.63 (95%CI 0.42–0.92) for females. The pooled HRs comparing ICIs versus chemotherapy were 0.74 (95%CI 0.67–0.81) for males and 0.83 (95%CI 0.73–0.95) for females. In squamous NSCLC, the pooled HRs comparing immunotherapy vs non-immunotherapy were 0.73 (95%CI 0.58–0.91) for males and 0.74 (95%CI 0.37–1.48) for females. In non-squamous NSCLC, the pooled HRs comparing immunotherapy versus non-immunotherapy were 0.62 (95%CI 0.71–0.94) for males and 0.59 (95%CI 0.39–0.89) for females.
Conclusion
Compared to chemotherapy, immunotherapy can improve the prognosis of patients with advanced NSCLC. Meanwhile, there are sex differences in the efficacy of immunotherapy.
KEY MESSAGE
Compared to chemotherapy, immunotherapy can improve the prognosis of patients with advanced NSCLC.
The most interesting thing in this study is that immunotherapy showed significant sex differences in the treatment of squamous NSCLC.
Keywords: Immunotherapy, non-small cell lung cancer, sex, meta, prognosis
1. Introduction
Immunotherapy is defined as the use of materials that augment and/or re-establish the immune system’s ability to prevent and fight disease [1]. It is well known that immune system functions and immune responses differ in male and female [2]. This is associated with complex interactions between genetic, hormones, behavioural traits, and symbiotic microbial composition. Previous studies have noted that this difference is also reflected in the response to immunotherapy with cancer patients [3]. In 2017, Botticelli et al. reported a trend towards increased benefits for male patients treated with immune-checkpoint inhibitors (ICIs). This meta-analysis included eight studies on melanoma, 6 on non-small cell lung cancer (NSCLC), 1 on renal cells, 1 on head and neck tumours and 1 on urothelial carcinoma [4]. As the therapeutic effects of ICIs varies between males and females, sex may have the potential to be a natural biomarker in solid cancers and the effect of sex differences on immunotherapy is worth exploring.
Increasing literature points to sex differences in the immune system. Sex-related differences in survival benefits were studied by Conforti et al. [5], demonstrating that men receive greater benefit from cancer immunotherapy than women. Wallis et al. [6] have reported conflicting results, who found no statistically significant association between patient sex and the magnitude of benefit from advanced cancer immunotherapy. However, the specific effect of sex on the efficacy of immunotherapy in patients with lung cancer still remains unclear.
Lung cancer is current the second most diagnosed tumour with its mortality rate ranks the first around the world. The vast majority of lung cancer is NSCLC [7]. In addition, NSCLC was found to be a tumour in the presence of a sexual dimorphism [8]. Therefore, it is very necessary to study the sex effect in therapeutic efficacy in NSCLC patients. The types of immunotherapy include vaccines, antibody therapies, ICIs, oncolytic virus therapy, chimeric antigen receptor T-cell therapy, etc. Among the ICIs, programmed cell death 1 (PD-1), programmed cell death ligand 1 (PD-L1), and cytotoxic T-lymphocyte protein 4 (CTLA-4) are the most well-represented [1]. Herein, this meta-analysis was conducted to study the therapeutic differences in ICIs (PD-1, PD-L1 or CTLA-4) between males and females in NSCLC patients in order to explore the application scheme of immunotherapy in clinical practice.
2. Methods
2.1. Retrieval strategy and inclusion criteria
This meta-analysis was performed under the guidance of the PRISMA guidelines and registered with PROSPERO (CRD42022298439). Phase 2 and 3 randomized controlled trials (RCT), published by PubMed, medline, embase, and Scopus, before 15 June 2022, and related to NSCLC and ICIs, were searched for our meta-analysis. Two researchers searched the databases independently. The retrieval word was ("nivolumab" OR "ipilimumab" OR "sintilimab" OR "tislelizumab" OR "cemiplimab" OR "camrelizumab" OR "BMS 936558" OR "BMS 936559" OR "pembrolizumab" OR "lambrolizumab" OR "MK 3475" OR "pidilizumab" OR "CT 011" OR "durvalumab" OR "MEDI 4736" OR "atezolizumab" OR "MPDL 3280a" OR "avelumab" OR "AMP 224" OR "PD-1" OR "PD-L1" OR "B7-H1" OR "CD274" OR "programmed death 1" OR "programmed death ligand 1" OR "CTLA-4 Antigen"[Mesh]) AND ("lung tumour" OR "lung cancer" OR "lung carcinoma" OR "lung neoplasm" OR "lung malignancy" OR "lung sarcoma" OR "Lung Neoplasms"[Mesh] OR "Carcinoma, Non-Small-Cell Lung" OR "squamous cell lung carcinoma" OR "lung adenocarcinoma " OR "large cell lung carcinoma"). In addition, we reviewed the References and Supplementary materials for the final searched articles.
All the final included studies should include the programmed cell death protein 1 (PD-1) or programmed cell death 1 ligand (PD-L1) or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors in the treatment of intervention group, while the control group contained no ICIs, and the prognosis according to the sex of the patients should be provided. In addition, the included studies needed to meet the following criteria. (a) Type of study: phase II/III RCTs; (b) patients: advanced or metastatic NSCLC that cannot be treated by surgery. The following types of studies were excluded. (a) Non-English articles; (b) studies containing cancers beyond NSCLC; (c) studies unable to obtain the full article; (d) studies with survival prognosis that not able to be analyzed. For studies that have published multiple reports, only the latest or most complete reports were chose for further analysis.
2.2. Data fetch
Two researchers independently extracted the following information from each study: (a) year of publication, author, study stage, line of treatment, treatment, and median follow-up time; (b) number of patients, sex, and tumour histological type; (c) the long-term survival prognosis of the males patients and female patients, respectively.
2.3. Quality evaluation
The quality of the included clinical trials was assessed through the Cochrane collaboration tool. Each eligible study was mainly evaluated in six aspects: (a) the sequence generation; (b) allocation concealment; (c) blinding; (d) incomplete outcome data; (e) selective outcome reporting; (f) free of other bias. Each section was rated as “low risk,” “high risk” or “unclear risk”. The Revman software was used to visualize the results of the article quality evaluation
2.4. Data analysis and statistical methods
The pooled hazard ratios (HR) and 95% confidence interval (CI) was used to assess the therapeutic outcomes between the intervention and control groups. Heterogeneity between studies was assessed by the I2 statistics and I2 value more than 50% is an indication of significant heterogeneity. Subgroup analyses were performed to investigate the sources of heterogeneity. Considering the complexity of baseline level and therapeutic regimen, random effects model was applied to improve the reliability of the results in this article. Sensitivity analysis was used to test the stability of the results. The Egger’s test was used to test if the included studies had a publication bias (p < .1). The above analysis was performed using the Stata software. All of the p-values reported were two-sided and p < .05 was considered as to be statistically different, unless otherwise stated.
3. Result
3.1. Included studies and their characteristics
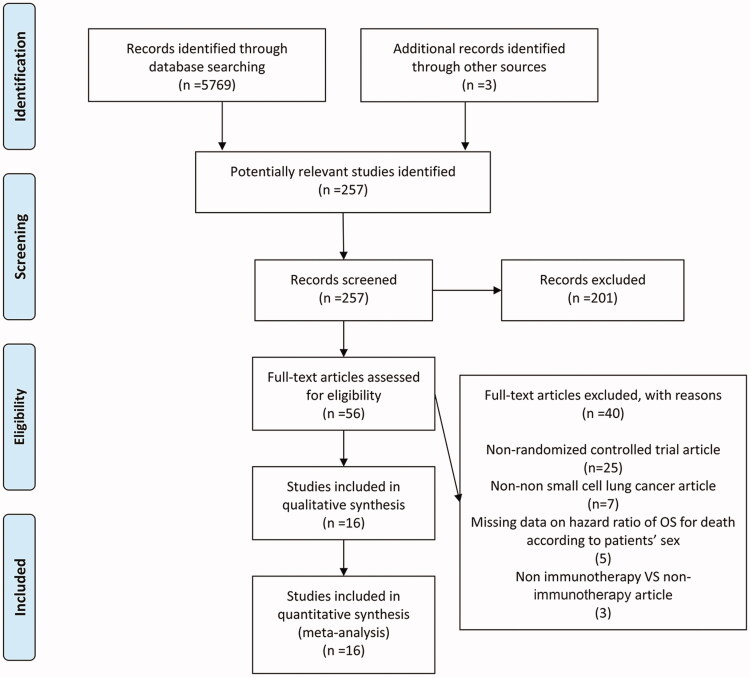
Under the guidance of the PRISMA guidelines, after obtaining 5569 studies through the search strategy, 257 potentially relevant articles were selected. After a summary and full-text review, 16 RCTs [9–24] met the inclusion criteria (Figure 1). Of these RCTs, 6 RCTs studied with chemotherapy plus ICIs vs chemotherapy, 9 RCTs with ICIs vs. chemotherapy, and 1 RCT with ICIs vs placebo. Meanwhile, these RCTs included 8 of PD-1 inhibitors (3 nivolumab, 5 pembrolizumab), 5 PD-L1 inhibitors (3 atezolizumab, 1 avelumab, 1 durvalumab), 1 CTLA-4 inhibitors (ipilimumab), and 2 PD-1 + CTLA-4 inhibitors (nivolumab + ipilimumab) (Table 1). Publication dates ranged from 2015 to 2021. All trials were phase 3 RCTs, of which 10 were in first-line treatment stages and others 6 were in non-first line. Three studies only included patients with squamous NSCLC, while 4 studies were only involved with patients with non-squamous NSCLC.
Figure 1.
A schematic flow for the selection of articles included in this meta-analysis.
Table 1.
Characteristics of included clinical trials in the meta-analysis.
| Author, year | Trial phase | Line | Histotype | Agent of immunotherapy | Number of patients |
Therapeutic regimen |
Number of inclusions |
Median follow-up time | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Treatment | Control | Treatment | Control | ||||||
| Brahmer J et al., 2015 | 3 | Non-first | Sq | PD-1 | 208 | 64 | Nivolumab | Docetaxel | 135 | 137 | 11+ months |
| Borghaei H et al., 2015 | 3 | Non-first | Non-sq | PD-1 | 319 | 263 | Nivolumab | Docetaxel | 292 | 290 | 18 months |
| Carbone DP et al., 2017 | 3 | First | All | PD-1 | 332 | 209 | Nivolumab | Chemotherapy | 271 | 270 | 13.5 months |
| Govindan R et al., 2017 | 3 | First | Sq | CTLA-4 | 635 | 114 | Ipilimumab + chemotherapy | Placebo + chemotherapy | 388 | 361 | 12.5 months |
| Fehrenbacher L et al., 2018 | 3 | Non-first | All | PD-L1 | 758 | 467 | Atezolizumab | Docetaxel | 613 | 612 | 21 months |
| Barlesi F et al., 2018 | 3 | Non-first | All | PD-L1 | 367 | 162 | Avelumab | Docetaxel | 264 | 265 | 17.8+ months |
| Gandhi L et al., 2018 | 3 | First | Non-sq | PD-1 | 363 | 253 | Pembrolizumab + chemotherapy | Placebo + chemotherapy | 410 | 206 | 10.5 months |
| Paz-Ares L et al., 2018 | 3 | First | Sq | PD-1 | 455 | 104 | Pembrolizumab + chemotherapy | Placebo + chemotherapy | 278 | 281 | 7.8 months |
| West H et al., 2019 | 3 | First | Non-sq | PD-L1 | 400 | 279 | Atezolizumab + chemotherapy | Chemotherapy | 451 | 228 | 18.5+ months |
| Reck M et al., 2019 | 3 | First | All | PD-1 | 187 | 118 | Pembrolizumab | Chemotherapy | 154 | 151 | 25.2 months |
| Hellmann MD et al., 2019 | 3 | First | All | PD-1 + CTLA-4 | 515 | 278 | Nivolumab + ipilimumab | Chemotherapy | 396 | 397 | 29.3+ months |
| Herbst RS et al., 2020 | 2/3 | Non-first | All | PD-1 | 634 | 399 | Pembrolizumab | Docetaxel | 690 | 343 | 42.6 months |
| Wu YL et al., 2021 | 3 | First | All | PD-1 | 224 | 38 | Pembrolizumab | Chemotherapy | 128 | 134 | 33.0 months |
| Nishio M et al., 2021 | 3 | First | Non-sq | PD-L1 | 384 | 194 | Atezolizumab + chemotherapy | Chemotherapy | 292 | 286 | 14.8 months |
| Faivre-Finn C et al., 2021 | 3 | Non-first | All | PD-L1 | 500 | 213 | Durvalumab | Placebo | 476 | 237 | 34.2 months |
| Paz-Ares L et al., 2021 | 3 | First | All | PD-1 + CTLA-4 | 504 | 215 | Nivolumab + ipilimumab + chemotherapy | Chemotherapy | 361 | 358 | 13.2 months |
sq: squamous; non-sq: non-squamous; PD-1: programmed cell death protein 1; PD-L1: programmed cell death 1 ligand 1; CTLA-4: cytotoxic T-lymphocyte-associated protein 4.
The number of patients included in each trial ranged between 262 and 1225. A total of 10,155 patients were included, containing 6785 male patients (66.8%) and 3370 female patients (33.2%). The intervention group included 5599 (55.1%) patients and 4556 (44.9%) patients were included in the control group. In all studies, the median follow-up time varies between 7.8 and 42.6 months. All studies reported an overall survival (OS)-related HR based on the sex of the patient.
Randomized treatment assignment sequences were generated in all trials, two among which were double-blind trials. In addition, control groups of 4 trials used placebo to rule out placebo effects potentially triggered by ICIs. The clinical trials were evaluated using the Cochrane collaboration tool and the results were showed in Supplementary Figures 1 and 2.
3.2. Treatment effect of ICIs in male and female
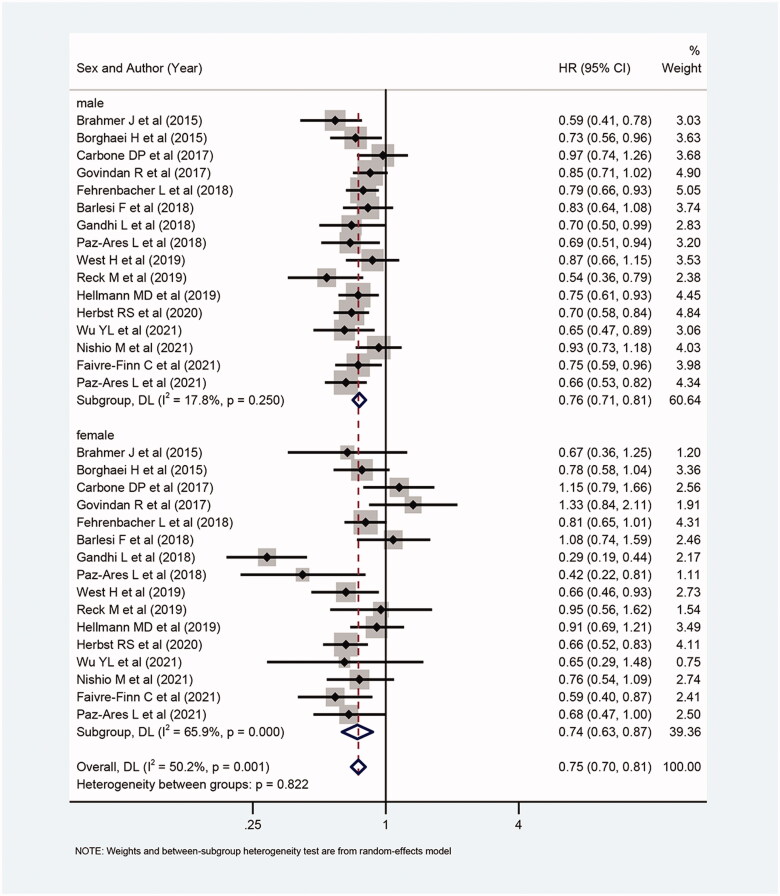
Overall, compared with patients not receiving ICIs (receiving chemotherapy or placebo only), the OS for patients receiving ICIs alone, combination (CTLA-4 + PD-1/L1) or with chemotherapy were all significantly prolonged (HR: 0.75, 95%CI 0.70–0.81; I2=50.2%; Figure 2). Meanwhile, both in males (HR: 0.76, 95%Cl 0.71–0.81; I2=17.8%; Figure 2) and females (HR: 0.74, 95%Cl 0.63–0.87; I2=65.9%; Figure 2), those who received ICIs (with or without chemotherapy) had longer OS than those who did not receive ICIs.
Figure 2.
Forest plot of comparison: overall survival of patients receiving ICIs alone or with chemotherapy versus patients not receiving ICIs (receiving chemotherapy or placebo only) (male: p < .001, female: p < .001, overall: p < .001).
3.3. Subgroup analysis
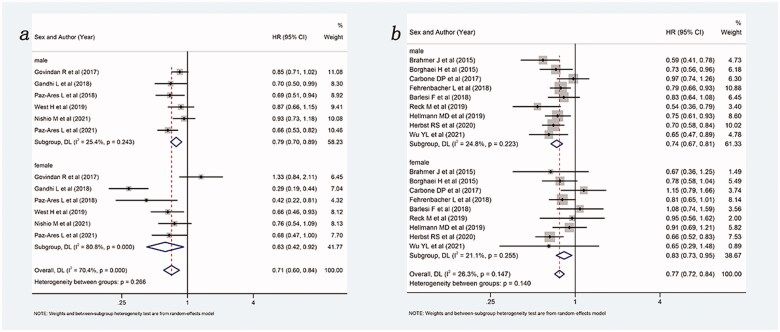
When control group was limited to chemotherapy, the results of the subgroup analysis based on whether the intervention group contains chemotherapy are shown in Figure 3. Overall, compared with chemotherapy alone, ICIs with (HR: 0.71, 95%Cl 0.60–0.84) or without (HR: 0.77, 95%Cl 0.72–0.84) chemotherapy were able to improve OS in NSCLC patients. For ICIs combined with chemotherapy, both males (HR: 0.79, 95%Cl 0.70–0.89) and females (HR: 0.63, 95%Cl 0.42–0.92) could benefit from ICIs combined with chemotherapy comparing with chemotherapy alone. For ICIs without combination with chemotherapy, both males (HR: 0.74, 95%Cl 0.67–0.81) and females (HR: 0.83, 95%Cl 0.73–0.95) could benefit from ICIs compared to chemotherapy alone. It seemed that females benefitted more from the ICIs & chemotherapy combination than males, while males benefitted more from ICIs without chemotherapy than females.
Figure 3.
Forest plot of comparison based on whether the intervention group contains chemotherapy. (a) Overall survival of patients receiving ICIs plus chemotherapy versus patients receiving chemotherapy alone (male: p < .001, female: p = .018, overall: p < .001). (b) Overall survival of patients receiving ICIs versus patients receiving chemotherapy alone (male: p < .001, female: p = .005, overall: p < .001).
A subgroup analysis based on the patient’s treatment stage was also performed, and the results are shown in Figure 4. Overall, ICIs with or without chemotherapy can improve OS, no matter in first-line (HR: 0.75, 95%Cl 0.87–0.85) or non-fist line (HR: 0.75, 95%Cl 0.69–0.80), compared with control group (chemotherapy or placebo). In the first line, both males (HR: 0.77, 95%Cl 0.69–0.85) and females (HR: 0.73, 95%Cl 0.56–0.96) could benefit from ICIs with or without chemotherapy compared with the control group. In the non-first line, both males (HR: 0.74, 95%Cl 0.68–0.81) and females (HR: 0.75, 95%Cl 0.65–0.68) also could benefit from ICIs with or without chemotherapy compared with the control group.
Figure 4.
Forest plot of comparison based on treatment stage. (a) Overall survival of patients receiving ICIs with or without chemotherapy versus patients not receiving ICIs (receiving chemotherapy or placebo only) in first line (male: p < .001, female: p = .023, overall: p < .001). (b) Overall survival of patients receiving ICIs with or without chemotherapy versus patients not receiving ICIs (receiving chemotherapy or placebo only) in non-first line (male: p < .001, female: p < .001, overall: p < .001).
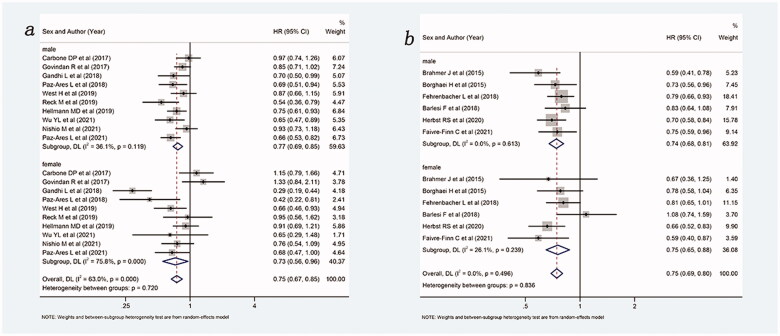
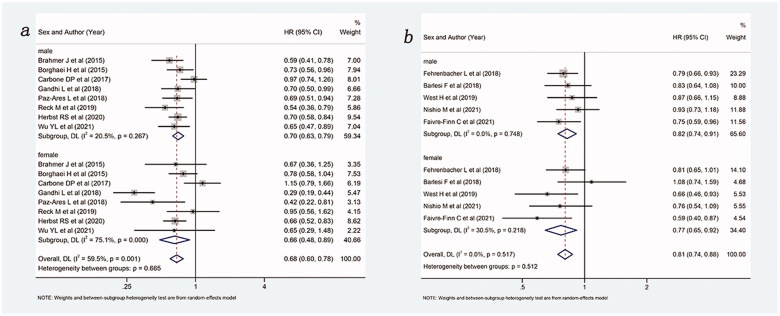
Then, a subgroup analysis of the treatment effects of PD-1 inhibitor and PD-L1 inhibitors was performed, and the results are shown in Figure 5. Overall, compared with control group, ICIs with or without chemotherapy improved OS, whether as PD-1 inhibitors (HR: 0.68, 95%Cl 0.60–0.78) or as PD-L1 inhibitors (HR: 0.81, 95%Cl 0.74–0.88). Among the PD-1 inhibitor, both males (HR: 0.70, 95%Cl 0.63–0.79) and females (HR: 0.66, 95%Cl 0.48–0.89) could benefit from ICIs with or without chemotherapy compared with the control group. Among the PD-L1 inhibitors, both males (HR: 0.82, 95%Cl 0.74–0.91) and females (HR: 0.77, 95%Cl 0.65–0.92) could also benefit from intervention group compared with the control group. It was important to note that treatment with PD-1 inhibitor appears superior to PD-L1 inhibitor in males and females.
Figure 5.
Forest plot of comparison based on the type of ICIs. (a) Overall survival of patients receiving PD-1 inhibitor with or without chemotherapy versus patients not receiving ICIs (receiving chemotherapy or placebo only) (male: p < .001, female: p = .006, overall: p < .001). (b) Overall survival of patients receiving PD-L1 inhibitor with or without chemotherapy versus patients not receiving ICIs (receiving chemotherapy or placebo only) (male: p < .001, female: p = .003, overall: p < .001).
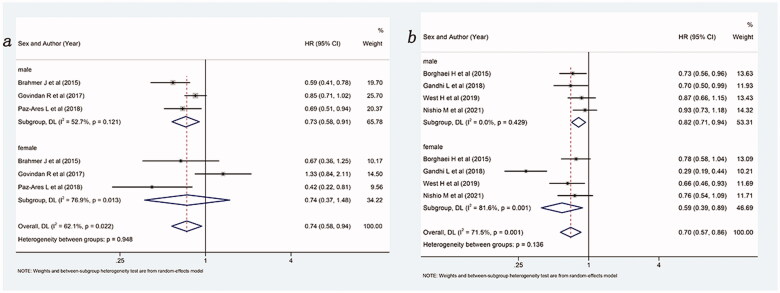
Lastly, a subgroup analysis based on the histological type of the included patients was performed, and the results are shown in Figure 6. Overall, compared with no ICIs, ICIs with or without chemotherapy were able to improve OS, whether for squamous (HR: 0.74, 95%Cl 0.58–0.94) or non-squamous (HR: 0.70, 95%Cl 0.57–0.86) NSCLC patients. In patients with non-squamous NSCLC, both males (HR: 0.82, 95%Cl 0.71–0.94) and females (HR: 0.59, 95%Cl 0.39–0.89) could benefit more from ICIs with or without chemotherapy compared with the control group. Notably, in squamous, males benefitted more from ICIs (with or without chemotherapy) than the control group (HR: 0.73, 95%Cl 0.58–0.91) while females did not (HR: 0.74, 95%Cl 0.37–1.48).
Figure 6.
Forest plot of comparison based on histological type. (a) Overall survival of patients receiving ICIs with or without chemotherapy versus patients not receiving ICIs (receiving chemotherapy or placebo only) in squamous NSCLC (male: p = .005, female: p = .400, overall: p = .015). (b) Overall survival of patients receiving ICIs with or without chemotherapy versus patients not receiving ICIs (receiving chemotherapy or placebo only) in non-squamous NSCLC (male: p = .005, female: p = .011, overall: p = .001).
3.4. Sensitivity analysis and publication bias
Sensitivity analysis showed results were stable in the HR of OS comparing immunotherapy (CTLA-4 or PD-1/L1 inhibitor alone, combination or with chemotherapy) to non-immunotherapy (receiving chemotherapy or placebo only) (Supplementary Figure 3). The Egger’s test showed that there was no statistically significant publication bias in the study findings included in this meta-analysis (p = .311, Supplementary Figure 4).
4. Discussion
Curing cancer through precision medicine is the overarching goal of a new wave of molecular and genomic therapies. Precision medicine relies on biomarker discovery and research [25]. Sex can also be considered as a biomarker since there are clear differences in genes and lifestyle habits between males and females [26,27]. In NSCLC, a kind of cancer with sexual dimorphism, the role of sex as potential biomarker may be particularly evident [28]. The sex differences between males and females with NSCLC are reflected in many aspects: (1) the median age of females at diagnosis is lower than males; (2) the tobacco contact history of females is generally less than that of males; (3) in females and males with similar tobacco exposure, lung cancer occurs earlier in females [29,30]; (4) the major histological subtype in females is adenocarcinoma, while in males is squamous; (5) females usually have better outcomes than males at all stages of diagnosis; (6) epidermal growth factor receptor (EGFR) mutations is more common in females [31,32]. However, in the existing clinical treatment options, sex is rarely used as the basis for choosing the treatment options. Therefore, the role of sex in the treatment of NSCLC needs to be further explored.
In this meta-analysis, although the ICIs, compared to chemotherapy, could improve OS in NSCLC, the females seemed to benefit more from chemotherapy plus ICIs than males, and males seemed to benefit more from ICIs alone than females. Similar results were also found by Conforti et al. [33], in whose study, females with advanced lung cancer experience a larger benefit from the addition of chemotherapy to an anti-PD-1 or PD-L1 than males while males show greater efficacy of anti-PD-1 alone than females. Since the single-agent immunotherapy included in Conforti study contained only the case of PD-1 inhibitors, our studies that including PD-1, PD-L1 and CTLA-4 inhibitors could be considered necessary complementary and complete. Moreover, our study included the larger number of NSCLC patients (10,155 patients), coming from 16 RCT pairs, which increased the credibility of our analysis.
In addition to the evidence from the clinical experiments, numerous preclinical studies have also revealed an association between sex differences and lymphocytes. For example, Liva et al. found that testosterone can act directly to increase IL-10 gene expression via the androgen receptor on CD4 + T lymphocytes [34]. The effects of ICIs depends on immune priming of peripheral lymphoid tissues. Besides, by analyzing patient data, Conforti et al. found that females responses to pembrolizumab may be less than males [35]. These may be the biological principles that support males patients may benefit more from ICIs alone than females.
As for another therapeutic regimen that is included in this article, the association of chemotherapy plus immunotherapy with better prognosis in female patients has ever been found in triple-negative breast cancer (TNBC). The clinical effect showed that the combination regimen of PD-1/L1 inhibitor plus chemotherapy had a higher success rate in metastatic TNBC (mTNBC) than ICIs alone [36]. Biological studies show that ICIs combined with chemotherapy have the potential to enhance the recognition and elimination of tumour cells by the immune system [37]. Squamous, the main type of NSCLC in male patients, responds poorer to chemotherapy, and lung adenocarcinoma, the main type of NSCLC in female patients, is more sensitive to chemotherapy. Therefore, the sex differences in ICIs plus chemotherapy treatment patterns in the two sexes may mainly stem from the difference in benefit in chemotherapy. Indeed, studies have attributed the poor immunotherapy outcomes observed in female patients to weaker antigenicity in females, and chemotherapy is thought could increase the mutational load of tumours and thus increase the antigenicity of tumour cells [26]. However, the results can only show that males and females benefit differently under different treatment regimens. It is not clear the immunotherapy regimen that females or males could benefit most is ICIs alone or combined with chemotherapy. Therefore, RCTs in males and females compared ICIs alone to ICIs combination chemotherapy may be warranted.
Besides whether immunotherapy should be combined with chemotherapy, whether immunotherapy should be used in the first line is also a question worth clinical attention [38]. By subgroup analysis according the line of treatment, it was found that whether in the first or non-first-line, males and females have a better benefit in immunotherapy (ICIs alone or together with chemotherapy), compared with the control group. Moreover, there were no significant sex differences in the performance of immunotherapy (ICIs alone or together with chemotherapy) in both first-and and non-first-line treatments. The study by Ruiz Patino et al. [39] also supports that immunotherapy with any treatment line of therapy can improve survival in patients with advanced metastatic NSCLC. However, from the perspective of clinical application, first-line patients are supposed to have better immune system function and stronger physical function than non-first-line patients, which may help lower the adverse effects and improve the treatment effect. Therefore, immunotherapy may be more meaningful in first-line treatment.
With the rapid development of immunotherapy, both in first and non-first-line therapy, there are different types of ICIs in clinical use. Among them, the most widely used and the most dominant types are PD-1 and PD-L1 inhibitors. By subgroup analysis according the inhibitor type, no significant difference in benefit using PD-1 or PD-L1 inhibitors. However, it can be found that PD-1 inhibitors appear to work better than PD-L inhibitors in both males and females. Theoretically, PD-1 antibodies can bind to the PD-1 protein on T cell membranes, which would block the binding between both PD-1 and PD-L1/PD-L2. However, the PD-L1 antibody can only interact with PD-L1 and specifically blocks the binding between PD-1 and PD-L1. Therefore, after using the anti-PD-L1 treatment, the interaction between PD-1 and PD-L2 may still inhibit T cells. This may explain the greater potential of PD-1 inhibitors than PD-L1 inhibitors in the treatment of NSCLC patients. Note that the large sample size gap between the PD-1 and PD-L1 subgroup analysis may influence result in the study. The number of PD-1 related studies is much larger than PD-L1 related studies because of the early launch time of PD-1. In this study, eight PD-1 studies were included, involving 8340 patients and five PD-L1 studies were included, involving 1315 patients. Therefore, richer clinical experimental data are needed to support more accurate and convincing results.
The most interesting thing in this study is that immunotherapy showed significant sex differences in the treatment of squamous NSCLC. Possible explanations were tried to be given in terms of both genetic and behavioural differences between the sexes. Females and males differ in early-stage transcriptomic biomarkers and cell-lineage gene of squamous NSCLC [40]. For instance, the sex-determining region Y-Box 2 (SOX2) [41] is a potential cell lineage gene highly expressed in the pathogenesis of squamous NSCLC. Those sex-related genes like Y-Box 2 (SOX2) may be the gene hierarchy responsible for sex differences. In terms of behavioural habits, males have more tobacco applicability than females. Studies have shown that in squamous patients, light smokers are associated with more female patients, more advanced tumours, and worse prognosis than gravity smokers [42,43]. Therefore, it may be the poor prognosis of squamous NSCLC in females that results in worse benefit from immunotherapy in females than in males. But the deeper biological principles remain to be explored.
Although female and male patients with squamous NSCLC showed significant differences in benefit from immunotherapy, it has to be admitted that great heterogeneity was found in the subgroup analysis for squamous females (I2=76.9%, p = .013). The experimental groups in the three included studies were nivolumab (Brahmer J), ipilimumab + chemotherapy (Govindan R), and pembrolizumab + chemotherapy (Paz-Ares L). It can be found that CTLA-4 inhibitors (ipilimumab) performed the worst in female squamous NSCLC treatment (HR: 1.33, 95%Cl 0.84–2.11), which may be the critical to outcomes. In some published studies, a higher benefit from anti-CTLA-4 was found in males compared to females [44]. The significant sex differences in the effects of anti-CTLA-4 may influence the conclusion that males benefit more from immunotherapy (ICIs alone or with chemotherapy) than females in squamous NSCLC. Therefore, it is necessary to replicate this finding in a larger cohort of squamous NSCLC patients.
Honestly, this study has some limitations. First, most RCTs did not report OS according to sex, which largely limited the number of RCTs that we included. Second, in the RCTs included in our study, the vast majority of non-immunotherapy control groups were chemotherapy, which narrowed the scope of non-immunotherapy in a practical sense.
5. Conclusion
To conclude, appropriate biomarkers, on the one hand, can help to facilitate a more effective selection of those patients who could truly benefit from ICIs. On the other hand, it may be beneficial to select the most appropriate treatment strategy for patients to achieve precision medicine. In our study, immunotherapy for squamous NSCLC performed significantly better in males than in females. It would be a good choice for future studies to explore different immunotherapy regimens for males and females, which may be beneficial to help patients choose the most appropriate treatment strategy.
Supplementary Material
Acknowledgement
The authors have no financial support to declare.
Glossary
Abbreviations
- CI
confidence interval
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- EGFR
epidermal growth factor receptor
- HR
hazard ratios
- ICIs
immune-checkpoint inhibitors
- NSCLC
non-small cell lung cancer
- OS
overall survival
- PD-1
programmed death 1
- PD-L1
programmed death ligand 1
- RCT
randomized controlled trials
- TNBC
triple-negative breast cancer
Author contributions
Dina Guo designed the research process. Jiali Liang and Jiaze Hong searched the database for corresponding articles and drafted the meta-analysis. Xin Tang and Xinyi Qiu extracted useful information from the articles above. Keying Zhu used statistical software for analysis. Liyuan Zhou used statistical software for analysis and polished this article. All authors had read and approved the manuscript and ensured that this was the case.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Abbott M, Ustoyev Y.. Cancer and the immune system: the history and background of immunotherapy. Semin Oncol Nurs. 2019;35(5):150923. [DOI] [PubMed] [Google Scholar]
- 2.Klein SL, Flanagan KL.. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. [DOI] [PubMed] [Google Scholar]
- 3.Klein SL, Morgan R.. The impact of sex and gender on immunotherapy outcomes. Biol Sex Differ. 2020;11(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Botticelli A, Onesti CE, Zizzari I, et al. The sexist behaviour of immune checkpoint inhibitors in cancer therapy? Oncotarget. 2017;8(59):99336–99346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conforti F, Pala L, Bagnardi V, et al. Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. The Lancet Oncol. 2018;19(6):737–746. [DOI] [PubMed] [Google Scholar]
- 6.Wallis CJ, Butaney M, Satkunasivam R, et al. Association of patient sex with efficacy of immune checkpoint inhibitors and overall survival in advanced cancers: a systematic review and meta-analysis. JAMA Oncol. 2019;5(4):529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–249. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y, Cho HJ, Stranger BE, et al. Sex dimorphism in response to targeted therapy and immunotherapy in non-small cell lung cancer patients: a narrative review. Transl Lung Cancer Res. 2022;11(5):920–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carbone DP, Reck M, Paz-Ares L, CheckMate 026 Investigators, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Govindan R. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol. 2017;35(30):3449–3457. [DOI] [PubMed] [Google Scholar]
- 13.Fehrenbacher L, von Pawel J, Park K, et al. Updated efficacy analysis including secondary population results for OAK: a randomized phase III study of atezolizumab versus docetaxel in patients with previously treated advanced non-small cell lung cancer. J Thorac Oncol. 2018;13(8):1156–1170. [DOI] [PubMed] [Google Scholar]
- 14.Barlesi F, Vansteenkiste J, Spigel D, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN lung 200): an open-label, randomised, phase 3 study. Lancet Oncol. 2018;19(11):1468–1479. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. [DOI] [PubMed] [Google Scholar]
- 16.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. [DOI] [PubMed] [Google Scholar]
- 17.West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924–937. [DOI] [PubMed] [Google Scholar]
- 18.Reck M. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non–small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37(7):537–546. [DOI] [PubMed] [Google Scholar]
- 19.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020–2031. [DOI] [PubMed] [Google Scholar]
- 20.Herbst RS. Long-term outcomes and retreatment among patients with previously treated, programmed death-ligand 1–positive, advanced non–small-cell lung cancer in the KEYNOTE-010 study. J Clin Oncol. 2020;38(14):1580–1590. [DOI] [PubMed] [Google Scholar]
- 21.Wu YL, Zhang L, Fan Y, et al. Randomized clinical trial of pembrolizumab vs chemotherapy for previously untreated Chinese patients with PD-L1-positive locally advanced or metastatic non-small-cell lung cancer: KEYNOTE-042 China study. Int J Cancer. 2021;148(9):2313–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishio M, Barlesi F, West H, et al. Atezolizumab plus chemotherapy for First-Line treatment of nonsquamous NSCLC: results from the randomized phase 3 IMpower132 trial. J Thorac Oncol. 2021;16(4):653–664. [DOI] [PubMed] [Google Scholar]
- 23.Faivre-Finn C, Vicente D, Kurata T, et al. Four-year survival with durvalumab after chemoradiotherapy in stage III NSCLC – an update from the PACIFIC trial. J Thorac Oncol. 2021;16(5):860–867. [DOI] [PubMed] [Google Scholar]
- 24.Paz-Ares L, Ciuleanu T-E, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198–211. [DOI] [PubMed] [Google Scholar]
- 25.Collins DC, Sundar R, Lim JSJ, et al. Towards precision medicine in the clinic: from biomarker discovery to novel therapeutics. Trends Pharmacol Sci. 2017;38(1):25–40. [DOI] [PubMed] [Google Scholar]
- 26.Wang S, Cowley LA, Liu X-S.. Sex differences in cancer immunotherapy efficacy, biomarkers, and therapeutic strategy. Molecules. 2019;24(18):3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cook MB, McGlynn KA, Devesa SS, et al. Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomarkers Prev. 2011;20(8):1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Lara V, Hernandez-Martinez J-M, Arrieta O.. Influence of estrogen in non-small cell lung cancer and its clinical implications. J Thorac Dis. 2018;10(1):482–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vidyullatha P. Lung cancer incidence in never smokers: genetic and gender basis. Gene Rep. 2016;4:19–207. [Google Scholar]
- 30.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. [DOI] [PubMed] [Google Scholar]
- 31.De Matteis S, Consonni D, Pesatori AC, et al. Are women who smoke at higher risk for lung cancer than men who smoke? Am J Epidemiol. 2013;177(7):601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958–967. [DOI] [PubMed] [Google Scholar]
- 33.Conforti F, Pala L, Bagnardi V, et al. Sex-based heterogeneity in response to lung cancer immunotherapy: a systematic review and meta-analysis. J Natl Cancer Inst. 2019;111(8):772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liva SM, Voskuhl RR.. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J Immunol. 2001;167(4):2060–2067. [DOI] [PubMed] [Google Scholar]
- 35.Conforti F, Pala L, Bagnardi V, et al. Sex-based differences of the tumor mutational burden and T-cell inflammation of the tumor microenvironment. Ann Oncol. 2019;30(4):653–655. [DOI] [PubMed] [Google Scholar]
- 36.Keenan TE, Tolaney SM.. Role of immunotherapy in triple-negative breast cancer. J Natl Compr Canc Netw. 2020;18(4):479–489. [DOI] [PubMed] [Google Scholar]
- 37.Leonetti A, Wever B, Mazzaschi G, et al. Molecular basis and rationale for combining immune checkpoint inhibitors with chemotherapy in non-small cell lung cancer. Drug Resist Updat. 2019;46:100644. [DOI] [PubMed] [Google Scholar]
- 38.Proto C, Ferrara R, Signorelli D, et al. Choosing wisely first line immunotherapy in non-small cell lung cancer (NSCLC): what to add and what to leave out. Cancer Treat Rev. 2019;75:39–51. [DOI] [PubMed] [Google Scholar]
- 39.Ruiz‐Patiño A, Arrieta O, Cardona AF, CLICaP, et al. Immunotherapy at any line of treatment improves survival in patients with advanced metastatic non‐small cell lung cancer (NSCLC) compared with chemotherapy (Quijote). Thorac Cancer. 2020;11(2):353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Q, Wang Y, Duan M, et al. Females and males show differences in early-stage transcriptomic biomarkers of lung adenocarcinoma and lung squamous cell carcinoma. Diagnostics. 2021;11(2):347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan P, Kadara H, Behrens C, et al. Sex determining region Y-Box 2 (SOX2) is a potential cell-lineage gene highly expressed in the pathogenesis of squamous cell carcinomas of the lung. PLOS One. 2010;5(2):e9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mo J, Hu X, Gu L, et al. Smokers or non-smokers: who benefits more from immune checkpoint inhibitors in treatment of malignancies? An up-to-date meta-analysis. World J Surg Onc. 2020;18(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okamoto T, Suzuki Y, Fujishita T, et al. The prognostic impact of the amount of tobacco smoking in non-small cell lung cancer—differences between adenocarcinoma and squamous cell carcinoma. Lung Cancer. 2014;85(2):125–130. [DOI] [PubMed] [Google Scholar]
- 44.Grassadonia A, Sperduti I, Vici P, et al. Effect of gender on the outcome of patients receiving immune checkpoint inhibitors for advanced cancer: a systematic review and meta-analysis of phase III randomized clinical trials. JCM. 2018;7(12):542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.