Abstract
The identification of patients who can benefit the most from the available preventive treatments is important in chronic migraine. We explored the rate of excellent responders to onabotulinumtoxinA in a multicenter European study and explored the predictors of such response, according to different definitions. A pooled analysis on chronic migraineurs treated with onabotulinumtoxinA and followed-up for, at least, 9 months was performed. Excellent responders were defined either as patients with a ≥75% decrease in monthly headache days (percent-based excellent responders) or as patients with <4 monthly headache days (frequency-based excellent responders). The characteristics of excellent responders at the baseline were compared with the ones of patients with a <30% decrease in monthly headache days. Percent-based excellent responders represented about 10% of the sample, whilst frequency-based excellent responders were about 5% of the sample. Compared with non-responders, percent-based excellent responders had a higher prevalence of medication overuse and a higher excellent response rate even after the 1st and the 2nd injection. Females were less like to be frequency-based excellent responders. Chronic migraine sufferers without medication overuse and of female sex may find fewer benefits with onabotulinumtoxinA. Additionally, the excellent response status is identifiable after the first cycle.
Keywords: chronic migraine, onabotulinumtoxinA, predictors of response, excellent responders
1. Introduction
Migraine is characterized by unilateral attacks of throbbing, pulsating headache, associated with nausea and vomiting, phonophobia and/or photophobia [1]. Chronic migraine (CM) is characterized by a headache occurring on ≥15 days per month for ≥3 months, displaying migraine features on ≥8 days per month [1]. CM affects about 2% of the general population, affecting especially middle-aged adults [2], thus imposing a significant burden on society [3]. Since this, the development of effective and safe treatment for CM is needed. Before the advent of the monoclonal antibodies (mAbs) acting on the calcitonin gene-related peptide (CGRP) or its receptor, the only drug specifically approved to prevent CM was onabotulinumtoxinA (BT-A) [4]. A large proportion of patients with CM, up to 70% within the first three cycles, respond well to BT-A with a ≥50% reduction in monthly headache days compared with the baseline [5]; however, the rate of ≥50% responders from real-life studies seem lower in the first 3 cycles (49.3%) [6], but a response to the BT-A may be attained even after 12 months—i.e., 5 treatment cycles—from treatment start [7]. Conversely, monoclonal antibodies targeting the CGRP pathway have a quick onset of action, even in CM [8,9,10,11,12,13,14,15]. Therefore, to better understand the place of BT-A in migraine prevention, it would be important to clearly identify patients’ clinical features potentially predicting a substantial response to the drug. An interesting population is that of patients with ≥75% decrease in monthly headache days compared with the baseline, i.e., excellent responders. Previous studies dealt with the determination of the predictors of response to BT-A, according to different definitions, but with conflicting results [16,17,18,19,20,21,22,23,24,25]. The main limitation of the current literature is that the excellent responders are a limited proportion of the treated patients; hence, large collaborations are needed to report reliable results. Furthermore, another possible definition of “excellent responders”, based on the absolute number of residual headache days, could be explored. Indeed, even a patient who reports a ≥75% decrease in monthly headache days could still have a great impairment, especially if the number of headache days at the baseline was very high, thus presenting the same limitations of the 50% of responders [25].
The aim of this study was to report the rate of excellent responders according to the percent decrease in headache frequency (percent-based) and a residual headache frequency (frequency-based) in a large European sample after 3, 6 and 9 months, corresponding to the first three cycles of BT-A treatment. Additionally, the rate of patients reporting no headache days at all, i.e., total responders, was also explored. Finally, the potential predictors of an excellent response to BT-A, according to the different definitions, were explored.
2. Materials and Methods
2.1. Study Design
The methods of this study have been previously published, since two more articles were derived from the analysis of the present dataset [26,27]; this is a pooled patient-level analysis of data from real-life studies on patients with CM treated with BT-A at 16 European headache centers. The centers potentially participating in the study were first identified through a MEDLINE search of the most recent publications on BT-A treatment for CM in Europe. Globally, 19 centers were initially selected and contacted by e-mail. Among them, two centers did not meet the inclusion criteria and one declined to participate in the study. A list of the headache centers participating in this study is available in supplemental Table S1. All centers have already performed, between 2010 and 2020, a real-life prospective study on patients with CM, diagnosed according to the International Classification of Headache Disorders 3rd edition (ICHD-3) criteria [1], aged ≥ 18 years and treated with BT-A according to the Phase 3 REsearch Evaluating Migraine Prophylaxis Therapy (PREEMPT) protocol [28,29]. Additionally, involved centers must:
Have obtained study approval by the local ethics committees and informed consent obtained from patients, if necessary, according to the local regulations;
Be able to share a database of collected data;
Have planned a follow-up of ≥9 months for all patients, irrespective of the treatment discontinuation.
Since all included centers followed the EHF guidelines to initiate BT-A in CM [30], there were no differences regarding its prescription.
The present analysis was approved by the Internal Review Board of the University of L’Aquila with protocol number 23/2020; approval was shared with all centers; moreover, local ethical approval to pool data was obtained from the centers, if needed. Patients did not need sign any additional informed consent for this study since no additional data with respect to those originally collected were required for the present analyses. All procedures were conducted in accordance with the latest version of the declaration of Helsinki.
2.2. Variables and Response Categories
The following variables were collected: age, sex, duration of migraine, duration of CM, presence of medication overuse (MO), duration of the MO, the mean number of monthly headache days (MHDs) in the last 3 months, the mean number of days per month in which patient took, at least, one medication (MDs) in the last 3 months, other preventive treatments co-assumed among BT-A. Regarding the last point, patients were allowed to continue, at a stable dose, the preventive treatment they were already taking when they started the BT-A. Hence, the effect of the preventative treatment used in combination with BT-A was not quantifiable. Response categories were defined by the percent reduction in mean MHDs during the course of each 3-months treatment cycle compared to the baseline, i.e., the mean of the three months before starting BT-A treatment. Patients who reported 0 headache days during one cycle were indicated as 100% responders, i.e., total responders. Percent-based excellent response was defined as a ≥75% decrease in MHDs from the baseline, as already done in other articles [19,23]. Frequency-based excellent response was defined as a mean of <4 MHDs during the third BT-A cycle, since under 4 MHDs over a 3-months period, the preventive treatment should not be prescribed [31]. Non-response was defined as patients reporting a <30% decrease in MHDs from the baseline [20]. The above-mentioned categories were calculated for every injection cycle, but the comparisons between the different class of responders were performed during the third BT-A cycle, since it is the temporal limit to stop BT-A in case of a poor response [7]. Patients discontinuing treatment before the third cycle or lost at follow-up were considered as non-responders, except if the discontinuation was due to the “positive stopping rule”, i.e., patients reporting a ≥50% response and choosing to withdraw BT-A before the third cycle; these data were included in the analysis using a “last observation carried forward” approach.
2.3. Statistical Analysis
Continuous variables were reported as mean ± standard deviation (SD), whilst categorical variables were reported as counts and percentages. Baseline features were compared between percent-based excellent responders and non-responders. The same calculations were made between frequency-based excellent responders and non-responders. A multivariate logistic model with backward elimination was performed with the variables significantly associated with the response status at the univariate analysis, in order to assess independent predictors of excellent response. The model was then checked for collinearity using the phi-correlation coefficient and the overall goodness of fit of the model was explored with the Pearson’s χ2 goodness of fit test. Receiving Operating Characteristic (ROC) curve analysis was used to explore the sensitivity and specificity of those parameters significantly associated with the responder status even in the multivariate analysis. All analyses used the intent-to-treat population, including all patients who started BT-A treatment, but variables were considered for the analyses only if available for, at least, two-thirds of patients. No imputation was done for missing data. Since the calculations were made on available data, no sample size calculations were made. P-values lower than 0.05 (two-tailed) were considered significant. All calculations were made with STATA IC 13.1 software.
3. Results
3.1. Baseline Features
Globally, data from 2879 patients were included, of whom 2351 (81.7%) were women. The mean age was 46.6 ± 12.32 years, whilst the mean migraine duration was 30.74 ± 12.84 years. Patients with MO were 2055 (71.4%). At the baseline, patients displayed MHDs of 23.82 ± 5.93 and a mean number of MDs of 19.47 ± 9.05; these data are summarized in Table 1.
Table 1.
Baseline characteristics of the analyzed sample.
| Variable | Value |
|---|---|
| Number of patients | 2879 |
| Age (years) | 46.6 ± 12.3 |
| Female sex | 2351 (81.7) |
| Migraine duration (years) | 30.7 ± 12.8 |
| CM duration (years) | 8.0 ± 8.0 |
| Medication overuse at baseline | 2055 (71.38) |
| MHDs | 23.8 ± 5.9 |
| MDs | 19.4 ± 9.1 |
3.2. Rates of Excellent Responders and Total Responders
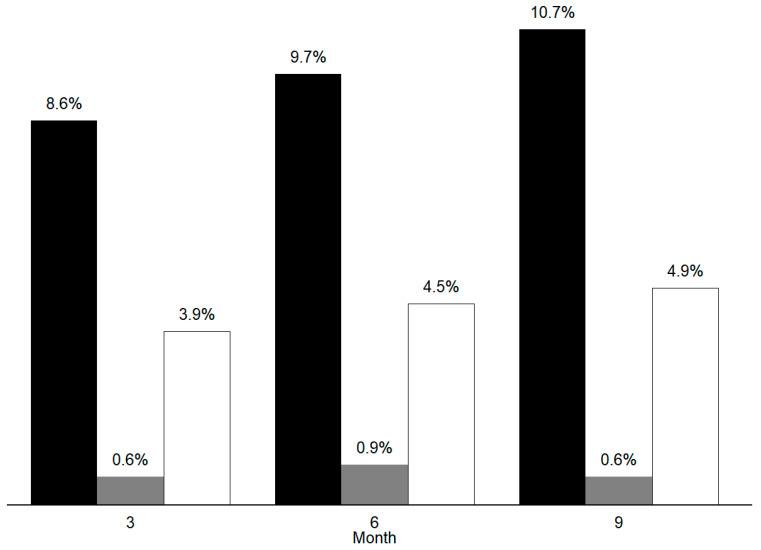
Total responders were 18 (0.6%) during the first, 26 (0.9%) during the second, and 18 (0.6%) during the third treatment cycle. Percent-based excellent responders were 248 (8.6%) during the first, 278 (9.7%) during the second, and 307 (10.7%) during the third treatment cycle. Considering any of the three BT-A cycles, 532 (18.5%) patients were classifiable as percent-based excellent responders. Eighty-four patients (2.9%) were classifiable as percent-based excellent responders during all three treatment cycles. Frequency-based excellent responders were 112 (3.9%) during the first, 130 (4.5%) during the second, and 140 (4.9%) during the third BT-A cycle. Frequency-based excellent responders in, at least, one cycle were 272 (9.5%), whilst 22 patients (0.76%) were classifiable as frequency-based excellent responders in all injection cycles. Non-responders were 1564 (54.32%) during the first, 1374 (47.72%) during the second, and 1376 (47.79%) during the third treatment cycle. Data regarding percent-based excellent responders, total responders and frequency-based excellent responders are graphically summarized in Figure 1.
Figure 1.
Overall number of percent-based excellent responders to onabotulinumtoxinA (black bars), the overall number of total responders (grey bars) and the overall number of the frequency-based excellent responders (white bars) during the first, second, and third onabotulinumtoxinA cycle.
3.3. Comparison between Percent-Based Excellent Responders and Non-Responders
Compared with non-responders, percent-based excellent responders displayed a higher prevalence of MO (71.7% vs. 63.8%, p = 0.001) and a higher medication consumption at the baseline (20.97 ± 9.45 vs. 18.10 ± 9.95, p < 0.001); moreover, percent-based excellent responders at the 9th month displayed a higher proportion of excellent responders even at the 3rd (52.85% vs. 47.15%, p < 0.001) and the 6th months (77.83% vs. 22.17%, p < 0.001). At the multivariate analysis, the presence of MO at the baseline and an excellent response during the first and the second BT-A cycles were significantly associated with percent-based excellent response during the third BT-A cycle. these data are summarized in Table 2. The Pearson’s χ2 test displayed a general goodness of fit of the entire model (Pearson’s χ2 = 509.6, p = 0.0093). The ROC curve analysis displayed an area under the curve of 0.7813.
Table 2.
Comparison between the percent-based excellent responders and the non-responders after the third BT-A cycle.
| Status at the 9th Month | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Baseline Characteristics |
Excellent
Responders (n = 307) |
Non-Responders
(n = 1376) |
p-Value | OR (95% CI) | p-Value | OR (95% CI) |
| Age | 46.68 ± 12.55 | 46.79 ± 12.89 | 0.887 | - | - | - |
| Female sex | 241/307 (%) | 1101/1376 (%) | 0.604 | 0.92 [0.68 ÷ 1.25] | - | - |
| Migraine duration | 28.20 ± 12.77 | 30.63 ± 13.74 | 0.056 | - | - | - |
| CM duration | 8.66 ± 9.27 | 8.34 ± 8.80 | 0.603 | - | - | - |
| MO | 220/307 (71.66%) | 878/1376 (63.81%) | 0.001 | 1.59 [1.20 ÷ 2.12] | 0.046 | 1.91 [1.01 ÷ 3.62] |
| Headache days | 25.71 ± 5.38 | 24.42 ± 6.16 | 0.001 | - | 0.106 | 1.02 [0.99 ÷ 1.06] |
| Medication days | 21.20 ± 9.58 | 18.10 ± 9.95 | <0.001 | - | 0.877 | 1 [0.97 ÷ 1.03] |
| Excellent responders et the 3rd month | 102/307 (52.85%) | 91/1376 (47.15%) | <0.001 | 1.95 [1.63 ÷ 2.27] | 0.047 | 1.63 [1.01 ÷ 2.64] |
| Excellent responders et the 6th month | 158/307 (77.83%) | 45/1376 (22.17%) | <0.001 | 3.45 [3.07 ÷ 3.82] | <0.001 | 22.14 [14.37 ÷ 34.10] |
Abbreviations: CM: chronic migraine; MO: medication overuse.
3.4. Comparison between Frequency-Based Excellent Responders and Non-Responders during the Third BT-A Cycle
At the third injection cycle, only the female sex resulted significantly associated with the frequency-based excellent responders, whilst none of the other variables was. In particular, the proportion of females was significantly lower in the frequency-based excellent responders. These data are summarized in Table 3.
Table 3.
Comparison between frequency-based excellent responders and non-responders during the third onabotulinumtoxinA cycle.
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Baseline Characteristics | Frequency-Based Excellent Responders (n = 140) | Non-Responders (n = 1376) | p-Value | OR (95% CI) | p-Value | OR (95% CI) |
| Age | 46.1 ± 12.9 | 46.79 ± 12.9 | 0.52 | - | Not applicable | |
| Female sex | 101/140 (72.1%) | 1101/1376 (80%) | 0.041 | 0.71 [0.41 ÷ 0.98] | ||
| Migraine duration | 28.8 ± 13.9 | 30.6 ± 13.7 | 0.345 | - | ||
| CM duration | 8.6 ± 8.3 | 8.3 ± 8.8 | 0.715 | - | ||
| MO | 68.6% | 65.2% | 0.103 | 1.39 [0.93 ÷ 2.06] | ||
| Headache Days | 23.4 ± 6.6 | 24.4 ± 6.2 | 0.064 | - | ||
| Medication Days | 19.7 ± 9.5 | 18.1 ± 9.9 | 0.104 | - | ||
Abbreviations: CM: chronic migraine; MO: medication overuse headache.
4. Discussion
According to the present study, the rate of percent-based excellent responders to BT-A was about 10% in every injection cycle and about 3% of patients were classifiable as percent-based excellent responders in all cycles. In a previous Spanish study, the proportion of percent-based excellent responders to BT-A was about 20% after 6 months and 25% after one year [20]; the higher number of HD and MD at baseline in the present sample may account for the observed discrepancies. Total responders were less than 1% during all BT-A cycles. Anyhow, the stability of this outcome suggests that BT-A action lasts in time, as already assessed in the open-label phase of the PREEMPT trial [29]; however, patients obtaining a 50% or even a 75% response compared with the baseline might indeed have a substantial number of days with headache and, consequently, a high headache-related disability: many patients would still have >8 debilitating HDs per month, configuring resistance to BT-A [6,25]. The proportion of frequency-based excellent responders was about 4% and, indeed, this criterion is far more restrictive. Our data show that frequency-based criteria identify a lower proportion of responders than percent-based criteria; this finding is in line with what was found in a real-life study on erenumab [26]. The clinical consequence of this finding is that many patients on treatment with BT-A could benefit from combination treatments for the prevention of migraine. Oral treatments are particularly suitable for combination with BT-A as the toxin has a local action without systemic absorption; therefore, it has no interaction with systemic treatments. Identifying patients with CM with an excellent response to BT-A has gained importance with the advent of the effective, safe, and specific mAbs acting on the CGRP pathway [31]; it is increasingly important to select patients who can have a substantial benefit from BT-A in order to better target treatments. In patients with CM in whom an excellent response to BT-A can be anticipated, starting and continuing the treatment is a suitable option, while in patients in whom an excellent response cannot be anticipated, strict monitoring could be advised, with the possibility of switching to other treatments [32], or adding new ones, such as an anti-CGRP mAb [33]. Anyway, the choice of combining 2 preventive treatments if often not supported by well-designed randomized-controlled trials [34] and, consequently, some authors suggest that it should only be cautiously considered only in the case of refractory CM [35]. Response to BT-A is assessed over 2–3 cycles [7], corresponding to 6–9 months, during which patients with CM might still suffer from frequent and debilitating headaches. Response to BT-A can have different definitions [36], each identifying different proportions of patients [37]. Excellent responders are an interesting group of patients, as they represent those who can continue treatment with BT-A without starting concurrent or alternative preventive treatments. The absolute number of headache days is, in our opinion, a more reliable way to quantify the benefit of migraine prevention; patients with <4 headache days per month do not require further migraine prevention according to common practice guidelines [31]. Notably, both groups of excellent responders may be distinguished from non-responders according to the baseline characteristics. The present study found that the presence of MO and the excellent responder status at 3 and 6 months were independent predictors of the percent-based excellent responder status in comparison with non-responders (Table 2). Unexpectedly, the proportion of MO sufferers at the baseline was higher in excellent responders than non-responders whilst, in other studies, the presence of MO was negatively associated with the BT-A response [20,24]. Data from animal models identify MO as a risk factor for peripheral sensitization of the trigeminal pathway [38], since an up-regulation of CGRP in the trigeminal ganglion has been found in rats [39]. In humans, CGRP has been linked with trigeminal-mediated pain [40] and its levels have been found higher in the peripheral blood of CM and MO sufferers [41]; moreover, BT-A has been found to decrease inter-ictal levels of CGRP in CM sufferers [42], thus making CGRP plasma levels as possible predictors of response to BT-A [17,19]; thus, patients with MO should be characterized by a higher release of CGRP by trigeminal C-fibers, which is blocked by BT-A [43]. Indeed, BT-A inhibits the SNARE complex and, therefore, blocks the exocytosis of CGRP from trigeminal C-fibers [43]. In this context, patients who suffer from MO and, hence, who have a higher CGRP release, should find higher benefits with BT-A; it should be noted that the significance of MO in the model was marginal and could be prone to bias. The absolute difference in MDs and in the prevalence of MO between excellent responders and non-responders was relatively low; therefore, we cannot exclude a spurious significance, even because of the high number of patients which could have increased the risk of falsely significant results. Another notable finding of our paper is that the early status of excellent responder was the strongest predictor of excellent response during the third BT-A cycle (Table 2); this finding is in line with a previous publication on the same subset which showed that BT-A response status during the second BT-A cycle could predict response during the third cycle [7]. Therefore, even when considering an excellent response to BT-A, it might not be necessary to wait for the third BT-A cycle to plan a switch to other migraine preventatives; this is a relevant finding in the era of anti-CGRP treatments [32].
The excellent responders at the 9th month displayed a higher proportion of excellent responders even at the 3rd and the 6th month; this suggests that the excellent response can be distinguished from a non-response even from the first injection cycle and, again, this witness that BT-A spreads rapidly [44] and that action lasts on time [45]. The HD and the MD were significantly associated with the responder status, but only at the univariate analysis and, consequently, they are not considerable as independent predictors of an excellent response versus non-response. Despite this, a large body of literature revealed that migraine frequency at the baseline was negatively associated with BT-A response [18,24]. Interestingly, our results partially confirm the ones obtained in a previous study from Pozo-Rosich’s group [24]. In that study, the factors independently associated with a percent-based excellent response at 6 months were daily headache frequency, the presence of MOH, and a higher number of HD at the baseline. The duration of migraine was found to be associated with an excellent response to the BT-A, but after 12 months [24]. The HD was also found to be associated with a poor response to BT-A in a study by Dominguez et al. [21] and our study seems to confirm this association. In particular, a higher number of HD at the baseline may negatively predict the response to BT-A compared to poor-responders; this suggests that a greater burden of CM at baseline may account for a lower response after 9 months of BT-A of treatment, in line with the available literature [18,20,22]. Again, the excellent responders’ group in the ninth month showed a higher number of excellent responders in the 6th month of treatment, confirming that the effect of the BT-A spreads in the first months [44] and is almost stable on time. The frequency-based excellent responders displayed a lower proportion of females at the baseline. The gender difference in BT-A response has been studied, but with conflicting results. In particular, a previous study failed to find a clear gender difference in the clinical response towards BT-A [27]. Despite its statistical significance, sex difference in the response towards BT-A is gravated by the great difference between men and women numerosity as well as a higher number of drop-outs among men; thus, the exact magnitude of this difference is difficultly detectable but, at least, questionable. Globally, this study allowed the description of a limited subgroup of patients with CM and an excellent response to BT-A in a large group of almost 3000 patients treated in clinical practice; this number ensures the reliability of our findings even if regarding a limited proportion of patients. Besides, we could analyze two different definitions of excellent response to BT-A, one based on traditional percent-based response status and one based on absolute headache frequency, which could be clinically significant; however, our study also has some limitations. We could not quantify the effect of the preventative treatment used in combination with BT-A, which was allowed as the present study was based on clinical practice. Although the data were collected prospectively, the study had a there was significant heterogeneity of data collection across centers. We minimized the collected data in order to limit missing information and inaccuracy; however, this led to missing important information, including the presence of cutaneous allodynia, the number of prior preventive treatment failures, BT-A dosage, and a number of medical comorbidities which may have affected response to BT-A [22,24,45]. A further study limitation is represented by the heterogeneity in the quantitative contribution of different centers, since large centers might have had a greater impact on the study than the smaller ones. The number of drop-outs may have also led to the underestimation of excellent responders as some of them stopped the treatment according to a “positive stopping rule” [46]. Furthermore, we did not distinguish migraine days from headache days, which could have added further detail to the outcome assessment. Lastly, we could not distinguish patients discontinuing BT-A because of ineffectiveness from those who discontinued because of non-compliance.
5. Conclusions
The present study suggested that the proportion of sustained percent-based excellent responders to BT-A is about 10% during the first three injection cycles, while the proportion of sustained frequency-based excellent responders is about 3%. Early excellent response to BT-A predicted response during the third BT-A cycle, which is a relevant finding when planning treatment strategies in clinical practice. Factors independently associated with percent-based excellent response included the presence of MO and a lower number of headache days per month at baseline. In brief, our data indicate that the more the impairment of patients at the baseline is, the less the therapeutic benefit with BT-A.
Abbreviations
| BT-A | onabotulinumtoxinA |
| CGRP | calcitonin gene-related peptide |
| CM | chronic migraine |
| CSD | cortical spreading depression |
| HIT-6 | 6 items headache impact test |
| ICHD-3 | International Classification of Headache Disorders 3rd edition |
| mAbs | monoclonal antibodies |
| MIDAS | Migraine Disability Assessment Questionnaire |
| MHDs | the mean number of monthly headache days in the last 3 months |
| MDs | the mean number of days per month in which patient took at least one medication (MDs) in the last 3 months |
| MO | medication overuse |
| PREEMPT | Phase 3 REsearch Evaluating Migraine Prophylaxis Therapy |
| ROC | Receiving Operating Characteristic |
| SD | standard deviation |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph191710975/s1, Table S1: Overview of the study centers.
Author Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Internal Review Board of the University of L’Aquila with protocol number 23/2020.
Informed Consent Statement
Patient consent was waived because patients had already given their written informed consent for study participation and data publication during the enrolment to the studies from which data were derived to perform the present study.
Data Availability Statement
The dataset analyzed during this study is available from the corresponding author on reasonable request.
Conflicts of Interest
Raffaele Ornello reports speaker fees from Novartis and Eli Lilly, travel grants from Teva, and support for publication from Novartis and Allergan. Simona Sacco reports personal fees from Allergan-AbbVie, AstraZeneca, Abbott, Teva, Novartis, and Eli Lilly. Alicia Alpuente has received honoraria for educational projects from Allergan-Abbvie, Novartis, and Teva. Patricia Pozo-Rosich has received honoraria as a consultant and speaker for Allergan-Abbvie, Almirall, Amgen, Biohaven, Chiesi, Eli Lilly, Medscape, Neurodiem, Novartis, and Teva; her research group has received research grants from Allergan, and has received funding for clinical trials from Alder, Allergan-Abbvie, electroCore, Eli Lilly, Novartis, and Teva; she is a trustee member of the board of the International Headache Society, member of the Council of the European Headache Society; she is on the editorial board of Revista de Neurologia, associate editor of Cephalalgia, Headache, Neurologia, and Frontiers in Neurology, and advisor for The Journal of Headache and Pain; she is a member of the Clinical Trials Standing Committee of the International Headache Society; she has edited the Guidelines for the Diagnosis and Treatment of Headache of the Spanish Neurological Society; she is the founder of www.midolordecabeza.org; she does not own stocks from any pharmaceutical company. Andrea Negro has received speaking honoraria and has served on the advisory boards of Allergan, Eli Lilly and Company, and Novartis. Paolo Martelletti has received speaker’s honoraria and has served on the advisory boards of Allergan, Eli Lilly and Company, Novartis, and Teva Pharmaceuticals; he is also an EMA Expert and has served as Editor-in-Chief of The Journal of Headache and Pain and as Editor-in-Chief of SN Comprehensive Clinical Medicine; he also declares royalties received from Springer Nature. Massimo Filippi is Editor-in-Chief of the Journal of Neurology and Associate Editor of Neurological Sciences; received compensation for consulting services and/or speaking activity from Bayer, Biogen Idec, Merck Serono, Novartis, Roche, Sanofi Genzyme, Takeda, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck Serono, Novartis, Teva Pharmaceutical Industries, Roche, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA). Elena Filatova reports speaker fees from Novartis and Teva. Nina Latysheva reports speaker fees from Novartis and Allergan. Carlo Baraldi has received travel grants and honoraria from Allergan, Novartis, Teva, and Ely Lilly. Licia Grazzi has received consultancy and advisory fees from Allergan, electroCore LLC, Novartis, and Eli Lilly, and collaborates in trials sponsored by Eli Lilly, Teva, and PharmInd. Simona Guerzoni has received travel grants and honoraria from Allergan, Eli Lilly, Teva, and Novartis. Luca Pani has been a scientific consultant to Acadia USA, BCG, Switzerland, Dialectica UK-Greece, EDRA-LSWR Publishing Company Italy, Ferrer Spain, Guidepoint UK, Inpeco SA Switzerland, Johnson and Johnson USA, Otsuka USA, Pfizer Global USA, PharmaMar Spain, Relmada Therapeutics USA, Sanofi-Genzyme, USA, Takeda USA, WCG-VeraSci USA. Sabina Cevoli has received honoraria as speaker, for participating on advisory boards, or for clinical investigation studies from Teva, Allergan, Novartis, Lilly, and Ibsa. Fayyaz Ahmed has received honorarium from Allergan for being on their advisory board and is paid to charitable organisations. Giorgio Lambru has received speaker honoraria and funding for travel, and has received honoraria for participation on advisory boards sponsored by Allergan, Novartis, Eli Lilly, and Teva; he has also received speaker honoraria and funding for travel from electroCore, Nevro Corp., and Autonomic Technologies. Anna P. Andreou has received speaker honoraria and funding for travel from Allergan, Eli Lilly, and eNeura, honoraria for participation on advisory boards sponsored by Allergan and Eli Lilly, sponsorship for educational purposes from eNeura, Allergan, Autonomic Technologies, and Novartis, and an equipment grant from eNeura. Antonio Russo has received speaker honoraria from Allergan, Lilly, Novartis, and Teva, and serves as an associate editor of Frontiers in Neurology (Headache Medicine and Facial Pain session). Marcello Silvestro has received speaker honoraria from Lilly, Novartis, and Teva. Ruth Ruscheweyh has received travel grants and/or honoraria from Allergan, Hormosan, Lilly, Novartis, and Teva. Fabrizio Vernieri has received travel grants, honoraria for advisory boards, and speaker panels from Allergan, Angelini, Lilly, Novartis, and Teva. Marcin Straburzynski has received personal fees from Novartis and Teva. Katharina Kamm has received travel grants and/ or honoraria from Lilly, Novartis, and Teva. Paola Torelli has received travel grants and/or honoraria from Allergan, Lilly, Novartis, and Teva. Anna Gryglas-Dworak has received honoraria as a speaker and travel grants from Allergan and honoraria as a speaker from Novartis. Ilaria Frattale, Anna Maria Miscio, Antonio Santoro, Nicoletta Brunelli, Bruno Colombo, Calogera Butera, and Marco Russo declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 2.Storvner L.J., Hagen K., Linde M., Steiner T.J. The global prevalence of headache: An update, with analysis of the influences of methodological factors on prevalence estimates. J. Headache Pain. 2022;23:34. doi: 10.1186/s10194-022-01402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burch R.C., Buse D.C., Lipton R.B. Migraine: Epidemiology, burden, and comorbidity. Neurol. Clin. 2019;37:631–649. doi: 10.1016/j.ncl.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Lantéri-Minet M., Duru G., Mudge M., Cottrell S. Quality of life impairment, disability and economic burden associated with chronic daily headache focusing on chronic migraine with or without medication overuse: A systematic review. Cephalalgia. 2011;31:837–850. doi: 10.1177/0333102411398400. [DOI] [PubMed] [Google Scholar]
- 5.Silberstein S.D., Dodick D.W., Aurora S.K., Diener H.C., DeGreyse R.E., Lipton R.B., Turkerl C.C. Per cent of patients with chronic migraine who responded per onabotulinumtoxinA treatment cycle: PREEMPT. J. Neurol. Neurosurg. Psychiatry. 2015;86:996–1001. doi: 10.1136/jnnp-2013-307149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ornello R., Ahmed F., Negro A., Miscio A.M., Santoro A., Alpuente A., Russo A., Silverstro M., Cevoli S., Brunelli N., et al. Early management of OnabotulinumtoxinA treatment in chronic migraine: Insights from a real-life European multicentre study. Pain Ther. 2021;10:637–650. doi: 10.1007/s40122-021-00253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frampton J.E., Silberstein S. OnabotulinumtoxinA: A review in the prevention of chronic migraine. Drugs. 2018;78:589–600. doi: 10.1007/s40265-018-0894-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vernieri F., Paolucci M., Altamura C., Pasqualetti P., Mastrangelo V., Pierangeli G., Cevoli S., D’Amico D., Grazzi L. Onabotulinumtoxin-A in chronic migraine: Should timing and definition of non-responder status be revised? Headache. 2019;59:1300–1309. doi: 10.1111/head.13617. [DOI] [PubMed] [Google Scholar]
- 9.Shwedt T., Reuter U., Tepper S., Ashina M., Kudrow D., Broessner G., Boudreau G.P., McAllister P., Vu T., Zhnag F., et al. Early onset of efficacy with erenumab in patients with episodic and chronic migraine. J. Headache Pain. 2018;19:92. doi: 10.1186/s10194-018-0923-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwedt T.J., Kuruppu D.K., Dong Y., Standley K., Yunes-Medina L., Pearlman E. Early onset of effect following galcanezumab treatment in patients with previous preventive medication failures. J. Headache Pain. 2021;22:15. doi: 10.1186/s10194-021-01230-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeshima T., Nakai M., Shibasaki Y., Ishida M., Kim B.K., Ning X., Koga N. Early onset of efficacy with fremanezumab in patients with episodic and chronic migraine: Subanalysis of two phase 2b/3 trials in Japanese and Korean patients. J. Headache Pain. 2022;23:24. doi: 10.1186/s10194-022-01393-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winner P.K., Spierings E.L.H., Yeung P.P., Aycardi E., Blankenbiller T., Grozinski-Wolff M., Yang R., Ma Y. Early onset of efficacy with Fremanezumab for the preventive treatment of chronic migraine. Headache. 2021;59:1743–1752. doi: 10.1111/head.13654. [DOI] [PubMed] [Google Scholar]
- 13.Lambru G., Hill B., Murphy M., Tylova I., Andreou A.P. A prospective real-world analysis of erenumab in refractory chronic migraine. J. Headache Pain. 2020;21:61. doi: 10.1186/s10194-020-01127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russo A., Silvestro M., Scotto di Clemente F., Trojsi F., Bisecco A., Bonavita S., Tessitore A., Tedeschi G. Multidimensional assessment of the effects of erenumab in chronic migraine patients with previous unsuccessful preventive treatments: A comprehensive real-world experience. J. Headache Pain. 2020;21:69. doi: 10.1186/s10194-020-01143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goadsby P.J., Dodick D.W., Martinez J.M., Ferguson M.B., Oakes T.M., Zhang Q., Skljarevski V., Aurora S.K. Onset of efficacy and duration of response of galcanezumab for the prevention of episodic migraine: A post-hoc analysis. J. Neurol. Neurosurg. Psychiatry. 2021;90:939–944. doi: 10.1136/jnnp-2018-320242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ornello R., Casalena A., Frattale I., Gabriele A., Affaiati G., Giamberardino M.A., Assetta M., Maddestra M., Marzoli F., Viola S., et al. Real-life data on the efficacy and safety of erenumab in the Abruzzo region, central Italy. J. Headache Pain. 2020;21:32. doi: 10.1186/s10194-020-01102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cernuda-Morollon E., Martinez-Camblor P., Ramon C., Larrosa D., Serrano-Pertierra E., Pascual J. CGRP and VIP levels as predictors of efficacy of Onabotulinumtoxin type A in chronic migraine. Headache. 2014;54:987–995. doi: 10.1111/head.12372. [DOI] [PubMed] [Google Scholar]
- 18.Lee M.J., Lee C., Choi H., Chung C.S. Factors associated with favourable outcome in botulinum toxin A treatment for chronic migraine: A clinic-based prospective study. J. Neurol. Sci. 2016;363:51–54. doi: 10.1016/j.jns.2016.01.054. [DOI] [PubMed] [Google Scholar]
- 19.Dominguez C., Vieites-Prado A., Perez-Mato M., Sobrino T., Rodriguez-Osorio X., Lopez A., Campos F., Martinez F., Castillo J., Leira R. CGRP and PTX3 as predictors of efficacy of Onabotulinumtoxin type A in chronic migraine: An observational study. Headache. 2018;58:78–87. doi: 10.1111/head.13211. [DOI] [PubMed] [Google Scholar]
- 20.Domínguez C., Pozo-Rosich P., Torres-Ferrús M., Hernández-Beltrán N., Jurado-Cobo C., González-Oria C., Santos S., Monzón M.J., Latorre G., Álvaro L.C., et al. OnabotulinumtoxinA in chronic migraine: Predictors of response. A prospective multicentre descriptive study. Eur. J. Neurol. 2018;25:411–416. doi: 10.1111/ene.13523. [DOI] [PubMed] [Google Scholar]
- 21.Domínguez C., Pozo-Rosich P., Leira Y., Leira R. Unilateral pain and shorter duration of chronic migraine are significant predictors of response to onabotulinumtoxin A. Eur. J. Neurol. 2018;25:e48. doi: 10.1111/ene.13570. [DOI] [PubMed] [Google Scholar]
- 22.Schiano di Cola F., Caratozzolo S., Liberini P., Rao R., Padovani A. Response predictors in chronic migraine: Medication overuse and depressive symptoms negatively impact Onabotulinumtoxin-A treatment. Front. Neurol. 2019;10:678. doi: 10.3389/fneur.2019.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreno-Mayordomo R., Ruiz M., Pascual J., Gallego de la Sacristana M., Vidriales I., Sobrado M., Cernuda-Morollon E., Gago-Veiga A.B., Garcia-Azorin D., Telleria J.J., et al. CALCA and TRPV1 genes polymorphisms are related to a good outcome in female chronic migraine patients treated with OnabotulinumtoxinA. J. Headache Pain. 2019;20:39. doi: 10.1186/s10194-019-0989-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alpuente A., Gallardo V.J., Torres-Ferrús M., Álvarez-Sabin J., Pozo-Rosich P. Short and mid-term predictors of response to onabotulinumtoxinA: Real-life experience observational study. Headache. 2020;60:677–685. doi: 10.1111/head.13765. [DOI] [PubMed] [Google Scholar]
- 25.Martínez-Pías E., Guerrero Á.L., Sierra Á., Trigo J., García-Azorín D. Daily headache in chronic migraine is a predictive factor of response in patients who had completed three sessions of OnabotulinumtoxinA. Toxins. 2021;13:432. doi: 10.3390/toxins13060432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ornello R., Baraldi C., Guerzoni S., Lambru G., Andreou A.P., Raffaelli B., Gendolla A., Barbanti P., Aurilia C., Egeo G., et al. Comparing the relative and absolute effect of erenumab: Is a 50% response enough? Results from the ESTEEMen study. J. Headache Pain. 2022;23:38. doi: 10.1186/s10194-022-01408-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ornello R., Ahmed F., Negro A., Miscio A.M., Santoro A., Alpuente A., Russo A., Silvestro M., Cevoli S., Brunelli N., et al. Is there a gender difference in the response to onabotulinumtoxinA in chronic migraine? Insights from a real-life European multicenter study on 2879 patients. Pain Ther. 2021;10:1605–1618. doi: 10.1007/s40122-021-00328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aurora S.K., Winner P., Freeman M.C., Spierings E.L., Heiring J.O., DeGreyse R.E., VanDenburgh A.M., Nolan M.E., Turkel C.C. OnabotulinumtoxinA for treatment of chronic migraine: Pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51:1358–1373. doi: 10.1111/j.1526-4610.2011.01990.x. [DOI] [PubMed] [Google Scholar]
- 29.Diener H.C., Dodick D.W., Aurora S.K., Turkel C.C., DeGreyse R.R., Lipton R.B., Silberstein S.D., Brin M.F., PREEMPT2 Chronic Migraine Study Group OnabotulinumtoxinA for treatment of chronic migraine: Results from the doule-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30:804–814. doi: 10.1177/0333102410364677. [DOI] [PubMed] [Google Scholar]
- 30.Bendsten L., Sacco S., Ashina M., Mitsikostas Dm Ahmed F., Pozo-Rosich P., Martelletti P. Guideline on the use of onabotulinumtoxinA in chronic migraine: A consensus statement from the Erueopan Headache Federation. J. Headache Pain. 2018;19:91. doi: 10.1186/s10194-018-0921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sacco S., Amin F.M., Ashina M., Bendsten L., Deligianni C.I., Gil-Gouveia R., Katsarava Z., MassenVanDenBrink A., Martelletti P., Mitsikostas D.D., et al. European Headache Federation guideline on the use of monoclonal antibodies targeting the calcitonin gene related peptide pathway for migraine prevention-2022 update. J. Headache Pain. 2022;23:67. doi: 10.1186/s10194-022-01431-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sacco S., Russo A., Geppetti P., Grazzi L., Negro A., Tassorelli C., Tedeschi G., Martelletti P. What is changing in chronic migraine treatment? An algorithm for onabotulinumtoxinA treatment by the Italian chronic migraine group. Expert. Rev. Neurother. 2020;20:1275–1286. doi: 10.1080/14737175.2020.1825077. [DOI] [PubMed] [Google Scholar]
- 33.Guerzoni S., Baraldi C., Pani L. The association between onabotulinumtoxinA and anti-CGRP monoclonal antibodies: A reliable option for the treatment of chronic migraine. Neurol. Sci. 2022;43:5687–5695. doi: 10.1007/s10072-022-06195-5. [DOI] [PubMed] [Google Scholar]
- 34.Marteletti P. Combination therapy in migraine: Asset or issue? Exp. Rev. Neurother. 2020;10:995–996. doi: 10.1080/14737175.2020.1821655. [DOI] [PubMed] [Google Scholar]
- 35.Martelletti P., Luciani M., Spuntarelli V., Bentivegna E. Deprescribing in migraine. Exp. Opin. Drug Saf. 2020;6:623–625. doi: 10.1080/14740338.2021.1907342. [DOI] [PubMed] [Google Scholar]
- 36.Sacco S., Lampl C., Maassen van den Brink A., Caponnetto V., Braschinsky M., Ducros A., Little P., Pozo-Rosich P., Reuter U., Ruiz de la Torre E., et al. Burden and attitude to resistant and refractory migraine: A survey from the European Headache Federation with the endorsement of the European Migraine and Headache Alliance. J. Headache Pain. 2021;22:39. doi: 10.1186/s10194-021-01252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ching J., Tinsley A., Rothrock J. Prognosis following discontinuation of OnabotulinumA therapy in “super-responding” chronic migraine patients. Headache. 2019;59:1279–1285. doi: 10.1111/head.13630. [DOI] [PubMed] [Google Scholar]
- 38.De Felice M., Ossipov M.H., Wang R., Dussor G., Lai J., Meng I.D., Chichorro J., Andrews J.S., Rakhit S., Maddaford S., et al. Triptan-induced enhacement of neuronal nitric oxide synthetase in trieminal ganglion dural afferents uinderlies increased responsiveness to potential migraine triggers. Brain. 2010;133:2475–2488. doi: 10.1093/brain/awq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buonvicino D., Urru M., Muzzi M., Ranieri G., Luceri C., Oteri C., Lapucci A., Chiarugi A. Trigeminal ganglion transcriptome analysis in 2 rat models of medication-overuse headache reveals coherent and widespread induction of pronociceptive gene expression patterns. Pain. 2018;159:1980–1988. doi: 10.1097/j.pain.0000000000001291. [DOI] [PubMed] [Google Scholar]
- 40.Frese A., Summ O., Evers S. CGRP release in an experimental human trigeminal pain model. Cephalalgia. 2021;412:1268–1271. doi: 10.1177/03331024211017250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greco R., De Icco R., Demartini C., Zanabodi A.M., Tumelero E., Sances G., Allena M., Tassorelli C. Plasma levels of CGRP and expression of specific microRNAs in blood cells of episodic and chronic migraine subjects: Towards the identification of a panel of peripheral biomarkers of migraine? J. Headache Pain. 2020;21:122. doi: 10.1186/s10194-020-01189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cernuda-Morollon E., Ramon C., Martinez-Camblor P., Serrano-Pertierra E., Larrosa D., Pascual J. OnabotulinumotxinA decreases interictal CGRP plasma levels in patients with chronic migraine. Pain. 2015;156:820–824. doi: 10.1097/j.pain.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 43.Burstein R., Blumenfeld A.M., Silberstein S.D., Adams A.M., Brin M.F. Mechanism of action of OnabotulinumtoxinA in chronic migraine: A narrative review. Headache. 2020;60:1259–1272. doi: 10.1111/head.13849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dodick D.W., Silberstein S.D., Lipton R.B., DeGryse R.E., Manack A.A., Diener H.C. Early onset of effect of onabtoulinumtoxinA for chronic migraine treatment: Analysis of PREEMPT data. Cephalalgia. 2019;39:945–956. doi: 10.1177/0333102418825382. [DOI] [PubMed] [Google Scholar]
- 45.Negro A., Curto M., Lionetto L., Martelletti P. A two years open-label prospective study of OnabotulinumtoxinA 195 U in medication overuse headache: A real-world experience. J. Headache Pain. 2015;17:1. doi: 10.1186/s10194-016-0591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hollier-Hann G., Curry A., Onischchenko K., Akehurst R., Ahmed F., Davies B., Keyzor I. Updated cost-effectiveness analysis of onabotulinumtoxinA for the prevention of headache in adults with chronic migraine who have previously received three or more preventive treatments in the UK. J. Med. Econ. 2020;23:113–123. doi: 10.1080/13696998.2019.1675417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset analyzed during this study is available from the corresponding author on reasonable request.