ABSTRACT
Respiratory syncytial virus (RSV) is the main cause of severe respiratory infections in young children. The need for global epidemiologic data regarding RSV has been increasingly recognized. RSV A infections are reported more frequently than RSV B. Nonetheless, the temporal distribution of infections caused by both RSV groups has not been investigated globally. A systematic review was carried out regarding published studies on RSV A and B epidemiology, as well as RSV G gene ectodomain sequence data available at GenBank. A total of 76,668 [45,990 (60%) RSV A and 30,678 (40%) RSV B] positive samples from 83 countries were identified and included in the analysis. Genotype assignment was obtained in 5,340 RSV A and 2,518 RSV B sequences. Two patterns of RSV circulation were observed: continuous seasons with RSV A predominance and alternate predominance of RSV A and B. These patterns were observed in all regions, but the predominant RSV group seldom coincided in all continents during a given year or season. The most frequently identified RSV A genotype was NA1 (including ON1 viruses) (76.30%), and the most frequently identified RSV B genotype was BA (70.65%). Multiple genotypes circulated simultaneously throughout the evolutionary history of RSV, but genotype diversity decreased after the year 2000. The classification of RSV group and genotype is important for the development of vaccines, as well as to understand viral dynamics. This study displays the global and continental RSV circulation patterns from the first report of human RSV infection until the end of 2020.
KEYWORDS: Respiratory syncytial virus, respiratory viruses, epidemiology, genotype, molecular epidemiology
Introduction
Respiratory syncytial virus (RSV) is a major cause of respiratory illness worldwide [1]. While children are affected most notably by this virus, reinfections occur throughout life, and it is also a major cause of severe respiratory illness in elderly adults [2,3]. RSV is classified into two major antigenic and genetic groups (RSV A and B) based on the characterization of the G protein gene; in addition, multiple genotypes have been identified within each of these groups [4,5]. A notable feature of currently circulating strains is the presence of a partial duplication of the G protein gene both in RSV A (described originally as ON1 genotype) and RSV B (described originally as BA genotype) [6,7]. Overall, RSV A viruses are detected more frequently among patients with acute respiratory infections compared to RSV B viruses, although seasonal and regional variations in the predominance of viruses of each group have been reported [8]; however, the global pattern of RSV A and B circulation has not been assessed previously. In addition, the impact of the emergence of new genotypes on the relative contribution of RSV A and B as a cause of respiratory illness has not been defined. To better understand the interaction between RSV A and RSV B circulation, we carried out a systematic review to describe the contribution of each group to respiratory infections on a global, hemispheric, and continental scale. Finally, we identified the predominant genotype during each year, based on sequences available in GenBank, to assess the impact of emergent viruses on the predominance between RSV A and RSV B viruses.
Materials and Methods
Information sources, search strategies, and selection criteria. We conducted a systematic review across the National Library of Medicine Medline database following the approach detailed in the PRISMA guidelines [9]. The search strategy developed for this study (Table 1) was applied to Medline on 1 April 2021. Article search was restricted to articles with publication dates up to December 2020. No publication status criteria or language restrictions were applied. We included studies that fulfilled any of the following inclusion criteria: a) Studies reporting community or hospital incidence, surveillance studies, studies reporting relevant samples (incidence rate, surveillance) or providing sufficient input data to enable the calculation of outcomes of interest, or studies that involved RSV detection methods; b) Studies that included data collected over 12 continuous months or studies that did not include data for 12 continuous months, but were restricted to periods of respiratory syncytial virus circulation; and c) Studies, which provided clearly separated results for RSV A and RSV B detections. Studies that met any of the following criteria were excluded from analysis: a) Studies that did not separate results for individual years (calendar) or winter seasons; b) Studies that presented data only for RSV A or RSV B; c) Studies that presented data repeated in other studies, including systematic reviews and meta-analyses; and d) Studies with insufficient detail to determine the location (country) where the study samples were collected. When two studies reported overlapping data, the study that presented the more complete information was selected. Selection process. Two reviewers (KCF and GRA) conducted the search independently, which included articles published in English, Chinese, Japanese, Korean, French, Portuguese, and Spanish languages. Search results were reviewed by title and when considered relevant, the abstract was analyzed; based on information presented in the abstract, articles with potentially relevant information were selected for full-text review. The final dataset comprised all articles that fulfilled the selection criteria during this review. Discrepant results between reviewers were solved by a joint review of the article for data extraction and analysis.
Table 1.
Search strategy for systematic review of global distribution of respiratory syncytial virus A and B infections
|
Database |
Search strategy |
Results |
| Medline (Ovid) | 1 Mesh term Respiratory Syncytial Viruses | 20,925 |
| 2 Mesh term Respiratory Syncytial Virus, human | ||
| 3 RSV mp. | ||
| 4 exp Respiratory Syncytial Virus | ||
| 5 Mesh term Multiplex Polymerase Chain Reaction | ||
| 6 respiratory viruses mp. | ||
| 7 1 or 2 or 3 or 4 | ||
| 8 5 and 6 | ||
| 9 7 or 8 | ||
| 10 limit year 2020 |
Data collection process. The following characteristics were extracted from each of the articles included in the final analysis: number of total RSV A and RSV B samples per year or season; country; hemisphere (North or South); continent (North America, South America, Europe, Africa, Asia, and Oceania). RSV circulation peaks during the fall and winter months; as a result, the seasonal distribution differs in the Northern Hemisphere (most cases occur between October and March) and the Southern Hemisphere (most cases occur between April and September) [10]; therefore, yearly data was recorded as calendar year or season, according to RSV circulation patterns for each country: data for countries in the Northern Hemisphere were recorded per season, defined as detection from July of a given year through June of the following year, and data for countries in the Southern Hemisphere were recorded per calendar year (January through December of each year); however, there were some exceptions, particularly for countries located within 15◦ N and 15◦ S latitude (Supplementary Table 1). Data were extracted by the two reviewers independently; when discrepancies were found, the study was reviewed jointly by the two reviewers to confirm the final data.
Synthesis methods. Data from each study were tabulated including the number of RSV A and RSV B detections observed for each season/year in the study country (or each of the countries included in the study, when data from several countries was included in an article). The number of RSV A, RSV B, and total detections were summarized for each country, and for each season/year. In addition, data for all countries within a continent, hemisphere, or globally were analyzed. In order to carry out the global analysis, data for a given season in the Northern Hemisphere were added to data of the following calendar year in the Southern Hemisphere, after analyzing if there was a predominant flow of RSV A/RSV B infections from the Northern Hemisphere to the Southern Hemisphere or vice versa. To this end, we analyzed the change in the proportion of RSV A/RSV B infections observed in a given season in the Northern Hemisphere when the data from the Southern Hemisphere (previous or subsequent year) were added; addition of data of the subsequent year in the Southern Hemisphere resulted in an average 2% variation in the proportion of RSV A/RSV B infections compared to the Northern Hemisphere alone, while addition of data of the previous year resulted in an average 17% variation. This suggests that there is a predominant flow of strains from the Northern Hemisphere to the Southern Hemisphere.
Genotype analysis. In addition, we sought to analyze if there was a temporal association between the emergence of novel RSV genotypes and changes in the predominance of RSV A or RSV B. To this end, we analyzed all sequences that included the RSV G gene ectodomain region available at GenBank until 31 December 2020. We selected to directly analyze sequences, rather than extracting genotype data from published articles because currently there is no established consensus for genotype classification; as a result, sequences with high homology might have been reported as different genotypes in the literature, and sequences reported as distinct genotypes in two publications might be identical [5]. Thus, the totality of RSV sequences was downloaded, including sequences from whole genomes, partial genomes, complete coding sequences, and partial coding sequences; these were analyzed for genotype assignment with a systematic approach as previously described [4,5]. The following information from each sequence was included for analysis: date (month/year) of sample collection; country of sample collection; RSV group (A or B); and genotype assignment. We aimed to assess data from each country as winter seasons or calendar year based on the pattern of RSV circulation, as described in the Data collection process section; however, for a large proportion of sequences, information regarding the month/year of sample collection was not available, precluding the possibility of assigning the corresponding winter season for infections reported from countries with the seasonal pattern of RSV circulation. Therefore, analysis of the number and proportion of sequences of each RSV group and genotype were summarized for each country, hemisphere, and globally for each calendar year.
Genotype assignment was carried out through sequence clustering with genotype reference sequences in a cladogram generated by a Maximum Likelihood analysis. All RSV ectodomain sequences available in GenBank on 31 December 2020, were downloaded and analyzed. In total, 14,988 out of 31,279 available sequences were considered for analysis, after exclusion of synthetic, chimeric, and reference sequences, as well as sequences from organisms different from RSV and sequences smaller than the length of the RSV G gene ectodomain (600 bp). A total of 7,265 RSV A and 4,307 RSV B G gene ectodomain sequences were identified through local BLAST runs; however, due to the presence of degenerate nucleotides, insertions, or deletions, only 5,340 RSV A and 2,518 RSV B sequences could be assigned to a specific genotype. Genotype assignment was based on phylogenetic analysis of 2,805 unique RSV A and 1,730 RSV B sequences under General Time Reversible nucleotide substitution model, discrete gamma distribution with invariant sites, and 1,000 bootstrapping iterations as previously described [4,5].
Results
Overall, our search strategy identified 20,925 publications in the literature; 14,635 were excluded after reviewing the title or abstract (Figure 1). The full text of the remaining 6,290 articles was reviewed and 692 fulfilled the inclusion criteria; however, 273 were excluded during extraction of the data or during analysis. The final dataset is comprised of 419 articles. The RSV detection methods varied between studies: 44 used antigenic detection methods (including the use of monoclonal antibody panels, immunofluorescence, or enzyme-linked immunosorbent assays), 368 used molecular detection methods (reverse transcription polymerase-chain reaction), and 7 used whole or partial gene sequencing. The complete list of articles included in this analysis is available in the Supplementary Material.
Figure 1.

Flow diagram of literature search. The total number of articles identified through the search strategy, the number of items excluded during the selection process (and reasons for exclusion), and the final number of articles included in the study are shown.
The study is intended to comprise all RSV positive samples identified as RSV A or B published in the literature in studies that reported the proportion of each group until the last day of 2020. The earliest sample included in our study was classified as RSV A and was isolated in Australia in the year 1961 [11]. Starting from that year, until the end of 2019, a total of 76,668 [45,990 (60%) RSV A and 30,678 (40%) RSV B] positive samples were found and included in the analysis. Our dataset included reports of RSV A and RSV B infections from 83 countries and two regions in all continents (a study from the Canary Islands was analyzed and recorded as a region separate from Spain, because of their geographical location outside of Europe; a study from Martinique was recorded as a region separate from France, because of its geographical location in America). Countries with the largest number of samples were China (n = 9,103; 5,379 RSV A and 3,724 RSV B), the United States of America (n = 8,973; 5,574 RSV A and 3,399 RSV B), Spain (n = 4,991; 2,268 RSV A and 2,723 RSV B), and South Korea (n = 4,292; 3,235 RSV A and 1,057 RSV B). The number of RSV A and RSV B viruses in each country and continent are shown in Supplementary Table 1.
The number of samples identified from countries in the Northern Hemisphere (seasonal pattern, including North America, Europe, and Asia) was larger (n = 63,980; 38,139 RSV A and 25,841 RSV B) than those from the Southern Hemisphere (yearly pattern, including South America, Africa, and Oceania) (n = 12,688; 7,851 RSV A and 4,837 RSV B). In addition, the number of samples available before 1980 was small [296 of 76,668 (0.4%) samples included in the study], particularly in the Southern Hemisphere (only 4 of these 296 were reported from countries South from the Equator), limiting the conclusions that may be drawn from this time period. The number of samples available for analysis during each season/year included in the study is shown in Figure 2.
Figure 2.
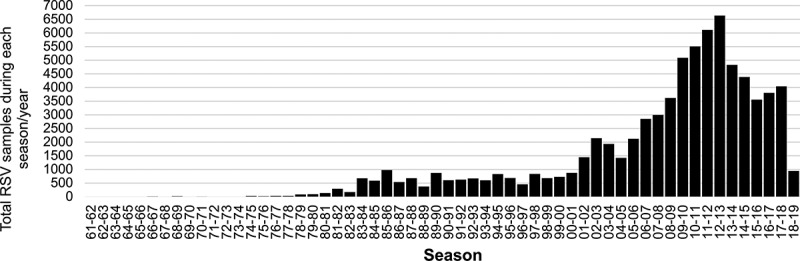
Total number of RSV samples during each season/year included in 419 articles selected from the literature. Samples reported from a given season in the Northern Hemisphere [for example, 1980–1981] and samples identified in the subsequent calendar year in the Southern Hemisphere [for example, 1981]) are shown in the same season. Total samples reported in the graph: 76,668 (63,980 for the Northern Hemisphere and 12,688 for the Southern Hemisphere).
The predominant RSV group (A or B) during each season (Northern Hemisphere), year (Southern Hemisphere), and worldwide (which included samples identified in a given season in the Northern Hemisphere [for example, 1980–1981] and samples identified in the subsequent calendar year in the Southern Hemisphere [for example 1981]) are shown in Figures 3 and 4.
Figure 3.
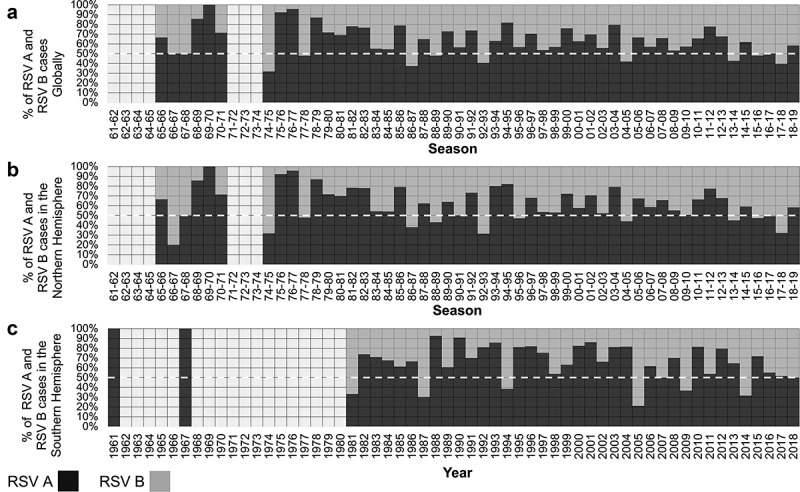
Proportion of RSV A and RSV B detections during each Global season (Northern + Southern hemispheres, 76,668 samples) (A), season (Northern hemisphere, 63,980 samples, 83.4%) (B), and year (Southern hemisphere 12,688 samples, 16.5%) (C). The dashed line (white) indicates 50% to identify seasons (or years) in which RSV A or RSV B predominate.
Figure 4.

Number of RSV A (black line) and RSV B (gray line) detections during each Global season (Northern + Southern hemispheres, 76,668 samples) (A), season (Northern hemisphere, 63,980 samples) (B), or year (Southern hemisphere, 12,688 samples) (C).
The co-circulation of both RSV groups was noted in all years; the only exception was the 69–70 season where only one RSV A positive sample was identified. RSV A was the predominant group (>50% detections) during most seasons/years. RSV B predominated during 11 (21.6%) of 51 seasons analyzed for the Northern Hemisphere (1966–67, 1974–75, 1977–78, 1986–87, 1988–89, 1992–93, 1995–96, 2004–05, 2013–14, 2015–16, and 2017–18) and in 6 (15%) of 40 years analyzed for the Southern Hemisphere (1981, 1987, 1994, 2005, 2009, and 2015). Globally, RSV B predominated during 9 (17.3%) of 52 seasons/years (1974–75, 1977–78, 1986–87, 1988–89, 1992–93, 2004–05, 2013–14, 2015–16, and 2017–18). The proportions of RSV A and RSV B were equal during the 1966–67, 1967–68, and 2016–17 seasons.
Two patterns across more than two consecutive seasons/years were observed: consecutive seasons with RSV A as the predominant virus and seasons/years with alternating predominance between RSV A and RSV B. On a global scale, three major periods of continued RSV A predominance were observed: a) the period between 1978–79 and 1985–86 seasons; b) the period between 1993–94 and 2003–04 seasons; and c) the period between 2005–06 and 2012–13 seasons (Figures 3 and 4). These periods were separated by years in which RSV B was the predominant virus (with an alternate predominance of RSV A) including the following: a) the period between 1986–87 and 1992–93 seasons (more notably in the Northern Hemisphere); b) the 2004–05 season (including 2005 in the Southern Hemisphere); and c) the period between 2013–14 and 2017–18 seasons.
An analysis of samples reported from each continent was also performed. In total, the number of samples identified was 9,072 in Africa (5,327 RSV A and 3,745 RSV B), 28,700 in Asia (18,185 RSV A and 10,515 RSV B), 21,092 in Europe (11,571 RSV A and 9,521 RSV B), 1,370 in Oceania (848 RSV A and 522 RSV B), 12,115 in North America (including the Caribbean) (7,444 RSV A and 4,671 RSV B), and 4,319 in South America (2,615 RSV A and 1,704 RSV B) (Figures 5 and 6). RSV circulation periods in North America and Europe are represented as winter seasons for the Northern Hemisphere, while RSV circulation periods in South America and Oceania are enclosed within each year corresponding to the winter seasons of the Southern Hemisphere. Africa and Asia include countries located in both hemispheres; therefore, RSV samples reported in countries located south of the Equator in those continents were added to the corresponding winter seasons of the Northern Hemisphere.
Figure 5.
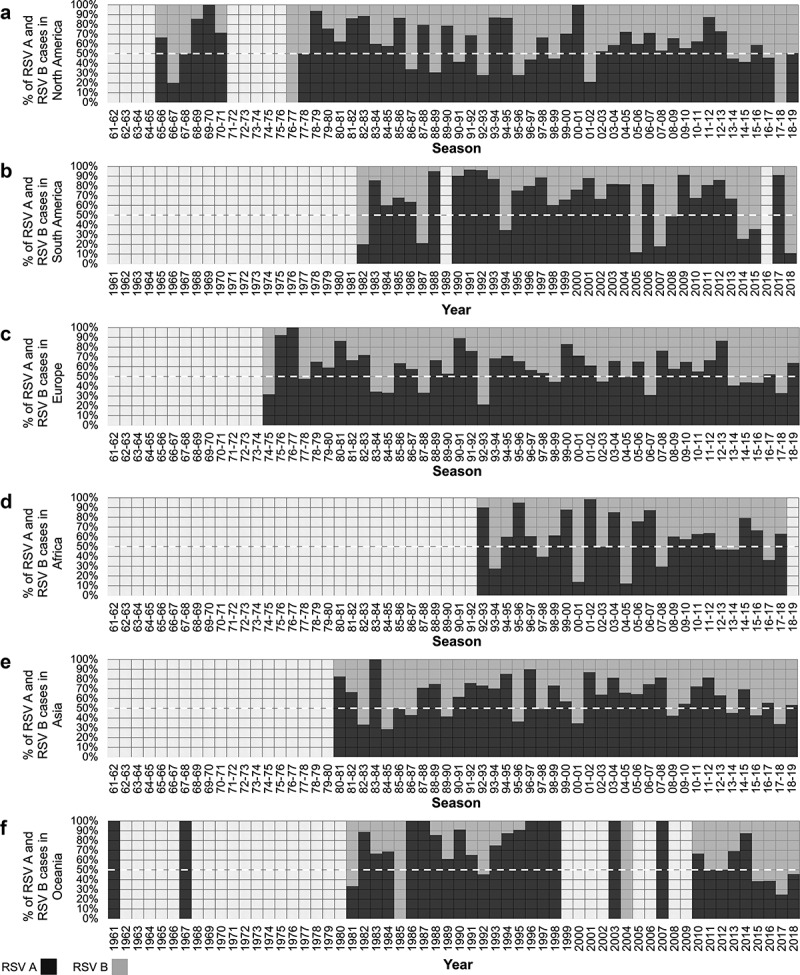
Proportion of RSV A and RSV B detections during each season or year in North America (A), South America (B), Europe (C), Africa (D), Asia (E), and Oceania (F). The dashed line (white) indicates 50% to identify seasons (or years) in which RSV A or RSV B predominate.
Figure 6.
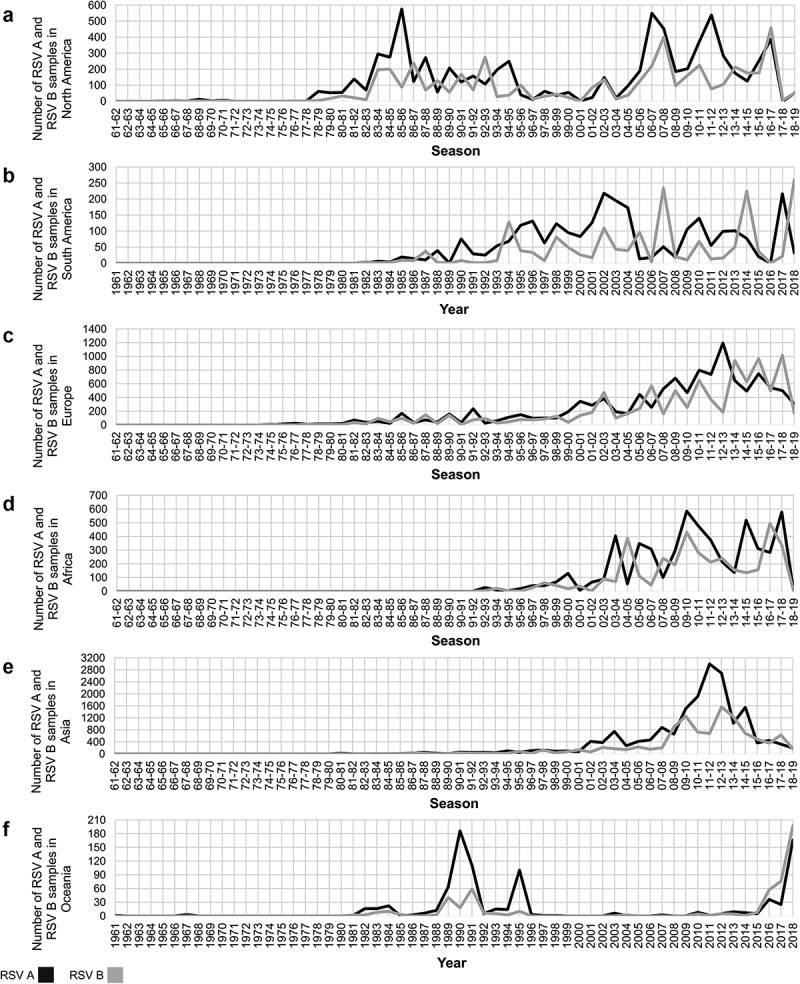
Number of RSV A (black line) and RSV B (gray line) detections during each season/year in North America (A), South America (B), Europe(C), Africa (D), Asia (E), and Oceania (F). Of note, the Y axis scale varies in each continent, based on the maximum number of detections reported in a given season/year.
In general, the predominant RSV group is not uniform during a given season/year across all continents. However, there were two periods in which RSV A was the predominant virus across all continents: the period encompassed between the 1978–79 and the 1980–81 seasons, and the period encompassed between the 2009–10 and the 2011–12 seasons (Figures 5 and 6). Of note, during several seasons/years there was at least one continent from which no reports were available, particularly prior to the 1992–93 season, when the first reports from Africa were available. Nevertheless, the same characteristics previously described on a global basis were observed in all continents: a) RSV A was the predominant group in most seasons; b) periods of several consecutive seasons/years in which RSV A is the predominant virus were observed, followed by years/seasons in which RSV A and RSV B alternated as the predominant group.
Genotype assignment of RSV sequences was carried out based on analysis of all ectodomain sequences available on GenBank as of 31 December 2020. Genotype assignment was achieved in 5,340 RSV A and 2,518 RSV B sequences. Partial or complete collection of data (year, month and country or year and country) was available for 4,919 RSV A (92.11%) and 2,518 RSV B (88.94%) sequences. The earliest sequence identified was assigned as GA1 (RSV A) and was collected in the United States of America in 1956. Analyzed sequences included data from 39 countries; Kenya accounted for the largest set of sequences (n = 2,757; 1,644 RSV A and 1,113 RSV B) followed by USA (n = 992; 693 RSV A and 299 RSV B) and China (n = 715; 584 RSV A and 131 RSV B). Countries were grouped by geographical location: the Northern Hemisphere accounted for the largest set of sequences (n = 6,322; 4,175 RSV A and 2,147 RSV B), whereas the Southern Hemisphere accounted for a much smaller set (n = 1,124; 742 RSV A and 382 RSV B). Availability of sequence data was discontinuous until 1976 in the case of the Northern Hemisphere and until 1987 in the case of the Southern Hemisphere; from those years onward, sequence data was available for all years (Supplementary Figure S1).
Globally, the most frequently identified genotypes were, in the case of RSV A, NA1 (n = 3,753, 76.30%), GA5 (n = 678, 13.78%), and GA2 (n = 253, 5.14%); the cumulative frequency of all the other genotypes (GA1, GA3, GA4, GA6, GA7, and SAA1) contributed to less than 5% of the sequences. Of note, ON1, a lineage that originated from genotype NA1 that carries a partial G gene duplication, accounted for a considerable number of sequences (n = 1,146; 23.30%). For RSV B, the genotypes most frequently isolated were BA (n = 1,779; 70.65%), BA-CC (n = 353; 14.02%), and SAB1 (n = 140; 5.56%); the cumulative frequency of all other genotypes (BA-C, CB1-THB, GB0, GB1, GB2, GB3, GB4, GB6, JAB1-NZB2, SAB4, URU2) contributed with less than 10% of RSV B sequences.
Multiple genotypes circulated simultaneously throughout the evolutionary history of RSV. Remarkably, in both the Northern and Southern Hemispheres, genotype diversity decreased dramatically in the early 2000s. By 2005, the BA genotype outnumbered all other RSV B genotypes; by 2007, the NA1 genotype showed a similar behavior becoming the predominant RSV A genotype (Figure 7 and Supplementary Figure S2). The most recent changes in genotype circulation include the emergence and global spread of ON1 as the predominant RSV A virus and the emergence and expansion of BA-CC, a genotype that originated from BA that also carries a partial G gene duplication. Currently, these are the predominant genotypes, and no other genotypes have been identified in the last 5 years in any continent (Figure 8 and Supplementary Figure S3).
Figure 7.
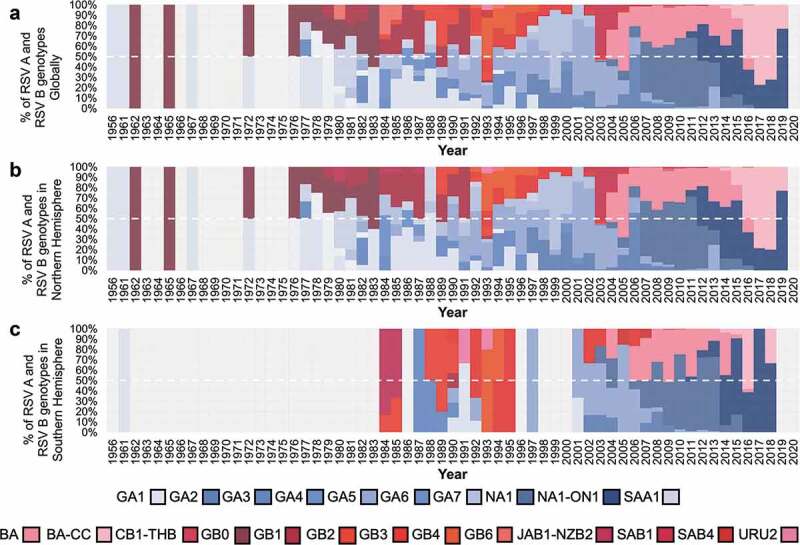
Proportion of RSV genotypes during each year Globally (A), in the Northern hemisphere (B), in the Southern hemisphere (C). Genotype distribution was based on analysis of all RSV G gene ectodomain sequences available in GenBank with sample collection date up until 31 December 2020.
Figure 8.
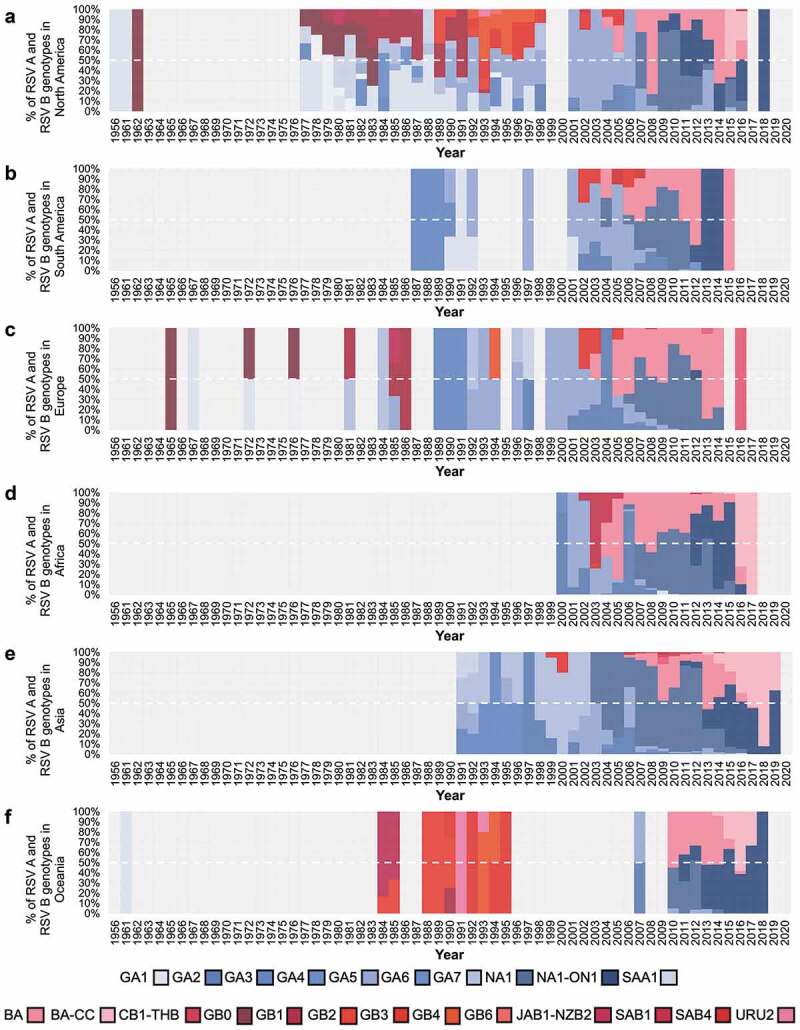
Proportion of RSV genotypes during each year in North America (A), South America (B), Europe (C), Africa (D), Asia (E), and Oceania (F). Genotype distribution was based on analysis of all RSV G gene ectodomain sequences available in GenBank with sample collection date up until 31 December 2020.
Discussion
In the present study, we have analyzed the contribution of RSV A and B viruses based on all published studies identified in the National Library of Medicine databases and sequences available in GenBank since the first report of this virus until December 2020. Overall, RSV A infections were most common, accounting for 60% of the infections reported in the literature and 66% of the sequences available in GenBank. Up to 83% of RSV cases included in reports in the literature and 85% of sequences correspond to infections detected in the Northern Hemisphere. This is explained, in part, due to a larger proportion of the human population living in countries located north of the Equator. However, this is also the result of a lower number of studies carried out in countries located south of the Equator, as noted in the number of RSV infections reported in each country (Supplemental Table 1). Also, most information regarding the predominance of RSV A or B infections has been accrued after the year 2000. Prior to 1970, there were only 39 samples reported in the literature indicating the RSV group (A or B), making it very difficult to assess the dominance of one group or another during each year. While in the 1970s a larger number of reports were available, most of them were restricted to countries in the Northern Hemisphere. In contrast, a significant number of infections were studied in the Southern Hemisphere until the 1980s and onward. No RSV infections were reported for the year 2020 in any article and, therefore, our results describe RSV circulation up to 2019. The absence of data in 2020 might have been explained due to the time required between sample collection and publishing of results; in addition, the reduction in detection of non-SARS-CoV-2 respiratory viruses during the COVID-19 pandemic might have also had an impact on this. Additional information is expected to become available in the coming years and, eventually, updated analyses will be required.
Overall, RSV A was the predominant group accounting for 59.6% of the infections reported in the Northern Hemisphere, 61.9% in the Southern Hemisphere, and 60% globally. RSV B was the predominant group in only nine seasons/years globally, and this RSV group was not identified as predominant in two or more consecutive seasons. Nevertheless, when results were analyzed on the continent, a larger proportion of RSV B infections compared to RSV A was noted during the last decade, including consecutive years/seasons of RSV B predominance in all continents (except Asia) in recent years. Of note, during the years at the end of this analysis RSV A and RSV B appeared to reach an apparent equilibrium associated with an increase in the overall RSV B prevalence. Also, analysis of sequences available at GenBank showed that RSV A NA1 (including viruses with partial duplication of the G gene referred to as ON1 viruses) has been the most frequently identified genotype since 2007; however, between 2016 and 2018, BA-CC, has been the predominant genotype worldwide.
Viral evolution, leading to novel genotypes, likely contributes to the shifting pattern in RSV A and RSV B dominance. Two patterns across several seasons were observed in the study: consecutive seasons with RSV A as the predominant virus and alternative predominance between RSV A and RSV B (which was more notable in the decades between 1980 and 2000). Of interest, during that period between 1980 and 2000 notable diversification of RSV A and B genotypes occurred [4,5]. As a result, during this period, the predominant genotype during a given year usually accounted for less than 50% of the sequences identified worldwide; in contrast, after the year 2000, a leading genotype usually accounted for at least half of all available sequences, and between 2010 and 2019 three genotypes (NA1, including ON1, BA, and BA-CC) accounted for 95% (4391/4608) of RSV viruses circulating worldwide.
Over the last 10 years, RSV strains with a partial duplication of the G gene have become the dominant circulating viruses. The emergence and predominance of these viruses occurred in RSV B first (BA strains) and has been reported worldwide [12,13]. As for RSV A, after the identification of ON1 viruses in Canada, strains with the partial duplication of the G gene became the predominant viruses in all countries [8,12,14–20]. Most recently, the BA-CC genotype has displaced BA as the leading RSV B genotype. The mechanisms leading to the predominance of RSV strains with the partial duplication of the G gene over other genotypes (such as increased virulence or immune evasion) are still under study.
A limitation of our analysis is the heterogeneity in the study design of the reports included in this review. Studies did not follow the same methodology and did not have the same objectives: some publications focused on epidemiological descriptions, while others focused on diagnostic methods. Also, many studies that included a large number of RSV infections were excluded because no distinction between A and B groups was carried out. Analysis of genotype diversity was based on sequences available at GenBank. Although many published studies report the frequencies of RSV genotypes detected in different countries, currently there is no consensus regarding criteria to define specific genotypes. We have previously found that sequences with high homology might be reported as different genotypes, and identical sequences might be reported as distinct genotypes in two studies [5]; as such, comparisons of genotype frequencies reported in the literature might lead to inaccurate results.
We also found that, despite there being many published studies regarding RSV epidemiology, a large proportion of available data for some continents has been acquired in one or two countries. For example, in our literature review, we identified a total of 9,072 RSV samples from Africa, and studies carried out in Kenya accounted for 3,795 (41%) of those samples; in contrast, the population of Kenya represents only 4% of the total population in Africa [21]. Also, for several countries, data is limited to results from a single study, with small sample size or carried out over a short period of time.
The present systematic review is, to our knowledge, the first global analysis of RSV A and B circulation patterns to include all published reports since this virus was first reported as a cause of respiratory infections in infants [22]. Despite the aforementioned limitations, our study provides a thorough description of the epidemiology of RSV A and B viruses. While RSV A encompasses the majority of infections caused by this virus, the emergence of RSV B novel genotypes, including BA and BA-CC, has led to a more balanced contribution of both groups as causes of respiratory infections in recent years. Continued RSV surveillance is required to determine the impact of the emergence of new genotypes on viral circulation, as well as on respiratory morbidity and mortality.
Supplementary Material
Funding Statement
This study was supported by Consejo Nacional de Ciencia y Tecnologia (Mexico) (Proyecto CONACYT-A1-S-38080). The results of this work were presented, in part, at XIX Congreso Latinomaericano de Infectologia Pediatrica SLIPE 13-15 October 2021, Buenos Aires, Argentina.
Disclosure statement
DEN has participated as a member of the speakers’ bureau of AbbVie and speakers’ bureau and advisory board for Sanofi Pasteur. All other authors declare that there are no competing interests regarding the publication of this manuscript.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].World Health Organization . Respiratory Syncytial Virus (RSV) disease. [Internet]. [cited 2021 Nov 16]. Available from: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/respiratory-syncytial-virus-disease
- [2].Bianchini S, Silvestri E, Argentiero A, et al. Role of respiratory syncytial virus in pediatric pneumonia. Microorganisms. 2020. Dec 21;8(12):2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tseng HF, Sy LS, Ackerson B, et al. Severe morbidity and short- and mid- to long-term mortality in older adults hospitalized with respiratory syncytial virus infection. J Infect Dis. 2020. Sep 14;222(8):1298–1310. [DOI] [PubMed] [Google Scholar]
- [4].Muñoz-Escalante JC, Comas-García A, Bernal-Silva S, et al. Respiratory syncytial virus A genotype classification based on systematic intergenotypic and intragenotypic sequence analysis. Sci Rep. 2019. Dec;9(1):20097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Muñoz-Escalante JC, Comas-García A, Bernal-Silva S, et al. Respiratory syncytial virus B sequence analysis reveals a novel early genotype. Sci Rep. 2021. Dec;11(1):3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Eshaghi A, Duvvuri VR, Lai R, et al. Genetic variability of human respiratory syncytial virus A strains circulating in Ontario: a novel genotype with a 72 nucleotide G gene duplication. Khudyakov YE, editor. PLoS ONE. 2012. Mar 28;7(3):e32807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Trento A, Galiano M, Videla C, et al. Major changes in the G protein of human respiratory syncytial virus isolates introduced by a duplication of 60 nucleotides. J Gen Virol. 2003. Nov 1;84(11):3115–3120. [DOI] [PubMed] [Google Scholar]
- [8].Esposito S, Piralla A, Zampiero A, et al. Characteristics and their clinical relevance of respiratory syncytial virus types and genotypes circulating in Northern Italy in five consecutive winter seasons. Tregoning JS, editor. PLoS ONE. 2015. Jun 5;10(6):e0129369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021. Dec;10(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Obando-Pacheco P, Justicia-Grande AJ, Rivero-Calle I, et al. Respiratory syncytial virus seasonality: a global overview. J Infect Dis. 2018. Apr 11;217(9):1356–1364. [DOI] [PubMed] [Google Scholar]
- [11].Zheng H, Peret TC, Randolph VB, et al. Strain-specific reverse transcriptase PCR assay: means to distinguish candidate vaccine from wild-type strains of respiratory syncytial virus. J Clin Microbiol. 1996. Feb;34(2):334–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yun KW, Choi EH, Lee HJ.. Molecular epidemiology of respiratory syncytial virus for 28 consecutive seasons (1990-2018) and genetic variability of the duplication region in the G gene of genotypes ON1 and BA in South Korea. Arch Virol. 2020. May;165(5):1069–1077. [DOI] [PubMed] [Google Scholar]
- [13].Trento A, Casas I, Calderón A, et al. Ten years of global evolution of the human respiratory syncytial virus BA genotype with a 60-nucleotide duplication in the G protein gene. J Virol. 2010. Aug;84(15):7500–7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ahmed A, Haider SH, Parveen S, et al. Co-circulation of 72bp duplication group A and 60 bp duplication group B Respiratory Syncytial Virus (RSV) strains in Riyadh, Saudi Arabia during 2014. Cormier SA, editor. PLoS ONE. 2016. Nov 11;11(11):e0166145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hibino A, Saito R, Taniguchi K, et al. Molecular epidemiology of human respiratory syncytial virus among children in Japan during three seasons and hospitalization risk of genotype ON1. Jin D-Y, editor. PLoS ONE. 2018. Jan 29;13(1):e0192085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Razanajatovo Rahombanjanahary NH, Rybkina K, Randriambolamanantsoa TH, et al. Genetic diversity and molecular epidemiology of respiratory syncytial virus circulated in Antananarivo, Madagascar, from 2011 to 2017: predominance of ON1 and BA9 genotypes. J Clin Virol. 2020. Aug;129:104506. [DOI] [PubMed] [Google Scholar]
- [17].Pretorius MA, van Niekerk S, Tempia S, et al. Replacement and positive evolution of subtype A and B respiratory syncytial virus G-protein genotypes from 1997–2012 in South Africa. J Infect Dis. 2013. Dec 15;208(suppl_3):S227–37. [DOI] [PubMed] [Google Scholar]
- [18].Agoti CN, Otieno JR, Gitahi CW, et al. Rapid spread and diversification of respiratory syncytial virus genotype ON1, Kenya. Emerg Infect Dis. 2014. Jun;20(6):950–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chi H, Hsiao K-L, Weng L-C, et al. Persistence and continuous evolution of the human respiratory syncytial virus in northern Taiwan for two decades. Sci Rep. 2019. Dec;9(1):4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Comas-García A, Noyola DE, Cadena-Mota S, et al. Respiratory syncytial virus-A ON1 genotype emergence in central Mexico in 2009 and evidence of multiple duplication events. J Infect Dis. 2018. Mar 13;217(7):1089–1098. [DOI] [PubMed] [Google Scholar]
- [21].United Nations, Department of Economic and Social Affairs, Population Division . World population prospects highlights. [Internet]; 2019. [cited 2021 Nov 16]. Available from: https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf
- [22].Chanock R, Roizman B, Myers R.. Recovery from infants with respiratory illness of a virus related to Chimpanzee Coryza Agent (CCA). Am J Epidemiol. 1957. Nov;66(3):281–290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


