Abstract
Maintaining stable breeding groups of rhesus macaques (Macaca mulatta) can be challenging due to the complex social dynamics and despotic nature of the species. Trauma from aggression is a common problem in rhesus colonies and can cause social disruption, strain veterinary and animal management resources, and potentially affect reproduction. Previous research has shown that increasing the number of non-natal adult males in a breeding group can improve group stability, reduce trauma, and increase reproduction. Here, we used mixed-effects regression models to examine the effects of sex ratio and other factors on trauma and reproduction at the Yerkes National Primate Research Center using a historical dataset made up of four large rhesus groups over an eleven-year period (2003–2013). As expected, sex ratio was a significant predictor for both trauma and reproduction. However, group age since formation was a stronger predictor of trauma frequency and the amount of space available was a slightly better predictor of reproduction than sex ratio or trauma. These results indicate that improving sex ratios can be a viable management strategy to reduce trauma and improve reproduction, particularly when it is difficult to manipulate the group compositions and/or their housing situations. Reducing trauma is a primary goal for rhesus breeding colonies, as it directly impacts the monkeys’ health and psychological well-being. Such improvements are necessary for the ethical treatment and care of the animals themselves, but also to reduce financial burdens and maintain a healthy colony for research purposes.
Keywords: animal welfare, reproduction, rhesus macaque, sex ratio, social stability, trauma
Introduction
Managing breeding colonies of non-human primates involves maintaining, as far as possible, naturalistic social groupings and physical environments that resemble that of wild populations, including managing group sizes and sex ratios, ‘migrations’ between groups, enrichment structures and feeding routines, etc. At research facilities, the goal is to maximise the animals’ physical health and psychological well-being to create a functional and productive colony that produces reliable and valid animal models for biomedical and behavioural research. A common issue for managing breeding colonies of rhesus macaques (Macaca mulatta) is high rates of aggression, which can have many negative consequences for the colony. While it is natural for rhesus macaques to use aggression in both wild and captive settings in social interactions (Lindburg 1971; Bernstein & Gordon 1974), chronic severe aggression, such as biting and wounding conspecifics, can negatively impact a group’s stability and the animals’ physical health and welfare (Alberts et al 1992; Judge et al 1994; Ha et al 2011; Beisner et al 2012). These negative effects can include increased stress, reduced reproductive health, acute and potentially chronic pain from the injury itself, and the social disruption and potential upheaval associated with the temporary or permanent removal of animals for veterinary treatment. Therefore, it is a high priority for colony managers to identify potential sources of severe aggression and find ways to mitigate it.
In this study, we investigated management-related factors that may contribute to two critical outcomes, trauma rates and reproductive success. Here, trauma refers to wounds received from a conspecific group member in an aggressive interaction that required veterinary intervention — that is, temporary removal from the group for medical treatment. In particular, we were interested in evaluating the effects of sex ratio on both outcomes, as it presents a potentially feasible management strategy compared to other approaches that may be more difficult to manipulate from a logistic and funding perspective (eg, drastic changes in group sizes and compositions, constructing new housing).
Sex ratio and aggression
Several studies, primarily from the California National Primate Research Center (CNPRC), demonstrate the importance of sex ratio to the stability of rhesus social groups (Beisner et al 2012). Wild troops of rhesus macaques show sex ratios of approximately 1–6 adult females to each adult male (Southwick et al 1961, 1965; Makwana 1978; Teas et al 1980; Seth & Seth 1985; Goldstein & Richard 1989; Sahi & Sharma 2004), whereas sex ratios in captive groups are often less balanced, ranging from 1–34 females per male (Beisner et al 2012; Stavisky et al 2018). A skewed sex ratio with many more females than males can affect aggression through at least two mechanisms. First, adult males, particularly high-ranking ones, and/or those with certain personality traits (eg equable), are usually the group members that intervene in conflicts and prevent aggression from escalating (‘policing’ behaviour), which helps reduce severe wounding events (Flack et al 2005, 2006; McCowan et al 2011; Beisner & McCowan 2013). Ha et al (2011) also demonstrated reduced contact aggression with male(s) present in groups of pigtail macaques (Macaca nemestrina). Therefore, not having enough males that can effectively police conflicts can lead to more aggression. Second, with fewer males, overt fighting among females for access to a male may be more likely (Zumpe & Michael 1987). Under these conditions, female aggression may increase unchecked, as more intra-female aggression is elicited with fewer males as sexual partners and there are not enough males to police those conflicts adequately (Oates-O’Brien et al 2010).
Managing social groups for appropriate sex ratios can go a long way toward improving stability in social groups (McCowan et al 2018). It is likely to help improve reproduction rates within groups, as well, as there are simply more males available for mating. Furthermore, numerous measures of reproductive output are compromised in macaque females that have experienced prolonged psychosocial stress and intra-group conflict (Cameron 1997; Ha et al 2011). For example, Cameron (1997) found that socially induced trauma resulting in pain, injury, energy restriction, and infection has been associated with suppression of reproductive hormone secretion and, if sustained, a suppression of fertility. Similarly, the psychological stress associated with subordinate status in female cynomolgus monkeys (Macaca fascicularis) adversely affects their ovarian function with increased cycle length, increased variability in cycling, and lower levels of progesterone and oestradiol (Kaplan et al 2010).
Aggression is associated with infant loss in rhesus macaques, as well (ie spontaneous abortions, stillbirths, infant mortality, and infant removal for medical reasons; Deutsch & Lee 1991; Dettmer et al 2015). Ha and colleagues (2011) showed that increased aggression within pigtail macaque groups is associated with increased probability of non-viable births and/or the need for veterinary intervention when a female gives birth. Dettmer and colleagues (2015) also showed long-term reduced reproductive output for entire matrilines that were involved in an overthrow (that is, when a lower-ranking matriline deposes a higher-ranking matriline). Matrilineal overthrow is characterised by severe aggression, significant morbidity and even mortality, which can lead to fewer breeding females present to give birth in subsequent years, further impacting a group’s potential reproductive output. Finally, infants that elevated post-natal emotional responsiveness, modified HPA axis regulation, elevated glucocorticoid output following maternal separation, and lower haematocrit levels compared to control infants (Herrington et al 2016). Therefore, reducing intra-group aggression via improved sex ratios may have a large positive impact on the animals’ reproductive health and psychological well-being.
Other group attributes and aggression
Aside from sex ratio, many other social and environmental factors can influence the degree of aggression a group displays. We investigated three additional variables that may affect aggression in the groups we studied: the number of years since initial group formation (group age), group size, and the amount of space available. Although these variables were of interest independently, they were considered covariates when testing our central hypothesis of whether sex ratio contributes significantly to trauma and reproduction. For this analysis, a few additional variables related to group composition were not available, but are of interest for future research (eg number and size of matrilines within groups, age ranges within and across matrilines, etc).
Group age
Although group age has not been thoroughly examined in the literature, particularly regarding changes in reproduction over time within groups, a few analyses showed a pattern of increased aggression as groups age (defined as the duration in years since the initial formation of a large group from the merging of smaller groups and individuals together). For example, rhesus groups at Yerkes that experienced matrilineal overthrows tended to be older than those that did not experience overthrows (Sánchez et al 2014). At the CNPRC, groups that had been together longer had higher rates of wounding, as well, possibly due to older groups having more matrilines that had lost their matriarchs or other adult females that had served as connections between more distant kin (Beisner et al 2011b). Given the effects of aggression on reproduction described above, it stands to reason that reproduction could also decline over time — although changes in group composition over time may mitigate that effect.
Group size
As with group age, the impact of group size on aggression has not been studied extensively. While group size was predictive of aggression in captive pigtail macaques (Ha et al 2011), group size did not affect rates of aggression among female rhesus macaques at the CNPRC (Beisner & Isbell 2011). It is possible the monopolisation of resources plays a role, as has been seen in wild populations where contest competition over defensible food sources increases with group size (Goss-Custard et al 1984; Grenier et al 1999; Mathy & Isbell 2001; Weir & Grant 2004; Vogel et al 2007; Asensio et al 2008). The size of wild primate groups also has the potential to influence reproductive success. Slower weaning, inter-birth intervals and infant survival rates have all been tied to larger group sizes in several species (Leaf monkey [Trachypithecus phayrei], Borries et al 2008; savannah baboon [Papio hamadryas], Altmann & Alberts 2003; Thomas’ langur [Presbytis thomasil], Steenbeek & van Schaik 2001). Among two wild groups of rhesus macaques, Liu et al (2018) found that the cost-benefit trade-offs between living in small or large groups, in terms of feeding competition and predator protection, could account for variation in reproductive success across group sizes.
Available space
Regarding the impact of available space on aggression in groups of primates, behavioural responses vary widely across studies and species. Early studies of rhesus monkeys in both wild and captive settings appeared to support a density-aggression relationship. For example, urban-dwelling rhesus monkeys with higher population density (ie more crowded conditions), had higher aggression rates than less-crowded rural groups; similarly, captive rhesus monkeys had higher aggression rates when they were housed in smaller areas (Southwick 1967, 1969). However, more recent studies on the amount of space available to groups of primates have produced inconsistent results. Specifically, higher density has produced increases in aggression (Erwin & Erwin 1976; Elton & Anderson 1977; Erwin 1979; Niewenhuijsen & de Waal 1982), decreases in aggression (Eaton et al 1981; Novak & Drewsen 1989; Bercovitch & Lebron 1991) or no changes in aggression (Dazey et al 1977; McGuire et al 1978; Demaria & Thierry 1989; Crast et al 2015). While Oates-O’Brien and colleagues (2010) found that matrilineal overthrows were associated (though not significantly) with less densely populated conditions in captivity, Judge and de Waal (1993) found that female, but not male, rhesus macaques increased aggression as crowding increased. In contrast, some primate groups increase affiliative behaviours under more crowded conditions, presumably to reduce tension and avoid conflict (McGuire et al 1978; Nieuwenhuijsen & de Waal 1982; Caws & Aureli 2003; Sannen et al 2004; Cordoni & Palagi 2007).
Studies with a variety of non-primate species and humans have demonstrated that increased population density results in reduced reproductive success (Dahlgren 1979; Arcese & Smith 1988; Jenkins et al 1999; Bonenfant et al 2002; Pettorelli et al 2002; Jones et al 2008). A possible mechanism is via the hypothalamic-pituitary-adrenal (HPA) axis activity, as greater population density is related to HPA activity. Dettmer and colleagues (2014) discovered that higher population density was associated with increased cortisol concentrations in captive rhesus macaques (a reliable indicator of integrated HPA-axis activity), so it is feasible that reproductive output could be suppressed under such conditions. Given that exposure to chronically elevated circulating glucocorticoids results in a host of health disparities in animals and humans alike, it is important to understand how population density may impact chronic stress in macaques, which in turn may negatively impact reproduction and infant survival.
Research goals and hypotheses
The overarching goal of this study was to inform management strategies to improve health and reproduction in rhesus macaque breeding colonies. More specifically, our primary goal was to evaluate sex ratio as a potential mechanism to do so. Our central hypothesis was that sex ratio would significantly contribute to both trauma and reproduction in rhesus groups. We investigated four representative groups of rhesus macaques at the Yerkes National Primate Research Center (YNPRC) from 2003–2013, where sex ratios ranged from eight to 34 breeding-aged females to each breeding-aged male and were persistently skewed throughout the studied time-frame (Figure 1). These ratios were concerning given the link between skewed sex ratio and wounding rates, even when sex ratios ranged from 0.6–10.7 adult females per adult male at the CNPRC (Beisner et al 2012). Additional potentially influential variables (reviewed above) were included as covariates when assessing the influence of sex ratio on trauma and reproduction. However, given the paucity of research on group age and size, and the inconsistent results seen in studies of available space, we did not make directional hypotheses regarding their effects on trauma and reproduction in the current sample.
Figure 1.
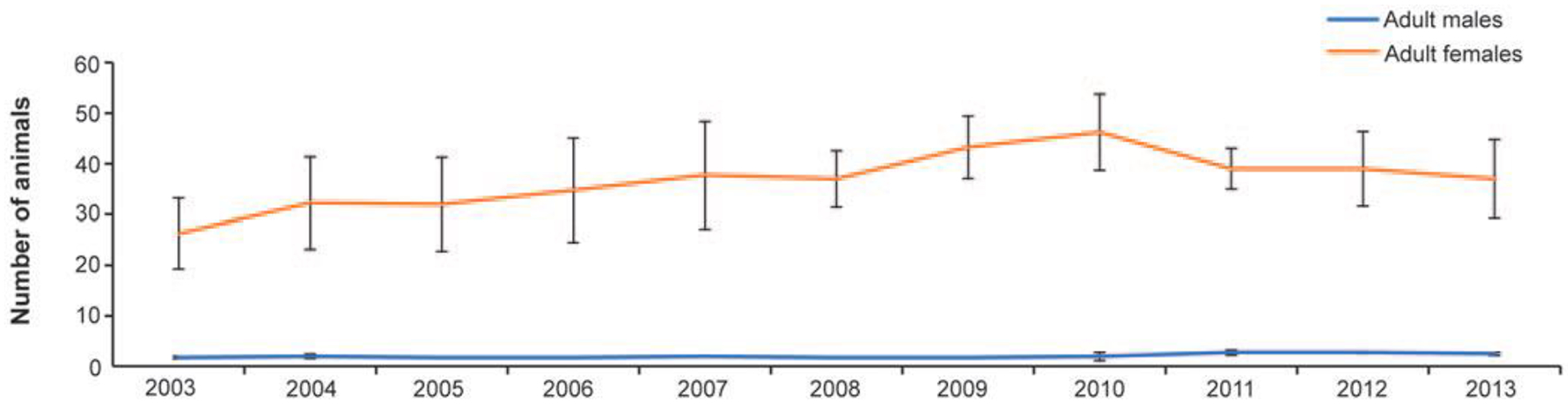
Average number of adult males (5+ years) and adult females (3+ years) across the four study groups of rhesus macaques at Yerkes from 2003–2013. Error bars represent standard error of the mean. Overall, the relationship between group size and group age was r = 0.27.
Materials and methods
Study animals and housing
We gathered archival data from 2003 to 2013 on each variable of interest for four large breeding groups of rhesus macaques housed at YNPRC (Table 1; data were gathered in 2015). These four groups were chosen randomly from over 20 breeding groups housed at the centre. The sample size was limited by the time-consuming process of manually compiling these archival data. The groups lived in indoor/outdoor compound-style enclosures of varying sizes across the selected time-frame, were composed of multiple matrilines and had been formed two to eight years prior to the start of the study time-frame (2003). These groups were formed by merging several smaller groups of monkeys together with colony management staff oversight. The range of group ages (years since formation) across the focal timeframe is 2–18 years since original formation (mean = 10) and group sizes ranged from 32–171 animals of all ages (mean = 85). During the time-frame, each group was moved for various management purposes and each lived in 3–6 different compounds. The overall space available ranged 808–4,875 m2, with a mean of 11 to 87 m2 per animal (mean = 38; Table 2). All groups were provided with Purina monkey chow (Standard LabDiet 5038 Monkey Diet, Purina, St Louis, MO, USA) and water ad libidum, a variety of produce, foraging enrichment, and diverse structural enrichment providing shade, visual barriers, and climbing opportunities. For each group over the studied time-frame, group sizes and composition varied, as occasionally animals were permanently removed for health or management purposes, died from natural causes, or (more rarely) socially induced trauma. Matrilineal overthrow was also a factor in group composition changes, which occurred at least once in each group (there were seven in total, out of 44 group-years), as colony managers sometimes remove individuals and/or an entire matriline to reduce risk of recurrence. These incidences were few and showed no pattern of increased trauma or reduced reproduction in those group-years, so were not investigated further.
Table 1.
Definitions of each variable. Data were collected on a yearly basis from 2003–2013 in four large breeding groups of rhesus macaques.
| Variable | Definition (per year) |
|---|---|
| Sex Ratio | The number of reproductively capable, breeding-aged females (3+ years) in the group divided by the number of breeding-aged males (5+ years) in the group each year (ages were determined at the start of each breeding season). |
| Group Age | The number of years since the group was originally formed. Group age was counted from the point when a larger group was initially formed from the deliberate merging of smaller groups and individuals. |
| Group Size | The maximum number of animals in the group each year. |
| Individual Space | Square meters per animal (housing size in square meters divided by maximum group size in each year). |
| Trauma Frequency | The number of times an animal of any age was temporarily removed from their group for veterinary treatment of a socially induced injury each year for each group. |
| Trauma Rate | Trauma frequency divided by the maximum group size in each year (trauma rate exceeded 1 when the number of traumas per year exceeded the number of animals in the group). |
| Reproductive Success | The percentage of breeding-aged females that gave birth to infants surviving past four months of age by the end of each calendar year. |
Table 2.
Descriptive statistics for variables included in the analyses, as well as additional variables of interest (N BAM = number of breeding-aged males; N BAF = number of breeding-aged females).
| Variable | Mean | Median | SD | Min | Max |
|---|---|---|---|---|---|
| Group age | 11 | 11 | 3.95 | 2 | 18 |
| Group size (n animals) | 86 | 84 | 33.48 | 32 | 171 |
| N BAM | 2 | 2 | 0.76 | 0 | 4 |
| N BAF | 37 | 36 | 14.89 | 15 | 68 |
| Housing size (m2) | 3299 | 3166 | 1643.05 | 808 | 4875 |
| Individual space (m2/animal) | 38 | 38 | 17.10 | 11 | 87 |
| Trauma frequency | 37 | 24 | 29.60 | 7 | 125 |
| Trauma rate (N trauma/group size) | 42 | 36 | 27.96 | 6 | 124 |
| Sex ratio (N BAF / N BAM) | 18 | 17 | 7.57 | 8 | 34 |
| Reproductive success (%) | 63 | 65 | 12.61 | 27 | 83 |
Data collection and analysis
For each group in each year, we collated the following continuous variables from archival records (defined below and in Table 1): sex ratio, group age, group size, individual space, trauma frequency, trauma rate, and reproductive success. Regarding the variable ‘individual space’, this is a measure of population density in an enclosure; specifically, it is the amount of space available to a group (eg m2), divided by the number of animals in that group (Erwin 1979). We note that ‘individual space’ has an inverse relationship to ‘spatial density’ (eg number of animals divided by m2; Erwin 1979). Both provide a measure of the degree of ‘crowding’ in a captive environment — that is, as individual space goes up, crowding declines; as spatial density increases, crowding increases. We decided to operationalise the amount of space available to our groups as individual space because our groups lived in large compounds. Therefore, it made more sense intuitively to measure the amount of space available to each animal, as if they were (hypothetically) spaced out evenly throughout the compound, as a proxy for the degree of crowding they might experience (or the lack thereof).
Collapsing across groups and years, the variables were summarised by mean (± SD), median, minimum and maximum values in Table 2. We ran bivariate Pearson correlations to determine the relationships between the predictor variables and the outcome measures, which were yearly frequency of socially induced trauma and yearly reproductive success, as well as the interrelationships among all variables (Table 3). Next, we evaluated predictors of trauma frequency and reproductive success in our groups using generalised linear mixed-effects and linear mixed-effects regression models, respectively (lme4 and multilevel packages; R Studio version 1.2.5033). We built models to test our central hypothesis that sex ratio contributes a significant amount of variance to both outcomes (trauma frequency and reproductive success), with the other variables serving as covariates. To avoid issues related to multicollinearity (redundant covariates), we avoided entering covariates with r > 0.60 in the same model. AIC scores were used to compare models and select the one that both evaluated sex ratio and included covariates that significantly contributed to the outcome of interest.
Table 3.
Full Pearson product moment correlation matrix with all variables of interest. Note that increased spatial density means more space available per animal.
| Group Age | Group Size | Individual Space | Trauma Frequency | Trauma Rate | Sex Ratio | |
|---|---|---|---|---|---|---|
| Group Size (N) | 0.27 | |||||
| Individual Space (m2/N) | 0.58*** | 0.06 | ||||
| Trauma Frequency | 0.53*** | 0.49*** | 0.11 | |||
| Trauma Rate (Freq/N) | 0.50*** | 0.04 | 0.18 | 0.85*** | ||
| Sex Ratio (#BAF/#BAM) | 0.13 | 0.66*** | −0.07 | 0.38* | 0.10 | |
| Reproductive Success | −0.40** | −0.14 | −0.45** | −0.28 | −0.33* | −0.27 |
p<0.05;
p<0.01;
p<0.00
For trauma frequency, we used a negative binomial distribution model because the trauma frequency was count data and over-dispersed (mean = 37 [± 29.6] and ranged from 7–125 across group-years; Table 2). For reproduction, we used mixed-effect model with a Gaussian distribution, as the distribution of the percentage of breeding-aged females with infants that survived past four months was close to normal. For both outcomes, we included group identification as a random-effects term, meaning we accounted for variance in the outcomes of interest that were due to group membership, allowing us to parse out the specific effects of other predictors more accurately. Because we had four groups and eleven years of data, we had 44 cases (groupyears) of data to analyse. One case was excluded from the trauma frequency analysis because no males were successfully introduced to the group for longer than a week or two at a time, and so we could not calculate a sex ratio. That case and one other was excluded from the reproductive success analysis because they resulted in zero reproductive output for the year (in the second case, males were not successfully introduced until after the breeding season).
Results
General findings
Overall, trauma increased and reproduction decreased over time (see Figures 2 and 3). In addition, throughout the study period, groups tended to get bigger, more skewed in their sex ratios, and groups tended to move into larger compounds with more space per individual (Table 3). The regression models showed that: (i) as groups aged and became more skewed in their sex ratios, the occurrence of trauma increased; and (ii) increases in trauma, along with larger living spaces and more skewed sex ratios, negatively impacted reproduction.
Figure 2.
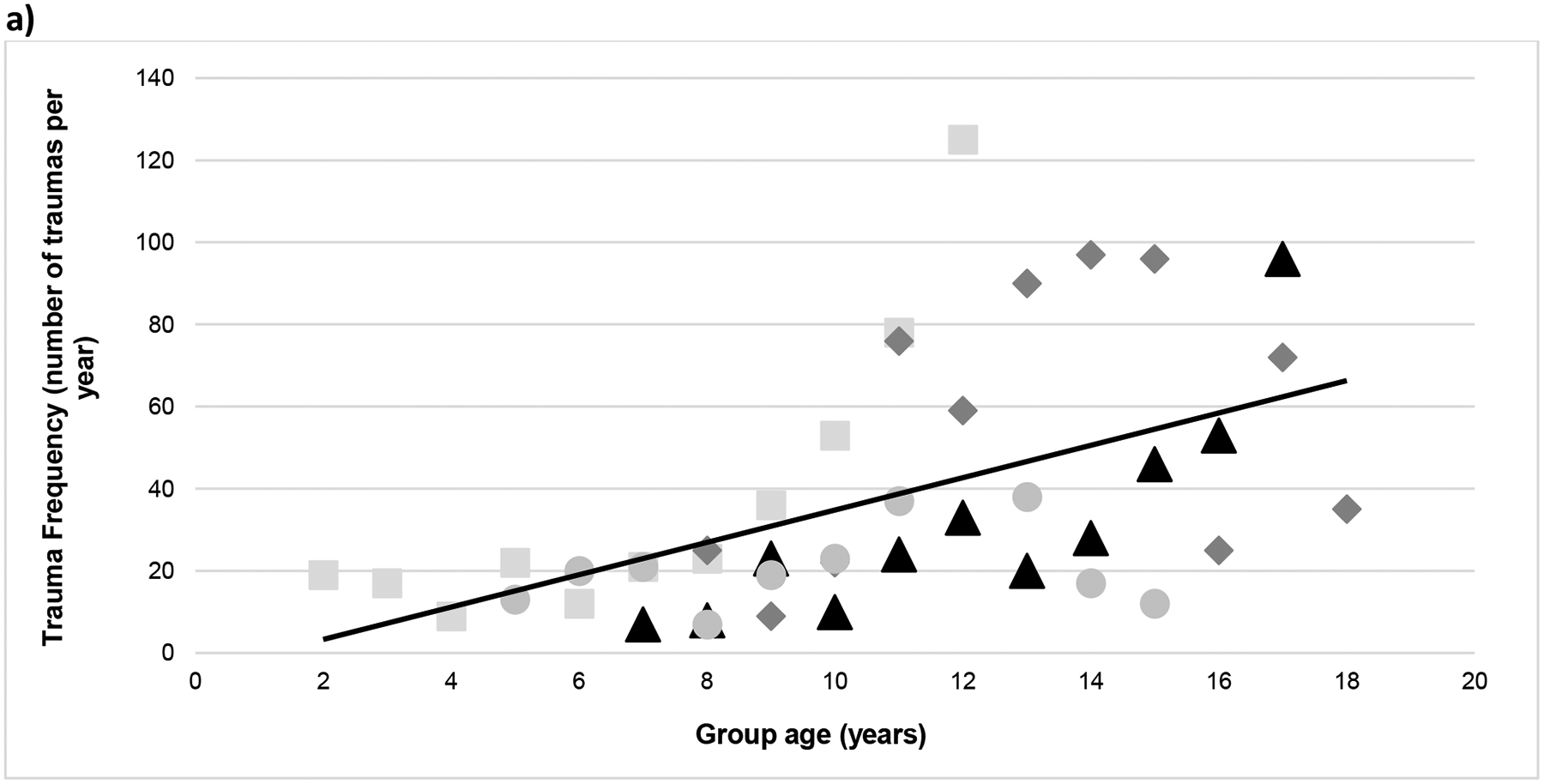
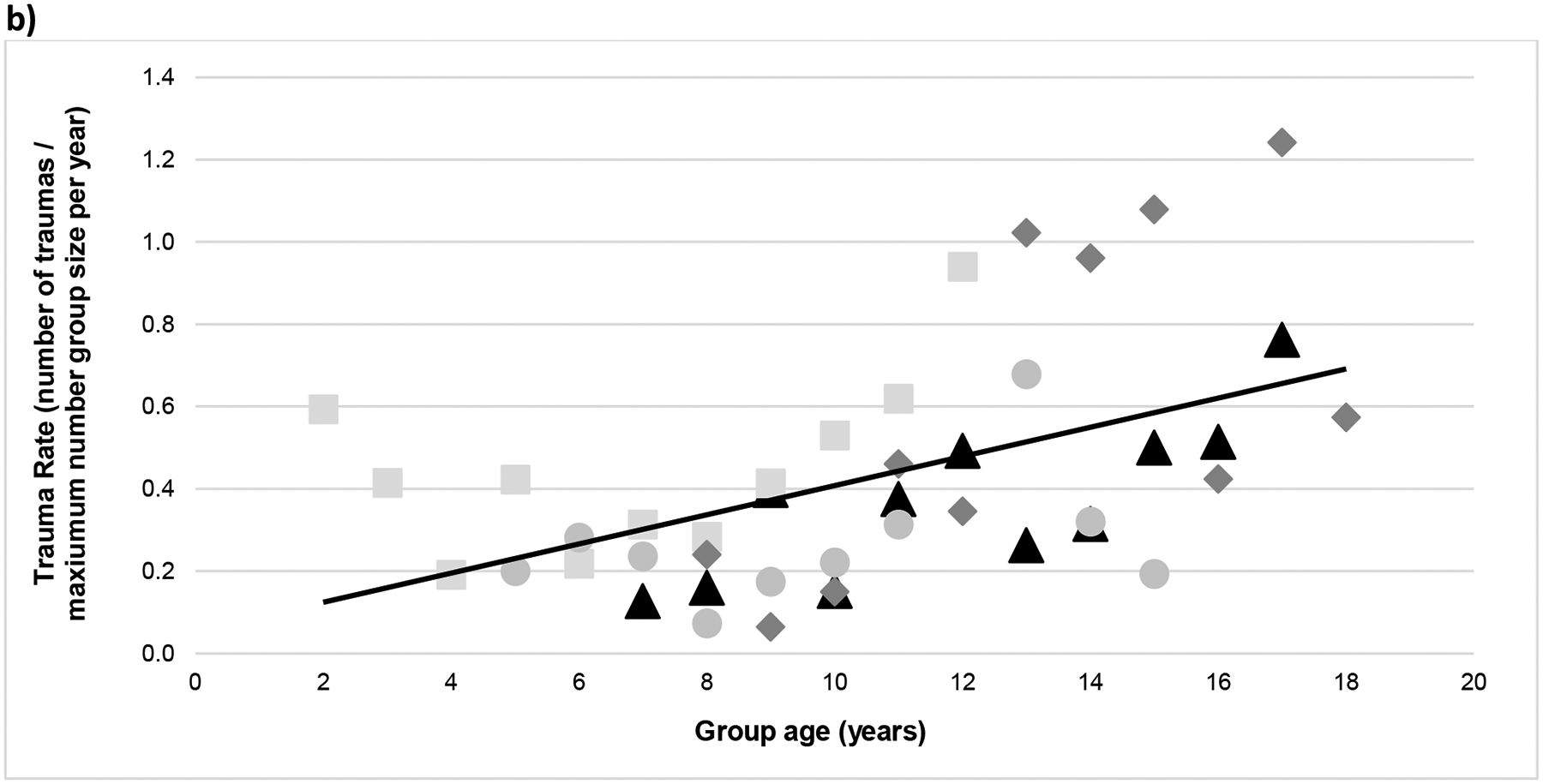
Yearly (a) trauma frequency and (b) trauma rate over the years since original group formation (each group is represented by a different symbol and shade). Overall, the relationship between trauma frequency and group age was r = 0.53 (p <0.001) and between trauma rate and group age r = 0.50 (p < 0.001).
Figure 3.
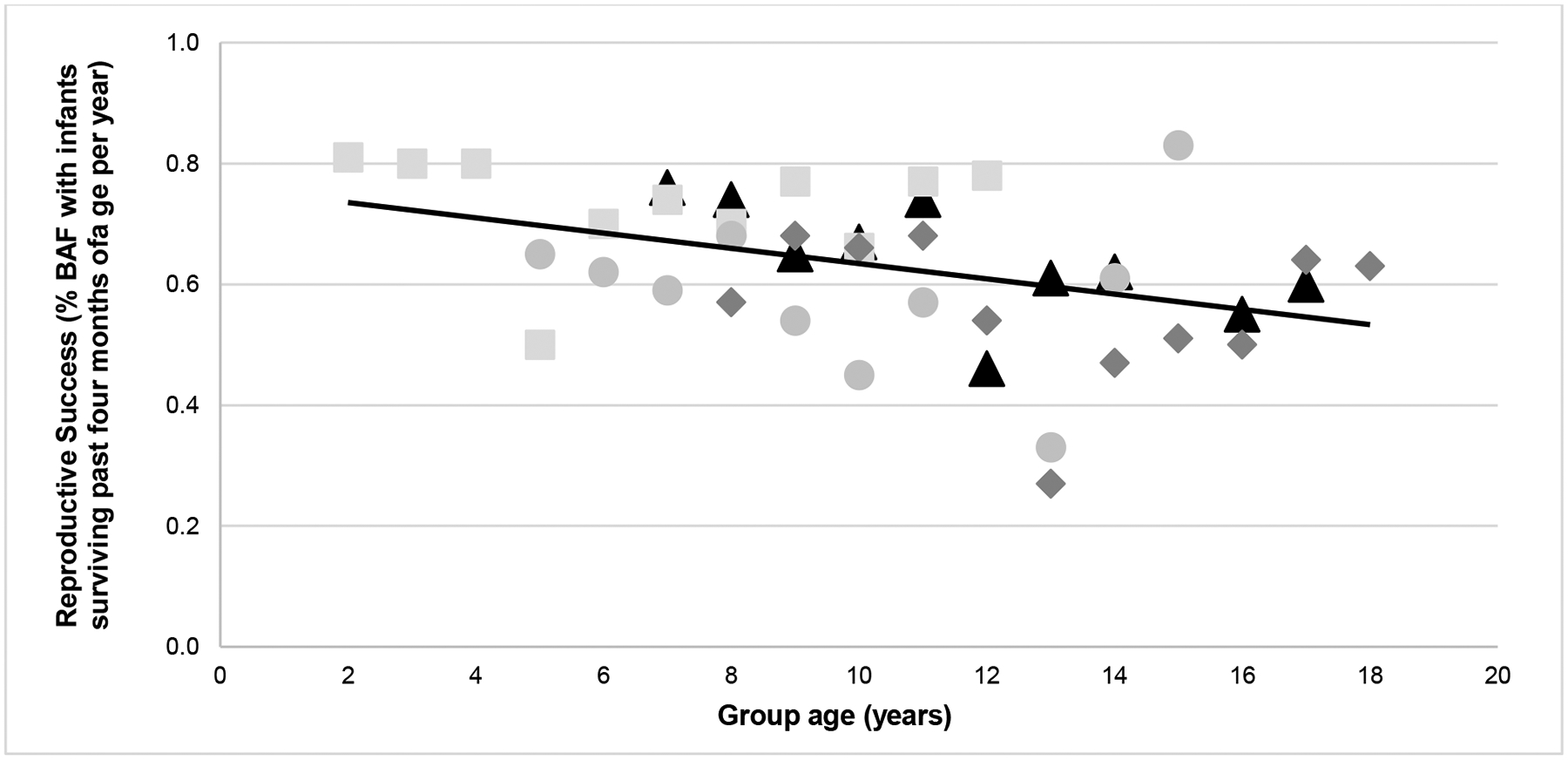
Yearly reproductive success over the years since original group formation (each group is represented by a different symbol and shade). Overall, the relationship between reproductive success and group age was r = −0.40 (p = 0.009).
Trauma frequency
Overall, the frequency of traumas per year increased as the groups got older, bigger, and had proportionally fewer adult males present. The final trauma model included group age and sex ratio (Pearson correlations with trauma for each were r = 0.53 and r = 0.38, respectively). Although sex ratio was a significant predictor, group age was a stronger predictor of trauma (group age: β = 0.138; P < 0.001; sex ratio: β = 0.030; P = 0.005; note that β coefficients are on the log scale, that is, in terms of the log odds; see Table 4). Group identification was included as a random factor, as it contributed measurable variability in trauma frequency (the ‘null model’ including just the random effects of group showed 7% variance, and a linear model used to calculate the Intra-class Correlation [ICC] showed group membership contributing 9% of the variance in trauma frequency). This demonstrated that the amount of trauma differed by group, however all groups tended to experience more trauma over the studied time-frame (Figures 2[a] and [b] show both trauma frequency and rates over time). Because of our specific interest in sex ratio, we avoided including group size with models that tested the effects of sex ratio because of their relatively high correlation (r = 0.66). This was also the reason for predicting trauma frequency instead of trauma rate (as it is calculated by dividing trauma frequency by group size). The strong correlation implies that group size is captured by the sex ratio metric to a large extent, since females and their offspring make up the bulk of all groups studied (Figure 1). Therefore, we were not able to evaluate its independent contribution with this dataset (note: including group size in the models with sex ratio resulted in ‘failure to converge’ warnings). The final trauma model was significantly different from a null model (AIC = 392.52 vs 364.90 for the final model, χ2 = 31.62; P < 0.001), indicating that the addition of group age and sex ratio improved the model significantly (see Table 5). Thus, while sex ratio had a significant effect on trauma frequency, the effect is in tandem with group size: larger groups had more skewed sex ratios which, in turn, had more traumas.
Table 4.
Results of mixed-effects regression models showing significant predictors of trauma and reproductive success in four large breeding groups over an 11-year period (2003–2013).
| Final model results* | β, P-value | Regression model specification |
|---|---|---|
| Trauma frequency = | Negative binomial regression model (glmer.nb function in R) | |
| Intercept | 1.420 | |
| Group age (years since formation) + | 0.138, p<0.001 | |
| Sex ratio (# breeding aged females/males) + | 0.030, p=0.005 | |
| Group (random effects term) | ||
| Reproductive Success = | Linear Gaussian regression model (lmer function in R) | |
| Intercept | 84.791 | |
| Individual space (meters per animal) + | −0.249, p=0.008 | |
| Trauma rate (# trauma/group size) + | −0.116, p=0.039 | |
| Sex ratio (# breeding aged females/males) + | −0.419, p=0.041 | |
| Group (random effects term) |
All models included a term accounting for variation in the dependent variable due to group membership. With the size of the dataset, we could only account for varying intercepts, not varying slopes as well.
Table 5.
Model Comparisons. For the trauma models, we used a negative binomial distribution and for the reproductive success models, we used a Gaussian distribution. The term 1|Group ID refers to the inclusion of group identification as a random effects term.
| AIC | Model | DV | Predictor Term 1 | Predictor Term 2 | Predictor Term 3 | Predictor Term 4 | Model comparison* |
|---|---|---|---|---|---|---|---|
| 392.5 | Null | Trauma Frequency (count per group-year) | 1|Group ID | p<.001 | |||
| 364.9 | Final | Group age | Sex Ratio | 1|Group ID | |||
| 370.1 | Alternate 1 | Group age | 1|Group ID | p=.007 | |||
| 388.2 | Alternate 2 | Sex Ratio | 1|Group ID | p<.001 | |||
| 362.9 | Alternate 3§ | Group age | Group Size | 1|Group ID | p<.001 | ||
| 332.6 | Null | Repro Success(% per group-year) | 1|group | p=.002 | |||
| 324.1 | Final | Indiv. space | Sex Ratio | Trauma rate | 1|Group ID | ||
| 325.9 | Alternate 1 | Indiv. space | Sex Ratio | 1|Group ID | p=.051 | ||
| 326.1 | Alternate 2 | Indiv. space | Trauma rate | 1|Group ID | p=.046 | ||
| 327.7 | Alternate 3 | Trauma rate | Sex Ratio | 1|Group ID | p=.017 | ||
| 328.2 | Alternate 4 | Indiv. space | 1|Group ID | p=.018 | |||
| 328.2 | Alternate 5 | Trauma rate | 1|Group ID | p=.017 | |||
| 332.4 | Alternate 6 | Sex Ratio | 1|Group ID | p=.002 |
Model comparisons show the P-value of a Chi-squared test comparing two models – the Null or Alternate to the final model (α = 0.05).
Note: Alternate model 3 generated a failure to converge warning, indicating the AIC value may not be reliable.
Reproductive success
Overall, reproduction declined as animals had more space per individual, had highly skewed sex ratios, and had higher rates of trauma. The final model for reproductive success included sex ratio, individual space, and yearly trauma rates (that is, trauma frequency adjusted by group size); Pearson correlations between each variable and reproductive success were: r = −0.45, r = −0.27, and r = – 0.33, respectively). Individual space was the strongest predictor, followed by sex ratio and trauma (individual space: β = −0.249; P = 0.008; sex ratio: β = −4.19; P = 0.041; trauma rate: β = −0.116; P = 0.039, respectively). Note the negative β coefficient for individual space, indicating that as individual space increased (that is, less crowded conditions), reproductive success declined (Table 4). The intra-class correlation (ICC) was 0.20, indicating group membership accounted for 20% of the variance in reproductive success. This indicates that the groups differed from one another in their reproductive success by a relatively large degree. Although the downward trends were not strong (overall r = – 0.40), one group had a more pronounced downward trend than the others (see Figure 3). Again, this final reproductive success model was significantly different from a null model accounting for just the random effects of group membership (AIC = 324 vs 333; χ2 = 14.58; P = 0.002), indicating the addition of the predictor variables improved the model significantly (Table 5).
Discussion
The results of this study supported our central hypothesis that sex ratio would significantly predict both trauma and reproduction in large breeding groups of rhesus macaques. We consider this to be corroborating evidence along with other studies (primarily from the CNPRC) that sex ratio is an important factor in group stability. Other managementrelated variables (group age, group size and available space per animal) were also predictive, and some more strongly than expected. Specifically, group age was a stronger predictor of trauma frequency per year than sex ratio, and individual space was a stronger predictor of reproduction than anticipated. Overall, the relationships indicate that over time, large female-skewed groups lived in very large enclosures, where the few males that were present had difficulty policing and controlling aggression. These conditions negatively affected trauma and reproduction.
Sex ratio and trauma
We found that more balanced sex ratios between adult females and males significantly predicted the yearly frequency of socially induced trauma when controlling for group age. That is, the more balanced the ratios, the fewer traumas (see Figure 4 for the association between sex ratio and trauma frequency by group; overall r = 0.38). The likely mechanism for this effect is that these sex ratio differences are associated with differences in social group stability (McCowan et al 2018), likely driven by the presence of sufficient numbers of adult males that are willing and able to successfully intervene in conflicts (Flack et al 2006; Beisner et al 2012). Beisner and colleagues (2012) also found that the most successful males were not genetically related to the alpha and beta matrilines, nor socially familiar with them. At the YNPRC, genetic variability is a primary goal when selecting males for ‘migrations’ to new groups, so our males are as unrelated as they can be to their new group-mates. In addition, they are unlikely to have any social ties to the resident females, and if they had met them before, it would have been years since they last interacted. Thus, our findings of skewed sex ratios associated with more frequent trauma is more likely due to the general paucity of adult males in the groups over long periods of time and, potentially, their prior social experience, personality, and experience during group integration, as well. This is the subject of current research with the goal of improving the quantity and quality of unrelated, adult males to the YNPRC rhesus breeding groups.
Figure 4.
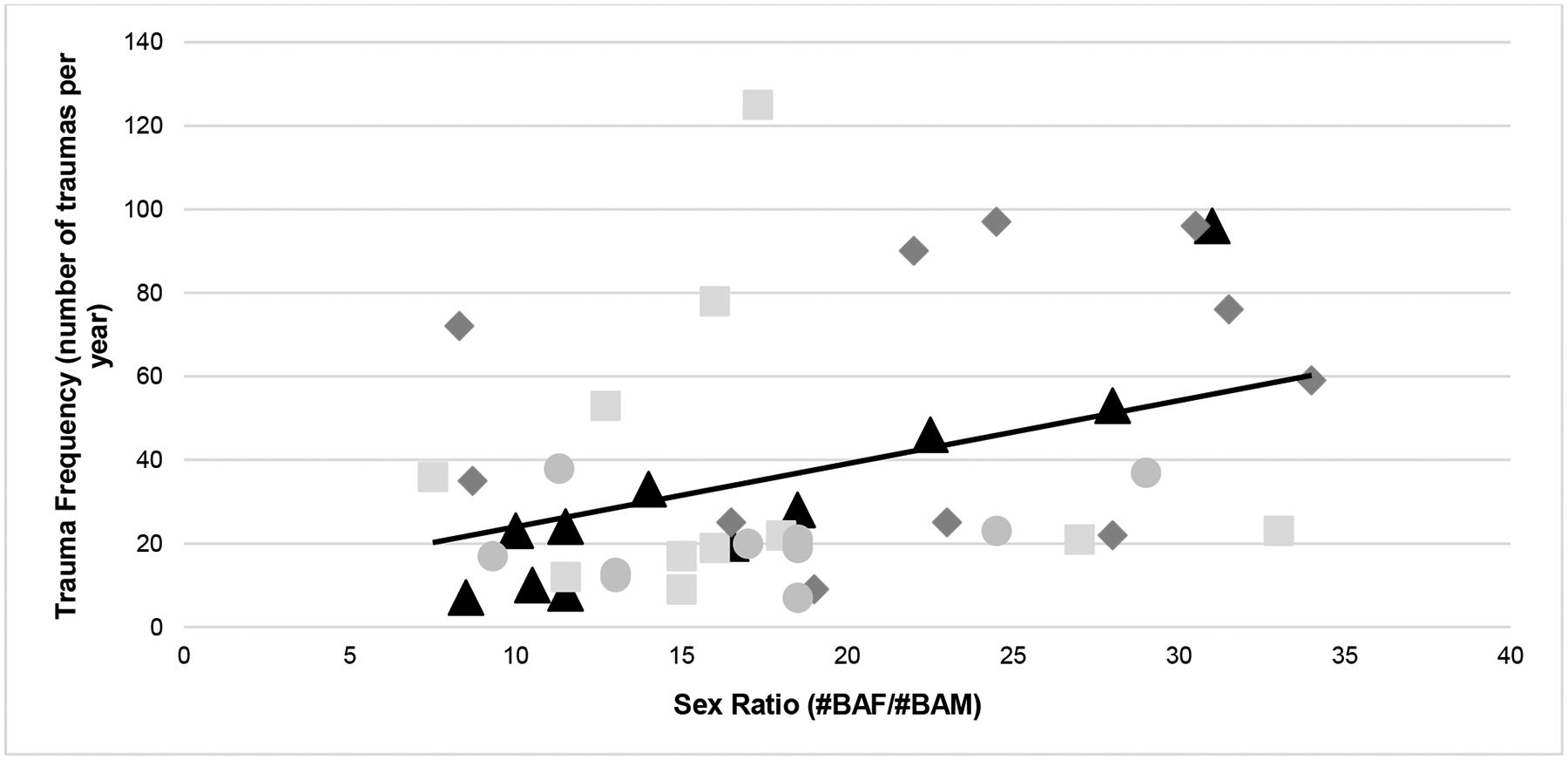
Yearly trauma frequency across sex ratios (groups represented by different symbols and shades; note that each group had 10–11 data-points across the studied time-frame). Overall, the relationship between trauma frequency and sex ratio was r = 0.38 (p = 0.013).
Another possibility is that groups with more balanced sex ratios are less likely to have as much aggressive competition among female macaques for sexual access to males (Zumpe & Michael 1987). Additionally, in contrast to Beisner and colleagues’ (2012) findings, group size correlated with trauma frequency in our dataset (r = 0.49; Table 3). Because group size was also so highly correlated with sex ratio (r = 0.66; Table 3), we were not able to disentangle its separate effects from sex ratio in our models. While we found that more balanced sex ratios predicted fewer traumas, these more balanced sex ratios were in the context of smaller groups. A larger group size may create more opportunities for socially induced traumas, but we would expect a more balanced sex ratio to mitigate that through male policing. In this study, there were not enough males in those larger groups to effectively intervene in conflict and reduce trauma occurrence. This is a clear limitation of this study and additional research is needed to better understand the independent and synergistic effects of these variables.
The results indicate that even in groups with more unbalanced sex ratios than those previously studied (eg at the CNPRC), there still is credence to the idea that improved sex ratios (fewer adult females per male) can help reduce wounding rates. Particularly when groups get very large, it is essential to scale up the numbers of adult males proportionally, as we found that very large rhesus groups that have only a few adult males present are clearly associated with increased trauma. Since wild groups are more likely than captive to have the optimal sex ratio for group stability, as membership adjusts through dispersal and other mechanisms (Beisner et al 2012), we expect the effect we observed may strengthen if sex ratios in the Yerkes groups were manipulated to approach the sex ratio of wild groups (1–6 adult females to each adult male; Southwick et al 1961, 1965; Makwana 1978; Teas et al 1980; Seth & Seth 1985; Goldstein & Richard 1989; Sahi & Sharma 2004).
One way to increase the number of adult males in a group is to introduce larger groups of unrelated males into the groups. Other strategies are to retain natal males in the groups, even after they gain sexual maturity, or to crossfoster very young male infants into non-natal groups; however, these approaches can lead to higher incidences of wounding associated with males who have grown up in the group (Beisner et al 2011a). Introducing new males into rhesus social groups is not an easy task given the strong matrilineal structure of rhesus society and the xenophobic nature of rhesus macaques (Bernstein et al 1974; Singh & Gupta 1980). Introductions of adult males are socially disruptive and may result in severe aggression directed toward the new males (Bernstein 1964; Bernstein & Draper 1964). This process results in varying levels of aggression from resident group members, which can affect how many males remain long-term. However, it is essential to find successful strategies to integrate unrelated adult males into groups of females such that the resulting breeding groups are socially stable and produce a satisfactory number of offspring that can be safely reared in the group. Upcoming research at the YNPRC will test various male introduction strategies to improve the sex ratios in our groups.
Sex ratio, trauma rates, and reproduction
We found that reproductive success was significantly predicted by sex ratio when controlling for trauma rates and individual space. Specifically, the more balanced the ratios, the better reproductive output in the groups (for the association between sex ratio and reproductive success by group; overall; r = −0.27, see Figure 5). Thus, highly skewed sex ratios appear to negatively impact the animal production goals for rhesus breeding programmes. There are at least two pathways through which this decrease in reproductive output may occur. First, with more balanced sex ratios, there are simply more males available for mating. Increasing the choice of males for females may positively affect mating and conception as some females may refuse to mate with a particular male (Manson 1995). The second pathway is through the negative effects of imbalanced sex ratios on socially induced wounding, observed in this study and others, and the psychosocial stress that likely occurs in result. High conflict within social groups is a psychosocial stressor that alters glucocorticoid levels (Cleveland et al 2004) and ultimately leads to reproductive dysfunction (Sade et al 1976; Dittus 1977, 1980; Adams et al 1985; Harcourt 1987; Cameron 1997; Kaplan et al 2010). Dysfunction can include the suppression of reproductive hormone levels, compromised ovarian function, increased incidence of spontaneous abortions, stillbirths, infant mortality and health problems requiring medical care (Deutsch & Lee 1991; Cameron 1997; Kaplan et al 2010; Ha et al 2011; Dettmer et al 2015).
Figure 5.
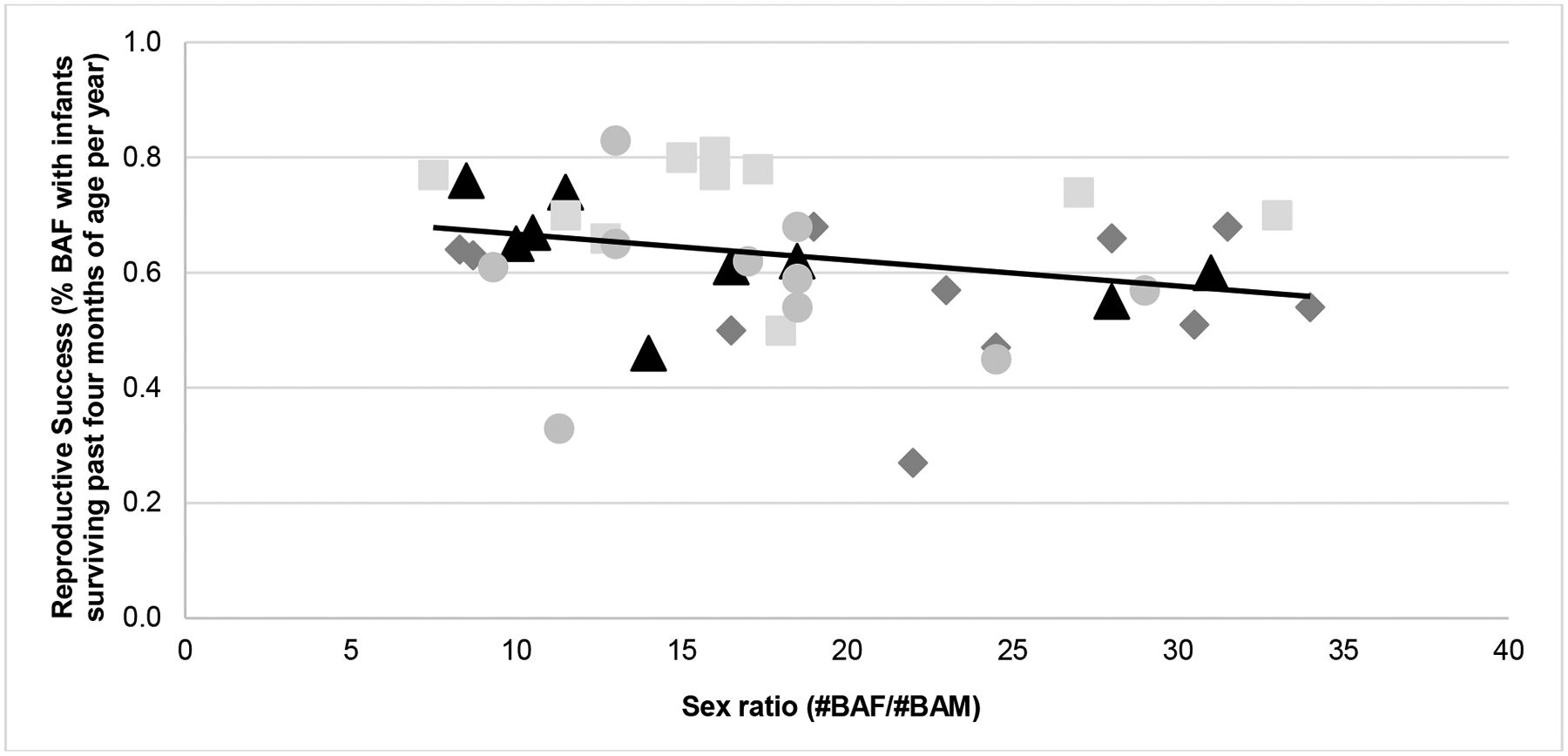
Yearly reproductive success across sex ratios (groups represented by different symbols and shades; note that each group had 10–11 data-points across the studied time-frame). The overall relationship between reproductive success and sex ratio was r = −0.27 (p = 0.081).
Altering sex ratios in rhesus breeding groups is a practical means of producing more animals, and there is a strong need for more non-human primates to meet the needs of biomedical research. The National Institutes of Health has recently published a ‘Nonhuman Primate Evaluation and Analysis’ (2018; p 1) which concluded that their
…results also indicated current shortages and projected future high demand for and consequent shortages of rhesus macaques… Infectious disease and behavioural and systems neuroscience research were, in general, the major drivers of NHP demand, particularly in regard to demand for rhesus macaques.
Among their potential solutions outlined is expanding rhesus macaque colonies by 10 to 25% to keep up with these projected needs (p 7–8). Changing sex ratios within breeding groups of rhesus macaques is one technique for meeting that objective.
Group age and trauma
Some of the additional factors we assessed, mainly as a way of controlling these potentially confounding variables, were also important influences on trauma and reproduction outcomes. Among all the factors we measured, group age (time since the group was formed) had the largest effect on trauma (holding sex ratio constant), with older groups experiencing higher yearly frequencies of injuries requiring veterinary treatment (note that the correlation between group age and sex ratio was small; r = 0.13). Although there are few previous studies of group age and wounding, a past Yerkes study corresponds with our findings, as they found that rhesus groups that experienced matrilineal overthrows were older than those that did not have overthrows (Sánchez et al 2014). A possible mechanism for the relationship between group age and trauma is that older groups also have lower group-mean matrilineal coefficients of relatedness, and generally have more matrilines which have lost their matriarchs; this genetic fragmentation is associated with higher wounding rates (Beisner et al 2011b). Genetic fragmentation may be an inevitable outcome for an ageing group, particularly if group membership is not deliberately manipulated to maintain it (Beisner et al 2011b).
At Yerkes, groups continue to grow in size and age with limited options for space and housing changes (ie the existing large, outdoor enclosures continue to be used). Given this practical limitation, a management strategy of moving groups to other spaces or splitting groups when they reach a certain age as a way to mitigate trauma is difficult to implement. However, when it is possible to do this, it should be considered for groups with high wounding rates. Colony managers at the Oregon National Primate Research Center have successfully used a strategy of disbanding macaque groups when instability within the hierarchy is predicted to put the safety of a large proportion of animals at risk. This instability may have resulted from recent trauma to highranking animals, or the planned removal of the dominant male or female for health reasons (A Heagerty, personal communication 2020). However, group splitting and/or removing problematic matrilines by colony managers can sometimes have unpredictable results and lead in some cases to more trauma. In that case, a more feasible management strategy for mitigating trauma would be manipulating group composition by increasing the number of adult males in groups, to bring skewed sex ratios into better alignment.
Available space and reproduction
Of all of the factors we assessed, individual space had the strongest relationship with reproductive output in our groups. We found that as groups had more space available per individual group member, fewer infants were produced. One possible explanation for our finding of declining reproduction with more space is that males and females are not spending time near one another, and hence breeding less frequently. However, anecdotally, we know that the females enthusiastically seek out males for mating when they are in oestrous, so that seems unlikely. Perhaps the larger spaces make it physically taxing for the males to patrol their groups and to breed with all of the available females. Unfortunately, we do not have behavioural data in this study, which would be useful to discern what might account for the reduced reproductive success. Certainly, more research is needed to fully understand the relationship between available space and reproduction in rhesus macaques.
Considerations for rhesus breeding colony managers
Since the purpose of holding the breeding groups of rhesus macaques in biomedical research facilities is to produce high quality monkeys that will be healthy animal models for biomedical and behavioural research, the impact of sex ratio on trauma rates and reproductive success is important to consider. Working to introduce larger numbers of males can be instrumental in improving group stability and reproduction, both of which are critical to the mission of these breeding colonies and essential to maintain the animals’ physical and psychological well-being. When logistically feasible, colony managers may also consider group age and housing situations as potential changes that might also positively impact wounding and reproduction rates. For example, with an abundance of caution, knowledge and expertise on the part of colony managers, splitting groups and moving them to smaller housing situations may lead to improved stability and reproduction. Alternatively, leaving groups that are growing in numbers in their current enclosure could be a minimally disruptive way to improve reproduction. As a heuristic for gauging the value of making changes in sex ratios, we have developed a hypothetical dataset from which to make projections regarding trauma, reproduction and sex ratio based on our final regression models. It is important to note that these are models based on YNPRC data where the sex ratios tended to be extremely skewed. Therefore, this information will apply most directly to other colonies with sex ratios in the range of those at YNPRC. Table 6 depicts a hypothetical group containing 32 adult females with varying numbers of adult males and provides our outcome measures for a group of each composition, holding the other significant covariates constant. The table illustrates the improvements in yearly trauma frequency and reproductive output that would be anticipated as the sex ratio is brought into better balance by adding adult males to the group. The anticipated yearly trauma frequency is reduced by about half by the presence of four adult males (1:8 ratio) and then continues a slow decline if additional males were to be added. The number of predicted infants produced annually increases by 10% until four males are present, and then makes smaller gains as the sex ratio continues to improve. These projections show the potential improvements colony managers might see if they are contemplating changes to the sex ratios in their groups. It is notable that these two advances occur together: improvements in sex ratio will lead to a larger number of infants being born, and it will improve the social environment within which those infants grow and develop.
Table 6.
Predicted values for trauma frequency and reproductive success using the final models for each outcome. (Note that we used the average group age, spatial density, and trauma rates in the equations to yield these predictions.)
| Hypothetical N males | Hypothetical N females | Hypothetical sex ratio | Predicted N trauma | Predicted Reproduction (%) | Predicted N infants |
|---|---|---|---|---|---|
| 1 | 32 | 32 | 46 | 57 | 18 |
| 2 | 32 | 16 | 28 | 64 | 20 |
| 3 | 32 | 11 | 24 | 66 | 21 |
| 4 | 32 | 8 | 22 | 67 | 22 |
| 5 | 32 | 6 | 21 | 68 | 22 |
| 6 | 32 | 5 | 21 | 68 | 22 |
| 7 | 32 | 5 | 20 | 69 | 22 |
| 8 | 32 | 4 | 20 | 69 | 22 |
Animal welfare implications
This study provides evidence that improving the balance of adult sex ratios in rhesus macaque groups will reduce the rate of socially induced wounding. Reducing wounding may be evidence of greater social group stability, and both factors support the welfare of the group members. Reducing the occurrence of socially induced injuries reduces the associated pain a monkey would experience, as well as the opportunity for infection and the need to remove monkeys from their groups for veterinary care. The latter exposes multiple group members to the additional stressors of social disruption related to the dominance hierarchy of the group, the separation and eventual reunion between family members, housing in an unfamiliar environment, and perhaps stressful veterinary procedures. Improving social group stability enhances welfare by increasing the certainty and predictability of social relationships and reducing the chances of matrilineal overthrow, which are stressful and often result in severe injuries. It is important that studies like this one inform decisions regarding the management of captive primates with the goal of maximising group stability, production of infants in safe social environments, and the well-being of the individual animals.
Acknowledgements
This work was funded by the National Institutes of Health Office of Research Infrastructure Programs, Office of the Director, grant R24OD020349 awarded to the YNPRC. We also acknowledge support from the NIH Office of Research Infrastructure Programs, Office of the Director, grant P51OD011132 to the YNPRC. We thank Kelly Bailey for help preparing and editing the manuscript and Brianne Beisner for consultation on the statistical analysis.
References
- Adams MR, Kaplan JR and Koritnik DR 1985. Psychosocial influences on ovarian endocrine and ovulatory function in Macaca fascicularis. Physiology and Behavior 35: 935–940. 10.1016/0031-9384(85)90262-8 [DOI] [PubMed] [Google Scholar]
- Alberts SC, Sapolsky RM and Altmann J 1992. Behavioral, endocrine, and immunological correlates of immigration by an aggressive male into a natural primate group. Hormones and Behavior 26: 167–178. 10.1016/0018-506X(92)90040-3 [DOI] [PubMed] [Google Scholar]
- Altmann J and Alberts SC 2003. Variability in reproductive success viewed from a life-history perspective in baboons. American Journal of Human Biology 15(3): 401–409. 10.1002/ajhb.10157 [DOI] [PubMed] [Google Scholar]
- Arcese P and Smith JNM 1988. Effects of population density and supplemental food on reproduction in song sparrows. Journal of Animal Ecology 57: 119–136. 10.2307/4768 [DOI] [Google Scholar]
- Asensio N, Korstjen AH, Schaffner CM and Aureli F 2008. Intragroup aggression, fission–fusion dynamics and feeding competition in spider monkeys. Behaviour 145: 983–1001. 10.1163/156853908784089234 [DOI] [Google Scholar]
- Beisner BA and Isbell LA 2011. Factors affecting aggression among females in captive groups of rhesus macaques. American Journal of Primatology 73: 1152–1159. 10.1002/ajp.20982 [DOI] [PubMed] [Google Scholar]
- Beisner BA, Jackson ME, Cameron A and McCowan B 2011a. Effects of natal male alliances on aggression and power dynamics in rhesus macaques. American Journal of Primatology 73: 790–801. 10.1002/ajp.20907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisner BA, Jackson ME, Cameron AN and McCowan B 2011b. Detecting instability in animal social networks: Genetic fragmentation is associated with social instability in rhesus macaques. PLoS One 6(1): e16365. 10.1371/journal.pone.0016365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisner BA, Jackson ME, Cameron A and McCowan B 2012. Sex ratio, conflict dynamics and wounding in rhesus macaques (Macaca mulatta). Applied Animal Behavior Science 137:137–147. 10.1016/j.applanim.2011.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisner BA and McCowan B 2013. Policing in nonhuman primates: Partial interventions serve a prosocial conflict management function in rhesus macaques. PLoS One 8(10): 1–13. 10.1371/journal.pone.0077369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercovitch FB and Lebron MR 1991. Impact of artificial fissioning and social networks on levels of aggression and affiliation in primates. Aggressive Behavior 17: 17–25. [DOI] [Google Scholar]
- Bernstein IS 1964. Role of the dominant male rhesus monkey in response to external challenges to the group. Journal of Comparative and Physiological Psychology 57: 404–406. 10.1037/h0041409 [DOI] [PubMed] [Google Scholar]
- Bernstein IS and Draper WA 1964. The behavior of juvenile rhesus monkeys in groups. Animal Behaviour 12(1): 84–91. 10.1016/0003-3472(64)90107-1 [DOI] [Google Scholar]
- Bernstein IS and Gordon TP 1974. The function of aggression in primate societies. American Scientist 62: 304–311 [PubMed] [Google Scholar]
- Bernstein IS, Gordon TP and Rose RM 1974. Factors influencing the expression of aggression during introductions to rhesus monkey groups. In: Holloway RL (ed) Primate Aggression, Territoriality, and Xenophobia: A Comparative Perspective pp 211–240. Academic Press: New York, USA [Google Scholar]
- Bonenfant C, Gaillard JM, Klein F and Loison A 2002. Sex and age-dependent effects of population density on life history traits of red deer Cervus elaphus in a temperate forest. Ecography 25: 446–458. 10.1034/j.1600-0587.2002.250407.x [DOI] [Google Scholar]
- Borries C, Larney E, Lu A, Ossi K and Koenig A 2008. Costs of group size: Lower developmental and reproductive rates in larger groups of leaf monkeys. Behavioral Ecology 19: 1186–1191. 10.1093/beheco/arn088 [DOI] [Google Scholar]
- Cameron JL 1997. Stress and behaviorally induced reproductive dysfunction in primates. Seminars in Reproductive Medicine 15: 37–45. 10.1055/s-2008-1067966 [DOI] [PubMed] [Google Scholar]
- Caws C and Aureli F 2003. Chimpanzees cope with temporary reduction of escape opportunities. International Journal of Primatology 24: 1077–1091. 10.1023/A:1026280329544 [DOI] [Google Scholar]
- Cleveland A, Westergaard GC, Trenkle MK and Higley JD 2004. Physiological predictors of reproductive outcome and mother– infant behaviors in captive rhesus macaque females (Macaca mulatta). Neuropsychopharmacology 29: 901–910. 10.1038/sj.npp.1300361 [DOI] [PubMed] [Google Scholar]
- Cordoni G and Palagi E 2007. Response of captive lowland gorillas (Gorilla gorilla gorilla) to different housing conditions: Testing the aggression-density and coping models. Journal of Comparative Psychology 121: 171–180. 10.1037/0735-7036.121.2.171 [DOI] [PubMed] [Google Scholar]
- Crast J, Bloomsmith MA and Jonesteller T 2015. Effects of changing housing conditions on mangabey behavior (Cercocebus atys): Spatial density, housing quality, and novelty effects. American Journal of Primatology 77: 1001–1014. 10.1002/ajp.22430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren BT 1979. The effects of population density on fecundity and fertility in the guppy, Poecilia reticulata. Journal of Fish Biology 15: 71–91. 10.1111/j.1095-8649.1979.tb03573.x [DOI] [Google Scholar]
- Dazey J, Kuyk K, Oswald M, Martenson J and Erwin J 1977. Effects of group composition on agonistic behavior of captive pigtail macaques, Macaca nemestrina. American Journal of Physical Anthropology 46: 73–76. 10.1002/ajpa.1330460110 [DOI] [Google Scholar]
- Demaria C and Thierry B 1989. Lack of effects of environmental changes on agonistic behavior patterns in a stabilizing group of stumptailed macaques (Macaca arctoides). Aggressive Behavior 15: 353–360. [DOI] [Google Scholar]
- Dettmer AM, Novak MA, Meyer JS and Suomi SJ 2014. Population density-dependent hair cortisol concentrations in rhesus monkeys (Macaca mulatta). Psychoneuroendocrinology 42: 59–67. 10.1016/j.psyneuen.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer AM, Woodward RA and Suomi SJ 2015. Reproductive consequences of a matrilineal overthrow in rhesus monkeys. American Journal of Primatology 77: 346–352. 10.1002/ajp.22350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch JC and Lee PC 1991. Dominance and feeding competition in captive rhesus monkeys. International Journal of Primatology 12: 615–628. 10.1007/BF02547673 [DOI] [Google Scholar]
- Dittus WP 1980. The social regulation of primate populations: a synthesis. In: Lindburg DG (ed) The Macaques: Studies in Ecology, Behavior and Evolution pp 263–286. Van Nostrand Reinhold Company: New York, USA. [Google Scholar]
- Dittus WPJ 1977. The social regulation of population density and age-sex distribution in the toque monkey. Behaviour 63: 281–322. 10.1163/156853977X00450 [DOI] [Google Scholar]
- Eaton GG, Modahl KB and Johnson DF 1981. Aggressive behavior in a confined troop of Japanese macaques: effects of density, season, and gender. Aggressive Behaviour 7: 145–164. [DOI] [Google Scholar]
- Elton RH and Anderson BV 1977. The social behavior of a group of baboons (Papio anubis) under artificial crowding. Primates 18: 225–234. 10.1007/BF02382961 [DOI] [Google Scholar]
- Erwin J 1979. Aggression in captive macaques: interaction of social and spatial factors. In: Erwin J, Maple TL and Mitchell G (eds) Captivity and Behavior pp 139–171. Van Nostrand: New York, USA [Google Scholar]
- Erwin N and Erwin J 1976. Social density and aggression in captive groups of pigtail monkeys (Macaca nemestrina). Applied Animal Ethology 2: 265–269. 10.1016/0304-3762(76)90059-6 [DOI] [Google Scholar]
- Flack JC, de Waal FBM and Krakauer DC 2005. Social structure, robustness, and policing cost in a cognitively sophisticated species. The American Naturalist 165: 129–139. 10.1086/429277 [DOI] [PubMed] [Google Scholar]
- Flack JC, Girvan M, de Waal FBM and Krakauer DC 2006. Policing stabilises construction of social niches in primates. Nature 439: 426–429. 10.1038/nature04326 [DOI] [PubMed] [Google Scholar]
- Goldstein SJ and Richard AF 1989. Ecology of rhesus macaques (Macaca mulatta) in northwest Pakistan. International Journal of Primatology 10(6): 531–567. 10.1007/BF02739364 [DOI] [Google Scholar]
- Goss-Custard J, Clarke R and Durell S 1984. Rates of food intake and aggression of oystercatchers (Haematopus ostralegus) on the most and least preferred mussel (Mytilus edulis) beds of the Exe Estuary. Journal of Animal Ecology 53(1): 233. 10.2307/4354 [DOI] [Google Scholar]
- Grenier D, Barrette C and Crete M 1999. Food access by white-tailed deer (Odocoileus virginianus) at winter feeding sites in Eastern Quebec. Applied Animal Behaviour Science 63(4): 323–337. 10.1016/S0168-1591(99)00017-9 [DOI] [Google Scholar]
- Ha JC, Alloway H and Sussman A 2011. Aggression in pigtailed macaque (Macaca nemestrina) breeding groups affects pregnancy outcome. American Journal of Primatology 73: 1169–1175. 10.1002/ajp.20984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcourt AH 1987. Dominance and fertility among female primates. Zoological Society of London 213: 471–487. 10.1111/j.1469-7998.1987.tb03721.x [DOI] [Google Scholar]
- Herrington JA, Del Rosso LA and Capitanio JP 2016. Biobehavioral consequences of prenatal exposure to a matrilineal overthrow and relocation in captive infant rhesus (Macaca mulatta) monkeys. American Journal of Primatology 78(9): 895–903. 10.1002/ajp.22557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins TM Jr, Diehl S, Kratz KW and Cooper SD 1999. Effects of population density on individual growth of brown trout in streams. Ecology 80: 941–956. 10.1890/0012-9658(1999)080[0941:EOPDOI]2.0.CO;2 [DOI] [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL and Daszak P 2008. Global trends in emerging infectious diseases. Nature 451: 990–994. 10.1038/nature06536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge P and de Waal FBM 1993. Conflict avoidance among rhesus monkeys: coping with short-term crowding. Animal Behaviour 46: 221–232. 10.1006/anbe.1993.1184 [DOI] [PubMed] [Google Scholar]
- Judge P, de Waal FBM, Paul KS and Gordon TP 1994. Removal of a trauma-inflicting alpha matriline from a group of rhesus macaques to control severe wounding. Laboratory Animal Science 44: 344–350 [PubMed] [Google Scholar]
- Kaplan JR, Chen H, Appt SE, Lees CJ, Franke AA, Berga SL, Wilson ME, Manuck SB and Clarkson TB 2010. Impairment of ovarian function and associated health-related abnormalities are attributable to low social status in premenopausal monkeys and not mitigated by a high-isoflavone soy diet. Human Reproduction 25: 3083–3094. 10.1093/humrep/deq288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindburg DG 1971. The rhesus monkey in north India: an ecological and behavioral study. In: Rosenblum LA (ed) Primate Behavior: Developments in Field and Laboratory Research pp 1–106. Academic Press: New York, USA. 10.1016/B978-0-12-534002-1.50007-9 [DOI] [Google Scholar]
- Liu B-J, Wu C-F, Garber PA, Zhang P and Li M 2018. Effects of group size and rank on mother-infant relationships and reproductive success in rhesus macaques (Macaca mulatta). American Journal of Primatology 80: e22881. 10.1002/ajp.22881 [DOI] [PubMed] [Google Scholar]
- Makwana SC 1978. Field ecology and behaviour of the rhesus macaque (Macaca mulatta): Group composition, home range, roosting sites, and foraging routes in the Asarori forest. Primates 19: 483–492. 10.1007/BF02373310 [DOI] [Google Scholar]
- Manson JH 1995. Do female rhesus macaques choose novel males? American Journal of Primatology 37: 285–296. 10.1002/ajp.1350370403 [DOI] [PubMed] [Google Scholar]
- Mathy J and Isbell L 2001. The relative importance of size of food and interfood distance in eliciting aggression in captive rhesus macaques (Macaca mulatta). Folia Primatologica 72(5): 268–277. 10.1159/000049948 [DOI] [PubMed] [Google Scholar]
- McCowan B, Beisner BA, Capitanio JP, Jackson ME, Cameron A, Seil S, Atwill ER and Fushing H 2011. Network stability is a balancing act of personality, power, and conflict dynamics in rhesus macaque societies. PLoS One 6: e22350. 10.1371/journal.pone.0022350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCowan B, Beisner BA and Hannibal D 2018. Social management of laboratory rhesus macaques housed in large groups using a network approach: A review. Behavioral Processes 156: 77–82. 10.1016/j.beproc.2017.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire MT, Cole SR and Crookshank C 1978. Effects of social and spatial density changes in Cercopithecus aethiops sabaeus. Primates 19: 615–631. 10.1007/BF02373630 [DOI] [Google Scholar]
- National Institutes of Health Office of Research Infrastructure Programs 2018. Non-human primate evaluation and analysis: Part 2 Report of the Expert Panel Forum on Challenges in Assessing Nonhuman Primate Needs and Resources for Biomedical Research. https://orip.nih.gov/sites/default/files/NHP%20Evaluation%20and%20Analysis%20Final%20%20Report%20-%20Part%202%20Final%20508%2021Dec2018_002.pdf
- Nieuwenhuijsen K and de Waal FBM 1982. Effects of spatial crowding on social behavior in a chimpanzee colony. Zoo Biology 1: 5–28. 10.1002/zoo.1430010103 [DOI] [Google Scholar]
- Novak MA and Drewsen KH 1989. Enriching the lives of captive primates: issues and problems. In: Segal EF (ed) Segal Housing, Care and Psychological Wellbeing of Captive and Laboratory Primates pp 161–182. Noyes Publications: Park Ridge, New Jersey, USA [Google Scholar]
- Oates-O’Brien RS, Farver TB, Anderson-Vicino KC, McCowan B and Lerche NW 2010. Predictors of matrilineal overthrows in large captive breeding groups of rhesus macaques (Macaca mulatta). Journal of the American Association for Laboratory Animal Science 49: 196–201 [PMC free article] [PubMed] [Google Scholar]
- Pettorelli N, Gaillard JM, Van Laere G, Duncan P, Kjellander P, Liberg O, Delorme D and Maillard D 2002. Variations in adult body mass in roe deer: the effects of population density at birth and of habitat quality. Proceedings of the Royal Society. London. B: Biological Sciences 269: 747–753. 10.1098/rspb.2001.1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade DS, Cushing K, Cushing P, Dunaif J, Figueroa A and Kaplan JR 1976. Population dynamics in relation to social structure on Cayo Santiago. Yearbook of Physical Anthropology 20: 253–262 [Google Scholar]
- Sahi DN and Sharma S 2004. An ecological and behavioural study on Macaca mulatta. In: Jammu JK, Gupta VK and Verma AK (eds) Perspectives in Animal Ecology and Reproduction pp 201–213. Daya Publishing House: New Delhi, India [Google Scholar]
- Sanchez DM, Herman R and Wallen K 2014. Matrilineal overthrows in captive groups of rhesus macaques (Macaca mulatta): a retrospective analysis. 37th Meeting of the American Society of Primatologists. 12–17 September 2014, Decatur, GA, USA [Google Scholar]
- Sannen A, Elsacker LV and Eens M 2004. Effect of spatial crowding on aggressive behavior in a bonobo colony. Zoo Biology 23: 383–395. 10.1002/zoo.20024 [DOI] [Google Scholar]
- Seth PK and Seth S 1985. Ecology and feeding behavior of the free ranging rhesus monkeys in India. Indian Anthropologist 15: 51–62 [Google Scholar]
- Singh SD and Gupta BS 1980. Xenophobic reactions of freeranging rhesus infant groups raised in natural habitat. Primates 21: 492–497. 10.1007/BF02373837 [DOI] [Google Scholar]
- Southwick C, Beg MA and Siddiqi MR 1965. Rhesus monkeys in North India. In: DeVore I (ed) Primate Behavior. Holt, Rinehart, and Winston: New York, USA [Google Scholar]
- Southwick CH 1967. An experimental study of intragroup agonistic behavior in rhesus monkeys (Macaca mulatta). Behaviour 28: 182–209. 10.1163/156853967X00235 [DOI] [PubMed] [Google Scholar]
- Southwick CH 1969. Aggressive behaviour of rhesus monkeys in natural and captive groups. In: Garattini S and Sigg EB (eds) Sigg Aggressive Behavior pp 32–43. John Wiley: New York, USA [Google Scholar]
- Southwick CH, Beg MA and Siddiqi MR 1961. A population survey of rhesus monkeys in India: transportation routes and forest areas. Ecology 42: 698–710. 10.2307/1933499 [DOI] [Google Scholar]
- Stavisky RC, Ramsey JK, Meeker T, Stovall M and Crane MM 2018. Trauma rates and patterns in specific pathogen free (SPF) rhesus macaque (Macaca mulatta) groups. American Journal of Primatology 80(3): e22742. 10.1002/ajp.22742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbeek R and van Schaik CP 2001. Competition and group size in Thomas’s langurs (Presbytis thomasil): The folivore paradox revisited. Behavioral Ecology and Sociobiology 49: 100–110. 10.1007/s002650000286 [DOI] [Google Scholar]
- Teas J, Richie T, Taylor H and Southwick CH 1980. Population patterns and behavioral ecology of rhesus monkeys in Nepal. In: Lindburg DG (ed) The Macaques: Studies in Ecology, Behavior, and Evolution pp 247–262. Van Nostrand Reinhold Company: New York, USA [Google Scholar]
- Vogel ER, Much S and Janson CH 2007. Understanding escalated aggression over food resources in white-faced capuchin monkeys. Animal Behaviour 74(1): 71–80. 10.1016/j.anbehav.2007.02.003 [DOI] [Google Scholar]
- Weir L and Grant JW 2004. The causes of resource monopolisation: Interaction between resource dispersion and mode of competition. Ethology 110(1): 63–74. 10.1046/j.1439-0310.2003.00948.x [DOI] [Google Scholar]
- Zumpe D and Michael RP 1987. Relation between the dominance rank of female rhesus monkeys and their access to males. American Journal of Primatology 13: 155–196. 10.1002/ajp.1350130206 [DOI] [PubMed] [Google Scholar]


