Abstract
Background
Immune activation persists despite suppressive antiretroviral therapy (ART) and may be affected by sex or body composition. We explored these relationships in a subset of participants who initiated ART in two large randomized trials.
Methods
Purposeful sampling selected participants who achieved virologic suppression on ART and either maintained weight within ± 0.5 kg/m2 or gained 2.6–6.4 kg/m2 from baseline to 96 weeks. We measured 7 markers of inflammation and immune activation at weeks 0 and 96. Multivariable linear regression explored associations of weight gain, sex, and pre-ART BMI with pre-ART and changes in biomarker concentrations.
Results
340 participants were selected; median pre-ART age 42 years, CD4+ cell count 273 cells/mm3, HIV-1 RNA 4.7 log10 copies/mL; 49% were women, 33% white, 42% black, and 24% Hispanic. Among participants with a normal pre-ART BMI, higher pre-ART levels of IL-6, sTNF-RI and RII, CXCL-10, sCD163 and hsCRP were associated with weight gain. Association of weight gain with week 96 changes of these biomarkers differed by sex; women who gained weight had smaller declines in most measured biomarkers compared to men who gained.
Conclusions
Among women, weight gain is associated with attenuated decline in several immune activation markers following ART initiation.
Clinical Trials Registration. NCT 00811954 and NCT 00811954.
Keywords: HIV, weight gain, immune activation, inflammation, sex differences
Among participants with HIV initiating antiretroviral therapy in 2 randomized controlled trials, women who gained weight had smaller declines in measures of immune activation compared to men who gained weight.
There are an estimated 37.9 million people living with human immunodeficiency virus (PWH), and an increasing number are overweight or obese [1, 2]. This is a cause for concern because obesity is a major risk factor for a number of important clinical comorbidities including cardiovascular disease, diabetes, fatty liver disease, and cognitive decline. The mechanism for these complications is under investigation, but immune activation is likely to be important.
Although levels of immune activation and inflammatory markers tend to decrease after the initiation of antiretroviral therapy (ART), they remain elevated when compared to age-matched controls despite suppressive ART [3, 4]. Several factors may contribute to persistent immune activation and inflammation including both modifiable (eg, tobacco use, obesity) and nonmodifiable factors (eg, thymic dysfunction, persistent antigen stimulation due to residual low-level viremia). Previous work has shown that obesity is associated with chronic inflammation with elevated levels of multiple inflammatory cytokines including interleukin 6 (IL-6), tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP) [5–7]. Higher circulating levels of these cytokines are associated with an increased risk of cardiovascular events, incident diabetes, and mortality among PWH [8–10].
Adipose tissue CD4+ T cells harboring latent virus may be a driver of the residual systemic inflammation and immune activation that occurs in spite of suppressive ART [11, 12]. While adipose tissue in lean individuals promotes an anti-inflammatory cytokine environment [13], obesity generates proinflammatory immune responses and it is adipose tissue T cells and macrophages that drive obesity-related inflammation [12, 14–16]. Studies evaluating the relationship between body composition and inflammatory cytokines in PWH have found associations between weight gain/higher body mass index (BMI) and higher levels of various markers including CRP, IL-6, soluble TNF-α receptor I (sTNF-RI), soluble CD14 (sCD14), and soluble CD163 (sCD163) [17, 18].
There is also evidence of an association between sex and immune activation in PWH. Prior studies have shown that women develop stronger immune (eg, interferon-α) responses than men to a number of different pathogens, and innate immune sensing of human immunodeficiency virus (HIV) is more robust in women than in men [19–21]. Additionally, several markers of immune activation and coagulation are higher in women with HIV than in men with HIV, both before and after initiation of ART. In one cohort, CRP concentrations decreased in men but tended to increase in women and sCD163 remained higher in women than men after 24 months of ART [22]. In another cohort, women had greater increases in TNF-α and more muted decreases in CRP and sCD14 as compared to men [23].
Previous work has shown that women with HIV gain more weight than men following the initiation of ART, independent of ART type [24–27]. However, it is unknown whether sex differences exist in the relationship between immune activation and changes in body composition after ART initiation.
We hypothesized that sex and weight gain/body composition would be associated with both pre-ART levels of systemic inflammation (IL-6, sTNF-RI and RII, C-X-C motif chemokine 10 [CXCL-10], and high-sensitivity CRP [hsCRP]) and monocyte activation (sCD14 and sCD163) and changes in these biomarkers following ART initiation and explored these relationships in a selected subset of women and men who had initiated ART in 2 large, randomized AIDS Clinical Trials Group (ACTG) trials (A5202 and A5257).
METHODS
Study Design and Population
We accessed participant-level data from 2 phase 3 ACTG ART initiation trials where data on BMI changes were available up to 96 weeks following ART initiation. We included data from ACTG 5202 (NCT 00118898, enrolled 2005–2007) [28] and ACTG 5257 (NCT 00811954, enrolled 2009–2011) [29].
A5202 randomized 1858 participants (17% women) to atazanavir/ritonavir or efavirenz combined with either abacavir/lamivudine or tenofovir disoproxil fumarate/emtricitabine. A5257 randomized 1809 participants (24% women) to either atazanavir/ritonavir, darunavir/ritonavir, or raltegravir combined with tenofovir disoproxil fumarate/emtricitabine. We included cis-gender participants from these studies who were not underweight (BMI > 18.5 kg/m2) prior to ART initiation, and who had achieved virologic suppression as measured by plasma HIV-1 viral loads <200 copies/mL at weeks 48 and 96, and excluded any women who became pregnant.
A purposeful sampling design selected participants who either maintained weight within ± 0.5 kg/m2 (maintainers) or gained 2.6–6.4 kg/m2 (gainers) from baseline to 96 weeks. This latter range of weight gain represents the observed 75th to 95th percentile in BMI changes over 96 weeks and the relative BMI change among participants in this group ranged from plus 6.5% to 33%. All women with available stored plasma samples who met criteria for 1 of these 2 subgroups (maintained or gained weight) were included in the analysis. A similar number of men were selected using stratified random sampling with strata defined by age (below or above the median observed age among the women) and parent study/ART regimen, so that there was not confounding of sex by age or ART regimen.
Participants who lost more than 0.5 kg/m2 from baseline (20% of women in the 2 parent trials), who gained between 0.5 and 2.6 kg/m2 over 96 weeks (33% of women in the 2 parent trials), and who experienced the most extreme (> 95th percentile) weight gain (5% of women in the 2 parent trials) were all excluded from this analysis.
The human experimentation guidelines of the Department of Health and Human Services were followed, and the institutional review board or ethics committees at each site provided approval. Informed consent, including permission to use biological materials, was obtained from all participants.
Laboratory Testing
Plasma specimens at weeks 0 and 96 were stored at −80°C in a repository and were not thawed until the time of testing. Each marker was batch tested at a single laboratory to minimize variability. Plasma levels of IL-6, sTNF-RI and II, CXCL-10, hsCRP, and sCD14 and sCD163 were measured using enzyme-linked immunosorbent assay (ELISA; R&D kits) at the Core Laboratory of the Clinical Research Unit at University Hospitals Cleveland Medical Center (G. A. M. Director).
Analyses
Weight was measured at weeks 0 and 96 and height at week 0 in both studies. BMI (kg/m2) was categorized as underweight (<18.5), normal (18.5–24.9), overweight (≥25–29.9), and obese (≥30).
Multivariable linear regression explored associations of weight gain category (gainers vs maintainers), sex, and pre-ART BMI category with outcomes of pre-ART biomarker concentrations (log10 transformed) and absolute changes in log10 transformed biomarker concentrations from baseline to week 96, adjusting for baseline age, race/ethnicity, ART regimen, pre-ART CD4 cell count, and pre-ART HIV-1 RNA.
P values of < .05 were determined a priori as a guideline for associations to highlight. All analyses were conducted using SAS software (version 9.4).
RESULTS
For this study, 340 participants were selected, 40% from A5202 and 60% from A5257. As shown in Table 1, median pre-ART age was 42 years, BMI was 25.56 kg/m2, CD4+ cell count 273 cells/mm3, and HIV-1 RNA 4.7 log10 copies/mL; 49% were women, 33% white, 42% black, and 24% Hispanic. The distribution of pre-ART BMI did not significantly differ between those who later maintained weight and those who later gained weight. Median pre-ART CD4 cell count was significantly lower among those who gained weight versus those who maintained weight (195 cells/mm3 vs 344 cells/mm3; P < .01). Similarly, median pre-ART HIV-1 RNA was significantly higher among those who gained weight versus those who maintained weight (4.84 log10 copies/mL vs 4.47 log10 copies/mL; P < .01). Participants with more advanced HIV disease as measured by viral load and CD4+ cell count had higher pre-ART biomarkers (data not shown).
Table 1.
Baseline Characteristics by Sex and Weight Gain
| Characteristic | Total (n = 340) | Women and Men Who Gained Weight, 2.6–6.4 kg/m2 (n = 196) | Women and Men Who Maintained Weight, ± 0.5 kg/m2 (n = 144) | P Value, Weight Gain | Women (n = 166) | Men (n = 174) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gained Weight (n = 96) | Maintained Weight (n = 70) | P Value, Weight Gain Within Women | Gained Weight (n = 100) | Maintained Weight (n = 74) | P Value, Weight Gain Within Men | |||||
| Clinical trial, n (%) | ||||||||||
| A5202 | 137 (40) | 82 (42) | 55 (38) | NAa | 42 (44) | 25 (36) | NAa | 40 (40) | 30 (41) | NAa |
| A5257 | 203 (60) | 114 (58) | 89 (62) | 54 (56) | 45 (64) | 60 (60) | 44 (59) | |||
| ART regimen, n (%) | ||||||||||
| ATV/r + NUCsb | 132 (39) | 74 (38) | 58 (40) | NAa | 34 (35) | 23 (33) | NAa | 40 (40) | 35 (47) | NAa |
| DRV/r + NUCsb | 66 (19) | 37 (19) | 29 (20) | 20 (21) | 17 (24) | 17 (17) | 12 (16) | |||
| EFV + NUCsb | 69 (21) | 43 (22) | 26 (18) | 26 (27) | 13 (19) | 17 (17) | 13 (18) | |||
| RAL + NUCsb | 73 (21) | 42 (21) | 31 (22) | 16 (17) | 17 (24) | 26 (26) | 14 (19) | |||
| Age, y, median (Q1, Q3) | 42 (33, 49) | 42 (34, 49) | 42 (33, 49) | NAa | 43 (35, 49) | 42 (34, 49) | NAa | 42 (33, 49) | 42 (31, 49) | NAa |
| Race/ethnicity, n (%) | ||||||||||
| White non-Hispanic | 111 (33) | 56 (29) | 55 (38) | .26 | 13 (14) | 16 (23) | .46 | 43 (43) | 39 (53) | .55 |
| Black non-Hispanic | 142 (42) | 89 (45) | 53 (37) | 56 (58) | 35 (50) | 33 (33) | 18 (24) | |||
| Hispanic | 81 (24) | 48 (24) | 33 (23) | 26 (27) | 18 (26) | 22 (22) | 15 (20) | |||
| Other | 6 (2) | 3 (2) | 3 (2) | 1 (1) | 1 (1) | 2 (2) | 2 (3) | |||
| Pre-ART BMI, kg/m2, median (Q1, Q3) | 25.6 (22.4, 29.0) | 25.4 (22.4, 29.5) | 25.9 (22.4, 29.0) | .70 | 27.1 (23.3, 32.8) | 27.7 (23.6, 32.3) | .69 | 24.1 (21.7, 27.6) | 24.2 (22.4, 27.1) | .74 |
| Pre-ART CD4, cells/mm3, median (Q1, Q3) | 273 (107, 394) | 195 (47, 337) | 344 (232, 460) | <.01 | 219 (56, 363) | 328 (231, 441) | <.01 | 174 (45, 299) | 361 (242, 465) | <.01 |
| Pre-ART HIV RNA, log10 copies/mL, median (Q1, Q3) | 4.70 (4.20, 5.09) | 4.84 (4.35, 5.35) | 4.47 (3.94, 4.80) | <.01 | 4.73 (4.00, 5.21) | 4..32 (3.72, 4.81) | <.01 | 4.95 (4.64, 5.43) | 4.54 (4.20, 4.80) | <.01 |
Abbreviations: 3TC, lamivudine; ABC, abacavir; ART, antiretroviral therapy; ATV/r, atazanavir/ritonavir; BMI, body mass index; DRV/r, darunavir/ritonavir; EFV, efavirenz; FTC, emtricitabine; HIV, human immunodeficiency virus; NA, not applicable; NUC, nucleos(t)ide analogue; RAL, raltegravir; TDF, tenofovir disoproxil fumarate.
aClinical trial, ART regimen, and age used in sampling design to enforce balance by these factors; therefore, no hypothesis testing performed.
bNUCS included TDF/FTC, or ABC/3TC (only with EFV or ATV/r).
Associations Between Pre-ART Inflammatory Marker Concentrations, Sex, Pre-ART BMI, and Weight Gain
In unadjusted models, pre-ART concentrations of sTNF-RI and sTNF-RII were different between sexes (estimated means of 0.05 log10 pg/mL lower in women than men). These associations persisted after adjustment for pre-ART BMI, other baseline covariates, and weight gain group as an average 0.04 log10 pg/mL lower in women than men (P = .01 and P = .02).
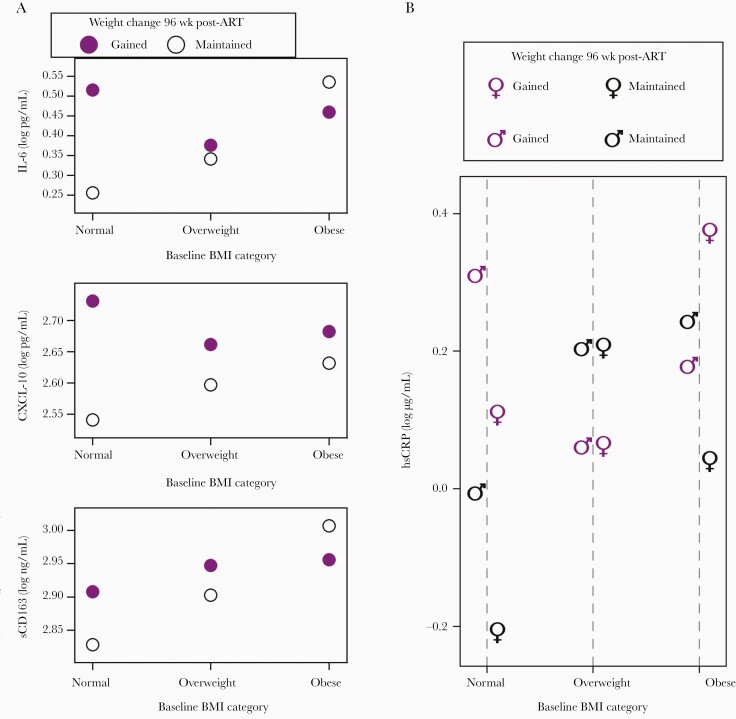
As shown in Table 2, there was a statistical interaction between sex and pre-ART BMI category for the association with pre-ART levels of hsCRP (P = .02). Among women, increasing pre-ART BMI category was associated with higher pre-ART hsCRP levels. This association was not observed among men.
Table 2.
Model-Based Mean Differences (95% CIs) of Pre-ART hsCRP Concentrations for Participants by Pre-ART BMI Category and Sex
| Sex Subgroup | Pre-ART BMI Category vs Normal Weight | hsCRP, log10 μg/mL |
|---|---|---|
| Women | Overweight | 0.18 (.01 to .36) |
| Obese | 0.46 (0.28 to .63) | |
| Men | Overweight | −0.02 (−.17 to .13) |
| Obese | 0.06 (−.18 to .29) |
Models adjusted for sex, age (by decade), race/ethnicity, pre-ART viral load, and CD4 cell count, and ART assignment. Bold text indicates a statistically significant value.
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; hsCRP, high-sensitivity C-reactive protein.
Among weight maintainers, higher pre-ART BMI weight category was associated with higher pre-ART biomarker levels for 4 of the 5 biomarkers (ie, all but CXCL-10; all interaction P values ≤ .03). We did not observe this association among the weight gainers (Table 3).
Table 3.
Model-Based Mean Differences (95% CIs) of Pre-ART Biomarker Concentrations for Participants Who Were Starting With Pre-ART Overweight or Obese BMI Versus Normal BMI, Separately by Subsequent Weight Gain on ART
| Weight Gain Subgroup Over 96 Weeks | Pre-ART BMI Category vs Normal Weight | IL-6, log10 pg/mL | sTNF-RII, log10 pg/mL | CXCL-10, log10 pg/mL | sCD163, log10 ng/mL | hsCRP, log10 μg/mL |
|---|---|---|---|---|---|---|
| Maintainers | Overweight | 0.09 (−.01 to .18) | 0.08 (.03 to .13) | 0.06 (−.03 to .14) | 0.08 (.01 to .14) | 0.31 (.14 to .49) |
| Obese | 0.28 (.16 to .40) | 0.17 (.10 to .23) | 0.09 (−.02 to .2) | 0.18 (.10 to .26) | 0.45 (.23 to .67) | |
| Gainers | Overweight | −0.14 (−.22 to −.05) | −0.03 (−.07 to .02) | −0.07 (−.15 to .01) | 0.04 (−.02 to .09) | −0.15 (−.30 to .01) |
| Obese | −0.06 (−.15 to .04) | −0.01 (−.06 to .04) | −.05 (−.14 to .04) | 0.05 (−.02 to .11) | 0.07 (−.11 to .24) |
Models adjusted for sex, age (by decade), race/ethnicity, pre-ART viral load and CD4 cell count, and ART assignment. Bold text indicates a statistically significant comparison.
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; sTNF-RII, soluble TNF-α receptor II.
To understand why pre-ART biomarkers were not different among those with pre-ART BMI categories in the overweight and obese categories versus the normal BMI category among gainers, we turned to the association between weight gain in the 96 weeks following ART initiation and pre-ART biomarker levels. As illustrated in Table 4, we observed that among the subgroup of people who had pre-ART BMIs in the normal range, gainers had higher biomarker levels than maintainers. But among those who were already overweight or obese pre-ART, biomarker levels were similar between gainers versus maintainers.
Table 4.
Model-based Mean Differences (95% CI) of Pre-ART Biomarker Concentrations for Participants Who Gained Weight Versus Maintained Weight
| Pre-ART BMI Subgroup | IL-6, log10 pg/mL | sTNF-RII, log10 pg/mL | CXCL-10, log10 pg/mL | sCD163, log10 ng/mL | hsCRP, log10 μg/mL |
|---|---|---|---|---|---|
| Normal weight | 0.26 (.17 to .35) | 0.12 (.07 to .16) | 0.19 (.11 to .27) | 0.08 (.02 to .14) | 0.32 (.16 to .48) |
| Overweight | 0.04 (−.06 to .13) | 0.01 (−.05 to .06) | 0.07 (−.02 to .15) | 0.05 (−.02 to .11) | 0.00 (−.32 to .03) |
| Obese | −0.08 (−.20 to .05) | −0.06 (−.13 to .01) | 0.05 (−.06 to .16) | −0.05 (−.13 to .03) | −0.07 (−.29 to .16) |
Models adjusted for sex, age (by decade), race/ethnicity, pre-ART viral load and CD4 cell count, and ART assignment. Bold text indicates a statistically significant comparison.
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; sTNF-RII, soluble TNF-α receptor II.
Among those with normal pre-ART BMI, weight gain was associated with higher pre-ART biomarker concentrations after adjusting for baseline characteristics including pre-ART viral load and CD4 cell count (Table 4).
Putting these 2 different sets of associations together, these data suggest that either preexisting excess weight (irrespective of weight gain following ART initiation) or being of normal weight but on a trajectory of weight gain during the first 96 weeks of ART were both associated with higher biomarker levels at the time of ART initiation. Figure 1 (and Supplementary Table 1) provide both model-based and observed means of log10 transformed biomarker concentrations to demonstrate these associations.
Figure 1.
Estimated biomarker levels before ART initiation. Estimates from final multivariable model with the following inputs across subgroups: age 40–49 years, white race, non-Hispanic ethnicity, pre-ART CD4 cell count of 272 cells/mm3, pre-ART plasma HIV-1 RNA of 4.65 log10 copies/mL, assigned ART regimen atazanavir/ritonavir plus emtricitabine/tenofovir disoproxil fumarate, and in models not showing subgroups by sex, male. Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CXCL-10, C-X-C motif chemokine 10; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; sCD163, soluble CD163.
Sex, Weight Gain, and Change in Inflammatory Marker Concentrations Over 96 Weeks
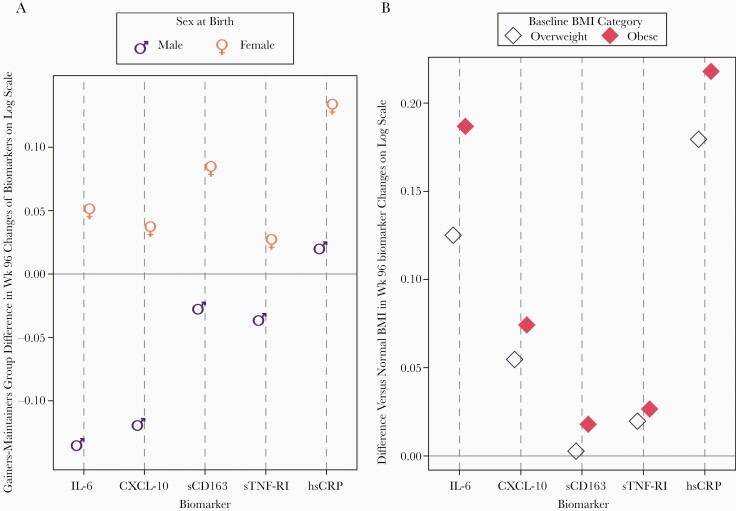
Figure 2A (and Supplementary Table 2) summarize the association of weight gain over 96 weeks with changes in markers of inflammation and immune activation over the same time period. For most biomarkers (IL-6, CXCL-10, sTNF-RI, sTNF-RII, and sCD163), these associations differed significantly by sex. Among women, weight gain was associated with some attenuation in decreases whereas weight gain in men was associated with larger decreases. For hsCRP where changes over time were increases from pre-ART levels, both weight gain and female sex were independently associated with larger increases over time. The association of weight gain with TNF-RII biomarker changes also varied by pre-ART BMI category (Table 5): larger TNF-RII decreases were observed among the subgroup with a normal pre-ART BMI.
Figure 2.
A, Estimated differences between weight gainers and maintainers by sex in biomarker level changes from pre-ART to week 96. Estimates from final multivariable model including the following covariates: race/ethnicity, age (decade), pre-ART CD4 count and plasma HIV-1 RNA, and pre-ART BMI group. B, Estimated differences between pre-ART BMI category (vs normal BMI) in biomarker level changes from pre-ART to week 96. Estimates from final multivariable model including the following covariates: sex, race/ethnicity, age (decade), pre-ART CD4 count, and plasma HIV-1 RNA. Differences were significantly larger than normal BMI for IL-6 and hsCRP. Untransformed units for biomarkers were pg/mL for IL-6, sTNF-RI, and CXCL-10; μg/mL for hsCRP; and ng/mL for sCD163. Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CXCL-10, C-X-C motif chemokine 10; HIV, human immunodeficiency virus; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; sCD163, soluble CD163; sTNF-RI, soluble TNF-α receptor I.
Table 5.
Estimated Mean Change From Baseline to Week 96 (95% CI) for Participants Who Gained Weight Versus Maintained Weight, by Pre-ART BMI Category
| Pre-ART BMI Subgroup | TNF-RII Interaction P = .05 |
|---|---|
| Normal | −0.06 (−.10 to −.01) |
| Overweight | 0.01 (−.04 to .06) |
| Obese | 0.03 (−.03 to .10) |
Other covariates in the model included sex, age, race/ethnicity, pre-ART CD4 cell count and plasma HIV-1 viral load, and ART assignment. Bold text indicates a statistically significant value.
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; sTNF-RII, soluble TNF-α receptor II.
Pre-ART BMI and Change in Inflammatory Marker Concentrations Over 96 Weeks
As shown in Figure 2B, higher BMI categories (ie, overweight and obese) were not associated with variation in changes in sTNF-RII, CXCL-10, or sCD163 over 96 weeks. For IL-6, both overweight and obese participants had significant differences in changes compared to normal weight participants. Most participants had increases in hsCRP over time (Figure 2B and Table 6), so the greater changes in overweight and obese participants represented larger increases in hsCRP compared to those with a normal pre-ART BMI.
Table 6.
Predicted Mean Changes From Pre-ART to Week 96 by Subgroups
| BMI Category | IL-6, log10 pg/mL | hsCRP, log10 μg/mL | ||||
|---|---|---|---|---|---|---|
| Women | Men | Women | Men | |||
| Gained Weight | Maintained Weight | Gained Weight | Maintained Weight | |||
| Normal | −0.11 | −0.16 | −0.25 | −0.12 | 0.11 | −0.05 |
| Overweight | 0.02 | −0.03 | −0.13 | 0.01 | 0.29 | 0.13 |
| Obese | 0.08 | 0.03 | −0.07 | 0.07 | 0.32 | 0.16 |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; IL-6, interleukin 6; hsCRP, high-sensitivity C-reactive protein.
Sex, Pre-ART BMI, and Change in Inflammatory Marker Concentrations Over 96 Weeks
Sex and pre-ART BMI were additive for changes in inflammatory biomarkers (Figure 2B and Table 6). The multivariable model showed that women with a higher baseline BMI were estimated to have the largest increases in hsCRP over time (mean of 0.32 for obese, 0.29 for overweight, log10 μg/mL increase from pre-ART level), even after controlling for weight gain over time. Specifically, while normal weight men were estimated (and observed) to have slight decreases in hsCRP over 96 weeks (Table 6), normal weight women were estimated to have increases in hsCRP (0.11 log10 μg/mL) that were similar in magnitude to the increases among overweight (0.13 log10 μg/mL), or obese (0.16 log10 μg/mL) men. As estimated, obese women had the largest increases in hsCRP compared to any other (sex and pre-ART BMI) subgroup.
Discussion
In this sample of participants from 2 large randomized ACTG trials of women and men who initiated first-line ART in the United States, there are several important findings. Individuals with more advanced HIV disease (ie, with higher viral loads and lower CD4 counts) had higher baseline levels of immune activation. Additionally, baseline weight was found to influence the relationship between the levels of immune activation and weight gain. Among those with a normal pre-ART BMI, higher levels of immune activation predicted who gained weight whereas, among those who were overweight or obese prior to ART initiation, higher levels of immune activation were less predictive. When given effective ART, levels of most of the inflammatory markers measured in this study (IL-6, TNF-RII, CXCL-10, and sCD163) decreased, although the changes often differed by sex and pre-ART BMI.
Among women, weight gain was associated with slight attenuation of decreases, but the opposite was true for men (weight gain was associated with slightly larger decreases). hsCRP was distinct from the other inflammatory markers as this marker did not routinely decrease following ART initiation and, among women, hsCRP increases occurred regardless of pre-ART BMI.
While pre-ART BMI was similar between gainers and maintainers, gainers had significantly lower pre-ART CD4+ T-cell counts and higher HIV-1 RNA levels. These findings have been demonstrated across multiple study populations [30–33]. Lower CD4 represents more advanced HIV disease and perhaps more exposure to the immune modulatory effects of HIV. Others have found associations between markers of immune activation and adiposity among PWH who are virally suppressed on ART [17]. The notion that this association also exists prior to ART initiation and is independent of baseline CD4+ cell count raises the possibility that these markers may be useful for predicting which patients are most likely to gain weight following ART initiation, particularly among patients with a normal pre-ART BMI, and is a novel finding.
We found compelling associations between sex, pre-ART BMI, and pre-ART inflammatory markers after adjusting for potential confounders. Higher pre-ART BMI category (overweight or obese) was associated with higher pre-ART hsCRP levels, and obese women had significantly higher baseline hsCRP levels than obese men. Further, for IL-6, sTNF-RI, sTNF-RII, sCD163, and hsCRP, among individuals who maintained weight following ART initiation, being overweight or obese prior to ART initiation was associated with significantly higher pre-ART inflammatory marker levels. However, this association was not observed among individuals who gained weight. Our data suggest that this is explained by the fact that among individuals with pre-ART BMI in the normal range, those who gained weight after ART initiation had significantly higher pre-ART inflammatory markers, even after adjusting for pre-ART CD4 count and HIV-1 RNA levels. The interactions between these variables may help explain some of the discordant findings in the literature to date regarding sex differences in baseline inflammatory markers [22, 23].
We observed an overall decrease in many markers of inflammation and immune activation [4, 34] after effective therapy for HIV. Important associations between weight gain and changes in biomarkers over 96 weeks demonstrated differences by sex and pre-ART BMI. Women who gained weight had smaller declines in biomarkers compared to women who maintained weight. Men who gained weight had larger declines in biomarkers than men who maintained weight. It may be that the pathological processes are different for men and women. Obese men had higher levels of immune activation pre-ART, and with treatment these tended to return to baseline. Men who maintained their weight did not experience a change in their markers of inflammation, perhaps reflecting a basal status: immune activation is not as pronounced in men.
Other investigators have evaluated the extent to which changes in weight influenced changes in inflammation following ART initiation. In a randomized trial of ART initiation in resource-limited settings weight gain was associated with an attenuated decrease in sCD14 [18]. Similar trends were seen among several other biomarkers in this study, but none reached statistical significance. It may be that in the setting of high immune activation, such as in female sex and as is observed in resource-limited settings [35], fat ensures that a state of immune activation persists. There are several potential reasons for this, and they are worthy of investigation. With respect to fat, we know that estrone is produced by fat cells, and it is known that estrogens modify the immune response. Recent work implicates the macrophage as the driver of the immune response in adipocytes [36], and it is known that estrogen receptor gene expression, which affects both monocyte and plasmacytoid dendritic cells, is regulated by estrogens [37].
Our study is not without limitations. This analysis was not designed to evaluate for differences by race and ethnicity. Further, participants in this study received a variety of initial ART regimens and although a purposeful sampling design was utilized to avoid confounding of sex by ART regimen, we were not able to evaluate the association between the various ART regimens (eg, integrase strand transfer inhibitor vs other) and markers of immune activation and inflammation in this analysis. Additionally, dual-energy absorptiometry or computed tomography findings were only available on 15% of the participants so we were unable to determine whether the changes in weight were due to changes in subcutaneous fat, visceral fat, or muscle. This is important to note because the distribution of weight gain among these compartments could impact the markers of inflammation and immune activation. We were not able to account for tobacco, marijuana, or other drug use in the majority of participants and this has been associated with chronic inflammation and immune activation. Also, we did not have information on diet or physical activity, both of which could impact changes in weight and inflammation. Finally, we did not control for sex-specific factors such as use of hormonal contraceptives, hormone replacement therapy, or feminizing hormone therapy that might impact changes in weight and inflammation. In spite of these limitations, the measurement of multiple inflammatory markers before and after the initiation of ART in a diverse population contributes to the clinical relevance and generalizability of these data. Future studies evaluating the association between sex and weight gain on inflammation would be enriched by including this information and also evaluating for differences by race and ethnicity, as well as ART class given the differential impacts noted in the current literature.
In summary, our findings are important because they show that individuals with preexisting excess weight and individuals on a trajectory of weight gain have higher pre-ART levels of immune activation and coagulation markers. Although initiating ART is associated with a reduction of immune activation, women who gain weight experience a slight attenuation in this reduction. Further research is needed to evaluate the associations between sex differences in weight gain and changes in immune activation and clinical outcomes following ART initiation.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. This paper was written by C. G. in her capacity as a United States government employee, but the views expressed in this paper do not represent those of the Department of State or the Centers for Disease Control and Prevention.
Financial support . This work was supported by the National Institutes of Health (grant numbers UM1 AI068636 to AIDS Clinical Trials Group [ACTG] and UM1 AI06834 to ACTG statistical and data management center); and the Office for Research on Women’s Health (supplement to grant number 1U01 AI069501).
Potential conflicts of interest. S. H. B. has received research funding to her institution from Gilead Sciences. G. A. M. has served as a consultant for Gilead, ViiV, Theratechnology, and Merck; and received grant support from Astellas, Tetraphase, and Roche. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Conference for Retroviruses and Opportunistic Infections, Seattle, WA, 4–7 March 2019.
Contributor Information
Sara H Bares, University of Nebraska Medical Center, Omaha, Nebraska, USA.
Laura M Smeaton, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
Sarah E Scott, University Hospitals Cleveland Medical Center and Case Western Reserve University School of Medicine, Cleveland, Ohio, USA.
Beth A Smith, University Hospitals Cleveland Medical Center and Case Western Reserve University School of Medicine, Cleveland, Ohio, USA.
Catherine Godfrey, Office of the Global AIDS Coordinator, Department of State, Washington, District of Columbia, USA.
Grace A McComsey, University Hospitals Cleveland Medical Center and Case Western Reserve University School of Medicine, Cleveland, Ohio, USA.
References
- 1. Crum-Cianflone N, Roediger MP, Eberly L, et al. ; Infectious Disease Clinical Research Program HIV Working Group . Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS One 2010; 5:e10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koethe JR, Jenkins CA, Lau B, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) . Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retroviruses 2016; 32:50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nabatanzi R, Bayigga L, Cose S, et al. Monocyte dysfunction, activation, and inflammation after long-term antiretroviral therapy in an African Cohort. J Infect Dis 2019; 220:1414–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wada NI, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lihn AS, Richelsen B, Pedersen SB, et al. Increased expression of TNF-alpha, IL-6, and IL-8 in HALS: implications for reduced adiponectin expression and plasma levels. Am J Physiol Endocrinol Metab 2003; 285:E1072–80. [DOI] [PubMed] [Google Scholar]
- 6. Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab 2007; 92:1023–33. [DOI] [PubMed] [Google Scholar]
- 7. Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006; 6:772–83. [DOI] [PubMed] [Google Scholar]
- 8. Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care 2010; 33:2244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuller LH, Tracy R, Belloso W, et al. ; INSIGHT SMART Study Group . Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr 2009; 51:268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wanjalla CN, McDonnell WJ, Koethe JR. Adipose tissue T cells in HIV/SIV infection. Front Immunol 2018; 9:2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang H, Youm YH, Vandanmagsar B, et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol 2010; 185:1836–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kohlgruber AC, LaMarche NM, Lynch L. Adipose tissue at the nexus of systemic and cellular immunometabolism. Semin Immunol 2016; 28:431–40. [DOI] [PubMed] [Google Scholar]
- 14. Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009; 15:914–20. [DOI] [PubMed] [Google Scholar]
- 15. Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009; 15:930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Winer S, Chan Y, Paltser G, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med 2009; 15:921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koethe JR, Jenkins CA, Furch BD, et al. Brief report: circulating markers of immunologic activity reflect adiposity in persons with HIV on antiretroviral therapy. J Acquir Immune Defic Syndr 2018; 79:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mave V, Erlandson KM, Gupte N, et al. ; ACTG PEARLS and NWCS 319 Study Team . Inflammation and change in body weight with antiretroviral therapy initiation in a multinational cohort of HIV-infected adults. J Infect Dis 2016; 214:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berghöfer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H. TLR7 ligands induce higher IFN-alpha production in females. J Immunol 2006; 177: 2088–96. [DOI] [PubMed] [Google Scholar]
- 20. Chang CC, Omarjee S, Lim A, et al. Chemokine levels and chemokine receptor expression in the blood and the cerebrospinal fluid of HIV-infected patients with cryptococcal meningitis and cryptococcosis-associated immune reconstitution inflammatory syndrome. J Infect Dis 2013; 208:1604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Souyris M, Mejía JE, Chaumeil J, Guéry JC. Female predisposition to TLR7-driven autoimmunity: gene dosage and the escape from X chromosome inactivation. Semin Immunopathol 2019; 41:153–64. [DOI] [PubMed] [Google Scholar]
- 22. Ticona E, Bull ME, Soria J, et al. Biomarkers of inflammation in HIV-infected Peruvian men and women before and during suppressive antiretroviral therapy. AIDS 2015; 29:1617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mathad JS, Gupte N, Balagopal A, et al. ; New Work Concept Sheet 319 and AIDS Clinical Trials Group A5175 (PEARLS) Study Teams . Sex-related differences in inflammatory and immune activation markers before and after combined antiretroviral therapy initiation. J Acquir Immune Defic Syndr 2016; 73:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bares SH, Smeaton LM, Xu A, Godfrey C, McComsey GA. HIV-infected women gain more weight than HIV-infected men following the initiation of antiretroviral therapy. J Womens Health 2018; 27:1162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kouanfack C, Mpoudi-Etame M, Omgba Bassega P, et al. ; NAMSAL ANRS 12313 Study Group . Dolutegravir-based or low-dose efavirenz-based regimen for the treatment of HIV-1. N Engl J Med 2019; 381:816–26. [DOI] [PubMed] [Google Scholar]
- 26. Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis 2020; 71:1379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019; 381:803–15. [DOI] [PubMed] [Google Scholar]
- 28. Daar ES, Tierney C, Fischl MA, et al. ; AIDS Clinical Trials Group Study A5202 Team . Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1. Ann Intern Med 2011; 154:445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lennox JL, Landovitz RJ, Ribaudo HJ, et al. ; ACTG A5257 Team . Efficacy and tolerability of 3 nonnucleoside reverse transcriptase inhibitor-sparing antiretroviral regimens for treatment-naive volunteers infected with HIV-1: a randomized, controlled equivalence trial. Ann Intern Med 2014; 161:461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Erlandson KM, Kitch D, Tierney C, et al. Weight and lean body mass change with antiretroviral initiation and impact on bone mineral density. AIDS 2013; 27:2069–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mallon PW, Miller J, Cooper DA, Carr A. Prospective evaluation of the effects of antiretroviral therapy on body composition in HIV-1-infected men starting therapy. AIDS 2003; 17:971–9. [DOI] [PubMed] [Google Scholar]
- 32. Shikuma CM, Zackin R, Sattler F, et al. ; AIDS Clinical Trial Group 892 Team . Changes in weight and lean body mass during highly active antiretroviral therapy. Clin Infect Dis 2004; 39:1223–30. [DOI] [PubMed] [Google Scholar]
- 33. Yuh B, Tate J, Butt AA, et al. Weight change after antiretroviral therapy and mortality. Clin Infect Dis 2015; 60:1852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De Pablo-Bernal RS, Ruiz-Mateos E, Rosado I, et al. TNF-α levels in HIV-infected patients after long-term suppressive cART persist as high as in elderly, HIV-uninfected subjects. J Antimicrob Chemother 2014; 69:3041–6. [DOI] [PubMed] [Google Scholar]
- 35. Rizzardini G, Trabattoni D, Saresella M, et al. Immune activation in HIV-infected African individuals. Italian-Ugandan AIDS cooperation program. AIDS 1998; 12: 2387–96. [DOI] [PubMed] [Google Scholar]
- 36. Bailin SS, Gabriel CL, Wanjalla CN, Koethe JR. Obesity and weight gain in persons with HIV. Curr HIV/AIDS Rep 2020; 17:138–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol 2015; 294: 63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.