Abstract
Objective
Investigations on neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (LMR) in patients with ischemic stroke are insufficient. We aimed to investigate the relationship of NLR and LMR with in-hospital clinical outcomes at different time points in ischemic stroke patients treated with intravenous tissues plasminogen activator (IV tPA).
Methods
We retrospectively enrolled patients who received IV tPA therapy within 4.5 hours from symptoms onset. Demographics, clinical characteristics, imaging measures, and the in-hospital clinical outcomes including early neurological improvement (ENI, defined as NIHSS score reduction within 24 hours ≥4 points or decreased to the baseline) and favorable functional outcome (defined as modified Rankin scale 0–1) were collected. Multivariable logistic regression analyses were performed to test whether NLR or LMR was an independent predictor for the in-hospital clinical outcomes.
Results
One hundred and two patients treated with IV tPA were included. NLR at 24 hours proved to be an independent predictor of ENI (adjusted OR=0.85, 95% CI=0.75–0.95, P=0.04). NLR at 48 hours and LMR at 48 hours proved to be independent predictors of mRS 0–1 at discharge (NLR at 48 hours: adjusted OR=0.64, 95% CI=0.49–0.83, P=0.01; LMR at 48 hours: adjusted OR=1.50, 95% CI=1.08–2.09, P=0.02). The AUC of NLR at 48 hours to predict favorable functional outcome at discharge was 0.79 (95% CI=0.70–0.88, P<0.001) and the optimal cut-off was 5.69 (sensitivity=0.52, specificity=0.63).
Conclusion
In our study, NLR at 24 hours was correlated with ENI. Both NLR and LMR at 48 hours were closely associated with favorable functional outcomes at discharge.
Keywords: stroke, thrombolysis, tissue plasminogen activator, neutrophil, inflammation
Introduction
Intravenous thrombolysis with tissue plasminogen activator (IV tPA) has proved its efficacy for patients with ischemic stroke to improve functional outcomes.1–3 However, in the real-world, registry cohort studies showed that about 40–50% of the patients treated with IV tPA failed to achieve favorable functional outcomes.4–6 Previously, factors including age, blood pressure, hyperglycemia, recanalization, and door-to-needle time delay were demonstrated to be predictors of functional outcomes after IV tPA.3,7–10
The inflammatory process will be initiated after the onset of ischemic stroke and is reported to be related to the progression and prognosis of ischemic stroke.11–13 Among the neuroinflammatory biomarkers, neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (LMR) were found to be significantly associated with clinical outcomes for patients treated with endovascular therapy14 or IV tPA.15 A pilot study showed that a higher level of NLR and lower level of LMR were observed in patients treated with IV tPA or bridging thrombectomy.16 In patients treated with IV tPA or bridging thrombectomy, higher NLR level was associated with higher risk of hemorrhagic transformation.17 Considering blood routine examination including neutrophil, lymphocyte, and monocyte count is served as a routine laboratory examination, NLR and LMR are accessible and quite convenient in clinical practice. However, most studies only collected NLR or LMR data at one time point and omit analysis on the variations of NLR or LMR between different time points.15,18–21
In this study, we aimed to investigate the relationship of repeated measures of NLR and LMR with in-hospital clinical outcomes. Furthermore, we sought to determine the optimal cut-offs and predictive values of NLR and LMR to predict the in-hospital clinical outcomes.
Methods
Study Design
Patients treated with IV tPA in the emergency department (ED) of Beijing Tiantan Hospital were screened for eligibility for our retrospective study. The study was approved by the ethics committee of Beijing Tiantan Hospital (No.: KY2019-019-05). The fully deidentified data on the patients enrolled in the current study and its retrospective study design enables this study, conducted under waiver of informed consent by the local institutional review board.
Participants
Patients treated with IV tPA were recruited consecutively from Oct 1, 2018 to Nov 5, 2020 and would be assessed for eligibility if they met the inclusion criteria:
diagnosed with acute ischemic stroke in our ED;
received IV tPA with alteplase (0.9 mg/kg) within 4.5 hours from symptoms onset; and
had complete information on blood routine examination of four time points including a) baseline before IV tPA (0 hours); b) 24 hours after IV tPA (24 hours); c) 48 hours after IV tPA (48 hours); and d) discharge.
Patients were excluded if they were diagnosed with infectious diseases, inflammatory diseases, immune system disorders, hematological diseases, or were receiving hormone/immunotherapy. Patients were also excluded if the neurological examination was not conducted at 24 hours or discharge.
Data Collection
Clinical data collection was conducted by a neurological physician (L.G.) blinded to the results of blood tests and imaging information. The clinical data collected included demographic information (age, sex), medical history (hypertension, diabetes, hyperlipidemia, atrial fibrillation, prior stroke, etc.), alcohol intake/smoking status, medication history (prior antiplatelet therapy of aspirin/clopidogrel), in-hospital antiplatelet therapy (aspirin/clopidogrel), and time delay before IV tPA (onset-to-needle time and door-to-needle time). Neurological impairment severity was assessed by the National Institute of Health Stroke Scale (NIHSS) score.22 Cause of ischemic stroke was classified according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification.23
Another physician (W.S.) who was blinded to medical information conducted the data collection of imaging and laboratory examination. Symptomatic intracerebral hemorrhage was categorized according to the National Institute of Neurological Disorders and Stroke (NINDS)1 and Safe Implementation of Thrombolysis in Stroke (SITS)4 standards. White matter hyperintensities severity was evaluated with the Fazekas scale on FLAIR MRI sequence.24 Peripheral blood samples were collected at four points (0 hours; 24 hours; 48 hours; and discharge) for blood routine examination including neutrophil counts, lymphocyte counts, and monocyte counts. NLR was calculated as neutrophil counts/lymphocyte counts. LMR was calculated as lymphocyte counts/monocyte counts.
Outcomes
In our study, in-hospital clinical outcomes included early neurological improvement (ENI) and favorable functional outcome at discharge. ENI was defined as NIHSS score reduction within 24 hours ≥4 points or decreased to the baseline.25 Favorable functional outcome was defined as modified Rankin Scale (mRS) 0–1 at discharge.
Statistical Analysis
For continuous variables, normally distributed data were displayed as mean±SD and compared using the Student’s t-test while non-normally distributed data were displayed as median (interquartile range) and compared using Mann–Whitney U-test. Shapiro test was used to test data distribution. Categorical variables were displayed as number (percentage) and compared using the χ2 tests or Fisher’s exact tests, as appropriate. Multivariable logistics regression analyses were conducted to test whether NLR or LMR was an independent predictor of the in-hospital clinical outcomes with odds ratio (OR) and 95% confidential intervals (95% CI). Considering potential collinearity between NLR and LMR, multivariable logistics regression analyses were performed using backward stepwise method. Adjusted confounders were variables with a P-value <0.05 in baseline comparisons. Subsequently, receiver operating characteristic (ROC) curves were used to assess the predictive ability and define the optimal cut-off values of NLR and LMR. Then, patients were categorized into the high-level group and low-level group by the optimal cut-off values of NLR and LMR, additional ordinal analyses were used to compare the distribution of mRS score at discharge between the high-level group and low-level group of NLR and LMR, respectively. All statistical analyses were performed with the SPSS 25.0. P-value <0.05 was considered statistically significant.
Results
From Oct 1, 2018 to Nov 5, 2020, 595 patients received IV tPA therapy in the ED of Beijing Tiantan Hospital, and 105 of them had complete blood sample data at four time points. The baseline comparison between included and excluded patients is summarized in Supplemental Table 1. Among the patients with complete blood sample data, 16 of them were diagnosed with infectious diseases, inflammatory diseases, immunity disorders, hematological diseases, or receiving hormone/immunological therapy. Another three patients were excluded for incomplete information on NIHSS score at 24 hours or mRS score at discharge. In the final study, 102 patients were included. Among the finally included patients, the average age was 64.01±13.02 and 65.69% (67/102) of them were male. The median time interval from ischemia onset to IV tPA was 174.50 (136.50–228.25) minutes. 33.33% (34/102) patients received bridging mechanical thrombectomy (MT) after IV tPA. The median admission NIHSS score was 9.4–13
Dynamic Change Trends at Different Time Points
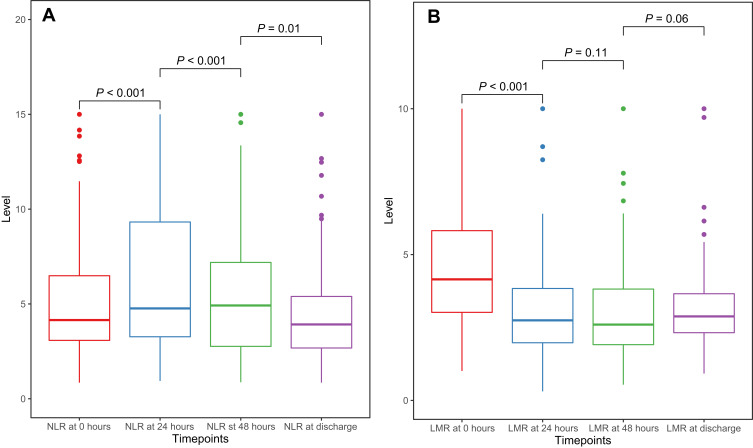
The dynamic change trends of NLR and LMR at different time points are displayed in Figure 1A and B. Among the four time points, the NLR level increased before 48 hours and dropped from 48 hours to discharge. (0 hours: 4.15; 24 hours: 4.77; 48 hours: 4.92; discharge: 3.92). LMR level decreased before 48 hours and elevated from 48 hours to discharge (0 hours: 4.15; 24 hours: 2.74; 48 hours: 2.60; discharge: 2.88).
Figure 1.
Boxplots to show dynamic change trends of NLR (A) and LMR (B).
Relationship of NLR and LMR with In-Hospital Outcomes
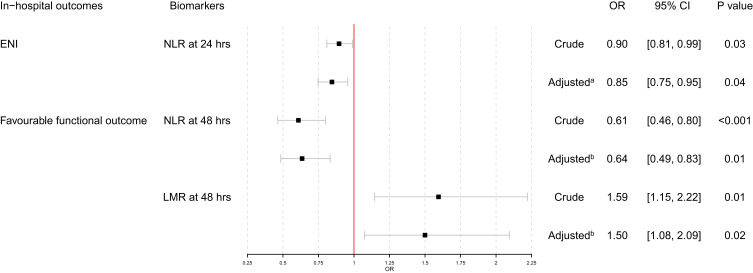
Table 1 summarized the comparison of baseline characteristics between ENI and non-ENI group. Compared to non-ENI group, ENI group showed higher admission NIHSS score (11.50 [8.25, 14.00] vs 6.50 [3.00, 11.25], P=0.01), lower NLR level at 24 hours (4.18 [2.96, 6.45] vs 5.62 [3.36, 11.64], P=0.04), higher LMR level at 24 hours (3.28 [2.56, 4.06] vs 2.45 [1.60, 3.54], P=0.01) and decreased NLR change between 0 hours and 24 hours (−0.08 [−0.54, 1.39] vs 1.13 [−0.22, 3.20], P=0.02). As shown in Figure 2, multivariable logistics regression models using stepwise method were conducted and NLR at 24 hours proved to be an independent predictor of ENI (crude OR=0.90, 95% CI=0.81–0.99, P=0.03; adjusted OR=0.85, 95% CI=0.75–0.95, P=0.04).
Table 1.
Baseline Characteristics Between No-ENI and ENI Group in Patients Treated with IV tPA
| Overall (n=102) | No-ENI (n=64) | ENI (n=38) | P-value | |
|---|---|---|---|---|
| Age, years (mean (SD)) | 64.01 (13.02) | 64.31 (13.30) | 63.50 (12.69) | 0.76 |
| Male, n (%) | 67 (65.69) | 46 (71.88) | 21 (55.26) | 0.09 |
| DNT time, minutes (median [IQR]) | 47.50 [35.00–64.00] | 47.50 [34.00–64.75] | 47.50 [37.25–64.00] | 0.80 |
| Time from symptom onset to IV-tPA, minutes (median [IQR]) | 174.50 [136.50–228.25] | 181.50 [150.00–231.25] | 156.00 [128.75–208.00] | 0.09 |
| Bridging mechanical thrombectomy, n (%) | 34 (33.33) | 19 (29.69) | 15 (39.47) | 0.31 |
| Pre-mRS score (%) | 0.30 | |||
| 0 | 81 (79.41) | 48 (75.00) | 33 (86.84) | |
| 1 | 8 (7.84) | 5 (7.81) | 3 (7.89) | |
| 2 | 6 (5.88) | 6 (9.38) | 0 (0.00) | |
| 3 | 4 (3.92) | 2 (3.12) | 2 (5.26) | |
| 4 | 2 (1.96) | 2 (3.12) | 0 (0.00) | |
| 5 | 1 (0.98) | 1 (1.56) | 0 (0.00) | |
| Admission NIHSS score (median [IQR]) | 9.00 [4.00–12.75] | 6.50 [3.00–11.25] | 11.50 [8.25–14.00] | 0.01 |
| Admission NIHSS score ≤5 (%) | 33 (32.35) | 29 (45.31) | 4 (10.53) | <0.001 |
| Hypertension, n (%) | 67 (65.69) | 45 (70.31) | 22 (57.89) | 0.20 |
| Atrial fibrillation, n (%) | 19 (18.63) | 13 (20.31) | 6 (15.79) | 0.57 |
| Diabetes mellitus, n (%) | 30 (29.41) | 23 (35.94) | 7 (18.42) | 0.06 |
| Hyperlipidemia, n (%) | 14 (13.73) | 7 (10.94) | 7 (18.42) | 0.29 |
| Prior stroke, n (%) | 27 (26.47) | 18 (28.12) | 9 (23.68) | 0.62 |
| Coronary artery disease, n (%) | 18 (17.65) | 13 (20.31) | 5 (13.16) | 0.36 |
| Prior antiplatelet therapy, n (%) | 20 (19.61) | 12 (18.75) | 8 (21.05) | 0.78 |
| Prior statin therapy, n (%) | 15 (14.71) | 10 (15.62) | 5 (13.16) | 0.73 |
| Smoking, n (%) | 59 (57.84) | 40 (62.50) | 19 (50.00) | 0.22 |
| Drinking, n (%) | 44 (43.14) | 30 (46.88) | 14 (36.84) | 0.32 |
| TOAST, n (%) | 0.78 | |||
| LAA | 67 (65.69) | 44 (68.75) | 23 (60.53) | |
| CE | 26 (25.49) | 16 (25.00) | 10 (26.32) | |
| SAA | 2 (1.96) | 1 (1.56) | 1 (2.63) | |
| Other | 4 (3.92) | 2 (3.12) | 2 (5.26) | |
| Unknown | 3 (2.94) | 1 (1.56) | 2 (5.26) | |
| Admission SBP level, mmHg (median [IQR]) | 144.00 [130.00–162.00] | 145.50 [130.25–162.00] | 140.00 [129.50–153.00] | 0.25 |
| Admission DBP level, mmHg (median [IQR]) | 82.50 [75.00–93.00] | 81.00 [75.00–95.00] | 83.00 [74.50–90.00] | 0.34 |
| Admission serum glucose level, mmol/L (median [IQR]) | 6.80 [5.55–8.50] | 7.40 [5.70–9.40] | 6.50 [5.43–7.33] | 0.11 |
| HbA1c level, % (median [IQR]) | 6.10 [5.80–6.60] | 6.10 [5.90–6.70] | 6.20 [5.70–6.50] | 0.61 |
| LDL level, mmol/L (mean (SD)) | 2.50 (0.86) | 2.57 (0.89) | 2.38 (0.80) | 0.34 |
| Cholesterol level, mmol/L (mean (SD)) | 4.11 (0.94) | 4.18 (0.95) | 4.00 (0.93) | 0.39 |
| CT hypointensity sign, n (%) | 22 (21.57) | 13 (20.31) | 9 (23.68) | 0.69 |
| MCA hyperintensity sign, n (%) | 9 (8.82) | 5 (7.81) | 4 (10.53) | 0.92 |
| Fazekas scale (median [IQR]) | 1.00 [1.00–2.00] | 1.00 [1.00–2.00] | 1.00 [1.00–1.00] | 0.74 |
| Large vessel occlusion, n (%) | 32 (31.37) | 19 (29.69) | 13 (34.21) | 0.63 |
| sICH-NINDS, n (%) | 9 (8.82) | 8 (12.50) | 1 (2.63) | 0.18 |
| sICH-SITS, n (%) | 4 (3.92) | 4 (6.25) | 0 (0.00) | 0.30 |
| NLR at 0 h, 10^9/L (median [IQR]) | 4.15 [3.08–6.49] | 4.27 [3.08–8.79] | 3.96 [3.09–5.27] | 0.33 |
| NLR at 24 h, 10^9/L (median [IQR]) | 4.77 [3.27–9.32] | 5.62 [3.36–11.64] | 4.18 [2.96–6.45] | 0.04 |
| LMR at 0 h, 10^9/L (median [IQR]) | 4.15 [3.02–5.82] | 4.00 [2.95–5.83] | 4.62 [3.25–5.70] | 0.74 |
| LMR at 24 h, 10^9/L (median [IQR]) | 2.74 [1.98–3.84] | 2.45 [1.60–3.54] | 3.28 [2.56–4.06] | 0.01 |
| NLR change between 0 hrs and 24 h, 10^9/L (median [IQR]) | 0.68 [−0.45–2.53] | 1.13 [−0.22–3.20] | −0.08 [−0.54–1.39] | 0.02 |
| LMR change between 0 hrs and 24 h, 10^9/L (median [IQR]) | −1.25 [−3.11–−0.01] | −1.26 [−3.31–−0.56] | −0.81 [−1.74–0.28] | 0.08 |
Abbreviations: ENI, early neurological improvement; DNT, door-to-needle; IV, intravenous thrombolysis; tPA, tissue plasminogen activator; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; TOAST, Trial of Org 10172 in Acute Stroke Treatment; LAA, large atherosclerosis artery; CE, cardiac embolism; SAA, small artery occlusion; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL, low density lipoprotein; MCA, middle cerebral artery; sICH, symptomatic intracerebral hemorrhage; NINDS, National Institute of Neurological Disorders and Stroke trial; SITS, Safe Implementation of Thrombolysis in Stroke; NLR, neutrophil-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio.
Figure 2.
Multivariable logistics regression analyses: (A) adjusted for admission NIHSS score; (B) adjusted for bridging MT, admission NIHSS score and large vessel occlusion.
As shown in Table 2, baseline characteristics comparison between mRS 0–1 group and mRS 2–6 group showed that the mRS 0–1 group had a lower proportion of bridging MT (11.11% vs 41.33%, P=0.01), lower admission NIHSS score (6.00 [3.00, 10.50] vs 9.00 [5.00, 14.00], P=0.04), lower proportion of large vessel occlusion (14.81% vs 37.33%, P=0.03), lower NLR level at 0 hours (3.41 [2.60, 5.03] vs 4.35 [3.42, 7.02], P=0.03), lower NLR level at 24 hours (3.11 [2.52, 4.52] vs 5.87 [3.84, 10.65], P=0.01), lower NLR level at 48 hours (2.62 [2.20, 4.48] vs 5.72 [3.59, 8.14], P<0.001), lower NLR level at discharge (2.57 [2.13, 3.92] vs 4.43 [3.08, 5.89], P<0.001), higher LMR level at 24 hours (3.45 [2.69, 4.92] vs 2.52 [1.80, 3.54], P=0.01), higher LMR level at 48 hours (3.70 [2.66, 4.31] vs 2.18 [1.75, 3.23], P<0.001), positive NLR change between 0 hours and 24 hours (−0.10 [−0.83, 0.82] vs 1.22 [−0.27, 3.27], P=0.01), more decreased LMR change between 0 hours and 24 hours (−0.27 [−1.22, 0.27] vs −1.33 [−3.40, −0.65], P=0.01), less decreased LMR change between 0 hours and discharge (−0.35 [−1.88, 0.33] vs −1.45 [−3.00, −0.35], P=0.03) and decreased LMR change between 48 hours and discharge (−0.20 [−1.03, 0.48] vs 0.47 [−0.36, 1.37], P=0.01). As shown in Figure 2, multivariable logistics regression analyses found that NLR at 48 hours and LMR at 48 hours proved to be independent predictors of mRS 0–1 at discharge (NLR at 48 hours: crude OR=0.61, 95% CI=0.46–0.80, P<0.001; adjusted OR=0.64, 95% CI=0.49–0.83, P=0.01; LMR at 48 hours: crude OR=1.59, 95% CI=1.15–2.22, P=0.01; adjusted OR=1.50, 95% CI=1.08–2.09, P=0.02).
Table 2.
Baseline Characteristics Between mRS 2–6 and mRS 0–1 at Discharge Group in Patients Treated with IV tPA
| Overall (n=102) | mRS 2–6 (n=75) | mRS 0–1 (n=27) | P-value | |
|---|---|---|---|---|
| Age (mean (SD)) | 64.01 (13.02) | 63.31 (12.94) | 65.96 (13.29) | 0.37 |
| Male, n (%) | 67 (65.69) | 50 (66.67) | 17 (62.96) | 0.73 |
| DNT time (median [IQR]) | 47.50 [35.00–64.00] | 48.00 [35.00–64.00] | 44.00 [35.50–68.50] | 0.99 |
| Time from symptom onset to IV-tPA, min (median [IQR]) | 174.50 [136.50–228.25] | 186.00 [149.00–229.50] | 160.00 [115.50–217.50] | 0.11 |
| Bridging mechanical thrombectomy, n (%) | 34 (33.33) | 31 (41.33) | 3 (11.11) | 0.01 |
| Pre-mRS score (%) | 0.91 | |||
| 0 | 81 (79.41) | 58 (77.33) | 23 (85.19) | |
| 1 | 8 (7.84) | 6 (8.00) | 2 (7.41) | |
| 2 | 6 (5.88) | 5 (6.67) | 1 (3.70) | |
| 3 | 4 (3.92) | 3 (4.00) | 1 (3.70) | |
| 4 | 2 (1.96) | 2 (2.67) | 0 (0.00) | |
| 5 | 1 (0.98) | 1 (1.33) | 0 (0.00) | |
| Admission NIHSS score (median [IQR]) | 9.00 [4.00–12.75] | 9.00 [5.00–14.00] | 6.00 [3.00–10.50] | 0.04 |
| Admission NIHSS score ≤5 (%) | 33 (32.35) | 22 (29.33) | 11 (40.74) | 0.28 |
| Hypertension, n (%) | 67 (65.69) | 48 (64.00) | 19 (70.37) | 0.55 |
| Atrial fibrillation, n (%) | 19 (18.63) | 13 (17.33) | 6 (22.22) | 0.58 |
| Diabetes mellitus, n (%) | 30 (29.41) | 23 (30.67) | 7 (25.93) | 0.64 |
| Hyperlipidemia, n (%) | 14 (13.73) | 10 (13.33) | 4 (14.81) | 1 |
| Prior stroke, n (%) | 27 (26.47) | 21 (28.00) | 6 (22.22) | 0.56 |
| Coronary artery disease, n (%) | 18 (17.65) | 12 (16.00) | 6 (22.22) | 0.67 |
| Prior antiplatelet therapy, n (%) | 20 (19.61) | 15 (20.00) | 5 (18.52) | 0.87 |
| Prior statin therapy, n (%) | 15 (14.71) | 12 (16.00) | 3 (11.11) | 0.77 |
| Smoking, n (%) | 59 (57.84) | 41 (54.67) | 18 (66.67) | 0.28 |
| Drinking, n (%) | 44 (43.14) | 31 (41.33) | 13 (48.15) | 0.54 |
| TOAST, n (%) | 0.29 | |||
| LAA | 67 (65.69) | 50 (66.67) | 17 (62.96) | |
| CE | 26 (25.49) | 18 (24.00) | 8 (29.63) | |
| SAA | 2 (1.96) | 2 (2.67) | 0 (0.00) | |
| Other | 4 (3.92) | 4 (5.33) | 0 (0.00) | |
| Unknown | 3 (2.94) | 1 (1.33) | 2 (7.41) | |
| Admission SBP level, mmHg (median [IQR]) | 144.00 [130.00–162.00] | 141.50 [129.25–161.00] | 145.00 [139.50–158.50] | 0.44 |
| Admission DBP level, mmHg (median [IQR]) | 82.50 [75.00–93.00] | 81.00 [75.00–93.00] | 86.00 [76.00–91.50] | 0.96 |
| Admission serum glucose level, mmol/L (median [IQR]) | 6.80 [5.55–8.50] | 6.95 [5.88–8.80] | 5.70 [5.05–7.35] | 0.07 |
| HbA1c level, % (median [IQR]) | 6.10 [5.80–6.60] | 6.20 [5.85–6.85] | 6.00 [5.75–6.30] | 0.24 |
| LDL level, mmol/L (mean (SD)) | 2.50 (0.86) | 2.42 (0.80) | 2.71 (0.97) | 0.16 |
| Cholesterol level, mmol/L (mean (SD)) | 4.11 (0.94) | 4.03 (0.93) | 4.33 (0.96) | 0.20 |
| CT hypointensity sign, n (%) | 22 (21.57) | 18 (24.00) | 4 (14.81) | 0.32 |
| MCA hyperintensity sign, n (%) | 9 (8.82) | 7 (9.33) | 2 (7.41) | 1 |
| Fazekas scale (median [IQR]) | 1.00 [1.00–2.00] | 1.00 [1.00–2.00] | 1.00 [1.00–1.00] | 0.94 |
| Large vessel occlusion, n (%) | 32 (31.37) | 28 (37.33) | 4 (14.81) | 0.03 |
| sICH-NINDS, n (%) | 9 (8.82) | 9 (12.00) | 0 (0.00) | 0.14 |
| sICH-SITS, n (%) | 4 (3.92) | 4 (5.33) | 0 (0.00) | 0.52 |
| NLR at 0 h, 10^9/L (median [IQR]) | 4.15 [3.08–6.49] | 4.35 [3.42–7.02] | 3.41 [2.60–5.03] | 0.03 |
| NLR at 24 h, 10^9/L (median [IQR]) | 4.77 [3.27–9.32] | 5.87 [3.84–10.65] | 3.11 [2.52–4.52] | 0.01 |
| NLR at 48 h, 10^9/L (median [IQR]) | 4.92 [2.76– 7.19] | 5.72 [3.59–8.14] | 2.62 [2.20–4.48] | <0.001 |
| NLR at discharge, 10^9/L (median [IQR]) | 3.92 [2.68–5.40] | 4.43 [3.08–5.89] | 2.57 [2.13–3.92] | <0.001 |
| LMR at 0 h, 10^9/L (median [IQR]) | 4.15 [3.02–5.82] | 4.57 [3.13–5.89] | 3.81 [2.89–5.28] | 0.38 |
| LMR at 24 h, 10^9/L (median [IQR]) | 2.74 [1.98–3.84] | 2.52 [1.80–3.54] | 3.45 [2.69–4.92] | 0.01 |
| LMR at 48 h, 10^9/L (median [IQR]) | 2.60 [1.91–3.82] | 2.18 [1.75–3.23] | 3.70 [2.66–4.31] | <0.001 |
| LMR at discharge, 10^9/L (median [IQR]) | 2.88 [2.32–3.66] | 2.88 [2.28–3.51] | 2.96 [2.75–4.91] | 0.10 |
| NLR change between 0 h and 24 h, 10^9/L (median [IQR]) | 0.68 [−0.45–2.53] | 1.22 [−0.27–3.27] | −0.10 [−0.83–0.82] | 0.01 |
| NLR change between 24 h and 48 h, 10^9/L (median [IQR]) | −0.35 [−2.47–0.36] | −0.40 [−2.71–0.55] | −0.34 [−1.41–0.05] | 0.99 |
| NLR change between 0 h and discharge, 10^9/L (median [IQR]) | −0.61 [−2.09–0.91] | −0.54 [−2.09–1.45] | −0.85 [−2.23–0.48] | 0.60 |
| NLR change between 48 h and discharge, 10^9/L (median [IQR]) | −0.75 [−2.46–0.91] | −0.83 [−2.94–0.99] | −0.15 [−0.90–0.56] | 0.13 |
| LMR change between 0 h and 24 h (median [IQR]) | −1.25 [−3.11–−0.01] | −1.33 [−3.40–−0.65] | −0.27 [−1.22–0.27] | 0.01 |
| LMR change between 24 h and 48 h, 10^9/L (median [IQR]) | −0.08 [−0.74–0.35] | −0.02 [−0.66–0.35] | −0.19 [−0.82–0.23] | 0.58 |
| LMR change between 0 h and discharge, 10^9/L (median [IQR]) | −1.29 [−2.75–0.02] | −1.45 [−3.00–−0.35] | −0.35 [−1.88–0.33] | 0.03 |
| LMR change between 48 h and discharge, 10^9/L (median [IQR]) | 0.16 [−0.56–1.07] | 0.47 [−0.36–1.37] | −0.20 [−1.03–0.48] | 0.01 |
Abbreviations: DNT, door-to-needle; IV, intravenous thrombolysis; tPA, tissue plasminogen activator; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; TOAST, Trial of Org 10172 in Acute Stroke Treatment; LAA, large atherosclerosis artery; CE, cardiac embolism; SAA, small artery occlusion; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL, low density lipoprotein; MCA, middle cerebral artery; sICH, symptomatic intracerebral hemorrhage; NINDS, National Institute of Neurological Disorders and Stroke trial; SITS, Safe Implementation of Thrombolysis in Stroke; NLR, neutrophil-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio.
ROC Curves and Ordinal Analyses
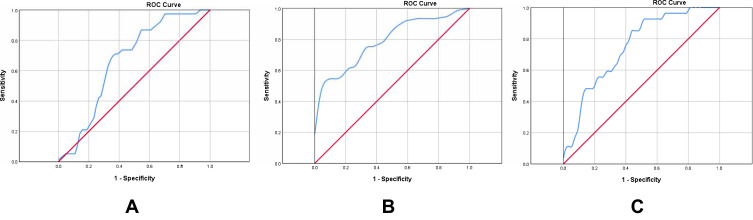
ROC curves were used to assess the predictive ability of in-hospital clinical outcomes and cut-off values of NLR and LMR (Figure 3). The area under curve (AUC) of NLR at 24 hours to predict ENI was 0.62 (95% CI=0.51–0.73, P=0.046). Considering the AUC of NLR at 24 hours to predict ENI was too low (<0.70), we abandoned further analysis on optimal cut-off. The AUC of NLR at 48 hours to predict favorable functional outcome at discharge was 0.79 (95% CI=0.70–0.88, P<0.001) and the optimal cut-off was 5.69 (sensitivity=0.52, specificity=0.63). The AUC of LMR at 48 hours to predict favorable functional outcome at discharge was 0.75 (95% CI=0.65–0.85, P<0.001) and the optimal cut-off was 2.48 (sensitivity=0.85, specificity=0.57).
Figure 3.
Receiver operating characteristic curves. (A) ROC curve for LMR at 24 hours to predict ENI; (B) ROC curve for NLR at 48 hours to predict favorable functional outcome at discharge; (C) ROC curve for LMR at 48 hours to predict favorable functional outcome at discharge.
Abbreviations: ROC, Receiver operating characteristic curves; LMR, lymphocyte-to-monocyte ratio; ENI, early neurological improvement; NLR, neutrophil-to-lymphocyte ratio.
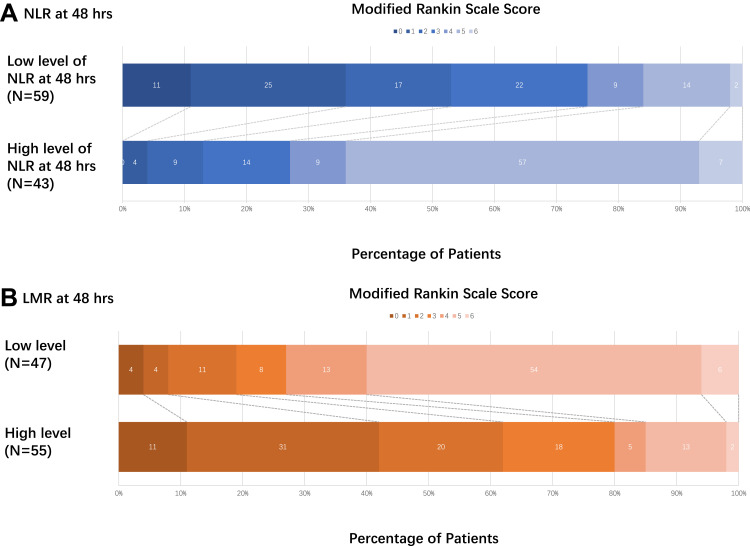
Ordinal analyses of mRS distribution between the high-level group and low-level group of NLR and LMR at 48 hours based on the optimal cut-off values derived from ROC were shown in Figure 4. For NLR at 48 hours, a high level of NLR at 48 hours represented poor functional outcome at discharge (common OR=0.09, 95% CI=1.54–3.18, P<0.001). For LMR at 48 hours, a high level of LMR at 48 hours represented favorable functional outcome at discharge (common OR=7.87, 95% CI=1.27−2.85, P<0.001).
Figure 4.
Distribution of mRS score at discharge between different level of NLR/LMR at 48 hours. Scores range from 0–6, with 0 indicating no symptoms, 1 no clinically significant disability, 2 slight disability (patients are able to look after their own affairs without assistance but are unable to carry out all previous activities), 3 moderate disability (patients require some help but are able to walk unassisted), 4 moderately severe disability (patients are unable to attend to bodily needs without assistance and are unable to walk unassisted), 5 severe disability (patients require constant nursing care and attention), and 6 death. (A) For NLR at 48 hours, high level of NLR at 48 hours represented poor functional outcome at discharge, common OR was 0.09 and 95% CI was 1.54–3.18 (P<0.001). (B) For LMR at 48 hours, high level of LMR at 48 hours represented favorable functional outcome at discharge, common OR was 7.87 and 95% CI was 1.27−2.85 (P<0.001).
Discussion
In our study, four time points repeated measures of NLR and LMR showed that NLR was highest at 48 hours, and LMR was lowest at 48 hours after IV tPA. NLR at 24 hours was an independent predictor for ENI, and both NLR at 48 hours and LMR at 48 hours independently predicted a favorable functional outcome at discharge in patients treated with IV tPA. The NLR at 48 hours and LMR at 48 hours with the optimal cut-off values 5.69 and 2.48, respectively, yielding good diagnostic abilities for favorable functional outcomes at discharge. Our study comprehensively elucidates dynamic changes of NLR and LMR at four different time points and emphasizes their clinical significance for ENI and favorable functional outcome at discharge in patients treated with IV tPA.
Our study found inverse dynamic change trends between NLR and LMR. NLR level increased from admission and reached the peak at 48 hours, while LMR level decreased from admission and hit bottom at 48 hours. This is consistent with a single-center, observational study which described an increase of NLR from admission to 24 hours, and a decrease from 24 hours to the 7-day.26 However, this observational study26 did not have the data of NLR at 48 hours, whereas our study reported a peak of NLR level was presented at 48 hours instead of 24 hours. Another retrospective study also demonstrated that post-tPA NLR was superior compared with baseline NLR in predicting clinical outcomes in patients treated with IV tPA, in accord with the dynamic trends in our study.27 Hence, NLR level increases after stroke onset up to 48 hours and decreases before discharge.
Reperfusion therapy including IV tPA and endovascular treatment demonstrated to achieve recanalization of larger vessel occlusion and improve functional outcome.3 However, recanalization of the large intracranial vessel may not improve microvascular reflow and neutrophils adhered to endothelium were reported to hamper the microvascular reflow and exacerbate the damage of blood–brain barrier (BBB) through matrix metalloproteinases-9 (MMP-9) and other neurotoxic cytokines.28,29 Hence, neuroinflammation and its downstream process deserved more investigations and potential treatment targets may lie in the neuroinflammation process for patients treated with reperfusion therapy. The neuroinflammation process in response to ischemia or hypoperfusion was initiated by astrocytes and microglial.30,31 Microglial in ischemic tissue initiated the secretion of inflammatory cytokine, including interleukin-1 and tumor necrosis factor-α (TNF-α) to recruit immune cells including neutrophils, lymphocytes, and monocytes.32,33 Recruited immune cells broke the integrity of BBB through reactive oxygen species (ROS) build-up and activation of MMP.28 Damaged endothelium caused by ischemia recruited more neutrophil into the parenchyma within 1 hour after ischemic stroke onset and last for about 14 days with a peak at 2–3 days, which was consistent with the dynamic change trends in our study.33 The neutrophils recruited into parenchyma caused damage to brain tissue and deteriorated neurological impairment.33 Lymphocyte was recruited into the parenchyma within days after stroke onset, and both protective and harmful functions were found of lymphocytes.28,34,35 Lymphocytes were proved to repair the inflammatory damage and secrete cytotoxic substances at the same time.36–38 This paradoxical influence of lymphocyte may be due to different subtypes.39,40 The recruitment of monocyte was initiated within 3–4 hours, earlier than lymphocytes, after stroke onset and monocytes cause harm to BBB through the expression of MMP-9.41 Besides, tPA could activate microglia and enhance the downstream neuroinflammatory to cause more damage to the brain tissue.42 The migration of neutrophils and release of MMP-9 from neutrophils could also be promoted via tPA and aggravated the destruction of BBB.43
In accordance with a previous retrospective study in 1,000 ischemic stroke patients,15 we also found that a higher level of NLR at 24 hours was correlated with a lower likelihood to achieve ENI. With regard to a favorable functional outcome at discharge, our study found a lower NLR level and a higher LMR level were associated with a higher proportion of mRS 0–1 at discharge. Several clinical studies and meta-analyses confirmed the predictive significance of functional outcomes in patients treated with IV tPA or endovascular thrombectomy, and that a high level of NLR was related to poor functional outcomes.21,44 In terms of short-term functional outcome, a retrospective study which enrolled 889 patients with intracranial atherosclerotic stenosis proved that admission NLR was related to 14-day mRS score with an AUC of 0.613.18 NLR was also proved to be predictive for discharge mRS score in patients with mild stroke (NIHSS <8).20 However, it is controversial whether admission NLR or NLR at other time points represented superior clinical significance. Most studies15,18–21 only included admission NLR and failed to conduct a further comparison between admission NLR and NLR at other time points. Our study investigated NLR level at four time points and found that NLR at 48 hours was the best predictor for functional outcome at discharge when compared with NLR at other time points. Our study also excluded patients without complete data on blood routine tests. The included patients tended to have a worse clinical status (more comorbidity and higher admission NIHSS score), higher proportion of MT, and be less likely to achieve favorable functional outcome at discharge. This may be explained by its relatively severe status, and more frequent blood tests were carried out to rigidly monitor the patients’ complications. However, the baseline characteristics in our study were comparable with other similar studies, suggesting favorable external validation of our study.15,26 The relationship of NLR and LMR with in-hospital clinical outcomes in ischemic stroke patients with less severity warrants further investigation.
In our study, we provided the optimal cut-off of NLR at 48 hours to predict favorable functional outcome at discharge was 5.69 and the optimal cut-off of LMR at 48 hours to predict favorable functional outcome at discharge was 2.48. For patients treated with endovascular thrombectomy, admission NLR above 5.9 was reported to predict 90-day functional outcome.45 For patients treated with IV tPA, the optimal cut-off value of post-tPA NLR to predict 90-day functional outcome was reported to be 4.8.27 Because we have too much missing data for 90-day functional outcome for this study, we used the discharge functional outcome instead. A single center, retrospective analysis showed that mRS at discharge was similar compared with 90-d mRS in patients treated with IV tPA.27 Discharge mRS was reported to be related to 90-d mRS.46–48 Another retrospective study also found that NLR was a predictor of both discharge mRS and 90-d mRS in patients treated with IV tPA.20
Our study has some potential limitations. First, the analyses were based on a database of a single decent stroke center in China. Potential selection and observational bias could not be ignored. However, blind assessment and video-training programs were conducted to reduce the potential bias. Besides, a multi-center cohort study may create additional bias, considering different laboratory machines, reagents, and testing kits of blood routine tests. Second, the sample size of our study was limited, however, our study only included patients treated with IV tPA and collected data at four different time points, while most studies with a larger sample size included patients treated with or without IV tPA and collected data at less timepoints. Considering tPA may have an influence on the neuroinflammatory process, a study population that included both patients treated with IV tPA and patients treated without IV tPA or collected biomarkers data at one point may increase heterogeneity and create additional bias to decrease the validation of the study conclusion. Third, mRS score at 90-days was absent in our study. In fact, face-to-face follow-up was unpractical considering the COVID-19 pandemic and population mobility in Beijing, the capital of China. Hence, we investigated the relationship of NLR and LMR with mRS score at discharge instead of mRS score at 90-days to investigate the influence of NLR and LMR on in-hospital outcomes.
Conclusion
NLR and LMR for 48 hours may be served as promising biomarkers for in-hospital functional outcomes.
Funding Statement
This work was supported by grants from Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2019-I2M-5-029), the National Natural Science Foundation (82171272), Beijing Municipal Science & Technology Commission (Z211100003521019), Beijing Hospitals Authority (PX2022019), Ministry of Finance of the People’s Republic of China [issued by Finance and Social Security [2015] Document No. 82; [2016] Document No. 50; [2017] Document No. 72; [2018] Document No. 48; [2019] Document No. 77; [2020] Document No. 75; [2021] Document No. 84, Ministry of Finance], and Beijing Hospitals Authority Innovation Studio of Young Staff Funding Support, code: 202112.
Ethics Statement
The study was approved by the ethics committee of Beijing Tiantan Hospital (No.: KY2019-019- 05). The fully deidentified data on the patients enrolled in the current study and its retrospective study design enabled this study to be conducted under waiver of informed consent by the local institutional review board. Our study also complied with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest in relation to this work to declare.
References
- 1.Group NIoNDaSr-PSS. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581–1587. doi: 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- 2.Bluhmki E, Chamorro Á, Dávalos A, et al. Stroke treatment with alteplase given 3·0–4·5 h after onset of acute ischaemic stroke (ECASS III): additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol. 2009;8(12):1095–1102. doi: 10.1016/S1474-4422(09)70264-9 [DOI] [PubMed] [Google Scholar]
- 3.Saver JL, Smith EE, Reeves MJ, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309(23):2480–2488. doi: 10.1001/jama.2013.6959 [DOI] [PubMed] [Google Scholar]
- 4.Wahlgren N, Ahmed N, Dávalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369(9558):275–282. doi: 10.1016/S0140-6736(07)60149-4 [DOI] [PubMed] [Google Scholar]
- 5.Wahlgren N, Ahmed N, Dávalos A, et al. Thrombolysis with alteplase 3–4·5 h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet. 2008;372(9646):1303–1309. doi: 10.1016/S0140-6736(08)61339-2 [DOI] [PubMed] [Google Scholar]
- 6.Ahmed N, Wahlgren N, Grond M, et al. Implementation and outcome of thrombolysis with alteplase 3–4·5 h after an acute stroke: an updated analysis from SITS-ISTR. Lancet Neurol. 2010;9(9):866–874. doi: 10.1016/S1474-4422(10)70165-4 [DOI] [PubMed] [Google Scholar]
- 7.Ahmed N, Wahlgren N, Brainin M, et al. Relationship of blood pressure, antihypertensive therapy, and outcome in ischemic stroke treated with intravenous thrombolysis: retrospective analysis from Safe Implementation of Thrombolysis in Stroke-International Stroke Thrombolysis Register (SITS-ISTR). Stroke. 2009;40(7):2442–2449. doi: 10.1161/STROKEAHA.109.548602 [DOI] [PubMed] [Google Scholar]
- 8.Ahmed NDA, Eriksson N, Ford GA, et al.; SITS Investigators. Association of admission blood glucose and outcome in patients treated with intravenous thrombolysis: results from the Safe Implementation of Treatments in Stroke International Stroke Thrombolysis Register (SITS-ISTR). Arch Neurol. 2010;67(9):1123–1130. doi: 10.1001/archneurol.2010.210 [DOI] [PubMed] [Google Scholar]
- 9.Kharitonova TV, Melo TP, Andersen G, Egido JA, Castillo J, Wahlgren N. Importance of cerebral artery recanalization in patients with stroke with and without neurological improvement after intravenous thrombolysis. Stroke. 2013;44(9):2513–2518. doi: 10.1161/STROKEAHA.111.000048 [DOI] [PubMed] [Google Scholar]
- 10.Strbian D, Ahmed N, Wahlgren N, et al. Trends in door-to-thrombolysis time in the safe implementation of stroke thrombolysis registry: effect of center volume and duration of registry membership. Stroke. 2015;46(5):1275–1280. doi: 10.1161/STROKEAHA.114.007170 [DOI] [PubMed] [Google Scholar]
- 11.Schuhmann MK, Stoll G, Bieber M, et al. CD84 links T cell and platelet activity in cerebral thrombo-inflammation in acute stroke. Circ Res. 2020;127(8):1023–1035. doi: 10.1161/CIRCRESAHA.120.316655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parikh NS, Merkler AE, Iadecola C. Inflammation, autoimmunity, infection, and stroke: epidemiology and lessons from therapeutic intervention. Stroke. 2020;51(3):711–718. doi: 10.1161/STROKEAHA.119.024157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoll G, Nieswandt B. Thrombo-inflammation in acute ischaemic stroke - implications for treatment. Nat Rev Neurol. 2019;15(8):473–481. doi: 10.1038/s41582-019-0221-1 [DOI] [PubMed] [Google Scholar]
- 14.Lux D, Alakbarzade V, Bridge L, et al. The association of neutrophil-lymphocyte ratio and lymphocyte-monocyte ratio with 3-month clinical outcome after mechanical thrombectomy following stroke. J Neuroinflammation. 2020;17(1):60. doi: 10.1186/s12974-020-01739-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong P, Liu Y, Gong Y, et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflammation. 2021;18(1):51. doi: 10.1186/s12974-021-02090-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Switonska M, Slomka A, Korbal P, et al. Association of neutrophil-to-lymphocyte ratio and lymphocyte-to-monocyte ratio with treatment modalities of acute ischaemic stroke: a pilot study. Medicina. 2019;55(7):342. doi: 10.3390/medicina55070342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Switonska M, Piekus-Slomka N, Slomka A, Sokal P, Zekanowska E, Lattanzi S. Neutrophil-to-lymphocyte ratio and symptomatic hemorrhagic transformation in ischemic stroke patients undergoing revascularization. Brain Sci. 2020;10(11):771. doi: 10.3390/brainsci10110771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ying Y, Yu F, Luo Y, et al. Neutrophil-to-lymphocyte ratio as a predictive biomarker for stroke severity and short-term prognosis in acute ischemic stroke with intracranial atherosclerotic stenosis. Front Neurol. 2021;12:705949. doi: 10.3389/fneur.2021.705949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SH, Jang MU, Kim Y, et al. The neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios predict reperfusion and prognosis after endovascular treatment of acute ischemic stroke. J Pers Med. 2021;11(8):696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu YL, Wu ZQ, Qu JF, et al. High neutrophil-to-lymphocyte ratio is a predictor of poor short-term outcome in patients with mild acute ischemic stroke receiving intravenous thrombolysis. Brain Behav. 2020;10(12):e01857. doi: 10.1002/brb3.1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Zhang M, Hao Y, et al. Early prediction of poor outcome despite successful recanalization after endovascular treatment for anterior large vessel occlusion stroke. World Neurosurg. 2018;115:e312–e21. doi: 10.1016/j.wneu.2018.04.042 [DOI] [PubMed] [Google Scholar]
- 22.Lyden PBT, Tilley B, Welch KM, et al. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke. 1994;25(11):2220–2226. doi: 10.1161/01.STR.25.11.2220 [DOI] [PubMed] [Google Scholar]
- 23.Adams HP, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 24.Fazekas FCJ, Hurtig HI, Hurtig HI, Zimmerman RA, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149(2):351–356. doi: 10.2214/ajr.149.2.351 [DOI] [PubMed] [Google Scholar]
- 25.Logallo N, Novotny V, Assmus J, et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a Phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol. 2017;16(10):781–788. doi: 10.1016/S1474-4422(17)30253-3 [DOI] [PubMed] [Google Scholar]
- 26.Weng Y, Hu J, Ren J, et al. Dynamic neutrophil-lymphocyte ratios predict short-term prognostic outcome of thrombolysis in patients with acute ischemic stroke. Neurotox Res. 2021;39(5):1678–1687. doi: 10.1007/s12640-021-00382-6 [DOI] [PubMed] [Google Scholar]
- 27.Chen CT, Li LH, Su PY, et al. Neutrophil-to-lymphocyte ratio in predicting neurologic outcome of patients with acute ischemic stroke treated with intravenous thrombolytics. J Chin Med Assoc. 2022;85(1):102–108. doi: 10.1097/JCMA.0000000000000599 [DOI] [PubMed] [Google Scholar]
- 28.Jian Z, Liu R, Zhu X, et al. The involvement and therapy target of immune cells after ischemic stroke. Front Immunol. 2019;10:2167. doi: 10.3389/fimmu.2019.02167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrovic-Djergovic D, Goonewardena SN, Pinsky DJ. Inflammatory Disequilibrium in Stroke. Circ Res. 2016;119(1):142–158. doi: 10.1161/CIRCRESAHA.116.308022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berchtold D, Priller J, Meisel C, Meisel A. Interaction of microglia with infiltrating immune cells in the different phases of stroke. Brain Pathol. 2020;30(6):1208–1218. doi: 10.1111/bpa.12911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu S, Lu J, Shao A, Zhang JH, Zhang J. Glial cells: role of the immune response in ischemic stroke. Front Immunol. 2020;11:294. doi: 10.3389/fimmu.2020.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khoshnam SE, Winlow W, Farzaneh M, Farbood Y, Moghaddam HF. Pathogenic mechanisms following ischemic stroke. Neurol Sci. 2017;38(7):1167–1186. doi: 10.1007/s10072-017-2938-1 [DOI] [PubMed] [Google Scholar]
- 33.Ao LY, Yan YY, Zhou L, et al. Immune cells after ischemic stroke onset: roles, migration, and target intervention. J Mol Neurosci. 2018;66(3):342–355. doi: 10.1007/s12031-018-1173-4 [DOI] [PubMed] [Google Scholar]
- 34.Planas AM. Role of immune cells migrating to the ischemic brain. Stroke. 2018;49(9):2261–2267. doi: 10.1161/STROKEAHA.118.021474 [DOI] [PubMed] [Google Scholar]
- 35.Rayasam A, Hsu M, Kijak JA, et al. Immune responses in stroke: how the immune system contributes to damage and healing after stroke and how this knowledge could be translated to better cures? Immunology. 2018;154(3):363–376. doi: 10.1111/imm.12918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xue J, Huang W, Chen X, et al. Neutrophil-to-lymphocyte ratio is a prognostic marker in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26(3):650–657. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 37.Kim JY, Kawabori M, Yenari MA. Innate inflammatory responses in stroke: mechanisms and potential therapeutic targets. Curr Med Chem. 2014;21(18):2076–2097. doi: 10.2174/0929867321666131228205146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Ren Q, Song Y, et al. Prognostic role of neutrophil-lymphocyte ratio in patients with acute ischemic stroke. Medicine. 2017;96(45):e8624. doi: 10.1097/MD.0000000000008624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liesz A, Suri-Payer E, Veltkamp C, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15(2):192–199. doi: 10.1038/nm.1927 [DOI] [PubMed] [Google Scholar]
- 40.Ren X, Akiyoshi K, Dziennis S, et al. Regulatory B cells limit CNS inflammation and neurologic deficits in murine experimental stroke. J Neurosci. 2011;31(23):8556–8563. doi: 10.1523/JNEUROSCI.1623-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma G, Pan Z, Kong L, Du G. Neuroinflammation in hemorrhagic transformation after tissue plasminogen activator thrombolysis: potential mechanisms, targets, therapeutic drugs and biomarkers. Int Immunopharmacol. 2021;90:107216. doi: 10.1016/j.intimp.2020.107216 [DOI] [PubMed] [Google Scholar]
- 42.Won S, Lee JK, Stein DG. Recombinant tissue plasminogen activator promotes, and progesterone attenuates, microglia/macrophage M1 polarization and recruitment of microglia after MCAO stroke in rats. Brain Behav Immun. 2015;49:267–279. doi: 10.1016/j.bbi.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 43.Ruhnau J, Schulze J, Dressel A, Vogelgesang A. Thrombosis, neuroinflammation, and poststroke infection: the multifaceted role of neutrophils in stroke. J Immunol Res. 2017;2017:5140679. doi: 10.1155/2017/5140679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang R, Wu X, Hu W, et al. Neutrophil-to-lymphocyte ratio predicts hemorrhagic transformation in ischemic stroke: a meta-analysis. Brain Behav. 2019;9(9):e01382. doi: 10.1002/brb3.1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brooks SD, Spears C, Cummings C, et al. Admission neutrophil-lymphocyte ratio predicts 90 day outcome after endovascular stroke therapy. J Neurointerv Surg. 2014;6(8):578–583. doi: 10.1136/neurintsurg-2013-010780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karamchandani RR, Strong D, Rhoten JB, et al. Age and discharge modified Rankin score are associated with 90-day functional outcome after basilar artery occlusion treated with endovascular therapy. Interv Neuroradiol. 2021;27(4):531–538. doi: 10.1177/1591019920987040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.ElHabr AK, Katz JM, Wang J, et al. Predicting 90-day modified Rankin Scale score with discharge information in acute ischaemic stroke patients following treatment. BMJ Neurol Open. 2021;3(1):e000177. doi: 10.1136/bmjno-2021-000177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson MP, Reeves MJ. Abstract 168: assessing the utility of the Modified Rankin Scale (mRS) at discharge to predict day 90 outcomes in acute stroke registries. Circulation. 2012;5:A168. [Google Scholar]