Abstract
There was inconsistent evidence regarding the use of matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry (MALDI‐TOF MS) for microorganism identification with/without antibiotic stewardship team (AST) and the clinical outcome of patients with bloodstream infections (BSI). In a systematic review and meta‐analysis, we evaluated the effectiveness of rapid microbial identification by MALDI‐TOF MS with and without AST on clinical outcomes. We searched PubMed and EMBASE databases from inception to 1 February 2022 to identify pre–post and parallel comparative studies that evaluated the use of MALDI‐TOF MS for microorganism identification. Pooled effect estimates were derived using the random‐effects model. Twenty‐one studies with 14,515 patients were meta‐analysed. Compared with conventional phenotypic methods, MALDI‐TOF MS was associated with a 23% reduction in mortality (RR = 0.77; 95% CI: 0.66; 0.90; I 2 = 35.9%; 13 studies); 5.07‐h reduction in time to effective antibiotic therapy (95% CI: −5.83; −4.31; I 2 = 95.7%); 22.86‐h reduction in time to identify microorganisms (95% CI: −23.99; −21.74; I 2 = 91.6%); 0.73‐day reduction in hospital stay (95% CI: −1.30; −0.16; I 2 = 53.1%); and US$4140 saving in direct hospitalization cost (95% CI: $‐8166.75; $‐113.60; I 2 = 66.1%). No significant heterogeneity sources were found, and no statistical evidence for publication bias was found. Rapid pathogen identification by MALDI‐TOF MS with or without AST was associated with reduced mortality and improved outcomes of BSI, and may be cost‐effective among patients with BSI.
Our meta‐analysis found that MALDI‐TOF MS significantly reduced the time to identify microorganisms, was able to prescribe antibiotics earlier, and that it decreased mortality rates, hospital stays, and medical costs.
INTRODUCTION
Bloodstream infection (BSI) is a major cause of sepsis affecting approximately millions of people worldwide each year (Laupland et al., 2007). The timeliness and appropriateness of antibiotic treatment are critical factors for patients' survival, which largely depend on the microbiology test results of blood cultures (Diekema et al., 2004; Peterson et al., 2001). Without culture‐based evidence for bloodstream infection, antimicrobial therapy cannot effectively treat the infections (Diekema et al., 2004; Fridkin et al., 2014; McNulty et al., 2011). Conventional blood culture methods may require an interval of 6–60 h before the infective pathogens are identified from positive blood cultures (Kirn & Weinstein, 2013; McGowan et al., 2000; Zadroga et al., 2013). Although Gram staining could provide a rapid report of the suspected pathogens, it does not allow a clear‐cut discrimination between contamination and true BSI. Therefore, broad‐spectrum empirical treatment based on clinical judgement remains the mainstay of treatment. However, it has been shown empirical antibiotics are suboptimal or unnecessary in 25%–50% of patients (Vora et al., 2015; Willemsen et al., 2007). Unnecessary antibiotics could lead to the development of antibiotic resistance, increased risk of Clostridiodes difficile or invasive fungal infection and excess healthcare cost (Costelloe et al., 2010; Fridkin et al., 2014; Hecker et al., 2003; MacDougall et al., 2005; Patel et al., 2008; Schultz et al., 2014). Suboptimal antibiotics adversely impact the outcome of sepsis (Brunkhorst et al., 2012; Campion & Scully, 2018; Suffredini & Munford, 2011). Each hour's delay in effective antibiotic results in an estimated 7.6% decrease in survival (Chaudhary et al., 2014; Funk & Kumar, 2011; Kumar et al., 2006; Seymour et al., 2017).
The microbial identification by matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry (MALDI‐TOF MS) accelerates the isolate identification process of blood cultures (Hettick et al., 2004; Holland et al., 1996; La Scola & Raoult, 2009). MALDI‐TOF contains two parts of technology. The matrix‐assisted laser desorption/ionization (MALDI) technology desorbs and ionizes large biomolecules from bacteria or fungi, while the recorded time of flight (TOF) in the magnetic chamber generates characteristic mass spectral fingerprints that are unique and ideal for an accurate microbial identification (Domon & Aebersold, 2006). MALDI‐TOF MS can identify the characteristics of molecules within a few minutes; therefore, it was soon applied to identify microorganisms detected in blood cultures (Bessède et al., 2011; Marklein et al., 2009). Since 2010, commercialized platforms for clinical use became available. The introduction of this technique in the clinical laboratory alongside the microbiology staffing and reporting has significantly shortened the time of microbial identification.
Although this strategy has the potential to increase the appropriateness of empirical antibiotic therapy and ultimately improve patients' survival, several previous studies showed inconsistent results on the efficiency measures and patients' clinical outcomes. Hence, there is a need to systematically summarize the current evidence on the clinical and economic impact of the MALDI‐TOF MS technology. The primary goal of this systematic review and meta‐analysis was to determine the effectiveness of MALDI‐TOF MS‐based pathogen identification with or without antibiotic stewardship in improving the efficiency of microbiology report and clinical outcomes.
EXPERIMENTAL PROCEDURES
Search strategy and selection criteria
A systematic literature review and meta‐analysis were conducted in accordance with PRISMA recommendations (Page et al., 2021). PubMed and EMBASE were searched from inception of the database to 1 February 2022, with the search terms (“matrix‐assisted laser desorption ionization time‐of‐flight mass spectrometry” OR “MALDI‐TOF” OR “mass spectrometry”) AND (“bloodstream infection” OR “BSI” OR “bacteremia” OR “blood culture” OR “septicemia” OR “sepsis”). Two authors (Y‐HS and ZC) independently assessed the eligibility of clinical studies. The searches and studies were not limited by publication date, country or language. To ensure the comprehensive acquisition of literature, independent supplemental manual searches were performed on the reference lists of retrieved articles and other minor databases, including Web of Science, Cochrane databases, China National Knowledge Infrastructures (CNKI), Latin American and Caribbean of Health Sciences Information System (LILACS) and African Index Medicus (AIM). Medical Subject Heading (MeSH) and EMBASE TREE tool (EMTREE) were used to guide the choice of appropriate search terms in other databases.
Inclusion and exclusion
Two reviewers independently identified articles eligible for in‐depth examination (Y‐HS, W‐TH). Studies were included if at least one of the following outcomes was analysed: (1) efficiency measures such as time to bacteriology identification or time to effective antibiotic therapy or time to optimal antibiotics; (2) clinical outcome measures such as length of hospital stay, length of intensive care unit (ICU) stay or mortality; and (3) economical measure such as total hospitalization costs. Relevant interventions were defined as the use of MALDI‐TOF MS for the identification of microorganism from blood culture with or without a dedicated antibiotic stewardship team. Studies using pre–post comparison or parallel comparison designs were deemed appropriate, whereas review articles, editorials, case studies, letters to the editor or studies without a comparison group were excluded. When multiple articles reported on the same study population, we included only the most detailed publication that met the inclusion criteria. Discrepancies on articles meriting inclusions between reviewers were resolved by a consensus meeting of three authors (C‐CL, YHS and W‐TH). Study selection is summarized in Figure 1.
FIGURE 1.
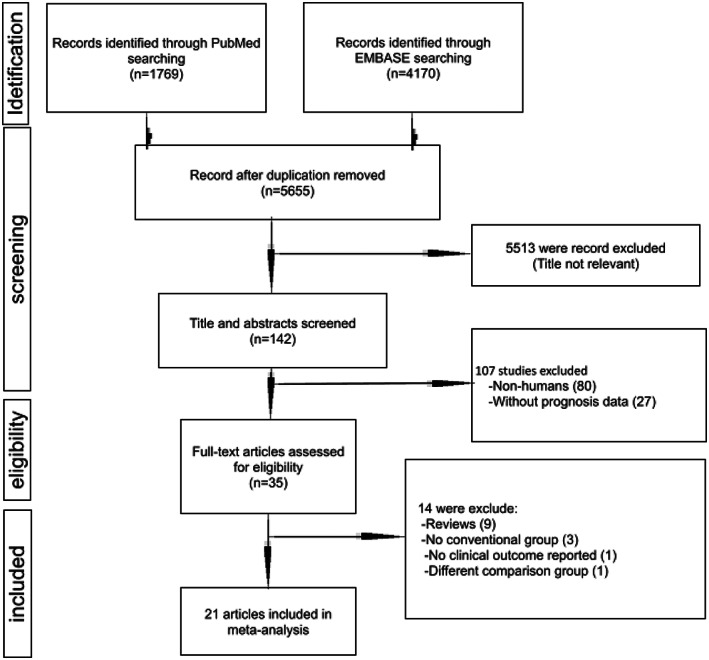
Study selection process
Data extraction and quality assessment
Data were extracted on first author's surname, country, year of publication, study design, study population, number of participants, algorithm for microbiology examination, and outcome measures, outcome before and after intervention according to the outcome definitions, crude and adjusted effect sizes if available and confidence intervals (CIs) of all continuous variables if available. Risk of bias was assessed independently by two authors (C‐HY and W‐TH) using Quality Assessment Tool for Before–After (Pre–Post) Studies With No Control Group of U.S. National Heart, Lung, and Blood Institute (2014 ). The tool was adopted in our study by removing four of 12 criteria that were not applicable to our specific study designs. Four criteria were regarding blinding (question 8), follow‐up (question 9), interrupted time‐series design (question 11) and individual‐level data adjustment (question 12). Our modified tool included eight assessment criteria (one point each, total of eight points; Table S1). Studies were classified as low quality (fewer than three points), moderate quality (3–5 points) or high quality (more than five points).
Statistical analysis
We followed the Cochrane Collaboration recommendations for meta‐analysis and PRISMA guidelines of observational studies in our data reporting (Higgins et al., 2019; Page et al., 2021). Heterogeneity was tested using the Cochran Q statistic (p < 0.10) and quantified with the I 2 statistic, which describes the variation of effect size that is attributable to heterogeneity across studies (Cochran, 1950; Higgins et al., 2003; Higgins & Thompson, 2002; Ioannidis et al., 2007). Because of the expected clinical heterogeneity in the reported outcomes across studies, summary estimates were calculated using the inverse‐variance weighting model according to the method of DerSimonian and Laird, which accounts for within‐ and between‐study heterogeneity (DerSimonian & Laird, 1986, 2015). Galbraith plots were used to visualize the impact of individual studies on the overall homogeneity test statistic (Galbraith, 1994). Publication bias was examined visually through funnel plots and statistically through Begg and Egger's tests (Begg & Mazumdar, 1994; Egger et al., 1997; Stuck et al., 1998). Meta‐regression was used for two purposes in this study. First, we performed a univariate meta‐regression analysis to identify whether trial‐level covariates were a significant source of heterogeneity. These covariates included mean age, bloodstream infection (general vs. specific pathogen), study quality, antibiotic stewardship team (presence or absence), time to pathogen identification and time to effective antibiotic treatment.
When the meta‐regression was significant, effect sizes were stratified on the same study characteristics so that they were available as separate estimates for each stratum. Second, we evaluated the potential effect modification by meta‐regression. We were interested to investigate whether the mortality reduction by MALDI‐TOF MS rapid organism identification algorithm would be modified by time reduced in bacteriology identification or time to effective antibiotic treatment. All meta‐analyses were performed using STATA 11, with the metan, metareg, and metabias, galbr, confunnel package, except the meta‐regression plot was created by the metafor package of R. p < 0.05 was considered statistically significant unless otherwise specified.
RESULTS
Study inclusion and quality assessment
Our literature search identified 1769 studies from PubMed and 4170 studies from EMBASE (Figure 1). After removing duplicates and applying inclusion and exclusion criteria for the abstract screening, 35 studies remained for full‐text review. A total of 21 studies were included in the final meta‐analysis (Table 1). Of the included studies, nine studies received six points on the quality assessment tool, due to compromising generalizability and a limited sample size (Carreno et al., 2016; Clerc et al., 2013; Mok et al., 2019; Nagel et al., 2014; Osthoff et al., 2017; Puckett et al., 2021; Shimamoto et al., 2021; Vlek et al., 2012; Wenzler et al., 2016). Ten studies received seven points (Delport et al., 2016; Huang et al., 2013; Jeon et al., 2018; Lo et al., 2020; Lockwood et al., 2016; López‐Pintor et al., 2021; Niwa et al., 2019; Perez et al., 2013, 2014; Zadka et al., 2019), two studies received eight points (Dixon et al., 2021; MacGowan et al., 2020), indicating high quality of study (Table S1). Overall, 14,515 patients were included for analysis. There were only two randomized controlled trials among the included studies (Dixon et al., 2021; MacGowan et al., 2020). However, both RCTs were actually the same study but with different analyses. Accordingly, only the primary study was included in the analysis.
TABLE 1.
Summary of studies comparing the clinical outcomes of patients with or without MALDI‐TOF
| Author (Location, Year) | Design | Setting and study population | Size | Intervention | Outcome definition and case ascertainment | Intervention period | Control period |
|---|---|---|---|---|---|---|---|
| Vlek (Netherlands, 2012) | Pre–post | Paediatric patients with BSI | 253 | MALDI‐TOF | Time to pathogen identification (h) | 16.4 | 45.2 |
| Time to optimal antibiotics (h) | 17.5 | 24 | |||||
| Huang (USA, 2013) | Pre–post | Patients with BSI | 501 | MALDI‐TOF with AST | Time to effective antibiotics (h) | 20.4 | 30.1 |
| Time to pathogen identification (h) | 55.9 | 84 | |||||
| 30‐day mortality (%) | 12.70 | 20.30 | |||||
| Hospital LOS (days) | 14.2 | 11.4 | |||||
| ICU LOS (days) | 8.3 | 14.9 | |||||
| Recurrent bacteraemia (%) | 2.00 | 5.90 | |||||
| Carreno (USA, 2016) | Pre–post | Patient with BSI | 219 | MALDI‐TOF and AST | Time to effective antibiotics (h) | 0.06 | 0.18 |
| Time to optimal antibiotics (h) | 0.12 | 0.3 | |||||
| Mortality (%) | 7.6 | 11.4 | |||||
| LOS hospital (days) | 10 | 9 | |||||
| LOS ICU (days) | 5 | 4 | |||||
| Clerc (Switzerland, 2013) | Prospective cohort | Patients with G‐BSI | 202 | MALDI‐TOF | Use of empiric antibiotics | 8.9% | 5.0% |
| Streamlining | 10.9% | 7.9% | |||||
| Spectrum broadening | 15.3% | 7.9% | |||||
| Delport (Canada, 2016) | Pre–post | Paediatric patients with BSI | 92 | MALDI‐TOF | Time to effective antibiotics (h) | 14.4 | 14.5 |
| Time to optimal antibiotics (h) | 78.33 | 68.8 | |||||
| Time to Gram stain result (h) | 27.2 | 29.4 | |||||
| Time to pathogen identification (h) | 75.7 | 87.2 | |||||
| Hospital LOS (h) | 508.8 | 582.5 | |||||
| Lockwood (USA, 2016) | Pre–post | Patients with G‐BSI | 346 | MALDI‐TOF and AST | Time to effective antibiotics (h) | 22 | 48 |
| Time to pathogen identification (h) | 6.5 | 32 | |||||
| 30‐day mortality (%) | 4.9 | 9.4 | |||||
| Time to optimal antibiotics (h) | 30 | 71 | |||||
| ICU LOS (days) | 3.7 | 2.3 | |||||
| Hospital LOS (days) | 6.4 | 6.4 | |||||
| Costs ($) | 22,473 | 24,116 | |||||
| Nagel (USA, 2014) | Pre–post | Patients with positive CoNS blood culture | 78 | MALDI‐TOF and AST | Time to effective antibiotics (h) | 23 | 37.7 |
| Time to optimal antibiotics (h) | 34.4 | 58.7 | |||||
| Time to pathogen identification (h) | 57 | 83.4 | |||||
| 30‐day mortality (%) | 3.1 | 21.7 | |||||
| ICU LOS (days) | 11 | 28 | |||||
| Hospital LOS (days) | 15 | 14 | |||||
| 30‐day readmission | 0 | 4.3 | |||||
| Recurrent bacteraemia (%) | 0 | 13 | |||||
| Osthoff (Switzerland, 2017) | Pre–post | Patients with BSI | 242 | MALDI‐TOF | Time to pathogen identification (h) | 28.2 | 49.7 |
| Duration of IV antibiotics (days) | 13.1 | 13.7 | |||||
| Duration of antibiotics (days) | 18.5 | 18.7 | |||||
| Hospital LOS (days) | 16.2 | 19 | |||||
| 30‐day mortality (%) | 8.3 | 16.5 | |||||
| In‐hospital mortality (%) | 7.4 | 12.4 | |||||
| Admission to ICU after BSI onset | 23.1 | 37.2 | |||||
| Perez (USA, 2013) | Pre–post | Patients with BSI | 219 | MALDI‐TOF | Time to effective antibiotics (h) | 24.4 | 47.1 |
| Time to optimal antibiotics (h) | 29 | 75 | |||||
| Time to pathogen identification (h) | 11.1 | 36.6 | |||||
| ICU LOS (days) | 4.9 | 6.1 | |||||
| Hospital LOS (days) | 8.1 | 9.9 | |||||
| Costs ($) | 26,162 | 45,709 | |||||
| Perez (USA, 2014) | Pre–post | Patients with antibiotic‐resistant G‐BSI | 269 | MALDI‐TOF | Time to effective antibiotics (h) | 29.3 | 46.7 |
| Time to optimal antibiotics (h) | 23.2 | 80.9 | |||||
| Time to pathogen identification (h) | 14.5 | 40.9 | |||||
| In‐hospital mortality (%) | 8 | 18.5 | |||||
| 30‐day mortality (%) | 8.9 | 21 | |||||
| 60‐day mortality (%) | 12.5 | 30.6 | |||||
| ICU LOS (days) | 7.3 | 12.5 | |||||
| Hospital LOS (days) | 10.8 | 16.2 | |||||
| Costs ($) | 52,693 | 78,991 | |||||
| Wenzler (USA, 2016) | Pre–post | Paediatric patients with A. baumannii pneumonia or BSI | 252 | MALDI‐TOF | Time to effective antibiotics (days) | 9 | 11 |
| 14‐day mortality (%) | 25 | 20 | |||||
| 30‐day readmission (%) | 8 | 9 | |||||
| Clinical cure at 7 days (%) | 34 | 15 | |||||
| Hospital LOS (days) | 11 | 13 | |||||
| Costs ($) | 42,872 | 49,402 | |||||
| Jeon (Korea, 2018) | Pre–post | Patients >18 y/o with positive blood cultures | 556 | MALDI‐TOF | ICU_LOS (days) | 14.7 | 16.8 |
| Time to pathogen identification (h) | 63.5 | 86.4 | |||||
| Time to effective therapy (h) | 23.2 | 27.4 | |||||
| 30‐day mortality (%) | 15.7 | 17.54 | |||||
| Recurrent bacteraemia (%) | 2.8 | 5.2 | |||||
| Niwa (Japan, 2019) | Pre–post | Patients with bloodstream infections and candida bloodstream infection | 366 | MALDI‐TOF | Time to pathogen identification (h) | 48.6 | 78.1 |
| Time to effective antibiotics (h) | 12.9 | 26.2 | |||||
| Time to optimal antibiotics (h) | 53.3 | 91.7 | |||||
| 30‐day mortality (%) | 5.4 | 9.4 | |||||
| Recurrent bacteraemia (%) | 5.1 | 5.5 | |||||
| Zadka (Israel, 2019) | Pre–Post | Patients with positive blood culture (bacteria only) | 4170 | MALDI‐TOF and AST | Hospital LOS (days) | 9.79 | 10.83 |
| In‐hospital mortality (%) | 18.3 | 20.9 | |||||
| Mok (Korea, 2019) | Pre–post | Patients with MDR bacteraemia | 187 | MALDI‐TOF | Time to pathogen identification (h) | 82.5 | 92.3 |
| Time to effective antibiotics (h) | 99.5 | 102.2 | |||||
| 28‐day mortality (%) | 35.6 | 40.2 | |||||
| Lo (Canada, 2020) | Retrospective cohort | Patients with Gram‐negative bacteraemia | 377 | MALDI‐TOF | Time to Gram stain result (h) | 18.42 | 20.29 |
| Time to pathogen identification (h) | 34.58 | 48.91 | |||||
| Time to appropriate prescription (h) | 50.34 | 66.71 | |||||
| Time to appropriate discontinuation (h) | 58.21 | 68.39 | |||||
| MacGowan (UK, 2020) | RCT | Adult patients with positive blood culture for bacteria or fungi | 5550 | MALDI‐TOF | Time to pathogen identification (h) | 38.5 | 55.2 |
| Time to effective antibiotics (h) | 24 | 13 | |||||
| Hospital LOS (d) | 15 | 15 | |||||
| 28‐day mortality (%) | 18.50 | 17.69 | |||||
| Dixon (UK, 2021) | RCT | Adult patients with positive blood culture for bacteria or fungi | 4486 | MALDI‐TOF | 28‐day mortality (%) | 20.57 | 17.69 |
| 28‐day cost (£) | 8139 | 8253 | |||||
| Puckett (USA, 2021) | Pre–Post | Patients aged 0–26y having a blood culture positive for monomicrobial organism | 131 | MALDI‐TOF and AST | Time to effective antibiotics (h) | 42.7 | 60.8 |
| Time to optimal antibiotics (h) | 53.2 | 72.9 | |||||
| Time to pathogen identification (h) | 35.4 | 42.3 | |||||
| Shimamoto (Japan, 2021) | Retrospective cohort | Patients with enterococcal BSI | 173 | MALDI‐TOF | Time from positive blood culture drawn to definitive antibiotic therapy (days) | 1 | 3 |
| Hospital LOS (days) | 19 | 16 | |||||
| Duration of antibiotic treatment | 11 | 11 | |||||
| 28‐day mortality (%) | 26.4 | 29.3 | |||||
| López‐Pintor (Spain, 2021) | Pre–post | Patients with positive blood cultures with GNB | 332 | MALDI‐TOF | Time from positive blood culture to antibiotic treatment (days) | 1.0 | 2.0 |
| 30‐day mortality (%) | 7.98 | 4.8 | |||||
| Hospital LOS (days) | 8 | 8 | |||||
| 30‐day readmission (%) | 15 | 10 |
The study characteristics are summarized in Table 1. Studies were conducted in nine countries between 2012 and 2021. The countries most represented were the USA (eight studies) (Carreno et al., 2016; Huang et al., 2013; Lockwood et al., 2016; Nagel et al., 2014; Perez et al., 2013, 2014; Puckett et al., 2021; Wenzler et al., 2016), Switzerland, Canada, Korea, Japan and the UK (two studies each) (Clerc et al., 2013; Osthoff et al., 2017); and the Netherlands, Israel and Spain (one study each) (López‐Pintor et al., 2021; Vlek et al., 2012; Zadka et al., 2019). Except three cohort studies and two RCT studies, the remaining 16 studies were one group pre–post studies.
Three studies (Delport et al., 2016; Vlek et al., 2012; Wenzler et al., 2016) were performed in children, while the other 18 studies were on adults (Carreno et al., 2016; Clerc et al., 2013; Dixon et al., 2021; Huang et al., 2013; Jeon et al., 2018; Lo et al., 2020; Lockwood et al., 2016; López‐Pintor et al., 2021; MacGowan et al., 2020; Mok et al., 2019; Nagel et al., 2014; Niwa et al., 2019; Osthoff et al., 2017; Perez et al., 2013, 2014; Puckett et al., 2021; Shimamoto et al., 2021; Zadka et al., 2019). In six studies, MALDI‐TOF MS bacterial identification was implemented with antibiotic stewardship (Carreno et al., 2016; Huang et al., 2013; Lockwood et al., 2016; Nagel et al., 2014; Puckett et al., 2021; Zadka et al., 2019).
Effect of rapid pathogen identification by MALDI‐TOF MS on mortality reduction
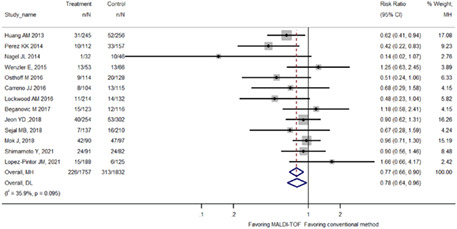
Pooled analysis of eligible studies showed that rapid pathogen identification by MALDI‐TOF was associated with reductions in the in‐hospital mortality of patient with BSI (23% reduction; RR 0.77, 95% CI: 0.66–0.90; Figure 2). The mortality reduction tended to be more pronounced when restricting analysis to adult studies (31% reduction; RR 0.69, 95% CI: 0.57–0.84; nine studies) or to studies co‐implemented with antibiotic stewardship programme (35% reduction; RR 0.65, 95% CI: 0.49–0.86; six studies) (Table 2). Different studies focused on different BSI populations, with some on Gram‐negative and others on Gram‐positive pathogens. As for studies that focused on all patients with BSI, the mortality reduction was similar to the overall pooled effect size (25% reduction; RR 0.75, 95% CI: 0.60–0.94; six studies). Eleven studies were using 28‐ or 30‐day mortality, and the mortality reduction was also similar to the overall effect size (24% reduction; RR 0.76, 95% CI: 0.64–0.89) (Table 2).
FIGURE 2.
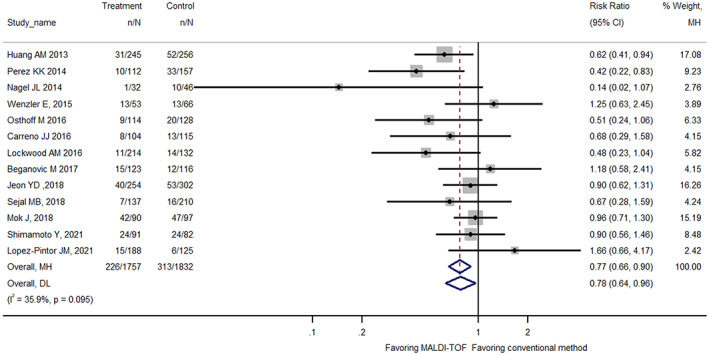
Forest plot comparing in‐hospital mortality between MALDI‐TOF MS bacterial identification and conventional methods among patients with bloodstream infection.
TABLE 2.
Summary risk ratios of mortality before and after the introduction of MALDI‐TOF for identification of pathogen from blood cultures
| Category | Number of studies | Summary estimate (95% CI) | I 2 (95% CI) | Meta‐regression, p‐Value | Publication bias (Egger's test, p‐Value) |
|---|---|---|---|---|---|
| Mortality | 13 | 0.77 (0.66, 0.90) | 35.9 (0.0, 65.7) | NA | 0.227 |
| Reporting 28‐ or 30‐day mortality | 11 | 0.76 (0.64, 0.89) | 41.1 (0.0, 69.5) | 0.522 | 0.181 |
| Purely adult population | 9 | 0.69 (0.57, 0.84) | 37.1 (0.0, 69.8) | 0.103 | 0.269 |
| MALDI‐TOF with AST | 6 | 0.65 (0.49, 0.86) | 26.7 (0.0, 70.6) | 0.189 | 0.799 |
| MALDI‐TOF without AST | 5 | 0.77 (0.61, 0.98) | 44.9 (0.0, 78.3) | 0.947 | 0.424 |
Abbreviations: AST, antibiotic stewardship team; BSI, bloodstream infection; MALDI‐TOF, matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry.
Other clinical and economic effect of MALDI‐TOF MS rapid pathogen identification
In addition to mortality, rapid pathogen identification by MALDI‐TOF mass spectrometry resulted in several other clinical and economic benefits. It reduced the time to effective antibiotic therapy (5.07 hours' reduction; 95% CI: −5.83 to −4.31, p < 0.0001), time to pathogen identification (22.86 hours' reduction; 95% CI: −23.99 to −21.74, p < 0.0001), length of hospital stay (0.73 days' reduction; 95% CI: −1.30 to −0.16, p = 0.012) and direct hospitalization cost (4140.18 US$ saving; 95% CI: −8166.75 to −113.6, p = 0.044). However, the reduction in length of ICU stay was not statistically significant (0.20 days' reduction; 95% CI: −0.38 to 0.79, p = 0.494) (Table 3).
TABLE 3.
Summary mean difference of continuous outcomes before and after the introduction of MALDI‐TOF for identification of pathogen from blood cultures
| Number of studies | Mean difference (95% confidence interval) | p‐value | I 2 | |
|---|---|---|---|---|
| Time to effective antibiotics (h) | 18 | −5.07 (−5.83, −4.31) | <0.0001 | 95.7 |
| Time to pathogen identification (h) | 14 | −22.86 (−23.99, −21.74) | <0.0001 | 91.6 |
| Length of hospital stay (days) | 12 | −0.73 (−1.30, −0.16) | 0.012 | 53.1 |
| Length of ICU stay (days) | 8 | 0.20 (−0.38, 0.79) | 0.494 | 87.1 |
| Direct hospitalization cost (US$) | 5 | −4140.18 (−8166.75, −113.60) | 0.044 | 66.1 |
Heterogeneity, effect modification and publication bias
The heterogeneity of the effect estimates for mortality reduction as quantified by I 2 was <30% for all subgroups. The heterogeneity among studies of different clinical and economic outcomes, however, was large. These outcomes were presented with continuous figures, and higher heterogeneity than the binomial outcomes was reasonably expected. We did not find statistical outliers to account for the major source of heterogeneity by the Galbraith plot (Figure 3). Furthermore, the potential sources of heterogeneity were assessed through meta‐regression, and no difference in any effect size estimate was found by specific study characteristics. To explore whether the mortality reduction by MALDI‐TOF MS was modified by reduced time to bacteriology identification or time to effective antibiotics therapy, we meta‐regressed the effect of mortality reduction (in the form of log relative risk) on the time reduction for all studies. There was a trend towards a more significant mortality reduction with more reduced time to effective antibiotic initiation (meta‐regression, p = 0.016, slope, −0.003, 95% CI: −0.006‐0.000); however, mortality reduction by MALDI‐TOF MS was not modified by reduced time to bacteriology identification (meta‐regression, p = 0.495, slope, −0.027, 95% CI: −0.076; 0.022) (Figure 4). There was no evidence of publication bias as the funnel plot looked symmetrical (Figure 5), and the p‐values for Begg and Egger's tests were >0.05 (Table 2).
FIGURE 3.
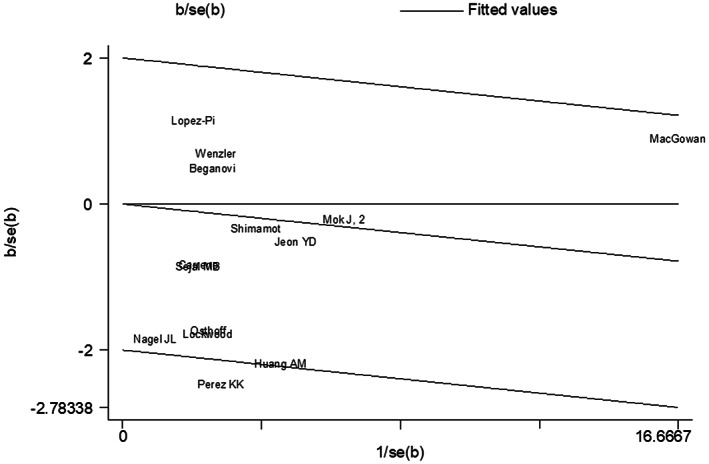
Galbraith plot of MALDI‐TOF MS bacterial identification associated with in‐hospital mortality as compared to conventional methods: Galbraith plot did not disclose any statistical outlier for the main analysis (in‐hospital mortality).
FIGURE 4.
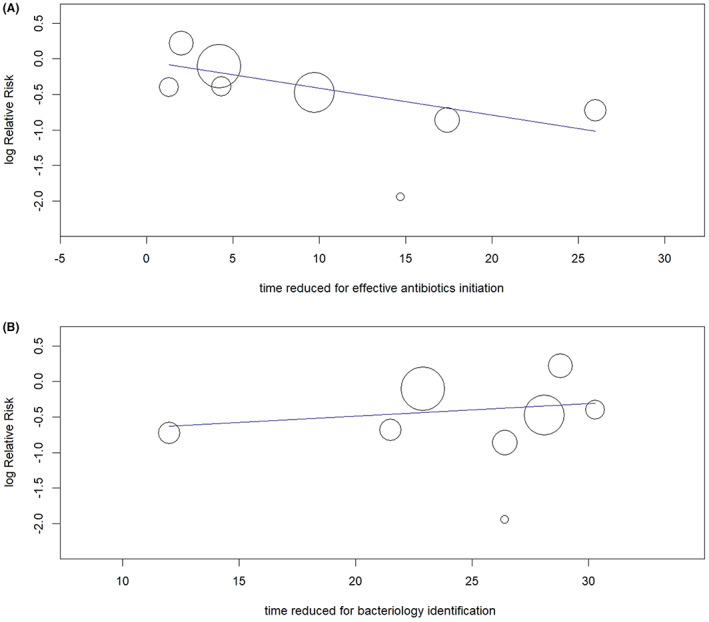
Meta‐regression to analyse the relationship between the mortality reduction by MALDI‐TOF (log risk ratios) to the time reduced for effective antibiotic initiation (Panel A) and time reduced for bacteriology identification (Panel B). The effect of MALDI‐TOF on mortality reduction was significantly modified by the time reduced for effective antibiotic initiation (p = 0.016) but not by the time reduced for bacteriology identification (p = 0.495).
FIGURE 5.
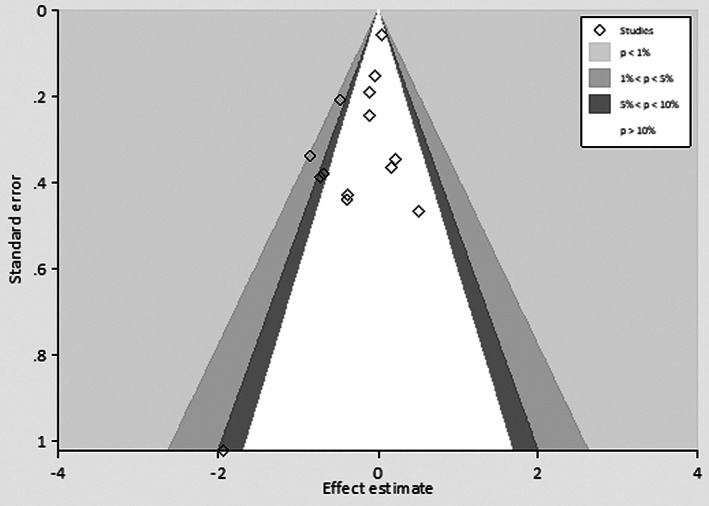
Funnel plot of effect estimate did not show signs of asymmetry, indicating no evidence of publication bias.
DISCUSSION
In this meta‐analysis comprising 21 studies with 14,515 patients, we showed that rapid pathogen identification by MALDI‐TOF MS was associated with a mean reduction of 22.86 h for time to pathogen identification and 5.07 h for time to effective antibiotic therapy. The reduction in time to effective antibiotic therapy resulted in a pronounced reduction (23% reduction) in the in‐hospital mortality among patients with BSI. The largest reductions were seen in studies co‐implemented with antibiotic stewardship programmes (35% reduction).
Shorter time to effective antibiotic therapy in BSI patients diagnosed with MALDI‐TOF MS was found significantly associated with lower mortality, ranging from 11% mortality reduction for 2.7‐h reduction in time to effective therapy (Mok et al., 2019) to 58% mortality reduction for 17.4‐h reduction in time to effective therapy (Perez et al., 2014). Although the technology of MALDI‐TOF MS itself is expensive, the application of MALDI‐TOF MS in the clinical settings as in this study, however, could shorten the length of hospital stay by 0.73 days with a resultant mean saving of U$ 4140.18 for each patient's hospitalization cost.
One study included in our analysis did not show improvement in mortality. Wenzler et al. (2016) evaluated the clinical and economic outcomes of MALDI‐TOF MS combined with stewardship intervention with A. baumannii pneumonia and/or bacteraemia in which the majority of AB isolates were resistant to at least three antibiotics. They found rapid organism identification by MALDI‐TOF MS was associated with a 19% increase in clinical cure (15% vs. 34%, p = 0.02) and a decreased length of stay (13 [range 8–18] vs. 11 [range 7–15] days, p = 0.02), despite no difference in 14‐day mortality (20% vs. 25%, p = 0.53). Unlike other included studies, Wenzler et al.’s study included a group of patients with BSI caused by multi‐drug‐resistant bacteria. MALDI‐TOF MS could not provide the drug sensitivity results and could only guide the empirical antibiotics. When A. baumannii was identified, intravenous and/or inhaled colistin plus minocycline was recommended by the antibiotic stewardship team. In addition, there is substantial evidence supporting the implementation of antibiotic stewardship programme as it results in mortality reduction in patients with systemic infection (Baur et al., 2017; Karanika et al., 2016; Schuts et al., 2016). In this study, we did not find a significant incremental value of antibiotics stewardship to the rapid pathogen identification by MALDI‐TOF MS. In fact, Beganovic M et al. performed the only one study that compared MALDI‐TOF with antibiotics stewardship to MALDI‐TOF MS alone (Beganovic et al., 2017). The study found the addition of antibiotics stewardship to MALDI‐TOF MS did not result in incremental benefits in mortality reduction but was associated with improvements in length of antibiotic therapy, ICU or hospital length of stay, recurrent bacteraemia and average direct cost (Beganovic et al., 2017). Previous studies showed that using molecular rapid diagnostic testing with the help of antibiotic stewardship can cause mortality reduction (Timbrook et al., 2017). Therefore, despite the non‐significant finding of mortality reduction in our study, it is important to emphasize antibiotic stewardship programmes have many other benefits that could not be replaced by the MALDI‐TOF MS rapid pathogen identification.
Over the past four decades, scientific research has focused on the development of novel therapeutic targets for sepsis (Suffredini & Munford, 2011). Despite this effort, no therapy aiming at these novel targets is currently licensed. The tremendous mortality reduction shown in this meta‐analysis demonstrated early microbiology diagnosis by MALDI‐TOF MS had more impact on the outcome of BSI than any of the previous therapeutic trials, such as early goal‐directed therapy, steroid therapy, combination antibiotic therapy, haemofiltration or organ support therapy. Results of the meta‐regression showed ‘time to effective antibiotics’ was the strongest driver of mortality reduction, which was consistent with previous findings. Kumar et al. showed each 1‐h delay in effective antibiotics was associated with around 8% increase in mortality among patients with septic shock. Seymour CM in a large observational study comprising 49,331 patients form 149 hospitals showed longer time to the administration of antibiotics (odds ratio, 1.04 per hour; 95% CI: 1.03 to 1.06; p < 0.001) but not a longer time to the completion of a bolus of intravenous fluids (odds ratio, 1.01 per hour; 95% CI: 0.99 to 1.02; p = 0.21) was associated with higher risk‐adjusted in‐hospital mortality (Seymour et al., 2017).
Results of this meta‐analysis confirmed the time to effective antibiotic therapy, rather than time to bacteriology identification, was the key to outcome improvement for BSI. There was a large time lag (average 17 h) between bacteriology identification and effective antibiotic initiation. Details of this time lag were not clearly reported in the included literature, but several clinical, operational or systematic factors may provide some insights into this knowledge–implementation gap. Among the clinical factors, MALDI‐TOF MS could characterize the pathogen species but could not accurately predict drug resistance. Several rapid tests based on nucleic acid amplification or immunolateral flow could help identify the mechanisms of resistance of the bacteria (Kostrzewa et al., 2013; Manukumar & Umesha, 2017). In addition, around 10%–15% of the infections were polymicrobial, which could not be accurately discriminated by MALDI‐TOF MS (Faron et al., 2017). Lastly, several reports also showed the inadequacy of current software in interpreting some of the blood culture samples. This weakness can potentially be improved by the complimentary use of multiplex polymerase chain reaction, which identified bacterial species and drug‐resistant genes simultaneously and could further shorten the time required for drug sensitivity test (Chang et al., 2013; Kumar et al., 2006; Peters et al., 2004). For the operational or systematic factors, lack of 24–7 MALDI‐TOF service, lack of prompt notification system to the treating physicians, lack of the antibiotic stewardship support system and lack of the hospital standard on the response time to the microbiology report, among many other aspects, have been reported (Alghamdi et al., 2019; Swaan et al., 2018).
Although most of the included studies were high‐quality pre–post design (Carreno et al., 2016; Delport et al., 2016; Huang et al., 2013; Jeon et al., 2018; Lockwood et al., 2016; López‐Pintor et al., 2021; Mok et al., 2019; Nagel et al., 2014; Niwa et al., 2019; Osthoff et al., 2017; Perez et al., 2013, 2014; Puckett et al., 2021; Vlek et al., 2012; Wenzler et al., 2016; Zadka et al., 2019), MacGowan et al. conducted the sole multicentre randomized controlled trial. According to the trial results, the introduction of MALDI‐TOF MS service reduced the time to pathogen identification by almost half (38.5 vs. 55.2 h, p < 0.0001); however, it did not reduce the time to effective antibiotics (24 vs. 13 h, p = 0.056) or 28‐day mortality by nearly half (18.5% vs. 17.7%, p = 0.42). This analysis and previous studies have shown that time to effective antibiotics is negatively correlated with mortality. Possibly, the paradoxical increase in time to effective antibiotics in the MALDI‐TOF MS group may account for the null effect on mortality reduction. The authors did not provide an explanation on the paradoxical increase of time to effective antibiotics in the intervention arm, but the presence of antibiotic stewardship teams in both intervention and control groups might have reduced the positive effect of shortened turnaround times for blood culture reports.
Aside from the trial, we think current evidence is sufficient for the promotion of the technology and systems that accelerate the BSI diagnosis and infective pathogen identification processes among patients with severe infections. The message of this meta‐analysis that MALDI‐TOF MS could shorten the hospital stay and reduce the direct medical cost should inform the policy and hospital management decision‐makers about priority and resource allocation. In addition, it is advisable for time to effective antibiotic therapy to be used as an important quality indicator for monitoring the hospital care quality for sepsis. Quality improvement campaign to narrow the time gap between infective pathogen identification and effective antibiotics initiation should also be promoted.
Strengths and limitations
This meta‐analysis had many strengths. To the best of our knowledge, this was the first systematic review on the effectiveness of rapid pathogen identification by MALDI‐TOF MS on both clinical and economical outcomes of BSI. The low heterogeneity of the mortality outcome estimates made the pooled effect estimates more reliable. A formal cost‐effectiveness analysis may be performed using pooled effect estimates from this study to assist with clinical or policy decisions. When planning future studies on the effect of rapid pathogen identification by MALDI‐TOF MS, it would be advisable to adhere to some reporting policies to enable comparison and generalization of results. Controlled interventional study designs with either parallel comparison or before‐and‐after comparison are favourable designs to provide reliable data. Recommended elements for data reporting include time to bacteriology identification, time to effective antibiotic therapy, length of hospital stay, length of ICU stay, 30‐day mortality and longer term mortality. Specific effects for Gram‐negative and Gram‐positive bacteria are also encouraged to report.
Our study had some limitations. The majority of the included studies had a pre–post design without a parallel comparison control group. The trend bias and confounding by time‐varying covariates associated with a pre–post design could not be totally excluded. The systematic improvement after the implementation of a new programme may exaggerate the benefit of a new intervention. Second, although our results of meta‐regression showed ‘time to effective antibiotics’ was the strongest driver of ‘mortality reduction’, the concomitant use of some rapid tools to detect the mechanisms of resistance was not evaluated in this study and should be further investigated in future. Studies have shown that diagnostic tools for rapid detection of resistance mechanisms, including T2 magnetic resonance (T2MR) assay, fluorescence in situ hybridization(FISH) and nucleic acid amplification technology, could have a strong impact on antimicrobial therapy. Third, there was only 1 study performed on children. Therefore, we could not analyse specific estimates for children. Similarly, only two studies provided a complete reporting for the effect on Gram‐positive or Gram‐negative bacterial BSI, while specific effect estimates for different types of pathogens were not possible. Fourth, significant heterogeneity between studies was detected in the continuous outcomes, which restricted our ability and confidence to generalize the results of these pooled effect estimates to all populations. The different discharge policy, operational parameters and charge rate that were not reported in the literature might have contributed to the heterogeneity in time, length of stay and cost measures. In addition, different age groups, different disease severity stages, co‐implementation of antibiotic stewardship and the focus on different bacteria might have also contributed to the heterogeneity. Last but not least, it is true that the standard of care in control groups varies across studies, and the effect of MALDI‐TOF on mortality reduction can only be determined when this standard is defined. However, there were few details on standard care reported in the included literature, making it impossible to define a standard care pattern that would benefit from MALDI‐TOF and antibiotic stewardship. In future studies, it should be encouraged to report the details of standard care protocol in the control groups.
In conclusion, our meta‐analysis showed that rapid pathogen identification by MALDI‐TOF MS in combination with or without antibiotic stewardship can greatly improve the outcome of BSI and may also be a cost‐effective procedure. The effect size of mortality reduction was larger than any prior pharmacological or treatment bundle interventional trial for sepsis patients. A formal multicentre randomized controlled trial is therefore needed to confirm the finding of these pre–post comparison studies. In addition, the significant association between time to effective antibiotic therapy and mortality reduction makes it an important indicator for monitoring the care quality of patients with BSI in a hospital. Overall, MALDI‐TOF is thought to be more effective and cost‐saving than standard practice, although further research is required before its widespread application can be recommended.
AUTHOR CONTRIBUTIONS
C‐HY interpreted the data, wrote the manuscript, and reviewed and edited the manuscript. Y‐HS and ZRC conducted literature reviews, collected data and provided critical feedback. W‐TH conducted literature reviews and statistical analysis, wrote the final draft and provided critical feedback. RAM wrote the final draft and provided critical feedback. WTJL and S‐CC interpreted data and provided critical feedback. C‐CL oversaw research, designed the study, conducted literature review, analysed the data, wrote the first draft and authorized the final manuscript. All authors reviewed and approved the manuscript.
FUNDING INFORMATION
This work was supported by Far Eastern Memorial Hospital, Taiwan (Grant FEMH‐2022‐C‐051). No funding bodies had any role in the study design, data collection and analysis, the decision to publish or the preparation of the manuscript.
CONFLICT OF INTEREST
The authors have no conflict of interests to declare.
ETHICS STATEMENT
Not required.
Supporting information
Appendix S1
Yo, C‐H , Shen, Y‐H , Hsu, W‐T , Mekary, R.A. , Chen, Z.R. & Lee, W‐T. et al. (2022) MALDI‐TOF mass spectrometry rapid pathogen identification and outcomes of patients with bloodstream infection: A systematic review and meta‐analysis. Microbial Biotechnology, 15, 2667–2682. Available from: 10.1111/1751-7915.14124
Contributor Information
Yi‐Hsuan Shen, Email: annie453300@gmail.com.
Chien‐Chang Lee, Email: hit3transparency@gmail.com, Email: cclee100@gmail.com.
Data availability statement
The datasets used in thecurrent study are available from the corresponding author (C‐CL) uponreasonable request.
REFERENCES
- Alghamdi, S. , Atef‐Shebl, N. , Aslanpour, Z. & Berrou, I. (2019) Barriers to implementing antimicrobial stewardship programmes in three Saudi hospitals: evidence from a qualitative study. Journal of global antimicrobial resistance, 18, 284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur, D. , Gladstone, B.P. , Burkert, F. , Carrara, E. , Foschi, F. , Döbele, S. et al. (2017) Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic‐resistant bacteria and Clostridium difficile infection: a systematic review and meta‐analysis. The Lancet Infectious Diseases, 17, 990–1001. [DOI] [PubMed] [Google Scholar]
- Beganovic, M. , Costello, M. & Wieczorkiewicz, S.M. (2017) Effect of matrix‐assisted laser desorption ionization‐time of flight mass spectrometry (MALDI‐TOF MS) alone versus MALDI‐TOF MS combined with real‐time antimicrobial stewardship interventions on time to optimal antimicrobial therapy in patients with positive blood cultures. Journal of Clinical Microbiology, 55, 1437–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg, C.B. & Mazumdar, M. (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics, 50, 1088–1101. [PubMed] [Google Scholar]
- Bessède, E. , Angla‐gre, M. , Delagarde, Y. , Sep Hieng, S. , Ménard, A. & Mégraud, F. (2011) Matrix‐assisted laser‐desorption/ionization BIOTYPER: experience in the routine of a University hospital. Clinical Microbiology and Infection, 17, 533–538. [DOI] [PubMed] [Google Scholar]
- Brunkhorst, F.M. , Oppert, M. , Marx, G. , Bloos, F. , Ludewig, K. , Putensen, C. et al. (2012) Effect of empirical treatment with moxifloxacin and meropenem vs meropenem on sepsis‐related organ dysfunction in patients with severe sepsis: a randomized trial. JAMA, 307, 2390–2399. [DOI] [PubMed] [Google Scholar]
- Campion, M. & Scully, G. (2018) Antibiotic use in the intensive care unit: optimization and de‐escalation. Journal of Intensive Care Medicine, 33, 647–655. [DOI] [PubMed] [Google Scholar]
- Carreno, J.J. , Lomaestro, B.M. , Jacobs, A.L. , Meyer, R.E. , Evans, A. & Montero, C.I. (2016) Assessment of time to clinical response in patients with sepsis treated before and after implementation of a matrix‐assisted laser desorption ionization time‐of‐flight blood culture identification algorithm. Infection Control and Hospital Epidemiology, 37, 916–923. [DOI] [PubMed] [Google Scholar]
- Chang, S.‐S. , Hsieh, W.‐H. , Liu, T.‐S. , Lee, S.‐H. , Wang, C.‐H. , Chou, H.‐C. et al. (2013) Multiplex PCR system for rapid detection of pathogens in patients with presumed sepsis ‐ a systemic review and meta‐analysis. PLoS One, 8, e62323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary, T. , Hohenstein, C. & Bayer, O. (2014) Die goldene stunde der sepsis. Med Klin ‐ Intensivmed Notfallmedizin, 109, 104–108. [DOI] [PubMed] [Google Scholar]
- Clerc, O. , Prod'hom, G. , Vogne, C. , Bizzini, A. , Calandra, T. & Greub, G. (2013) Impact of matrix‐assisted laser desorption ionization time‐of‐flight mass spectrometry on the clinical management of patients with Gram‐negative bacteremia: a prospective observational study. Clin Infect Dis Off Publ Infect Dis Soc Am, 56, 1101–1107. [DOI] [PubMed] [Google Scholar]
- Cochran, W.G. (1950) The comparison of percentages in matched samples. Biometrika, 37, 256–266. [PubMed] [Google Scholar]
- Costelloe, C. , Metcalfe, C. , Lovering, A. , Mant, D. & Hay, A.D. (2010) Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta‐analysis. BMJ, 340, c2096. [DOI] [PubMed] [Google Scholar]
- Delport, J.A. , Strikwerda, A. , Armstrong, A. , Schaus, D. & John, M. (2016) Quality of care is improved by rapid short incubation MALDI‐ToF identification from blood cultures as measured by reduced length of stay and patient outcomes as part of a multi‐disciplinary approach to bacteremia in pediatric patients. PLoS One, 11, e0160618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian, R. & Laird, N. (1986) Meta‐analysis in clinical trials. Controlled Clinical Trials, 7, 177–188. [DOI] [PubMed] [Google Scholar]
- DerSimonian, R. & Laird, N. (2015) Meta‐analysis in clinical trials revisited. Contemporary Clinical Trials, 45, 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekema, D.J. , Lee, K. , Raney, P. , Herwaldt, L.A. , Doern, G.V. & Tenover, F.C. (2004) Accuracy and appropriateness of antimicrobial susceptibility test reporting for bacteria isolated from blood cultures. Journal of Clinical Microbiology, 42, 2258–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, P. , Hollingworth, W. , Pike, K. , Reynolds, R. , Stoddart, M. & MacGowan, A. (2021) Cost‐effectiveness of rapid laboratory‐based mass‐spectrometry diagnosis of bloodstream infection: evidence from the RAPIDO randomised controlled trial. BMJ Open, 11, e044623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domon, B. & Aebersold, R. (2006) Mass spectrometry and protein analysis. Science, 312, 212–217. [DOI] [PubMed] [Google Scholar]
- Egger, M. , Smith, G.D. , Schneider, M. & Minder, C. (1997) Bias in meta‐analysis detected by a simple, graphical test. BMJ, 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faron, M.L. , Buchan, B.W. & Ledeboer, N.A. (2017) Matrix‐assisted laser desorption ionization‐time of flight mass spectrometry for use with positive blood cultures: methodology, performance, and optimization. Journal of Clinical Microbiology, 55, 3328–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridkin, S. , Baggs, J. , Fagan, R. , Magill, S. , Pollack, L.A. , Malpiedi, P. et al. (2014) Vital signs: improving antibiotic use among hospitalized patients. Morbidity and Mortality Weekly Report, 63, 194–200. [PMC free article] [PubMed] [Google Scholar]
- Funk, D.J. & Kumar, A. (2011) Antimicrobial therapy for life‐threatening infections: speed is life. Critical Care Clinics, 27, 53–76. [DOI] [PubMed] [Google Scholar]
- Galbraith, R.F. (1994) Some applications of radial plots. Journal of the American Statistical Association, 89, 1232–1242. [Google Scholar]
- Hecker, M.T. , Aron, D.C. , Patel, N.P. , Lehmann, M.K. & Donskey, C.J. (2003) Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Archives of Internal Medicine, 163, 972–978. [DOI] [PubMed] [Google Scholar]
- Hettick, J.M. , Kashon, M.L. , Simpson, J.P. , Siegel, P.D. , Mazurek, G.H. & Weissman, D.N. (2004) Proteomic profiling of intact mycobacteria by matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry. Analytical Chemistry, 76, 5769–5776. [DOI] [PubMed] [Google Scholar]
- Higgins, J.P.T. & Thompson, S.G. (2002) Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine, 21, 1539–1558. [DOI] [PubMed] [Google Scholar]
- Higgins, J.P.T. , Thompson, S.G. , Deeks, J.J. & Altman, D.G. (2003) Measuring inconsistency in meta‐analyses. BMJ, 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J.P.T. , Thomas, J. , Chandler, J. , Cumpston, M. , Li, T. , Page, M.J. et al. (2019) Cochrane handbook for systematic reviews of interventions, 2nd edition. Chichester, UK: John Wiley & Sons. [Google Scholar]
- Holland, R.D. , Wilkes, J.G. , Rafii, F. , Sutherland, J.B. , Persons, C.C. , Voorhees, K.J. et al. (1996) Rapid identification of intact whole bacteria based on spectral patterns using matrix‐assisted laser desorption/ionization with time‐of‐flight mass spectrometry. Rapid Communications in Mass Spectrometry, 10, 1227–1232. [DOI] [PubMed] [Google Scholar]
- Huang, A.M. , Newton, D. , Kunapuli, A. , Gandhi, T.N. , Washer, L.L. , Isip, J. et al. (2013) Impact of rapid organism identification via matrix‐assisted laser desorption/ionization time‐of‐flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clinical Infectious Diseases, 57, 1237–1245. [DOI] [PubMed] [Google Scholar]
- Ioannidis, J.P.A. , Patsopoulos, N.A. & Evangelou, E. (2007) Uncertainty in heterogeneity estimates in meta‐analyses. BMJ, 335, 914–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon, Y.D. , Seong, H. , Kim, D. , Ahn, M.Y. , Jung, I.Y. , Jeong, S.J. et al. (2018) Impact of matrix‐assisted laser desorption/ionization time of flight mass spectrometric evaluation on the clinical outcomes of patients with bacteremia and fungemia in clinical settings lacking an antimicrobial stewardship program: a pre‐post quasi experimental study. BMC Infectious Diseases, 18, 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanika, S. , Paudel, S. , Grigoras, C. , Kalbasi, A. & Mylonakis, E. (2016) Systematic review and meta‐analysis of clinical and economic outcomes from the implementation of hospital‐based antimicrobial stewardship programs. Antimicrobial Agents and Chemotherapy, 60, 4840–4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn, T.J. & Weinstein, M.P. (2013) Update on blood cultures: how to obtain, process, report, and interpret. Clinical Microbiology and Infection, 19, 513–520. [DOI] [PubMed] [Google Scholar]
- Kostrzewa, M. , Sparbier, K. , Maier, T. & Schubert, S. (2013) MALDI‐TOF MS: an upcoming tool for rapid detection of antibiotic resistance in microorganisms. Proteomics Clinical Applications, 7, 767–778. [DOI] [PubMed] [Google Scholar]
- Kumar, A. , Roberts, D. , Wood, K.E. , Light, B. , Parrillo, J.E. , Sharma, S. et al. (2006) Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Critical Care Medicine, 34, 1589–1596. [DOI] [PubMed] [Google Scholar]
- La Scola, B. & Raoult, D. (2009) Direct identification of bacteria in positive blood culture bottles by matrix‐assisted laser desorption ionisation time‐of‐flight mass spectrometry. PLoS One, 4, e8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laupland, K.B. , Gregson, D.B. , Flemons, W.W. , Hawkins, D. , Ross, T. & Church, D.L. (2007) Burden of community‐onset bloodstream infection: a population‐based assessment. Epidemiology and Infection, 135, 1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo, C.K.‐F. , Mertz, D. , Yamamura, D. & Loeb, M. (2020) Assessing impact of MALDI mass spectroscopy on reducing directed antibiotic coverage time for Gram‐negative organisms. PLoS One, 15, e0228935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood, A.M. , Perez, K.K. , Musick, W.L. , Ikwuagwu, J.O. , Attia, E. , Fasoranti, O.O. et al. (2016) Integrating rapid diagnostics and antimicrobial stewardship in two community hospitals improved process measures and antibiotic adjustment time. Infection Control and Hospital Epidemiology, 37, 425–432. [DOI] [PubMed] [Google Scholar]
- López‐Pintor, J.M. , Sánchez‐López, J. , Navarro‐San Francisco, C. , Sánchez‐Díaz, A.M. , Loza, E. & Cantón, R. (2021) Real life clinical impact of antimicrobial stewardship actions on the blood culture workflow from a microbiology laboratory. Antibiotics, 10, 1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall, C. , Powell, J.P. , Johnson, C.K. , Edmond, M.B. & Polk, R.E. (2005) Hospital and community fluoroquinolone use and resistance in Staphylococcus aureus and Escherichia coli in 17 US hospitals. Clinical Infectious Diseases, 41, 435–440. [DOI] [PubMed] [Google Scholar]
- MacGowan, A. , Grier, S. , Stoddart, M. , Reynolds, R. , Rogers, C. , Pike, K. et al. (2020) Impact of rapid microbial identification on clinical outcomes in bloodstream infection: the RAPIDO randomized trial. Clinical Microbiology and Infection, 26, 1347–1354. [DOI] [PubMed] [Google Scholar]
- Manukumar, H.M. & Umesha, S. (2017) MALDI‐TOF‐MS based identification and molecular characterization of food associated methicillin‐resistant Staphylococcus aureus . Scientific Reports, 7, 11414–11416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklein, G. , Josten, M. , Klanke, U. , Müller, E. , Horré, R. , Maier, T. et al. (2009) Matrix‐assisted laser desorption ionization‐time of flight mass spectrometry for fast and reliable identification of clinical yeast isolates. Journal of Clinical Microbiology, 47, 2912–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan, K.L. , Foster, J.A. & Coffin, S.E. (2000) Outpatient pediatric blood cultures: time to positivity. Pediatrics, 106, 251–255. [DOI] [PubMed] [Google Scholar]
- McNulty, C.A.M. , Lasseter, G.M. , Charlett, A. , Lovering, A. , Howell‐Jones, R. , Macgowan, A. et al. (2011) Does laboratory antibiotic susceptibility reporting influence primary care prescribing in urinary tract infection and other infections? The Journal of Antimicrobial Chemotherapy, 66, 1396–1404. [DOI] [PubMed] [Google Scholar]
- Mok, J. , Jo, E.‐J. , Eom, J.S. , Kim, M.‐H. , Kim, K.U. , Park, H.‐K. et al. (2019) Clinical efficacy of matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry in patients with multidrug‐resistant bacteremia: a single‐center study in Korea. The Korean Journal of Internal Medicine, 34, 1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel, J.L. , Huang, A.M. , Kunapuli, A. , Gandhi, T.N. , Washer, L.L. , Lassiter, J. et al. (2014) Impact of antimicrobial stewardship intervention on coagulase‐negative Staphylococcus blood cultures in conjunction with rapid diagnostic testing. Journal of Clinical Microbiology, 52, 2849–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (2014). Quality Assessment Tool for Before‐After (Pre‐Post) Studies With No Control Group. Available online at: https://www.nhlbi.nih.gov/health‐topics/study‐quality‐assessment‐tools [Accessed 28 November 2019].
- Niwa, T. , Yonetamari, J. , Hayama, N. , Fujibayashi, A. , Ito‐Takeichi, S. , Suzuki, K. et al. (2019) Clinical impact of matrix‐assisted laser desorption ionization‐time of flight mass spectrometry combined with antimicrobial stewardship interventions in patients with bloodstream infections in a Japanese tertiary hospital. International Journal of Clinical Practice, 73, e13332. [DOI] [PubMed] [Google Scholar]
- Osthoff, M. , Gürtler, N. , Bassetti, S. , Balestra, G. , Marsch, S. , Pargger, H. et al. (2017) Impact of MALDI‐TOF‐MS‐based identification directly from positive blood cultures on patient management: a controlled clinical trial. Clinical Microbiology and Infection Off Publ Eur Soc Clin Microbiol Infect Dis, 23, 78–85. [DOI] [PubMed] [Google Scholar]
- Page, M.J. , Moher, D. , Bossuyt, P.M. , Boutron, I. , Hoffmann, T.C. , Mulrow, C.D. et al. (2021) PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ, 372, n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, G. , Huprikar, S. , Factor, S.H. , Jenkins, S.G. & Calfee, D.P. (2008) Outcomes of carbapenem‐resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infection Control and Hospital Epidemiology, 29, 1099–1106. [DOI] [PubMed] [Google Scholar]
- Perez, K.K. , Olsen, R.J. , Musick, W.L. , Cernoch, P.L. , Davis, J.R. , Land, G.A. et al. (2013) Integrating rapid pathogen identification and antimicrobial stewardship significantly decreases hospital costs. Archives of Pathology & Laboratory Medicine, 137, 1247–1254. [DOI] [PubMed] [Google Scholar]
- Perez, K.K. , Olsen, R.J. , Musick, W.L. , Cernoch, P.L. , Davis, J.R. , Peterson, L.E. et al. (2014) Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic‐resistant Gram‐negative bacteremia. The Journal of Infection, 69, 216–225. [DOI] [PubMed] [Google Scholar]
- Peters, R.P. , van Agtmael, M.A. , Danner, S.A. , Savelkoul, P.H. & Vandenbroucke‐Grauls, C.M. (2004) New developments in the diagnosis of bloodstream infections. The Lancet Infectious Diseases, 4(12), 751–760. [DOI] [PubMed] [Google Scholar]
- Peterson, L.R. , Hamilton, J.D. , Baron, E.J. , Tompkins, L.S. , Miller, J.M. , Wilfert, C.M. et al. (2001) Role of clinical microbiology laboratories in the management and control of infectious diseases and the delivery of health care. Clin Infect Dis Off Publ Infect Dis Soc Am, 32, 605–611. [DOI] [PubMed] [Google Scholar]
- Puckett, L.M. , Rajkotia, P. , Coppola, L. , Baumgartner, L. , Roberts, A.L. , Maldonado, Y. et al. (2021) Impact of direct from blood culture identification of pathogens paired with antimicrobial stewardship interventions in a pediatric hospital. Journal of Pediatric Pharmacology and Therapeutics, 26, 802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz, L. , Lowe, T.J. , Srinivasan, A. , Neilson, D. & Pugliese, G. (2014) Economic impact of redundant antimicrobial therapy in US hospitals. Infection Control and Hospital Epidemiology, 35, 1229–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuts, E.C. , Hulscher, M.E.J.L. , Mouton, J.W. , Verduin, C.M. , Stuart, J.W.T.C. , Overdiek, H.W.P.M. et al. (2016) Current evidence on hospital antimicrobial stewardship objectives: a systematic review and meta‐analysis. The Lancet Infectious Diseases, 16, 847–856. [DOI] [PubMed] [Google Scholar]
- Seymour, C.W. , Gesten, F. , Prescott, H.C. , Friedrich, M.E. , Iwashyna, T.J. , Phillips, G.S. et al. (2017) Time to treatment and mortality during mandated emergency care for sepsis. The New England Journal of Medicine, 376, 2235–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamoto, Y. , Araie, H. , Itoh, K. , Shigemi, H. , Yamauchi, T. & Iwasaki, H. (2021) MALDI‐TOFMS‐oriented early definitive therapy improves the optimal use of antibiotics for Enterococcus spp. bloodstream infection. Journal of Infection and Chemotherapy, 27, 393–396. [DOI] [PubMed] [Google Scholar]
- Stuck, A.E. , Rubenstein, L.Z. & Wieland, D. (1998) Bias in meta‐analysis detected by a simple, graphical test. Asymmetry detected in funnel plot was probably due to true heterogeneity. BMJ, 316, 469 author reply 470‐471. [PMC free article] [PubMed] [Google Scholar]
- Suffredini, A.F. & Munford, R.S. (2011) Novel therapies for septic shock over the past 4 decades. JAMA, 306, 194–199. [DOI] [PubMed] [Google Scholar]
- Swaan, C. , van den Broek, A. , Kretzschmar, M. & Richardus, J.H. (2018) Timeliness of notification systems for infectious diseases: a systematic literature review. PLoS One, 13(6), e0198845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbrook, T.T. , Morton, J.B. , McConeghy, K.W. , Caffrey, A.R. , Mylonakis, E. & LaPlante, K.L. (2017) The effect of molecular rapid diagnostic testing on clinical outcomes in bloodstream infections: a systematic review and meta‐analysis. Clinical Infectious Diseases, 64, 15–23. [DOI] [PubMed] [Google Scholar]
- Vlek, A.L.M. , Bonten, M.J.M. & Boel, C.H.E. (2012) Direct matrix‐assisted laser desorption ionization time‐of‐flight mass spectrometry improves appropriateness of antibiotic treatment of bacteremia. PLoS One, 7, e32589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora, N.M. , Kubin, C.J. & Furuya, E.Y. (2015) Appropriateness of gram‐negative agent use at a tertiary care hospital in the setting of significant antimicrobial resistance. Open Forum Infectious Diseases, 2, ofv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzler, E. , Goff, D.A. , Mangino, J.E. , Reed, E.E. , Wehr, A. & Bauer, K.A. (2016) Impact of rapid identification of Acinetobacter Baumannii via matrix‐assisted laser desorption ionization time‐of‐flight mass spectrometry combined with antimicrobial stewardship in patients with pneumonia and/or bacteremia. Diagnostic Microbiology and Infectious Disease, 84, 63–68. [DOI] [PubMed] [Google Scholar]
- Willemsen, I. , Groenhuijzen, A. , Bogaers, D. , Stuurman, A. , van Keulen, P. & Kluytmans, J. (2007) Appropriateness of antimicrobial therapy measured by repeated prevalence surveys. Antimicrobial Agents and Chemotherapy, 51, 864–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadka, H. , Raykhshtat, E. , Uralev, B. , Bishouty, N. , Weiss‐Meilik, A. & Adler, A. (2019) The implementation of rapid microbial identification via MALDI‐ToF reduces mortality in gram‐negative but not gram‐positive bacteremia. European Journal of Clinical Microbiology & Infectious Diseases, 38, 2053–2059. [DOI] [PubMed] [Google Scholar]
- Zadroga, R. , Williams, D.N. , Gottschall, R. , Hanson, K. , Nordberg, V. , Deike, M. et al. (2013) Comparison of 2 blood culture media shows significant differences in bacterial recovery for patients on antimicrobial therapy. Clinical Infectious Diseases, 56, 790–797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The datasets used in thecurrent study are available from the corresponding author (C‐CL) uponreasonable request.


