ABSTRACT
Recently, small extracellular vesicles (sEVs) secreted in vivo from chronic lymphocytic leukemia (CLL) preclinical murine models were characterized. Leukemia microenvironment sEV (LME-sEVs) selectively target CD8+ T-cells, inducing exhaustion and hampering anti-tumor immune response. Additionally, a sEV-related gene expression correlated with patient treatment-free survival, overall survival and clinical parameters.
KEYWORDS: Small extracellular vesicles, exosomes, CLL, tumor microenvironment, tumor immune escape, sEV-based biomarkers
With a size between 30 and 150 nm, small extracellular vesicles (sEVs) are involved in multiple hematological malignancies by supporting tumor development and progression through direct interaction with tumor cells, as well as by reshaping composition and behavior of the surrounding microenvironment, including immune cells.1 Chronic lymphocytic leukemia (CLL), the most common leukemia in western countries, is a perfect example of microenvironment signal addiction.2 Indeed, CLL progression is dependent on tight interactions with the highly immune-suppressed microenvironment. In this instance, we have previously shown that sEV components confer direct proliferative and survival advantages to CLL cells. In addition, conversion of bone marrow mesenchymal stem cells (BM-MSCs) into cancer-associated fibroblast (CAF)-like cells, through miRNA contained in CLL-derived sEVs (miR-150, -155 and -146a), further supports CLL progression due to cytokine and proangiogenic factor release. Concerning immuno-modulation, CLL sEV-derived miR-155 drives monocyte conversion into myeloid-derived suppressor cells (MDSCs), increasing CLL migration, Tregs and MDSCs recruitment and reducing CD8+ T-cell proliferation.3 Similarly, we also showed that the CLL-derived sEV Y RNA hY4 mediates pro-tumorigenic phenotypes in monocytes by upregulating PD-L1 at the surface and increasing the release of pro-inflammatory cytokines. Smallwood and colleagues demonstrated that CLL sEV-derived miR-363 enhances CLL growth and survival by increasing CD4+ T-cell migration and interaction with leukemia cells.4 Finally, it was recently shown that CLL-derived sEVs alter CD4+ and CD8+ T-cell leading to an expansion of regulatory T-cell subsets and exhaustion, respectively.5
Despite the increasing amount of information acquired in the last decade, our and other studies on the subject were mainly achieved using in vitro culture systems with cell lines or primary cells,6 like the vast majority of the literature on sEVs, raising the question of the true sEV relevance in vivo. To illustrate this idea, a recent review published in Science describing the biology, function and biomedical applications of sEVs – especially in cancer – highlighted that experimental setup using murine models and more physiological conditions are needed in order to fully understand the role of sEVs in vivo. Indeed, it currently remains unclear whether more closely physiological levels of sEVs have actual regulatory or pathological functions in vivo.7
Small EV release and composition are dynamic and input-dependent processes,8 highly influenced by microenvironment characteristics and extracellular stimuli. Thus, with the aim to uncover the complexity and role of sEVs in leukemia progression in vivo, we developed a protocol to isolate and purify sEVs directly from the leukemic microenvironment (LME-sEVs) of the widely used pre-clinical CLL murine model (Eµ-TCL1).9 For the first time, we characterized the totality of the sEVs released in the leukemia microenvironment, derived from CLL and surrounding cells alike. Compared with sEVs isolated from healthy controls (HCME-sEVs), LME-sEVs contain specific miRNAs and proteins, and display multiple immune checkpoint ligand combinations on their surface, playing a key role in CLL development. In this setup, LME-sEVs rapidly and selectively target CD8+ T-cells in vivo, leading to profound transcriptomic, proteomic and metabolic changes. This translates into an efficient sEV-mediated immune escape and into disease progression (Figure 1(a)). Indeed, when sEV release is genetically impaired in CLL (TCL1-RAB27DKO model), immune evasion fails to occur and the disease progression rapidly breaks off.9 On the other hand, when LME-sEVs are reintroduced in the same experimental setup, CLL progression is restored leading to full leukemic development (Figure 1(b)). Interestingly, CLL immune clearance appears to be majorly performed by CD8+ T-cells. In fact, when TCL1-RAB27DKO cells are transferred in CD8+ T-cell-depleted mice, they successfully recapitulate the disease (Figure 1(c)). At the same time, injection of CD8+ T-cells treated ex vivo with LME-sEVs reduced T lymphocytes ability to control disease progression in vivo (Figure 1(c)). These data strongly highlight how CLL requires to downregulate CD8+ T-cells, rapidly establishing an immune suppressive microenvironment from the onset of the disease development.
Figure 1.
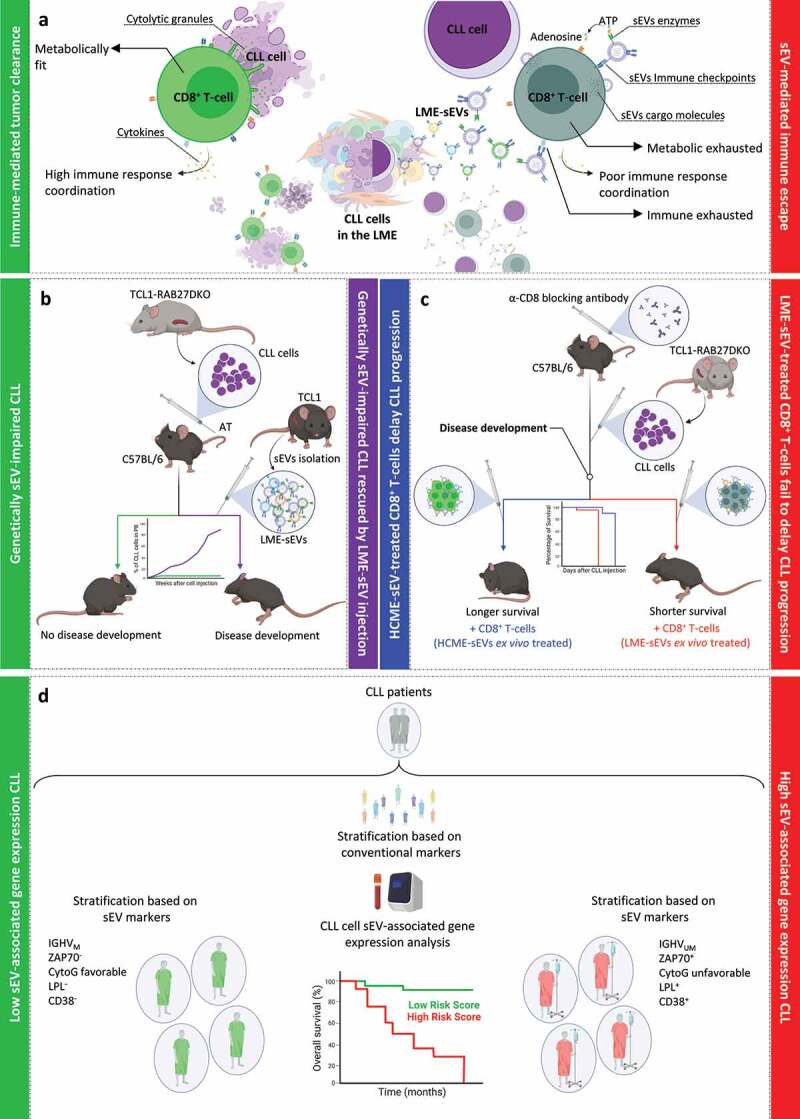
Small EV-mediated immune escape in CLL microenvironment supports leukemia growth. (a) In the absence of LME-sEVs (left side), CD8+ T-cells rapidly interact with CLL cells fully eliminating them. In this state, CD8+ T-cells are metabolically fit, release cytotoxic granules directly to the target and signal with cytokines to other immune cells. On the other hand, in the presence of LME-sEVs (right side), CD8+ T-cell activity is affected by multiple sEV components, including, immune checkpoint interaction, and transfer of proteins and miRNAs. In this instance, CD8+ T-cells are metabolically exhausted, unable to properly release cytotoxic granules and show a reduced cytokine polyfunctionality. (b) Adoptive transfer (AT) of genetically sEV-impaired CLL (TCL1-RAB27DKO) cells into immunocompetent mice triggers CD8+ T-cell-mediated tumor clearance, leading to complete eradication of the tumor clone (green line). Injection of leukemia microenvironment (LME) sEVs, isolated from leukemic mice (TCL1), leads to rescue of the disease development (violet line). (c) Depletion of CD8+ T-cells using neutralizing antibodies allows TCL1-RAB27DKO cells to recapitulate the disease in immunocompetent mice. During the disease development, injection of either HCME- or LME-sEV-treated CD8+ T-cells influences mice survival. (d) Analysis of sEV-related gene expression in CLL patient cells proved useful as prognosis biomarker. Combined expression of multiple sEV-related genes segregated CLL subgroups typically characterized by unfavorable prognosis markers. CytoG: cytogenetic factors.
Small EVs represent a complex delivery system where multiple cargo and surface components can affect target cells in different degrees. In our setup in particular, LME-sEVs are highly heterogeneous, given the wide range of cells releasing them. For these reasons, we hypothesized that the effect on CD8+ T-cells must have been mediated by multiple sEV effector molecules. Overall, LME-sEVs show a plethora of strategies to damp effector T-cell activity, including, but not limited to, the transfer of both conventional (e.g. PD-L1 and GAL-9) and metabolic (IL4I1) immune checkpoints, and typical CLL EV-associated miRNAs (e.g. miR-155 and -150). These factors, together with other molecules such as the ectonucleotidases CD39 and CD73, strongly altered CD8+ T-cell cytotoxicity, metabolism and proliferation ex vivo and in vivo. In particular, CD8+ T-cells appeared to be highly exhausted (increased PD1, TIM3, LAG3 and ICOS expression), functionally impaired (reduced GzmB and perforin-1 production), unable to orchestrate an appropriate immune response (reduced IL-2, IFN-γ and increased TNF-α production) and have an impaired proliferation due to the increased levels of adenosine and reduced pentose phosphate pathway activity.9
Interestingly, we achieved a major rescue of the CD8+ T-cell immune phenotype by targeting multiple LME-sEV components. Specifically, sEV transfection with a mix of anti-miR-150, -155 and -378a antagomiRs, but not with single antagomiRs, showed a normalization of GzmB and perforin-1 production. Finally, incubation of sEVs with blocking antibodies against PD-L1, GAL9, VISTA and MHC-II reduced PD1, TIM3 and LAG3 expression, and restored perforin-1 level (but not GzmB) in treated CD8+ T-cells.9
Given the striking importance of sEVs in CLL pathogenesis and immune escape, we analyzed the expression of selected sEV-related genes (e.g. RAB27a and PDCD6IP) in a large cohort of 144 CLL patients, and identified gene signatures correlating with treatment-free survival, overall survival, and with clinical parameters routinely used in CLL for diagnosis and prognosis (Figure 1(c)). In particular, our results demonstrated that high expression of sEV-related genes in CLL cells correlates with poor prognosis and reduced overall survival for patients.9
Overall, our data demonstrate the importance of sEVs in CLL progression in vivo, highlighting the role of various sEV components and interactions with the target cells. Indeed, by altering the transfer or blocking only one of these elements, we failed to rescue the phenotype, indicating that multiple components should be inhibited at once. These data, together with the possible use of sEV-related genes as prognostic markers, could lead to the use of sEVs as biomarkers and therapeutic targets in CLL and, potentially, other B-cell malignancies.9,10
Funding Statement
This work was supported by grants from the Luxembourg National Research Fund (FNR) and Fondation Cancer, Luxembourg to EG, EM and JP (PRIDE15/10675146/CANBIO, INTER/DFG/16/11509946, C20/BM/14582635 and C20/BM/14592342) and from Fonds National de la Recherche Scientifique (FNRS)-Télévie to EV (7.4509.20).
Disclosure statement
The authors declare no conflict of interest.
Author contributions
E.G., E.V., J.P. and E.M. contributed to the writing of the manuscript and the conception of the figures.
References
- 1.Silvestri G, Trotta R, Stramucci L, Ellis JJ, Harb JG, Neviani P, Wang S, Eisfeld A-K, Walker CJ, Zhang B, et al. Persistence of drug-resistant leukemic stem cells and impaired NK cell immunity in CML patients depend on MIR300 antiproliferative and PP2A-activating functions. Blood Cancer Discov. 2020;1(1):48–3. doi: 10.1158/0008-5472.BCD-19-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caligaris-Cappio F, Bertilaccio MT, Scielzo C.. How the microenvironment wires the natural history of chronic lymphocytic leukemia. Semin Cancer Biol. 2014;24:43–48. doi: 10.1016/j.semcancer.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Bruns H, Bottcher M, Qorraj M, Fabri M, Jitschin S, Dindorf J, Busch L, Jitschin R, Mackensen A, Mougiakakos D, et al. CLL-cell-mediated MDSC induction by exosomal miR-155 transfer is disrupted by vitamin D. Leukemia. 2017;31(4):985–988. doi: 10.1038/leu.2016.378. [DOI] [PubMed] [Google Scholar]
- 4.Smallwood DT, Apollonio B, Willimott S, Lezina L, Alharthi A, Ambrose AR, De Rossi G, Ramsay AG, Wagner SD. Extracellular vesicles released by CD40/IL-4-stimulated CLL cells confer altered functional properties to CD4+ T cells. Blood. 2016;128(4):542–552. doi: 10.1182/blood-2015-11-682377. [DOI] [PubMed] [Google Scholar]
- 5.Böttcher M, Böttcher-Loschinski R, Kahlfuss S, Aigner M, Gießl A, Mackensen A, Schlötzer-Schrehardt U, Tüting T, Bruns H, Mougiakakos D, et al. CLL-derived extracellular vesicles impair T-cell activation and foster T-cell exhaustion via multiple immunological checkpoints. Cells. 2022;11(14):2176. doi: 10.3390/cells11142176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wierz M, Pierson S, Gargiulo E, Guerin C, Moussay E, Paggetti J. Purification of leukemia-derived exosomes to study microenvironment modulation. Methods Mol Biol. 2019;1884:231–245. [DOI] [PubMed] [Google Scholar]
- 7.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478). doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmulli R, van Niel G. To be or not to be … secreted as exosomes, a balance finely tuned by the mechanisms of biogenesis. Essays Biochem. 2018;62(2):177–191. doi: 10.1042/EBC20170076. [DOI] [PubMed] [Google Scholar]
- 9.Gargiulo E, Viry E, Morande PE, Largeot A, Gonder S, Xian F, Ioannou N, Benzarti M, Kleine Borgmann FB, Mittelbronn M, et al. Extracellular vesicle secretion by leukemia cells in vivo promotes CLL progression by hampering antitumor T-cell responses. Blood Cancer Discovery. 2022. doi: 10.1158/2643-3230.BCD-22-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gargiulo E, Morande PE, Largeot A, Moussay E, Paggetti J. Diagnostic and therapeutic potential of extracellular vesicles in B-cell malignancies. Front Oncol. 2020;10:580874. doi: 10.3389/fonc.2020.580874. [DOI] [PMC free article] [PubMed] [Google Scholar]


