ABSTRACT
Acute kidney injury (AKI) occurs in ~20% of patients receiving immune checkpoint inhibitor (ICI) therapy; however, only 2–5% will develop ICI-mediated immune nephritis. Conventional tests are nonspecific in diagnosing disease pathology and invasive procedures (i.e. kidney biopsy) may not be feasible. In other autoimmune renal diseases, urinary immune cells correlated with the pathology or were predictive of disease activity. Corresponding evidence and analysis are absent for ICI-mediated immune nephritis. We report the first investigation analyzing immune cell profiles of matched kidney biopsies and urine of patients with ICI-AKI. We demonstrated the presence of urinary T cells in patients with immune nephritis by flow cytometry analysis. Clonotype analysis of T cell receptor (TCR) sequences confirmed enrichment of kidney TCRs in urine. As ICI therapies become standard of care for more cancers, noninvasively assessing urinary immune cells of ICI therapy recipients can facilitate clinical management and an opportunity to tailor ICI-nephritis treatment.
KEYWORDS: Immune checkpoint inhibitor, immune-related adverse event, acute kidney injury, acute interstitial nephritis, immune nephritis, urinary immune cells, T cell receptor clonotype
1. Introduction
Immune checkpoint inhibitor (ICI) therapy can be a highly effective cancer treatment option, but its use is often limited by the development of autoimmune side effects targeting normal tissues, termed immune-related adverse events (irAEs). While the frequency of acute kidney injury (AKI) in patients receiving ICI therapy is over 15%, immune nephritis is observed in 2–5% of the patients experiencing ICI-associated irAEs.1,2 The most common pathology associated with immune nephritis is acute interstitial nephritis (AIN), an inflammatory renal lesion characterized by a T-lymphocytic tubulointerstitial infiltrates.2 However, other autoimmune pathologies such as glomerulonephritis (GN) and vasculitis can also develop.3 Unlike patients suffering from AKI mediated by other causes, those with ICI-AKI (i.e. AIN, GN, and vasculitis) directly benefit from immune suppressive therapies.4 Differentiating ICI-associated immune nephritis from other causes of AKI (e.g. dehydration, obstruction, sepsis, etc.) herein referred to as non-ICI AKI can be complicated since conventional blood and urine tests are nonspecific in identifying AKI etiology. The risk of major bleeding associated with kidney biopsy in cancer patients further poses a challenge due to elevated risk of morbidity and mortality.5–8 A means to noninvasively screen for ICI-immune nephritis will enable timely diagnosis, improve patient management, and obviate complication risks from invasive diagnostic procedures (i.e. kidney biopsy).
In patients with healthy kidneys, T lymphocytes are not routinely detected in the urine; when detected, the presence of urinary T lymphocytes has been associated with immune mediated kidney diseases such as lupus nephritis, acute T cell mediated rejection in kidney transplant, and glomerulonephritis.9–11 In such reports, the urinary immune cells correlated with the disease pathology or were found to be predictive of disease activity.9–11 For ICI-associated immune nephritis, corresponding evidence and analysis is absent because studies specifically aimed at understanding the underlying mechanism are limited and the majority of the data evaluating ICI-associated AKI including immune nephritis are largely based on retrospective epidemiological investigations.4,12 We postulated that similar to lupus nephritis, cases of ICI-associated immune nephritis may yield urinary T cells commensurate with renal pathology.
In this study, we present five patients who developed AKI on ICI therapy and underwent a diagnostic kidney biopsy. Paired urine specimens were obtained for all five cases, and one paired blood sample was also collected. An abundance of urinary T cells was present in all cases of ICI-immune nephritis, whereas sparse T cells were isolated in the non-ICI AKI case. Clonotype analysis of the T cells isolated from the ICI-immune nephritis urine specimen matched the clonotype of the T cells in the paired kidney biopsy specimen. These observations have broad diagnostic and prognostic implications for patients receiving ICI therapy who develop acute renal dysfunction.
2. Methods
2.a. Study design and participants
We obtained urine, blood and residual kidney tissue biopsy specimens in adult patients who developed AKI after receiving ICI therapy. All urine specimens were obtained prior to kidney biopsy. All patients underwent a clinically indicated kidney biopsy that was evaluated by a renal pathologist to establish a pathological diagnosis. Residual biopsy specimen was used for analysis. Only one patient was able to undergo blood collection due to the delay in z-code activation for billing. All patients received either programmed cell death protein 1 (PD-1) inhibitors: pembrolizumab or nivolumab, programmed death-ligand 1 (PD-L1) inhibitors: durvalumab or combined PD-1 with cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors: ipilimumab. This study was approved by the MD Anderson Cancer Center Institutional Review Board in accordance with the Declaration of Helsinki under approval number LAB03-0320 and PA19-0084. All participants provided written informed consent.
2.b. Histochemistry
FFPE kidney biopsy tissue (4 μm sections) were deparaffinized in xylene, and rehydrated through a graded alcohol series. Sections were then stained with Mayer’s Hematoxylin, rinsed with water and counterstained with Eosin. This was followed by dehydration through a graded alcohol series, cleared with xylene and mounting of coverslips.
2.c. Multiplex immunofluorescence staining and image analysis
Multiplex immunofluorescence (mIF) staining was performed as previously described.13–15 Briefly, FFPE sections were stained using a mIF panel containing antibodies against: CD3 (clone D7A6E, Cell Signaling Technology), CD8 (clone C8/144B, Thermo Fisher Scientific), CD4 (clone EPR6855, Abcam), and CD20 (Clone L26, DAKO) and their respective fluorophore in the Opal 7 kit (Akoya Biosciences, Waltham, MA).16 The slides were scanned using the Vectra/Polaris 3.0.3 (Akoya Biosciences), and 8 regions of interest (ROIs) were selected by a pathologist. Density of cell phenotypes expressed as cells/mm2 were quantified using InForm 2.6.0 image analysis software (Akoya Biosciences). The final results are expressed as cell densities from the total area analyzed (n/mm2). All the data was consolidated using R studio 3.5.3 (Phenopter 0.2.2 packet, Akoya Biosciences).
2.d. Isolation of urine and peripheral blood mononuclear cells
Single void urine samples were processed to isolate mononuclear cells within a 2-h window of collection. Ficoll-Histopaque (Sigma-Aldrich, St. Louis, MO) density gradient centrifugation method was used to isolate mononuclear cells. A fraction of urinary cells was stained for flow cytometric analysis cells and the remaining cells were used for genomic DNA extraction for TCR analysis. Cells isolated from peripheral blood were also used for TCR analysis.
2.e. Flow cytometric analysis of lymphocytes in urine
A cocktail of anti-human mAbs (Biolegend, San Diego, CA) containing anti-CD3 (Pacific Blue, 1:50), anti-CD4 (PE, 1:100), anti-CD8 (APC-Cy7, 1:100) and anti-CD19 (PE-Cy5.5, 1:100) was used for phenotyping of mononuclear cells isolated from urine. Freshly isolated cells (2,500–50,000) were stained with the cocktail of mAbs. SYTOX™ Green Dead Cell Stain (Life Technologies, Thermo Fisher Scientific, Waltham, MA) was used for exclusion of dead cells. Stained samples were acquired on Novocyte Flow Cytometer (Agilent, Santa Clara, CA) and data were analyzed using FlowJo (version 10.7.1) software (BD Biosciences, Ashland, OR). For appropriate gating strategy, normal donor PBMC simultaneously stained with the same antibody cocktail were used as controls.
2.f. DNA extraction and T cell receptor (TCR) sequencing
Genomic DNA was extracted from FFPE tissue and mononuclear cells using DNeasy Blood & Tissue Kit (Qiagen GmbH, Hilden, Germany). Survey level sequencing of TCRβ CDR3 regions was performed by Adaptive Biotechnologies Corp. ImmunoSEQ assay (Adaptive Biotechnologies, Seattle, WA). Genomic DNA was amplified, followed by high-throughput sequencing. The absolute abundance of each unique TCR-β CDR3 region was analyzed. The fraction of T cells was calculated by normalizing TCR-β template counts to the total amount of DNA usable for sequencing.17
2.g. Data sources
Data on patient demographics, comorbidities, medications, cancer type and stage and laboratory test results were obtained through the electronic health record system. Events were graded according to the AKI guidelines using Kidney Disease: Improving Global Outcomes (KDIGO) classification.18
3. Results
3.a. Clinical characteristics of the cases
We evaluated five patients who developed AKI while receiving ICI therapy. Table 1 summarizes the demographic and clinical characteristics of the patients. All five patients had Grade 2 or 3 AKI at the time of kidney biopsy. Cases 1–4 had biopsy proven immune mediated renal lesions associated with ICI therapy. ICI-associated AIN was observed with Cases 1, 2 and 3; Case 4 had ICI-associated granulomatous necrotizing vasculitis without glomerular involvement; Case 5 had non-ICI AKI, where renal pathology revealed hypertensive (HTN) nephrosclerosis. Three of the five patients (Case 2–4) were diagnosed with metastatic malignant melanoma and received combination therapy with ipilimumab with nivolumab. Case 1 was diagnosed with squamous cell carcinoma of the lung and received adjuvant therapy with durvalumab. Case 5 was diagnosed with squamous cell carcinoma of the oropharynx and received pembrolizumab. Urinalysis (UA) results from the ICI-mediated immune nephritis cases (Cases 1–4) were positive for the presence of WBCs except for Case 3 (S. Table 1). The UA for Case 3 was negative for both WBCs and RBCs. All five cases had a urine protein-to-creatinine ratio of less than 1 g/g.
Table 1.
Clinical characteristics of the patients on immune checkpoint inhibitor therapy who developed acute kidney injury.
| Case Ref. |
Malignancy Age/sex |
Co-morbidities | non-ICI drugs associated with AIN | ICI therapy Number of ICI cycles |
Other IRAEs | AKI: Cr change* AKI stage† |
UA UPCR |
Renal pathology IFTA% GS% |
Cancer Response |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Stage 3c Squamous cell ca left lung 77/M |
HTN COPD Afib CAD/CABG CKD 4 |
Pantoprazole | Durvalumab 3 cycles |
Pneumonitis | Cr 2.45 > 8.56 AKI stage 3 |
6–10 RBC/HPF 21–50 WBC/HPF 0.31 g/g |
AIN IFTA 40% GS 56% |
PFS 20 months |
| Case 2 | Stage 4 Malignant Melanoma 48/M |
None | None | Ipilimumab, Nivolumab 3 cycles |
Rash Colitis |
Cr 0.91 > 3.16 AKI stage 3 |
19 WBC/HPF 4 RBC/HPF 0.58 g/g |
AIN IFTA 10% GS 6% |
No evidence of disease |
| Case 3 | Stage Ib Malignant Melanoma of esophagus 77/M |
Adenoca esophagus HTN T2DM Hypothyroidism |
Omeprazole | Ipilimumab, Nivolumab 7 cycles |
Colitis | Cr 1.07 > 2.43 AKI stage 2 |
0 RBC/HPF 0 WBC/HPF 0.42 g/g |
AIN IFTA 10% GS 16% |
No evidence of disease |
| Case 4 | Stage 4 Uveal Melanoma 70 F |
T2DM Afib CVA |
Omeprazole | Ipilimumab + Nivolumab 2 cycles |
Dermatitis Hypothyroid Hypophysitis Colitis |
Cr 1.45 > 4.89 AKI stage 3 |
6 RBC/HPF 31 WBC/HPF 0.80 g/g |
Vasculitis without glomerular involvement IFTA 30% GS 50% | PFS 6 months |
| Case 5 | Stage 4 Squamous cell ca oropharynx 41/M |
Hypothyroidism | None | Pembrolizumab 24 cycles |
Colitis | Cr 0.83 > 1.89 AKI stage 2 |
0 RBC/HPF 0 WBC/HPF 0.06 g/g |
HTN nephrosclerosis IFTA 5% GS 5% |
PFS 27 months |
Afib, atrial fibrillation; AIN, acute interstitial nephritis; AKI, acute kidney injury; ATI acute tubular injury; CABG, coronary artery bypass graft; CAD, coronary artery disease; CIN chronic interstitial nephritis; COPD, chronic obstructive pulmonary disease; Cr, serum creatinine (mg/dl); CVA, cerebrovascular accident; GS, glomerular sclerosis; HLD, hyperlipidemia; HPF, high power field; HTN, hypertension; ICI, immune checkpoint inhibitor; IFTA, interstitial fibrosis tubular atrophy; IRAE, immune-related adverse events; M, male; F, female; T2DM, type 2 diabetes mellitus; UA, urinalysis; UPCR, urine protein to creatinine ratio.
*baseline serum creatinine and peak serum creatinine; †AKI stage by KDIGO criteria
3.b. T cell dense infiltration in AIN and flow cytometry of urinary immune cells.
Representative renal pathology images are shown in Figure 1. Consistent with the diagnosis of AIN, histologic analysis of Case 1 demonstrated a dense infiltration of CD3+ T cells (Figure 1b). Further immune cell characterization revealed CD20+ B cells (density 44 cells/mm2) and abundant total CD3+ T cells with a density of 1259 cells/mm2. The CD4+ to CD8+ T cell ratio was 1.5 to 1 (Figure 1e). Histologic analysis of Case 4 with ICI-vasculitis demonstrated CD20+ B cell density of 142 cells/mm2 and a total CD3+ T cell density of 667 cells/mm2 with a CD4+ to CD8+ ratio of 2.6 to 1 (Figure 1c). Few CD20+ and CD3+ cells were observed in Case 5 with HTN nephrosclerosis (Figure 1d).
Figure 1.
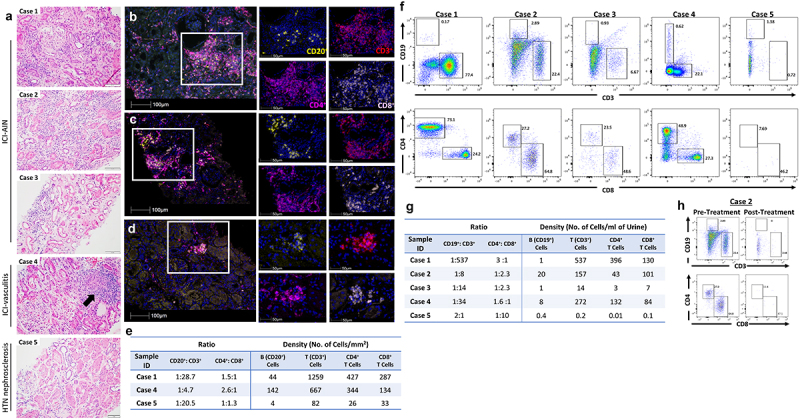
Pathologic representative images. (a) Photomicrographs of hematoxylin and eosin (H&E)-stained renal tissue sections, 20x magnification. Case 1–3: active tubulointerstitial nephritis with lymphocytic tubulitis. Case 4: necrotizing vasculitis with granuloma formation (black arrow). Case 5: renal cortex without significant inflammation. (B, C, D) Multiplex immunofluorescence (mIF) staining with immune-oncology panel composed of anti-CD20 (yellow), anti-CD3 (red), anti-CD4 (magenta) and anti-CD8 (pink) antibodies for Case 1 (b), Case 4 (c) and Case 5 (d). mIF composite images (left) 20x magnification and details (right) 40x magnification. (e) Ratio of B to T cells and CD4+T to CD8+T cells and density of cells per mm2. Flow cytometric analysis was performed on mononuclear cells isolated from urine and frequencies and density of B (CD19+) and T (CD3+) cells in viable mononuclear cell fraction and CD4+ T and CD8+ T cells in T cell fraction were assessed. (f) Phenotypic profile of lymphocytes isolated from urine of Cases 1–5. (g) Ratio of B to T cells and CD4+ T to CD8+ T cells and density of cells in urine samples. (h) Phenotypic profile of lymphocytes isolated from urine of Case 2 pre- and post-corticosteroid therapy.
In diseases such as lupus nephritis and renal transplant rejection, studies have shown that urinary immune cells can be detected in patients with immune-mediated kidney disease and may correlate with the course of the disease. Since ICI-associated renal lesions are characterized by the infiltration of kidney tissue with immune cells, we analyzed the urine of these patients for the presence of immune cells. Flow cytometric analysis of mononuclear cells isolated from freshly voided urine enabled determination of immune cell composition (Figure 1f). In Case 1–4 urinary CD3+ T cells and CD19+ B cells were present. The percentage and ratio of CD4+ and CD8+ T cell subsets were also assessed (Figure 1g). Contrary to the UA observations where WBCs were not detected, for Case 3 flow cytometry analysis revealed the presence of both T (CD4+ and CD8+) and B lymphocytes. In the non-ICI AKI case (Case 5) a negligible number of T and B cells were observed with flow cytometry analysis. In another AKI case (Case 6) in a patient on ICI therapy, urine flow cytometry analysis revealed negligible number of T and B cells, the patient had complete renal recovery and renal biopsy was not performed (S. Figure 1). Further analysis of a follow-up urine specimen upon completion of AIN therapy with corticosteroid on Case 2 showed a marked reduction in urinary T lymphocytes which coincided with the improvement in his serum creatinine from a peak creatinine of 3.16 mg/dl to 1.03 mg/dl (Figure 1h). These findings support further studies in the application of noninvasive urine-based screening for timely diagnosis and patient management of ICI-immune nephritis.
3.c. TCR clonotype analysis
Since T cells were detected in both kidney biopsy and urine, we performed a paired TCR analysis of kidney biopsy and urine specimen to determine the presence and abundance of shared TCR repertoire. Analysis of the productive rearrangements (i.e. the count of unique rearrangements in the sample that produce a functional protein receptor) revealed a total of 3,347 urinary TCR sequences in Case 1 and approximately 42.2% of the urinary TCR sequences overlapped with the TCR sequences from the kidney biopsy (Figure 2a). The top 25 kidney TCR sequences represented 57.6% of the top 100 productive TCR sequences (Figure 2d). These 25 TCR sequences were identified in the urine and represented 30.0% of the top 100 productive urine TCR sequences. In Case 4 (Figure 2c), approximately 29.7% or 667 urinary TCR sequences were shared with TCR sequences from the kidney. The top 25 kidney TCR sequences were similarly represented in the urine (66.3% kidney and 41.9% urine, Figure 2f).
Figure 2.
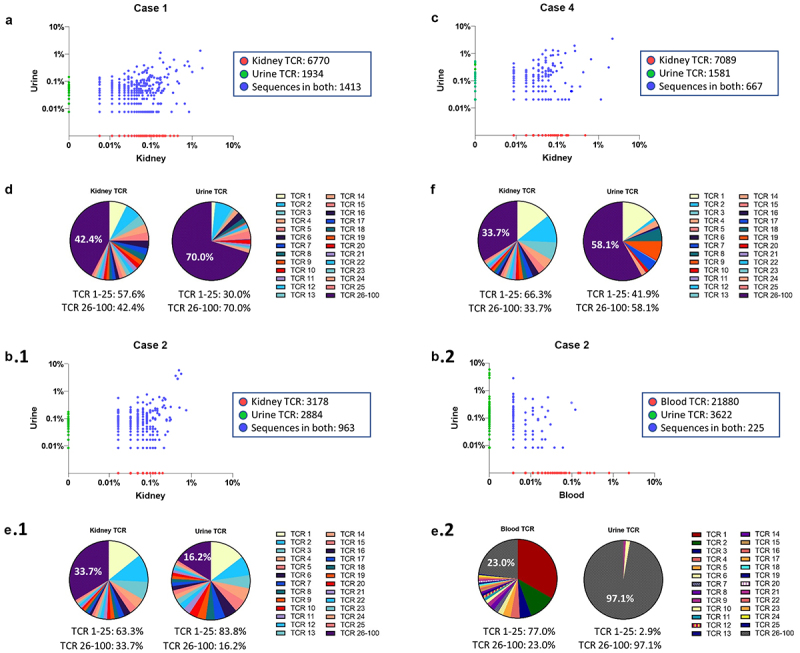
Composition of TCR repertoires in Case 1, 2 and 4. (A, B, C) Scatterplot of TCRβ sequences in paired specimens. TCRβ sequences shared in paired specimens (blue dots). Non-shared TCRβ sequences are located on x-axis (red dots) or y-axis (green dots). (a) In Case 1, a total of 3,347 urine TCRβ sequences were detected (3,347 TCRβ = 1,413 shared TCRβ sequences + 1,934 TCRβ sequences unique to urine) where 42.2% urine TCRβ sequences were also found in the kidney TCRβ sequences. (B.1 and B.2) In Case 2, 25% urine TCRβ sequences (963 shared TCRβ sequences out of a total of 3,847 urine TCRβ sequences) were also found in kidney TCRβ sequences, but only 5.8% of TCRβ sequences in urine (225 shared TCRβ sequences out of a total of 3,847 urine TCRβ sequences) were found in blood TCRβ sequences. (c) In Case 4, approximately 29.7% of urine TCRβ sequences (667 shared TCRβ sequences out of 2,248 total urine TCRβ sequences) were shared with kidney TCRβ sequences. (D, E.1, F) Pie charts display the frequency of the top 25 productive kidney TCRβ sequences as a percent of the top 100 kidney TCRβ sequences. The top 25 kidney TCRβ sequences are identified in the urine and displayed as a percent of the top 100 urine TCRβ sequences. (E.2) The frequency of the top 25 blood TCRβ sequences are displayed as a percent of the top 100 TCRβ blood sequences. The top 25 blood TCRβ sequences are identified in the urine and displayed as a percent of the top 100 urine TCRβ sequences.
For Case 2, a total of 3,847 urinary TCR sequences were detected in the urine of which 963 (approximately 25%) of the urine TCR sequences were also shared with the TCR repertoire of the kidney (Figure 2b). In comparison only 225 (approximately 5.8%) urine TCR sequences were shared with the TCR repertoire of T cells in the peripheral blood (Figure 2b). Of the 963 TCR clones that were shared between urine and kidney, 88% or 846 of these TCR sequences were not found in blood. The frequency of the top 25 kidney TCRs account for more than 83% of urine TCRs (Figure 2e), and the top 25 blood TCRs were found in <3% of urine TCRs (Figure 2e) suggesting that kidney TCR sequences are preferentially enriched in the urine.
To confirm the enrichment of the kidney (as opposed to blood) TCRs in urine for Case 2, we performed a statistical test to compare the difference in TCR distribution between kidney and urine versus (vs) blood and urine. To simplify the comparison, we focused only on the top 100 TCRs in kidney and top 100 TCRs in blood, since the remaining TCRs in both specimens had negligible frequencies. A total of eight shared TCRs between kidney and blood were removed since they do not contribute to the comparison. This left 184 TCRs (184 TCRs = (100 kidney TCRs – 8 shared TCRs) + (100 blood TCRs – 8 shared TCRs)). The count of TCRs for each category by specimen type was collapsed accordingly, resulting in a 3 × 2 contingency table. Since two of the cells had a zero count, a continuity correction (i.e., addition) of 1 count was applied to each of the 3 × 2 = 6 cells (S. Table 2).
We then performed a logistic regression analysis with a TCR falling into the top 100 kidney or blood TCRs (i.e., column variable) as the outcome variable, and specimen type (kidney, blood, or urine, with urine being the reference level, or row variable) as the independent variable. We tested whether the log odds ratio comparing the TCR distribution between kidney and urine is the same as the log odds ratio comparing the TCR distribution between blood and urine. The difference in these log odds ratios was statistically significantly different from zero (difference in log odds ratio = 13.72, 95% CI: 10.94 to 16.50; z statistic = 9.70, Wald test p-value <2e-16), confirming that the urine is enriched with kidney TCRs as compared to blood TCRs in Case 2 (S. Table 3).
4. Discussion
To our knowledge, this is the first study that examines immune cell phenotype and repertoire in matched renal biopsies and urine in ICI-triggered immune nephritis. We used immunophenotyping analysis to describe the detection of urinary T lymphocytes in patients with biopsy-proven ICI-associated immune nephritis. Clonotype analysis demonstrated that urinary TCRs are statistically significantly enriched for a TCR repertoire found predominantly in the infiltrating T cells in kidney tissue compared to blood TCRs of a patient with ICI-AIN.
Recent studies have shown that analyzing urine from patients with kidney disease provides a useful, noninvasive source of information on the etiology of kidney injury. In conditions such as acute allograft rejection, active lupus nephritis and glomerulonephritis, increased levels of T cells in the urine of patients have been shown to mirror intrarenal inflammation.9–11 Here we show that an abundance of urinary T cells can be detected in patients with ICI-mediated inflammatory lesions (Cases 1–4). Further, flow cytometric analysis reveals the presence of urinary lymphocytes that were not previously detectable by urine sediment analysis alone (Case 3). We also observed in Case 2 that following corticosteroid therapy for treatment of AIN, urinary T lymphocyte counts decreased substantially, commensurate with an improvement in the serum creatinine level suggesting that longitudinal monitoring of urinary T lymphocyte counts in patients with ICI-immune nephritis may serve as an indicator of treatment response.
T cells present in the urine could be derived from kidney infiltrating T cells or circulating T cells in the blood and similarities in TCR repertoire profile comparing kidney and urine vs. blood and urine may reveal the origin of urinary T cells.19 Longitudinal analysis studies comparing TCR repertoire of graft infiltrating lymphocytes and blood have shown that biopsy sampling underestimates the entire infiltrating lymphocyte population of the organ.20 Thus, although kidney biopsy represents a much-restricted repertoire compared to blood, our analysis confirmed that the urine is enriched with kidney TCRs as compared to blood TCRs in Case 2. The identification of antigens shared between tumors and healthy tissue is increasingly recognized in patients who develop irAEs.21,22 The lack of available tumor tissue precluded us from conducting this comparison. Future studies are necessary to determine whether these T cells recognize shared tumor and kidney antigens.
The ability to draw definitive conclusions from this pilot study is limited by the small sample size and absence of baseline urine profiling prior to the development of AKI. While urinary T lymphocytes may be present in patients with chronic kidney disease, we do not, however, expect urinary T cells in patients with preserved kidney function.23 To gain a more comprehensive understanding of the urine immune landscape and perform hypothesis-testing, future analyses will include paired peripheral blood mononuclear cells and/or tumor specimens along with additional control cases (e.g. patients with preexisting chronic kidney disease).
Studies in lupus nephritis and glomerulonephritis have established that renal infiltration with specific immune cells relates to disease manifestations and treatment responses.9,10 As ICI-based therapies become standard of care for more cancers, it is essential to transition from empirical management practices such as corticosteroid therapy for all patients with potential ICI-related AKI to selectively limiting treatment to only the 2–5% of patients who have ICI-associated immune nephritis.4,12 Although diagnosis relying on biopsy is recommended, it is not always feasible due to elevated risk of bleeding and resultant morbidity and mortality.24 Profiling for renal immune cells can facilitate clinical decision-making by improving accuracy of diagnosis and enabling monitoring of patients during and after treatment. Our approach of noninvasively assessing urinary immune cells of ICI therapy recipients using flow cytometry can facilitate risk stratification of renal disease prior to biopsy and provides an opportunity to tailor the treatment of ICI-associated nephritis. A more sensitive qualitative and quantitative evaluation method for determining urinary cell composition than standard UA, this flow cytometry-based screening method can assist in confirming a diagnosis of renal irAEs and its potential role in prediction of clinical outcomes of ICI therapies similar to those with ICI-colitis.25 Under current management practices, diagnosis of immune nephritis in patients responding to ICI therapy can lead to discontinuation of these anti-tumor therapy and deprive patients of continued benefit from these therapies. Studies have reported that fewer than 20% of the patients redevelop AIN with ICI retreatment.12 Regular assessment of urinary immune cells offers an option for close monitoring of development of AIN in these patients if they continue to receive therapy.
Our study endorses the clinical feasibility of performing urine cell isolation on human urine samples as a method to monitor for ICI-immune nephritis. Future studies will include independent validation and/or verification of the application of this approach for monitoring disease and assessing ICI-immune nephritis therapy response.
5. Conclusion
As the landscape of use of ICIs expands to treat more cancer types, and its efficacy improves with novel combinatory regimens, the detection and timely management of ICI-associated irAEs will significantly impact the ability of oncologists to care for patients. These findings have both broad diagnostic and prognostic implication for renal immune related adverse events. In summary, we report monitoring of urinary cell populations as a potential method for monitoring ICI-nephritis.
6. List of abbreviations
AIN, acute interstitial nephritis; AKI, acute kidney injury; CTLA-4 cytotoxic T-lymphocyte-associated protein 4; FFPE, formalin-fixed, paraffin-embedded; ICI, immune checkpoint inhibitor; mIF, multiplex immunofluorescence; irAE, immune-related adverse event; pBMC, peripheral blood mononuclear cell; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; TCR, T cell receptor; UA, urinalysis.
Supplementary Material
Acknowledgments
We thank the patients and their families for providing permission to report adverse events and for their participation and contribution to research. The Multiplex Immunofluorescence and Image Analysis Laboratory from the Department of Translational Molecular Pathology, thanks their members who contribute daily to quality mIF. We thank the pathology team from the laboratory at Translational Molecular Pathology Department that works with image analysis.
Funding Statement
Y.L. is partially supported by the National Cancer Institute Grant P50CA217674. J.P.L. is partially supported by the National Cancer Institute and the National Center for Advancing Translational Sciences of the NIH P30CA016672 and CCTS UL1TR003167. Cassian Yee (C.Y.2.) is Member of Parker Institute of Cancer Immunotherapy. J.S.L. is supported by the NIH/NIDDK (K08 DK119466). This research is also supported by the Division of Internal Medicine Immuno-Oncology Toxicity Award Program of the University of Texas MD Anderson Cancer Center (PI: J.S.L.) and the University Cancer Foundation via the Institutional Research Grant program at the University of Texas MD Anderson Cancer Center (PI: J.S.L.). The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health through Cancer Center Support Grant P30CA016672.
Authors’ contributions
Data acquisition was performed by S.S., L.C.C., E.R.P., A.T. and J.S.L. L.C.C., E.R.P., and A.T. performed the histological examination, provided images and description of the biopsied kidney specimens. L.C.C participated in the mIF image analysis. E.R.P. designed the mIF panel design and interpretation of the mIF data. J.P.L. provided TCR sequence analysis support. Chao Yang (C.Y.1.) and Y.L. provided statistical analysis of the TCR clonotypes. L.C.C., E.R.P., A.T., C.Y.1., Y.L., and J.P.L. contributed in writing the manuscript. The manuscript was prepared by S.S., C.Y.2., and J.S.L. All authors reviewed the manuscript at all stages. All the authors contributed to the quality control data, analysis, interpretation of data and writing and final proof of paper. All authors read and approved the final manuscript.
Data availability statement
All data generated or analyzed during this study are included in this published article.
Disclosure statement
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This study was approved by the institutional review board in accordance with the principles of the Declaration of Helsinki.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/2162402X.2022.2124678
References
- 1.Meraz-Munoz A, Amir E, Ng P, Avila-Casado C, Ragobar C, Chan C, Kim J, Wald R, Kitchlu A.. Acute kidney injury associated with immune checkpoint inhibitor therapy: incidence, risk factors and outcomes. J Immunother Cancer. 2020;8(1):e000467. doi: 10.1136/jitc-2019-000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, Le DT, Lipson EJ, Glezerman IG, Wolchok J, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016;90(3):638–8. doi: 10.1016/j.kint.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mamlouk O, Selamet U, Machado S, Abdelrahim M, Glass WF, Tchakarov A, Gaber L, Lahoti A, Workeneh B, Chen S, et al. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: single-center experience. J Immunother Cancer. 2019;7(1):2. doi: 10.1186/s40425-018-0478-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta S, Cortazar FB, Riella LV, Leaf DE. Immune Checkpoint Inhibitor Nephrotoxicity: update 2020. Kidney360. 2020;1(2):130–140. doi: 10.34067/KID.0000852019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halimi J-M, Gatault P, Longuet H, Barbet C, Bisson A, Sautenet B, Herbert J, Buchler M, Grammatico-Guillon L, Fauchier L, et al. Major bleeding and risk of death after percutaneous native kidney biopsies: a French nationwide cohort study. Clin J Am Soc Nephrol. 2020;15(11):1587–1594. doi: 10.2215/CJN.14721219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker ML, Yamamoto Y, Perazella MA, Dizman N, Shirali AC, Hafez N, Weinstein J, Simonov M, Testani JM, Kluger HM, et al. Mortality after acute kidney injury and acute interstitial nephritis in patients prescribed immune checkpoint inhibitor therapy. J ImmunoTherapy of Cancer. 2022;10(3):e004421. doi: 10.1136/jitc-2021-004421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atwell TD, Spanbauer JC, McMenomy BP, Stockland AH, Hesley GK, Schleck CD, Harmsen WS, Welch TJ. The timing and presentation of major hemorrhage after 18,947 image-guided percutaneous biopsies. AJR Am J Roentgenol. 2015;205(1):190–195. doi: 10.2214/AJR.14.13002. [DOI] [PubMed] [Google Scholar]
- 8.Kang E, Park M, Park PG, Park N, Jung Y, Kang U, Kang HG, Kim DK, Oh K-H, Joo KW, et al. Acute kidney injury predicts all-cause mortality in patients with cancer. Cancer Med. 2019;8(6):2740–2750. doi: 10.1002/cam4.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopetschke K, Klocke J, Griessbach AS, Humrich JY, Biesen R, Dragun D, Burmester G-R, Enghard P, Riemekasten G. The cellular signature of urinary immune cells in Lupus nephritis: new insights into potential biomarkers. Arthritis Res Ther. 2015;17:94. doi: 10.1186/s13075-015-0600-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goerlich N, Brand HA, Langhans V, Tesch S, Schachtner T, Koch B, Paliege A, Schneider W, Grützkau A, Reinke P, et al. Kidney transplant monitoring by urinary flow cytometry: biomarker combination of T cells, renal tubular epithelial cells, and podocalyxin-positive cells detects rejection. Sci Rep. 2020;10(1):796. doi: 10.1038/s41598-020-57524-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakatsume M, Xie Y, Ueno M, Obayashi H, Goto S, Narita I, Homma N, Tasaki K, Suzuki Y, Gejyo F, et al. Human glomerulonephritis accompanied by active cellular infiltrates shows effector T cells in urine. J Am Soc Nephrol. 2001;12(12):2636–2644. doi: 10.1681/ASN.V12122636. [DOI] [PubMed] [Google Scholar]
- 12.Gupta S, Short SAP, Sise ME, Prosek JM, Madhavan SM, Soler MJ, Ostermann M, Herrmann SM, Abudayyeh A, Anand S, et al. Acute kidney injury in patients treated with immune checkpoint inhibitors. J ImmunoTherapy of Cancer. 2021;9(10):e003467. doi: 10.1136/jitc-2021-003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parra ER, Zhai J, Tamegnon A, Zhou N, Pandurengan RK, Barreto C, Jiang M, Rice DC, Creasy C, Vaporciyan AA, et al. Identification of distinct immune landscapes using an automated nine-color multiplex immunofluorescence staining panel and image analysis in paraffin tumor tissues. Sci Rep. 2021;11(1):4530. doi: 10.1038/s41598-021-83858-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parra ER, Ferrufino-Schmidt MC, Tamegnon A, Zhang J, Solis L, Jiang M, Ibarguen H, Haymaker C, Lee JJ, Bernatchez C, et al. Immuno-profiling and cellular spatial analysis using five immune oncology multiplex immunofluorescence panels for paraffin tumor tissue. Sci Rep. 2021;11(1):8511. doi: 10.1038/s41598-021-88156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parra ER, Uraoka N, Jiang M, Cook P, Gibbons D, Forget M-A, Bernatchez C, Haymaker C, Wistuba II, Rodriguez-Canales J, et al. Validation of multiplex immunofluorescence panels using multispectral microscopy for immune-profiling of formalin-fixed and paraffin-embedded human tumor tissues. Sci Rep. 2017;7(1):13380. doi: 10.1038/s41598-017-13942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parra ER, Jiang M, Solis L, Mino B, Laberiano C, Hernandez S, Gite S, Verma A, Tetzlaff M, Haymaker C, et al. Procedural requirements and recommendations for multiplex immunofluorescence tyramide signal amplification assays to support translational oncology studies. Cancers (Basel). 2020;12(2):255. doi: 10.3390/cancers12020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leick M, Gittelman RM, Yusko E, Sanders C, Robins H, DeFilipp Z, Nikiforow S, Ritz J, Chen Y-B. T cell clonal dynamics determined by high-resolution tcr-β sequencing in recipients after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2020;26(9):1567–1574. doi: 10.1016/j.bbmt.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17(1):204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu M, Zhang GY, Walters G, Sartor M, Watson D, Knight JF, Alexander SI. Matching T-cell receptors identified in renal biopsies and urine at the time of acute rejection in pediatric renal transplant patients. Am J Transplant. 2004;4(11):1859–1868. doi: 10.1111/j.1600-6143.2004.00587.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim JY, Lei Z, Maienschein-Cline M, Chlipala GE, Balamurugan A, McDiarmid SV, Azari K, Yang OO. Longitudinal analysis of the T-cell receptor repertoire in graft-infiltrating lymphocytes following hand transplantation. Transplantation. 2021;105(7):1502–1509. doi: 10.1097/TP.0000000000003535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ibraheim H, Perucha E, Powell N. Pathology of immune-mediated tissue lesions following treatment with immune checkpoint inhibitors. Rheumatology. 2019;58(Supplement_7):vii17–vii28. doi: 10.1093/rheumatology/kez465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J ImmunoTherapy of Cancer. 2019;7(1):306. doi: 10.1186/s40425-019-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abedini A, Zhu YO, Chatterjee S, Halasz G, Devalaraja-Narashimha K, Shrestha R, S. Balzer M, Park J, Zhou T, Ma Z, et al. Urinary single-cell profiling captures the cellular diversity of the kidney. J Am Soc Nephrol. 2021;32(3):614–627. doi: 10.1681/ASN.2020050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, Gerber DE, Hamad L, Hansen E, Johnson DB, et al. Society for immunotherapy of cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J ImmunoTherapy of Cancer. 2021;9(6):e002435. doi: 10.1136/jitc-2021-002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abu-Sbeih H, Ali FS, Qiao W, Lu Y, Patel S, Diab A, Wang Y. Immune checkpoint inhibitor-induced colitis as a predictor of survival in metastatic melanoma. Cancer Immunol Immunother. 2019;68(4):553–561. doi: 10.1007/s00262-019-02303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


