Abstract
Pneumocystis jirovecii pneumonia (PCP), caused by fungal species named P. jirovecii, is a frequent opportunistic infection in those with human immunodeficiency virus infection. However, PCP has been documented in immunocompetent patients. This study aims to determine if P. jirovecii detection occurs in asthma patients following coronavirus disease 2019 (COVID-19) in a Jordanian cohort. Another aim was to evaluate a method of TaqMan quantitative polymerase chain reaction (qPCR) assay to detect P. jirovecii, from sputum samples. The nasopharyngeal swabs were used to detect SARS-CoV-2 and sputum samples were tested for P. jirovecii using real-time qPCR assay. Beta-tubulin (BT) and dihydrofolate reductase (DHFR) genes were the directed targets of P. jirovecii. The results showed that the mean qPCR efficiencies of BT and DHFR were 96.37% and 100.13%, respectively. Three out of 31 included patients (9.7%) had a positive P. jirovecii. All of the three patients had used oral corticosteroids (OCS) in the past 2 months due asthma exacerbation and were treated with OCS for COVID-19. This is the first study based in Jordan to demonstrate that P. jirovecii and COVID-19 can coexist and that it is important to maintain a broad differential diagnosis, especially in immunocompromised patients. Chronic lung disease can be a risk factor for the P. jirovecii colonization possibly due to corticosteroid’s immunosuppression.
Keywords: Asthma, COVID-19, developing country, Pneumocystis jirovecii, polymerase chain reaction
INTRODUCTION
A current global pandemic of coronavirus infectious disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Infection with this virus results in a wide range of symptoms, from asymptomatic illness to severe acute respiratory distress syndrome (ARDS), interstitial pneumonia associated with respiratory failure, and even death [1,2]. Comorbidities, including respiratory disease, were documented as contributing factors for more severe form of the disease and worse prognosis [3-5].
Other respiratory viruses, for example, seasonal/pandemic influenza exhibit varying degrees of coinfection with bacteria and fungi [6]. In addition, coinfection has been linked to more severe consequences during the pandemic [6].
While clinical care is primarily directed at diagnosing and managing COVID-19, there is emerging evidence that this disease can be complicated with developing concurrent pulmonary infections including fungal superinfection [7-10]. The coinfection with other pathogens can increase the disease symptoms and mortality, in addition to making the diagnosis and management more difficult [11]. As a result, there is a clinical need for rigorous examination of coinfection in COVID-19 patients as invasive fungal infections are not uncommon in COVID-19 patients [12].
Pneumocystis jirovecii pneumonia (PCP or PJP) caused by fungal species of P. jirovecii is a frequent opportunistic infection in those with human immunodeficiency virus (HIV) infection [8]. Although subclinical infection (colonization) with P. jirovecii reaches 9% in COVID-19 patients hospitalized in intensive care units (ICUs), only a few cases of PCP have been reported thus far, almost exclusively in immunocompromised patients [12-14]. However, PCP has been documented in immunocompetent individuals with COVID-19 [13-16].
Despite being not typically immunosuppression state, chronic lung disease can be a risk factor for the P. jirovecii colonization possibly as a result of structural lung destruction, corticosteroid’s immunosuppression, and smoking [17]. The previous studies based on animals propose an association between P. jirovecii infection and both of the obstructive pulmonary diseases; chronic obstructive lung disease (COPD); and asthma [18,19]. While oral corticosteroids (OCSs) are considered an independent PCP risk factor and usually used to treat asthma exacerbation, PCP is unusual in patients with asthma without any other identified risk factors [20].
At the moment, it is uncertain whether or not patients with asthma and COVID-19 are susceptible to P. jirovecii colonization in Jordan as there are lack of any regional publications about P. jirovecii and it is not tested routinely. Moreover, because most of the previous studies used nested PCR that associated with high chance of contamination, P. jirovecii prevalence might be overestimated in other countries. The purpose of this study was therefore to determine the prevalence of P. jirovecii coinfections in asthmatic patients with COVID-19 and to assess a method of TaqMan quantitative polymerase chain reaction (qPCR) assay in detecting P. jirovecii, from sputum samples.
MATERIALS AND METHODS
Study design and participants
Among asthmatic adult patients with confirmed COVID-19 not requiring hospitalization from April 1, 2021, to November 1, 2021, we analyzed all sputum samples for P. jirovecii detection. During the virus pandemic, there was no microscopic diagnosis performed. Individuals with any other respiratory condition, such as COPD or cystic fibrosis, were excluded from the study.
The medical records and/or the patient or his relative interview were used to gather the medical history and demographic data.
Nucleic acid extraction and SARS-CoV-2 detection
The nasopharyngeal swabs were used to detect SARS-CoV-2. The total nucleic acid extraction was performed using the automated BIOBASE nucleic acid extraction kit employing the magnetic beads method (Biobase Biodustry [Shandong] co. ltd). Following extraction, the eluted nucleic acid samples were utilized to detect SARS-CoV-2. The SARS-CoV-2 reverse transcriptase-qPCR assay was performed according to the CDC methodology utilizing the TIANLONG: Real-time PCR System with 48-well block equipment and the LiliFTM COVID-19 Multi Real-time RT-PCR Kit. A SARS-CoV-2 assay result was classified as positive if the ribonuclease P (RNP) gene and either the N1 or N2 gene were detected, and as negative if only the RNP gene was identified. RNP was utilized to determine the sample quality and to detect PCR inhibition. Data related to primers and probe sequence for SARS-CoV-2, PCR conditions, as well as positive and negative controls are available and adapted from the previous studies [21,22].
P. jirovecii detection
All sputum samples were tested for P. jirovecii. Beta-tubulin (BT) and dihydrofolate reductase (DHFR) genes were the directed targets of P. jirovecii. These sputum samples were collected 10 days after the onset of COVID-19 symptoms.
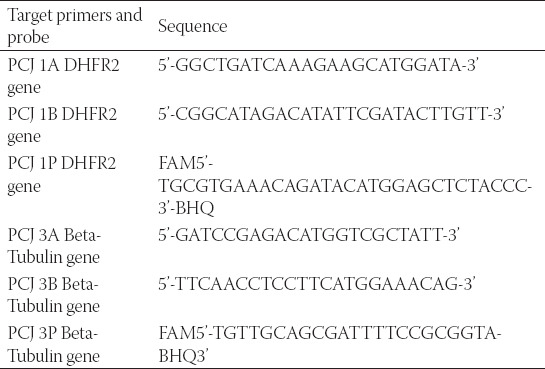
P. jirovecii purified DNA templates of cell-conditioned medium from cultured primary epithelial cells were used for real-time qPCR of the calibration data. All real-time qPCR assays were TaqMan assays. Table 1 lists the primers and probes sequences (Eurofins, UK). Ten-fold serial dilutions of the four concentrations of the standard DNA (1000, 100, 10, and 1 copies/μL) with five replicates were performed to make the standard curve for P. jirovecii. The amplification reaction of the five replicates was conducted for each of the four concentrations of the standard DNA using 90 μL (10-fold serial dilutions) of a specific type of buffer (a “blocking” nucleic acid; lambda DNA, yeast tRNA, salmon sperm DNA) (Sigma-Aldrich, UK).
TABLE 1.
Primer and probe sequences of Pneumocystis jirovecii targets
We used 9 μL of master-mix (Roche Diagnostics, UK) (Table 2) and 6 μL P. jirovecii DNA template for each replicate of the four concentrations, and this final step was done in a duplicate so a total of 40 wells, for each target gene (both BT and DHFR genes), each with 15 μL. We used four negative control samples for each target gene.
TABLE 2.
Master mix preparation for Pneumocystis jirovecii

The absolute quantification was used to analyze the samples in a similar principle of a previous recent publication [23]. For Ct values ≤40 cycles, a positive result was recorded, whereas a negative result was recorded in the lack of a Ct value and for Ct values greater than 40 cycles. In addition, a positive RNP value with Ct ≤37 cycles was required. When RNP levels exceed 37 cycles, the sample is frozen, thawed, re-extracted, and re-tested. For the second test, samples with RNP values larger than 37 cycles would be excluded from analysis due to either low quality or the presence of an inhibitor of the PCR.
Six microliters from each patient’ specimen were pipetted into the 48 wells-plate after dispensing 9 mL of the master-mix. Plates were sealed using sealing foil, then centrifuged at 1500 rotations per minute and placed in the TIANLONG: Real-Time PCR System. The TaqMan PCR program consisted of 50°C for 15 min, 95°C for 5 min, 45 cycles of 95°C for 10 s, and then 60 °C for 1 min, with a final step of 40°C for 15 s.
Ethical statement
The approval of the study was gained from the Research Ethics Board of Applied Science Private University, Amman, Jordan (2021-PHA-35) and from Al-Rayhan Medical Center (2021-IRB-9-2). Informed consent was obtained from every patient.
Statistical analysis
Data were analyzed using SPSS Statistics version 24 (IBM Corporation, USA). All data are presented as mean (standard deviation) or number (percentage).
RESULTS
Study population and characteristics
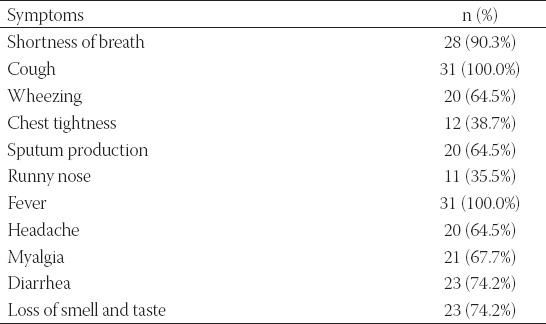
During the study period and among patients with asthma and COVID-19, 31 had a sputum sample for P. jirovecii analysis. Patients’ demographic data and clinical variables are described in Table 3. The patients mean age (SD) was 45.35 (12.020). Among those 31 asthmatic patients, 19 (61.3%) were female. Overall, 4 (12.9%) have diabetes mellitus. Around three-quarters were non-smokers (77.4%). More than one-half of the included participants were second-hand smokers (64.5%). The majority (90.3%) of the included asthmatic patients did not take any dose of the COVID-19 vaccine at the time of enrolment. The inhaled corticosteroid/long-acting beta-2 agonist was the used medication for all of the participants. During the study period, supplemental oxygen was used for 17 patients (54.8%) (Table 3). None of the study participants required hospitalization during the 14 days follow-up as all of the included patients were classified to have moderate/non-severe COVID-19 with non-severe pneumonia according to the World Health Organization classification [24,25]. The most frequently reported symptoms at the initial visit were cough and fever, as reported in all of the patients (100.0%) (Table 4).
TABLE 3.
Demographics and clinical variables for the study participants (n=31)
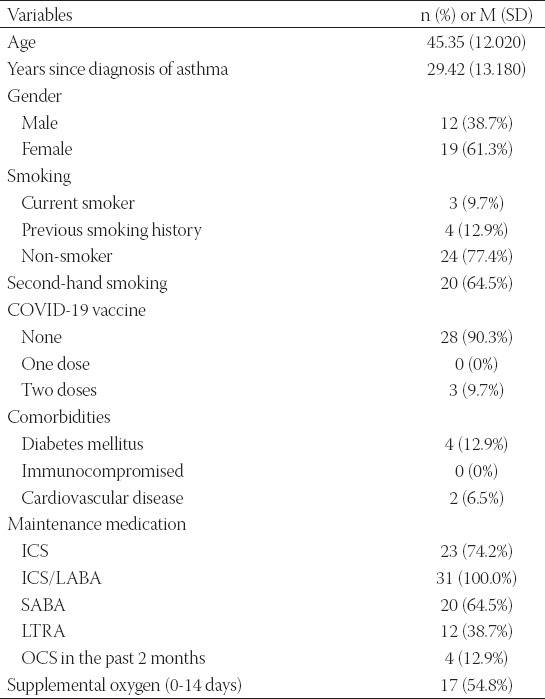
TABLE 4.
Presented complains at the initial visit
Three patients (9.7%) had a positive P. jirovecii qPCR. All of the three patients had used OCS in the past 2 months due asthma exacerbation. Diabetes was found in two of the three patients. Furthermore, OCS for COVID-19 was administered to these three patients. None of the included patients required mechanical ventilation and admission to the ICU. One patient died within 2 months of his COVID-19 diagnosis. Trimethoprim-sulfamethoxazole or other alternatives were not used to treat any of the patients. All of the patients were treated with broad-spectrum antimicrobial agents.
P. jirovecii calibration data
The means of the Ct values with standard deviation were calculated for all the concentrations and replicates of P. jirovecii calibration data (Table 5). The mean (SD) of the correlation coefficient (r2) was −0.998 (0.001) for BT, and −0.996 (0.001) for DHFR targets.
TABLE 5.
Pneumocystis jirovecii calibration data
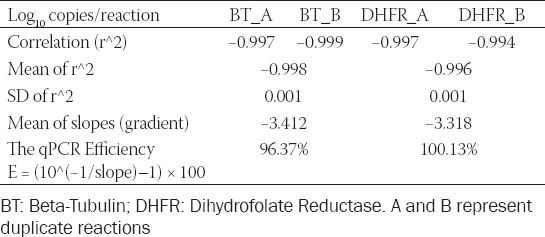
By plotting the log of target DNA concentrations against Ct values, the standard curve was created; linear regressions from each of the replicate dilution series were used. Figure 1 depicts the calibration curves for P. jirovecii. The amplification efficiency (E) of the absolute quantification is the mean efficiency derived by E = (10-Slope–1) × 100. The results showed that the mean PCR efficiencies of BT and DHFR were 96.37% and 100.13%, respectively.
FIGURE 1.
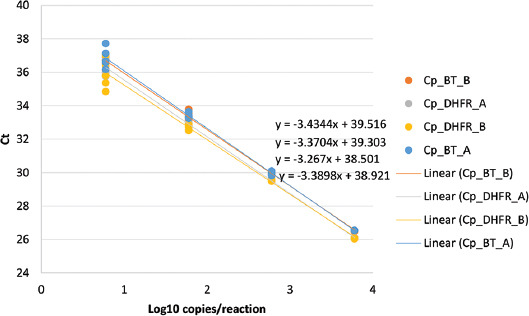
Pneumocystis jirovecii Calibration Curve. A and B represent duplicate.
DISCUSSION
The primary objective of this study was to ascertain the prevalence of P. jirovecii in Jordanian asthmatic patients following SARS-CoV-2 infection. We identified three cases of P. jirovecii using qPCR in sputum samples from 31 (9.7%) COVID-19 asthmatic patients. Concerning risk factors, all of these three patients used OCS in the preceding 10-14 days for COVID-19 and asthma exacerbation treatment. Two patients had a favorable prognosis, but one died within 2 months of the COVID-19 incident.
P. jirovecii is an opportunistic microorganism that is most prevalent in immunocompromised persons. Even though PCP was formerly linked with severe HIV infection, it, now, affects individuals who are immunocompromised for other reasons, including those who require corticosteroids [26,27].
The prevalence of positive P. jirovecii qPCR in patients with COVID-19 is unknown. A recent study reported positive P. jirovecii qPCR in 2/145 (1.4%) COVID-19 patients in ICU [16]. Another study discovered a detection of P. jirovecii PCR in 10/108 (9.3 %) of COVID-19 patients presenting with ARDS [13]. Numerous cases of SARS-CoV-2 coinfection with P. jirovecii have also been recorded in patients with recognized risk factors for PCP [1,15,16,28-31]. In one example, a patient who had been diagnosed with COVID-19 and treated with tocilizumab and glucocorticoids was later diagnosed with PCP. This raised the hypothesis, similar to this study, that immunomodulatory treatment for COVID-19 may have contributed to the development of PCP [31].
The diagnosis of PCP in COVID-19 cases, as well as the distinction between infection and colonization, are extremely difficult to make [16]. COVID-19 patients in the ICU may develop PCP as a result of mechanical ventilation, corticosteroid medication, or the presence of a cytokine storm [1]. However, radiographic similarities exist between the two illnesses, with the appearance of cysts or tiny reticular alterations on computed tomography (CT) scan indicating pneumocystosis [16,28,29]. It is possible that overdiagnosis of PCP in colonized patients will occur as a result of the high sensitivity of PCR, and distinguishing colonization from PCP can be difficult, particularly in immunocompetent patients [13,16,29]. In most cases, no direct examination is performed on patients with COVID-19 infection. In our study, we did not use staining due to the estimated risk of aerosolization during COVID-19. However, staining is quite useful for visualizing cysts or trophozoite forms and, therefore, for differentiating infection from colonization. This could be critical in determining the presence of these coinfections accurately.
Due to the high negative predictive value of the serum fungal marker (1→3)-β-D-Glucan (BG) assay, it may be used to rule out infection in some cases [13,32]. However, confirmation of the diagnosis requires further testing and well-matched clinical features. In addition to mycological criteria, the diagnosis of infection must be based on a number of other factors, including clinical deterioration, immunosuppression, severe lymphocytopenia, serum BG, and lactate dehydrogenase assays, and response to therapy [16]. PCP and moderate-to-severe COVID-19 have a number of clinical characteristics in common, making it difficult to distinguish between the two diseases. Both conditions are characterized by the presence of fever, cough, and hypoxia [33] and the presence of a wide range of radiographic findings, including diffuse ground-glass opacities [34]. The similarities between the two infections may be due to shared pathogenic mechanisms and interactions with pulmonary surfactant [35], which have been hypothesized. As a result of these and other similar findings, PCP is increasingly being recognized as a COVID-19 mimicker in critically ill patients [14].
A single-center case series included five patients diagnosed with PCP, one of whom had classical risk factors for PCP and the others were immunocompetent before the onset of COVID-19. Surprisingly, none of the patients with confirmed that PCP had detectable BG in repeated serum samples; these findings were explained by the fact that, while serum BG has a high negative predictive value for PCP diagnosis, its role is primarily recognized in HIV patients, and its sensitivity may be lower in immunocompetent hosts [36]. Each patient received a minimum of 2 weeks of high-dose corticosteroid. This is the most likely risk factor for PCP development in this cohort, along with CD4+ lymphopenia, which has been observed in the majority of patients affected by COVID-19 and is associated with a poor prognosis, particularly in younger patients [37,38].
Concurrent infection with SARS-CoV-2 and P jirovecii can create diagnostic difficulties. While most hospital laboratories now offer COVID-19 testing using nasopharyngeal swabs with a rapid turnaround time [39,40], PCP is more difficult to diagnose. Due to its increased sensitivity, in patients with severe hypoxia, bronchial alveolar lavage (BAL) fluid stays the standard method for PCP diagnosis [41]; however, doing a bronchoscopy to collect, a BAL specimen is an invasive procedure that is not always plausible in severely hypoxic patients and due to the possibility of SARS-CoV-2 aerosolization, greater procedural vigilance is required.
Corticosteroids for severe COVID-19 would, further, defer identification of cooccurring PCP, as these individuals may possibly improve temporarily as a result of steroids’ documented beneficial effect on severe PCP. Tocilizumab, under investigation as a possible COVID-19 treatment, has been related with PCP in the management of rheumatoid arthritis [42]. Accordingly, health-care professionals should be aware of the possibility of PCP.
PCP has a more subacute course than COVID-19 [43-45]. In comparison to COVID-19, PCP symptoms might continue many weeks before a diagnosis is made, with a median duration to diagnosis of around 1 week from disease beginning [2,46].
The part of the purpose of this research was to evaluate a method of TaqMan qPCR assay to detect P. jirovecii, from sputum samples of asthma patients infected with SARS-CoV-2. The findings of the assay targeting P. jirovecii were highly linear over the replicated dilution series (as observed from r2 values) and the data from independent replicates were highly reproducible. The use of the nested PCR in most of the previous studies and the high chance of contamination associated with the nested PCR may contribute to the inconsistent P. jirovecii prevalence. Whereas the assay in this study uses TaqMan real-time qPCR chemistry, so accurate detection and quantification of the microbial targets are more likely, as appropriate calibrations are available. Real-time qPCR avoids the cross-contamination and allows target quantification, unlike the nested PCR assay. In a multicenter study, in which four laboratories involved, it was demonstrated that there is a high risk of contamination with the nested PCR [47]. Using the rapid molecular methods, such as qPCR, should increase the yield of microbial detection and provide a better insight of the microbial association with asthma exacerbation [48,49]. The qPCR can detect small amounts of nucleic acids from the target pathogens; it does not rely on the viability of the pathogen which is probably less affected by previous antibiotic treatment compared with the culture-based approaches [50].
Although the findings of this study did not confirm a significant association of P. jirovecii and asthma exacerbation compared with the baseline, it could not be ruled out that P. jirovecii continued in the lower respiratory tract (LRT) from a preceding exacerbation. Further, work needs to be performed to solve the debatable issue whether P. jirovecii recovered from the LRT of patients with asthma indicate colonization, infection, or persistence. The previous research has established a link between P. jirovecii colonization and chronic lung illnesses such as asthma [19]. Patients with stable asthma show a greater enrichment of P. jirovecii in BAL fluid [51] and higher sera titers against whole cell P. jirovecii antigens [19], implying that they are more exposed to P. jirovecii than healthy persons. However, there are a number of P. jirovecii antigens that are highly immunogenic but do not confer protection against infection [52,53].
Our study is not without limitations. To begin, only 31 sputum specimens from COVID-19 patients were examined for P. jirovecii coinfection; however, all of the patients included had asthma, hence increasing the statistical power. PCP is difficult to diagnose in COVID-19, based on a single P. jirovecii qPCR result and the lack of additional tests to confirm coinfections in this study can be justified by the limitations of the other tests, especially during the pandemic and lack of a strong recommendation of other test for non-HIV patients. HIV markers were not provided for each patient, because HIV test is not commonly done in Jordan nor accepted by the patients. However, none of the included patients were with suspected HIV clinically. Finally, one patient in our study had a very poor prognosis, but it is unknown if this death is attributable to P. jirovecii or if the presence of P. jirovecii is only a marker of immunosuppression and severe form of COVID-19 infection.
Our research adds geographical data on the discovery of P. jirovecii in individuals with asthma and COVID-19. These coinfections are uncommon but substantial; consequently, PCP should be investigated in patients with moderate-severe COVID-19 who experience deterioration of their disease. Additional research is needed to elucidate the epidemiology of P. jirovecii detection in asthmatic and COVID-19 patients, in addition to the identification and management of this pathogen.
CONCLUSION
This is the first study based in Jordan to demonstrate that P. jirovecii detection and COVID-19 can coexist and that it is important to maintain a broad differential diagnosis, especially in immunocompromised patients.
COVID-19 and PCP share some characteristics that may make identification more difficult. As a result, further diagnostic testing should be done in individuals with COVID-19 who have clinical data compatible with PCP and a possible coinfection. This is especially true for immunocompromised individuals.
An important risk factor for PCP development in COVID-19 patients is the use of corticosteroids for asthma exacerbations and/or the presence of COVID-19, and further study is needed to identify these risk factors and predictors of PCP development and to find the best preventative and therapeutics strategy.
ACKNOWLEDGMENTS
A very special thank you goes out to the Alrayhan Medical Center and Basheer Alshammari.
Footnotes
Conflict of interests: The authors declare no conflicts of interests.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Gangneux JP, Bougnoux ME, Dannaoui E, Cornet M, Zahar JR. Invasive fungal diseases during COVID-19:We should be prepared. J Mycol Med. 2020;30(2):100971. doi: 10.1016/j.mycmed.2020.100971. https://doi.org/10.1016/j.mycmed.2020.100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 aHospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9. doi: 10.1001/jama.2020.1585. https://doi.org/10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y, Yang Q, Chi J, Dong B, Lv W, Shen L, et al. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019:A systematic review and meta-analysis. Int J Infect Dis. 2020;99:47–56. doi: 10.1016/j.ijid.2020.07.029. https://doi.org/10.1016/j.ijid.2020.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanyaolu A, Okorie C, Marinkovic A, Patidar R, Younis K, Desai P, et al. Comorbidity and its Impact on Patients with COVID-19. SN Compr Clin Med. 2020;2(8):1069–76. doi: 10.1007/s42399-020-00363-4. https://doi.org/10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 apatients with COVID-19 in China:A nationwide analysis. Eur Respir J. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joseph C, Togawa Y, Shindo N. Bacterial and viral infections associated with influenza. Influenza Other Respir Viruses. 2013;7(2):105–13. doi: 10.1111/irv.12089. https://doi.org/10.1111/irv.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baddley JW. Covid-19 aassociated pulmonary aspergillosis:Do we have the capacity to improve outcomes? Clin Infect Dis. 2021;74(1):92–4. doi: 10.1093/cid/ciab259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong WH, Saha BK, Ramani A, Chopra A. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 apneumonia . Infection. 2021;49(4):591–605. doi: 10.1007/s15010-021-01602-z. https://doi.org/10.1007/s15010-021-01602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chong WH, Neu KP. Incidence, diagnosis and outcomes of COVID-19-associated pulmonary aspergillosis (CAPA):A systematic review. J Hosp Infect. 2021;113:115–29. doi: 10.1016/j.jhin.2021.04.012. https://doi.org/10.1016/j.jhin.2021.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chong WH, Chieng H, Tiwari A, Beegle S, Feustel PJ, Ghalib S, et al. Incidence and risk factors for secondary pulmonary infections in patients hospitalized with coronavirus disease 2019 apneumonia . Am J Med Sci. 2022;363(6):476–83. doi: 10.1016/j.amjms.2021.04.007. https://doi.org/10.1016/j.amjms.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Liao B, Cheng L, Peng X, Xu X, Li Y, et al. The microbial coinfection in COVID-19. Appl Microbiol Biotechnol. 2020;104(18):7777–85. doi: 10.1007/s00253-020-10814-6. https://doi.org/10.1007/s00253-020-10814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koehler P, Bassetti M, Chakrabarti A, Chen SCA, Colombo AL, Hoenigl M, et al. Defining and managing COVID-19-associated pulmonary aspergillosis:The 2020 aECMM/ISHAM consensus criteria for research and clinical guidance . Lancet Infect Dis. 2021;21(6):e149–62. doi: 10.1016/S1473-3099(20)30847-1. https://doi.org/10.1016/s1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alanio A, Dellio S, Voicu S, Bretagne S, Metagned B. The presence of Pneumocystis jirovecii in critically ill patients with COVID-19. J Infect. 2021;82(4):84–123. doi: 10.1016/j.jinf.2020.10.034. https://doi.org/10.1016/j.jinf.2020.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman H, Snell LB, Simons R, Douthwaite ST, Lee MJ. Coronavirus disease 2019 aand Pneumocystis jirovecii pneumonia:A diagnostic dilemma in HIV. AIDS. 2020;34(8):1258–60. doi: 10.1097/QAD.0000000000002571. https://doi.org/10.1097/qad.0000000000002571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menon AA, Berg DD, Brea EJ, Deutsch AJ, Kidia KK, Thurber EG, et al. ACase of COVID-19 aand Pneumocystis jirovecii Coinfection. Am J Respir Crit Care Med. 2020;202(1):136–8. doi: 10.1164/rccm.202003-0766LE. https://doi.org/10.1164/rccm.202003-0766le. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blaize M, Mayaux J, Luyt CE, Lampros A, Fekkar A. Covid-19-related respiratory failure and lymphopenia do not seem associated with pneumocystosis. Am J Respir Crit Care Med. 2020;202(12):1734–36. doi: 10.1164/rccm.202007-2938LE. https://doi.org/10.1164/rccm.202007-2938le. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris A, Norris KA. Colonization by Pneumocystis jirovecii and its role in disease. Clin Microbiol Rev. 2012;25(2):297–317. doi: 10.1128/CMR.00013-12. https://doi.org/10.1128/cmr.00013-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shipley TW, Kling HM, Morris A, Patil S, Kristoff J, Guyach SE, et al. Persistent pneumocystis colonization leads to the development of chronic obstructive pulmonary disease in a nonhuman primate model of AIDS. J Infect Dis. 2010;202(2):302–12. doi: 10.1086/653485. https://doi.org/10.1086/653485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eddens T, Campfield BT, Serody K, Manni ML, Horne W, Elsegeiny W, et al. ANovel CD4+ T cell-dependent murine model of pneumocystis-driven asthma-like pathology. Am J Respir Crit Care Med. 2016;194(7):807–20. doi: 10.1164/rccm.201511-2205OC. https://doi.org/10.1164/rccm.201511-2205oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sepkowitz KA. Pneumocystis carinii pneumonia without acquired immunodeficiency syndrome:Who should receive prophylaxis? Mayo Clin Proc. 1996;71(1):102–3. doi: 10.4065/71.1.102. https://doi.org/10.4065/71.1.102. [DOI] [PubMed] [Google Scholar]
- 21.Lu X, Sakthivel SK, Wang L, Lynch B, Dollard SM. Enhanced throughput of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) real-time RT-PCR panel by assay multiplexing and specimen pooling. J Virol Methods. 2021;293:114149. doi: 10.1016/j.jviromet.2021.114149. https://doi.org/10.1016/j.jviromet.2021.114149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mondal S, Feirer N, Brockman M, Preston MA, Teter SJ, Ma D, et al. Adirect capture method for purification and detection of viral nucleic acid enables epidemiological surveillance of SARS-CoV-2. Sci Total Environ. 2021;795:148834. doi: 10.1016/j.scitotenv.2021.148834. https://doi.org/10.1016/j.scitotenv.2021.148834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alsayed A, Al-Doori A, Al-Dulaimi A, Alnaseri A, Abuhashish J, Aliasin K, et al. iInfluences of bovine colostrum on nasal swab microbiome and viral upper respiratory tract infections-a case report. Respir Med Case Rep. 2020;31:101189. doi: 10.1016/j.rmcr.2020.101189. https://doi.org/10.1016/j.rmcr.2020.101189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim WS. Pneumonia-overview. Reference Module Biomed Sci. 2020;2:185–197. [Google Scholar]
- 25.World Health Organization. Living guidance for clinical management of COVID-19:Living guidance. Geneva: World Health Organization; 2021. [Google Scholar]
- 26.Chew LC, Maceda-Galang LM, Tan YK, Chakraborty B, Thumboo J. Pneumocystis jirovecii pneumonia in patients with autoimmune disease on high-dose glucocorticoid. J Clin Rheumatol. 2015;21(2):72–5. doi: 10.1097/RHU.0000000000000215. https://doi.org/10.1097/rhu.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 27.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 acases of 2019 novel coronavirus pneumonia in Wuhan, China:A descriptive study . Lancet. 2020;395(10223):507–13. doi: 10.1016/S0140-6736(20)30211-7. https://doi.org/10.1016/s0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mang S, Kaddu-Mulindwa D, Metz C, Becker A, Seiler F, Smola S, et al. Pneumocystis jirovecii pneumonia and severe acute respiratory syndrome coronavirus 2 acoinfection in a patient with newly diagnosed HIV-1 infection . Clin Infect Dis. 2021;72(8):1487–9. doi: 10.1093/cid/ciaa906. https://doi.org/10.1093/cid/ciaa906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Francesco MA, Alberici F, Bossini N, Scolari F, Pascucci F, Tomasoni G, et al. Pneumocystis jirevocii and SARS-CoV-2 aco-infection:A common feature in transplant recipients? Vaccines (Basel) 2020;8(3):E544. doi: 10.3390/vaccines8030544. https://doi.org/10.3390/vaccines8030544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Permpalung N, Kittipibul V, Mekraksakit P, Rattanawong P, Nematollahi S, Zhang SX, et al. Acomprehensive evaluation of risk factors for Pneumocystis jirovecii pneumonia in adult solid organ transplant recipients:A systematic review and meta-analysis. Transplantation. 2021;105(10):2291–306. doi: 10.1097/TP.0000000000003576. https://doi.org/10.1097/tp.0000000000003576. [DOI] [PubMed] [Google Scholar]
- 31.Cai S, Sun W, Li M, Dong L. A complex COVID-19 acase with rheumatoid arthritis treated with tocilizumab . Clin Rheumatol. 2020;39(9):2797–802. doi: 10.1007/s10067-020-05234-w. https://doi.org/10.1007/s10067-020-05234-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammarström H, Grankvist A, Broman I, Kondori N, Wennerås C, Gisslen M, et al. Serum-based diagnosis of pneumocystis pneumonia by detection of pneumocystis jirovecii DNA and 1,3-b-d-glucan in hiv-infected patients:A retrospective case control study. BMC Infect Dis. 2019;19(1):658. doi: 10.1186/s12879-019-4289-4. https://doi.org/10.1186/s12879-019-4289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parker A, Shaw J, Karamchand S, Lahri S, Schrueder N, Chothia MY, et al. HIV and SARS-CoV-2 aco-infection:The diagnostic challenges of dual pandemics. S Afr Med J. 2020;110(6):473–5. doi: 10.7196/SAMJ.2020.v110i6.14825. https://doi.org/10.7196/samj.2020.iav110i6.14825. [DOI] [PubMed] [Google Scholar]
- 34.Hanfi SH, Lalani TK, Saghir A, McIntosh LJ, Lo HS, Kotecha HM. COVID-19 aand its mimics:What the radiologist needs to know. J Thorac Imaging. 2021;36(1):W1–10. doi: 10.1097/RTI.0000000000000554. https://doi.org/10.1097/rti.0000000000000554. [DOI] [PubMed] [Google Scholar]
- 35.Kanduc D, Shoenfeld Y. On the molecular determinants of the SARS-CoV-2 aattack. Clin Immunol. 2020;215:108426. doi: 10.1016/j.clim.2020.108426. https://doi.org/10.1016/j.clim.2020.108426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.RECOVERY Collaborative. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. https://doi.org/10.1056/nejmoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun HB, Zhang YM, Huang LG, Lai QN, Mo Q, Ye XZ, et al. The changes of the peripheral CD4+lymphocytes and inflammatory cytokines in patients with COVID-19. PLoS One. 2020;15(9):0239532. doi: 10.1371/journal.pone.0239532. https://doi.org/10.1371/journal.pone.0239532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nasim S, Kumar S, Azim D, Ashraf Z, Azeem Q. Corticosteroid use for 2019-nCoV infection:A double-edged sword. Infect Control Hosp Epidemiol. 2020;41(10):1244–5. doi: 10.1017/ice.2020.165. https://doi.org/10.1017/ice.2020.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel R, Babady E, Theel ES, Storch GA, Pinsky BA, St G, eorge K, et al. Report from the American society for microbiology COVID-19 ainternational summit, 23 March 2020:Value of diagnostic testing for SARS-CoV-2/COVID-19. mBio. 2020;11(2):e00722-20. doi: 10.1128/mBio.00722-20. https://doi.org/10.1128/mbio.00722-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Younes N, Al-Sadeq DW, Al-Jighefee H, Younes S, Al-Jamal O, Daas HI, et al. Challenges in laboratory diagnosis of the novel coronavirus SARS-CoV-2. Viruses. 2020;12(6):E582. doi: 10.3390/v12060582. https://doi.org/10.3390/v12060582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masur H, Brooks JT, Benson CA, Holmes KK, Pau AK, Kaplan JE, et al. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents:Updated guidelines from the centers for disease control and prevention, national institutes of health, and HIV medicine association of the infectious diseases society of America. Clin Infect Dis. 2014;58(9):1308–11. doi: 10.1093/cid/ciu094. https://doi.org/10.1093/cid/ciu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubiano C, Tompkins K, Sellers SA, Bramson B, Eron J, Parr JB, et al. Pneumocystis and severe acute respiratory syndrome coronavirus 2 acoinfection:A case report and review of an emerging diagnostic dilemma. Open Forum Infect Dis. 2021;8(1):ofaa633. doi: 10.1093/ofid/ofaa633. https://doi.org/10.1093/ofid/ofaa633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutinaa-OsOcampo E, Villamizar-Pela R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19:A systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. https://doi.org/10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 ain china. N Engl J Med. 2020;382(18):1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White PL, Price JS, Backx M. Therapy and Management of Pneumocystis jirovecii Infection. J Fungi (Basel) 2018;4(4):E127. doi: 10.3390/jof4040127. https://doi.org/10.3390/jof4040127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 anovel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Apfalter P, Assadian O, Blasi F, Boman J, Gaydos CA, Kundi M, et al. iReliability of nested PCR for detection of Chlamydia pneumoniae DNA in atheromas:Results from a multicenter study applying standardized protocols. J Clin Microbiol. 2002;40(12):4428–34. doi: 10.1128/JCM.40.12.4428-4434.2002. https://doi.org/10.1128/jcm.40.12.4428-4434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Desai H, Eschberger K, Wrona C, Grove L, Agrawal A, Grant B, et al. iBacterial colonization increases daily symptoms in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(3):303–9. doi: 10.1513/AnnalsATS.201310-350OC. https://doi.org/10.1513/annalsats.201310-350oc. [DOI] [PubMed] [Google Scholar]
- 49.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359(22):2355–65. doi: 10.1056/NEJMra0800353. https://doi.org/10.1056/nejmra0800353. [DOI] [PubMed] [Google Scholar]
- 50.Murdoch DR. Molecular genetic methods in the diagnosis of lower respiratory tract infections. APMIS. 2004;112((11-12)):713–27. doi: 10.1111/j.1600-0463.2004.apm11211-1202.x. https://doi.org/10.1111/j.1600-0463.2004.iaapm11211-1202.x. [DOI] [PubMed] [Google Scholar]
- 51.Goldman DL, Chen Z, Shankar V, Tyberg M, Vicencio A, Burk R. Lower airway microbiota and mycobiota in children with severe asthma. J Allergy Clin Immunol. 2018;141(2):808–11e7. doi: 10.1016/j.jaci.2017.09.018. https://doi.org/10.1016/j.jaci.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 52.Gingo MR, Lucht L, Daly KR, Djawe K, Palella FJ, Abraham AG, et al. Serologic responses to pneumocystis proteins in HIV patients with and without Pneumocystis jirovecii pneumonia. J Acquir Immune Defic Syndr. 2011;57(3):190–6. doi: 10.1097/QAI.0b013e3182167516. https://doi.org/10.1097/qai.0b013e3182167516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rayens E, Noble B, Vicencio A, Goldman DL, Bunyavanich S, Norris KA. Relationship of Pneumocystis antibody responses to paediatric asthma severity. BMJ Open Respir Res. 2021;8(1):000842. doi: 10.1136/bmjresp-2020-000842. https://doi.org/10.1136/bmjresp-2020-000842. [DOI] [PMC free article] [PubMed] [Google Scholar]


