Abstract
Aims:
To examine the associations of average weight and weight velocity in three growth periods from birth through adolescence with type 2 diabetes incidence.
Methods:
Child participants representing three growth periods- pre-adolescence (birth to ~8 years), early adolescence (~8 to ~13 years), and late adolescence (~13 to ~18 years) -were selected from a 43-year longitudinal study of American Indians. Age-sex-height-standardized average weight z score and weight z score velocity (change/year) were computed in each period. Participants were followed for up to 25 years from the end of each growth period until they developed diabetes. Associations of average weight z score or weight z score velocity with diabetes incidence were determined with sex-, birth date- and maternal diabetes-adjusted Poisson regression models.
Results:
Among 2,100 participants representing the pre-adolescence growth period, 1,558 representing the early adolescence period, and 1,418 representing the late adolescence period, there were 290, 315, and 380 incident diabetes cases, respectively. During the first 10 years of follow-up, diabetes incidence rate ratio was 1.72 (95% CI 1.40–2.11)/standard deviation (SD) of log10weight z score mean in pre-adolescence, 2.09 (1.68–2.60)/SD of log10weight z score mean in early adolescence, and 1.85 (1.58–2.17)/SD of log10weight z score mean in late adolescence. Diabetes incidence rate ratio was 1.79 (1.49–2.17)/SD of log10weight z score velocity pre-adolescence, 1.13 (0.91–1.41)/SD log10weight z score velocity in early adolescence, and 1.29 (1.09–1.51)/SD log10weight z score velocity in late adolescence. There were strong correlations in the average weight z scores and weak correlations in the weight z score velocities between successive periods.
Conclusions:
Higher weight and accelerated weight gain in all growth periods associates with increased type 2 diabetes risk. Importantly, higher weight and greater weight velocity pre-adolescence jointly associate with the highest type 2 diabetes risk.
Keywords: pediatric obesity, growth, child health, diabetes mellitus, Type 2, incidence study
Introduction
The incidence and prevalence of obesity are increasing among children and adolescents in the United States1,2 and coincide with an increasing type 2 diabetes incidence among youth and young adults.3,4 Excessive body weight in childhood or adolescence often persists into adulthood,5 thereby increasing the risk of early-onset type 2 diabetes.6,7 Given the significant long-term complications and mortality associated with early-onset type 2 diabetes,8,9 careful monitoring of growth in childhood and adolescence is important. However, available evidence on the impact of variability in weight gain at critical growth periods in childhood and adolescence on type 2 diabetes development is limited by a paucity of serial anthropometric data for weight tracking from early childhood or of prospective biochemical data for ascertaining type 2 diabetes incidence.6,10–19
Pima Indians living in the Southwestern United States have a high prevalence of obesity and type 2 diabetes, especially among children and young adults,20,21 with considerable long-term burden associated with these comorbidities.22 In the present study, we use longitudinal data obtained from Pima children and adults to identify critical growth periods during childhood and adolescence that are associated with type 2 diabetes incidence. We hypothesized that both higher average weight and greater weight velocity in childhood and adolescence associate with greater type 2 diabetes risk, and that these associations differ by the growth period.
Methods
Study design and participants
A longitudinal open cohort study of diabetes and related complications was conducted from 1965 through 2007 among residents of the Gila River Indian Community (GRIC) in Arizona aged ≥ 5 years. Diabetes in this community is thought to be exclusively type 2, as available data from both child and adult residents of this community with diabetes suggest no autoimmunity (i.e. islet cell antibodies including GAD65Ab) typical of type 1 diabetes or insulin dependence even in those with very young onset.23,24
Participants were invited to undertake standardized research examinations approximately every two years. Although participants in the longitudinal study were aged ≥5 years, data on events preceding participation in the longitudinal study (e.g. weight at birth) were obtained from secondary sources (e.g. medical records and birth certificates) when available. Because participants entered the biennial study at different ages and the time points at which research examinations occurred were not uniform in all participants, there were limited serial data in childhood. Therefore, to maximize the available data, the present study used data obtained at four time points in childhood (Fig. 1): birth, age ~8 years (range 7–9 years at the last birthday, median 8.4), age ~13 years (range 12–14, median 13.2), and age ~18 years (range 17–19, median 18.3). If a participant had more than one examination in the window defining a time point, only the examination closest to the midpoint age was used. These time points were used to define three sequential growth periods: pre-adolescence (birth to ~8 years), early adolescence (~8 to ~13 years), and late adolescence (~13 to ~18 years; Fig. 1).
Fig. 1.
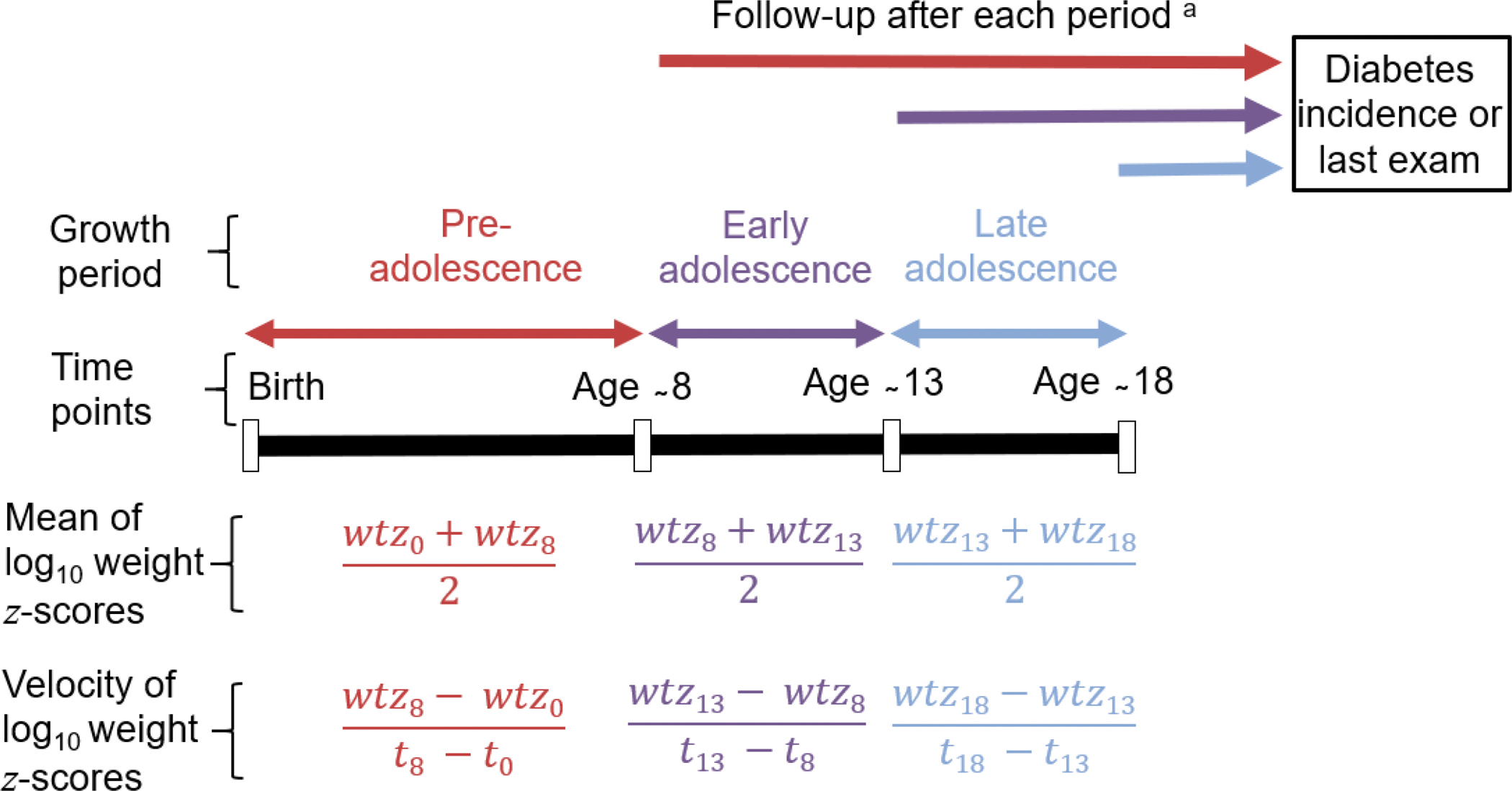
Study design and calculation of average weight z scores and weight velocity z scores. Age ~8 years ranged from 7–9, median 8.4; Age ~13 years ranged from 12–14, median 13.2; Age ~18 years ranged from 17–19, median 18.3; wtz is the log10weight z score at a time point.
a Given that analyses were conducted separately for each growth period, a participant representing more than one growth period could be followed for varying lengths of time as the start point for follow-up varies by growth period.
Although the period of adolescence is typically defined as being between 10–19 years of age, this growth period varies across populations and contexts.25 Data are limited on age at puberty among American Indians. Therefore, we selected the age range of 7–9 years as the endpoint for pre-adolescence given that the mean age at menarche among Pima Indian girls is 12 years26 and that menarche is 2–3 years after the onset of the first signs of puberty. Coincidentally, most of the available data points in early childhood were concentrated in this age range. We selected age ranges 12–14 years and 17–19 years as end points for early and late adolescence respectively to maximize available data points in adolescence while reflecting the variability in growth at this stage of childhood.
Participants were included in the analysis for a growth period if they had measurements of height and weight at the beginning and the end of the period; if they did not have diabetes during the period (ascertained using OGTTs at the research examinations including examinations defining the growth periods); and if they had at least one research examination after the period. Participants could be included in the analysis for more than one growth period. The study was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases. Minors (aged <18 years) gave assent, and written informed consent was obtained from their parents. Participants aged ≥18 years provided written informed consent.
Data collection
At each research examination, height was measured to the nearest centimeter and weight to the nearest kilogram while participants were dressed in light clothing without shoes. Birthweights were obtained from birth certificates and review of medical records. A 75-gram oral glucose tolerance test (OGTT) was performed at each examination. Diabetes was diagnosed by World Health Organization criteria when a 2-hour plasma glucose concentration was ≥11.1 mmol/l (200 mg/dl),27 or when a diagnosis was documented during routine clinical care between research examinations. More recently adopted tests for diabetes, such as the fasting plasma glucose and glycated hemoglobin, were not used in this analysis because they were not used throughout the longitudinal study that began in 1965. The same methods were used to ascertain maternal diabetes prior to the birth of the participant, i.e. diagnosis was by OGTT or documented clinical diagnosis rather than by self-report. A subgroup of 2,630 participants was assessed for functional mutation of melanocortin-4 receptor (MC4R), a common genetic cause of both obesity and type 2 diabetes in childhood and adolescence in this study population.26 The MC4R variants have a larger effect on obesity traits among Pima Indians than other known genetic variants associated with obesity in childhood and adolescence.
Statistical analysis
Characteristics of participants were summarized with descriptive statistics, including median, 25th (Q1) and 75th (Q3) centiles for non-normally distributed variables. The objective of this analysis was to estimate the associations with diabetes incidence of two growth parameters at different points in childhood - standardized weight at a point and standardized weight velocity at an interval leading to that point. To achieve this, we modeled weight-for-height rather than body mass index (BMI). This is because modeled weight-for-height is more appropriate for modeling growth in children than BMI as the latter leaves residual confounding by height.28 Therefore, weights in childhood and adolescence were standardized to the study population to obtain z scores by (1) identifying sex-specific age- and height-adjusted regression models that provide the best fit for weight, i.e. log10weight=β0 + β1age + β2height for males and females; and (2) standardizing residuals from each model to a mean=0 and standard deviation (SD)=1. Because data on gestational age and length at birth were unavailable, birthweight was standardized to have a mean=0 and SD=1, separately by sex.
Standardized measures of weight at a point and of weight velocity during an interval leading to that point are strongly correlated thereby limiting the ability to clearly delineate their individual and joint associations. Given that this correlation can be considerably minimized by using the average and the difference of two scores,29,30 we analyzed both the average weight z score and the weight z score velocity of each participant for each growth period (Fig. 1). The average weight z score was the z score of the mean of log10weight z scores at the points defining a growth period, while the weight z score velocity was the z score of the difference in log10weight z scores divided by the length (in years) of the growth period (Fig. 1). Growth was modeled using this approach because of limited data at regular age intervals in childhood in this study.
Although there is some correlation introduced by using some of the same measures to calculate the average weight z score or weight velocity z score velocity for the growth periods (Fig. 1), these overlapping measures were not included in the same model. In other words, analyses of diabetes incidence were conducted separately for each growth period, and thus, separately for each group of participants representing the growth periods. Each person was followed from the examination that defined the end of the corresponding growth period until diabetes incidence or the last research examination, whichever came first (Fig. 1). Although some participants had a follow-up period >25 years, analyses were restricted to up to 25 years of follow-up because of limited (<1000 person-years) follow-up afterwards which may reduce the precision of the incidence rate estimates; and to ensure better balance in the length of follow-up between groups than when follow-up is extended beyond 25 years. Given that analyses were conducted separately for each group, a participant representing more than one group could be followed for varying lengths of time as the start point for follow-up varied by growth period.
Diabetes incidence rate was calculated per 1000 person-years of follow-up. Poisson regression was used to model diabetes incidence rate ratios (IRRs) for the average weight z score or weight z score velocity in a growth period with the offset term, i.e. log (person-years), accounting for different length of follow-up. Models were adjusted for sex, birth date and maternal diabetes. For each growth period, average weight z score and weight z score velocity were analyzed in separate models (to estimate their individual associations) and then jointly in the same model (to estimate their joint associations). In some models (particularly those estimating the IRR for average weight z scores), associations of the average weight z score or weight z score velocity with diabetes IRR, were non-proportional (i.e. non-constant) over time. Therefore, time was split into 5-year intervals follow-up and IRRs were estimated with increasingly longer 5-year intervals follow-up. For example, a participant who was censored at the 18th year of follow-up will be included in the analysis for <5 years, <10 years, <15 years, and <20 years.
All models were tested for potential interactions of the exposure variables with sex, birth date or maternal diabetes by including product terms. Models were also tested for any quadratic (non-linear) associations of the weight variables with type 2 diabetes incidence.
A bootstrap analysis was performed to account for the partial overlap of participants representing the growth periods and for any potential imbalance of covariates between groups and for any correlation arising from using some of the same measures to calculate the average weight z score or weight velocity z score for the growth periods. Briefly, we drew 10,000 random samples of participants in the longitudinal analysis with replacement to obtain a bootstrapped estimate of the standard error of the difference in risk estimates between the growth periods; this standard error was used to assess the significance of the difference. This approach assumes that the bootstrapped statistic approximates a normal distribution.31
A few sensitivity analyses were performed. To use all available data points in childhood in these participants, we estimated weight z score velocities using the linear spine mixed-effects model.32 This multilevel approach involved using piecewise linear splines with knots at 9 years (at the last birthday) and 14 years (at the last birthday). The positions of the knots were selected to obtain linear slopes of log10weight z scores corresponding to the growth periods analyzed in the initial (simple) growth modeling approach, i.e. birth-9 years, 9–14 years, and 14–19 years. The estimated slopes, which varied between participants, were standardized to mean=0 and SD=1 and were subsequently used as continuous variables in Poisson regression models to estimate diabetes IRRs. The slope in a growth period was adjusted for the average weight z score in that period and other covariates, such as, sex, birth date and maternal diabetes. For more details on these analyses, please see the SAS syntax in the methods section of the ESM.
To test whether risk estimates were influenced by exposure to maternal diabetes, models were restricted to participants without such exposure. Similarly, models were also restricted to participants without functional mutations in the MC4R gene to test any influence of such mutations. Finally, models were adjusted for birthweight in analyses restricted to participants with data on birthweight.
All analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC). A two-sided p <0.05 was considered statistically significant.
Results
Characteristics of participants
This study comprised 2,100 child participants representing the pre-adolescence growth period, 1,558 representing the early adolescence period, and 1,418 representing the late adolescence period. Overall, the present analysis included 3,107 participants with some participants representing more than one growth period. Participants representing the late adolescence were more often females (63.3%) than those representing the pre-adolescence period (52.2%) and the early adolescence period (55.2%; Table 1). Exposure to maternal diabetes was similarly common among participants representing the growth periods. There is balance in the median follow-up period to the end point for this analysis. However, participants representing pre-adolescence had a greater median number of follow-up visits (n=6) than those representing older growth periods (n=3; Table 1). The percentage of subsequent diabetes diagnoses made in follow-up at research examinations (as opposed to from clinical data) was comparable for the different growth periods (59.3% for pre-adolescence, 56.8% for early adolescence and 54.2% for late adolescence).
Table 1.
Participant characteristics by growth period
| Pre-adolescence a (n=2100) | Early adolescence a (n=1558) | Late adolescence a (n=1418) | |
|---|---|---|---|
|
| |||
| Female (%) | 1097 (52.2) | 860 (55.2) | 898 (63.3) |
| Birthweight (kg) b | 3.45 (3.15, 3.75) | 3.45 (3.15, 3.75) | 3.44 (3.13, 3.75) |
| Mean ± SD weight (kg) | 19.2 ± 5.7 | 49.8 ± 14.3 | 72.6 ± 19.4 |
| Mean ± SD weight velocity (kg/year) | 3.7 ± 1.2 | 6.5 ± 2.4 | 3.6 ± 2.5 |
| Maternal diabetes (%) | 264 (12.8) | 196 (12.6) | 143 (10.1) |
| Median (Q1, Q3) years of follow-up | |||
| Up to the end of the longitudinal study | 12.7 (6.4, 30.0) | 14.6 (6.1, 24.9) | 14.3 (5.8, 23.2) |
| When analyses were restricted to up to 25 years | 11.2 (6.1, 22.1) | 11.5 (5.3, 20.8) | 9.8 (4.3, 18.5) |
| Median (Q1, Q3) follow-up visits | |||
| Up to the end of the longitudinal study | 6 (3, 11) | 3 (2, 6) | 3 (2, 6) |
| When analyses were restricted to up to 25 years | 5 (3, 10) | 3 (2, 5) | 3 (1, 5) |
Q, quartile; SD, standard deviation
Pre-adolescence is birth to ∼8yr, early adolescence is ∼8yr to ∼13yr, and late adolescence is ∼13yr to ∼18yr (see Methods for precise definitions of the three growth periods)
Data are available for 1244 participants representing the early adolescence period and 990 participants representing the late adolescence period.
Among participants representing more than one growth period, there were significant correlations in the average weight z scores between successive periods, i.e. r=0.67 between weight z scores in pre-adolescence and early adolescence, r=0.92 between early adolescence and late adolescence, and r=0.49 between pre-adolescence and late adolescence (Fig. 2). By contrast, only weight z score velocities in early and late adolescence showed significant but weak negative correlation (r=−0.19).
Fig. 2.
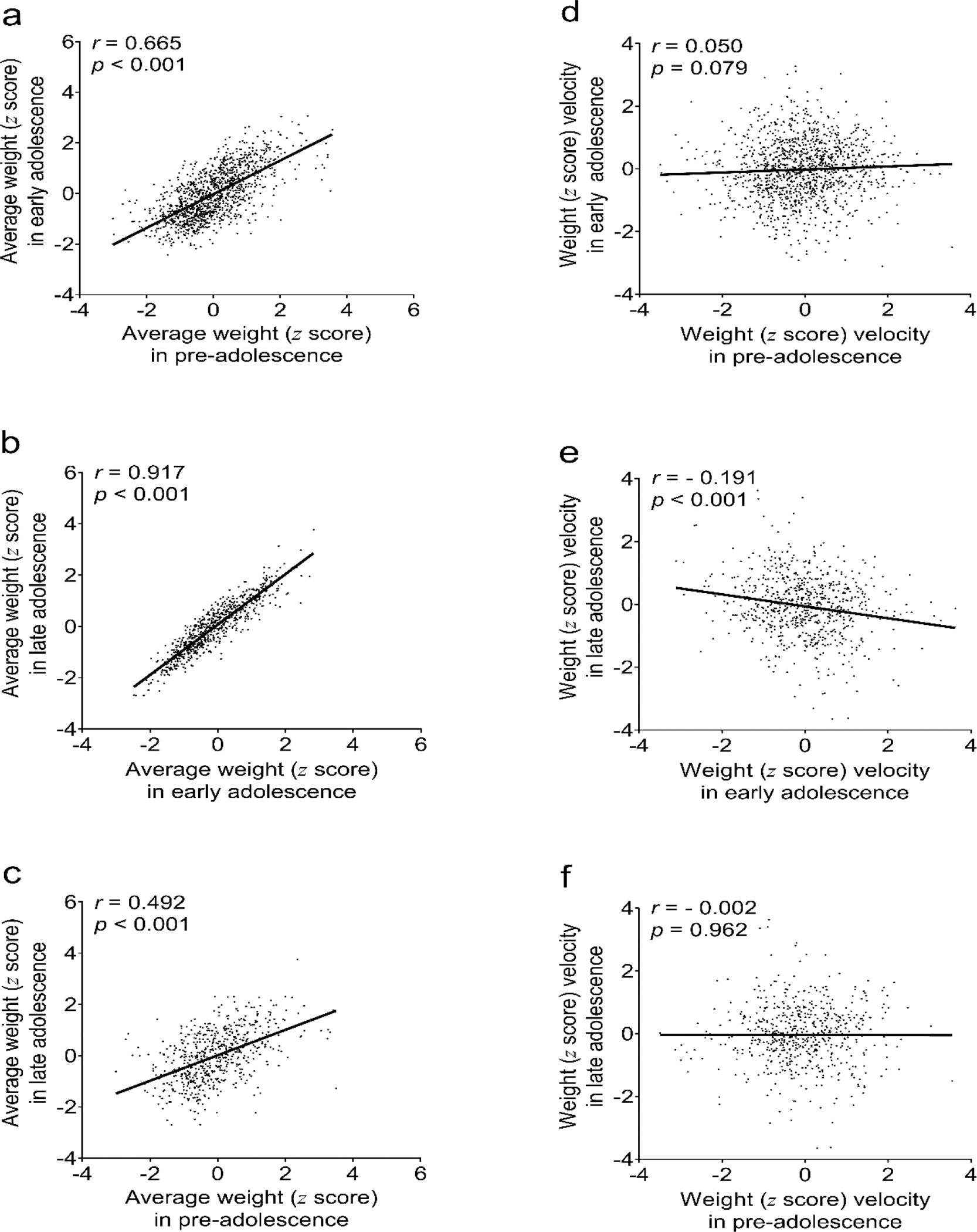
Correlations of pre-, early, and late adolescence age-sex-height-standardized average weight z score (a-c) and weight z score velocity (d-f).
r, Pearson’s correlation coefficient; p, p-value of the correlation coefficient. number of participants analyzed: 1244 for (a) and (d), 725 for (b) and (e), and 595 for (c) and (f). Pre-adolescence is birth to ~8yr, early adolescence is ~8yr to ~13yr, and late adolescence is ~13yr to ~18yr (see Methods for precise definitions of the three growth periods).
Diabetes incidence
During up to 25 years of follow-up from the end of the growth periods, there were 290 incident cases of diabetes among participants representing the pre-adolescence growth period (10.1 per 1000 person-years), 315 in those representing the early adolescence growth period (15.2 per 1000 person-years), and 380 in those representing the late adolescence growth period (22.0 per 1000 person-years; Table 1). Overall, there were 607 unique incident diabetes cases of which 63% were females. At the first research examination with diabetes, the mean age was 28 years and 89% were obese (BMI ≥30 kg/m2; ESM Table 1). Incidence rate estimates became less precise when analyses were extended beyond 25 years of follow-up because fewer participants had >25 years of follow-up (ESM Table 2).
Adjusted for sex, birth date, and maternal diabetes, greater average weight z score in each growth period associated with increased diabetes incidence during the first 10 years of follow-up (Fig. 3a; ESM Table 3), i.e. IRR 1.72 per SD of log10weight z-score pre-adolescence (95% CI 1.40–2.11), IRR 2.09/SD of log10weight z-score in early adolescence (CI 1.68–2.60), and IRR 1.86/SD of log10weight z-score in late adolescence (CI 1.58–2.17). Although these associations declined steadily over time (ESM Fig. 1a–c), they persisted for up to 25 years of follow-up (Fig. 3b). Comparing associations across the growth periods, there was no significant between-growth period difference in the association of the average weight z score with diabetes incidence (p ≥0.05).
Fig. 3.

Sex-, date of birth- and maternal diabetes-adjusted individual association by growth period of average weight z score with type 2 diabetes incidence during (a) the first 10 years and (b) up to 25 years of follow-up; and of weight z score velocity with type 2 diabetes incidence during (c) the first 10 years and (d) up to 25 years of follow-up.
IRR, incidence rate ratio.
Pre-adolescence is birth to ~8yr, early adolescence is ~8yr to ~13yr, and late adolescence is ~13yr to ~18yr (see Methods for precise definitions of the three growth periods).
Weight z score velocity pre-adolescence significantly associated with diabetes incidence during the first 10 years of follow-up (IRR 1.79/SD of log10weight z score velocity, 95% CI 1.49–2.17; Fig. 3c; ESM Table 3), with this significant association persisting for up to 25 years of follow-up (Fig. 3d; ESM Fig. 1d). Weight z score velocity in early adolescence had no significant effect on subsequent diabetes incidence during the first 10 years of follow-up (IRR 1.13/SD of log10weight z score velocity, 95% CI 0.91–1.41; Fig. 3c) but had a significant but relatively weak association when incidence was extended to up to 25 years of follow-up (IRR 1.16/SD of log10weight z score velocity, CI 1.05–1.30; Fig. 3d; ESM Fig. 1e). Similarly, compared to the weight z score velocity pre-adolescence, the cumulative association of weight z score velocity in late adolescence with diabetes incidence was weak during the first 10 years of follow-up (IRR 1.29/SD of log10weight z score velocity, CI 1.09–1.51) and up to 25 years of follow-up (IRR 1.19/SD of log10weight z score velocity, CI 1.07–1.32; Fig. 3c–d; ESM Fig. 1f). Overall, weight z score velocity in pre-adolescence had a significantly greater association with diabetes incidence than those in early adolescence (p<0.001) and late adolescence (p=0.016).
For each growth period, the pattern and magnitude of individual associations of the average weight z score and weight z score velocity with diabetes incidence (Fig. 3; ESM Table 3) were similar to those of their joint associations (ESM Table 4). There were no significant interactions of sex, birth date, or maternal diabetes with either the average weight z score or weight z score velocity in any analyses. Similarly, there was no significant non-linear association between the weight variables and type 2 diabetes incidence.
Sensitivity analysis
Findings from the linear spine mixed-effects model, adjusted for average weight z score, sex, birth date and maternal diabetes, were generally similar to those of the initial (simple) modeling approach with weight z score velocity pre-adolescence more strongly associated with type 2 diabetes incidence than weight z score velocities in early and late adolescence (ESM Tables 5). However, the linear spine mixed-effects model, unlike the simple model, showed a significant association between weight z score velocity in early adolescence and type 2 diabetes incidence during the first 10 years of follow-up (IRR 1.34/SD of log10weight z score, CI 1.07–1.68) and up to 25 years of follow-up (IRR 1.25/SD of log10weight z score, CI 1.07–1.37).
Results were substantially similar when models were restricted to participants without exposure to maternal diabetes (ESM Table 6) or when models were restricted to participants with no known MC4R deficiency (data not shown). Similarly, no meaningful difference was observed between results of analyses adjusted for birthweight compared to models not adjusted for this covariate (ESM Table 7); and in results of models comprising weight z scores (Fig. 3) compared with those comprising BMI z scores (ESM Fig. 2).
Discussion
Statement of principal findings
In this longitudinal analysis, higher weight in all growth periods significantly associated with greater type 2 diabetes incidence, as did accelerated weight gain in pre- and late- adolescence. Importantly, higher weight and greater weight velocity pre-adolescence jointly associated with the highest type 2 diabetes risk; these associations were similar, but weaker, for higher weights and greater weight velocities in early and late adolescence.
Strengths and weaknesses of the study
The strength of this study is the prospective ascertainment of diabetes using OGTTs. In addition, all anthropometric and biochemical measurements were performed at the same center and in the same laboratory for the entire 43-year study. Importantly, our longitudinal data permit the identification of critical and clinically meaningful growth periods.
Our study did not adjust birthweight for length at birth and gestational age, or adjust analyses for age at puberty, diet, physical activity, or socioeconomic status because of insufficient data on these variables. Similarly, due to limited serial research examination in childhood, growth was modelled using a simple approach that used weight measures from selected data points rather than all available data points. However, conclusions were not substantially affected when all available data were used in a linear spline mixed-effects model. We were unable to use other analytic approaches such as growth curve modelling (requiring more data) or age-period-cohort analysis (requiring larger sample sizes at age intervals). There is potential for selection bias given the greater proportion of females among participants representing the late adolescence period, although there is no evidence that risk was modified by sex. However, our estimates should be interpreted in the context that administrative censoring limited the proportion of participants completing all growth periods, i.e. children who were younger in the early years of the longitudinal study had longer potential follow-up than those who entered the study later, closer to the end of the study. Pima Indians have a high prevalence of obesity and early-onset type 2 diabetes.19,20 However, prior findings on childhood obesity and type 2 diabetes in this population have generally been replicated in other populations.
Strengths and weaknesses in relation to other studies
Our findings on the association between childhood weight and type 2 diabetes incidence agree largely with results of previous studies, although direct comparisons are difficult given the inconsistencies in the growth periods examined, lack of longitudinal data on type 2 diabetes incidence, and differences in analytical approach. Similar to our findings, greater BMI z score at ages 4 to 19 years consistently associated with higher prevalence of prediabetes or type 2 diabetes in adulthood in the Bogalusa Heart study.12 Moreover, being overweight at age 13 years (BMI ≥21.8 kg/m2) and persistent overweight at ages 7 years (BMI ≥17.4 kg/m2) and 13 years associated with greater type 2 diabetes among adult Danish men (30–60 years).6 However, contrary to our findings, being overweight at 7 years unadjusted for subsequent weight was not associated with type 2 diabetes incidence in the Danish study. The contrasting results may be due to differences in the analytic method as BMI was categorized and diabetes incidence was assessed from age ≥30 years in the Danish study. In the present study, weight was analyzed as a continuous variable while diabetes incidence was assessed from childhood. In a larger and gender diverse Danish study,13 although there were no significant interactions of childhood BMI with covariates, analyses were stratified by BMI, sex, birth cohort, and age at diagnosis, thereby complicating the interpretation of findings. We previously showed the additive effects of post-natal BMI, birthweight, maternal diabetes, and genotype on type 2 diabetes incidence.33 In the present analysis, birthweight, maternal diabetes, and genotype did not significantly add to or modify the association between the child weight variables and type 2 diabetes incidence. Similarly, no such interactions were reported in other studies.6,12,13
The observed association between greater weight gain in childhood and type 2 diabetes risk, especially the relatively stronger association of weight gain in early childhood, agrees with findings from a study of female nurses in the United States, although, in that study, childhood adiposity was assessed by recall of somatotypes or pictorial diagrams.14 By contrast, our findings differ from those of the Bogalusa Heart study in which weight gain in adolescence (aged 10–19 years) and not in pre-adolescence (aged 5–9 years) significantly associated with the prevalence of prediabetes or type 2 diabetes in adulthood.12 In a Swedish study, weight change from ages 2–11 years significantly associated with type 2 diabetes (odds ratio 1.20) but not impaired glucose tolerance in adulthood (1.06), and the association was weaker when these outcomes were combined (1.12).15 Similarly, there was no significant association between weight gain in early childhood and the prevalence of hyperglycemia in other studies.16,17 The contrasting results may be due to the difference in analytic approach. In the present study, risk was evaluated from childhood based on the time at risk of diabetes. By contrast, in other studies,16,17 risk was evaluated based on diabetes prevalence in adulthood. Given that individuals with greater weight gain in early childhood are more likely to develop type 2 diabetes before adulthood, the association between weight gain in childhood and type 2 diabetes risk is more efficiently estimated if based on time at risk. In some studies,15,17–19 weight was adjusted for or weight gain was calculated from adult weight obtained close to the time of type 2 diabetes ascertainment rather than measures that clearly preceded type 2 diabetes onset, thereby making reliable comparison with our estimates difficult. Concurrent weight, being closer to diabetes diagnosis, usually accounts for most of the variability in type 2 diabetes incidence thereby weakening the association of weight variables preceding diabetes incidence.
Meaning of the study: possible explanations and implications for clinicians and policy makers
This study identifies higher weight and greater weight change in pre-adolescence to be associated with the greatest type 2 diabetes incidence. There may be several possible explanations for this finding. Children who attained a higher weight, or gained weight excessively during pre-adolescence, are exposed to adiposity induced chronic inflammatory state for a longer duration, thereby increasing their metabolic risk. Indeed, for participants representing more than one growth period, the high correlations in their longitudinal weights suggests that participants who were heavier in pre-adolescence were also heavier in adolescence, i.e. weight tracked from younger to older ages, in agreement with a previous report in this population.21 Longer duration of obesity increases type 2 diabetes incidence both in the current study population34 and in a nationally representative sample.35 Therefore, the strong association of excess weight, regardless of the period attained, with greater type 2 diabetes incidence coupled with the weaker association of weight velocities in early and late adolescence with later type 2 diabetes risk suggest that excess weight-related type 2 diabetes risk is driven largely by greater weight velocity pre-adolescence and the effect of continuing weight gain thereafter.
Preadolescence is also a period when physiological “adiposity rebound” occurs with a second rise in BMI. Although we did not assess the age of adiposity rebound in the current study, others have shown an association between early onset of adiposity rebound and metabolic dysfunction including diabetes.36,37 Pre-pubertal adiposity could also elicit an early rise in insulin resistance before the expression of physical and biochemical changes typically associated with puberty, thereby increasing the risk for type 2 diabetes.38
Unanswered questions and future research
Pre-adolescence is an important growth period for preventing and managing obesity and reducing the incidence of type 2 diabetes. Lifestyle interventions aimed at weight loss and increased physical activity reduce the incidence of type 2 diabetes in adults.39 This suggests that similar interventions for weight management in children and adolescents might be effective in preventing type 2 diabetes, although this hypothesis has not yet, to our knowledge, been tested.
Supplementary Material
Table 2.
Incidence rates of diabetes by growth period and by 5-year follow-up intervals
| Length of follow-up | Early childhood a (n=2100) |
Early adolescence a (n=1558) |
Late adolescence a (n=1418) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | n | PY | I | Cases | n | PY | I | Cases | n | PY | I | |
|
| ||||||||||||
| < 5 years | 32 | 2100 | 9820.6 | 3.3 | 33 | 1558 | 7116.7 | 4.6 | 59 | 1418 | 6361.5 | 9.3 |
| 5 to < 10 years | 45 | 1711 | 7199.0 | 6.3 | 48 | 1220 | 5216.9 | 9.2 | 96 | 1060 | 4447.6 | 21.6 |
| 10 to < 15 years | 49 | 1196 | 5104.1 | 9.6 | 68 | 893 | 3856.7 | 17.6 | 90 | 742 | 3189.4 | 28.2 |
| 15 to < 20 years | 78 | 870 | 3768.5 | 20.7 | 87 | 662 | 2777.6 | 31.3 | 76 | 528 | 2113.1 | 36.0 |
| 20 to < 25 years | 86 | 652 | 2713.8 | 31.7 | 79 | 450 | 1726.7 | 45.8 | 59 | 325 | 1191.4 | 49.5 |
| Total | 290 | 28606.0 | 10.1 | 315 | 20694.6 | 15.2 | 380 | 17303.0 | 22.0 | |||
n, number of participants followed; PY, person-years in a 5-year interval of follow-up; I, diabetes incidence rate in cases per 1000 person-years.
Pre-adolescence is birth to ∼8yr, early adolescence is ∼8yr to ∼13yr, and late adolescence is ∼13yr to ∼18yr (see Methods for precise definitions of the three growth periods).
Research in context.
What is already known about this subject?
Excessive body weight in childhood and adolescence increases the risk of early-onset type 2 diabetes and associated complications.
Limited evidence exists on critical growth periods in childhood and adolescence that predispose to the development of type 2 diabetes.
What is the key question?
What are the critical growth periods during childhood and adolescence that are associated with type 2 diabetes incidence?
What are the new findings?
Prospective analyses revealed that higher weight and accelerated weight gain in all growth periods significantly associated with a greater risk of type 2 diabetes.
Higher weight and greater weight velocity pre-adolescence jointly associated with the highest risk for type 2 diabetes.
Impact on clinical practice
Pre-adolescence is an important growth period for preventing and managing obesity and reducing type 2 diabetes incidence.
Acknowledgements
We are grateful to the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Phoenix clinic staff and to the residents of the Gila River Indian Community in Arizona for their participation in the study. This research was supported by the Intramural Research Program of the NIDDK.
Funding
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviations
- MC4R
Melanocortin-4 receptor
- OGTT
Oral glucose tolerance test
- IRR
Incidence rate ratio
Footnotes
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Twitter Hashtag: Weight tracking in childhood and adolescence and type 2 diabetes risk (Please include the screenshot of the title and author list).
Data availability
Owing to privacy concerns of the small population of volunteers, data are not available for distribution.
References
- 1.Cunningham SA, Kramer MR, Narayan KMV. Incidence of childhood obesity in the United States. New Engl J Med 2014;370:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the United States, 1999–2012. JAMA Pediatr 2014;168:561–566. [DOI] [PubMed] [Google Scholar]
- 3.Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic risks and severity of obesity in children and young adults. N Engl J Med 2015;373:1307–1317. [DOI] [PubMed] [Google Scholar]
- 4.Mayer-Davis EJ, Dabelea D, Lawrence JM. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med 2017;377:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo SS, Wu W, Chumlea WC, Roche AF. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr 2002;76:653–658. [DOI] [PubMed] [Google Scholar]
- 6.Bjerregaard LG, Jensen BW, Angquist L, Osler M, Sorensen TIA, Baker JL. Change in overweight from childhood to early adulthood and risk of type 2 diabetes. New Engl J Med 2018;378:1302–1312. [DOI] [PubMed] [Google Scholar]
- 7.Tanamas SK, Reddy SP, Chambers MA, et al. Effect of severe obesity in childhood and adolescence on risk of type 2 diabetes in youth and early adulthood in an American Indian population. Pediatr Diabetes 2018;19:622–629. [DOI] [PubMed] [Google Scholar]
- 8.Constantino MI, Molyneaux L, Limacher-Gisler F, et al. Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care 2013;36:3863–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Saeed AH, Constantino MI, Molyneaux L, et al. An inverse relationship between age of type 2 diabetes onset and complication risk and mortality: the impact of youth-onset type 2 diabetes. Diabetes Care 2016;39:823–829. [DOI] [PubMed] [Google Scholar]
- 10.Crowther NJ, Cameron N, Trusler J, Gray IP. Association between poor glucose tolerance and rapid post natal weight gain in seven-year-old children. Diabetologia 1998;41:1163–1167. [DOI] [PubMed] [Google Scholar]
- 11.Bhargava SK, Sachdev HS, Fall CH, et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med 2004;350:865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang T, Xu J, Li S, et al. Trajectories of childhood BMI and adult diabetes: the Bogalusa Heart Study. Diabetologia 2019;62:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmermann E, Bjerregaard LG, Gamborg M, Vaag AA, Sorensen TIA, Baker JL. Childhood body mass index and development of type 2 diabetes throughout adult life-A large-scale danish cohort study. Obesity 2017;25:965–971. [DOI] [PubMed] [Google Scholar]
- 14.Yeung EH, Zhang C, Louis GM, Willett WC, Hu FB. Childhood size and life course weight characteristics in association with the risk of incident type 2 diabetes. Diabetes Care 2010;33:1364–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eriksson JG, Osmond C, Kajantie E, Forsen TJ, Barker DJ. Patterns of growth among children who later develop type 2 diabetes or its risk factors. Diabetologia 2006;49:2853–2858. [DOI] [PubMed] [Google Scholar]
- 16.Wibaek R, Vistisen D, Girma T, et al. Body mass index trajectories in early childhood in relation to cardiometabolic risk profile and body composition at 5 years of age. Am J Clin Nutr 2019;110:1175–1185. [DOI] [PubMed] [Google Scholar]
- 17.Norris SA, Osmond C, Gigante D, et al. Size at birth, weight gain in infancy and childhood, and adult diabetes risk in five low- or middle-income country birth cohorts. Diabetes Care 2012;35:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park MH, Sovio U, Viner RM, Hardy RJ, Kinra S. Overweight in childhood, adolescence and adulthood and cardiovascular risk in later life: pooled analysis of three british birth cohorts. PLoS One 2013;8:e70684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juonala M, Magnussen CG, Berenson GS, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med 2011;365:1876–1185. [DOI] [PubMed] [Google Scholar]
- 20.Pavkov ME, Hanson RL, Knowler WC, Bennett PH, Krakoff J, Nelson RG. Changing patterns of type 2 diabetes incidence among Pima Indians. Diabetes Care 2007;30:1758–1763. [DOI] [PubMed] [Google Scholar]
- 21.Vijayakumar P, Wheelock KM, Kobes S, et al. Secular changes in physical growth and obesity among southwestern American Indian children over four decades. Pediatr Obes 2018;13:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA 2006;296:421–426. [DOI] [PubMed] [Google Scholar]
- 23.Dabelea D, Palmer JP, Bennett PH, Pettitt DJ, Knowler WC. Absence of glutamic acid decarboxylase antibodies in Pima Indian children with diabetes mellitus. Diabetologia 1999;42:1265–1266. [DOI] [PubMed] [Google Scholar]
- 24.Knowler WC, Bennett PH, Bottazzo GF, Doniach D. Islet cell antibodies and diabetes mellitus in Pima Indians. Diabetologia 1979;17:161–164. [DOI] [PubMed] [Google Scholar]
- 25.Sawyer SM, Azzopardi PS, Wickremarathne D, Patton GC. The age of adolescence. Lancet Child Adolesc Health 2018;2:223–228. [DOI] [PubMed] [Google Scholar]
- 26.Thearle MS, Muller YL, Hanson RL, et al. Greater impact of melanocortin-4 receptor deficiency on rates of growth and risk of type 2 diabetes during childhood compared with adulthood in Pima Indians. Diabetes 2012;61:250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO Study Group on Diabetes Mellitus and World Health Organization. Diabetes mellitus: report of a WHO study group: diabetes mellitus. Geneva: World Health Organization; 1985. [Google Scholar]
- 28.Araujo J, Ramos E, Mishra GD, Severo M. The use of weight adjusted for height rather than body mass index to assess growth trajectory: Results from a population-based cohort. Stat Med 2019;38:855–865. [DOI] [PubMed] [Google Scholar]
- 29.Oldham PD. A note on the analysis of repeated measurements of the same subjects. J Chronic Dis 1962;15:969–977. [DOI] [PubMed] [Google Scholar]
- 30.Blomqvist N On the relation between change and initial value. J Am Stat Assoc 1977;72:746–749. [Google Scholar]
- 31.Puth M-T, Neuhauser M, Ruxton GD. On the variety of methods for calculating confidence intervals by bootstrapping. J Anim Ecol 2015;84:892–897. [DOI] [PubMed] [Google Scholar]
- 32.Howe LD, Tilling K, Matijasevich A, et al. Linear spline multilevel models for summarising childhood growth trajectories: A guide to their application using examples from five birth cohorts. Stat Methods Med Res 2016;25:1854–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olaiya MT, Wedekind LE, Hanson RL, et al. Birthweight and early-onset type 2 diabetes in American Indians: differential effects in adolescents and young adults and additive effects of genotype, BMI and maternal diabetes. Diabetologia 2019;62:1628–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Everhart JE, Pettitt DJ, Bennett PH, Knowler WC. Duration of obesity increases the incidence of NIDDM. Diabetes 1992;41:235–240. [DOI] [PubMed] [Google Scholar]
- 35.The NS, Richardson AS, Gordon-Larsen P. Timing and duration of obesity in relation to diabetes: findings from an ethnically diverse, nationally representative sample. Diabetes Care 2013;36:865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eriksson JG, Forsén T, Tuomilehto J, Osmond C, Barker DJ. Early adiposity rebound in childhood and risk of type 2 diabetes in adult life. Diabetologia 2003;46:190–194. [DOI] [PubMed] [Google Scholar]
- 37.Rolland-Cachera MF, Deheeger M, Maillot M, Bellisle F. Early adiposity rebound: causes and consequences for obesity in children and adults. Int J Obes 2006;30:S11–17. [DOI] [PubMed] [Google Scholar]
- 38.Jeffery AN, Metcalf BS, Hosking J, Streeter AJ, Voss LD, Wilkin TJ. Age before stage: insulin resistance rises before the onset of puberty: a 9-year longitudinal study (EarlyBird 26). Diabetes Care 2012;3:536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Owing to privacy concerns of the small population of volunteers, data are not available for distribution.


