Abstract
Background:
129Xe gas-transfer MRI provides regional measures of pulmonary gas-exchange in adults and separates xenon in interstitial lung tissue/plasma (barrier) from xenon in red blood cells (RBC). The technique has yet to be demonstrated in pediatric populations or conditions.
Purpose/Hypothesis:
To perform an exploratory analysis of 129Xe gas-transfer MRI in children.
Study Type:
Prospective.
Population:
77 human volunteers (38 males, age = 17.7±15.1 years, range 5–68 years, 16 healthy). Four pediatric disease cohorts.
Field Strength/Sequence:
3T, 3D-radial 1-point Dixon FFE UTE.
Assessment:
Breath hold compliance was assessed by quantitative signal-to-noise and dynamic metrics. Whole-lung means and standard deviations were extracted from gas-transfer maps. Gas-transfer metrics were investigated with respect to age and lung disease. Clinical pulmonary function tests were retrospectively acquired for reference lung disease severity.
Statistical Tests:
Wilcoxon rank-sum tests to compare age and disease cohorts, Wilcoxon signed-rank tests to compare pre- and post-breath hold vitals, Pearson correlations between age and gas-transfer metrics, and limits-of-normal with a binomial exact test to compare fraction of subjects with abnormal gas-transfer. P ≤ 0.05 was considered significant.
Results:
80% of pediatric subjects successfully completed 129Xe gas-transfer MRI. Gas-transfer parameters differed between healthy children and adults, including ventilation (0.75 and 0.67) and RBC:barrier ratio (0.31 and 0.46) which also correlated with age (ρ = −0.76, 0.57, respectively). Bone marrow transplant subjects had impaired ventilation (90% of reference) and increased dissolved 129Xe standard deviation (242%). Bronchopulmonary dysplasia subjects had decreased barrier-uptake (69%). Cystic fibrosis subjects had impaired ventilation (91%) and increased RBC-transfer (146%). Lastly, childhood interstitial lung disease subjects had increased ventilation heterogeneity (113%). Limits-of-normal provided detection of abnormalities in additional gas-transfer parameters.
Data Conclusion:
Pediatric 129Xe gas-transfer MRI was adequately successful and gas-transfer metrics correlated with age. Exploratory analysis revealed abnormalities in a variety of pediatric obstructive and restrictive lung diseases.
Keywords: Gas-transfer, 129Xe, pediatric, exchange, pulmonary, interstitial lung disease
Introduction:
The inhalation of hyperpolarized 129Xe gas for breath-hold MRI has been shown to be safe and well tolerated in adults and children for ventilation and diffusion imaging(1–3). During the breath hold, xenon not only fills the airspace of the lung but also diffuses through the lung interstitium into the plasma and transiently binds to hemoglobin in red blood cells (RBCs)(4). The magnetic resonance chemical shifts of 129Xe occur near 0 ppm (air space), 197 ppm (interstitium and plasma, sometimes referred to as TP for tissue/plasma or barrier, as it is the diffusion barrier of gas exchange), or 218 ppm (red-blood cells, RBC)(5, 6). 129Xe gas-transfer MRI was developed to regionally quantify the amount of xenon in these three compartments(7, 8). The dissolved-phase signals are typically normalized by the amount of xenon in the gas phase to quantify the spatial distribution of barrier-uptake and RBC-transfer(9). Barrier-uptake and RBC-transfer have been shown to correlate with diffusing capacity of carbon monoxide in the lung (DLCO) in adults with and range of cardiopulmonary conditions, including idiopathic pulmonary fibrosis, radiation-induced pneumonitis and fibrosis due, pulmonary arterial hypertension, chronic thromboembolic pulmonary hypertension, and emphysema/chronic obstructive pulmonary disease (COPD) due to alpha-1 antitrypsin deficiency(7, 10). However, 129Xe gas-transfer imaging in any pediatric population has not been demonstrated.
Extending 129Xe gas-transfer MRI to pediatric subjects is non-trivial, due to the relatively long breath hold required, ∼16 seconds, which can be challenging for very young children—especially those with pulmonary disease. Additionally, most of the work in 129Xe gas-transfer MRI to date has focused on diseases and conditions that manifest later in adulthood, and these data are increasingly referenced to and interpreted using MRI data from healthy subjects. However, these reference data were acquired from adult subjects, and respiratory physiology—including gas exchange—is known to vary with age(11, 12). Thus, the aims of this study were to 1) determine the feasibility of 129Xe gas-transfer MRI in pediatric subjects and 2) assess the potential utility/applicability of this method to assess pulmonary pathology in pediatric-onset diseases.
Materials and Methods:
Human Subjects:
All subjects were imaged according to a protocol approved by the local Institutional Review Board (2014–5279) and FDA IND (123,577) after obtaining informed consent (adult subjects) or parental consent and subject assent (children) between October 2019 and December 2020. All subjects referred to our center during this time frame were evaluated for inclusion to this study.
Inclusion criteria were age ≥5 years and clinical stability with no recent medication changes or pulmonary exacerbations for subjects with lung disease. In addition to standard MRI exclusion criteria (e.g., claustrophobia, incompatible implants), subjects were excluded if they displayed symptoms of current respiratory infection (loose or productive cough or wheeze), experienced chest tightness within the previous week, had baseline pulse oximetry less than 95%, or were pregnant or lactating. Vital signs (SpO2 and heart rate) were monitored and recorded during xenon breath holds.
Additionally, the study’s feasibility and applicability objectives had inclusion criteria as shown in Figure 1. Repeated scans in the same subject were not included when assessing feasibility metrics. The remaining subjects were then split into a pediatric cohort (ages 5-<19) and an adult. To assess applicability, the inclusion criteria for healthy subjects were the same. For subjects with known or suspected pulmonary disorders, they needed to be under 19 years of age, had useable data (determined quantitatively by fits to signal dynamics and SNR, see below), and be diagnosed with a disease with at least 4 subjects (Figure 1). The subjects were then separated into a healthy pediatric cohort and a cohort of children with known or suspected pulmonary disorders (bone-marrow transplantation (BMT), history of bronchopulmonary dysplasia (BPD), cystic fibrosis (CF), and childhood interstitial lung diseases (chILD)). The demographics, retrospective clinical measures, and vital signs during Xe MRI can be found in Table 1. BMT patients are at risk to develop pulmonary complications such as bronchiolitis obliterans syndrome (BOS), and the BMT cohort were enrolled in this study to develop 129Xe MRI for early detection of post-BMT lung injury. The chILD cohort was being imaged to obtain novel information on lung function and structure for this group of rare diseases.
Figure 1:
Breakdown of all subjects and 129Xe gas-transfer MRI scan sessions. This study was split into two components, a feasibility study, and an applicability study. Adults were included for benchmark comparisons for the pediatric feasibility study. Healthy adults were included in the applicability cohort to assess potential differences in gas-transfer metrics due to age.
Table 1:
Demographics for each cohort within the feasibility and applicability groups of this study. Values are reported as percent for discrete variables and medians with the first and third quantiles for continuous variables. P-values are with respect to the corresponding pediatric cohort for the feasibility groups and with respect to the pediatric healthy group for the applicability group. Significant differences are bolded.
| N | % Male | % Healthy | Age | FEV1 % | FVC % | FEV1/FVC% | DLCO % | SpO2 Drop | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Median, N [Q1-Q3] (P-value) | ||||||||||
|
| ||||||||||
| Feasibility Cohorts |
Pediatric | 45 | 55.6 | 15.6 | 10.0 [8.3–13.7] |
86, N=34 [71–101] |
88, N=34 [72–104] |
85, N=34 [78–90] |
77, N=15 [66–101] |
4, N=44 [2–7] |
| Adult | 32 | 40.6 (0.20) |
28.1 (0.18) |
29.7
[22.8–44.4] (<0.01) |
90, N=13 [84–100] (0.17) |
98, N=13 [81–109] (0.15) |
80, N=13 [77–85] (0.06) |
98, N=5 [52–110] (0.48) |
7, N=27
[4–8] (0.02) |
|
|
| ||||||||||
| Pediatric Applicability Cohorts |
Healthy | 5 | 60 | − | 8.8 [7.0–11.1] |
110, N=1 [N/A] |
115, N=1 [N/A] |
86, N=1 [N/A] |
N/A, N=0 | 3, N=5 [2–6] |
| BMT | 12 | 58 (0.45) |
− | 12.6 [8.5–17.4] (0.28) |
83, N=12 [61–100] (0.05) |
82, N=12 [65–96] (0.05) |
89, N=12 [79–93] (0.34) |
77, N=8 [67–84] (N/A) |
9, N=12
[6–16] (0.02) |
|
| BPD | 4 | 50 (0.69) |
− | 7.4 [5.7–9.0] (0.52) |
77, N=2 [65–88] (0.11) |
86, N=2 [74–97] (0.11) |
82, N=2 [81–82] (0.11) |
109, N=2 [92–125] (N/A) |
2, N=4 [2–4] (0.20) |
|
| CF | 8 | 63 (0.56) |
− | 9.9 [9.2–11.6] (0.44) |
91, N=8 [83–107] (0.17) |
102, N=8 [92–106] (0.22) |
79, N=8 [76–86] (0.22) |
125, N=2 [125–125] (N/A) |
6, N=8 [3–6] (0.15) |
|
| chILD | 5 | 40 (0.46) |
− | 13.5 [7.2–16.4] (0.84) |
73, N=5 [59–85] (0.07) |
79, N=5 [63–90] (0.07) |
84, N=5 [78–88] (0.19) |
76, N=3 [57–93] (N/A) |
3, N=5 [0–13] (0.46) |
|
MRI Methods:
129Xe gas-transfer MRI was collected using a published sequence on either of two 3T MR scanners (Achieva or Ingenia, Philips Healthcare, Best, The Netherlands)(13). A custom-built 129Xe dual-loop coil or a quadrature vest coil (Clinical MR Solutions, Brookfield, WI) were used for 129Xe acquisitions, and the scanner’s body coil was used for 1H acquisitions(14). (Previous testing found no significant differences between scanner or coils. See Supp. Info.) The protocol consisted of three breath hold acquisitions: Xe sequence calibration, Xe gas-transfer imaging, and structural imaging with a breath hold of air(7).
For subjects less than 18 years old, 129Xe gas was dosed at 1/6th predicted total lung capacity for imaging, up to 1 L, with a predictive equation that used gender and height as inputs, while subjects 18 years old and older were dosed at 1L(15). The calibration dose used the same volume, but with consisted of ∼25% xenon mixed with ultrapure N2. The calibration dose permitted accurate flip angles, echo time, and frequencies when imaging xenon(16). Practice inhalations of air were performed outside and inside the scanner, prior to the Xe breath hold, to improve compliance.
To image gas-transfer, 129Xe was selectively excited using a 0.65-ms sinc pulse (central lobe only). Data were collected using a 3D-radial UTE sequence. Dissolved xenon and gaseous xenon imaging were interleaved, with the selective excitations and acquisitions, switching between the dissolved (7143 Hz, flip angle = 20°) and gaseous (0 Hz, flip angle = 0.5°) 129Xe. Additional 129Xe imaging parameters were: Field of view (FOV) = 3253 mm3, matrix = 563, Echo Time (TE) = TE90 (time to echo with 90° phase separation of dissolved xenon components, ∼0.46 ms), Repetition time (TR) = 15 ms (effectively due to interleaves), dwell time = 19.1 us, 950 radial projections (for each frequency offset), and 59 points per projection.
129Xe spectra were acquired of dissolved and gaseous 129Xe, with identical frequencies, flip angles, TEs and TRs as the 129Xe imaging. The 129Xe spectra were completed following imaging but before exhalation. Additional 129Xe spectra parameters were: TE = TE90 (∼0.46 ms), dwell time = 19.1 us, and 789 points per acquisition. The total breath hold duration was 16 seconds.
The proton 3D-radial UTE image was acquired during a second 16 s breath hold of room air (same volume as xenon). 1H imaging parameters were: FOV = 3253 mm3, matrix = 1123, TR/TE = 2.1/0.15 ms, dwell time = 9.0 us, 7060 radial projections, and 117 points per projection with a 5° rf block pulse.
Image Reconstruction:
Prior to image reconstruction, the first 60 projections from all 129Xe imaging data sets were omitted. The choice of 60 projections was determined empirically (M.W, 5 years of experience) by inspecting the duration of initial rapid signal decay across all subjects, ages, and disease states. Gas-phase contamination was removed from the dissolved-phase signals using a published method(13). Images were reconstructed using an open-source reconstruction package with iterative density compensation in MATLAB(17, 18).
Reconstruction kernel sharpness settings were optimized to provide signal-to-noise ratios ≥10 (see data analysis for calculation). When reconstructing dissolved-phase 129Xe images, a broad kernel (0.1) was required to achieve sufficient SNR. This kernel was also used for gas-phase 129Xe images which normalize the dissolved-phase images. For dedicated ventilation images, a sharper kernel (0.3) was used to provide the specified SNR, while retaining sufficiently high true resolution (assessed by M.W, Z.C., L.W., and J.W. with 5, 17, 12, and 22 years of experience, respectively).
Image Post-processing:
Coil inhomogeneity was removed from the 1H images and 129Xe ventilation images using N4 bias correction(19). 1H image bias correction parameters were: shrink factor = 4, spline distance of 6 voxels, 25×50 iterations, and bias field FWHM = 0.25. The 129Xe ventilation bias correction consisted of multiple steps. The first step included removal of linear, quadratic, and cubic biases with a shrink factor of 1, 25 iterations, and bias field FWHM of 0.75. This was followed by anterior-to-posterior, right-to-left, and head-to-foot bias corrections with cubic splines separated by 8 voxels in the specified orientation (constant in other two dimensions), a shrink factor of 1, 25 iterations, and a bias field FWHM of 0.5. The last step used a shrink factor of 2, cubic splines separated by 28 voxels, 50 iterations, and a bias field FWHM of 0.25.
The 1H images were registered to the 129Xe ventilation image using MATLAB 2019b’s 3D affine image registration. Masks were generated automatically using in-house code and manually edited as needed to remove airways and stomach (M.W. with 5 years of experience). The number of masks requiring manual corrections was recorded. The total dissolved-phase images were separated into barrier and RBC images using the 1-point Dixon method(9). The gaseous, total dissolved-phase, barrier, and RBC images were corrected for flip angle and corrected for T2* decay. Quantitative maps were obtained by dividing two images: dissolved (total dissolved normalized to gas), barrier-uptake (barrier normalized to gas), RBC-transfer (RBC normalized to gas), and RBC:barrier (RBC normalized to barrier) images.
Data Analysis:
Pulmonary function tests were used to provide clinical measures of lung function and disease severity. As the tests were acquired from clinical records, the predicted values were modeled according to the standard reference equations used clinically at our institution. FEV1% and FVC% were considered abnormal if less than 80% of their predicted value. FEV1/FVC% was considered abnormal if less than 70% of predicted and DLCO% was considered abnormal if less than 75% of predicted.
Subject compliance on the gas-transfer data was assessed by two methods: 1) quantifying post-imaging spectra SNR and 2) quantifying deviations from non-exponential signal decay. Subjects whose post-imaging spectra lacked sufficient signal to perform a successful 1-point Dixon decomposition were excluded from the applicability cohorts, which was determined if the dissolved-phase spectra FID-intensity was less than mean noise + 5 × the standard deviation of the noise (noise determined from data >10 ms from start of acquisition, last 25 points). Subjects with sufficient SNR were considered “quantifiable”. Single exponential decays were fit to the magnitude of the k0 points to assess signal dynamics. If the resulting coefficient of determination (R2) was < 0.975, those data were noted as an incomplete breath hold but still permitted in the applicability study. Subjects with a complete breath hold were deemed “high-quality”.
SNR of the 129Xe images were estimated by manual (M.W., 5 years of experience) selection of regions of interest within the lung and background signal, prior to any bias correction. The mean signal within the lung was divided by the root mean square of the background noise according to (20).
Statistical Analysis:
Non-parametric tests were used, despite their generally lower statistical power, due to small sample sizes and Shapiro–Wilk tests indicating non-normality for many of the metrics. Wilcoxon rank-sum tests were used to compare group medians. Wilcoxon sign-rank tests were used for paired comparisons. Pearson correlations were used for assessing the correlation between gas-transfer metrics with age. Should the gas-transfer metrics correlate with age, the healthy comparison cohort will be reduced to only pediatric subjects. Limits of normality were calculated from the healthy cohort using a Z-score of ±1.96. Subjects outside the limits of normality were deemed to have significant gas-transfer abnormalities. A two-sided exact binomial test was used to determine if the percentage of subjects with abnormal gas-transfer in a disease cohort was significantly larger than the healthy cohort. All statistics were considered significant if P ≤ 0.05.
Results:
129Xe gas-transfer MRI was acquired 86 times in 77 subjects, (9 repeat subjects). The pediatric subjects (healthy and with known or suspected pulmonary disorders) had a mean age of 11±4 years (range 5.04–18.54) while the adults had a mean age of 31±6 years (range 19.07–68.35). The pediatric subject cohorts were divided into 5 cohorts (5 healthy subjects, 12 BMT, 4 BPD, 8 CF, and 5 chILD). For all pediatric diseases with clinical PFTs, 67% had normal FEV1%, 70% had normal FVC%, 96% had normal FEV1/FVC%, and 75% had normal DLCO. Interestingly, all CF subjects had normal respiratory function according to the spirometric measures. The other cohorts had ∼50% of subjects with normal respiratory function.
Feasibility:
Table 1 summarizes subject demographics, pulmonary function tests, and saturation of peripheral oxygen (SpO2) changes related to the Xe breath-hold. Children displayed only transient decreases in SpO2, −5.6±5.7%, significantly different than the adults (−6.7±3.8%). Only the BMT subjects had a significantly different reduction compared to the healthy children (−9%). SpO2 returned to baseline quickly for all participants (< 30 s). Thus, a single 16 s, anoxic 129Xe breath hold was well tolerated and safe in children, regardless of disease state.
High-quality and quantifiable 129Xe gas-transfer images were obtained from 80% (36 of 45) pediatric subjects imaged. The remaining 9 subjects either performed incomplete breath holds (N = 6), had low spectral SNR due to non-compliant breath hold (N = 1), or both (N = 2). In comparison, 100% of adults were successfully imaged. Gas-phase contamination was measured to be 6.8±2.9%, with a phase separation of 89.3±5.9° between the RBC and barrier signals.
Figure 2 shows example spectra and gas-phase dynamics from both compliant and non-compliant subjects. Subjects who were non-compliant with the breath hold (e.g., exhaled or shifted during the imaging) displayed non-exponential signal decay during image acquisition. Compliant breath holds had R2 = 0.997±0.005 with a minimum of 0.980 while incomplete breath holds had R2 = 0.942±0.026 with a maximum of 0.973. Figure 3 shows example 129Xe images from 3 children that represent successful, incomplete, and non-compliant breath holds.
Figure 2:
Examples illustrating the definition of low-SNR and/or incomplete breath hold (BH). a.) Example dissolved-phase spectra (0 Hz corresponds to excitation/receive frequency; left-to-right, the peaks correspond to RBC, barrier, and gas peaks) acquired at the end of the imaging session during the same breath hold. Spectra with SNR too low to accurately fit the data were excluded from the application cohorts (red, N = 3) while the rest were compliant (blue, N = 74, 7 shown here). b.) Examples of the gas-phase dynamics, via the first data point along a projection, during the breath hold of xenon gas. Dynamics which do not fit the expected exponential decay well (R2 < 0.975) were determined to have an incomplete breath hold (red, N = 8, R2 = 0.942±0.026) while the others were considered compliant (blue, N = 69, R2 = 0.997±0.005, 7 shown here).
Figure 3:
Example gas-transfer images from pediatric subjects who were compliant with a good breath hold technique (left column), with a compliant but incomplete breath hold (middle column), and with too low of SNR in the corresponding 129Xe spectra (right column). Ventilation and gas-phase-normalization images were reconstructed from the same data using different gridding kernel sharpness. The subject with an incomplete breath hold shows a noticeable artifact in the shape of a crosshatch pattern. Note the low-SNR subject still had reasonable image quality, but their data could not be accurately decomposed using 1-point Dixon due to lack of signal at the end of the breath hold/in the spectra.
Within the full healthy cohort (i.e., combined adult and pediatric), ventilation SNR was 16.4±3.8, gas-phase (for signal normalization) SNR was 43.9±12.7, barrier SNR 47.7±20.1, and RBC SNR was 24.0±7.7. Of the healthy subjects, the lowest ventilation image SNR was 10.8 and the lowest dissolved SNR was 8.5 for an RBC image. SNR difference between healthy children and adults was not significant (P = 0.1–0.3, most significant (gas-phase SNR) confidence intervals [−7.6, 27.0]). Comparing the SNR from healthy pediatric subjects and children with known or suspected pulmonary disease, SNR was not significantly different (P = 0.1–1.0 with smaller differences than those between children and adults).
Applicability:
Medians and interquartile ranges were measured for the two cohorts (Table 2 with distributions shown in Figure 4a-d). When comparing the pediatric healthy cohort to the adult healthy cohort, the ranked medians were significantly higher for ventilation mean (0.75 and 0.69), lower for ventilation standard deviation (0.14 and 0.17), and RBC:barrier mean (0.29 and 0.43). RBC-transfer mean and standard deviation did not have a significant difference (P = 0.06, 0.08, respectively) but had median values which did not fall in the other cohort’s interquartile range. For ventilation, the medians [interquartile range] were 0.75 [0.74–0.76] and 0.69 [0.64–0.71], respectively, for the pediatric healthy cohort and adult healthy cohort. For barrier-uptake, the medians were 8.2×10−3 [7.9–9.4×10−3] and 8.3×10−3 [7.2–8.6×10−3], respectively. For RBC-transfer, the medians were 2.1×10−3 [2.1–2.7×10−3] and 3.3×10−3 [3.0–3.9×10−3], respectively. For RBC:barrier, the medians were 0.29 [0.26–0.32] and 0.43 [0.39–0.55], respectively.
Table 2:
Healthy subject means and standard deviations from their gas-exchange maps for the healthy pediatric and adult cohorts described by the median and interquartile range for their age group. Wilcoxon rank-sum tests show significant differences in medians and are bold.
| Ventilation | Dissolved (×10–3) | Barrier-uptake (×10–3) | RBC-transfer (×10–3) | RBC: Barrier | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Std. Dev. | Mean | Std. Dev. | Mean | Std. Dev. | Mean | Std. Dev. | Mean | Std. Dev. | |
| Median [Q1-Q3] | ||||||||||
|
| ||||||||||
| Pediatric Healthy N = 5 |
0.75
[0.74–0.76] |
0.14
[0.13–0.14] |
9.06 [8.30–9.52] |
1.32 [0.77–1.56] |
8.17 [7.89–9.35] |
1.30 [0.76–1.59] |
2.13 [2.12–2.74] |
0.62 [0.56–0.74] |
0.29
[0.26–0.32] |
0.09 [0.07–0.09] |
| Adult Healthy N = 9 |
0.69
[0.64–0.71] |
0.17
[0.16–0.19] |
9.05 [7.51–9.33] |
1.15 [1.04–1.51] |
8.32 [7.20–8.62] |
1.06 [0.97–1.47] |
3.26 [2.96–3.90] |
0.82 [0.72–1.05] |
0.43
[0.39–0.55] |
0.13 [0.08–0.15] |
| P-value | 0.003 | 0.002 | 0.369 | 0.369 | 0.274 | 0.369 | 0.063 | 0.081 | 0.027 | 0.081 |
Figure 4:
Healthy cohort distributions (left column, a-d) for the three different age groupings illustrating the change in expected distribution shape and intensity for the four main gas-exchange metrics. (Right column, e-h) Scatter plots showing the mean and standard deviation for each healthy subject’s gas-transfer maps as a function of age. Trends are noticeable for many of the metrics. Dashed lines are intended to be guides to the eye and are linear-least squares fit using a second-order polynomial to the data.
Figure 4e-h shows the mean signal intensity for each gas-transfer parameter with respect to age. Ventilation mean has a negative correlation (ρ = −0.76) with age while standard deviation increases with age (ρ = 0.79). Additionally, RBC:barrier ratio has a significant positive correlation with age (ρ = 0.56).
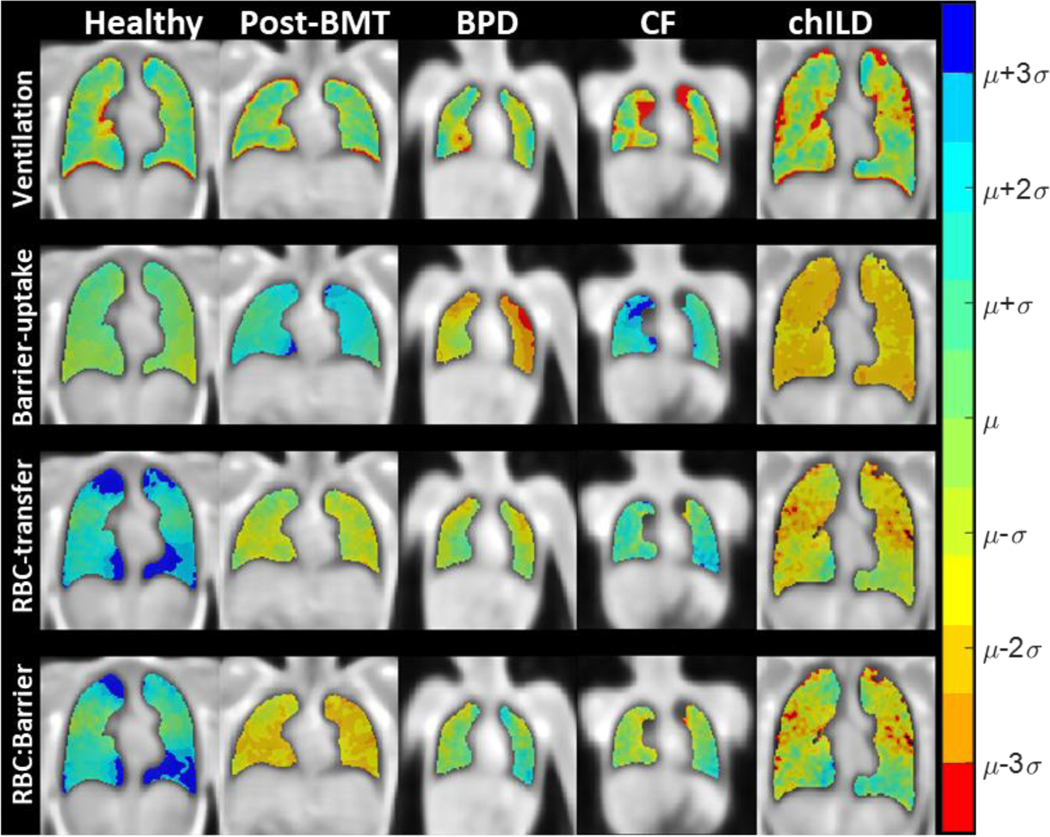
Figure 5 shows gas-transfer maps for a healthy pediatric subject and the 4 pediatric application groups. Subjects chosen to best illustrate their cohorts’ mean signals. Of note, the healthy subject shown in Figure 5 is 18 years old and thus illustrates the elevated RBC-transfer and RBC:barrier values when compared to the younger children, in line with age correlation. Post-BMT subjects exhibited impaired ventilation (0.75±0.01 and 0.69±0.07), increased variation in barrier-uptake (1.3±0.4×10−3 and 1.7±1.1×10−3) and a corresponding, but insignificant, decrease in RBC:barrier (P = 0.23). Former BPD subjects showed negligible ventilation defects (P = 0.07) with significant decreases in barrier-uptake (8.2±1.4×10−3 and 5.8±1.1×10−3). CF subjects had impaired ventilation (0.75±0.01 and 0.69±0.06) with increases in barrier-uptake (8.2±1.4×10−3 and 10.9±3.3×10−3) and RBC-transfer (6.2±1.6×10−4 and 7.9±1.6×10−4). Subjects with chILD had heterogenous ventilation (0.75±0.01 and 0.70±0.04), with a broad variation in the other metrics. Table 3 and Figure 6 show the quantitative measures from using 129Xe gas-transfer MRI to detect pediatric gas-transfer abnormalities. The limits of normality calculated from the healthy pediatric subjects were used to determine the subjects with clinically significant disease and can be found in Table 4. Using these limits, many of the gas-transfer metrics were able to detect an increased percentage of abnormal subjects compared to healthy pediatric subjects (BMT, 8 metrics; BPD, 1 metric; CF, 5 metrics; chILD, 4 metrics). BMT subjects had abnormal ventilation (83% of subjects), dissolved xenon (58%), barrier-uptake (58%), RBC-transfer (33%), and RBC:barrier (25%). BPD subjects had decreased dissolved xenon (50% of subjects). CF subjects had abnormalities in ventilation (75% of subjects), dissolved xenon (38%), barrier-uptake (50%), and RBC-transfer (50%). Subjects diagnosed with chILD demonstrated abnormalities in ventilation (80% of subjects), dissolved xenon (40%), and barrier-uptake (40%).
Figure 5:
Example gas-transfer maps for an 18-year-old healthy pediatric subject and a representative subject from each of the pediatric disease applicability groups. Maps are colored in relation to the mean (μ) and standard deviation (σ) of the healthy pediatric cohort. Mean derived from the median value of each subjects mean while the standard deviation was derived from the 3rd quartile of each subject’s standard deviation. See Table 3 for values. Note the increased RBC-transfer and RBC:barrier for the healthy subject due to their older age for the pediatric cohort.
Table 3:
Median and interquartile range of the extracted gas-transfer map means and standard deviations for the pediatric cohorts. Significant differences are in bold. P-values are reported with respect to the pediatric healthy cohort.
| Median [IQR] (P-value) | Ped. Healthy N = 5 | BMT N = 12 | BPD N = 4 | CF N = 8 | chILD N = 5 | |
|---|---|---|---|---|---|---|
|
| ||||||
| Ventilation | Mean | 0.75 [0.74–0.76] |
0.69
[0.66–0.72] (0.002) |
0.73 [0.72–0.75] (0.071) |
0.69
[0.64–0.74] (0.014) |
0.70 [0.66–0.74] (0.059) |
| Std. Dev. | 0.14 [0.13–0.14] |
0.17
[0.16–0.17] (0.003) |
0.13 [0.12–0.14] (0.500) |
0.18
[0.15–0.21] (0.004) |
0.15
[0.14–0.15] (0.038) |
|
| Dissolved (×10−3) |
Mean | 9.06 [8.30–9.52] |
11.39 [7.43–16.87] (0.123) |
6.34
[5.02–7.82] (0.014) |
11.50
[9.66–16.18] (0.029) |
8.76 [6.94–11.84] (0.458) |
| Std. Dev. | 1.32 [0.77–1.56] |
1.84
[1.23–2.20] (0.046) |
0.83 [0.50–1.27] (0.164) |
1.75
[1.21–2.00] (0.039) |
1.65 [0.86–2.43] (0.301) |
|
| Barrier-uptake (×10−3) |
Mean | 8.17 [7.89–9.35] |
10.88 [7.04–16.17] (0.146) |
5.81
[4.74–7.24] (0.014) |
10.88 [9.14–15.07] (0.054) |
8.49 [6.53–11.64] (0.458) |
| Std. Dev. | 1.30 [0.76–1.59] |
1.72
[1.23–2.25] (0.046) |
0.82 [0.50–1.21] (0.231) |
1.66 [1.15–1.99] (0.072) |
1.68 [0.89–2.42] (0.125) |
|
| RBC-transfer (×10−3) |
Mean | 2.13 [2.12–2.74] |
2.58 [1.78–3.84] (0.337) |
2.26 [1.44–2.74] (0.231) |
3.36
[2.90–4.94] (0.029) |
2.01 [1.18–2.49] (0.125) |
| Std. Dev. | 0.62 [0.56–0.74] |
0.90 [0.61–1.17] (0.070) |
0.51 [0.42–0.56] (0.071) |
0.79 [0.66–0.96] (0.054) |
0.73 [0.51–0.86] (0.377) |
|
| RBC:Barrier | Mean | 0.29 [0.26–0.32] |
0.24 [0.17–0.42] (0.230) |
0.34 [0.30–0.44] (0.071) |
0.31 [0.29–0.37] (0.232) |
0.24 [0.14–0.31] (0.087) |
| Std. Dev. | 0.09 [0.07–0.09] |
0.09 [0.06–1.14] (0.417) |
0.09 [0.07–1.08] (0.312) |
0.08 [0.07–0.91] (0.442) |
0.10 [0.08–1.03] (0.232) |
|
Figure 6:
Box plots showing the gas-transfer metrics for the pediatric healthy and disease cohorts. Left: mean signal intensities for each subject. Right: standard deviation for each subject. The red line corresponds to the median, the box ends correspond to the 25th and 75th percentile and the whiskers correspond to the ∼99th percentile. Statistically significant differences in mean are shown with *(P < 0.05) or **(P < 0.01). Additional statistical comparisons can be found in Table 3.
Table 4:
Percent of subjects with an abnormal mean or standard deviation for a given gas-transfer parameter as determined by the upper and lower limits of normal (in square brackets) for the healthy pediatric cohort. P-values (in parenthesis) are reported with respect to the pediatric healthy cohort and bolded when significant.
| Healthy Pediatric N = 5 [LLN - ULN] | BMT N = 12 % abnormal (P-value) | BPD N = 4 % abnormal (P-value) | CF N = 8 % abnormal (P-value) | chILD N = 5 % abnormal (P-value) | |
|---|---|---|---|---|---|
|
| |||||
| Ventilation | Mean [0.73–0.77] |
83%
(<0.001) |
25% (0.343) |
75%
(<0.001) |
80%
(<0.001) |
| Std. Dev. [0.10–0.17] |
50%
(<0.001) |
0% (1.000) |
63%
(<0.001) |
0% (1.000) |
|
| Dissolved (×10−3) |
Mean [6.3–11.8] |
58%
(<0.001) |
50%
(0.027) |
38%
(0.011) |
40%
(0.043) |
| Std. Dev. [0.5–2.2] |
25%
(0.035) |
25% (0.343) |
0% (1.000) |
40%
(0.043) |
|
| Barrier-uptake (×10−3) |
Mean [5.4–10.1] |
58%
(<0.001) |
25% (0.343) |
50%
(0.001) |
20% (0.407) |
| Std. Dev. [0.4–2.2] |
25%
(0.035) |
25% (0.343) |
0% (1.000) |
40%
(0.043) |
|
| RBC-transfer (×10−3) |
Mean [0.8–3.4] |
33%
(0.004) |
0% (1.000) |
50%
(0.001) |
0% (1.000) |
| Std. Dev. [0.3–0.9] |
42%
(<0.001) |
0% (1.000) |
25% (0.103) |
20% (0.407) |
|
| RBC:Barrier | Mean [0.12–0.45] |
25%
(0.035) |
25% (0.343) |
0% (1.000) |
20% (0.407) |
| Std. Dev. [0.04–0.13] |
17% (1.000) |
0% (1.000) |
0% (1.000) |
0% (1.000) |
|
Discussion:
Assessment of feasibility is complex as incomplete breath holds may lead to minor artifacts in the barrier and RBC images and changes in lung volume, which may affect the interpretation and quantitative analysis(21). For subjects with a non-compliant breath hold and low SNR, the 1-pt Dixon decomposition into barrier and RBC images is inaccurate since the RBC-to-barrier ratio cannot be fit accurately to the low-SNR data. Additionally, the necessary costs to perform 129Xe gas-transfer MRI could make the technique less feasible from the provider point of view.
Gas exchange abnormalities are ubiquitous in a wide range of pediatric lung diseases(22). However, patient-specific data are often unavailable, because DLCO is often not clinically ordered for subjects under 10 years of age at our institution, due to reduced subject compliance and increased variation in healthy measurements in younger children(12). Thus, 129Xe gas-transfer MRI in younger children has the potential to provide much needed clinical insight into early lung disease. To this end, the feasibility (safety and success rate) of 129Xe gas-transfer MRI has been demonstrated in children as young as age 5. To provide context for these data, the MRI-derived metrics were compared to those from adults obtained using the same acquisition parameters.
The safety profile of gas-transfer imaging in children agrees with previously-published safety results of 129Xe ventilation and diffusion imaging in adults and children(1–3).The success rate in children was 80% with most instances of poor the compliance occurring in those 8 years of age or younger. While 80% is a good success rate, it will need to be improved for clinical adaptation and for younger subjects. To achieve greater compliance in younger children, breath hold duration could be shortened by determining the minimum percentage of data-and thus breath hold duration-required to yield quantitatively reproducible images. More importantly, adapting the sequence that enables shorter breath holds, as demonstrated by Niedbalski, et. al. with a ∼10 second acquisition, would be beneficial(23). Together, those two changes could potentially reduce the breath hold to only ∼6 s.
This study implemented the 1-point Dixon approach with a 16 s breath hold duration in order to compare the data to published data in adults(9, 16, 23, 24). Multi-echo, IDEAL (Iterative decomposition of water and fat with echo asymmetry and least-squares estimation), and chemical-shift imaging approaches are promising and more robust as these techniques avoid the 1-point Dixon decomposition assumptions but are more sophisticated and typically require more compromise between breath hold duration, more undersampling artifacts, or coarser resolutions(10, 25–28). Additionally, there are limited references/data published for these emerging approaches especially if comparisons to multiple sites is needed. Of note, the calibration scan performed prior to gas-transfer MRI could provide adequate RBC:barrier ratios and permit 1-point Dixon decomposition for subjects who had inadequate SNR in the spectra at the end of the breath hold. However, uncertainty in their data would still be present due to the low compliance with the protocol and the decreased SNR of the imaging portion. Additionally, data from incomplete breath holds could be truncated but was not explored here as this could introduce undersampling artifacts and changes in quantitative maps. Thus, alternative approaches to increasing compliance should be considered with respect to the MRI sequence and the administration of the Xe.
Comparisons between healthy adults and healthy children showed differences in MRI-derived gas-exchange metrics. Due to the differences in gas-exchange metrics between pediatric and adult subjects, healthy subject comparisons must consider age matching with narrow age ranges. If both adults and children are included in the reference healthy cohort, reduced sensitivity and specificity to detect abnormalities are likely to occur due to the age variation of imaging metric distributions for healthy subject. This is not surprising as lung and vasculature development is known to continue through childhood(11, 29, 30). Similarly, age dependence has been reported in ventilation images where decreased ventilation in older subjects has been observed(31). While the limits of normal from the pediatric healthy cohort are narrower than those from a combined pediatric and adult cohort, the pediatric normal ranges could still be narrower. This is due to the remaining large variation in gas-exchange parameters, even after the exclusion of adults, as the largest change in median signal per year often occurs in the 5- to 25-year-old range. Thus, future studies should account for potential age biases to improve sensitivity and specificity. This could be completed by either only using age-matched healthy cohorts or by creating a model to account for the age dependence like those done for pulmonary-function tests(11, 12).
An exploratory analysis of 4 pediatric conditions was conducted to assess gas-transfer MRI and its application to pediatric lung disease. PFT values were provided retrospectively for subjects when available around their scan date. While spirometry was acquired for most subjects with disease, that was not the case for the healthy subjects. Additionally, DLCO is not standard of care for many of these subjects, even though it is expected to be the most strongly correlated PFT with gas-transfer MRI(24). Despite these limitations, the provided PFT values suggest subjects were on the milder side of disease severity.
129Xe gas-transfer MRI provided additional insight into disease and pathology, which is not obtained via 129Xe ventilation MRI alone. For post-BMT subjects, the average barrier-uptake heterogeneity was increased with a significant number of subjects exhibiting a complex mixture of ventilation impairment, increased barrier-uptake, and increased RBC-transfer, consistent with our understanding of those pulmonary complications(32, 33). Thus, the increased barrier-uptake observed in the post-BMT cohort may be related to inflammatory changes. For subjects with history of BPD, despite having normal ventilation, decreased barrier-uptake signal was observed, consistent with suspected alveolar simplification (for which there is no simple clinical gold standard)(34). For CF, despite having normal PFTs, many subjects exhibited ventilation impairment, increased barrier-uptake, and increased RBC-transfer, potentially associated with inflammatory changes in CF. The increased RBC-transfer in CF is an interesting finding but may be related to a compensatory mechanism. Ventilation heterogeneity was observed for the average subject in the chILD cohort, which suggests an obstructive process. Several of the chILD subjects were also considered abnormal for the total amount of dissolved xenon and heterogenous barrier-uptake. However, within the chILD cohort, there was a mixture of both elevated and decreased metrics, indicative of the variety of heterogenous clinical presentations in this cohort and the lack of statistical significance when comparing medians. Additional studies with larger cohorts are needed to understand the underlying etiology of these 129Xe gas-transfer MRI findings.
The analysis and sensitivity for the BMT and chILD cohorts are further confounded by the broad inter-subject clinical heterogeneity within these cohorts. Neither of these cohorts focused exclusively on one single lung disease, unlike the BPD and CF cohorts; however, there was some inter-subject heterogeneity within those groups as well. Undergoing a bone-marrow transplantation does not necessarily lead to any gas-transfer abnormalities(32). Therefore, many of these subjects had no known pulmonary involvement/issues. However, the associated chemotherapy and radiation is toxic to the lung and these subjects were being followed due to their risk of developing pulmonary complications including infection, inflammation, and bronchiolitis obliterans syndrome(35, 36). This inclusion of subjects without known/confirmed gas-transfer abnormalities broaden the distribution within the BMT cohort and reduces the ability to determine significant differences in gas-exchange metrics versus healthy individuals but does illustrate the potential for gas-transfer MRI to screen for pulmonary morbidity. Similarly, chILD is not a single disease but a heterogeneous group of rare-lung diseases, each with unique clinical presentation and pathophysiology, for example, alveolar simplification (and a corresponding decrease in the barrier-uptake) or inflammation/fibrosis (and an increased barrier-uptake)(37–39). Thus, this leads to broad distributions in the chILD cohort with no significant changes in the median signals outside of ventilation. However, these changes could be useful for obtaining a more specific diagnosis or in determining optimal treatments.
Limitations
First, after separating the pediatric subjects into specific cohorts, the cohort sizes were small (as low as 4–5 subjects), weakening statistical power of subgroup analysis. Additionally, since accounting for potential age dependencies was necessary through the creation of a pediatric healthy cohort, the statistical power was further weakened due to a smaller pediatric healthy cohort. This will be overcome in the future by developing an approach for age-dependent parameters, using similar methods to those developed by the Global Lung Initiative to model healthy pulmonary function testing distributions as a function of age, height, gender, and ethnicity(11, 12). Additionally, subjects whose breath holds were incomplete were still included in the analysis; further work will be necessary to understand the effect incomplete breath holds have on the quantitative markers. Moreover, the BMT and chILD cohorts have a wide range of disease pathology and severity, confounding the analysis. In this preliminary study, these results were only compared and not correlated with current clinical assessments; in future studies concurrent pulmonary function tests, specifically DLCO, would be of interest to better assess the sensitivity of 129Xe gas-transfer MRI versus clinical standards. Lastly, this study was performed at a single site and a single field strength and did not take into consideration other Xe imaging such as a dedicated ventilation scan.
Conclusion
129Xe gas-transfer MRI is feasible in pediatric subjects with and without known/suspected gas-transfer abnormalities. These results suggest significant age-dependent changes in the 129Xe gas-transfer parameters that must be considered in future studies. All the pediatric disease cohorts considered here had gas-transfer abnormalities, indicating 129Xe gas-transfer MRI provides novel and useful information about the spatial distribution of gas-exchange abnormalities in pediatric lung diseases.
Supplementary Material
Acknowledgments
Grant Support: NIH R01 HL131012, NIH R01 HL143011, CCRF 308530, NIH R00 HL138255, CFF NAREN19R0, NIH T32 HL007752, and NIH R01 HL126771.
References:
- 1.Driehuys B, Martinez-Jimenez S, Cleveland ZI, et al. : Chronic obstructive pulmonary disease: Safety and tolerability of hyperpolarized 129Xe MR imaging in healthy volunteers and patients. Radiology 2012; 262:279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shukla Y, Wheatley A, Kirby M, et al. : Hyperpolarized 129Xe Magnetic Resonance Imaging. Tolerability in Healthy Volunteers and Subjects with Pulmonary Disease. Acad Radiol 2012; 19:941–951. [DOI] [PubMed] [Google Scholar]
- 3.Walkup LL, Thomen RP, Akinyi TG, et al. : Feasibility, tolerability and safety of pediatric hyperpolarized 129Xe magnetic resonance imaging in healthy volunteers and children with cystic fibrosis. Pediatr Radiol 2016; 46:1651–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tilton RF, Kuntz ID: Nuclear magnetic resonance studies of xenon-129 with myoglobin and hemoglobin. Biochemistry 1982; 21:6850–6857. [DOI] [PubMed] [Google Scholar]
- 5.Cleveland ZI, Cofer GP, Metz G, et al. : Hyperpolarized 129Xe MR imaging of alveolar gas uptake in humans. PLoS One 2010; 5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaushik SS, Freeman MS, Yoon SW, et al. : Measuring diffusion limitation with a perfusion-limited gas-Hyperpolarized 129Xe gas-transfer spectroscopy in patients with idiopathic pulmonary fibrosis. J Appl Physiol 2014; 117:577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z, He M, Bier E, et al. : Hyperpolarized 129Xe gas transfer MRI: the transition from 1.5T to 3T. Magn Reson Med 2018; 80:2374–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patz S, Hersman FW, Muradian I, et al. : Hyperpolarized 129Xe MRI: A viable functional lung imaging modality? Eur J Radiol 2007; 64:335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Robertson SH, Wang J, et al. : Quantitative analysis of hyperpolarized 129Xe gas transfer MRI. Med Phys 2017; 44:2415–2428. [DOI] [PubMed] [Google Scholar]
- 10.Collier GJ, Eaden JA, Hughes PJC, et al. : Dissolved 129Xe lung MRI with four-echo 3D radial spectroscopic imaging: Quantification of regional gas transfer in idiopathic pulmonary fibrosis. Magn Reson Med 2021; 85:2622–2633. [DOI] [PubMed] [Google Scholar]
- 11.Stanojevic S, Wade A, Stocks J, et al. : Reference ranges for spirometry across all ages: A new approach. Am J Respir Crit Care Med 2008; 177:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanojevic S, Graham BL, Cooper BG, et al. : Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J 2017; 50. [DOI] [PubMed] [Google Scholar]
- 13.Willmering MM, Cleveland ZI, Walkup LL, Woods JC: Removal of off-resonance xenon gas artifacts in pulmonary gas-transfer MRI. Magn Reson Med 2021; 86:907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loew W, Thomen RP, Giaquinto R, et al. : A dual loop T/R-xenon coil for homogenous excitation with improved comfort and size. In Proc 24th Annu Meet ISMRM. Singapore; 2016. [Google Scholar]
- 15.Stocks J, Quanjer PH: Reference values for residual volume, functional residual capacity and total lung capacity: ATS Workshop on Lung Volume Measurements Official Statement of the European Respiratory Society. Eur Respir J 1995; 8:492–506. [DOI] [PubMed] [Google Scholar]
- 16.Niedbalski PJ, Hall CS, Castro M, et al. : Protocols for multi‐site trials using hyperpolarized 129 Xe MRI for imaging of ventilation, alveolar‐airspace size, and gas exchange: A position paper from the 129 Xe MRI clinical trials consortium. Magn Reson Med 2021; 86:2966–2986. [DOI] [PubMed] [Google Scholar]
- 17.Pipe JG, Menon P: Sampling density compensation in MRI: Rationale and an iterative numerical solution. Magn Reson Med 1999; 41:179–186. [DOI] [PubMed] [Google Scholar]
- 18.Robertson SH, Virgincar RS, He M, Freeman MS, Kaushik SS, Driehuys B: Optimizing 3D noncartesian gridding reconstruction for hyperpolarized 129Xe MRI-focus on preclinical applications. Concepts Magn Reson Part A Bridg Educ Res 2015; 44:190–202. [Google Scholar]
- 19.Tustison NJ, Avants BB, Cook PA, et al. : N4ITK: Improved N3 Bias Correction. IEEE Trans Med Imaging 2010; 29:1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Constantinides CD, Atalar E, McVeigh E: Signal-to-noise measurements in magnitude images from NMR phased arrays. In Proc 19th Annu Int Conf IEEE Eng Med Biol Soc ‘Magnificent Milestones Emerg Oppor Med Eng (Cat No97CH36136). Volume 1. IEEE; 1997:456–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahn AD, Kammerman J, Evans M, et al. : Repeatability of regional pulmonary functional metrics of Hyperpolarized 129Xe dissolved-phase MRI. J Magn Reson Imaging 2019; 50:1182–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pagtakhan RD, Chernick V: Respiratory Failure in the Pediatric Patient. Pediatr Rev 1982; 3:247–256. [Google Scholar]
- 23.Niedbalski PJ, Lu J, Hall CS, et al. : Utilizing flip angle/TR equivalence to reduce breath hold duration in hyperpolarized 129 Xe 1‐point Dixon gas exchange imaging. Magn Reson Med 2021(June):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Rankine L, Bier EA, et al. : Using hyperpolarized 129Xe gas-exchange MRI to model the regional airspace, membrane, and capillary contributions to diffusing capacity. J Appl Physiol 2021; 130:1398–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zanette B, Santyr G: Accelerated interleaved spiral-IDEAL imaging of hyperpolarized 129Xe for parametric gas exchange mapping in humans. Magn Reson Med 2019; 82:1113–1119. [DOI] [PubMed] [Google Scholar]
- 26.Doganay O, Chen M, Matin T, et al. : Magnetic resonance imaging of the time course of hyperpolarized 129 Xe gas exchange in the human lungs and heart. Eur Radiol 2019; 29:2283–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mata J, Guan S, Qing K, et al. : Evaluation of regional lung function in pulmonary fibrosis with xenon-129 mri. Tomography 2021; 7:452–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hahn AD, Kammerman J, Fain SB: Removal of hyperpolarized 129Xe gas-phase contamination in spectroscopic imaging of the lungs. Magn Reson Med 2018; 80:2586–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stein JM, Walkup LL, Brody AS, Fleck RJ, Woods JC: Quantitative CT characterization of pediatric lung development using routine clinical imaging. Pediatr Radiol 2016; 46:1804–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahlknecht U, Kaiser S: Age-related changes in peripheral blood counts in humans. Exp Ther Med 2010; 1:1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He M, Driehuys B, Que LG, Huang YCT: Using Hyperpolarized 129Xe MRI to Quantify the Pulmonary Ventilation Distribution. Acad Radiol 2016; 23:1521–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panoskaltsis-Mortari A, Griese M, Madtes DK, et al. : An official American Thoracic Society Research Statement: Noninfectious lung injury after hematopoietic stem cell transplantation: Idiopathic pneumonia syndrome. Am J Respir Crit Care Med 2011; 183:1262–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeuchi Y, Miyagawa-Hayashino A, Chen F, et al. : Pleuroparenchymal fibroelastosis and non-specific interstitial pneumonia: Frequent pulmonary sequelae of haematopoietic stem cell transplantation. Histopathology 2015; 66:536–544. [DOI] [PubMed] [Google Scholar]
- 34.Polverino F, Hysinger EB, Gupta N, et al. : Lung MRI as a Potential Complementary Diagnostic Tool for Early COPD. Am J Med 2020; 133:757–760. [DOI] [PubMed] [Google Scholar]
- 35.Walkup LL, Myers K, Nelson AS, et al. : Stabilization and Reversibility of Early Lung Abnormalities Post-HSCT Detected with Serial Hyperpolarized 129Xe MRI. Biol Blood Marrow Transplant 2019; 25:S259–S260. [Google Scholar]
- 36.Walkup LL, Myers K, El-Bietar J, et al. : Xenon-129 MRI detects ventilation deficits in paediatric stem cell transplant patients unable to perform spirometry. Eur Respir J 2019; 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hime NJ, Zurynski Y, Fitzgerald D, et al. : Childhood interstitial lung disease: A systematic review. Pediatr Pulmonol 2015; 50:1383–1392. [DOI] [PubMed] [Google Scholar]
- 38.Kurland G, Deterding RR, Hagood JS, et al. : An official american thoracic society clinical practice guideline: Classification, evaluation, and management of childhood interstitial lung disease in infancy. Am J Respir Crit Care Med 2013; 188:376–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guillerman RP: Imaging of childhood interstitial lung disease. Pediatr Allergy, Immunol Pulmonol 2010; 23:43–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.