Abstract
Background and Objectives
Age is the largest risk factor for dementia. However, dementia is not universal, even among the oldest-old age groups. Following contemporary neuropathologic guidelines, our objectives were to describe the key neuropathologic lesions and their associations with antemortem cognition in oldest-old individuals.
Methods
Participants were those enrolled in The 90+ Study, a longitudinal, population-based study of aging/dementia in the oldest old, who agreed to postmortem brain examination. All autopsied brains as of December 2020 were evaluated for the prevalence of Alzheimer disease neuropathologic change (ADNC) and non-ADNC neuropathologic comorbidities. Associations between neuropathologic lesions or the total neuropathologic burden score (sum of the individual scores) and cognition were assessed using multinomial logistic regression and multiple linear regression. Separate regression analyses evaluated relationships between limbic-predominant age-related TDP-43 encephalopathy (LATE-NC) and hippocampal sclerosis (HS) or ADNC/primary age-related tauopathy (PART). Resistance, or failure to develop ADNC/PART, and resilience, inferred from higher-than-expected cognitive functioning, were evaluated in the presence or absence of non-ADNC neuropathologic features.
Results
The most common neuropathologic features in the sample (n = 367) were ADNC/PART related. Increased dementia odds were associated with elevated total neuropathologic burden (odds ratio [OR] 1.5, 95% CI 1.3–1.7, p < 0.0001), β-amyloid (OR 1.6, 95% CI 1.2–2.0, p < 0.0001), neurofibrillary tangles (OR 2.6, 95% CI 1.7–4.1, p < 0.0001), and LATE-NC (OR 2.3, 95% CI 1.7–3.1, p < 0.0001), correcting for multiple comparisons. LATE-NC was associated with dementia with (OR 6.1, 95% CI 2.0–18.7, p = 0.002) and without (OR 5.0, 95% CI 2.6–9.7, p < 0.0001) co-occurring HS and increased the odds of dementia among participants with ADNC (OR 5.0, 95% CI 2.7–9.2, p < 0.0001). Resistance to moderate/severe ADNC/PART was rare (3%), but resilience to ADNC/PART was not (55%). Resilience was rarer in the presence of non-ADNC comorbid lesions, particularly LATE-NC. Among those with moderate/severe ADNC/PART, dementia odds increased with each non-ADNC comorbid lesion (e.g., 1 lesion: OR 2.4, 95% CI 1.3–4.5, p < 0.005; 2 lesions: OR 5.9, 95% CI 2.8–12.3, p < 0.0001).
Discussion
These results highlight the importance of non-ADNC neuropathologic comorbidity, predominantly LATE-NC, to cognition in the oldest old. Given the cumulative effects of non-ADNC comorbid neuropathologic abnormalities, reducing their prevalence, especially LATE-NC, will be vital to the ultimate goal of reducing dementia burden in the oldest-old individuals.
Specific neuropathologic abnormalities have been shown in several populations to account for the majority of cognitive impairment associated with aging: Alzheimer disease neuropathologic change (ADNC), Lewy body disease (LBD), microvascular brain injury (μVBI), limbic-predominant age-related TDP-43 encephalopathy neuropathologic change (LATE-NC), cerebral amyloid angiopathy (CAA), and hippocampal sclerosis (HS).1-5 ADNC is the most common disease underlying severe cognitive decline and impairment in individuals older than 65 years.6 However, the role of multiple non-ADNC neuropathologic comorbidities in the development of cognitive impairment is increasingly appreciated,7,8 and previous reports suggest that the strongest correlate of cognitive impairment is the aggregate neuropathologic burden of ADNC and non-ADNC lesions.4,5
Age is considerably the largest risk factor for severe cognitive impairment and dementia.9 Despite the increased susceptibility to dementia with brain aging, however, dementia is not considered a normal outcome.10 In the context of our expanding grasp of the additive influences of neuropathologic burden on the development of dementia, the study of individuals who remain cognitively healthy and independent well into the later decades becomes pivotal for the consideration of potential prevention or treatment. Whether such individuals are largely resistant to the development of neuropathologic abnormalities, inferred from lower-than-expected observable neuropathologic change in the presence of known risk factors, or resilient to observed neuropathologic change, inferred from a higher level of cognitive functioning than expected despite high neuropathologic burden, is an important question guiding such investigations.11 Early reports from large-scale autopsy cohorts support that ADNC alone (even severe) may be insufficient to cause clinical dementia symptoms in some individuals,12 results supported by subsequent clinicopathologic investigations.13-15 These studies include individuals who survive to various ages, however, which complicates establishing whether some individuals are truly resistant to neuropathologic burden or merely never reached an age at which such burden becomes clinically apparent. The study of those who survive into the ninth decade of life and later, currently the fastest expanding age cohort in the United States,16 may reveal whether individuals who remain dementia free also remain largely free from substantial neuropathologic burden or conversely fail to develop clinical dementia symptoms despite dementia-associated brain injury.
Prior autopsy data from The 90+ Study, a longitudinal, population-based study of aging and dementia in the oldest old, support that multiple neuropathologic etiologies underlie cognitive impairment and dementia.17 Since these initial investigations, updated neuropathologic consensus guidelines have been introduced, thus limiting comparisons between The 90+ Study and other cohorts.4,18,19 To provide more direct comparison with other large clinicopathologic cohorts and explore the concepts of resistance and resilience in the oldest old, we re-evaluated brain autopsies from The 90+ Study following contemporary consensus neuropathologic guidelines. Our objectives were to (1) describe the prevalence of individual harmonized neuropathologic lesions and total neuropathologic burden, (2) determine the associations between neuropathologic scores and antemortem cognition and establish which neuropathologic changes have the strongest associations with cognitive impairment, (3) establish the degree of resistance to the development of neuropathologic abnormalities despite advanced age, the strongest known risk factor for brain disease, and (4) examine the level of resilience to moderate/severe neuropathologic burden in the oldest old.
Methods
Participants
Participants were those enrolled in The 90+ Study, an ongoing, longitudinal, population-based study of aging and dementia in the oldest old, who agreed to postmortem brain examination.20 The 90+ Study, started in 2003, enrolled surviving participants of the Leisure World Cohort Study, a mailed health survey that began in 1981 and was sent to all residents in a retirement community in Southern California. Participants were invited to enroll in The 90+ Study if they were aged 90 years or older and agreed to the study procedures. Participants were followed at 6-month intervals with neurologic and physical examinations, cognitive testing, functional assessments, and study partner interviews. For the current study, we included all brain autopsies completed as of December 31, 2020.
Standard Protocol Approvals, Registrations, and Patient Consents
All participants or their designated surrogates provided written consent to participate in the study. Procedures were reviewed and approved by the Institutional Review Board at the University of California, Irvine.
Neuropathologic Indices
Neuropathologic index scores were assigned as follows: (1) β-amyloid (Aβ) plaque score (0 = none; 1 = Thal phase 1 or 2; 2 = Thal phase 3; 3 = Thal phase 4 or 5)18,19,21; (2) neurofibrillary tangle (NFT) score (0 = none, 1 = Braak stage I or II, 2 = Braak stage III or IV, 3 = Braak stage V or VI)18,19,22; (3) National Institute on Aging-Alzheimer's Association (NIA-AA) “ABC” score, which incorporates Thal phase, Braak staging, and the Consortium to Establish a Registry for AD staging for neuritic plaques (0 = none; 1 = low; 2 = intermediate; 3 = high)18,19; (4) CAA (0 = none; 1 = mild; 2 = moderate; 3 = severe)23; (5) LBD (0 = none; 1 = brainstem predominant; 2 = limbic [transitional]; 3 = neocortical [diffuse])24; (6) LATE-NC (0 = no TDP-43 inclusions; 1 = amygdala only; 2 = plus hippocampus; 3 = plus middle frontal gyrus)25; (7) μVBI, defined according to the number of lesions observed in a defined set of standard sections (0 = no microinfarcts; 1 = low [1 microinfarct]; 2 = moderate [2 microinfarcts], and 3 = severe [≥3 microinfarcts])18,19; and (8) HS, classified in 3 levels (0 = absent; 1 = present in either right or left hippocampi; 3 = present in both right and left hippocampi).18,19 “ADNC/PART” refers to Aβ and/or NFT-bearing diseases (AD or primary age-related tauopathy [PART]). The total neuropathologic burden score was calculated by adding the individual neuropathologic scores (ADNC/PART and non-ADNC). The total non-ADNC comorbid number score was determined by the total number of non-ADNC neuropathologic lesions (LATE-NC, LBD, μVBI, and HS) present, whereas the total comorbid burden score was the sum of all non-ADNC comorbid scores.
Clinical Assessments
Assessments included neurologic examination, assessment of functional activities, clinical dementia rating, and a comprehensive neuropsychological test battery (measuring global cognition, verbal learning/memory, language, attention/working memory, and construction). To limit the possibility of interval cognitive progression, only participants with evaluations within 6 months of death were included in analyses examining associations between cognitive test performance and neuropathologic lesion scores. As a result, many participants did not have complete cognitive scores. Thus, only the measures with the least amount of missingness were included: (1) global cognition (Mini-Mental State Examination [MMSE]26 and Modified Mini-Mental State Examination [3MS]27), (2) verbal learning and memory (California Verbal Learning Test II-Short Form [CVLT-II]28), and (3) semantic verbal fluency.29
Each participant was assigned a final cognitive diagnosis at the time of death during a multidisciplinary consensus conference using all available clinical information and blinded to neuropathologic diagnoses.20 Participants were classified as having no cognitive impairment, cognitive impairment, no dementia (CIND), or dementia. Dementia diagnosis was made according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition diagnostic criteria.30 CIND was assigned when cognitive or functional impairments were present but did not meet dementia criteria.31
Covariates
For all regression models with cognitive diagnosis as the dependent variable, age at death, gender, and education (<12th grade, high school graduate, vocational school/some college, college graduate, or advanced degree) were chosen a priori and retained as covariates in alignment with prior analyses. For models in which cognitive test scores were entered as the dependent variable, age at time of testing, interval between the final cognitive testing and death, gender, and education were entered as covariates. APOE genotype was available for a subsample; thus, separate sensitivity analyses were conducted entering APOE genotype (ε2/-, ε3/3, or ε4/-; participants with ε2/4 genotype were omitted).
Statistical Analyses
For our first objective of describing the prevalence of individual neuropathologic lesions and total neuropathologic burden, we calculated percentages of each lesion and the total neuropathologic burden score. Pairwise correlations between individual ordinal neuropathologic scores and between neuropathologic scores and demographic features were assessed using Spearman's rank-order correlations or χ2 tests. For our second objective of examining associations between neuropathologic scores and cognition, we tested associations between cognitive test performance (dependent variable) and neuropathologic scores (independent variable) with separate multiple linear regression analyses. We used the robust variance estimator, which is equivalent to the bootstrap variance estimator and thus robust to the nonconstant residual variance appreciated across cognitive measures. We then used multinomial logistic regression analyses, with cognitive diagnosis (no cognitive impairment, CIND, or dementia) as the dependent variable and neuropathologic score as the independent variable. We evaluated associations between cognition and LATE-NC and HS (LATE-NC, LATE + HS, HS only, or no LATE-NC/HS) or ADNC/PART (LATE-NC + ADNC/PART or ADNC/PART without LATE-NC) through calculating group proportions, multinomial logistic regression for cognitive diagnosis, and multiple linear regression for cognitive test performance as described above. For our third objective of establishing the frequency of resistance to ADNC/PART, we first reported the proportions of individuals with no or low evidence of ADNC/PART and non-ADNC comorbid lesions. We used ordinal logistic regression to examine the relationship between NFT or Aβ and non-ADNC comorbid lesions, with NFT or Aβ as the dependent variable and non-ADNC comorbid lesion number (0, 1, 2, or 3+) or non-ADNC comorbid burden (LBD, μVBI, LATE-NC, and HS combined score) as the independent variable. For our final objective of describing resilience to moderate/severe ADNC/PART, we reported the percentage of participants who did not receive a final diagnosis of CIND/dementia. We then used multinomial logistic regression to measure the association between cognition and non-ADNC comorbid burden among those with moderate/severe ADNC/PART, with final cognitive diagnosis (no cognitive impairment, CIND, or dementia) as the dependent variable and non-ADNC comorbid number or comorbid burden score as the independent variable. Bonferroni adjustment was used to control the familywise type I error set a priori at 0.05 for all analyses involving multiple testing. All analyses were performed using Stata/SE 15.1.
Data Availability
Data for all the analyses and results reported in this article were acquired from The 90+ Study. Data not published within the article will be shared by request of any qualified investigator.
Results
Neuropathologic Burden in the 90+ Cohort
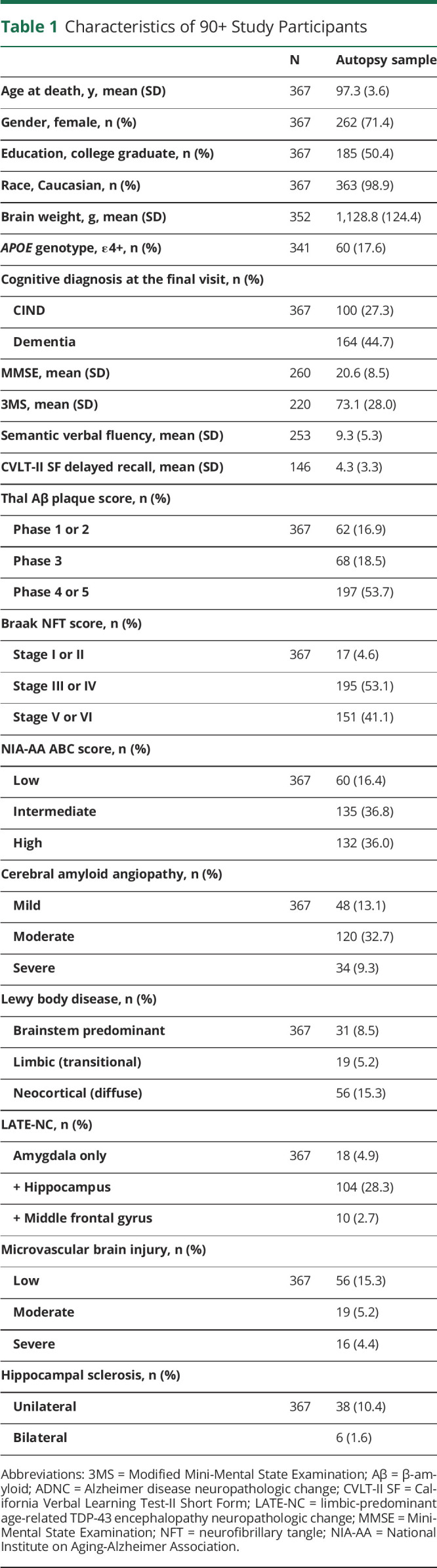
Study sample (n = 367) characteristics are summarized in Table 1. The mean age at death was 97 years (±4); 71% were female, 50% held a college degree or higher, and 45% were diagnosed with dementia. The mean postmortem interval was 8 hours (±10). The most common pathologic changes were NFTs (99%), Aβ plaques (89%), and CAA (55%), followed by LATE-NC (36%), LBD (29%), μVBI (25%), and HS (12%). None of the LATE-NC cases were diagnosed with frontotemporal lobar degeneration. Age at death, gender, education, brain weight, and postmortem interval were not significantly associated with any of the neuropathologic variables. The correlation between age at death and brain weight was weak but statistically significant (r = ‐ 0.23, p < 0.0001). APOE e4 genotype was associated with AB plaques (χ2 = 13.0, p = 0.005) and CAA (χ2 = 24.1, p < 0.001). There were no statistically significant correlations between APOE e2 and any neuropathologic variables.
Table 1.
Characteristics of 90+ Study Participants
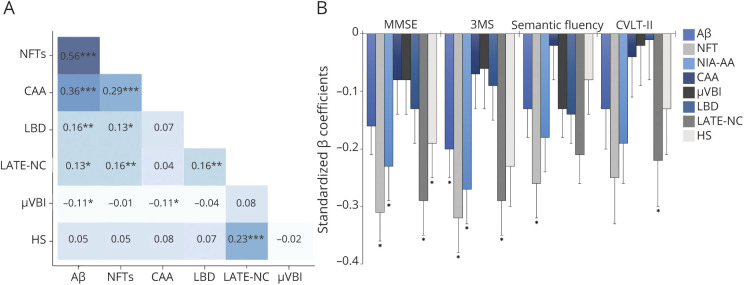
Among the individual neuropathologic lesions, the strongest associations were among the ADNC-related variables (Figure 1A). All 202 participants with CAA pathology had NFTs, Aβ plaques, or both, and 84% of CAA cases met NIA-AA pathologic criteria for AD diagnosis. Given this substantial overlap, CAA was not considered a non-ADNC comorbid lesion. The remaining individual neuropathologic scores (Aβ plaques, NFTs, LBD, μVBI, HS, and LATE-NC) were combined for a total neuropathologic burden score (possible range = 0–17).
Figure 1. Neuropathologic Associations in the 90+ Study Cohort.
Spearman rank-order correlations between individual neuropathologic scores (***p < 0.0001, **p < 0.01, and *p < 0.05) (A) and associations between individual neuropathologic lesion types and cognitive test scores, based on separate multiple linear regression analyses, with cognitive test score as the dependent variable and lesion type as the predictor, controlling for age at visit, interval between visit and death, gender, and education. Standardized β coefficients are reported to facilitate cross-measure comparison. Asterisks represent statistical significance after correcting for multiple comparisons (B). 3MS = Modified Mini-Mental State Examination; Aβ = β-amyloid; CAA = cerebral amyloid angiopathy; CVLT-II = California Verbal Learning Test-II; HS = hippocampal sclerosis; LATE-NC = limbic-predominant age-related TDP-43 encephalopathy neuropathologic change; LBD = Lewy body disease; μVBI = microvascular brain injury; MMSE = Mini-Mental State Examination; NFT = neurofibrillary tangle; NIA-AA = National Institute on Aging-Alzheimer Association.
Clinicopathologic Correlations
To examine the relationship between cognitive test scores proximal to death and total neuropathologic burden, a subset of participants who had cognitive testing within 6 months of autopsy was analyzed (n = 260). Individual neuropathologic scores were variably associated with the cognitive measures; the most consistent after correcting for multiple comparisons were NFTs and LATE-NC (Figure 1B). Nonstandardized regression coefficients for these analyses are provided in eTable 1 (links.lww.com/WNL/C136). To evaluate potential bias associated with excluding those without cognitive data, means and distributions of demographic and neuropathologic variables were compared with the overall sample (eTable 2).
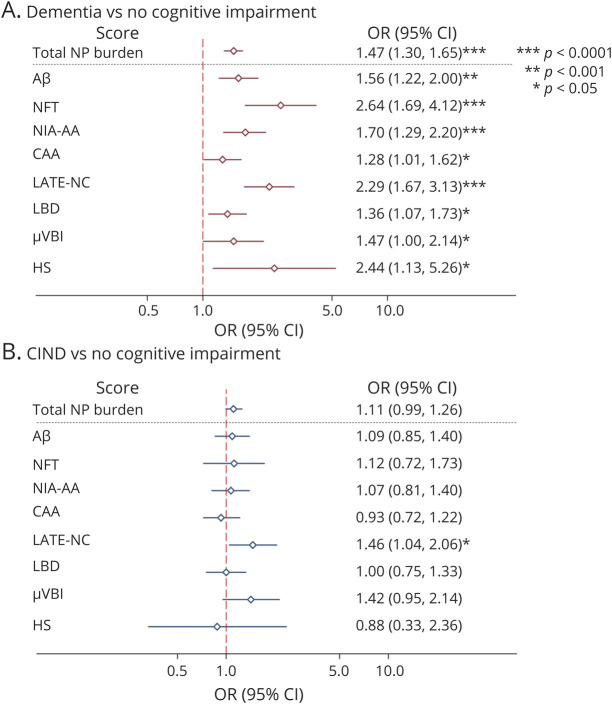
In the total sample, elevated total neuropathologic burden score, Aβ, NFT, NIA-AA score, and LATE-NC were significantly associated with increased odds of a final diagnosis of dementia after correcting for multiple comparisons (Figure 2A). Only LATE-NC was associated with increased odds of the final diagnosis of CIND, although this failed to meet statistical significance after correcting for multiple comparisons (Figure 2B). Sensitivity analyses were conducted for the sample with the APOE genotype available (n = 331) including the APOE genotype as a covariate; the odds ratios did not substantially change (eMethods and eFigure 1, links.lww.com/WNL/C136). The APOE genotype was not independently associated with final cognitive diagnosis.
Figure 2. Odds of Dementia or CIND Diagnosis in the Presence of Neuropathologic Lesions.
Odds ratios and 95% CIs for dementia (A) and CIND (B) vs. no cognitive impairment, estimated from multinomial logistic regression analyses controlling for age at death, gender, and education. The total neuropathologic burden scale was 0–17; all other scores were scaled 0–3, except for HS, which was scaled 0–2. Data are presented on a logarithmic scale. Aβ = β-amyloid; CAA = cerebral amyloid angiopathy; CIND = cognitive impairment, no dementia; HS = hippocampal sclerosis; LATE-NC = limbic-predominant age-related TDP-43 encephalopathy neuropathologic change; LBD = Lewy body disease; μVBI = microvascular brain injury; NFT = neurofibrillary tangle; NIA-AA = National Institute on Aging-Alzheimer Association; NP = neuropathologic; OR = odds ratio.
Associations Between LATE-NC and HS/ADNC
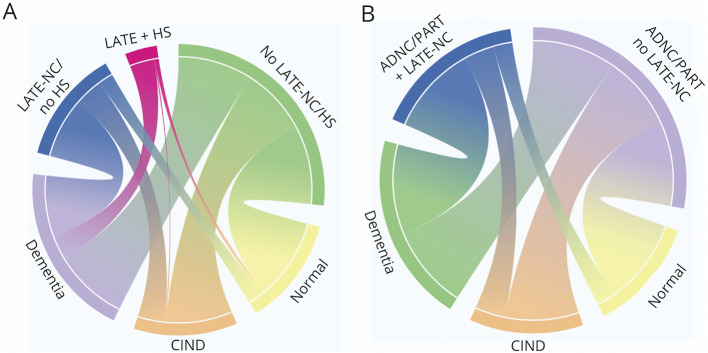
HS was present in 22% of participants with LATE-NC (LATE + HS). Eighty-three percent of participants with LATE + HS had dementia, compared with 59% of participants with LATE-NC/no HS and 34% of participants without LATE-NC/HS (Figure 3A). Both LATE + HS (odds ratio [OR] 6.06, 95% CI 1.96–18.71, p = 0.002) and LATE-NC/no HS (OR 4.98, 95% CI 2.56–9.69, p < 0.0001) were associated with increased odds of dementia compared with those without LATE-NC/HS. HS alone was not associated with increased dementia odds; however, the group size was small (n = 15). LATE + HS, LATE/no HS, and HS alone were not associated with CIND after correcting for multiple comparisons. LATE + HS and LATE-NC/no HS were associated with worse performance as compared to participants without LATE-NC/HS on the MMSE (LATE-NC: B = −4.20, 95% CI −6.49 to −1.91, p < 0.001, LATE + HS: B = −7.62, 95% CI −12.26 to −2.99, p = 0.001) and 3MS (LATE-NC: B = −11.00, 95% CI −19.32 to −2.68, p = 0.009, LATE + HS: B = −26.46, 95% CI −44.76 to −8.17, p = 0.005). LATE + HS was associated with lower CVLT-II delayed recall (B = −3.58, 95% CI −6.34 to −0.83, p = 0.01), and LATE-NC/no HS was associated with reduced verbal fluency (B = −2.17, 95% CI −3.59 to −0.76, p = 0.003).
Figure 3. Chord Diagrams Representing Connections Between Neuropathologic Lesions and Final Cognitive Diagnosis.
The weights of the connections represent the proportions of participants with LATE-NC/no HS, LATE + HS, and no LATE-NC/HS who were diagnosed with no cognitive impairment, CIND, or dementia (A) and the proportions of participants with moderate/severe ADNC/PART with and without LATE-NC who were diagnosed with no cognitive impairment, CIND, or dementia (B). Owing to the small sample size (n = 15), HS without LATE-NC is not included in the diagram; roughly equal numbers were diagnosed with no cognitive impairment, CIND, and dementia (n = 4, 6, and 5, respectively). Colors are presented only to distinguish between categories. ADNC = Alzheimer disease neuropathologic change; CIND = cognitive impairment, no dementia; HS = hippocampal sclerosis; LATE-NC = limbic-predominant age-related TDP-43 encephalopathy neuropathologic change; PART = primary age-related tauopathy.
Moderate/severe ADNC/PART was present in 98% of LATE-NC and 100% of LATE + HS cases. Sixty-four percent of participants with both LATE-NC and moderate/severe ADNC/PART were diagnosed with dementia, compared with 35% of participants with ADNC/PART with no LATE-NC (Figure 3B). Moderate/severe ADNC/PART + LATE-NC was associated with increased dementia odds as compared to moderate/severe ADNC/PART with no LATE-NC (OR 5.02, 95% CI 2.73–9.22, p < 0.0001), as well as worse performance on the MMSE (B = −4.79, 95% CI −6.99 to −2.59, p < 0.0001), 3MS (B = −14.10, 95% CI −22.39 to −5.81, p = 0.001), CVLT-II SF delayed recall (B = −1.70, 95% CI −2.98 to −0.42, p = 0.009), and verbal fluency (B = −1.94, 95% CI −3.29 to −0.60, p < 0.005).
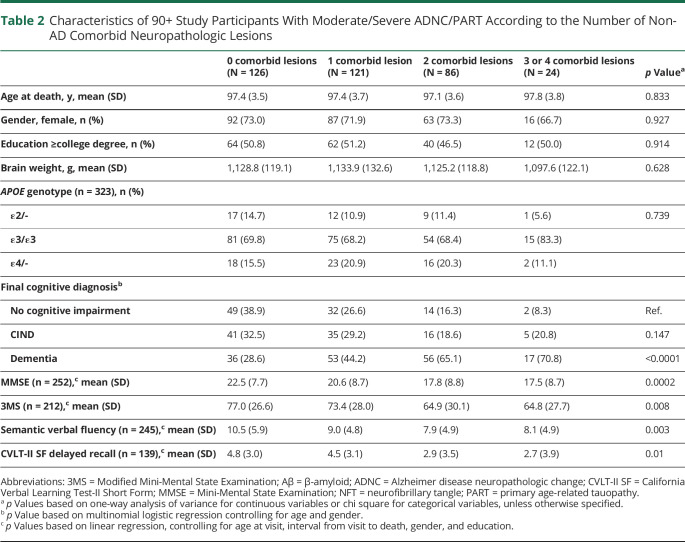
Resistance and Resilience
Resistance
Only 1 person of 367 90+ Study brain donors had no evidence of any Aβ plaques or NFTs; a female participant aged 91 years at the time of her death, who also had no evidence of any non-ADNC pathologic features examined. Similarly, resistance to moderate/severe ADNC/PART was seen in only 3% of the total autopsied cohort (10/367). Conversely, 36% of the sample was fully resistant to the non-ADNC comorbid lesions studied. We next examined those participants with moderate/severe ADNC/PART (Aβ plaques or NFT scores of 2 or 3) (n = 357) according to the number of non-ADNC comorbid lesions. There were no significant differences in age at death, education, gender, APOE genotype, or brain weight between groups (Table 2). However, odds of higher NFTs increased with the number of comorbid diseases (OR 1.3, 95% CI 1.1–1.7, p = 0.010), and the total burden of non-ADNC comorbidity was positively associated with Aβ (OR 1.1, 95% CI 1.0–1.3, p = 0.017) and NFTs (OR 1.2, 95% CI 1.1–1.3, p = 0.005), suggesting a general vulnerability to brain injury from multiple neuropathologic abnormalities in a subset of the oldest old with higher ADNC/PART.
Table 2.
Characteristics of 90+ Study Participants With Moderate/Severe ADNC/PART According to the Number of Non-AD Comorbid Neuropathologic Lesions
Resilience
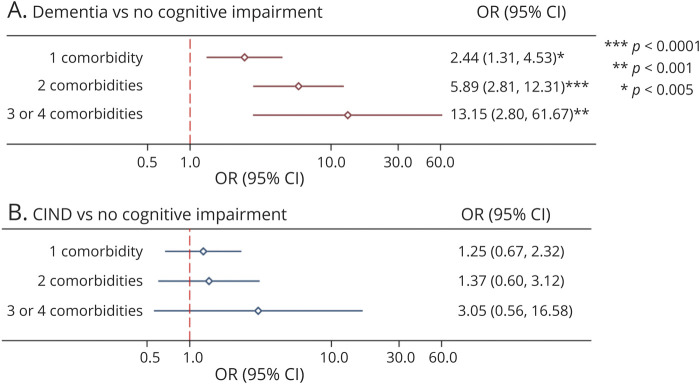
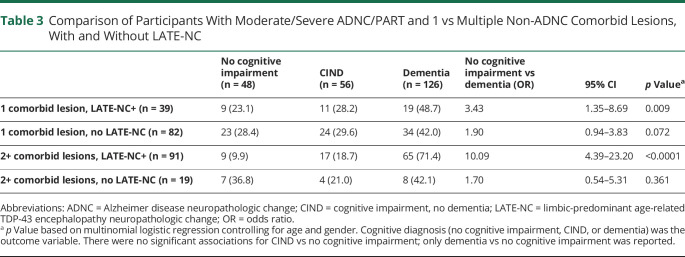
Despite substantial ADNC/PART in the cohort, 55% did not receive a final diagnosis of dementia, and 28% remained free from any detected cognitive impairment. Among those with moderate/severe ADNC/PART and no non-ADNC comorbid lesions (n = 126), 39% had no cognitive impairment and thus had apparent resilience to ADNC/PART. Conversely, only 16% of individuals with moderate/severe ADNC/PART who had 2 non-ADNC comorbid lesions (n = 86) and 8% who had 3 or 4 non-ADNC comorbid lesions (n = 24) were resilient to their observed neuropathologic burden. MMSE, 3MS, and semantic verbal fluency scores were also negatively associated with the number of non-ADNC comorbid lesions (Table 2). A diagnosis of dementia was positively associated with the number of non-ADNC comorbidities (OR 2.3, 95% CI 1.7–3.2, p < 0.0001) and with total non-ADNC comorbid burden (OR 1.5, 95% CI 1.3–1.8, p < 0.0001). Estimated odds of a final diagnosis of dementia vs no cognitive impairment increased with each non-ADNC comorbidity (Figure 4A), whereas odds of a final diagnosis of CIND vs no cognitive impairment did not (Figure 4B). Further investigation yielded that the presence of LATE-NC is largely responsible for the increase in odds of dementia among participants with moderate/severe ADNC/PART for both single lesion and multiple lesion comorbidities (Table 3).
Figure 4. Odds of Dementia and CIND According to the Number of Non-ADNC Comorbid Lesions in Participants With Intermediate/High ADNC.
Odds ratios and 95% CIs for dementia (A) and CIND (B) vs. no cognitive impairment, estimated from multinomial logistic regression analyses controlling for age at death, gender, and education. The dependent variable was final cognitive diagnosis (no impairment, CIND, or dementia). Data are presented on a logarithmic scale. ADNC = Alzheimer disease neuropathologic change; CIND = cognitive impairment, no dementia; OR = odds ratio.
Table 3.
Comparison of Participants With Moderate/Severe ADNC/PART and 1 vs Multiple Non-ADNC Comorbid Lesions, With and Without LATE-NC
Discussion
These results extend our understanding of the individual and cumulative effects of the most common dementia-related neuropathologic abnormalities in the oldest old. We found that although the most prevalent pathologic lesions at autopsy were NFTs or Aβ, the presence of LATE-NC was prominently associated with increased odds of dementia diagnosis. Despite the near ubiquity of ADNC/PART, a substantial proportion of participants in this oldest-old age group remained dementia free, especially those without non-ADNC comorbid neuropathologic abnormalities.
A primary purpose of this article was to re-evaluate the entire brain autopsy cohort from The 90+ Study using a consistent and comprehensive set of current consensus neuropathologic guidelines, which includes and approximately doubles the previously analyzed smaller sample size (n = 185).32 We report here a greater proportion of participants meeting the NIA-AA consensus guidelines for neuropathologic diagnosis of AD than previously described in 90+ Study participants (73% vs 50%)17,32 and in younger autopsy cohorts that used the same methods,4,33 but consistent with results from the Religious Orders Study and Rush Memory and Aging Project which found that intermediate/high ADNC peaks in the 10th decade at ∼76%, plateauing thereafter.34 In addition, consistent with prior studies of this and other cohorts, we found that non-ADNC comorbid pathologic lesions were common, such that 64% of the cohort had 1 or more non-ADNC comorbid pathologic changes.4,17,32,35 However, we report here a higher rate of neocortical LBD (15% vs 1%) and a lower rate of vascular brain injury (25% vs 75%) than reported previously in this cohort, although the previous vascular brain injury definition included CAA, discussed further below.32 Furthermore, we did not find the association between the APOE ε2 allele and ADNC reported previously in this cohort.36 Such differences may result from the inclusion of a substantially larger sample and/or use of updated neuropathologic consensus guidelines.
Of interest, within this oldest-old cohort, we did not find associations between age and any neuropathologic abnormalities. Although this is consistent with previously published estimated probabilities for the oldest old in terms of Aβ, NFTs, and LATE-NC, it is inconsistent with estimates that HS and VBI continue to rise in the oldest-old age groups.34 Failure to find an association here could be due to survival effects inherent in enrolling a sample enduring to the oldest-old age group, particularly when considering the low total prevalence of microvascular abnormalities.
To evaluate the influence of neuropathologic load in the cohort, we calculated a neuropathologic burden score as we have done with previous autopsy cohorts.4 In this at-risk group, the aggregate neuropathologic score was associated with dementia diagnosis and cognitive test performance, as we reported in younger cohorts. However, we found that specific neuropathologic abnormalities (NFTs and LATE-NC) were consistently associated with dementia and reduced performance on cognitive measures, whereas other neuropathologic lesions were more variably associated with dementia and cognitive test performance. This suggests that non-ADNC comorbid influences on cognition in the oldest old may differ from younger autopsy cohorts in which, although all indices were associated with cognition, none of the neuropathologic scores for individual lesions were as highly associated with neuropsychological test performance as was the aggregate score.4,37
Of interest in this cohort is the prominent negative association between LATE-NC and cognition. LATE-NC increases with age and is associated with worse cognition.38 Potential interactions between LATE-NC and other neuropathologic abnormalities complicate this relationship, however. For example, particularly as LATE-NC increases, there is a known association with HS that may mediate the relationship with cognition and has led to the hypothesis of both direct (resulting from TDP-43 proteinopathy alone) and indirect (resulting from TDP-43–associated HS) routes to dementia.5,25,39 Here, we found that although LATE-NC with and without comorbid HS was associated with dementia, a higher proportion of participants with LATE + HS had dementia compared with LATE-NC/no HS, along with lower performance on global cognitive tasks among those with LATE + HS. This supports the dual pathway hypothesis described above as well as previous conjecture that LATE + HS may represent a later stage of LATE-NC.25
Similarly, the nature of the association between LATE-NC and Aβ/NFTs may differ by cohort, suggesting possible moderation by gender, age, race/ethnicity, or other factors.5,40 Nonetheless, there is increasing evidence that processes that lead to ADNC and LATE-NC act synergistically to negatively affect cognition, particularly with advancing age, and that some cases of LATE-NC result “downstream” from AD pathology.25,35,41,42 Here, we demonstrate that ADNC/PART plus LATE-NC is more strongly associated with dementia than ADNC/PART alone, lending support to this assertion. Given the current difficulty in discerning the clinical manifestations of LATE-NC and ADNC/PART,25 these results underscore the need for identification of biomarkers to detect LATE-NC during life to adequately address the current mandate to identify biological treatment targets and advance a precision medicine approach for AD and related disorders.
Our results also offer interesting information pertaining to CAA, often described as a potential link between cerebrovascular and ADNC pathways.43 Previous reports noted associations between CAA and cognition that were either independent from or interacted with ADNC to produce worse cognitive outcomes and often largely vascular cognitive phenotypes.44-46 Although our results suggest a close alignment between CAA and ADNC, higher CAA was weakly associated with lower microvascular lesion scores. Furthermore, we did not find any associations between cognitive test performance and CAA, and increased CAA was not associated with increased odds of dementia diagnosis. It is important that the 90+ Study cohort has a lower prevalence of CAA than reported in a previous community-based neuropathology study (55% vs 79%) that found that both dementia risk and cognitive test performance were associated with CAA over and above ADNC.45 Given the increased risk of recurrent intracerebral hemorrhage and mortality incurred by CAA, it is possible that these findings in this oldest-old cohort are the result of selection bias.47
Inferred resistance to dementia-associated neuropathologic change is defined as a lack of observed specific neuropathologic change on autopsy as compared to what is expected given the person's age and other risk factors.11 As expected, complete resistance to ADNC/PART in the oldest old was negligible. Indeed, there was only a single case identified with no evidence of any ADNC or PART, which underscores the rarity of complete resistance to cerebral Aβ amyloidosis and/or neurofibrillary degeneration in people aged 90 years and older. As with prior autopsy studies,4,17,35,38,48 we found that resistance to non-ADNC dementia-associated lesions is less likely as ADNC/PART severity increases. Apparent resilience to dementia-associated brain injury can be inferred from cognitive function that is better than expected given the observed neuropathologic burden. Despite the high level of ADNC/PART and non-ADNC comorbid pathologic lesions seen in this cohort, 55% never met the criteria for dementia. Consistent with a recent meta-analysis in which the authors report that a substantial proportion of community-dwelling participants with ADNC sufficient to warrant a diagnosis of clinical AD do not develop dementia, over half of 90+ Study participants with moderate/severe ADNC/PART were not diagnosed with dementia before death.49 Again, this differed according to whether or not non-ADNC comorbid lesions were present: 39% of those with moderate/severe ADNC/PART alone remained free from any cognitive impairment (including CIND or dementia), compared with 16% of those with both moderate/severe ADNC/PART and 2 non-ADNC comorbid diseases and 8% of those with 3 or 4 non-ADNC comorbid diseases. Taken together, these results suggest that those with higher ADNC/PART pathology are less likely to be resistant to other non-AD lesions, which in turn decreases the likelihood of resilience to neuropathologic burden. These findings support what we have previously observed in other cohorts: that much of what may be termed resilience may instead represent resistance to comorbid brain disease.11 Our results further support that in people aged 90 years and older, resistance to LATE-NC may represent a protective factor against the development of dementia.
The current study has important limitations. First, our analyses are limited to the oldest old, thus limiting study generalizability. However, the value of studying those who survived into the 10th decade and beyond provides important information that may be obfuscated in studies that include younger participants (particularly because these rarely include sufficient sample sizes in the oldest-old age groups). A second factor limiting generalizability of these findings is our highly educated and predominantly Caucasian sample. Future work will need to focus on broadening study inclusion to provide more generalizable results and to fully address the role of education and cognitive reserve on neuropathologic change. Third, for our analyses examining resistance and resilience, we focused on the lesion type: either NFTs or Aβ deposition. In this sample, NFTs were highly prevalent and were the neuropathologic change most consistently associated with cognition, outperforming the combined NIA-AA severity score in predicting dementia odds and cognitive test performance. Thus, we opted for analyzing the impact of NFTs (ADNC and PART cases) or Aβ deposition (ADNC but not PART cases). Finally, we did not have full cognitive test scores for all individuals proximal to death. This limited our ability to associate individual lesions with specific cognitive functions, which would provide more information as to the potential real-word effects of specific lesion types.
This study supports the importance of non-ADNC neuropathologic comorbidities, especially LATE-NC, to cognition in 90+ Study participants. Despite the high prevalence of ADNC in older cohorts and the historical tendency to focus on ADNC as the primary driver of cognitive decline in aging, there is increasing appreciation of the role of non-ADNC comorbid pathologic abnormalities in aging and dementia. Our results show that a substantial proportion of participants aged 90+ years with moderate/severe ADNC/PART fail to develop the debilitating symptoms of dementia. Thus, treatments that focus solely on reducing or reversing ADNC/PART in the brain may not be sufficient to adequately treat cognitive impairment in older individuals50 and may have undermined, at least in part, some of the failed AD clinical trials to date. Instead, these data support the multiple hit model for severe cognitive impairment in the oldest old. In particular, further investigation into the pathophysiologic events that lead to LATE-NC accumulation in older adults will be vital to the development of treatment and prevention strategies for cognitive decline. Given the cumulative effects of multiple non-ADNC comorbid neuropathologic abnormalities, biomarkers that identify and interventions that reduce each of these neuropathologic abnormalities are vital to the ultimate goal of limiting the consequences of common neuropathologic lesions in the oldest old.
Acknowledgment
APOE genotyping by the National Centralized Repository for Alzheimer's Disease and Related Dementias (NCRAD), which receives government support under a cooperative agreement grant (U24AG021886) awarded by the National Institute on Aging (NIA), was used in this study. The authors sincerely thank their research participants and their families for their participation in this study. They also thank the testers and examiners of The 90+ Study.
Glossary
- 3MS
Modified Mini-Mental State Examination
- Aβ
β-amyloid
- AD
Alzheimer disease
- ADNC
Alzheimer disease neuropathologic change
- CAA
cerebral amyloid angiopathy
- CIND
cognitive impairment, no dementia
- CVLT-II
California Verbal Learning Test II
- HS
hippocampal sclerosis
- LATE-NC
limbic-predominant age-related TDP-43 encephalopathy neuropathologic change
- LBD
Lewy body disease
- MMSE
Mini-Mental State Examination
- OR
odds ratio
- μVBI
microvascular brain injury
- NFT
neurofibrillary tangle
- NIA-AA
National Institute on Aging-Alzheimer's Association
- PART
primary age-related tauopathy
- TDP-43
transactive response DNA-binding protein of 43 kDa
Appendix. Authors
Footnotes
CME Course: NPub.org/cmelist
Study Funding
This work was supported by funding from the NIH (UF1 AG057707, UF1 AG053983-01, R01AG021055, and P50AG016573).
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Boyle PA, Yu L, Leurgans SE, et al. Attributable risk of Alzheimer's dementia attributed to age-related neuropathologies. Ann Neurol. 2019;85(1):114-124. doi: 10.1002/ana.25380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flanagan M, Larson EB, Latimer CS, et al. Clinical-pathologic correlations in vascular cognitive impairment and dementia. Biochim Biophys Acta. 2016;1862(5):945-951. doi: 10.1016/j.bbadis.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson PT, Alafuzoff I, Bigio EH, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71(5):362-381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White LR, Edland SD, Hemmy LS, et al. Neuropathologic comorbidity and cognitive impairment in the Nun and Honolulu-Asia Aging Studies. Neurology. 2016;86(11):1000-1008. doi: 10.1212/WNL.0000000000002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flanagan ME, Cholerton B, Latimer CS, et al. TDP-43 Neuropathologic Associations in the Nun Study and the Honolulu-Asia Aging Study. J Alzheimers Dis. 2018;66(4):1549-1558. doi: 10.3233/JAD-180162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol. 2009;68(1):1-14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson PT, Abner EL, Schmitt FA, et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. 2010;20(1):66-79. doi: 10.1111/j.1750-3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197-2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 9.2020 Alzheimer's disease facts and figures. Alzheimers Dement. 2020;16(3):391-460. doi: 10.1002/alz.12068. [DOI] [Google Scholar]
- 10.Nelson PT, Head E, Schmitt FA, et al. Alzheimer's disease is not “brain aging”: neuropathological, genetic, and epidemiological human studies. Acta Neuropathol. 2011;121(5):571-587. doi: 10.1007/s00401-011-0826-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montine TJ, Cholerton BA, Corrada MM, et al. Concepts for brain aging: resistance, resilience, reserve, and compensation. Alzheimers Res Ther. 2019;11(1):22. doi: 10.1186/s13195-019-0479-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277(10):813-817. [PubMed] [Google Scholar]
- 13.Boyle PA, Wilson RS, Yu L, et al. Much of late life cognitive decline is not due to common neurodegenerative pathologies. Ann Neurol. 2013;74(3):478-489. doi: 10.1002/ana.23964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jellinger KA, Attems J. Challenges of multimorbidity of the aging brain: a critical update. J Neural Transm (Vienna). 2015;122(4):505-521. doi: 10.1007/s00702-014-1288-x. [DOI] [PubMed] [Google Scholar]
- 15.SantaCruz KS, Sonnen JA, Pezhouh MK, Desrosiers MF, Nelson PT, Tyas SL. Alzheimer disease pathology in subjects without dementia in 2 studies of aging: the Nun Study and the Adult Changes in Thought Study. J Neuropathol Exp Neurol. 2011;70(10):832-840. doi: 10.1097/NEN.0b013e31822e8ae9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Census Bureau. 2017 National Population Projections Tables. Main Series. Table 2: Projected Age and Sex Composition of the Population: 2017-2060. US Census Bureau, Population Division, 2018. [Google Scholar]
- 17.Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM. Multiple pathologies are common and related to dementia in the oldest-old: the 90+ Study. Neurology. 2015;85(6):535-542. doi: 10.1212/WNL.0000000000001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement. 2012;8(1):1-13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123(1):1-11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corrada MM, Berlau DJ, Kawas CH. A population-based clinicopathological study in the oldest-old: the 90+ study. Curr Alzheimer Res. 2012;9(6):709-717. doi: 10.2174/156720512801322537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791-1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 22.Nagy Z, Yilmazer-Hanke DM, Braak H, Braak E, Schultz C, Hanke J. Assessment of the pathological stages of Alzheimer's disease in thin paraffin sections: a comparative study. Dement Geriatr Cogn Disord. 1998;9(3):140-144. doi: 10.1159/000017038. [DOI] [PubMed] [Google Scholar]
- 23.Vonsattel JP, Myers RH, Hedley-Whyte ET, Ropper AH, Bird ED, Richardson EP Jr. Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol. 1991;30(5):637-649. doi: 10.1002/ana.410300503. [DOI] [PubMed] [Google Scholar]
- 24.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88-100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019;142(6):1503-1527. doi: 10.1093/brain/awz099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314-318. [PubMed] [Google Scholar]
- 28.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2nd ed. The Psychological Corporation, 2000. [Google Scholar]
- 29.Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK. Norms for letter and category fluency: demographic corrections for age, education, and ethnicity. Assessment. 1999;6(2):147-178. doi: 10.1177/107319119900600204. [DOI] [PubMed] [Google Scholar]
- 30.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association, 1994. [Google Scholar]
- 31.Graham JE, Rockwood K, Beattie BL, et al. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349(9068):1793-1796. doi: 10.1016/S0140-6736(97)01007-6. [DOI] [PubMed] [Google Scholar]
- 32.Robinson JL, Corrada MM, Kovacs GG, et al. Non-Alzheimer's contributions to dementia and cognitive resilience in the 90+ Study. Acta Neuropathol. 2018;136(3):377-388. doi: 10.1007/s00401-018-1872-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aiello Bowles EJ, Crane PK, Walker RL, et al. Cognitive resilience to Alzheimer's disease pathology in the human brain. J Alzheimers Dis. 2019;68(3):1071-1083. doi: 10.3233/JAD-180942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farfel JM, Yu L, Boyle PA, et al. Alzheimer's disease frequency peaks in the tenth decade and is lower afterwards. Acta Neuropathol Commun. 2019;7(1):104. doi: 10.1186/s40478-019-0752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Latimer CS, Burke BT, Liachko NF, et al. Resistance and resilience to Alzheimer's disease pathology are associated with reduced cortical pTau and absence of limbic-predominant age-related TDP-43 encephalopathy in a community-based cohort. Acta Neuropathol Commun. 2019;7(1):91. doi: 10.1186/s40478-019-0743-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berlau DJ, Corrada MM, Head E, Kawas CH. APOE epsilon2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology. 2009;72(9):829-834. doi: 10.1212/01.wnl.0000343853.00346.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Latimer CS, Keene CD, Flanagan ME, et al. Resistance to Alzheimer disease neuropathologic changes and apparent cognitive resilience in the nun and Honolulu-Asia aging studies. J Neuropathol Exp Neurol. 2017;76(6):458-466. doi: 10.1093/jnen/nlx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boyle PA, Yang J, Yu L, et al. Varied effects of age-related neuropathologies on the trajectory of late life cognitive decline. Brain. 2017;140(3):804-812. doi: 10.1093/brain/aww341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nag S, Yu L, Capuano AW, et al. Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann Neurol. 2015;77(6):942-952. doi: 10.1002/ana.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cykowski MD, Takei H, Van Eldik LJ, et al. Hippocampal sclerosis but not normal aging or Alzheimer disease is associated with TDP-43 pathology in the basal forebrain of aged persons. J Neuropathol Exp Neurol. 2016;75(5):397-407. doi: 10.1093/jnen/nlw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kapasi A, Yu L, Boyle PA, Barnes LL, Bennett DA, Schneider JA. Limbic-predominant age-related TDP-43 encephalopathy, ADNC pathology, and cognitive decline in aging. Neurology. 2020;95(14):e1951-e1962. doi: 10.1212/WNL.0000000000010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tome SO, Gomes LA, Li X, Vandenberghe R, Tousseyn T, Thal DR. TDP-43 interacts with pathological tau protein in Alzheimer's disease. Acta Neuropathol. 2021;141(5):795-799. doi: 10.1007/s00401-021-02295-2. [DOI] [PubMed] [Google Scholar]
- 43.Keage HA, Carare RO, Friedland RP, et al. Population studies of sporadic cerebral amyloid angiopathy and dementia: a systematic review. BMC Neurol. 2009;9:3. doi: 10.1186/1471-2377-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arvanitakis Z, Leurgans SE, Wang Z, Wilson RS, Bennett DA, Schneider JA. Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann Neurol. 2011;69(2):320-327. doi: 10.1002/ana.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boyle PA, Yu L, Nag S, et al. Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology. 2015;85(22):1930-1936. doi: 10.1212/WNL.0000000000002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfeifer LA, White LR, Ross GW, Petrovitch H, Launer LJ. Cerebral amyloid angiopathy and cognitive function: the HAAS autopsy study. Neurology. 2002;58(11):1629-1634. doi: 10.1212/wnl.58.11.1629. [DOI] [PubMed] [Google Scholar]
- 47.Yilmaz P, Ikram MA, Ikram MK, et al. Application of an imaging-based sum score for cerebral amyloid angiopathy to the general population: risk of major neurological diseases and mortality. Front Neurol. 2019;10:1276. doi: 10.3389/fneur.2019.01276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu L, Boyle PA, Leurgans S, et al. Effect of common neuropathologies on progression of late life cognitive impairment. Neurobiol Aging. 2015;36(7):2225-2231. doi: 10.1016/j.neurobiolaging.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Azarpazhooh MR, Avan A, Cipriano LE, et al. A third of community-dwelling elderly with intermediate and high level of Alzheimer's neuropathologic changes are not demented: a meta-analysis. Ageing Res Rev. 2020;58:101002. doi: 10.1016/j.arr.2019.101002. [DOI] [PubMed] [Google Scholar]
- 50.Brookmeyer R, Kawas CH, Abdallah N, Paganini-Hill A, Kim RC, Corrada MM. Impact of interventions to reduce Alzheimer's disease pathology on the prevalence of dementia in the oldest-old. Alzheimers Dement. 2016;12(3):225-232. doi: 10.1016/j.jalz.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for all the analyses and results reported in this article were acquired from The 90+ Study. Data not published within the article will be shared by request of any qualified investigator.