Abstract
Introduction Cancer immunotherapy targeting the programmed cell death ligand 1 (PD-L1) and programmed cell death-1 (PD-1) axis has revolutionized cancer therapy. PD-L1 also serves as a predictive marker for such therapy. To assess the potential of such therapy in any cancer, the positivity of PD-1 and PD-L1 in such cancers needs to be assessed. However, such studies for breast cancer are lacking in South Asia. We aimed to estimate the positivity of PD-L1 and PD-1 receptors in breast cancer and its various clinicopathological groups in our patient population.
Materials and Methods We studied the immunoexpression of PD-1 and PD-L1 in 103 histologically proven invasive carcinoma breast cases from October 2018 to April 2019. The percent positivity of PD-1 and PD-L1 with 95% confidence intervals (CI) was estimated for all the cases as well as groups defined by stage, grade, molecular subtype, hormone receptor status, K i -67, and age.
Results PD-1 positivity was seen in 72 (69.9%) cases (95% CI: 60.1–78.6). PD-L1 immunoexpression was seen in 61 (59.2%) cases (95% CI: 49.1–68.8) in immune cells and in 39 (37.9%) cases (95% CI: 28.5–50.0) in tumor cells. No significant association was found between PD-1, PD-L1 and age, overall clinical stage, grade, size, estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2, and K i -67. Moderate-to-high PD-1 and PD-L1 immunopositivity was seen in all subtypes of breast cancer.
Conclusion PD-1 and PD-L1 is expressed in all subgroups of breast carcinoma. Patients in all such groups are amenable to immunotherapy, provided they are found suitable otherwise.
Keywords: programmed cell death 1 ligand 1, programmed cell death 1 receptor, breast carcinoma
Introduction
During cancer progression, immune responses play a pivotal role. Inhibition of immune responses favors cancer progression. Tumor clearance is enhanced through host immune responses by inhibiting programmed cell death 1 (PD-1) and its ligand (programmed cell death ligand 1: PD-L1) receptors. 1 2 PD-L1 expression has been studied in a variety of cancers 1 3 4 with evidence of correlations to various clinicopathological features. Immune checkpoint inhibitors have now been U.S. Food and Drug Administration (FDA) approved in many of these cancers.
Recently, atezolizumab (PD-L1 inhibitor) and Abraxane chemotherapy as a combination therapy has been approved for the treatment of patients with PD-L1-positive, unresectable locally advanced or metastatic triple-negative breast cancer. 5 It is highly likely that patients having other breast cancer subtypes may also benefit from such immune checkpoint inhibitors. Prior to applying individualized treatment protocol guidelines, we need to study the prevalence of expression of immune checkpoint markers in a population of breast cancer patients to provide guidance about their potential utility. We also need to establish the level of expression in breast cancer and its different clinicopathological subgroups to have an idea of whether immunotherapy will be particularly useful in different groups. Therefore, we have conducted this study to estimate the expression of PD-1 and PD-L1 in breast cancer and its different clinicopathological subgroups. To the best of our knowledge, such a study has not been performed in South Asia and will additionally provide local guidance for testing in a South Asian context.
Materials and Methods
This was an observational study done in the Department of Pathology and Laboratory Medicine & Integrated Breast Cancer Center, of our Institute, after obtaining ethical approval from the Institutional Ethics Committee. One hundred and three consecutive histopathologically proven needle core biopsies and mastectomy invasive carcinoma specimens from October 2018 till April 2019 with adequate tissue for further workup were included in the study. Clinical details were noted from case files and radiology department. Patients on chemotherapy and radiotherapy before biopsy, recurrent tumors, necrotic cores with no viable tumor cells and incompletely worked up case were not included in the study.
Hematoxylin and eosin-stained sections of study cases were observed for lymphocyte load in reference to tumor tissue present. Four-micron thick paraffin embedded tissue sections were subjected to immunohistochemistry with following primary antibodies (prediluted) on positive charged slides along with control showing appropriate staining. For PD-1, Clone: EP239; Isotype: Monoclonal, Rabbit IgG, make: Path-in-Situ; and for PD-L1, Clone: SP-263; Isotype: Monoclonal, Rabbit IgG, make: Ventana were used.
Sections were examined under low power (10 × ) and higher power (20× and 40 × ) fields to observe immunoreactivity. Expression of PD-1 was studied in lymphocytes, while PD-L1 was studied in both tumor cells and immune cells. Immunoexpression of PD-1 was considered positive in membrane and cytoplasm of lymphocytes with a cutoff of at least 1% in lymphocytes. For PD-L1, partial or complete membranous staining in greater than 1% of tumor cells was considered positive for tumor cells, and cytoplasmic and membranous staining in greater than 1% of immune cells was considered as positive for immune cells. A cutoff of ≥ 1% was considered positive for PD-1 and PDL-1 in both tumor cell membrane and immune cells.
Estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (Her2neu), and K i -67 were assessed on immunohistochemistry. For ER and PR, tumors showing greater than 1% positivity were considered positive. Scoring for Her2neu was done as per American Society of Clinical Oncology/College of American Pathologists guidelines 2018. 6 Molecular surrogate subtyping was done according to St. Gallen Consensus recommendations of 2013. 7 Grading of tumor was done using the Nottingham System. Clinical tumor, node, metastasis staging was done based on American Joint Committee on Cancer, 8th edition 2018 8 recommendations.
The prevalence of PD1 and PDL1 positivity in the overall study population and clinicopathological parameters was assessed as a proportion along with exact 95% confidence intervals (CIs). The association of the expression with ER, PR, grade, and stage was studied by the Fisher's exact test, while that with K i -67, size, and age was assessed by the Mann–Whitney U test. Statistical analysis was done using R statistical environment version 3.5.0.
Results
One hundred and three consecutive breast carcinoma cases were studied. PD-1 positivity was seen in 72 (69.9%) cases (95% CI: 60.1–78.6). PD-L1 immunoexpression was seen in 61 (59.2%) cases (95% CI: 49.1–68.8) in immune cells and in 39 (37.9%) cases (95% CI: 28.5–50.0) in tumor cells. PD-L1 tumor cell positivity showed a statistically significant association with nodal status, distant metastasis, and ER status when using unadjusted p -values ( Table 1 ). PD-1 and PD-L1 positivity by immune cell positivity was not significantly associated with any of the variables studied (age, size, node status, distant metastasis, clinical stage, ER, PR, Her2neu, K i -67%, grade, and molecular subtype). PD-1 and PD-L1 by either staining method showed a moderately high proportion of positivity in all groups of breast cancer. When adjustment for multiple comparisons was used by the Holm method, no variable retained its statistical significance. There was a significant statistical association between PD-1 in immune cells and PD-L1 in immune cells and in tumor cells ( p < 0.001 by the Fisher's exact test between PD-1 and PD-L1 in immune cells, p = 0.004 between PD-1 and PD-L1 in tumor cells, p = 0.002 between PD-L1 in immune cells and PD-L1 in tumor cells). Fig. 1 gives representative examples of PD-L1 positivity in immune cells and membranous positivity in tumor cells.
Table 1. Association of PD-1, PD-L1 in tumor cells and PD-L1 in immune cells with various parameters studied.
| Parameter | All cases | PD-1 | PD-L1 in tumor cells | PD-L1 in immune cells | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | p -Value | Positive | Negative | p -Value | Positive | Negative | p -Value | |||
| Age in years | 51 (25–82) | 52.5 (25, 78) | 48 (30, 82) | 0.1 | 45 (25, 81) | 52.5 (30, 82) | 0.1 | 52 (25, 81) | 50.5 (30, 82) | 0.5 | |
| Size in cm | 3.4 (0.8–17) | 3.3 (0.8, 17) | 4.4 (1.9, 10.6) | 0.2 | 3.5 (0.8, 16.5) | 3.2 (1.0, 17) | 1 | 3.4 (0.8, 17) | 3.2 (1.0, 10.6) | 0.5 | |
| Lymph node | Positive | 67 | 45 | 22 | 0.5 | 20 | 47 | 0.03 | 35 | 32 | 0.06 |
| Negative | 36 | 27 | 9 | 19 | 17 | 26 | 10 | ||||
| Distant metastases | Present | 14 | 8 | 6 | 0.3 | 1 | 13 | 0.02 | 9 | 5 | 0.8 |
| Absent | 89 | 64 | 25 | 38 | 51 | 52 | 37 | ||||
| Estrogen receptor a | Positive | 63 | 41 | 22 | 0.2 | 19 | 44 | 0.04 | 37 | 26 | 0.8 |
| Negative | 39 | 31 | 8 | 20 | 19 | 24 | 15 | ||||
| Progesterone receptor a | Positive | 51 | 34 | 17 | 0.5 | 16 | 35 | 0.2 | 28 | 23 | 0.4 |
| Negative | 51 | 38 | 13 | 23 | 28 | 33 | 18 | ||||
| Her2neu a | Positive | 39 | 25 | 14 | 0.2 | 15 | 24 | 0.7 | 22 | 17 | 0.4 |
| Negative | 54 | 42 | 12 | 22 | 32 | 35 | 19 | ||||
| Equivocal | 9 | 5 | 4 | 2 | 7 | 4 | 5 | ||||
| K i -67 in % | 25% (2–95%) | 25 (2, 95) | 27.5 (3, 95) | 0.9 | 30 (5, 90) | 25 (2, 95) | 0.3 | 30 (3, 90) | 20 (2, 95) | 0.1 | |
| Molecular subtype a | Triple negative | 18 | 13 | 5 | 0.3 | 11 | 7 | 0.1 | 10 | 8 | 0.3 |
| Luminal A | 13 | 10 | 3 | 4 | 9 | 5 | 8 | ||||
| Luminal B | 51 | 32 | 19 | 15 | 36 | 33 | 18 | ||||
| Her2neu enriched | 20 | 17 | 3 | 9 | 11 | 13 | 7 | ||||
| Grade a | 1 | 6 | 3 | 3 | 0.2 | 2 | 4 | 0.9 | 3 | 3 | 0.8 |
| 2 | 26 | 16 | 10 | 11 | 15 | 15 | 11 | ||||
| 3 | 67 | 50 | 17 | 25 | 42 | 42 | 25 | ||||
| Stage | I | 5 | 4 | 1 | 0.6 | 2 | 3 | 0.06 | 4 | 1 | 0.6 |
| II | 38 | 29 | 9 | 16 | 22 | 24 | 14 | ||||
| III | 46 | 31 | 15 | 20 | 26 | 24 | 22 | ||||
| IV | 14 | 8 | 6 | 1 | 13 | 9 | 5 | ||||
Abbreviations: ER, estrogen receptor; Her2neu, human epidermal growth factor receptor 2; IHC, immunohistochemistry; PD-1, programmed cell death-1; PD-L1, programmed cell death ligand 1; PR, progesterone receptor.
Note: Age, size, and K i -67 are presented as median with range in parentheses. For age, size, and K i -67 index, the Mann–Whitney U test was used, and for the other variables, the Fisher's exact test was used to compute the statistical significance.
In four cases, grade could be assessed; in one case IHC for ER, PR, Her2neu, and molecular subtyping could not be done.
Fig. 1.
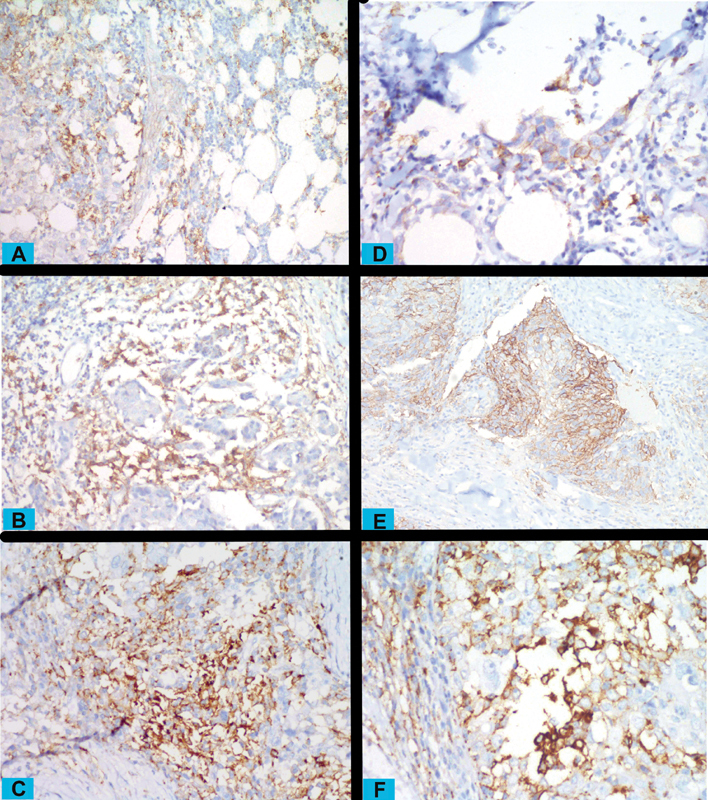
Programmed cell death ligand 1 (PD-L1)–positive immune cells in the tumoral stroma and infiltrating the tumor at low ( A ), moderate ( B ), and high ( C ) intensity (magnification ×200 for A , B , C ). Also, tumors cells having membranous PD-L1 positivity at low ( D ), moderate ( E ), and high ( F ) intensity (magnification ×400, ×200, and ×200 for D , E , F ).
Discussion
All subgroups have moderate expression of PD-1 and PD-L1. No statistically significant association after adjustment for multiple comparisons of either PD-1 or PD-L1 expression (by both tumor cell and immune cell positivity) was found with any specific type or group of breast cancer. This underlines that patients of all subgroups may be important candidates for immunotherapy. Others have found that PD-L1 shows increased expression in triple-negative breast cancers. 3 We feel that this difference may be explained based on either immunohistochemistry antibody differences or tumor location. At the same time, the positivity rate in our study for triple-negative breast cancers is comparable to similar other studies. While the efficacy of immune checkpoint inhibitors in breast cancers has mainly been evaluated just on triple-negative breast cancers, our results reflect that the potentially important benefits for immunotherapy may be trialed in all breast cancer groups and not just the triple-negative patients.
PD-L1 expression in tumor cells, but not immune cells, does seem to have a mild association with lymph node positivity, distant metastasis, and ER status. The statistical significance (by unadjusted p -values) reached in these three cases was associated with a mild-to-moderate effect size, supporting claims that PD-L1 may be associated with prognosis. Some studies have found a positive significant association of PD-L1 with ER, PR, and Her2 status. 9 10 11 The statistical significance is however lost when adjusted for multiple comparisons due to the high number of comparisons done due to the exploratory nature of this study. To draw a robust conclusion about association with prognostic factors, a follow-up-focused confirmatory study is required. Nonetheless, there is a relatively high proportion of PD-L1 cases in all subgroups defined by nodal status as well as ER status to suggest that these factors should not negatively influence a decision to treat such groups by immunotherapy provided other eligibility criteria are met.
Evaluation of PD-L1 immunoexpression is challenging due to lack of specific antibodies for use and their validation in formalin fixed paraffin embedded tissues in addition to different cutoff used and evaluation methods in different tumors depending upon the immune checkpoint inhibitor approved. To date, no standardized assays for companion diagnostics have been approved in immunohistochemistry for breast cancer, except SP-142 assay approved in 2019 for triple-negative breast cancer for the drug atezolizumab, but there are other clones that may be potentially useful including SP-263, 22C3, and 28–8 belonging to different companies and having different staining characteristics. 9 12
Similar differences appear in defining the area to be examined for PD-L1 positivity. While tumoral immune cell positivity by SP-142 clone is a valid method as a companion diagnostic for atezolizumab in triple-negative breast cancer, there are no standardized guidelines for the assessment of PD-L1 in other subtypes of breast cancer. Issues have also been raised about the lower sensitivity of the SP-142 assay compared with the other assays, and the difficulty to accurately validate the immune cell positivity the same assay. 13 Hence, we assessed PD-L1 separately by both tumor cell positivity and immune cell positivity. The assay we used (SP-263) is also more sensitive compared with the SP-142 assay. 13 While we found in this study that all patients who were positive for tumor cells were also positive for cells, with a strong association between tumor cell positivity and tumoral immune cell positivity for PD-L1, there were some cases of immune cell positivity while being tumor cell negative. Therefore, in subsequent trials, for immune checkpoint inhibitors, both tumor cell and immune cell positivity need to be tested for optimal identification of suitable patients for therapy.
We faced some difficulties with PD-L1 assessment. Immunoexpression of PD-L1 is heterogeneous and thus evaluation of PD-L1 expression by counting positive cells in hotspots may be appropriate. 14 15 Difficulty was encountered in evaluating PD-L1 immunoexpression in cases with PD-L1 positive tumor cells in a background of necrosis and in cases with PD-L1 positivity in tumor cells along with PD-L1 positive immune cells in background.
This is one of the first studies done in an Indian setting, and thus may be useful for setting guidelines for treatment in this subcontinent. Only a few studies have been done on PD-1 and PD-L1 in primary and metastatic breast cancer elsewhere in the world. 9 16 FDA approval of targeted therapy in breast cancer has opened new avenue for cancer management in breast by immunotherapy. The relatively high prevalence of PD-1 and PD-L1 in breast cancer cases supports routine screening of these biomarkers as this may significantly improve overall survival and outcome in these patients.
The most significant limitation of this study is that clone SP-263 was used instead of clone SP-142 that has been approved in triple-negative breast cancer. However, the usefulness of SP-142 clone is not given for other types of breast cancer, especially since the SP-142 clone itself has been found in lung cancers to have poor reproducibility compared with the other PD-L1 clones as well as having lesser sensitivity for detecting tumor cell positivity. 17 The clone SP-263 has a better reproducibility with the other clones and thus is likely to be a better indicator of “true” or “consensus” PD-L1 positivity. Whatever the method used to assess the PD-L1 status in this and other observational studies, the differences in PD-L1 positivity among the used PD-L1 clones need to be kept in mind and more than one clone needs to be studied according to specific reproducible criteria for reliable treatment. We conclude that PD-1 and PD-L1 show a moderately high degree of positivity in breast cancer across all subgroups in our setting in Northern India, and thus selected patients from all clinicopathological subgroups should be eligible for immunotherapy.
Footnotes
Conflict of Interest None.
References
- 1.Zhang H, Ave H. Advances of FDA approved Drugs that target PD-1 and PD-L1 for cancer immunotherapy. Am J Cancer Sci. 2019;7(01):18–31. [Google Scholar]
- 2.Bardhan K, Anagnostou T, Boussiotis V A. The PD1: PD-L1/2 pathway from discovery to clinical implementation. Front Immunol. 2016;7:550. doi: 10.3389/fimmu.2016.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gatalica Z, Snyder C, Maney T. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2965–2970. doi: 10.1158/1055-9965.EPI-14-0654. [DOI] [PubMed] [Google Scholar]
- 4.Shien K, Papadimitrakopoulou V A, Wistuba I I. Predictive biomarkers of response to PD-1/PD-L1 immune checkpoint inhibitors in non-small cell lung cancer. Lung Cancer. 2016;99:79–87. doi: 10.1016/j.lungcan.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.IMpassion130 Investigators . Schmid P, Rugo H S, Adams S. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(01):44–59. doi: 10.1016/S1470-2045(19)30689-8. [DOI] [PubMed] [Google Scholar]
- 6.ESMO Guidelines Committee. (clinicalguidelines@esmo.org) . Cardoso F, Kyriakides S, Ohno S. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30(08):1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 7.Panel members . Goldhirsch A, Winer E PEP, Coates A SAS. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24(09):2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hortobagyi G N, Connolly J L, D'Orsi C J. 8th edition. Chicago, Illinois: American College of Surgeons (ACS); 2018. Breast; pp. 589–636. [Google Scholar]
- 9.Tawfik O, Kimler B F, Karnik T, Shehata P. Clinicopathological correlation of PD-L1 expression in primary and metastatic breast cancer and infiltrating immune cells. Hum Pathol. 2018;80(80):170–178. doi: 10.1016/j.humpath.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Beckers R K, Selinger C I, Vilain R. Programmed death ligand 1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology. 2016;69(01):25–34. doi: 10.1111/his.12904. [DOI] [PubMed] [Google Scholar]
- 11.Bae S B, Cho H D, Oh M H. Expression of programmed death receptor ligand 1 with high tumor-infiltrating lymphocytes is associated with better prognosis in breast cancer. J Breast Cancer. 2016;19(03):242–251. doi: 10.4048/jbc.2016.19.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karnik T, Kimler B F, Fan F, Tawfik O. PD-L1 in breast cancer: comparative analysis of 3 different antibodies. Hum Pathol. 2018;72(72):28–34. doi: 10.1016/j.humpath.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Rimm D L, Han G, Taube J M. Reanalysis of the NCCN PD-L1 companion diagnostic assay study for lung cancer in the context of PD-L1 expression findings in triple-negative breast cancer. Breast Cancer Res. 2019;21(01):72. doi: 10.1186/s13058-019-1156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nawaz S, Heindl A, Koelble K, Yuan Y. Beyond immune density: critical role of spatial heterogeneity in estrogen receptor-negative breast cancer. Mod Pathol. 2015;28(06):766–777. doi: 10.1038/modpathol.2015.37. [DOI] [PubMed] [Google Scholar]
- 15.Taube J M, Klein A, Brahmer J R. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cimino-Mathews A, Thompson E, Taube J M. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol. 2016;47(01):52–63. doi: 10.1016/j.humpath.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rimm D L, Han G, Taube J M. A Prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol. 2017;3(08):1051–1058. doi: 10.1001/jamaoncol.2017.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]


