Abstract
pRm1132f isolated from Sinorhizobium meliloti is a group III rolling-circle-replicating (RCR) plasmid. At least seven of eight open reading frames in the nucleotide sequence represented coding regions. The minimal replicon contained a rep gene and single- and double-stranded origins of replication. Detection of single-stranded plasmid DNA confirmed that pRm1132f replicated via an RCR mechanism.
Sinorhizobium meliloti bacteria are gram-negative soil bacteria that fix nitrogen in symbiotic association with Medicago and Melilotus spp. In a previous study (14), an unusually small (7.2 kb) cryptic plasmid, pRm1132f, from S. meliloti strain 1132 was used to construct cloning vectors which were subsequently found to be destabilized upon cloning of a 12-kb DNA fragment. To ascertain the reason for this observed instability and to enhance our understanding of the replication, genomic organization, and function of S. meliloti cryptic plasmids, pRm1132f was characterized by nucleotide sequencing, cDNA hybridization, and deletion analysis.
Bacterial strains, growth conditions, plasmids, and recombinant DNA techniques.
Bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli bacteria were grown on Luria broth, and S. meliloti bacteria were grown on TY or TP medium (21). Plasmids resident in E. coli were introduced into S. meliloti by triparental mating (10). Standard recombinant DNA cloning techniques and Southern hybridization were carried out as described by Sambrook et al. (22).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | F− (φ80ΔlacZΔM15) endA1 recA1 hsdR17 supE44 thi-1 λ− gyrA96 relAIΔ (lacZYA-argF)U169 | 26 |
| HB101 | F−hsdS20 (rB− mB−) Δ(gpt-proA) 62 leu supE44 λ− ara-14 galK2 lacY1 Δ (merC-mrr) rpsL20 (Strr) xyl-5 mtl-1 recA13 | 5 |
| MT607 | pro-82 thi-1 hsdR17 supE44 rec A56 | 13 |
| S. meliloti | ||
| 1132 | Wild-type S. meliloti | 6 |
| 2011 | S. meliloti SU47 (RCR2011) | Rothamsted. Experimental station |
| 2011Rf-2 | Rif mutant of 2011 nod+ nif+; Rifr | This study |
| Plasmids | ||
| pBSL15 | 3.8-kb plasmid: Apr Kmr | 1 |
| pFD1001 | pRm1132f cloned into the PstI site of pSUP301; Kmr | 14 |
| pFD1192 | EcoRI/HindIII fragment of pRm1132f cloned into EcoRI/HindIII-digested pBR322 containing the oriT of pRK2; Tcr | 14 |
| pNRRM01 | pRm1132f cloned into the EcoRI site of pUC19, orientation +; Apr | This study |
| pNRRM2 | pRm1132f cloned into the EcoRI site of pUC19, orientation −; Apr | This study |
| pNRRa | pRm1132f amplified fragment (nt 2408–4501) cloned into SmaI-digested pBSL15, excised with PstI, and cloned into pSUP301; Kmr | This study |
| pNRSP1 | pRm1132f linearized with EcoRI and cloned into pSPT18 transcription vector; Apr | This study |
| pRK600 | pRK2013 npt::Tn9 Cmr Nm-Kms | 13 |
| pRm1132f | 7.2-kb plasmid from S. meliloti 1132 | 6 |
| pSPT18 | Transcription vector; Apr | Roche |
| pSUP301 | pACYC177 with oriT of RP4, ColE1 replicon; Apr Kmr | 24 |
| pSWEH | S1 nuclease-treated 2.4-kb EcoRI/HindIII fragment of pRm1132f and PstI-linearized pSUP301 followed by blunt-end ligation; Kmr | This study |
| pSWHC | S1 nuclease-treated 2.4-kb HindIII/ClaI fragment of pRm1132f and linearized PstI pSUP301 followed by blunt-end ligation; Kmr | This study |
nt, nucleotide.
DNA sequencing and analysis.
Nested deletions were made in pNRRM1 and pNRRM2 (Table 1) using a Pharmacia kit. Nucleotide sequencing of both DNA strands was carried out by the University of Laval Sequencing Service, Quebec, Canada. DNA sequences were analyzed with the University of Wisconsin Genetics Computer software package (version 10) and DNAMAN (Lynon Biosoft Quebec). Database searches were carried out with GenBank (release 113), EMBL (release 59.0), and SWISSPROT.
The plasmid, pRm1132f, consisted of 7,212 bp with a GC content of 65%, and FRAME analysis (2) revealed the presence of eight open reading frames (ORFs) (Fig. 1). The predicted protein encoded by ORF2 (235 amino acids) shared 28.5% identity with the product of the homocitrate synthase gene from Frankia (EMBL accession no. P54610), and the ORF3 predicted protein (430 amino acids) shared 30 to 34% identity (42 to 46% similarity) with the initiator-replication (Rep) proteins of the group III (cluster III) rolling-circle-replicating (RCR) plasmids, as shown by comparison with an RCR plasmid replicon database (http://www.essex.ac.uk/bs/staff/osborn/DPR_home.htm). The putative protein encoded by ORF4 (335 amino acids) shared 24% identity (31% similarity) with the Mob protein of the group III (cluster I) RCR plasmid, pTA1040, from Bacillus subtilis in the RCR plasmid replicon database. No significant homology was found in the databases for the predicted proteins of the remaining ORFs.
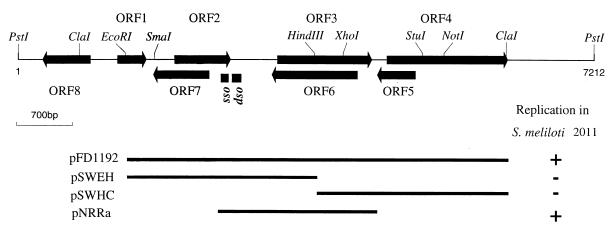
FIG. 1.
Arrangement of ORFs in pRm1132f and the minimum replicon. For determination of the minimal replicon, the plasmid was subcloned into either pSUP301 or pBR322, and the composite plasmid was transferred in S. meliloti 2011 by mating in the presence of appropriate antibiotic selection pressure.
ORF3 transcriptional start site.
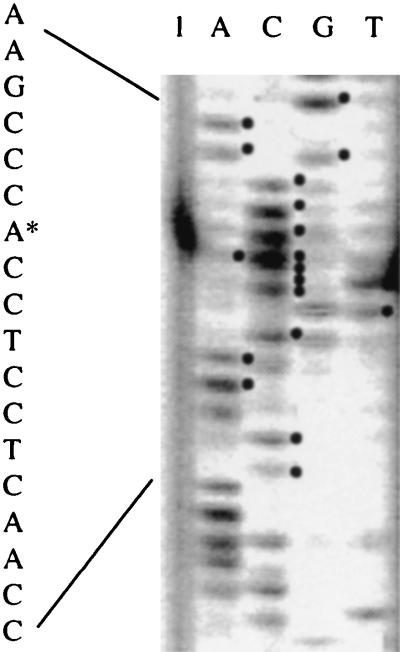
The transcriptional start site for the putative rep gene (ORF3) was determined by primer extension analysis (22) using the fluorescence (IRD800)-labeled primer X-100 (5′GTTTCGAGACGGTCAGATGGT-3′) from LI-COR (Lincoln, Nebr.). DNA sequencing was carried out according to the LI-COR protocol. To align the transcriptional start sites, the same primer was also used in conjunction with template DNA (pNRRM1) in a sequencing reaction. A single transcriptional start site with a GTG start codon was located at nucleotide 3241 of the plasmid sequence (Fig. 2).
FIG. 2.
Determination of the ORF3 transcriptional start site by the primer X-100 and using plasmid pNRRM1 as a template. The relevant sequence is shown on the left, with the asterisk indicating the position of the extension product.
dso and sso.
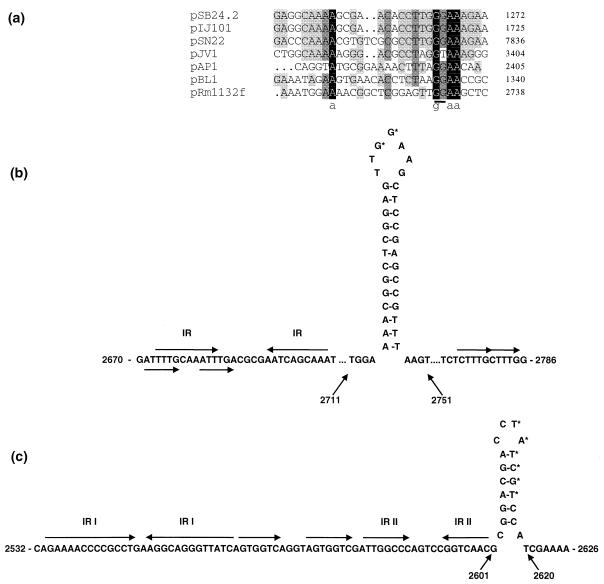
The double-stranded origins of replication (dso) of RCR plasmids are usually present either within or upstream of the rep gene in a region of strong secondary structure (8, 11, 18). Nucleotide sequences resembling the dso of a number of group III (cluster III) RCR plasmids in the RCR plasmid replicon database were present upstream of the putative rep gene of pRm1132f (Fig. 3a). The putative dso of pRm1132f contained imperfect repeat (IR) and direct repeat (iteron) nucleotide sequences (Fig. 3b) which have been implicated in the binding of the Rep protein prior to nicking of the duplex strand (8, 11, 18). Iterons at replication origins have also been implicated in copy number control of some plasmids (7). The single-stranded origins of replication (sso) of RCR plasmids exist in a region of strong secondary structure (8, 11, 17), as is the case for the putative sso of pRm1132f. A conserved six-nucleotide sequence, 5′-TAGCGt/a-3′, present in ssoA-type origins was shown to function as a transcriptional terminator for the synthesis of an RNA primer (19, 20). In pRm1132f, a TATCGT motif containing five of six nucleotides of the consensus sequence was located in the loop of a hairpin structure immediately upstream of the dso (Fig. 3c). The putative sso contained two imperfect repeat sequences (IR I and IR II) and a direct repeat sequence which may act as sites of attachment for host encoded factors during the conversion of single-stranded DNA (ssDNA) to double-stranded DNA (dsDNA).
FIG. 3.
sso and dso of pRm1132f. (a) Alignment of nucleotides in the dso of pRm1132f and group III RCR plasmids (from the RCR plasmid replicon database; see the text) pSB24.2 (4), pIJ101 (17), pSN22 (16), pJV1 (23), pAP1 (3), and pBL1 (12). The conserved GG dinucleotide (underlined) indicates the position of the nick site in pSN22, pJV1, and pIJ101 (23, 25). (b and c) Predicted secondary structures of the dso and sso, respectively; for the dso, a conserved GG dinucleotide in the loop of a hairpin structure is indicated by asterisks; for the sso, the nucleotide sequence TATCGT contains five of six of the nucleotides of the consensus sequence 5′-TAGCGt/a-3′ present in ssoA origins (19, 20). Direct repeats (unmarked arrows) and imperfect inverse repeats (IR) are indicated.
cDNA detection.
cDNA hybridization was used to determine whether the ORFs within the nucleotide sequence of pRm1132f represented coding regions. ORF-specific oligonucleotide primers (Table 2), total RNA from S. meliloti strain 1132, and reverse transcriptase were used to generate 32P-labeled cDNA probes according to the procedure of Han et al. (15). ORF-specific DNA prepared by PCR using the above-described primers was subjected to agarose electrophoresis, blotted onto a nylon membrane, and hybridized to the labeled cDNA probes. Hybridization of cDNA was observed with all of the ORF-specific DNAs tested, indicating that the respective ORFs represented coding regions (Fig. 4); ORF6-specific DNA could not be tested, since it was completely overlapped by ORF3. To the best of our knowledge, substantial overlap of genes on opposing plasmid DNA strands has not been observed, and further experiments are required to confirm that ORF6 represents a coding region.
TABLE 2.
Oligonucleotide primers for ORF-specific DNA
| ORFa | Primer | Nucleotide no. in plasmid sequence |
|---|---|---|
| ORF1 (F) | 5′-CCGAAAGCCTGACATATCG-3′ | 1242 |
| ORF1 (R) | 5′-GAGCGAACAGCCCTTTCTG-3′ | 1562 |
| ORF2 (F) | 5′-CATGCATGGGCGATCGACA-3′ | 2007 |
| ORF2 (R) | 5′-GCCAATCGACCACTACCTG-3′ | 2568 |
| ORF3 (F) | 5′-ATCGCGTGGGAAATGGCAGG-3′ | 3789 |
| ORF3 (R) | 5′-TTTCAGGCGGACCCTCTG-3′ | 4415 |
| ORF4 (F) | 5′-TATCGGGAGGCCATGAG-3′ | 5119 |
| ORF4 (R) | 5′-CTCGGGCACTGCGAATAT-3′ | 6059 |
| ORF5 (F) | 5′-CATCTCCAGAGCCAACGA-3′ | 4502 |
| ORF5 (R) | 5′-GTGCTGGTCCTTCCGCAC-3′ | 4783 |
| ORF7 (F) | 5′-ATTGACGGACCGAAGCGA-3′ | 1685 |
| ORF7 (R) | 5′-GGACGTCTGTCAGCGGAT-3′ | 2161 |
| ORF8 (F) | 5′-GACGAGCTCGGAACCGAA-3′ | 425 |
| ORF8 (R) | 5′-AACAGCTTGCAGCAGGCAC-3′ | 738 |
F, forward; R, reverse.
FIG. 4.
Hybridization of 32P-labeled cDNA with ORF-specific DNA of pRm1132f. cDNA probes prepared with ORF-specific oligonucleotide primers (see Table 2), total RNA from S. meliloti 1132, and reverse transcriptase were hybridized to ORF-specific DNA electrophoresed on agarose gels and blotted onto nylon filters. ORF-specific DNAs are numbered as indicated. Three separate hybridizations were carried out with cDNA probes derived from ORF1 (left panel), ORFs 2 to 4 (middle panel), and ORFs 5, 7, and 8 (right panel).
Minimal replicon.
To determine the minimum plasmid replicon, DNA fragments of pRm1132f were ligated into either pBR322 or pSUP301 and tested for survival in S. meliloti 2011 in the presence of antibiotic selection (Fig. 1). The minimum replicon (pNRRa) consisted of the Rep protein, dso, and sso. Deletion of the sso of RCR plasmids results in plasmid instability (8), as was found to be the case for pRm1132f (data not shown).
Detection of ssDNA.
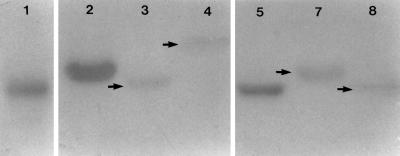
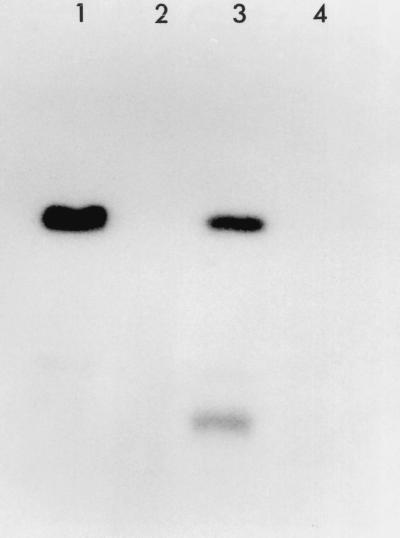
To confirm that pRm1132f was an RCR plasmid, 10 μg of genomic DNA from mid-log-phase cells of S. meliloti 1132 grown on TY medium was treated without and with (100 U) S1 nuclease. The DNA was then subjected to agarose gel electrophoresis and transferred to a nylon membrane without prior denaturation. Hybridization with 32P-labeled EcoRI-restricted pRm1132f DNA indicated that S. meliloti strain 1132 accumulated single-stranded plasmid DNA (Fig. 5). When rifampin and erythromycin (100 μg/ml of each) were added to the cells prior to harvesting, a lower-molecular-weight hybridizing band was evident, probably representing supercoiled ssDNA. Pretreatment of the DNA with S1 nuclease resulted in a loss of the hybridizing bands and confirmed that they consisted of ssDNA. The membrane-bound DNA was also hybridized to the 32P-labeled strand-specific riboprobes complementary to each of the two DNA strands. Hybridization was observed only with the riboprobe complementary to the plus strand (data not shown), indicating that the ssDNA was generated from the plus strand. Under normal growth conditions, the accumulation of RCR plasmid ssDNA indicates that the plasmid sso is operating inefficiently in its bacterial host (11). Despite the accumulation of pRm1132f ssDNA observed in this study, the plasmid is stably maintained in the parental strain, S. meliloti 1132. This was also reported to be the case for the RCR plasmids, pRF22F and pC194, which were not segregationally unstable in B. subtilis despite the accumulation of ssDNA (9).
FIG. 5.
Detection of ssDNA intermediates in the replication of pRm1132f. Genomic DNA from S. meliloti 1132 was blotted onto a nylon membrane without denaturation and hybridized to a 32P-labeled pRm1132f DNA probe. DNA without (lane 1) and with (lane 2) S1 nuclease treatment prior to electrophoresis is shown. DNA from cells incubated with rifampin and erythromycin prior to harvesting, without (lane 3) and with (lane 4) S1 nuclease treatment, is shown.
To date, the presence of RCR plasmids in many gram-positive and in some gram-negative bacteria has been documented in an RCR plasmid replicon database (see above). The present study indicating the presence of an RCR plasmid in a rhizobial species suggests that these plasmids are more widespread than originally thought, and this finding has implications with regard to the horizontal spread of RCR plasmids among soil bacteria.
Nucleotide sequence accession number.
The nucleotide sequence of pRm1132f has been deposited in GenBank (accession no. AF327371).
Acknowledgments
We thank Serge Laberge for sequencing the products of primer extension.
Footnotes
Contribution no. 679 of the Soils and Crops Research and Development Centre.
REFERENCES
- 1.Alexeyev M F. Three kanamycin resistance gene cassettes with different polylinkers. Biotechniques. 1995;18:52–54. [PubMed] [Google Scholar]
- 2.Bibb M J, Findley P R, Johnson M W. The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein codon sequences. Gene. 1984;30:157–166. doi: 10.1016/0378-1119(84)90116-1. [DOI] [PubMed] [Google Scholar]
- 3.Billington S J, Jost B H, Songer J G. The Arcanobacterium (Actinomyces) pyogenes plasmid pAP1 is a member of the pIJ101/pJV1 family of rolling circle replication plasmids. J Bacteriol. 1998;180:3233–3236. doi: 10.1128/jb.180.12.3233-3236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolotin A P, Sorokin A V, Aleksandrov N N, Danilenko V N, Kozlov Y I. Nucleotide sequence of DNA of the actinomycete plasmid pSB24.2. Dokl Biochem. 1986;283:260–263. [PubMed] [Google Scholar]
- 5.Boyer H W, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 6.Bromfield E S P, Thurman N P, Whitwill S T, Barran L R. Plasmids and symbiotic effectiveness of representative phage types from two indigenous populations of Rhizobium meliloti. J Gen Microbiol. 1987;133:3457–3466. [Google Scholar]
- 7.Chattoraj D K. Control of plasmid DNA replication by iterons: no longer paradoxical. Mol Microbiol. 2000;37:467–476. doi: 10.1046/j.1365-2958.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 8.Del Solar G, Giraldo R, Ruiz-Echevarria M J, Espinosa M, Diaz-Orejas R. Replication and control of circular bacterial plasmids. Microbiol Mol Biol Rev. 1998;62:434–464. doi: 10.1128/mmbr.62.2.434-464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devine K M, Hogan S T, Higgins D G, McConnell D. Replication and stability of Bacillus plasmid pBAA1. J Bacteriol. 1989;171:1166–1172. doi: 10.1128/jb.171.2.1166-1172.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ditta G, Stanfield S, Corbin D, Helinski D R. Broad host range DNA cloning system for Gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci USA. 1980;77:7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinosa M, Cohen S, Couturier M, del Solar G, Diaz-Orejas R, Giraldo R, Janniere L, Miller C, Osborn M, Thomas C. Plasmid replication and copy number control. In: Thomas C M, editor. The horizontal gene pool. Bacterial plasmids and gene spread. Amsterdam, The Netherlands: Harwood Academic Publishers; 2000. pp. 1–47. [Google Scholar]
- 12.Fernandez-Gonzalez C, Cadenas R F, Noirot-Gros M F, Martin J F, Gil J A. Characterization of a region of plasmid pBL1 of Brevibacterium lactofermentum involved in replication via the rolling circle model. J Bacteriol. 1994;176:3154–3161. doi: 10.1128/jb.176.11.3154-3161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finan T M, Kunkel B, De Vos G F, Signer E R. Second symbiotic megaplasmid in Rhizobium meliloti carrying exopolysaccharide and thiamine synthesis genes. J Bacteriol. 1986;167:66–72. doi: 10.1128/jb.167.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Froissard D, Bromfield E S P, Whitwill S, Barran L R. Construction and properties of cloning vectors based on a 7.2 kb Rhizobium meliloti cryptic plasmid. Plasmid. 1995;33:226–231. doi: 10.1006/plas.1995.1024. [DOI] [PubMed] [Google Scholar]
- 15.Han T, Bausch C, Richmond C, Blattner F R, Conway T. Functional genomics: expression analysis of Escherichia coli growing on minimal and rich medium. J Bacteriol. 1999;181:6425–6440. doi: 10.1128/jb.181.20.6425-6440.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kataoka M Y, Kyose M, Michisuji Y, Horiguchi T, Seki T, Yoshida T. Complete nucleotide sequence of the Streptomyces nigrifaciens plasmid, pSN22: genetic organization and correlation with genetic properties. Plasmid. 1994;32:55–69. doi: 10.1006/plas.1994.1044. [DOI] [PubMed] [Google Scholar]
- 17.Kendall K J, Cohen S N. Complete nucleotide sequence of the Streptomyces lividans plasmid pIJ101 and correlation of the sequence with genetic properties. J Bacteriol. 1988;170:4634–4651. doi: 10.1128/jb.170.10.4634-4651.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan S A. Rolling-circle replication of bacterial plasmids. Microbiol Mol Biol Rev. 1997;61:442–455. doi: 10.1128/mmbr.61.4.442-455.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramer M G, Khan S A, Espinosa M. Lagging-strand replication from the ssoA origin of plasmid pMV158 in Streptococcus pneumoniae: in vivo and in vitro influences of mutations in two conserved ssoA regions. J Bacteriol. 1998;180:83–89. doi: 10.1128/jb.180.1.83-89.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramer M G, Khan S A, Espinosa M. Plasmid rolling circle replication: identification of the RNA polymerase-directed primer RNA and requirement for DNA polymerase I for lagging strand synthesis. EMBO J. 1997;18:5784–5795. doi: 10.1093/emboj/16.18.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rastogi V K, Bromfield E S P, Whitwill S T, Barran L R. A cryptic plasmid of indigenous Rhizobium meliloti possesses reiterated nodC and nifE genes and undergoes DNA rearrangement. Can J Microbiol. 1992;38:563–568. [Google Scholar]
- 22.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 23.Servin-Gonzalez L, Sampieri A, Cabello J, Galvan L, Juarez V, Castro C. Sequence and functional analysis of the Streptomyces phaeochromogenes plasmid pJV1 reveals a modular organization of Streptomyces plasmids that replicate by rolling circle. Microbiology. 1995;141:2499–2510. doi: 10.1099/13500872-141-10-2499. [DOI] [PubMed] [Google Scholar]
- 24.Simon R, Priefer U, Puhler A. Vector plasmids for in vivo and in vitro manipulation of gram-negative bacteria. In: Puhler A, editor. Molecular genetics of bacterial-plant interactions. Berlin, Germany: Springer Verlag; 1983. [Google Scholar]
- 25.Suzuki I, Seki T, Yoshida T. Nucleotide sequence of a nicking site of the Streptomyces plasmid pSN22, replicating by the rolling circle mechanism. FEMS Microbiol Lett. 1997;150:283–288. doi: 10.1111/j.1574-6968.1997.tb10382.x. [DOI] [PubMed] [Google Scholar]
- 26.Woodcock D M, Crowther P J, Doherty J, Jefferson S, DeCruz E, Noyer-Weidner M, Smith S S, Michael M Z, Graham M W. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 1989;17:3469–3478. doi: 10.1093/nar/17.9.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]