Abstract
Objectives
To present a new short self-test, called the OSA wellness scale (OWS), for assessing the health-related quality of life (HRQoL) changes in obstructive apnea syndrome (OSA) patients treated with mandibular advancement device (MAD).
Methods
51 OSA patients (8 women and 43 men, mean age 52.3) treated with a fully customizable MAD device (Protrusor) were retrospectively enrolled. Each patient received a home sleep apnea testing (HSAT) at baseline (T0) and after three months of MAD treatment (T1). Two self-test evaluations, the Epworth sleepiness scale (ESS), and OWS were also submitted at T0 and T1. The OWS was a short self-test of 8 questions for evaluating the daytime HRQoL. Patients gave an assessment from 0 to 3 for each question. At the end of the questionnaire, the patients had a score from 0 to 24, resulting from the sum of all 8 scores. The higher the score, the greater the patient's perceived state of discomfort.
Results
At T1, a significant decrease in the oxygen desaturation index (ODI) and apnea-hypopnea index (AHI) was shown (p < 0.0001), while no significant changes in body mass index (BMI) were found. Both the ESS and the OWS records showed a significant reduction in daytime sleepiness and HRQoL (p < 0.0001).
Conclusion
The OWS could be a useful method to verify and numerically compare the perceived quality of life in OSA patients, before and after MAD therapy.
1. Introduction
Obstructive sleep apnea (OSA) is a respiratory disease characterized by repeated episodes of upper airway collapse and obstruction during sleep associated with arousal from sleep, with or without oxygen desaturation [1]. The obstruction may be complete (apnea) or partial (hypopnea) during sleep. It has long been recognized that men have greater vulnerability than women toward developing OSA [1]. The ratio of men to women is in the range from 5 to 8 : 1 because women are hormonally protected until menopause, after which the relationship tends to be equal [2]. The consequences of OSA are related to two important aspects: sleep time quality modification and respiratory gas exchange alteration [3]. The complete overnight laboratory polysomnographic evaluation (PSG) is required in severe cases and remains the gold standard for OSA evaluation [4]. Considering the continuous increase in sleep disorders, sleep laboratories and their diagnostic strategies are in high demand, and alternative methods are proposed in the literature for the screening and diagnosis of OSA, such as home sleep apnea testing (HSAT) [5]. The HSAT is a level III diagnostic tool, that can be performed in home settings without sleep technologists, representing a less expensive diagnostic option for clinical diagnosis of OSA. Continuous night arousals alter the physiologic sleep cycle, changing sleep quality, daytime life, and health-related quality of life (HRQoL) [6]. Nocturnal arousals also generate a disturbing unrefreshing sleep, that causes daytime sleepiness, head and neck pain, loss of concentration, anxiety or depression, weakness, and sleep bruxism [7, 8]. Untreated obstructive sleep apnea (OSA) requires medical intervention and is related to reduced work performance and work-related injuries. The economic burden associated with untreated OSA is significant [9]. The economic impact of untreated OSA patients is related to the limitation of work performance, and to the increased number of occupational injuries, motor vehicle crashes, and frequent hospitalizations for cardiovascular pathologies (strokes and heart attacks). OSA is comparable to other chronic diseases and is very difficult to describe exactly its economic effects, but is estimated that OSA patients cause a huge economic burden on society [10].
The impact of OSA is related to quality of life (QLI) modifications in undiagnosed or untreated patients [11]. The International Society for Quality of Life Research (ISOQOL) suggests that health-related quality of life (HRQoL) is the health aspect of quality of life that focuses on people's level of ability, daily functioning, and ability to experience a fulfilling life that includes the subjective perception of physical, social, and psychological functioning [12]. The physiologic parameters are clearly evaluated by HSAT or PSG, but the diagnosis of OSA is complete only if it has included the acquisition of data regarding the psychosocial aspect of the patient [13]. Various tools to measure HRQoL and self-evaluation of daytime sleepiness have been used in epidemiologic studies and clinical trials assessing OSA management [3]. The most used HRQoL tests are the sleep apnea quality of life index (SAQLI), The Medical Outcomes Study Short Form 36 Element Healthy Survey (SF-36), Maugeri obstructive sleep apnea syndrome questionnaire (MOSAS), Quebec sleep questionnaire (QSQ), obstructive sleep apnea patient-oriented severity index (OSAPOSI), functional outcomes of sleep questionnaire, the Pittsburgh sleep quality index (PSQI), and Epworth sleepiness scale (ESS) [14, 15].
In the present paper, the authors present a new short health quality life test, called the “OSA wellness scale (OWS),” to evaluate the HRQoL modifications in OSA patients treated with a mandibular advancement device (MAD).
2. Materials and Methods
51 patients (8 women and 43 men, mean age 52.3) with a diagnosis of OSA were retrospectively enrolled in the present study from patients treated at the Department of Orthodontics, University of Foggia, Italy, in chronological order from March 2017 to November 2020. All the procedures of this research protocol have adhered to the Declaration of Helsinki and have been approved by the Ethics Committee of the University of Foggia (Approval no. 43/CE/2019). Written consent was signed by each patient.
2.1. Criteria for Patients Selection
The inclusion criteria were as follows: age greater than 20 years old, body mass index (BMI) lower than 34 kg/m2, OSA diagnosis confirmed by nocturnal polysomnography, and night treatment with a fully customizable MAD-type device.
Exclusion criteria were as follows: smoking habit, neurological disorders, or previous cervical trauma.
2.2. Instrumental Evaluation
Each patient received a home sleep apnea testing (HSAT) at baseline (T0). A second HSAT was performed after three months of treatment (T1) with a fully customizable MAD device (Protrusor® Dr. Burlon) [16]. The data from the HSAT recordings were used for manual scoring according to the American Academy of Sleep Medicine (AASM) criteria from 2012 [16]. The extracted data are described in detail in Table 1.
Table 1.
Data and statistical analysis of the extracted records.
| BMI and TO | BMI and T1 | ODI TO | ODI and T1 | AHI and T0 | AHI and T1 | ESS and T0 | ESS and T1 | OWS and T0 | OWS and T1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Number of values | 51 | 51 | 51 | 51 | 51 | 51 | 51 | 51 | 51 | 51 |
| Mean | 26.07 | 26.40 | 18.05 | 6.547 | 20.79 | 6.900 | 8.392 | 4.314 | 8.039 | 4.137 |
| Std. Deviation | 3.594 | 3.359 | 14.66 | 7.211 | 15.47 | 6.859 | 5.142 | 2.494 | 4.280 | 3.060 |
| Std. Error of mean | 0.5033 | 0.4703 | 2.053 | 1.010 | 2.166 | 0.9605 | 0.7201 | 0.3492 | 0.5993 | 0.4284 |
| Lower 95% CI of mean | 25.06 | 25.46 | 13.92 | 4.519 | 16.44 | 4.971 | 6.946 | 3.612 | 6.835 | 3.277 |
| Upper 95% CI of mean | 27.08 | 27.35 | 22.17 | 8.575 | 25.14 | 8.829 | 9.838 | 5.015 | 9.243 | 4.998 |
| Normality test | Yes | Yes | No | No | No | No | No | Yes | No | No |
| p | ns | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||
BMI = body mass index; ODI = oxygen desaturation index; AHI = apnea-hypopnea index (AHI); ESS = Epworth sleepiness scale; OWS = OSA wellness scale.
2.3. Self-Test Evaluation
For each patient, two self-test evaluations were submitted Epworth sleepiness scale (ESS) and OWS. The ESS is a patient's self-evaluation about daytime sleepiness but no information about the HQRoL modifications can be shown [17]. The OWS is a new patient emotive self-evaluation about the daytime HQRoL. Both EES and OWS were done before MAD treatment and after three months of treatment.
2.4. Description of OWS
All patients were asked to complete the OWS questionnaire. The OWS is a short self-test to evaluate the daytime HRQoL subdivided into 8 questions (Figure 1). For each question, patients choose from 0 to 3 : 0 if the response is “never”, 1 if the response is “few times,” 2 if the response is “quite often,” and 3 if the response is “always”. At the end of the questionnaire, the patient has a score from 0 to 24, resulting from the sum of all 8 scores. The higher the score, the greater the patient's perceived state of discomfort.
Figure 1.

OSA wellness scale (OWS).
Question 1 is used to evaluate the patient's drug assumption; question 2 evaluates the sleep quality time; the last 4 questions are used to evaluate the patient psychological aspect.
2.5. Statistical Analysis
Data were evaluated using Shapiro–Wilk normality test with a confidence level of 95%. A parametric Test (paired t-Test) was used for values that passed the normality test; a nonparametric (Wilcoxon Test) was used for values that unpassed the normality test (Tables 1 and 2).
Table 2.
The mean difference of each evaluated parameter.
| BMI T0 and T1 | ODI T0 and T1 | AHI T0 and T1 | ESS T0 and T1 | OWS T0 and T1 | |
|---|---|---|---|---|---|
| Number of values | 51 | 51 | 51 | 51 | 51 |
| Mean | 0.3345 | −11.50 | −13.89 | −4.078 | −3.902 |
| Std. Deviation | 1.366 | 12.76 | 13.35 | 4.408 | 3.874 |
| Std. Error of mean | 0.1913 | 1.787 | 1.869 | 0.6173 | 0.5425 |
| Passed normality test (alpha = 0.05)? | No | No | No | No | No |
BMI = body mass index; ODI = oxygen desaturation index; AHI = apnea-hypopnea index (AHI); ESS = Epworth sleepiness scale; OWS = OSA wellness scale.
3. Results
Data and statistical evaluation were shown in Table 1. The mean difference of each evaluated parameters was shown in Table 2. Patients presented no significant modification of body mass index (BMI) after the MAD treatment (+0.3345; p=n.s.). A statistically significant modification was found in both the apnea-hypopnea index (AHI) and oxygen desaturation index (ODI) (p < 0.0001), which are the physiological parameters for assessing oxygen saturation. AHI decreased by −13.89 e/h (p < 0.0001); ODI decreased by −11.50 (p < 0.0001). After three months of treatment with MAD, the patients improved their daytime sleepiness and HRQoL. Both the ESS and the OWS records showed a significant reduction of −4,078 (p < 0.0001) and −3.902 (p < 0.0001) (Figure 2).
Figure 2.
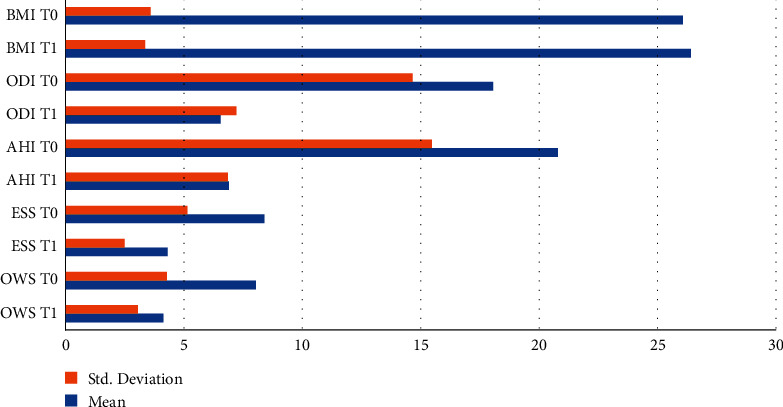
Bar chart of the evaluated records at T0 and T1.
4. Discussion
In the present paper, the authors evaluated the changes in the physiologic oxygen parameters (i.e., AHI and ODI reduction) and the modifications of HRQoL, using a new simple self-test (OWS), for assessing the efficacy of MAD treatment in OSA patients. The MAD therapy is effective in patients who have an upper airway obstruction in sleep time prevalently in the supine position, or in patients who refuse the CPAP or upper airway surgery [1]. The forward and vertical movement of the mandible increases the upper airway area, improving the oxygen saturation (SO2) and inducing a reduction of AHI and ODI, recorded by HSAT or PSG [18]. The increase in oxygen saturation may reduce the risk of cardiovascular disease and stroke, as suggested for other OSA therapy such as CPAP or upper airway surgery [19]. A second main effect of upper airway modification is the reduction of night arousals. The reduction of night arousals has an influence on daytime sleepiness and quality of life [1]. Several types of research have actually focused on the importance of HRQoL. The HRQoL describes the somatic, mental and social well-being status [20]. The self-evaluation is the most used criterion to evaluate the social effect of the treatment [21]. The Medical Outcomes Study Short Form 36 Element Healthy Survey (SF-36) is one of the most used questionnaires showing reduced subjective health status and HRQoL, in patients with OSA [22]. The SF-36 is designed for use in clinical practice and research, health policy evaluations, and general population surveys. This questionnaire is widely used, consisting of 36 questions to evaluate the health condition and the relative burden of disease [23]. The score of the SF-36 has a range from 0 to 100: zero represents the worst and 100 represents the best HRQoL. A norm-based score of more or less than 50 represents a better or worse HRQoL respectively, compared to the average general population. The eight norm-based domains are united into one physical and one mental aggregated health scale [24]. In order not to discourage patients from answering so many questions and to acquire the answers more quickly, burden a shorter test has been used by many authors. A short form of SF-36 is the SF-12 questionnaire. The developers of the SF-36 have consequently suggested that a 12-item subset of the items may accurately reproduce the two summary component scores which can be derived from the SF-36 (the physical component summary score (PCS) and mental health component summary score (MCS)) [25]. Other self-report questionnaires have focused on psychological distress [26]. Many authors have emphasized a relation between nonphysiologic sleep and distress [27]. Psychological distress is defined as a state of emotional suffering characterized by symptoms of depression, anxiety, hyperarousal, and psychophysiological tension that may be expressed through somatic symptoms like insomnia, headaches, muscular pain, lack of energy, and exhaustion [28]. Psychological discomfort or psychiatric disorders are caused by sleep disorders both in the young and adult populations [29]. Standardized scales, such as the Maugeri sleep quality and distress inventory (MaSQuDI ± 17), measure and monitor sleep-related distress in patients with insomnia, obstructive sleep apnea syndrome (OSAS), central hypersomnia, and behavioral sleep disorders (BSD), a macrocategory that includes unusual nocturnal behaviors such as rapid eye movement (REM) behavior disorders, parasomnia, periodic limb disorders, restless legs syndrome, nocturnal eating disorders, and sleep-related eating disorders [28].
In the present paper, the authors have evaluated the changes in HRQoL using the new OWS test, in OSA patients treated with MAD. This type of test has 8 questions to assess the HRQoL and distress, before and after treatment. The OWS test is simple and focuses on the real modifications of the HRQoL and distress. The OWS test also gives information about the drugs taken by the patient. This aspect is very important to evaluate the initial psychological discomfort of the patient before starting with every type of OSA treatment (both CPAP and MAD). The most widely used self-tests do not give this type of information. A recent study found that 40 mg of controlled-release oral morphine did not worsen OSA in men, challenging traditional thinking that OSA is worsened by opioids. However, a positive effect on OSA is associated with a direct effect on central nervous system respiratory depression. So, clinicians should be cautious in giving excess opiates and sedatives, given the increased risks [30].
In the present paper, the authors have shown lower scores in OWS questionnaires completed by the same patients after treatment. The improvement of functional parameters improves happiness, the HRQoL, and reduces daytime sleepiness. The OSA wellness scale (OWS) is a practical, quick and inexpensive tool that is easy to understand and is aimed at all walks of life. It may be used by the clinicians for the initial screening or for the final post-treatment check. In addition to the Epworth scale, anamnesis, and polysomnography (or cardio-respiratory monitoring), this test could provide a broader view of the impact of OSA pathology on the state of the general malaise of the apneic patient; and thus, could help the clinician not exclude from therapy an OSA patient with low apnea/hypopnea (AHI) who psychologically perceives the same discomfort as an OSA patient with high apnea/hypopnea (AHI). The title “OSA wellness scale (OWS)” is deliberately presented in a positive form to avoid altering the patient's judgment in advance, predisposing him negatively. The questionnaire takes into consideration various situations of daily life, including the possible intake of drugs, and, for each of them, the subject must establish a vote whose sum quantifies the degree of discomfort of the subject himself. It is an 8-item test that can be self-administered or hetero-administered. In the presented study, all clinical cases are self-administered to avoid external alterations. Each item is rated on a scale from 0 (“never”) to 3 (“always”). The sum of the answers given in numerical form gives the final score, which can range from 0 to 24. The higher the score, the greater the patient's perceived state of discomfort. In other words, the lower the overall score, the lower the state of discomfort perceived by the patient, so a low score is a sign of improvement in quality of life-related to health.
5. Conclusion
The OSA wellness scale (OWS) should be considered as a method to verify and numerically compare the patients' perception of the quality of life, both before and after therapy, in OSA patients treated with MAD.
Further studies should be suggested to create a larger sample for the validation of the test.
Acknowledgments
The authors would like to thank Carlotta Burlon for her help during the research and preparation of the manuscript.
Data Availability
The individual data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Wang G., Goebel J. R., Li C., Hallman H. G., Gilford T. M., Li W. Therapeutic effects of CPAP on cognitive impairments associated with OSA. Journal of Neurology . 2020;267(10):2823–2828. doi: 10.1007/s00415-019-09381-2. [DOI] [PubMed] [Google Scholar]
- 2.Orth M., Kotterba S., Rasche K., Walther J. W., Schultze-Werninghaus G., Duchna H. W. [Sleep apnoea in women?--The forgotten gender] Pneumologie . 2007;61(11):725–729. doi: 10.1055/s-2007-980128. [DOI] [PubMed] [Google Scholar]
- 3.Pauletto P., Reus J. C., Bolan M., et al. Association between obstructive sleep apnea and health-related quality of life in untreated adults: a systematic review. Sleep and Breathing . 2021;25(4):1773–1789. doi: 10.1007/s11325-021-02323-1. [DOI] [PubMed] [Google Scholar]
- 4.Laratta C. R., Ayas N. T., Povitz M., Pendharkar S. R. Diagnosis and treatment of obstructive sleep apnea in adults. Canadian Medical Association Journal . 2017;189(48):E1481–E1488. doi: 10.1503/cmaj.170296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller J. N., Schulz P., Pozehl B., Fiedler D., Fial A., Berger A. M. Methodological strategies in using home sleep apnea testing in research and practice. Sleep and Breathing . 2018;22(3):569–577. doi: 10.1007/s11325-017-1593-3. [DOI] [PubMed] [Google Scholar]
- 6.Hodoba D., Perusic D., Zdravkovic V., Goldoni V., Durrigl V. Sleep-waking cycle disturbance in patients with obstructive sleep apnea syndrome. Neurologia Croatica . 1991;40(4):293–305. [PubMed] [Google Scholar]
- 7.Sweetman A., Lack L., McEvoy R. D., et al. Effect of depression, anxiety, and stress symptoms on response to cognitive behavioral therapy for insomnia in patients with comorbid insomnia and sleep apnea: a randomized controlled trial. Journal of Clinical Sleep Medicine . 2021;17(3):545–554. doi: 10.5664/jcsm.8944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim D. H., Lee S. H., Lee S. H. Sleep bruxism episodes in patients with obstructive sleep apnea syndrome determined by in-laboratory polysomnography. Applied Sciences . 2020;10(23):p. 8587. doi: 10.3390/app10238587. [DOI] [Google Scholar]
- 9.AlGhanim N., Comondore V. R., Fleetham J., Marra C. A., Ayas N. T. The economic impact of obstructive sleep apnea. Lung . 2008;186(1):7–12. doi: 10.1007/s00408-007-9055-5. [DOI] [PubMed] [Google Scholar]
- 10.Ruttenberg R. The social and economic impact of chronic obstructive pulmonary disease on maintenance-of-way railroad workers. Journal of Occupational and Environmental Medicine . 2020;62(1):58–63. doi: 10.1097/jom.0000000000001757. [DOI] [PubMed] [Google Scholar]
- 11.Yazici O., Hatipoglu O. N. Evaluation of quality of life, anxiety, and depression in the spouses of patients with obstructive sleep apnea syndrome. Nigerian Journal of Clinical Practice . 2019;22(4):516–520. doi: 10.4103/njcp.njcp_500_18. [DOI] [PubMed] [Google Scholar]
- 12.She R., Yan Z., Hao Y., et al. Health-related quality of life after first-ever acute ischemic stroke: associations with cardiovascular health metrics. Quality of Life Research . 2021;30(10):2907–2917. doi: 10.1007/s11136-021-02853-x. [DOI] [PubMed] [Google Scholar]
- 13.Kapur V. K., Auckley D. H., Chowdhuri S., et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of sleep medicine clinical practice guideline. Journal of Clinical Sleep Medicine . 2017;13(03):479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moyer C. A., Sonnad S. S., Garetz S. L., Helman J. I., Chervin R. D. Quality of life in obstructive sleep apnea: a systematic review of the literature. Sleep Medicine . 2001;2(6):477–491. doi: 10.1016/s1389-9457(01)00072-7. [DOI] [PubMed] [Google Scholar]
- 15.Suurna M. V., Steffen A., Boon M., et al. Impact of body mass index and discomfort on upper airway stimulation: ADHERE registry 2020 update. The Laryngoscope . 2021;131(11):2616–2624. doi: 10.1002/lary.29755. [DOI] [PubMed] [Google Scholar]
- 16.Berry R. B., Budhiraja R., Gottlieb D. J., et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. deliberations of the sleep apnea definitions task force of the American Academy of sleep medicine. Journal of Clinical Sleep Medicine . 2012;08(05):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahin M. S., Kizilirmak D. Changes at mean platelet volume and platelet distribution width levels after septoplasty and its correlation with Epworth sleepness scale. Journal of Craniofacial Surgery . 2017;28(1):71–73. doi: 10.1097/scs.0000000000003207. [DOI] [PubMed] [Google Scholar]
- 18.Burlon G., Tepedino M., Laurenziello M., et al. Evaluation of factors that influence the success rate of OSA treatment with a customised adjustable MAD device - a retrospective study. Acta Otorhinolaryngologica Italica . 2020;40(4):297–303. doi: 10.14639/0392-100x-n0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciavarella D., Tepedino M., Burlon G., et al. Respiratory and cardiovascular parameters evaluation in OSA patients treated with mandibular advancement device. Applied Sciences . 2020;10(22):8175–8177. doi: 10.3390/app10228175. [DOI] [Google Scholar]
- 20.Karimi M., Brazier J. Health-related quality of life, and quality of life: what is the difference? PharmacoEconomics . 2016;34(7):645–649. doi: 10.1007/s40273-016-0389-9. [DOI] [PubMed] [Google Scholar]
- 21.Lacasse Y., Godbout C., Series F. Health-related quality of life in obstructive sleep apnoea. European Respiratory Journal . 2002;19(3):499–503. doi: 10.1183/09031936.02.00216902. [DOI] [PubMed] [Google Scholar]
- 22.Ware J. E., Sherbourne C. D. The MOS 36-ltem short-form health survey (SF-36) Medical Care . 1992;30(6):473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Kuhn E., Schwarz E. I., Bratton D. J., Rossi V. A., Kohler M. Effects of CPAP and mandibular advancement devices on health-related quality of life in OSA: a systematic review and meta-analysis. Chest . 2017;151(4):786–794. doi: 10.1016/j.chest.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 24.Blake C., Codd M. B., O’Meara Y. M. The short form 36 (SF-36) health survey: normative data for the Irish population. Irish Journal of Medical Science . 2000;169(3):195–200. doi: 10.1007/bf03167695. [DOI] [PubMed] [Google Scholar]
- 25.Jenkinson C., Layte R., Jenkinson D., et al. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? Journal of Public Health . 1997;19(2):179–186. doi: 10.1093/oxfordjournals.pubmed.a024606. [DOI] [PubMed] [Google Scholar]
- 26.LaGrotte C., Fernandez-Mendoza J., Calhoun S. L., Liao D., Bixler E. O., Vgontzas A. N. The relative association of obstructive sleep apnea, obesity and excessive daytime sleepiness with incident depression: a longitudinal, population-based study. International Journal of Obesity . 2016;40(9):1397–1404. doi: 10.1038/ijo.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seixas A. A., Nunes J. V., Airhihenbuwa C. O., et al. Linking emotional distress to unhealthy sleep duration: analysis of the 2009 national health interview survey. Neuropsychiatric Disease and Treatment . 2015;11:2425–2430. doi: 10.2147/ndt.s77909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrone E., Sguazzin C., Bertolotti G., et al. Development and validation of the Maugeri sleep quality and distress inventory (MaSQuDI-17) PLoS One . 2017;12(7) doi: 10.1371/journal.pone.0180743.e0180743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sivertsen B., Salo P., Mykletun A., et al. The bidirectional association between depression and insomnia: the HUNT study. Psychosomatic Medicine . 2012;74(7):758–765. doi: 10.1097/psy.0b013e3182648619. [DOI] [PubMed] [Google Scholar]
- 30.Wang D., Yee B. J., Grunstein R. R. Does sleep apnea worsen the adverse effects of opioids and benzodiazepines on chronic obstructive pulmonary disease? Annals of the American Thoracic Society . 2019;16(10):1237–1238. doi: 10.1513/annalsats.201907-504ed. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The individual data used to support the findings of this study are available from the corresponding author upon request.


