Abstract
Background
We conducted this study to assess the effect of disease-modifying therapies (DMTs) on coronavirus disease (COVID-19) susceptibility and severity in people with multiple sclerosis (MS).
Methods
Available studies from PubMed, Scopus, EMBASE, Web of Science, and gray literature, including reference lists and conference abstracts, were searched from December 1, 2019, to July 26, 2021. We included cross-sectional, case-control, and cohort studies assessing the association of DMTs with risk of contracting COVID-19 or its outcomes in MS patients on univariate or multivariate regression analyses. We conducted a network meta-analysis (NMA) to compare the risk of COVID-19 and developing severe infection across DMTs.
Results
Out of the initial 3893 records and 1883 conference abstracts, a total of 10 studies were included. Pairwise comparisons showed that none of the DMTs meaningfully affect the risk of acquiring infection. There was significant total heterogeneity and inconsistency across this NMA. In comparison with no DMT, dimethyl fumarate (0.62 (0.42, 0.93)), fingolimod (0.55 (0.32, 0.94)), natalizumab (0.50 (0.31, 0.81)), and interferon (0.42 (0.22, 0.79)) were associated with a decreased risk of severe COVID-19; but, rituximab was observed to increase the risk (1.94 (1.20, 3.12)). Compared to rituximab or ocrelizumab, all DMTs were associated with a decreased risk. Pairwise comparisons showed no differences across other DMTs. Interferon and rituximab were associated with the lowest and highest risks of severe COVID-19.
Conclusion
Our study showed an increased risk of severe COVID-19 in patients on rituximab and ocrelizumab. No association with COVID-19 severity across other DMTs was observed.
1. Introduction
The outbreak of Coronavirus Disease-2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to a newly emerging pandemic. This globally spreading virus affects people in different ways, with manifestations ranging from no symptoms to hospitalization and death due to acute respiratory distress syndrome (ARDS) [1, 2]. More than one year after the outbreak of COVID-19, the number of reported COVID-19 cases exceeds 150 million, with more than 3.5 million deaths [3].
Multiple sclerosis (MS) is one of the most common demyelinating diseases in the central nervous system (CNS), affecting generally young female adults. MS patients often receive immunosuppressive agents which put those at greater risk of developing viral and bacterial infections [4–6]. This raised a question regarding whether people living with MS were at higher risk of COVID-19 and were more likely to develop severe symptoms when infected than the general population. A recent systematic review has suggested a mortality rate of 3.5% among MS patients considering suspected/confirmed COVID-19 cases, which is slightly higher than the rate of 2.2% among the general population [2, 7]. This study showed that patients on anti-CD20 agents had highest rates of hospitalization and mortality than those on other DMTs. Moreover, studies suggested an increased risk of developing the infection in MS patients on anti-CD20 agents [8, 9].
Knowledge of the association between disease-modifying therapies (DMTs) and COVID-19 susceptibility/severity is necessary to provide the best care for patients during the pandemic and could be important for policymakers to adopt vaccine strategies. However, the current evidence is inconsistent and unclear. Therefore, this study was conducted to present the current evidence regarding the effect of DMTs on COVID-19 susceptibility and severity in people living with MS.
2. Method
2.1. Inclusion and Exclusion Criteria
Studies were included according to the following criteria: population (participants), outcomes, and study types. The population (participants) consists of suspected or confirmed COVID-19 patients with a previous diagnosis of MS. Outcomes are the association of each specific DMT with COVID-19 susceptibility and outcomes reported based on univariate or multivariate regression analyses. The included study types are cross-sectional, case-control, and cohort studies. Studies with the following characteristics were excluded: (a) studies did not compare DMTs with each other; (b) studies pooled DMTs based on the mechanism of action (immune cell depleting medications or immune-cell trafficking inhibitors) or risk of systemic infection (no risk, mild, risk, or high risk); (c) nonpeer-reviewed articles; (d) non-English studies; (e) review articles and systematic review; and (f) qualitative studies.
2.2. Information Source and Search Strategy
We comprehensively searched electronic databases including PubMed, Scopus, EMBASE, and Web of Science from December 1, 2019, to July 26, 2021. The following search words were adapted: ((coronavirus OR Wuhan coronavirus OR novel coronavirus OR coronavirus disease OR COVID-19 OR 2019 novel coronavirus infection OR 2019-nCOV OR severe acute respiratory syndrome coronavirus 2 OR SARS-CoV-2) AND (Multiple Sclerosis OR (Sclerosis, Multiple) OR (Sclerosis, Disseminated) OR Disseminated Sclerosis OR (Multiple Sclerosis, Acute Fulminating)). We also screened the reference lists of identified articles, review studies, or other relevant documents for inclusion in the study. In addition, we also searched the online library and abstracts of the following congresses: 8th American and European Committees for Treatment and Research in Multiple Sclerosis (ACTRIMS-ECTRIMS 2020), 145th Annual Meeting American Neurological Association, Annual meeting America Academy of Neurology 2021, and 6th Congress of the European Academy of Neurology, and to identify eligible studies that have not been published. We conducted this systematic review following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10].
2.3. Study Selection
Two researchers (MB and SB) independently screened the titles and abstracts of retrieved studies to identify the eligible studies. Then, the full text of the potentially eligible studies was reviewed. Disagreement regarding the study selection was resolved by consulting with a third investigator (AAS).
2.4. Quality Assessment
Two reviewers (OM and MB) independently evaluated the quality of the included studies using the Newcastle-Ottawa scale (NOS) quality tests [11]. Different checklists were used based on the study design. The third investigator solved any discrepancies (AAS) in quality assessment. We rated the quality of included studies by giving stars to three parameters of selection, comparability, and outcome according to the NOS guidelines (Supplementary file (available here)). Cross-sectional studies were categorized to very good, good, satisfactory, and unsatisfactory quality. Cohort studies were categorized as good, fair, and poor quality.
2.5. Data Extraction
Two researchers (MSH and GP) independently carried out the extraction of data. The following information was extracted from each eligible publication: first author's name, initial publication date, location of study, scenario of study, type of study, total number of MS patients, number of MS patients with confirmed/suspected COVID-19, and odds ratios (ORs) and their confidence intervals (95% CIs) of association between following DMTs and COVID-19 susceptibility or severity: interferon (IFN), glatiramer acetate (GA), dimethyl fumarate (DMF), teriflunomide (TRF), fingolimod (FNG), natalizumab (NTZ), rituximab (RTX), ocrelizumab (OCR), cladribine (CLA), and no DMT.
2.6. Data Synthesis
We conducted a network meta-analysis (NMA) on the risk of developing COVID-19 and its severity to assess the relative impacts of various DMTs. Model heterogeneity was estimated by I-square (I2) and tau-squared (τ2). The Q statistic (Qtotal) was decomposed to assess the heterogeneity (within study designs (Qwithin)) and inconsistency (between study designs (Qbetween)). League table was utilized to indicate all direct and indirect pairwise comparisons using ORs and their 95% CIs. ORs less than 1 indicated that the DMT reduced the risk of COVID-19 susceptibility or severity relative to the comparator DMT. A P score and net rank plot were also applied for ranking all DMTs based on their network estimates. A higher P score indicates a greater risk of COVID-19 susceptibility or severity. We did not perform sensitivity analysis based on the quality of studies since small number of included papers. However, only one included study had unsatisfied quality. We performed no publication bias test since less than 10 studies were included in each NMA [12]. The data were analyzed in Stata 14 software (Stata Corporation, College Station, Texas, USA) and R software (version 4.0.2, R Foundation for Statistical Computing, Vienna, Austria) using netmeta package.
3. Results
3.1. Study Selection
A total of 3893 records were initially identified according to the research strategy. After duplicate removal, 2140 retrieved studies were screened in the title and abstract. Among 213 records reviewed in the full text, 10 published articles met inclusion criteria. Out of 1883 conference abstracts, none met inclusion criteria. Finally, a total of 10 studies were included in this systematic review. The PRISMA flow chart shows the process of study selection (Figure 1).
Figure 1.
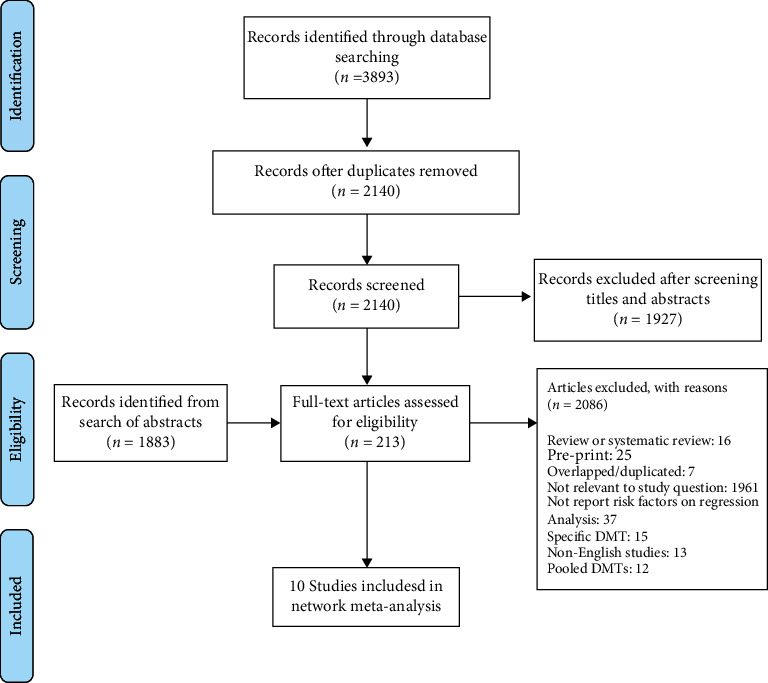
Study flow diagram.
3.2. Characteristics of Studies Included
The characteristics of included studies are summarized in Tables 1(a) and 1(b). Five studies with 36912 MS patients consisting of 616 cases of suspected/confirmed COVID-19 investigated association of DMTs with COVID-19 susceptibility [9, 13–16]. Five studies, including 3639 MS patients with COVID-19, evaluated association of DMTs with COVID-19 severity [17–21]. Two of the included studies were cross-sectional [13, 17], 7 were cohort [9, 14–16, 18–21], and one was pharmacovigilance [9]. Four studies reported data from the USA [9, 16, 17, 21], two from Spain [15, 20], and one from each of Italy, [18] Iran, [13] and Sweden [19]. One study was multicentric from Europe [14].
The risk of bias judgment for each included study is presented in Supplementary Tables 1 and 2. Respecting the quality of cross-sectional studies, one was very good [17] and another one was unsatisfactory [13]. Regarding cohort studies, the qualities of 4 included studies were good and 4 were fair.
3.3. Network Meta-Analysis
3.3.1. COVID-19 Susceptibility
The network graphs and forest plots for the association of DMTs with the risk of acquiring COVID-19 are presented in Figures 2 and 3. Based on univariate analysis, three studies assessing the association of DMTs with the risk of COVID-19 were included in the NMA [13–15]. In comparison with no DMT, natalizumab (OR = 4.25, 95% CI: 1.34, 13.46; P score = 0.83) and anti-CD20 agents (OR = 3.17, 95% CI: 1.38, 7.25; P score = 0.72) were associated with higher risk of infection (Figure 3(a)). Ranking of the risk of infection identified dimethyl fumarate as the best, indicating lowest risk of developing infection, and natalizumab as the worst among DMTs. No significant results were found for other comparisons (Table 2(a)). There was a disagreement between direct and indirect comparison of no DMT with platform therapies (rituximab and glatiramer acetate). In direct comparison, no DMT was associated with a decreased risk of infection compared to platform therapies (OR = 0.39, 95% CI: 0.17, 0.92); but, no significant difference in indirect model was found (OR = 0.45, 95% CI: 0.19, 1.02). The total heterogeneity in NMA was not significant (τ2 = 0.072 and I2 = 19.4%, Qtotal = 7.44, P = 0.282). There was no significant inconsistency between study designs (Qbetween = 7.86, P = 0.249).
Figure 2.
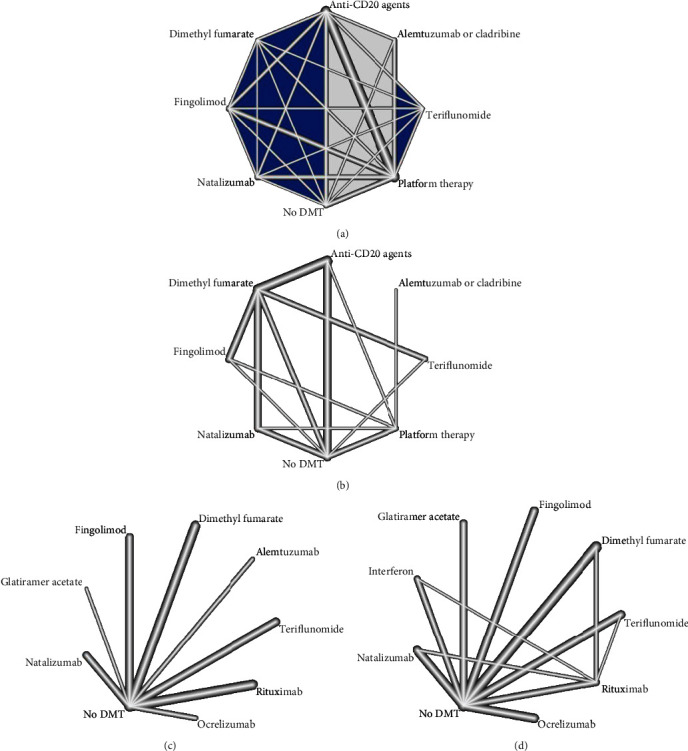
Network plots the effect of DMTs on the risk of acquiring COVID-19 and its severity. Platform therapy: interferon and glatiramer acetate; anti-CD20 agents: rituximab and ocrelizumab. (a) Risk of acquiring infection based on a univariate model. (b) Risk of acquiring infection based on a multivariate model. (c) Risk of severe infection based on a univariate model. (d) Risk of severe infection based on a multivariate model.
Figure 3.
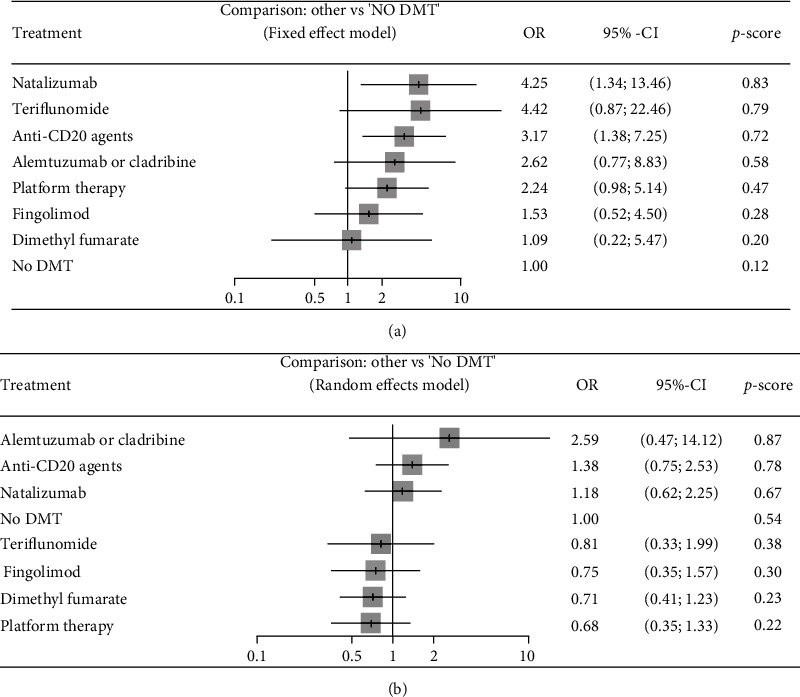
Forest plots of comparisons between DMTs and no DMTs for risk of acquiring COVID-19. Platform therapy: interferon and glatiramer acetate; anti-CD20 agents: rituximab and ocrelizumab. (a) Results of univariate analyses. (b) Results of multivariate analyses. P score ranges from zero to 1. A higher P score indicates a greater risk of being infected with COVID-19.
Three studies assessing the association between DMTs and risk of COVID-19 based on multivariate analysis were included in the NMA [9, 14, 16]. Pairwise comparisons showed that none of the DMTs had a worse effect on the risk of infection than another drug and no DMT (Table 2(b)). Ranking of the risk of infection identified interferon/glatiramer acetate as the best, indicating lowest risk, and alemtuzumab/cladribine as the worst among DMTs (Figure 3(b)). There was a disagreement between the direct and indirect comparisons of anti-CD20 agents with dimethyl fumarate. In direct comparison, the anti-CD20 agent's arm was associated with an increased risk of infection compared to dimethyl fumarate (OR = 3.25, 95% CI: 1.46, 7.24); but, we found no significant difference in the indirect model (OR = 1.88, 95% CI: 0.33, 10.73). We observed significant total heterogeneity (τ2 = 0.135 and I2 = 51.7%, Qtotal = 14.48, P = 0.043). There was significant inconsistency between study designs (Qbetween = 14.48, P = 0.043).
3.3.2. COVID-19 Severity
The network graphs and forest plots for the association of DMTs with COVID-19 severity are presented in Figures 2 and 4. Based on univariate analysis, two studies assessing the association of DMTs with COVID-19 severity were included in the NMA [20, 21]. In the comparison of DMTs with no DMT, natalizumab was associated with a decreased risk of severe infection (OR = 0.28, 95% CI: 0.10, 0.81; P score = 0.08) (Figure 4(a)). For other comparisons, dimethyl fumarate (OR = 0.29, 95% CI: 0.09, 0.90) and natalizumab (OR = 0.14, 95% CI: 0.04, 0.51) were associated with lower risk of severe infection than rituximab (Table 3(a)). Additionally, natalizumab decreased the risk of severe infection (OR = 0.22, 95% CI: 0.05, 0.91) compared to teriflunomide. Ranking of the risk of severe infection identified natalizumab as the best and rituximab as the worst among DMTs. No significant total heterogeneity was detected (τ2 = 0.0 and I2 = 0%, Qtotal = 2.06, P = 0.841). Additionally, there was no significant heterogeneity within designs (Qwithin = 2.06, P = 0.841).
Figure 4.
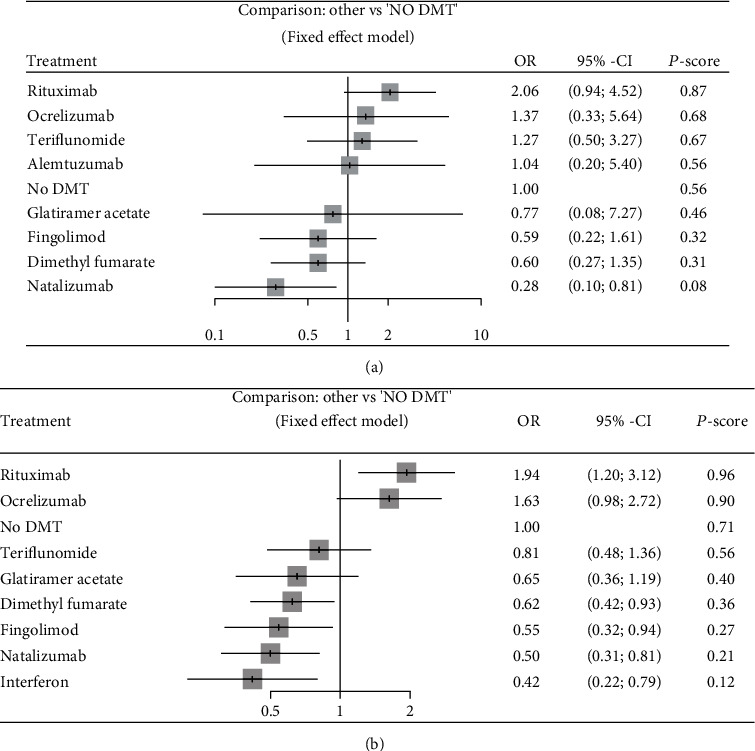
Forest plots of comparisons between DMTs and no DMTs for severity of COVID-19. (a) Results of univariate analyses. (b) Results of multivariate analyses. P score ranges from zero to 1. A higher P-score indicates a greater risk of developing severe COVID-19 infection.
Four studies assessing the association of DMTs with COVID-19 severity based on multivariate analysis were included in the NMA [17–20]. In comparison with no DMT, dimethyl fumarate (OR = 0.62, 95% CI:0.42, 0.93; P score = 0.36), fingolimod (OR = 0.55, 95% CI:0.32, 0.94; P score = 0.27), natalizumab (OR = 0.50, 95% CI:0.31, 0.81; P score = 0.21), and interferon (OR = 0.42, 95% CI:0.22, 0.79; P score = 0.12) were associated with a decreased risk of developing severe COVID-19. However, rituximab increased the risk of severe infection compared to no DMT (OR = 1.94, 95% CI: 1.20, 3.12). Compared to rituximab and ocrelizumab, all DMTs were associated with a decreased risk of severe infection. Only the difference between teriflunomide and ocrelizumab was not significant. There were two disagreements between direct and indirect results for rituximab vs. teriflunomide and rituximab vs. interferon. Although rituximab was associated with an increased risk of severe disease compared to teriflunomide in the indirect model, no significant difference was found in the direct comparison (OR = 0.93, 95% CI: 0.19, 4.57). In the indirect comparison, interferon reduced the risk of severe infection compared to rituximab, but this reduction was not significant in the direct comparison (OR = 0.49, 95% CI: 0.10, 2.46). Ranking of the risk of severe infection identified interferon as the best and rituximab as the worst among DMTs (Figure 4(b)). No significant total heterogeneity was detected (τ2 = 0.139 and I2 = 0.373%, Qtotal = 23.36, P = 0.077). There was significant heterogeneity within designs (Qwithin = 19.93, P = 0.046), though no significant inconsistency was detected (Qbetween = 3.43, P = 0.489).
4. Discussion
This study is aimed at summarizing the existing evidence on association of DMTs with COVID-19 susceptibility and severity in patients with MS. The finding of this network meta-analysis showed that patients on rituximab and ocrelizumab, and no DMT was at greater risk of severe COVID-19 infection compared to other MS patients. We observed no substantial difference across DMTs in the risk of developing severe infection.
When we ranked DMTs, interferon was associated with the lowest risk of acquiring COVID-19 and developing severe infection. This finding was also reported by Sormani et al. [22] that Italian MS patients on interferon were less likely to develop severe COVID-19 than those on other DMTs. These results were expected since interferon is not immunosuppressive and has anti-inflammatory and antiviral effects [23–26]. Protective effect of interferon against the SARS-CoV and MERS-CoV [27, 28], discovering autoantibodies against type I interferons in critically ill COVID-19 patients [29], and inhabitation effect of this agent on SARS-CoV-2 replication [30] suggested interferon as therapeutic candidate for COVID-19 [29, 31, 32]. However, the effectiveness of interferon on COVID-19 severity among general population in clinical trials remains unclear [33–35].
The harmful and beneficial effects of moderate and high effective DMTs on COVID-19 severity are still in dispute. Dimethyl fumarate, teriflunomide, and fingolimod decrease lymphocyte counts resulting in reduced viral clearance which may theoretically increase risk of severe COVID-19 infection [36–38]. Moreover, natalizumab limits viral clearance from the central nervous system [39] which could negatively affect the outcome of COVID-19 infection. However, experts and international recommendations suggested that these medications would not increase the risk of severe infection and may even have beneficial effects [40–42]. This network meta-analysis showed that none of the interferon, glatiramer acetate, dimethyl fumarate, teriflunomide, and natalizumab had a worse outcome compared to another one. All DMTs were also independently associated with a reduced risk of severe infection compared to no DMT, except anti-CD20 agents. This finding suggests that these medications are not likely to increase the risk of severe COVID-19 and are safe for using within the pandemic. Because of a lack of data, we could not examine the effect of alemtuzumab and cladribine on COVID-19 severity.
In the comparison of each specific DMT with no DMT, rituximab was associated with the highest risk of developing severe COVID-19 infection, followed by ocrelizumab. Observed increased risk of severe illness in patients treated with rituximab and ocrelizumab goes in line with studies on other autoimmune diseases [43–45]. Although the exact reason for this association is elusive, it is suggested that patients who treated with anti-CD20 monoclonal antibodies experience decreased antibody production, which can lead to an impaired immune response to SARS-CoV-2 [46–48]. Rituximab can also cause a decrease in CD4+ and CD8+ counts [49], which play a substantial role in response to SAR-CoV2 [50].
The results of primary studies showed a stronger association between rituximab and COVID-19 severity than ocrelizumab [17, 20, 22]. This difference could be related to the antibody-dependent cell-mediated cytotoxic effects and immunogenicity of these drugs [51, 52] or some confounders such as characteristics of patients and duration of treatment. The NMA on both univariate and multivariate results showed a decreased risk of developing severe COVID-19 in patients on ocrelizumab compared to rituximab. However, the differences were not substantially significant.
The NMA on univariate results identified lowest risk of developing COVID-19 in MS patients who received no DMT. However, the NMA on adjusted or multivariate results showed that platform therapies, fingolimod, dimethl fumarate, and teriflunomide had better outcome than no DMT. This inconsistency may be due the patients' characteristics. Most MS patients who received no DMTs are elderly and have advanced terminal stage. These patients are less involved in high-risk activities such as traveling, working outside the home, and spending a long time in social interaction. As a result, they may stay at home and not be in close contact with COVID-19 cases, which could decrease the risk of developing COVID-19.
One major issue in early research concerned the risk of acquiring COVID-19 in those who treated with anti-CD20 agents. Epidemiological and pharmacovigilance data suggested a higher risk of developing COVID-19 in MS patients on these agents [8, 9, 13]. However, some studies found no association between anti-CD20 medications and risk of the infection [14, 16, 53]. The suggested reasons for increased risk of acquiring infection in these agents are similar with those mentioned for increased risk of developing severe COVID-19. Although the pooled univariate results showed a higher risk of infection in patients treated with anti-CD20 agents than patients receiving no DMT, no notable difference between DMTs was detected after pooling multivariate analyses. These findings should be interpreted with caution since there was a high level of heterogeneity in NMA on multivariate analyses. Further work needs to be done to investigate the effect of DMTs on the risk of COVID-19 infection.
Our study has some limitations. First, we excluded non-English studies from the study. Second, there are differences in primary studies' health policies and medical care practices, which can affect our results. Third, we combined the quantitative findings of primary studies that used different adjustment methods. Fourth, the definition of COVID-19 susceptibility and severity varied among primary studies. Fifth, a limited number of studies included in quantitative analyses could dominate the estimates. Sixth, the primary used a different primary comparator (no therapy and no DMT). Seventh, this review is based on the current published articles, some of which were relatively small or did not have the necessary statistical power. Therefore, caution must be used when interpreting the association of DMTs with COVID-19 susceptibility or severity.
In conclusion, our study showed that MS patients on anti-CD20 agents are at greater risk of developing severe COVID-19 infection compared to those who received other DMTs and no DMT. It seems that other DMTs did not increase the risk of severe infection and are safe to continue during COVID-19 pandemic. We believed that our results are helpful to design appropriate programs to identify high-risk patients early and adapt vaccination strategies.
Table 1.
(a) Characteristics of studies assessing association of DMTs with COVID-19 susceptibility
| Author | Scenario of study | Type of study | Country reporting | Total MS patients | Number of suspected/confirmed COVID-19 cases | Definition of COVID-19 suspected or confirmed group | Analytical method used | Study quality |
|---|---|---|---|---|---|---|---|---|
| Sahraian et al., [30] | Contacted MS patients who were managed in the MS Clinic of Sina Hospital, Iran | Cross-sectional | Iran | 4647 | 68 | Patients were asked about COVID-19-related symptoms, CFT scan findings, PCR test, and hospitalization. | Univariate logistic regression | Unsatisfactory |
| Dalla Costa et al., [14] | A questionnaire sent to MS patients across Europe | Cohort | European multicentric | 399 | 52 | Patients experiencing fever or anosmia/ageusia+any other COVID-19 symptoms, or respiratory symptoms+two other COVID-19 Symptoms |
Univariate and multivariate penalized likelihood logistic regression models | Good |
| Reder et al., [9] | Using the IBM Explorys real-world dataset | Pharmacovigilance | USA | 30478 | 344 | Patients with PCR-confirmed COVID-19 were considered COVID-19 positive; all others were considered COVID-19 negative. | Logistic regression adjusted for patient age, sex, BMI, comorbidities, and race | Good |
| Zabalza et al., [15] | Self-administered survey sent to patients were followed in Multiple Sclerosis Centre of Catalonia (Cemcat). Suspected COVID-19 cases were interviewed by phone. | Cohort | Spain | 758 | 48 | (1) Patients with fever, dyspnoea, persistent cough, or (2) sudden onset of anosmia, ageusia or dysgeusia, or (3) radiological images compatible with COVID-19 were considered suspected cases. Patients with a positive SARS-CoV-2 PCR were considered confirmed cases | Univariable and multivariable logistic regressions | Good |
| Levin et al., [16] | Online surveys using the Research Electronic Data Capture (REDCap) platform was sent to patients MS or a related disorder across USA | Cohort | USA | 630 | 104 | (1) Patients with cough or shortness of breath, or (2) any two of the following: fever, muscle pain, sore throat, and new loss of taste or smell | Multivariate logistic regressions | Fair |
(b) Characteristics of studies assessing association of DMTs with COVID-19 severity
| Author | Scenario of study | Type of study | Country reporting | Total MS patients with COVID-19 | Number of severe cases | Definition of COVID-19 severity | Analytical method used | Study quality |
|---|---|---|---|---|---|---|---|---|
| Salter et al., [17] | Registry of MS and patients with confirmed or suspected COVID-19 in North America (COViMS Registry) | Cross-sectional | North America | 1626 | 333∗ | (a) Requiring hospitalization only (b) ICU and/or required ventilator support (c) Death |
Multivariable multinomial logistic regression | Very good |
| Sormani et al., [18] | Collected data of MS patients who had been in contact with their neurologist because of a confirmed or suspected COVID-19 (MUSC-19 registry) | Cohort | Italy | 844 | 136 | (a) No need for hospitalization or documented diagnosis of pneumonia (b) Diagnosis of pneumonia or hospitalization (c) Death or ICU admission |
Univariate and multivariate ordinal logistic regressions | Fair |
| Spelman et al., [19] | Registry of Swedish MS patients with suspected and confirmed COVID-19 infection (SMSreg) | Cohort | Sweden | 476 | 73 | (a) Not requiring hospitalization (b) Hospitalization, ICU, or death |
Weighted logistic regression with IPTW approach to adjust confounders | Fair |
| Moreno-Torres et al., [20] | Registry of MS and patients with confirmed or highly suspected COVID-19 across Madrid | Cohort | Spain | 219 | 51 | (a) No need for hospitalization (b) Requiring hospitalization |
Univariate and multivariate logistic regression models with an L1 penalty (Lasso regression) | Good |
| Klineova et al., [21] | Patients with MS or related CNS disorders with suspected or confirmed COVID-19 in New York or surrounded city (NYCNIC registry) | Cohort | USA | 474 | 58 | (a) Not requiring hospitalization (b) Hospitalization, ICU, or death |
Univariable and multivariable logistic regressions | Fair |
∗Only hospitalized patients. ICU: intensive care unit.
Table 2.
League table showing the results of network meta-analysis comparing the effect of DMTs on the risk of getting COVID-19.
(a) Results from univariate analyses
| ALZ or CLA | 0.27 (0.06, 1.28) |
. | . | . | 1.29 (0.24, 6.90) |
1.07 (0.41, 2.80) |
. |
|---|---|---|---|---|---|---|---|
| 0.83 (0.30, 2.27) |
Anti-CD20 agents | 3.11 (0.75, 12.94) |
2.56 (0.91, 7.20) |
0.70 (0.17, 2.98) |
2.86
(1.21, 6.77) |
1.32 (0.84, 2.07) |
0.77 (0.18, 3.24) |
| 2.40 (0.43, 13.3) |
2.90 (0.70, 12.03) |
DMF | 0.82 (0.15, 4.52) |
0.23 (0.03, 1.64) |
1.33 (0.19, 9.47) |
0.55 (0.13, 2.43) |
0.25 (0.03, 1.78) |
| 1.71 (0.51, 5.69) |
2.07 (0.94, 4.54) |
0.71 (0.15, 3.45) |
FNG | 0.28 (0.05, 1.53) |
1.61 (0.29, 8.84) |
0.74 (0.34, 1.60) |
0.30 (0.05, 1.66) |
| 0.62 (0.17, 2.17) |
0.74 (0.31, 1.82) |
0.26 (0.05, 1.32) |
0.36 (0.12, 1.08) |
NTZ | 5.85 (0.81, 42.25) |
1.97 (0.85, 4.56) |
1.09 (0.15, 7.94) |
| 2.62 (0.77, 8.83) |
3.17
(1.38, 7.25) |
1.09 (0.22, 5.47) |
1.53 (0.52, 4.50) |
4.25
(1.34, 13.46) |
No DMT |
0.39
(0.17, 0.92) |
0.19 (0.03, 1.34) |
| 1.17 (0.45, 3.02) |
1.41 (0.92, 2.17) |
0.49 (0.11, 2.06) |
0.68 (0.32, 1.45) |
1.90 (0.82, 4.38) |
0.45 (0.19, 1.02) |
Platform therapy | 0.45 (0.10, 1.99) |
| 0.59 (0.11, 3.33) |
0.72 (0.17, 3.01) |
0.25 (0.03, 1.78) |
0.35 (0.07, 1.70) |
0.96 (0.19, 5.00) |
0.23 (0.04, 1.15) |
0.51 (0.12, 2.18) |
TRF |
(b) Results from multivariate analyses
| ALZ or CLA | . | . | . | . | . | 3.78 (0.79, 18.00) |
. |
|---|---|---|---|---|---|---|---|
| 1.88 (0.33, 10.73) |
Anti-CD20 agents |
3.25
(1.46, 7.24) |
. | . | 0.91 (0.39, 2.12) |
1.27 (0.31, 5.13) |
. |
| 3.64 (0.65, 20.46) |
1.88 (0.33, 10.73) |
DMF | 0.87 (0.37, 2.05) |
0.71 (0.30, 1.69) |
1.07 (0.40, 2.85) |
. | 1.12 (0.44, 2.89) |
| 3.48 (0.59, 20.33) |
3.64 (0.65, 20.46) |
0.96 (0.48, 1.90) |
FNG | . | 0.68 (0.16, 2.82) |
0.94 (0.25, 3.57) |
. |
| 2.19 (0.38, 12.49) |
3.48 (0.59, 20.33) |
0.60 (0.32, 1.14) |
0.63 (0.27, 1.49) |
NTZ | 1.27 (0.51, 3.19) |
2.25 (0.60, 8.42) |
. |
| 2.59 (0.47, 14.12) |
2.19 (0.38, 12.49) |
0.71 (0.41, 1.23) |
0.75 (0.35, 1.57) |
1.18 (0.62, 2.25) |
No DMT | 1.69 (0.66, 4.38) |
0.65 (0.15, 2.92) |
| 3.78 (0.79, 18.00) |
2.59 (0.47, 14.12) |
1.04 (0.50, 2.18) |
1.09 (0.48, 2.49) |
1.73 (0.80, 3.76) |
1.46 (0.75, 2.84) |
Platform therapy | . |
| 3.18 (0.49, 20.81) |
3.78 (0.79, 18.00) |
0.87 (0.39, 1.97) |
0.91 (0.32, 2.58) |
1.46 (0.54, 3.94) |
1.23 (0.50, 2.99) |
0.84 (0.30, 2.39) |
TRF |
On the upper triangle, the effect size are direct comparisons; the effect sizes presented on lower triangle are network meta-analyses (indirect comparison). Comparisons should be read from left to right (example for upper triangle: OR (95% CI) of developing COVID-19 in anti-CD20 agents compared to DMF is 3.25 (1.46, 7.24); example for lower triangle: OR (95% CI) of developing COVID-19 in the ALZ or CLA group compared to anti-CD20 agents is 1.88 (0.33, 10.73). Platform therapy: interferon and glatiramer acetate; anti-CD20 agents: rituximab and ocrelizumab. ALZ: alemtuzumab; CLA: cladribine; DMF: dimethyl fumarate; FNG: fingolimod; NTZ: natalizumab; TRF: teriflunomide; DMT: disease-modifying therapy.
Table 3.
League table showing the results of network meta-analysis comparing the effect of DMTs on the severity of COVID-19.
(a) Results from univariate analyses
| ALZ | . | . | . | . | 1.04 (0.20, 5.40) |
. | . | . |
|---|---|---|---|---|---|---|---|---|
| 1.74 (0.28, 10.92) |
DMF | . | . | . | 0.60 (0.27, 1.35) |
. | . | . |
| 1.75 (0.26, 12.01) |
1.01 (0.28, 3.65) |
FNG | . | . | 0.59 (0.22, 1.61) |
. | . | . |
| 1.35 (0.08, 21.89) |
0.78 (0.07, 8.47) |
0.77 (0.07, 9.01) |
GA | . | 0.77 (0.08, 7.27) |
. | . | . |
| 3.65 (0.52, 25.72) |
2.10 (0.56, 7.91) |
2.09 (0.49, 8.85) |
2.71 (0.23, 32.21) |
NTZ |
0.28
(0.10, 0.81) |
. | . | . |
| 1.04 (0.20, 5.40) |
0.60 (0.27, 1.35) |
0.59 (0.22, 1.61) |
0.77 (0.08, 7.27) |
0.28
(0.10, 0.81) |
No DMT | 0.73 (0.18, 3.01) |
0.49 (0.22, 1.07) |
0.78 (0.31, 2.02) |
| 0.76 (0.09, 6.67) |
0.44 (0.09, 2.24) |
0.43 (0.08, 2.45) |
0.56 (0.04, 7.99) |
0.21 (0.04, 1.21) |
0.73 (0.18, 3.01) |
OCR | . | . |
| 0.51 (0.08, 3.14) |
0.29
(0.09, 0.90) |
0.29 (0.08, 1.03) |
0.37 (0.03, 4.04) |
0.14
(0.04, 0.51) |
0.49 (0.22, 1.07) |
0.67 (0.13, 3.36) |
RTX | . |
| 0.82 (0.12, 5.45) |
0.47 (0.14, 1.63) |
0.47 (0.12, 1.84) |
0.60 (0.05, 6.91) |
0.22
(0.05, 0.91) |
0.78 (0.31, 2.02) |
1.08 (0.20, 5.89) |
1.62 (0.47, 5.52) |
TRF |
(b) Results from multivariate analyses
| DMF | . | . | . | . |
0.62
(0.40, 0.95) |
. |
0.34
(0.12, 0.98) |
. |
|---|---|---|---|---|---|---|---|---|
| 1.14 (0.58, 2.22) |
FNG | . | . | . |
0.55
(0.32, 0.94) |
. | . | . |
| 0.95 (0.46, 1.96) |
0.84 (0.38, 1.87) |
GA | . | . | 0.65 (0.36, 1.19) |
. | . | . |
| 1.49 (0.70, 3.14) |
1.31 (0.57, 3.01) |
1.56 (0.65, 3.74) |
IFN | . |
0.36
(0.18, 0.72) |
. | 0.49 (0.10, 2.46) |
. |
| 1.24 (0.67, 2.30) |
1.09 (0.53, 2.24) |
1.30 (0.61, 2.80) |
0.83 (0.38, 1.84) |
NTZ |
0.51
(0.31, 0.86) |
. |
0.23
(0.07, 0.78) |
. |
|
0.62
(0.42, 0.93) |
0.55
(0.32, 0.94) |
0.65 (0.36, 1.19) |
0.42
(0.22, 0.79) |
0.50
(0.31, 0.81) |
No DMT | 0.61 (0.37, 1.02) |
0.39
(0.20, 0.75) |
1.37 (0.80, 2.36) |
|
0.38
(0.20, 0.73) |
0.34
(0.16, 0.70) |
0.40
(0.18, 0.88) |
0.26
(0.11, 0.58) |
0.31
(0.15, 0.62) |
0.61 (0.37, 1.02) |
OCR | . | . |
|
0.32
(0.18, 0.57) |
0.28
(0.14, 0.58) |
0.34
(0.16, 0.73) |
0.22
(0.10, 0.46) |
0.26
(0.14, 0.48) |
0.52
(0.32, 0.83) |
0.84 (0.42, 1.69) |
RTX | 0.93 (0.19, 4.57) |
| 0.77 (0.40, 1.47) |
0.67 (0.32, 1.42) |
0.80 (0.37, 1.77) |
0.52 (0.23, 1.17) |
0.62 (0.31, 1.24) |
1.23 (0.74, 2.06) |
2.01 (0.97, 4.15) |
2.39
(1.22, 4.66) |
TRF |
On the upper triangle, the effect size are direct comparisons; the effect sizes presented on lower triangle are network meta-analyses (indirect comparison). Comparisons should be read from left to right (example for upper triangle: OR (95% CI) of developing a severe COVID-19 in DMF compared to no DMT is 0.62 (0.40, 0.95); example for lower triangle: OR (95% CI) of developing a severe COVID-19 in DMF compared to FNG is 1.14 (0.58, 2.22). DMF: dimethyl fumarate; FNG: fingolimod; GA: glatiramer acetate; IFN: interferon; NTZ: natalizumab; TRF: teriflunomide; DMT: disease-modifying therapy; RTX: rituximab; OCR: ocrelizumab.
Acknowledgments
Aram Zabeti received funding from NIH, PCORI, Genentech, Chugai, Medimmune, and Biogen.
Contributor Information
Omid Mirmosayyeb, Email: omid.mirmosayyeb@gmail.com.
Vahid Shaygannejad, Email: v.shaygannejad@gmail.com.
Data Availability
All supporting the results of this study can be found within the tables, figures, and manuscript of the present study.
Disclosure
A preprint of this study is available via the link below and is added to the reference list of this manuscript as reference number 2: https://www.medrxiv.org/content/10.1101/2021.06.11.21258765v1
Conflicts of Interest
Aram Zabeti reports compensation for lectures given for Biogen, Celgene/Bristol Myers Squibb, Genentech-Roche, Novartis, Mark-Serono, Teva Pharma, Sanofi-Genzyme, and Viela Bio/Horizon. The remaining authors declared no conflict of interest related to the study.
Authors' Contributions
Conceptualization was performed by MB, OM, VS, AZ, and SB. Methodology was done by MB, SB, SH, AAS OM, and VS. Literature search was done by MB, OM, MSH, GP, and SB. Writing—original draft preparation was done by MB, SB, SH, and AZ. Writing—review and editing was done by MB, ES, OM, VS, and SB. Performing meta-analysis and network meta-analysis was done by ES and AAS. Supervision was done by VS and AZ.
Supplementary Materials
Table S1: quality assessment of the included cross-sectional studies based on the NOS checklist. Table S2: quality assessment of the included cohort studies based on the NOS checklist. Pages 3-5: PRISMA 2020 checklist.
References
- 1.Wu Z., McGoogan J. M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. Journal of the American Medical Association . 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Barzegar M., Houshi S., Hashemi M. S., et al. Factors associated with COVID-19 susceptibility and severity in patients with multiple sclerosis: a systematic review. 2011. https://www.medrxiv.org/content/10.1101/2021.06.11.21258765v1 . [DOI] [PMC free article] [PubMed]
- 3.Organization WH. Situation reports. Weekly epidemiological update on COVID-19-1 June 2021. https: // http://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. (Date Accessed: 3 June 2021)
- 4.Winkelmann A., Loebermann M., Reisinger E. C., Hartung H.-P., Zettl U. K. Disease-modifying therapies and infectious risks in multiple sclerosis. Nature Reviews Neurology . 2016;12(4):217–233. doi: 10.1038/nrneurol.2016.21. [DOI] [PubMed] [Google Scholar]
- 5.Luna G., Alping P., Burman J., et al. Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA neurology . 2020;77(2):184–191. doi: 10.1001/jamaneurol.2019.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mirmosayyeb O., Bagherieh S., Shaygannejad V. Acute CNS demyelination in a subject with cerebellar ataxia following the first dose of COVID-19 vaccine; a case report. Human Vaccines & Immunotherapeutics . 2021;17(11):4099–4101. doi: 10.1080/21645515.2021.1971920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barzegar M., Mirmosayyeb O., Gajarzadeh M., et al. COVID-19 among patients with multiple sclerosis. Neurology (R) neuroimmunology & neuroinflammation . 2021;8(4) doi: 10.1212/NXI.0000000000001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Safavi F., Nourbakhsh B., Azimi A. R. B-cell depleting therapies may affect susceptibility to acute respiratory illness among patients with multiple sclerosis during the early COVID-19 epidemic in Iran. Multiple sclerosis and related disorders . 2020;43, article 102195 doi: 10.1016/j.msard.2020.102195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reder A. T., Centonze D., Naylor M. L., et al. COVID-19 in patients with multiple sclerosis: associations with disease-modifying therapies. CNS Drugs . 2021;35(3):317–330. doi: 10.1007/s40263-021-00804-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D., Liberati A., Tetzlaff J., Altman D. G., Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine . 2009;6(7, article e1000097) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology . 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 12.Higgins J. P., Thomas J., Chandler J., et al. Cochrane Handbook for Systematic Reviews of Interventions . John Wiley & Sons; 2019. [DOI] [Google Scholar]
- 13.Sahraian M. A., Azimi A., Navardi S., Ala S., Moghadasi A. N. Evaluation of the rate of COVID-19 infection, hospitalization and death among Iranian patients with multiple sclerosis. Multiple sclerosis and related disorders . 2020;46, article 102472 doi: 10.1016/j.msard.2020.102472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalla Costa G., Leocani L., Montalban X., et al. Real-time assessment of COVID-19 prevalence among multiple sclerosis patients: a multicenter European study. Neurological Sciences . 2020;41(7):1647–1650. doi: 10.1007/s10072-020-04519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zabalza A., Cárdenas-Robledo S., Tagliani P., et al. COVID-19 in multiple sclerosis patients: susceptibility, severity risk factors and serological response. European Journal of Neurology . 2021 Oct;28(10):3384–3395. doi: 10.1111/ene.14690. [DOI] [PubMed] [Google Scholar]
- 16.Levin S. N., Venkatesh S., Nelson K. E., et al. Manifestations and impact of the COVID-19 pandemic in neuroinflammatory diseases. Annals of clinical and translational neurology . 2021;8(4):918–928. doi: 10.1002/acn3.51314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salter A., Fox R. J., Newsome S. D., et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis. JAMA neurology . 2021;78(6):699–708. doi: 10.1001/jamaneurol.2021.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sormani M. P., De Rossi N., Schiavetti I., et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Annals of Neurology . 2021;89(4):780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spelman T., Forsberg L., McKay K., Glaser A., Hillert J. Increased rate of hospitalisation for COVID-19 amongst rituximab treated multiple sclerosis patients: a study of the Swedish MS registry. Multiple Sclerosis Journal . 2021 doi: 10.2139/ssrn.3801769. [DOI] [PubMed] [Google Scholar]
- 20.Moreno-Torres I., Meca Lallana V., Costa-Frossard L., et al. Risk and outcomes of covid-19 in patients with multiple sclerosis. European Journal of Neurology . 2021;28(11):3712–3721. doi: 10.1111/ene.14990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klineova S., Harel A., Farber R. S., et al. Outcomes of COVID-19 infection in multiple sclerosis and related conditions: one-year pandemic experience of the multicenter New York COVID-19 Neuroimmunology Consortium (NYCNIC) Multiple Sclerosis and Related Disorders . 2021;55, article 103153 doi: 10.1016/j.msard.2021.103153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sormani M. P., Salvetti M., Labauge P., et al. DMTs and Covid‐19 severity in MS: a pooled analysis from Italy and France. Annals of Clinical and Translational Neurology . 2021;8(8):1738–1744. doi: 10.1002/acn3.51408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samuel C. E. Antiviral actions of interferons. Clinical microbiology reviews . 2001;14(4):778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katze M. G., He Y., Gale M. Viruses and interferon: a fight for supremacy. Nature Reviews Immunology . 2002;2(9):675–687. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- 25.Lin F.-c., Interferons Y. H. A. Interferons: success in anti-viral immunotherapy. Cytokine & growth factor reviews . 2014;25(4):369–376. doi: 10.1016/j.cytogfr.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kopitar-Jerala N. The role of interferons in inflammation and inflammasome activation. Frontiers in immunology . 2017;8:p. 873. doi: 10.3389/fimmu.2017.00873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hensley L. E., Fritz E. A., Jahrling P. B., Karp C., Huggins J. W., Geisbert T. W. Interferon-β 1a and SARS coronavirus replication. Emerging infectious diseases . 2004;10(2):317–319. doi: 10.3201/eid1002.030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hart B. J., Dyall J., Postnikova E., et al. Interferon-β and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell-based assays. The Journal of general virology . 2014;95(3):571–577. doi: 10.1099/vir.0.061911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bastard P., Rosen L. B., Zhang Q., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science (New York, NY) . 2020;370:p. 6515. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vanderheiden A., Ralfs P., Chirkova T., et al. Type I and type III interferons restrict SARS-CoV-2 infection of human airway epithelial cultures. Journal of virology . 2020;94(19, article e00985) doi: 10.1128/JVI.00985-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bastard P., Rosen L. B., Zhang Q., et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science (New York, NY) . 2020;370:p. 6515. doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jalkanen J., Hollmén M., Jalkanen S. Interferon beta-1a for COVID-19: critical importance of the administration route. Critical Care . 2020;24(1):1–3. doi: 10.1186/s13054-020-03048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Consortium WST. Repurposed antiviral drugs for COVID-19—interim WHO SOLIDARITY trial results. New England journal of medicine . 2021;384(6):497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li C., Luo F., Liu C., et al. Effect of a genetically engineered interferon-alpha versus traditional interferon-alpha in the treatment of moderate-to-severe COVID-19: a randomised clinical trial. Annals of Medicine . 2021;53(1):391–401. doi: 10.1080/07853890.2021.1890329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feld J. J., Kandel C., Biondi M. J., et al. Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial. The Lancet Respiratory Medicine . 2021;9(5):498–510. doi: 10.1016/S2213-2600(20)30566-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Comi G., Miller A. E., Benamor M., Truffinet P., Poole E. M., Freedman M. S. Characterizing lymphocyte counts and infection rates with long-term teriflunomide treatment: pooled analysis of clinical trials. Multiple Sclerosis Journal . 2020;26(9):1083–1092. doi: 10.1177/1352458519851981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehta D., Miller C., Arnold D. L., et al. Effect of dimethyl fumarate on lymphocytes in RRMS: implications for clinical practice. Neurology . 2019;92(15):e1724–e1738. doi: 10.1212/WNL.0000000000007262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakhaei-Nejad M., Barilla D., Lee C.-H., Blevins G., Giuliani F. Characterization of lymphopenia in patients with MS treated with dimethyl fumarate and fingolimod. Neurology-Neuroimmunology Neuroinflammation . 2018;5(2):p. e432. doi: 10.1212/NXI.0000000000000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niino M., Bodner C., Simard M. L., et al. Natalizumab effects on immune cell responses in multiple sclerosis. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society . 2006;59(5):748–754. doi: 10.1002/ana.20859. [DOI] [PubMed] [Google Scholar]
- 40.Berger J. R., Brandstadter R., Bar-Or A. COVID-19 and MS disease-modifying therapies. Neurology-Neuroimmunology Neuroinflammation . 2020;7(4):p. e761. doi: 10.1212/NXI.0000000000000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korsukewitz C., Reddel S. W., Bar-Or A., Wiendl H. Neurological immunotherapy in the era of COVID-19 -- looking for consensus in the literature. Nature reviews Neurology . 2020;16(9):493–505. doi: 10.1038/s41582-020-0385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng C., Kar I., Chen C. K., et al. Multiple sclerosis disease-modifying therapy and the COVID-19 pandemic: implications on the risk of infection and future vaccination. CNS Drugs . 2020;34(9):879–896. doi: 10.1007/s40263-020-00756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Avouac J., Drumez E., Hachulla E., et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. The Lancet Rheumatology . 2021;3(6):e419–e426. doi: 10.1016/S2665-9913(21)00059-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sparks J. A., Wallace Z. S., Seet A. M., et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: results from the COVID-19 Global Rheumatology Alliance physician registry. Annals of the Rheumatic Diseases . 2021;80(9):1137–1146. doi: 10.1136/annrheumdis-2021-220418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strangfeld A., Schäfer M., Gianfrancesco M. A., et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Annals of the rheumatic diseases . 2021;80(7):930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehta P., Porter J. C., Chambers R. C., Isenberg D. A., Reddy V. B-cell depletion with rituximab in the COVID-19 pandemic: where do we stand? The Lancet Rheumatology . 2020;2(10):e589–e590. doi: 10.1016/S2665-9913(20)30270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barun B., Bar-Or A. Treatment of multiple sclerosis with anti-CD20 antibodies. Clinical immunology . 2012;142(1):31–37. doi: 10.1016/j.clim.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Barmettler S., Ong M.-S., Farmer J. R., Choi H., Walter J. Association of immunoglobulin levels, infectious risk, and mortality with rituximab and hypogammaglobulinemia. JAMA network open . 2018;1(7):e184169–e184169. doi: 10.1001/jamanetworkopen.2018.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liossis S.-N. C., Sfikakis P. P. Rituximab-induced B cell depletion in autoimmune diseases: potential effects on T cells. Clinical immunology . 2008;127(3):280–285. doi: 10.1016/j.clim.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Cao X. COVID-19: immunopathology and its implications for therapy. Nature reviews immunology . 2020;20(5):269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sorensen P. S., Blinkenberg M. The potential role for ocrelizumab in the treatment of multiple sclerosis: current evidence and future prospects. Therapeutic advances in neurological disorders . 2016;9(1):44–52. doi: 10.1177/1756285615601933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dörner T., Burmester G. R. New approaches of B-cell-directed therapy: beyond rituximab. Current opinion in rheumatology . 2008;20(3):263–268. doi: 10.1097/BOR.0b013e3282f5e08d. [DOI] [PubMed] [Google Scholar]
- 53.Mirmosayyeb O., Shaygannejad V., Bagherieh S., Hosseinabadi A. M., Ghajarzadeh M. Prevalence of multiple sclerosis (MS) in Iran: a systematic review and meta-analysis. Neurological Sciences . 2022;43(1):233–241. doi: 10.1007/s10072-021-05750-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: quality assessment of the included cross-sectional studies based on the NOS checklist. Table S2: quality assessment of the included cohort studies based on the NOS checklist. Pages 3-5: PRISMA 2020 checklist.
Data Availability Statement
All supporting the results of this study can be found within the tables, figures, and manuscript of the present study.


