Abstract
About one out of eight people to convalesce from COVID-19 suffer from the so called Long COVID, a syndrome of non-specific symptoms with unclear pathogenesis. In a recent study published in Cell Long COVID participants reporting respiratory symptoms had low cortisol levels. In an as yet unpublished analysis from Yale University low plasma cortisol levels discriminated Long COVID from asymptomatic convalescent or healthy non-infected controls. Although various immune perturbations were present in Long COVID, low levels of cortisol were prominent and strikingly, depression and anxiety were increased. It has become clear that Long COVID features may be similar to those described in myalgic encephalomyelitis/chronic fatigue syndrome, post-SARS sickness syndrome, and various chronic stress syndromes which have been linked to hypocortisolemia. Notably, lack of response of the hypothalamic–pituitary–adrenal axis to hypocortisolemia shows a suppressed axis in Long COVID. We suggest that the inability of hypothalamic–pituitary–adrenal axis to recover after the acute illness, perhaps due to protracted stress in predisposed individuals, may represent the pathogenetic basis of the Long COVID-associated clinical and immunological manifestations.
Keywords: Long Covid-19, Pathophysiology, Hypocortisolemia, Adrenals, Chronic fatigue syndrome, Hypothalamic-pituitary-adrenal axis
1. Long COVID: A frequent syndrome with unclear pathogenesis
Currently, the number of people infected with SARS-CoV-2 worldwide exceeds 500 million [source: Johns Hopkins University & Medicine. Coronavirus Resource Centre. 2022. https://coronavirus.jhu.edu/ (accessed August 29, 2022)] and will continue to rise for the years to come [1]. A significant number of convalescent COVID-19 individuals, with the vast majority of them having a history of mild acute disease, do not make a full recovery and suffer from “Long” COVID for months to years. This post-acute infection clinical syndrome includes non-specific symptoms, such as fatigue, breathlessness, headache, chest pain, abdominal symptoms, myalgias and cognitive impairment [2]. The prevalence of Long COVID varies greatly between reports; the median proportion of patients with self-reported psychosomatic symptoms post-acute COVID-19 accounted for 72.5% in a systematic review of uncontrolled studies [3]. Despite the growing research interest focusing on the nature, prevalence, and management of Long COVID, the underlying pathogenetic mechanisms remain essentially unclear [4].
In the “Post-Hospitalization COVID-19 study”, a prospective, longitudinal cohort study recruiting adults discharged from hospital across the UK, 2468 participants had attended a 5-month visit, 924 participants returned for a 1-year visit and 807 participants attended both visits [5]. At 5 months, about 25% had fully recovered, 20% were not feeling sure, whereas 55% had not recovered. Responses were similar between 5 months and one year. Notably, the majority of participants had C-reactive protein (CRP) levels below 5 mg/L, and the proportions of participants with CRP below 5 mg/L were almost similar in the 3 groups (79%, 76% and 73%, respectively). In multivariable analysis, female sex, body mass index 30 kg/m2 or greater and prior invasive mechanical ventilation were all independent factors associated with being less likely to recover at one year after discharge from the hospital [5].
In the only well-controlled longitudinal cohort study published so far, almost one eighth of the convalescent non-hospitalized individuals suffered from at least one core symptom linked to Long COVID, namely chest pain, difficulties with breathing, pain when breathing, painful muscles, ageusia or anosmia, tingling sensation at the extremities, lump in the throat, feeling hot and cold alternately, as well as “having heavy arms or legs”, and general tiredness, 90–150 days post infection [6]. Pathogenetic insights were not provided by this study, thus, the basic biology and a mechanistic understanding of Long COVID, including possible immune perturbations related to persistent virus infection, latent reactivation, viral remnants, or autoimmunity, remain obscure.
2. Hypocortisolemia in Long COVID: An unexpected finding?
To explore potential factors that may predict Long COVID Su et al. correlated patient symptoms with in-depth profiling of blood cells and plasma components during acute COVID-19 and after 2–3 months, in a recent longitudinal multiomics study. Interestingly, they found that plasma cortisol levels were significantly lower in the subgroup of participants still reporting respiratory symptoms 3 months later, together with higher titers of autoantibodies to interferon [7]. The possibility that hypocortisolemia had resulted from a previous direct viral adrenal injury seems unlikely. Although some studies that were performed at the beginning of the pandemic reported virus-related adrenal hemorrhages and infarctions, a post-mortem study in 40 COVID-19 patients did not confirm cellular damage in their adrenal glands [8].
We have previously demonstrated higher diurnal salivary cortisol secretion in 52 COVID-19 patients than age- and sex-matched healthy controls. While the cortisol levels in the morning did not differ between the two groups, COVID-19 patients exhibited high evening and nocturnal cortisol secretion. Interestingly, individual serum IL-6 levels (6-fold higher than controls) strongly correlated with nocturnal cortisol hypersecretion [9]. To confirm that acute viral infection-driven cortisol hypersecretion was transient, salivary cortisol measurements were repeated 180 days post infection in 20 of these patients. While morning cortisol levels remained unchanged, nocturnal levels were almost 3-fold lower compared to those during COVID-19 (mean ± SD of 0.086 ± 0.057 versus 0.277 ± 0.243 μg/dL, p = 0.001, respectively, by paired t-test) and similar to those of 20 healthy controls (0.087 ± 0.069 μg/dL) None of these 20 individuals reported Long COVID-associated symptoms at the time of sampling (unpublished data).
Fascinating results from an as yet unpublished exploratory cross-sectional analysis involving 215 individuals showed that a low plasma cortisol level was indeed a robust distinctive feature between Long COVID patients and asymptomatic convalescent individuals or healthy uninfected controls [10]. Among the 99 participants with Long COVID, all reporting dramatically worsened quality of life, 68% were women, whereas 87% and 13% had a history of mild acute disease or had been hospitalized, respectively. In a period of more than 400 days post-acute infection, the plasma cortisol levels in patients with Long COVID were almost half of those found in matched controls independently of age, sex, sample collection time, and body mass index. Based on machine learning tools, cortisol levels alone were the most significant predictor for Long COVID classification. Moreover, the levels of depression and anxiety were highly increased in Long COVID compared to controls. Intriguingly, despite hypocortisolemia in Long COVID, ACTH levels were comparable to controls. Whether this impaired compensatory response by the hypothalamic-pituitary-adrenal (HPA) axis in Long COVID was associated with increased stress was not evaluated [10].
In the same study, immune perturbations in Long COVID were reported, albeit the differences compared to controls were by far less impressive than those observed for cortisol levels [10]. Elevated humoral responses against SARS-CoV-2 and Epstein-Barr virus, as well as significant differences in plasma levels of interleukins 6 and 8, and various chemokines were observed in Long COVID participants comparing to 15 convalescent and 25 healthy controls. A number of changes in circulating leukocytes were also noted, including elevated percentages (not absolute numbers) in the peripheral blood of non-classical monocytes, activated B- cells, IL-4/IL-6 secreting CD4 T cells, as well as of exhausted T-cells in 20–30% of patients. The authors emphasized the fact that the level of T-cells exhaustion was significantly associated with the increased humoral reactivity to Epstein-Barr virus [10]. Notably, the combination of low cortisol levels, T-cell exhaustion and augmented reactivity to Epstein-Barr virus has been also observed in patients with myalgic encephalomyelitis/chronic fatigue (ME/CFS) syndrome, a condition that also follows viral infection [11]. Along this line, patients who fulfil ME/CFS criteria account for about 45% in three different Long COVID cohorts [[12], [13], [14]].
3. Clinical and immune manifestations in Long COVID may be secondary to protracted stress-induced hypocortisolemia
We and others have reported a relative hypo-responsiveness of the HPA axis with persistently low cortisol levels in chronic stress syndromes, such as fibromyalgia [[15], [16], [17], [18]] and chronic fatigue syndrome [19]. These patients demonstrate significantly lower mean 24-h urinary free cortisol [15,19], and low diurnal salivary cortisol levels [17] compared to normal subjects, along with a relative unresponsiveness of the adrenal gland to exogenous CRH and/or ACTH stimulation [[15], [17], [18]]. Moreover, the hypocortisolemia and hypoactivation of the HPA axis reported in Long COVID [10] is also reminiscent of what was previously described in the post-SARS (severe acute respiratory syndrome) “sickness syndrome”-type condition characterized by symptoms such as malaise, anorexia, fatigue and myalgias [20].
In conditions such as acute viral infections, activation of the HPA axis associated with the elevation of proinflammatory cytokines and, consequently, an increased secretion of cortisol, operates as a negative feedback system to prevent uncontrolled and eventually harmful sequalae of inflammatory mechanisms and tissue damage [20,21]. Following a period of intense stress, however, there may be glucocorticoid-induced suppression of the HPA axis lasting varying times [22]. In this case, long-term “inadequate” cortisol secretion may unleash its inhibitory effect on immune system activation, possibly resulting in immune dysregulation and expression of sickness-syndrome manifestations. The magnitude and duration of post-stress sustained hypocortisolism in these conditions, may then determine whether an individual will develop a post-acute infection sickness syndrome. This concept is compatible with the clinical picture of the intrinsic hypocortisolemia of primary adrenal insufficiency (Addison's disease) which is also characterized by various sickness-syndrome manifestations, including fatigue, muscle weakness poor concentration, “brain fog”, nausea, loss of body hair, as well as by depression and anxiety [23]. Moreover, Addison's disease is associated with immune perturbations, including high IL-6 levels, due to failure of cortisol to appropriately suppress the increased secretion of proinflammatory cytokines [23,24].
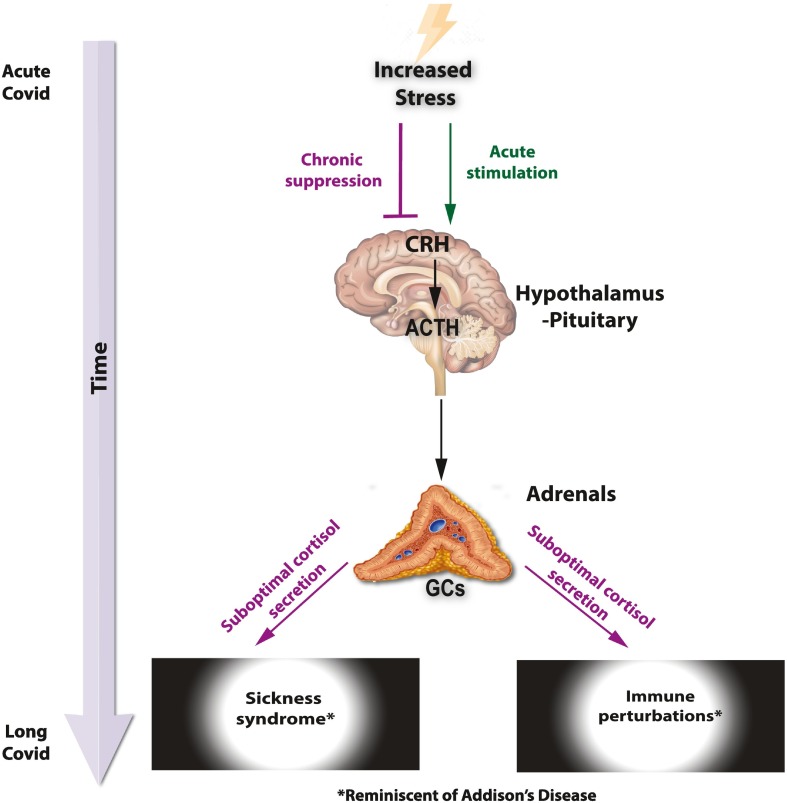
We suggest that Long COVID, as well as other post-viral infection sickness syndromes or sickness syndromes associated with chronic inflammatory diseases in predisposed individuals [11,21], are characterized by suppressed responsiveness of the HPA axis. The protracted subsequent lack of axis recovery due to a post-illness state of hypocortisolism in these individuals may be the underlying pathophysiologic mechanism responsible for, at least, some of sickness syndrome manifestations and the immune perturbations seen in Long COVID ( Fig. 1 ).
Fig. 1.
Suggested pathogenesis of Long COVID based on a protracted stress-induced hypocortisolemia. (CRH, Corticotropin-releasing hormone; ACTH, adrenocorticotropic hormone; GCs, glucocorticoids).
Why some people are more prone to develop Long COVID, and whether HPA axis stimulants or exogenously administered glucocorticoids could have a role in the management of some of these individuals, warrant further investigation. Predisposed individuals may be those with an intrinsically compromised adrenal reserve that fails to compensate for intense or long standing increased stress, as seen in patients with immune-mediated and inflammatory diseases, such as rheumatoid arthritis [25,26].
To improve our understanding of Long COVID, which is crucial for the proper management of these individuals, we propose that assessment of cortisol secretion, preferably diurnal measurements of free cortisol in saliva, should be considered for those individuals who do not fully recover after an even mild COVID-19 episode. Clear knowledge of the responsible pathophysiology should add further reassurance and self-confidence to the suffering people.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
None.
Data availability
No data was used for the research described in the article.
References
- 1.COVID is here to stay: countries must decide how to adapt. Nature. 2022;601:165. doi: 10.1038/d41586-022-00057-y. [DOI] [PubMed] [Google Scholar]
- 2.Brightling C.E., Evans R.A. Long COVID: which symptoms can be attributed to SARS-CoV-2 infection? Lancet. 2022;400:411–413. doi: 10.1016/S0140-6736(22)01385-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nasserie T., Hittle M., Goodman S.N. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehandru S., Merad M. Pathological sequelae of long-haul COVID. Nat. Immunol. 2022;23:194–202. doi: 10.1038/s41590-021-01104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.PHOSP-COVID Collaborative Group Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study. Lancet Respir. Med. 2022;10:761–775. doi: 10.1016/S2213-2600(22)00127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballering A.V., van Zon S.K.R., Olde Hartman T.C., Rosmalen J.G.M., I. Lifelines Corona Research Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet. 2022;400:452–461. doi: 10.1016/S0140-6736(22)01214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su Y., Yuan D., Chen D.G., Ng R.H., Wang K., Choi J., Li S., Hong S., Zhang R., Xie J., Kornilov S.A., Scherler K., Pavlovitch-Bedzyk A.J., Dong S., Lausted C., Lee I., Fallen S., Dai C.L., Baloni P., Smith B., Duvvuri V.R., Anderson K.G., Li J., Yang F., Duncombe C.J., McCulloch D.J., Rostomily C., Troisch P., Zhou J., Mackay S., DeGottardi Q., May D.H., Taniguchi R., Gittelman R.M., Klinger M., Snyder T.M., Roper R., Wojciechowska G., Murray K., Edmark R., Evans S., Jones L., Zhou Y., Rowen L., Liu R., Chour W., Algren H.A., Berrington W.R., Wallick J.A., Cochran R.A., Micikas M.E., Unit I.S.-S.C.-B., Wrin T., Petropoulos C.J., Cole H.R., Fischer T.D., Wei W., Hoon D.S.B., Price N.D., Subramanian N., Hill J.A., Hadlock J., Magis A.T., Ribas A., Lanier L.L., Boyd S.D., Bluestone J.A., Chu H., Hood L., Gottardo R., Greenberg P.D., Davis M.M., Goldman J.D., Heath J.R. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185:881–895 e820. doi: 10.1016/j.cell.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanczkowski W., Evert K., Stadtmuller M., Haberecker M., Laks L., Chen L.S., Frontzek K., Pablik J., Hantel C., Beuschlein F., Kurth T., Gruber S., Aguzzi A., Varga Z., Bornstein S.R. COVID-19 targets human adrenal glands. Lancet Diabetes Endocrinol. 2022;10:13–16. doi: 10.1016/S2213-8587(21)00291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yavropoulou M.P, Filippa M.G., Mantzou A., Ntziora F., Mylona M., Tektonidou M.G., Vlachogiannis N.I., Paraskevis D., Kaltsas G.A., Chrousos G.P., Sfikakis P.P. Alterations in cortisol and interleukin-6 secretion in patients with COVID-19 suggestive of neuroendocrine-immune adaptations. Endocrine. 2022;75:317–327. doi: 10.1007/s12020-021-02968-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein J., Wood J., Jaycox J., et al. Distinguishing features of Long COVID identified through immune profiling. medRxiv preprint. 2022 doi: 10.1101/2022.08.09.22278592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clayton E.W. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: an IOM report on redefining an illness. JAMA. 2015;313:1101–1102. doi: 10.1001/jama.2015.1346. [DOI] [PubMed] [Google Scholar]
- 12.Mancini D.M., Brunjes D.L., Lala A., Trivieri M.G., Contreras J.P., Natelson B.H. Use of cardiopulmonary stress testing for patients with unexplained dyspnea post-coronavirus disease. JACC Heart Fail. 2021;9:927–937. doi: 10.1016/j.jchf.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kedor C., Freitag H., Meyer-Arndt L., Wittke K., Hanitsch L.G., Zoller T., Steinbeis F., Haffke M., Rudolf G., Heidecker B., Bobbert T., Spranger J., Volk H.D., Skurk C., Konietschke F., Paul F., Behrends U., Bellmann-Strobl J., Scheibenbogen C. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat. Commun. 2022;13:5104. doi: 10.1038/s41467-022-32507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonilla H., Quach A., Tiwari A.E., et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is common in post-acute sequelae of SARS-CoV-2 infection (PASC): Results from a post-COVID-19 multidisciplinary clinic. medRxiv preprint. 2022 doi: 10.1101/2022.08.03.22278363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crofford L.J., Pillemer S.R., Kalogeras K.T., Cash J.M., Michelson D, Kling M.A., Sternberg E.M., Gold P.W., Chrousos G.P., Wilder R.L. Hypothalamic-pituitary-adrenal axis perturbations in patients with fibromyalgia. Arthritis Rheum. 1994;37:1583–1592. doi: 10.1002/art.1780371105. [DOI] [PubMed] [Google Scholar]
- 16.Gruber L.M., Nanda S., Nippoldt T., Chang A.Y., Bancos I. Secondary Adrenal Insufficiency and Growth Hormone Deficiency in Patients with Fibromyalgia. J Pain Res. 2021;14:1323–1329. doi: 10.2147/JPR.S302291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin Y.J., Ko Y.C., Chow L.H., Hsiao F.J., Liu H.Y., Wang P.N., Chen W.T. Salivary cortisol is associated with cognitive changes in patients with fibromyalgia. Sci Rep. 2021;11:1311. doi: 10.1038/s41598-020-79349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griep E.N., Boersma J.W., de Kloet E.R. Altered reactivity of the hypothalamic-pituitary-adrenal axis in the primary fibromyalgia syndrome. J Rheumatol. 1993;20:469–474. [PubMed] [Google Scholar]
- 19.Demitrack M.A., Dale J.K., Straus S.E., Laue L., Listwak S.J., Kruesi M.J., Chrousos G.P., Gold P.W. Evidence for impaired activation of the hypothalamic-pituitary-adrenal axis in patients with chronic fatigue syndrome. J Clin Endocrinol Metab. 1991;73:1224–1234. doi: 10.1210/jcem-73-6-1224. [DOI] [PubMed] [Google Scholar]
- 20.Leow M.K., Kwek D.S., Ng A.W., Ong K.C., Kaw G.J., Lee L.S. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS) Clin Endocrinol (Oxf) 2005;63:197–202. doi: 10.1111/j.1365-2265.2005.02325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chrousos G.P., Kaltsas Kaltsas. Post-SARS sickness syndrome manifestations and endocrinopathy: how, why, and so what? Clin Endocrinol (Oxf) 2005;63:363–365. doi: 10.1111/j.1365-2265.2005.02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chrousos, G.P., Gold P.W. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. doi: 10.1001/jama.1992.03480090092034. [DOI] [PubMed] [Google Scholar]
- 23.Charmandari E., Nicolaides N.C., Chrousos G.P. Adrenal insufficiency. Lancet. 2014;383:2152–2167. doi: 10.1016/S0140-6736(13)61684-0. [DOI] [PubMed] [Google Scholar]
- 24.Papanicolaou D.A., Tsigos C., Oldfield E.H., Chrousos G.P. Acute glucocorticoid deficiency is associated with plasma elevations of interleukin-6: does the latter participate in the symptomatology of the steroid withdrawal syndrome and adrenal insufficiency? J Clin Endocrinol Metab. 1996;81:2303–2306. doi: 10.1210/jcem.81.6.8964868. [DOI] [PubMed] [Google Scholar]
- 25.Filippa M.G., Tektonidou M.G., Mantzou A., Kaltsas G.A., Chrousos G.P., Sfikakis P.P., Yavropoulou M.P. Adrenocortical dysfunction in rheumatoid arthritis: A narrative review and future directions. Eur J Clin Invest. 2022;52:e13635. doi: 10.1111/eci.13635. [DOI] [PubMed] [Google Scholar]
- 26.Yavropoulou M.P., Filippa M.G., Panopoulos S., Spanos E., Spanos G., Tektonidou M.G., Sfikakis P.P. Impaired adrenal cortex reserve in patients with rheumatic and musculoskeletal diseases who relapse upon tapering of low glucocorticoid dose. Clin Exp Rheumatol. 2022;4:1789–1792. doi: 10.55563/clinexprheumatol/x78tko. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.