Abstract
Sodium-glucose cotransporter 2 (SGLT2) inhibitor have become widely used in patients with diabetes, heart failure, and kidney disease to improve clinical outcomes and diminish hospitalizations. They have also been associated with increased serum magnesium levels in patients with type 2 diabetes. The use of SGLT2 inhibitors resulted in improved magnesium homeostasis in a series of patients with refractory hypomagnesemia with urinary magnesium wasting. However, the role of SLGT2 inhibitors in patients with hypomagnesemia without urinary magnesium wasting remains unexplored. We report 2 cases with refractory hypomagnesemia without significant urinary magnesium wasting and dramatically improved serum magnesium levels after the initiation of SGLT2 inhibitors. Case 1 achieved independence from weekly intravenous magnesium infusions and reached sustainably greater serum magnesium levels with decreased oral magnesium supplementation and increased urinary fractional excretion of magnesium. Case 2 demonstrated improved serum magnesium levels with reduced oral magnesium supplementation without significant reduction in urinary fractional excretion of magnesium. These findings not only expand the use of SGLT2 inhibitors but also open the door for further studies to better understand the pathophysiology of how magnesium homeostasis is altered with inhibition of SGLT2.
Index Words: Empagliflozin, hypomagnesemia, magnesium, SGLT2 inhibitors, type 2 diabetes
Introduction
Sodium-glucose cotransporter 2 (SGLT2) inhibitors diminish proximal tubule glucose reabsorption. They were initially developed to improve glycemic control in patients with type 2 diabetes but have emerged to have multiple extra glycemic benefits that include reducing hospitalization rates in patients with heart failure, diminishing the progression of chronic kidney disease, improving albuminuria, and reducing major cardiovascular events, and clinical trials have consistently demonstrated lower mortality.1 Recent data from randomized clinical trials have shown that SGLT2 inhibitors increase serum magnesium (Mg2+) levels from 0.04 mmol/L to 0.1 mmol/L as a class effect in patients with type 2 diabetes with or without hypomagnesemia.2,3,4 Additionally, SGLT2 inhibitors were found to be beneficial in a report of 3 patients with refractory hypomagnesemia with variable urinary Mg2+ wasting.5 However, their role in patients without overt urinary Mg2+ wasting is unclear. We report a dramatic response in improving serum Mg2+ levels after the addition of SGLT2 inhibitors in 2 patients with type 2 diabetes and refractory hypomagnesemia without evidence of significant urinary Mg2+ wasting.
Case 1
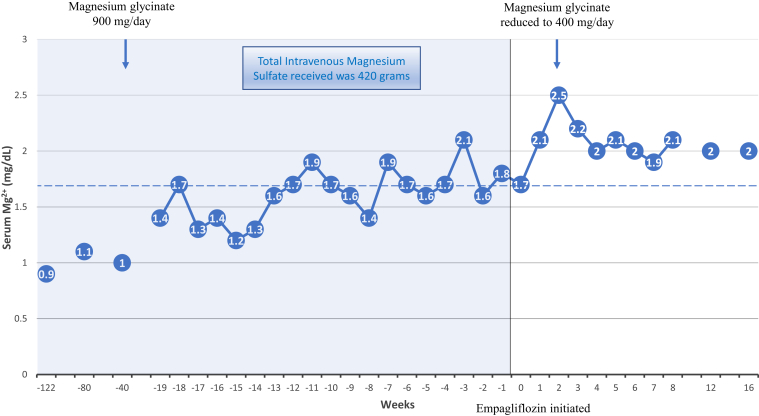
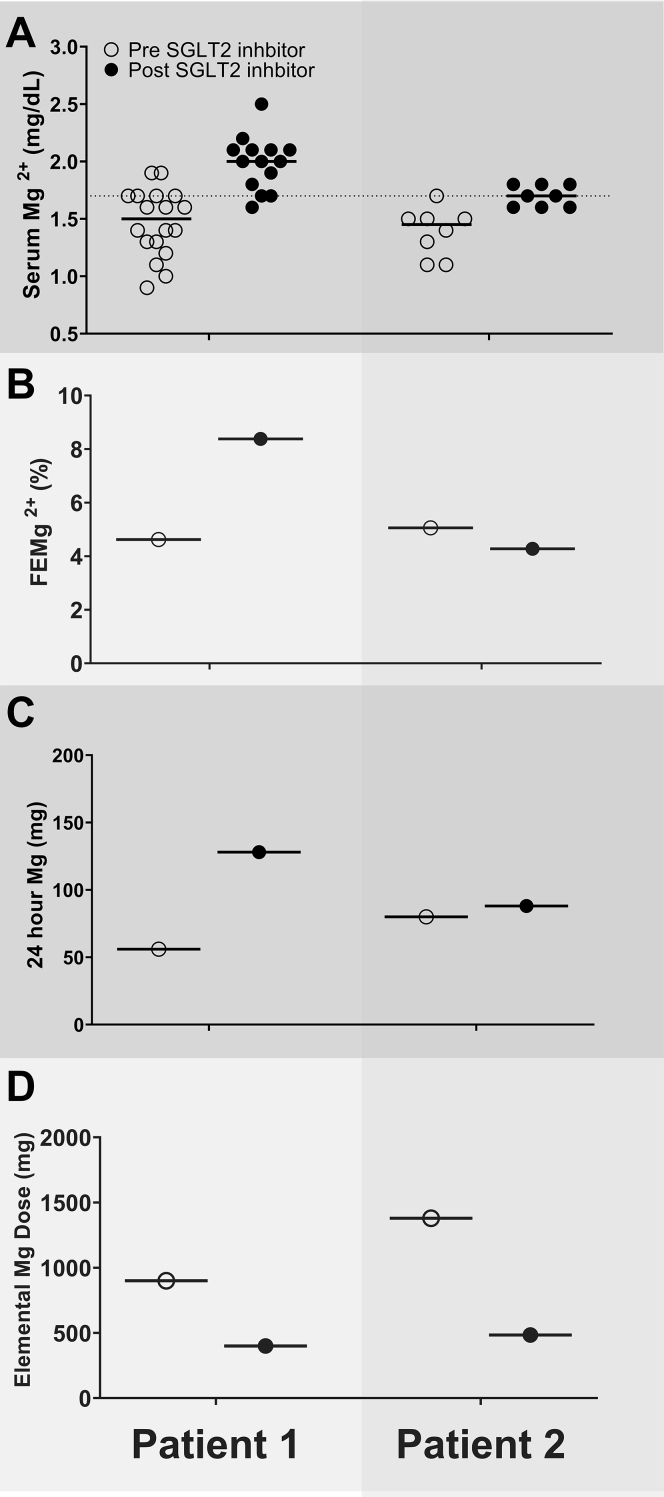
A woman in her late 70s with a history of type 2 diabetes, transcatheter sapien aortic valve replacement for severe aortic stenosis, atrial fibrillation, hypertension, chronic constipation, and chronic hypomagnesemia was referred owing to a history of severe hypomagnesemia and dependence on weekly intravenous magnesium sulfate (MgSO4), which compromised her quality of life. The patient’s hypomagnesemia was noticed during her daily intake of polyethylene glycol for over 2 years and had persisted even after discontinuing all medications for constipation. Her colonoscopy was unremarkable to identify any etiology for hypomagnesemia. Proton pump inhibitors were discontinued. Additionally, she was diagnosed with atrial fibrillation during a visit to the emergency department (ED) during an episode of severe hypomagnesemia. The patient could not tolerate oral magnesium oxide at 800 mg/day and was initiated on 4 g of intravenous MgSO4 every 2 weeks due to recurrent episodes of severe symptomatic hypomagnesemia resulting in multiple ED visits. However, her Mg2+ remained uncontrolled, with a serum Mg2+ level of 0.9 mg/dL during another ED visit and a 2-week Holter monitor suggestive of 50 episodes of supraventricular tachycardia (4 beats or more). Her intravenous MgSO4 infusion schedule was increased to a weekly regimen. The patient had received a total of 420 g of intravenous MgSO4 over 2 years before referral to nephrology. She was initiated on oral magnesium glycinate 900 mg/day. Later, empagliflozin 10 mg daily was initiated with a dramatic improvement in her serum Mg2+ level, gaining her complete independence from any intravenous infusion of MgSO4 over the next 4 months and allowing a reduction in her oral magnesium glycinate supplements from 900 mg to 400 mg daily without any further episodes of hypomagnesemia (Figs 1, 2; Table S1). Fractional excretion of Mg (FEMg) obtained 12 months pre-empagliflozin and 4 months post-empagliflozin initiation was 4.62% and 8.37%, respectively (Table 1, Fig 2, Table S2).
Figure 1.
Case 1. Historical serum magnesium levels with respect to before and after initiation of empagliflozin. The solid blue background represents a period of dependence on intravenous magnesium sulfate (IV MgSO4), varying from once a week to twice a month dosing. The empty white background represents independence from IV MgSO4. The dashed line at serum magnesium level of 1.7 mg/dL represents a lower limit of normal.
Figure 2.
A comparison of serum magnesium levels (A), fractional excretion of magnesium (FEMg) (B), 24-hour urine magnesium levels (C), and supplemental dose of oral magnesium supplements (D) before and after initiation of empagliflozin. The solid red line represents average magnesium levels for respective columns. The dashed line at serum magnesium level of 1.7 mg/dL represents a lower limit of normal. Note: Patient 1 was dependent on intravenous magnesium sulfate infusions, which were discontinued after initiation of empagliflozin. Abbreviation: SGLT2, sodium-glucose cotransporter 2.
Table 1.
Characteristics, Metabolic Profile, and Changes with SGLT2 Inhibitors.
| Case 1 | Case 2 | |
|---|---|---|
| Sex, age (y) | Female, 78 | Male, 73 |
| Comorbid conditions | Type 2 diabetes, transcatheter sapien aortic valve replacement for severe aortic stenosis, atrial fibrillation, hypertension, chronic constipation | Type 2 diabetes, benign prostate hypertrophy, dyslipidemia, hypertension, and gastroesophageal reflux disease |
| Therapy for hypomagnesemiaa | Maximum tolerated oral magnesium, intravenous MgSO4 4 to 6 grams weekly | Maximum tolerated oral magnesium, multiple ED visits requiring intravenous MgSO4 |
| SGLT2 inhibitor | empagliflozin 10 mg/day | empagliflozin 12.5 mg/day |
| Pre-Empagliflozin | Post-Empagliflozin | Pre-Empagliflozin | Post-Empagliflozin | |
|---|---|---|---|---|
| HbA1c (%) | 9.5 | 8.5 | 7.7 | 7.6 |
| PTH (pg/mL) | 40 | 63 | 37 | NA |
| 25-hydroxy vitamin D (ng/mL) | 38 | 31 | 43 | NA |
| Calcium (mg/dL) | 9.2 | 9.4 | 9.4 | 9.5 |
| Albumin (mg/dL) | 4.0 | 4.2 | 3.9 | NA |
| Creatinine (mg/dL) | 0.80 | 0.98 | 1.2 | 1.2 |
| 24-hour urine Mg2+ (mg) | 56 | 128 | 80 | 88 |
| FEMgb | 4.62% | 8.37% | 5.05% | 4.27% |
Note: FEMg was calculated as 100 × (uMg × sCr)/(0.7 × sMg × uCr), where uMg and uCr represent urinary magnesium and creatinine concentrations measured in 24-hour urine collections, and sMg and sCr represent serum magnesium and creatinine levels, respectively.
Abbreviations: ED, emergency department; HbA1c, hemoglobin A1c; MgSO4, magnesium sulfate; NA, not available; PTH, parathyroid hormone; SGLT2, sodium-glucose cotransporter 2.
Details of medications present during 24-hour urine collections can be found in Table S1.
Details of 24-hour urine study calculations of creatinine clearance, fractional excretion of magnesium (FEMg), and the filtered load of magnesium, pre-SGLT2 inhibitor use and post-SGLT2 inhibitor use can be found in Table S2.
Case 2
A man in his early 70s with a history of type 2 diabetes and gastroesophageal reflux disease was referred for evaluation of chronic hypomagnesemia refractory to magnesium oxide 400 mg/800 mg/400 mg 3 times a day and magnesium oxide extended-release capsules 140 mg twice a day (1,379 mg elemental Mg2+ daily). Despite such oral Mg2+ supplementation, the patient had multiple ED visits for symptomatic hypomagnesemia associated with ventricular arrhythmia requiring intravenous Mg2+ supplementation. Before referral, the patient had undergone an unremarkable workup that included upper and lower endoscopy studies, omeprazole was discontinued 5 months before the pre- or post-SGLT2 inhibitor urine studies, and metformin dose was reduced with a resolution of diarrhea. However, hypomagnesemia persisted (serum Mg2+, 1.1-1.5 mg/dL). The patient was subsequently started on empagliflozin 12.5 mg daily with a sustained increase in serum Mg2+ levels and a notable decrease in oral Mg2+ supplementation to magnesium oxide 400 mg twice a day (484 elemental Mg2+ daily). No repeat ED visits for hypomagnesemia or arrhythmia were noted since the initiation of empagliflozin during the follow-up of 8 months. FEMg obtained during the 6 months pre- and post-empagliflozin initiation was 5.05% and 4.27%, respectively. Moreover, pre-SGLT2 inhibitor 24-hour urinary Mg2+ was 80 mg and post-SGLT2 inhibitor 24-hour urinary Mg2+ was 88 mg (Table 1, Fig 2, Table S2). This was despite the reduction in oral Mg2+ supplements from 1,379 mg to 484 mg of elemental Mg2+, a 67% reduction) (Table S1).
Discussion
Previous studies have shown that SGLT2 inhibitors are associated with increased serum Mg2+ levels as a class effect in patients with type 2 diabetes.2,4 A post hoc analysis of 10 randomized clinical trials showed more correction of hypomagnesemia by dapagliflozin compared with placebo (77% in dapagliflozin vs 30% placebo corrected hypomagnesemia) in patients with type 2 diabetes.3 Improvement in chronic hypomagnesemia was also seen in a study of 50 kidney transplant recipients receiving SGLT2 inhibitors.6 Ray et al5 reported dramatic improvement in serum Mg2+ levels after initiating SGLT2 inhibitor therapy in 3 patients with refractory hypomagnesemia associated with urinary Mg2+ wasting and type 2 diabetes. However, it should be noted that patient 2 in this series, the patient without any identified genetic hypomagnesemic disorder, had correction of hypomagnesemia even without improvement in urinary Mg2+ wasting (based on the assessment of FEMg before and after treatment).5 To the best of our knowledge, we report the first 2 cases that show a dramatic and beneficial effect of SGLT2 inhibitors in patients with refractory hypomagnesemia without overt urinary Mg2+ wasting.
The etiology of hypomagnesemia appears primarily nonrenal combined with an inappropriate compensatory kidney response. Both patients were dependent on high-dose maintenance Mg2+ replacement therapy, and the low 24-hour urinary Mg2+ levels (56 mg in case 1 and 80 mg in case 2) cannot attribute the severe and refractory hypomagnesemia to a primarily kidney etiology. However, the FEMg levels were noted to be marginally above (4.62% for case 1 and 5.05% for case 2) the expected levels for nonrenal causes of hypomagnesemia (0.5%-2.7%).7 This is significant when associated with low serum Mg2+ levels and suggests an inappropriate kidney response to hypomagnesemia. Thus, kidney wasting combined with nonrenal losses, potentially gastrointestinal, are likely responsible in both cases.
Several mechanisms potentially contribute to this nonrenal effect of SGLT2 inhibitors to improve Mg2+ homeostasis. We considered the possibility that improved glycemic control and enhanced insulin resistance would lead to reduced insulin levels, favoring Mg2+ redistribution from the intracellular to the extracellular compartment.8 However, a previous study comparing SGLT2 inhibitors with sulfonylureas in patients with type 2 diabetes did not support this hypothesis.9 Moreover, in our 2 patients, glycemic control was relatively unchanged, whereas dramatic improvements in refractory hypomagnesemia were observed. Vitamin D supplementation has been associated with increased intestinal absorption of Mg2+ and improvements in serum Mg2+ levels.10 Relatively unchanged levels of 25-hydroxy vitamin D, parathyroid hormone, and calcium in patient 1 and no evidence of vitamin D deficiency/supplementation in patient 2 make this an unlikely explanation.
Another potential contribution includes increased Mg2+ absorption in the intestine or reabsorption in the kidney, possibly by enhancing transient receptor potential cation channel subfamily M, member 6 (TRPM6) mediated transport in the intestine and/or the kidney. Mg2+ absorption in the intestine is regulated by 2 different but parallel systems, an active transcellular transport via TRPM6/7 and a passive paracellular pathway. The physiologic amount of intraluminal Mg2+ in the intestine is primarily transported via the active transcellular route, whereas the passive paracellular Mg2+ absorption linearly increases with rising intraluminal concentrations.11 To explain the novel finding of our cases that SGLT2 inhibitors contributed to improving hypomagnesemia in our patients without significantly reducing FEMg, we speculate increased intestinal absorption of Mg2+, potentially via increased TRPM6 mediated by an active transcellular pathway. A group of researchers from Taiwan showed that dapagliflozin enhanced TRPM6-mediated transepithelial Mg2+ transport in renal tubule cells in an animal model.12 We should note that SGLT2 is expressed primarily in the luminal membrane of the S1 and S2 segments of the proximal tubule, whereas TRMP6 channels are situated primarily in the distal convoluted tubule of the kidney.13,14 The mechanism of how SGLT2 inhibitors affect TRPM6 channels remains an area of further investigation; however, local or systemic effects could mediate this outcome. The binding of insulin to its receptors results in the increased insertion of TRPM6 in the plasma membrane.8 Thus, one could speculate that reduced insulin resistance with SGLT2 inhibitors could potentially increase intestinal absorption of Mg2+. However, the relatively unchanged glycemic control in patient 2 and the immediate effect of SGLT2 inhibitors on serum Mg2+ levels make this an unlikely explanation. Data on the impact of SGLT2 inhibitors on hypomagnesemia among patients without type 2 diabetes will be essential to understand this better. The authors of the Taiwanese study suggested further study is needed to assess if dapagliflozin modulates intestinal Mg2+ absorption by TRPM6 transport.12 If confirmed, such treatment could impact the management of patients with impaired intestinal Mg2+ absorption, especially in the patients with hypomagnesemia with secondary hypocalcemia, an autosomal recessive disease with a defect in TRPM6 responsible for poor intestinal absorption of Mg2+ that leads to catastrophic seizures weeks to months after birth and requires lifelong oral Mg2+ supplements.11 Hereby, we propose that further investigations into the role of SGLT2 inhibitors in patients with hypomagnesemia without significant urinary Mg2+ loss are vital.
Lastly, although the patients were not observed to have overt urinary Mg2+ wasting, the FEMg levels suggested an inappropriate kidney response to hypomagnesemia, as discussed earlier. Thus, SGLT2 inhibitors could have potentially benefited our patients by reducing kidney wasting of Mg2+, but this was not clearly demonstrated in follow-up urine studies in these two cases. In conclusion, SGLT2 inhibitors can be considered to treat refractory hypomagnesemia without overt urinary Mg2+ wasting. Further studies to understand their role in the management of hypomagnesemia are needed.
Article Information
Authors’ Full Names and Academic Degrees
Chintan V. Shah, MD, T. Scott Robbins, MD, and Matthew A. Sparks, MD
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Patient Protections
The authors declare that they have obtained consent from the patients reported in this article for publication of the information about them that appears within this Case Report and any associated supplementary material.
Peer Review
Received May 23, 2022. Evaluated by 2 external peer reviewers, with direct editorial input from the Editor-in-Chief. Accepted in revised form July 20, 2022.
Footnotes
Complete author and article information provided before references.
Table S1. Medications during 24-hour urine collections.
Table S2. 24-hour urine study calculations of creatinine clearance, fractional excretion of magnesium (FEMg), and the filtered load of magnesium, pre-and-post-SGLT2 inhibitor use.
Supplementary Material
Table S1-S2.
References
- 1.Brown E., Wilding J.P.H., Alam U., Barber T.M., Karalliedde J., Cuthbertson D.J. The expanding role of SGLT2 inhibitors beyond glucose-lowering to cardiorenal protection. Ann Med. 2021;53(1):2072–2089. doi: 10.1080/07853890.2020.1841281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang H., Zhang X., Zhang J., et al. Elevated serum magnesium associated with SGLT2 inhibitor use in type 2 diabetes patients: a meta-analysis of randomised controlled trials. Diabetologia. 2016;59(12):2546–2551. doi: 10.1007/s00125-016-4101-6. [DOI] [PubMed] [Google Scholar]
- 3.Toto R.D., Goldenberg R., Chertow G.M., et al. Correction of hypomagnesemia by dapagliflozin in patients with type 2 diabetes: a post hoc analysis of 10 randomized, placebo-controlled trials. J Diabetes Complications. 2019;33(10) doi: 10.1016/j.jdiacomp.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J., Huan Y., Leibensperger M., Seo B., Song Y. Comparative effects of sodium-glucose cotransporter 2 inhibitors on serum electrolyte levels in patients with Type 2 diabetes: A pairwise and network meta-analysis of randomized controlled trials. Kidney360. 2022;3(3):477–487. doi: 10.34067/KID.0006672021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ray E.C., Boyd-Shiwarski C.R., Liu P., Novacic D., Cassiman D. SGLT2 inhibitors for treatment of refractory hypomagnesemia: a case report of 3 patients. Kidney Med. 2020;2(3):359–364. doi: 10.1016/j.xkme.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song C.C., Brown A., Winstead R., et al. Early initiation of sodium-glucose linked transporter inhibitors (SGLT-2i) and associated metabolic and electrolyte outcomes in diabetic kidney transplant recipients. Endocrinol Diab Metab. 2020;4(2) doi: 10.1002/edm2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Bommel E.J.M., Geurts F., Muskiet M.H.A., et al. SGLT2 inhibition versus sulfonylurea treatment effects on electrolyte and acid-base balance: secondary analysis of a clinical trial reaching glycemic equipoise: tubular effects of SGLT2 inhibition in type 2 diabetes. Clin Sci (Lond) 2020;134(23):3107–3118. doi: 10.1042/CS20201274. [DOI] [PubMed] [Google Scholar]
- 8.Al-Daghri N.M., Alkharfy K.M., Khan N., et al. Vitamin D supplementation and serum levels of magnesium and selenium in type 2 diabetes mellitus patients: gender dimorphic changes. Int J Vitam Nutr Res. 2014;84(1-2):27–34. doi: 10.1024/0300-9831/a000190. [DOI] [PubMed] [Google Scholar]
- 9.Schlingmann K.P., Waldegger S., Konrad M., Chubanov V., Gudermann T. TRPM6 and TRPM7—gatekeepers of human magnesium metabolism. Biochim Biophys Acta. 2007;1772(8):813–821. doi: 10.1016/j.bbadis.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Hsu Y.J., Hoenderop J.G., Bindels R.J. TRP channels in kidney disease. Biochim Biophys Acta. 2007;1772(8):928–936. doi: 10.1016/j.bbadis.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Srinivasan Sridhar V., Ambinathan J.P.N., Kretzler M., et al. Renal SGLT mRNA expression in human health and disease: a study in two cohorts. Am J Physiol Ren Physiol. 2019;317(5):F1224–F1230. doi: 10.1152/ajprenal.00370.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng H.Y., Kuo W.H., Tain Y.L., Leung F.F., Lee W.C., Lee C.T. Effect of dapagliflozin and magnesium supplementation on renal magnesium handling and magnesium homeostasis in metabolic syndrome. Nutrients. 2021;13(11):4088. doi: 10.3390/nu13114088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gommers L.M.M., Hoenderop J.G.J., Bindels R.J.M., de Baaij J.H.F. Hypomagnesemia in type 2 diabetes: a vicious circle? Diabetes. 2016;65(1):3–13. doi: 10.2337/db15-1028. [DOI] [PubMed] [Google Scholar]
- 14.Elisaf M., Panteli K., Theodorou J., Siamopoulos K.C. Fractional excretion of magnesium in normal subjects and in patients with hypomagnesemia. Magnes Res. 1997;10(4):315–320. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1-S2.