Abstract
Two linked mutations affecting glutamate dehydrogenase (GDH) formation (gdh-1 and rev-2) had been isolated at a locus near the trp cluster in Klebsiella aerogenes. The properties of these two mutations were consistent with those of a locus containing either a regulatory gene or a structural gene. The gdhA gene from K. aerogenes was cloned and sequenced, and an insertion mutation was generated and shown to be linked to trp. A region of gdhA from a strain bearing gdh-1 was sequenced and shown to have a single-base-pair change, confirming that the locus defined by gdh-1 is the structural gene for GDH. Mutants with the same phenotype as rev-2 were isolated, and their sequences showed that the mutations were located in the promoter region of the gdhA gene. The linkage of gdhA to trp in K. aerogenes was explained by postulating an inversion of the genetic map relative to other enteric bacteria. Strains that bore high-copy-number clones of gdhA displayed an auxotrophy that was interpreted as a limitation for α-ketoglutarate and consequently for succinyl-coenzyme A (CoA). Three lines of evidence supported this interpretation: high-copy-number clones of the enzymatically inactive gdhA1 allele showed no auxotrophy, repression of GDH expression by the nitrogen assimilation control protein (NAC) relieved the auxotrophy, and addition of compounds that could increase the α-ketoglutarate supply or reduce the succinyl-CoA requirement relieved the auxotrophy.
In the enteric bacterium Klebsiella aerogenes, glutamate dehydrogenase (GDH) carries out the NADPH-dependent synthesis of glutamate from α-ketoglutarate and ammonia. It is one of only two enzymes in the cell capable of net assimilation of ammonia into glutamate (for a review, see reference 29). The other enzyme, glutamate synthase (GOGAT), uses glutamine in place of ammonia and thus functions in conjunction with glutamine synthetase, the enzyme responsible for assimilating ammonia into glutamine. GDH is a hexameric protein composed of six identical subunits (30) and should be coded for by a single structural gene, gdhA. Glutamate plays two different roles in cellular metabolism: it is the source of 85% of the nitrogen in cellular material, and it plays a role in osmoprotection (16, 34). Thus, it seemed reasonable that multiple regulatory loci might exist. nac (which codes for the nitrogen assimilation control protein [NAC]) is one such locus. When K. aerogenes is grown in nitrogen-deficient medium, gdhA is strongly repressed by NAC, which is itself under the control of the global Ntr system (20, 31).
In 1974, Brenchley and Magasanik (6) described a mutant of K. aerogenes that had reduced levels of GDH activity, but they were not able to determine if the mutation responsible, gdh-1 (then called gdhD1), was in the structural gene for GDH. In 1976, Bender et al. (5) showed that the gdh-1 mutation was linked to the trp operon, in contrast to the Escherichia coli gdhA gene, which is not linked to trp (14). They further identified a class of GDH-overproducing mutants (e.g., rev-2) that led to a fourfold increase in the total GDH activity but was still responsive to regulation by NAC (although not so strongly as was the wild type). The rev-2 mutation was tightly linked to the locus defined by gdh-1. The fact that the genetic maps of E. coli and K. aerogenes are similar, coupled with the fact that regulatory mutations were isolated at this site, led us to question whether gdh-1 did in fact lie in gdhA, the structural gene for GDH.
Cloning of gdhA+.
K. aerogenes strain KB2560 (gltB200 gdh-1 and lysogenic for Mu cts62 hP1#1) lacks both GOGAT and GDH activities and cannot grow without exogenous glutamate (7). An in vivo cloning procedure (13) was used to generate plasmids that enabled KB2560 to grow in the absence of glutamate (the strains and plasmids used in this work are listed in Table 1). Roughly half of these clones contained apparent gltB (GOGAT) clones and the others contained apparent gdh clones. A 2.4-kb PstI restriction fragment from one of the GDH clones was subcloned into pUC19 and tested for complementation. This plasmid, pGDH4, restored GDH activity to a gdh-1 strain. The DNA sequence of the PstI fragment was determined and found to contain an open reading frame (ORF) with near identity (99% at the nucleotide level, 100% at the amino acid level) to the partial gdhA sequence previously reported for K. aerogenes (22, 35). In addition, this ORF was 81% identical at the nucleotide level (90% at the amino acid level) to the gdhA sequence from E. coli (21, 33). Thus, the gdhA+ gene was able to restore GDH activity to a strain carrying the gdh-1 mutation.
TABLE 1.
Strains and plasmids used in this work
| Strain or plasmid | Genotype or relevant characteristicsa | Source or reference |
|---|---|---|
| KC895b | gltB200 ntr-45 | 20 |
| KC1043 | Wild type | 2 |
| KB2560b | gltB200 gdhA1 Mu cts hP1#1 | Mu lysogen of KB630c |
| KC2637 | gdhA1 | This laboratory |
| KC2668 | Δ[bla]-2 | 17 |
| KC2863 | gdh-3 | This work |
| KB2907b | gltB200 gdhA1 Mu cts hP1#1 nac-306::Tn5tac1 | This work |
| KC3183 | gdhA2::Ω | This work |
| KC3228 | KC2668/pGDH4 | This work |
| KC3902 | KC2668/pCB515 | This work |
| KC4356 | KC2668/pCB513 | This work |
| KC4358 | KC2637/pCB644 | This work |
| KC5100 | gdhA12d | This work |
| pUC19 | High-copy-number cloning vector | Gibco-BRL |
| pACYC184 | Medium-copy-number cloning vector | 8 |
| pGB2 | Low-copy-number cloning vector | 9 |
| pGDH4 | gdhA+ cloned into pUC19 | This work |
| pGDH5 | Ω cloned into HpaI site (in gdhA) of pGDH4 | This work |
| pS4BC | Mu clone of gdhA+ | This work |
| pCB513 | gdhA+ cloned into pACYC184 | This work |
| pCB515 | gdhA+ cloned into pGB2 | This work |
| pCB644 | gdhA1 cloned into pGDH4 context | This work |
| pCB725 | gdhAp-lacZ fusion in pRJ800 | This work |
| pCB1205 | gdh-3-lacZ fusion in pRJ800 | This work |
Unless otherwise noted, all of the strains were derived from MK53 and carry the hutC515 and dadA1 alleles (17, 27). KC strains differ from KB strains in that they have been cured of the plasmid pPN100, which encodes (among other things) Smr.
Not derived from MK53, therefore hutC+ and dad+.
KB630 (gltB200 gdhA1) is a P1-sensitive version of MK261 (6).
Generated with a one-step gene inactivation method using PCR products (10). This allele replaces the promoter of gdhA from position −116 to +73 (which includes the first four nucleotides of the ORF) with a kanamycin resistance cassette.
gdhA is linked to trp.
Since the nature of the gdh-1 mutation was unknown, it was necessary to construct an authentic gdhA mutation for genetic mapping. The streptomycin- and spectinomycin-resistant (Sm Sp) Omega (Ω) cartridge (26) was cloned into a unique HpaI site within the gdhA gene of pGDH4. This inactivated gdhA gene (gdhA2::Ω) was crossed onto the K. aerogenes chromosome and replaced the resident wild-type gene. The resulting strain had low levels of GDH, comparable to those of strains carrying gdh-1 (Table 2). Another mutant, gdhA12 (in which the promoter and first four nucleotides of gdhA were replaced with a kanamycin resistance cassette) also displayed low but nonzero levels of GDH. It thus appeared that the residual activity observed resulted from an enzyme other than GDH, and the low levels of GDH observed in a gdh-1 mutant are not inconsistent with this mutation mapping to gdhA. The loss of GDH activity in strains carrying gdhA2::Ω was cotransducible with the Sm Sp resistance and was tightly linked to gdh-1. In addition, gdhA2::Ω, like gdh-1, was linked to trp but not to nas (data not shown). The linkage of gdhA to trp in K. aerogenes can be explained by an inversion of a chromosomal region relative to the same region in Salmonella enterica serovar Typhimurium LT-2. This inversion is similar to the inversions found in this area of E. coli and S. enterica serovar Enteritidis (4, 19). However, the K. aerogenes inversion appears to be smaller: gdhA, nar (or nas), and dad remain outside the boundaries of the inversion, but trp and pyrF remain inside.
TABLE 2.
Effects of gdh mutations on GDH activities
| Strain | Genotype | Enzymea | Sp act (nmol/min/mg) inb:
|
|
|---|---|---|---|---|
| −N | +N | |||
| KC1043 | gdhA+ | GDH | 45 | 379 |
| KC2637 | gdhA1 | GDH | ND | 14 |
| KC3183 | gdhA2::Ω | GDH | ND | 8 |
| KC5100 | gdhA12 | GDH | ND | 12 |
| KC2863 | gdh-3 | GDH | 579 | 1,420 |
| KC2668/pCB725 | gdhAp-lacZ | LacZ | ND | 860 |
| KC2668/pCB1205 | gdh-3p-lacZ | LacZ | ND | 2,710 |
Specific activities are averages of at least two independent experiments. +N, nitrogen-rich medium (glucose minimal medium supplemented with both ammonium sulfate and glutamine); −N, nitrogen-limited medium (glucose minimal medium supplemented with only glutamine (0.2%) as the sole nitrogen source). Cells were grown and prepared for enzyme assays as described previously (31). ND, not determined. β-Galactosidase activity is reported for promoter-lacZ fusions of the particular gdhA allele cloned into the plasmid pRJ800.
The gdh-1 mutation lies within gdhA.
To confirm that gdh-1 was an allele of gdhA, we tested for complementation between gdh-1 and gdhA2::Ω. The cloned gdhA2::Ω (pGDH5) failed to complement gdh-1, but prototrophic recombinants arose at significant frequencies when this plasmid was present in a gltB200 gdh-1 strain. All the plasmids tested in this manner that carried the region from bp 226 to 303 of the structural gene (as well as flanking DNA) yielded recombinants at a frequency similar to that of pGDH5. The plasmid pCB584, which contained only this region and no additional flanking DNA, also yielded recombinants, but at a lower frequency. This was presumably due to the small amount of homologous DNA contained in the fragment. Thus, the only sequence information needed to correct the deficiency in GDH caused by gdh-1 lies within this region (which corresponds to amino acids 76 to 101 of the polypeptide).
A 700-bp fragment of gdhA that includes the region with gdh-1 was cloned twice from independent PCR experiments, and the DNA sequences were determined. In both cases, a single nucleotide change (G to A at position 281 with respect to the ORF) was the only change detected. This would result in a glycine-to-glutamate change at position 94 in the amino acid sequence of GDH. Thus, the original gdh mutation, gdh-1, defines the structural gene in K. aerogenes and can be renamed gdhA1.
A regulatory mutation affecting gdhA expression.
Another mutation affecting GDH formation (rev-2) that had been isolated previously had higher (but still regulated) levels of GDH under all conditions tested and was also linked to trp (5). The simplest explanation for rev-2 was that it was an up-promoter mutation at gdhA or a structural mutation in gdhA that increased the specific activity of the enzyme. However, rev-2 might have defined a regulatory gene near gdhA. The original rev-2 isolate had been lost, so we used the same selection to isolate seven independent mutants with the same phenotype as the original rev-2 strain. This mutant was isolated as a glutamate-independent revertant of an Ntr-constitutive gltB strain (KC895, ntr-45 gltB200). The parent is a glutamate auxotroph due to the lack of GOGAT activity and the repression of gdhA by the Ntr system (via NAC). Most glutamate prototrophs resulted from mutations that lay in either ntrC or nac and affected the nitrogen regulation of many operons. In contrast, rev-2-like mutants were specific for GDH expression. The gdh-3 mutation was typical of the seven mutations isolated in this study in that it was linked to trp and resulted in increased levels of GDH that were still regulated by nitrogen (Table 2).
Our attempts to clone the gdh-3 allele by multicopy complementation of gdhA1 were unsuccessful. Therefore, we tested directly whether the gdh-3 mutation was a promoter mutation affecting gdhA. The 5′ region of the gdhA gene from the gdh-3 strain was amplified by PCR and cloned in front of a promoterless lacZ (pCB1205). This construct showed an increased level of β-galactosidase expression compared with the wild-type gdhA promoter lacZ fusion (Table 2). The DNA sequence of this region was determined and contained a single nucleotide change of G to A at position −14 relative to the start of transcription (Fig. 1a) of wild-type gdhA (as determined below). An identical analysis of the other six independently isolated mutants revealed the same nucleotide change.
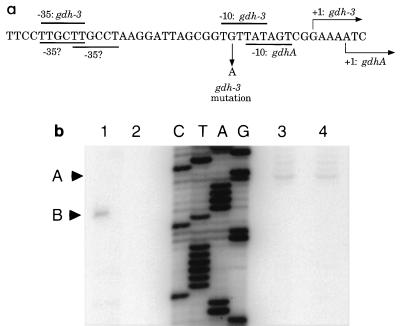
FIG. 1.
Mapping the start of transcription of gdhA and gdh-3. (a) Nucleotide sequence of the gdhA promoter region. Putative −10 and −35 regions and the start of transcription, as shown by primer extension analysis, are indicated. (b) Primer extension analysis (24) of gdhA and gdh-3. A, major start site for gdh-3; B, start site for gdhA. Total RNA was isolated from cells grown under nitrogen excess conditions (GN supplemented with 0.2% glutamine) or nitrogen limitation conditions (glucose minimal medium supplemented with 0.2% glutamine). Lane 1, KC1043 (wild type) with nitrogen excess; lane 2, KC1043 with nitrogen limitation; lane 3, KC2863 (gdh-3) with nitrogen excess; lane 4, KC2863 with nitrogen limitation.
Given the location of the single nucleotide change in the gdh-3 promoter region, it seemed likely that the mutation created a novel promoter with greater strength than that of wild-type gdhA. Primer extension analysis was performed on both a wild-type strain and a gdh-3 strain under nitrogen excess and nitrogen-limiting conditions (Fig. 1b). This analysis showed that the start of transcription had changed, with the primary site 4 nucleotides upstream of the wild-type initiation point but with weaker possible initiation at adjacent sites as well (Fig. 1b, lane 1 versus lane 3). The wild-type start of transcription appeared to be abolished in this mutant. The experiment also reflected the strong nitrogen regulation of gdhA transcription; no transcript was detected from the wild-type promoter under nitrogen-limiting conditions (Fig. 1b, lane 1 versus lane 2). The detection of a transcript from the gdh-3 promoter under nitrogen-limiting conditions was similar to the enzyme assay data; the gdh-3 allele responds to nitrogen limitation, but the effect is significantly less than that seen for the wild type (Table 2).
The phenotype of the gdh-3 allele can be explained by the creation of a new −10 region for the gdhA promoter. The change created a close match to a −10 recognition region that is spaced 16 nucleotides away from a possible −35 binding region of the wild-type promoter (Fig. 1a). The proposed −10 region in the wild-type promoter is spaced 19 nucleotides from this −35 region and 15 nucleotides from another possible −35 region. Thus, the proposed novel −10 site would result in a promoter that has more favorable spacing between the consensus hexamers than does the wild-type promoter (28).
We were surprised to discover that all seven of our independent isolates bore the same mutational change. Perhaps this is a hot spot for spontaneous mutation, or it may reflect the fact that the selection depends on relief of NAC-mediated repression of gdhA. Clearly, GDH formation in the gdh-3 strain under nitrogen limitation was much higher than in the wild type. Moreover, the relative strength of the NAC-mediated repression was much weaker in this strain (ca. 2.5-fold) than in the wild type (at least eightfold). A simple explanation for the reduced repression by NAC is that a stronger binding site for RNA polymerase made the polymerase a better competitor for the site. But repression of gdhA by NAC is complex, and other explanations remain possible.
Overproduction of GDH activity leads to auxotrophy.
Our inability to isolate gdh-3 clones via the in vivo cloning protocol (which uses a high-copy-number origin of replication) led us to suspect that overproduction of GDH activity might prevent growth in minimal medium. However, our original gdhA+ clone was present in high copy numbers. Closer analysis of strains freshly transformed with either pS4BC (high-copy-number gdhA+ Mu plasmid) or pGDH4 (high-copy-number gdhA+ pUC plasmid) revealed that these strains could not grow on glucose ammonia minimal medium (GN) (Table 3) and that secondary mutations which suppressed this growth defect occurred at a high frequency (5 × 10−4 for a wild-type strain carrying pGDH4). The growth defect was relieved by the addition of glutamate to the medium. This auxotrophy was not seen when the (inactive) gdhA1 allele replaced gdhA+ on the high-copy-number plasmid pCB644. In 1976, Struhl and Magasanik described a mutant of K. aerogenes that they explained had a reduced ability to form succinyl-coenzyme A (CoA) from α-ketoglutarate (32). The similarity between the phenotypes of that mutant and our GDH overproducers was striking and led us to the hypothesis that the overproducers converted most of the available α-ketoglutarate to glutamate. This in turn would lead to a limitation for succinyl-CoA. Three sets of observations supported this hypothesis. First, enzymatically active GDH was required for the phenotype. Second, conditions that reduced GDH production reduced the severity of the phenotype. Third, conditions that reduced the demand for succinyl-CoA and/or α-ketoglutarate also reduced the severity of the phenotype.
TABLE 3.
Effects of overexpression of GDH on the ability of strains to use different nitrogen sources
| Strain and plasmidb | Growth observed on indicated mediaa
|
|||||
|---|---|---|---|---|---|---|
| GN
|
GN Glt
|
G Ser
|
||||
| No IPTG | 1 mM IPTGc | No IPTG | 1 mM IPTG | No IPTG | 1 mM IPTG | |
| KC2668, pCB644 | ++ | ++ | ++ | ++ | ++ | ++ |
| KB2907, pCB644 | − | − | ++ | ++ | − | − |
| KB2907, pGDH4 | −d | ++ | ++ | ++ | + | − |
| KB2907, pS4BC | − | ++ | ++ | ++ | + | − |
Growth phenotypes were scored after 24 h at 30°C on solid media (G, glucose; N, ammonium sulfate; Glt, glutamate; Ser, serine) that did or did not include IPTG as indicated. −, no growth; +, slow growth; ++, full growth.
KC2668 is wild type, while KB2907 contains the gltB200 gdh-1 nac-306::Tn5tac1 alleles. pCB644 contains gdhA1 cloned in high copy numbers, while pGDH4 and pS4BC contain gdhA+ cloned in high copy numbers.
IPTG was used as an inducer of nac expression from the nac-306::Tn5tac1 (tacp::nac fusion) allele present in KB2907.
Suppression of the phenotype occurred at a high frequency.
The severity of the auxotrophy reflected the copy number of gdhA+ in the cell. A wild-type strain that contained no additional copies of gdhA+ (KC2668) had a doubling time of 57 min (Table 4). The presence of the low- or medium-copy-number clones increased the doubling time to 66 or 235 min, respectively, and the presence of the high-copy-number clone (pGDH4) prevented growth entirely. An equivalent high-copy-number plasmid which contained the nonfunctional gdhA1 allele had little impact on growth (Table 4; compare results for pCB644 and pGDH4). In order to confirm that pCB644 produced the same amount of polypeptide (albeit inactive) as pGDH4, the protein profiles of strains bearing the wild-type and mutant clones were compared. Both strains contained large amounts of a 45-kDa protein (which probably corresponds to a GDH monomer), and their amounts of polypeptide appeared to be identical (data not shown). Thus, the auxotrophy induced by the overproduction of GDH was linked to the enzyme's activity, not to overproduction of polypeptide.
TABLE 4.
Doubling times and GDH activities of Klebsiella strains containing multicopy levels of gdhA+
| Strain | Relevant genotype | Mediuma | GDHb sp act (nmol/min/mg) | Doubling time (min) |
|---|---|---|---|---|
| KC2668 | Wild type | GN | 492 | 57 |
| GN Glt | 569 | 55 | ||
| G Gln | 76 | 72 | ||
| G Ser | 43 | 202 | ||
| CN | 212 | 64 | ||
| Gly N | 266 | 72 | ||
| GN Lys | 249 | 64 | ||
| GN Met | 663 | 56 | ||
| GN Lys, Met | ND | 61 | ||
| GN Glt, Lys, Met | 120 | 51 | ||
| KC3228 | pGDH4 (high-copy-number gdhA+) | GN | 30,500c | NA |
| GN Glt | 30,700 | 102d | ||
| G Gln | 8,950 | 108 | ||
| G Ser | 6,920 | 213 | ||
| CN | 9,420 | 70 | ||
| Gly N | NA | No growth | ||
| GN Glt, Lys | 19,100 | 67 | ||
| GN Glt, Met | 34,900 | 76 | ||
| GN Lys, Met | ND | 165 | ||
| GN Glt, Lys, Met | 12,100 | 56 | ||
| KC4356 | pCB513 (medium-copy-number gdhA+) | GN | 12,000 | 235 |
| GN Glt | 11,300 | 83 | ||
| KC3902 | pCB515 (low-copy-number gdhA+) | GN | 2,120 | 66 |
| GN Glt | 2,360 | 59 | ||
| KC4358 | pCB644 (high-copy-number gdhA1) | GN | 14 | 65 |
| GN Glt | 14 | 65 |
Cells were grown as described previously (31) in W4 minimal media supplemented as follows: G, 0.4% glucose; N, 0.2% ammonium sulfate; Glt, 0.4% l-glutamate; Gln, 0.2% l-glutamine; Ser, 0.2% l-serine; C, 0.4% citrate; Gly, 0.4% glycerol; Lys, 0.01% l-lysine; Met, 0.01% l-methionine.
GDH specific activities are averages of at least two independent experiments. Assays were performed on whole cells or extracts as described previously (20). ND, value not determined; NA, value not applicable.
KC3228 fails to grow in glucose ammonia minimal media. This GDH value was obtained by washing cells that had been grown in the presence of glutamate and inoculating them at a high cell density in GN medium. The culture was allowed to incubate for several hours before cells were harvested and GDH levels were determined.
Doubling times for the strain in GN Glt medium were variable but averaged 102 min.
Many of the tested growth conditions that allowed KC3228 (high-copy-number gdhA+) to grow in minimal medium also reduced the amount of GDH activity present in the cell. Repression of gdhA transcription was the most straightforward explanation for the reduction of GDH activity. The transcriptional regulator NAC has been shown to repress gdhA from K. aerogenes (20, 31). In KB2907, where the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible tac promoter controls nac expression, the addition of IPTG was enough to allow the strain to grow on GN (Table 3). In addition, when KC3228 was grown with glutamine or serine as the limiting nitrogen source, GDH levels were reduced roughly fourfold (Table 4) and the strain was able to grow. The addition of lysine to the medium also reduced GDH levels approximately twofold. The mechanism of this repression was unclear but was apparently linked to transcription, since gdhA promoter fusions to lacZ also reflected this lysine-dependent repression (data not shown).
Our hypothesis was that the large amounts of GDH in the cell depleted the α-ketoglutarate levels in the cell, but a direct test of this hypothesis was complicated by the fact that K. aerogenes does not transport α-ketoglutarate. However, the replacement of glucose with citrate or succinate as the sole carbon and energy source circumvented this complication. These compounds feed into the tricarboxylic acid cycle and increase the flux into α-ketoglutarate and succinyl-CoA (1, 11). Under these conditions, overexpression of GDH did not affect the growth rate. Furthermore, addition of lysine and methionine, which need succinyl-CoA for synthesis (12, 23), reduced the demand for succinyl-CoA and the severity of the phenotype. However, the addition of both lysine and methionine was not sufficient to restore the full growth rate to a GDH overproducer. This is consistent with the observation that the addition of these two amino acids did not fully restore wild-type growth to α-ketoglutarate dehydrogenase mutants of E. coli (15).
By comparing GDH activities and growth rates in strains KC3228 and KC4356, it was possible to show that lysine and methionine reduced the severity of the phenotype independent of repression effects. This was most clearly shown by comparing three cultures (Table 4) that each had about 12,000 U of GDH activity per mg: KC3228 (high-copy-number gdhA+) grown in GN supplemented with glutamate, lysine, and methionine and KC4356 (medium-copy-number gdhA+) grown in either GN or GN supplemented with glutamate. While all three conditions provided roughly the same amount of GDH, the doubling time of KC4356 decreased from 235 min to 83 min with the addition of glutamate, and KC3228 with all three supplements grew faster still, doubling every 56 min. The effect of methionine alone is easily seen by examining the results for strain KC3228. In GN supplemented with glutamate, the strain had 30,700 U of GDH activity per mg and a doubling time of 102 min. The addition of methionine to the medium maintained high levels of GDH (34,900 U/mg), but the strain doubled faster (a doubling time of 76 min). The effect of lysine was harder to isolate because of the twofold repression caused by the addition of lysine to the medium. Nevertheless, strain KC4356 grown without lysine had high levels of GDH (11,300 U/mg) and had a doubling time of 83 min, while strain KC3228 grown in the presence of lysine had even higher levels of GDH (19,100 U/mg) yet grew faster, doubling every 67 min. Thus the addition of lysine, methionine, or both appeared to reduce the requirement for succinyl-CoA and allow faster growth.
Other growth conditions relieved the auxotrophy, but these conditions reduced both the total GDH activity and the demand for succinyl-CoA and α-ketoglutarate. For example, growth with serine as the sole nitrogen source severely limits the rate at which ammonia is supplied to the cell. This in turn limits the amount of α-ketoglutarate that GDH can convert to glutamate, thus slowing the drain on the α-ketoglutarate supply. However, when ammonia is limiting for K. aerogenes, GDH formation is severely repressed. Nevertheless, it is clear that when strain KC3228 was grown with serine as the sole nitrogen source, it grew as well as wild-type K. aerogenes, despite the fact that it had 160 times as much GDH as the wild type. Thus, restricting ammonia, a substrate of the GDH reaction, had an effect independent of repression.
Finally, it is not surprising that the gdhA1 mutation of K. aerogenes is enzymatically inactive. The gdhA1 allele of E. coli affects a lysine critical for catalytic activity (K92); this mutant GDH can still form hexamers but does not have enzymatic activity (18). The gdhA1 allele of K. aerogenes changes the glycine at position 94 to a glutamate; such a severe change close to an active-site residue would be expected to have an effect on enzymatic activity.
Nucleotide sequence accession number.
The DNA sequence of a 2.4-kb PstI restriction fragment from a gdh strain cloned in this study has been deposited in the GenBank nucleotide sequence database under accession no. AF332586.
Acknowledgments
We thank Robert Helling for critical review of the manuscript.
This work was supported by Public Health Service grant GM 47156 from the National Institutes of Health to R.A.B.
REFERENCES
- 1.Amarasingham C R, Davis B D. Regulation of α-ketoglutarate dehydrogenase formation in Escherichia coli. J Biol Chem. 1965;240:3664–3668. [PubMed] [Google Scholar]
- 2.Baldauf S L, Cardani M A, Bender R A. Regulation of the galactose-inducible lac operon and the histidine utilization operons in pts mutants of Klebsiella aerogenes. J Bacteriol. 1988;170:5588–5593. doi: 10.1128/jb.170.12.5588-5593.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball C A, Osuna R, Ferguson K C, Johnson R C. Dramatic changes in Fis levels upon nutrient upshift in Escherichia coli. J Bacteriol. 1992;174:8043–8056. doi: 10.1128/jb.174.24.8043-8056.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender R A. Variations on a theme by Escherichia. In: Neidhardt F C, Curtis III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 4–9. [Google Scholar]
- 5.Bender R A, Macaluso A, Magasanik B. Glutamate dehydrogenase: genetic mapping and isolation of regulatory mutants of Klebsiella aerogenes. J Bacteriol. 1976;127:141–148. doi: 10.1128/jb.127.1.141-148.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenchley J E, Magasanik B. Mutants of Klebsiella aerogenes lacking glutamate dehydrogenase. J Bacteriol. 1974;117:544–550. doi: 10.1128/jb.117.2.544-550.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenchley J E, Prival M J, Magasanik B. Regulation of the synthesis of enzymes responsible for glutamate formation in Klebsiella aerogenes. J Biol Chem. 1973;248:6122–6128. [PubMed] [Google Scholar]
- 8.Chang A C, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Churchward G, Belin D, Nagamine Y. A pSC101-derived plasmid which shows no sequence homology to other commonly used cloning vectors. Gene. 1984;31:165–171. doi: 10.1016/0378-1119(84)90207-5. [DOI] [PubMed] [Google Scholar]
- 10.Datsenko K A, Wanner B L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray C T, Wimpenny J W, Mossman M R. Regulation of metabolism in facultative bacteria. II. Effects of aerobiosis, anaerobiosis and nutrition on the formation of Krebs cycle enzymes in Escherichia coli. Biochim Biophys Acta. 1966;117:33–41. doi: 10.1016/0304-4165(66)90149-8. [DOI] [PubMed] [Google Scholar]
- 12.Greene R C. Biosynthesis of methionine. In: Neidhardt F C, Curtis III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 542–560. [Google Scholar]
- 13.Groisman E A, Casadaban M J. Mini-Mu bacteriophage with plasmid replicons for in vivo cloning and lac gene fusing. J Bacteriol. 1986;168:357–364. doi: 10.1128/jb.168.1.357-364.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helling R B. The glutamate dehydrogenase structural gene of Escherichia coli. Mol Gen Genet. 1990;223:508–512. doi: 10.1007/BF00264460. [DOI] [PubMed] [Google Scholar]
- 15.Herbert A A, Guest J R. Biochemical and genetic studies with lysine + methionine mutants of Escherichia coli: lipoic acid and α-ketoglutarate dehydrogenase-less mutants. J Gen Microbiol. 1968;53:363–381. doi: 10.1099/00221287-53-3-363. [DOI] [PubMed] [Google Scholar]
- 16.Ingraham J L, Marr A G. Effect of temperature, pressure, pH, and osmotic stress on growth. In: Neidhardt F C, Curtis III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 1570–1578. [Google Scholar]
- 17.Janes B K, Bender R A. Alanine catabolism in Klebsiella aerogenes: molecular characterization of the dadAB operon and its regulation by the nitrogen assimilation control protein. J Bacteriol. 1998;180:563–570. doi: 10.1128/jb.180.3.563-570.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones K M, McPherson M J, Baron A J, Mattaj I W, Riordan C L, Wootton J C. The gdhA1 point mutation in Escherichia coli K12 CLR207 alters a key lysine residue of glutamate dehydrogenase. Mol Gen Genet. 1993;240:286–289. doi: 10.1007/BF00277068. [DOI] [PubMed] [Google Scholar]
- 19.Liu S L, Hessel A, Sanderson K E. The XbaI-BlnI-CeuI genomic cleavage map of Salmonella enteritidis shows an inversion relative to Salmonella typhimurium LT-2. Mol Microbiol. 1993;10:655–664. doi: 10.1111/j.1365-2958.1993.tb00937.x. [DOI] [PubMed] [Google Scholar]
- 20.Macaluso A, Best E A, Bender R A. Role of the nac gene product in the nitrogen regulation of some NTR-regulated operons of Klebsiella aerogenes. J Bacteriol. 1990;172:7249–7255. doi: 10.1128/jb.172.12.7249-7255.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McPherson M J, Wootton J C. Complete nucleotide sequence of the Escherichia coli gdhA gene. Nucleic Acids Res. 1983;11:5257–5266. doi: 10.1093/nar/11.15.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mountain A, McPherson M J, Baron A J, Wootton J C. The Klebsiella aerogenes glutamate dehydrogenase (gdhA) gene: cloning, high-level expression and hybrid enzyme formation in Escherichia coli. Mol Gen Genet. 1985;199:141–145. doi: 10.1007/BF00327523. [DOI] [PubMed] [Google Scholar]
- 23.Patte J C. Biosynthesis of threonine and lysine. In: Neidhardt F C, Curtis III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 528–541. [Google Scholar]
- 24.Pomposiello P J, Bender R A. Activation of the Escherichia coli lacZ promoter by the Klebsiella aerogenes nitrogen assimilation control protein (NAC), a LysR family transcription factor. J Bacteriol. 1995;177:4820–4824. doi: 10.1128/jb.177.16.4820-4824.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pomposiello P J, Janes B K, Bender R A. Two roles for the DNA recognition site of the Klebsiella aerogenes nitrogen assimilation control protein. J Bacteriol. 1998;180:578–585. doi: 10.1128/jb.180.3.578-585.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 27.Prival M J, Magasanik B. Resistance to catabolite repression of histidase and proline oxidase during nitrogen-limited growth of Klebsiella aerogenes. J Biol Chem. 1971;246:6288–6296. [PubMed] [Google Scholar]
- 28.Record M T, Jr, Reznikoff W S, Craig M L, McQuade K L, Schlax P J. Escherichia coli RNA polymerase (Eς70), promoters, and the kinetics of the steps of transcription initiation. In: Neidhardt F C, Curtis III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 792–820. [Google Scholar]
- 29.Reitzer L J. Ammonia assimilation and the biosynthesis of glutamine, glutamate, aspartate, asparagine, l-alanine, and d-alanine. In: Neidhardt F C, Curtis III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 391–407. [Google Scholar]
- 30.Sakamoto N, Kotre A M, Savageau M A. Glutamate dehydrogenase from Escherichia coli: purification and properties. J Bacteriol. 1975;124:775–783. doi: 10.1128/jb.124.2.775-783.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwacha A, Bender R A. The product of the Klebsiella aerogenes nac (nitrogen assimilation control) gene is sufficient for activation of the hut operons and repression of the gdh operon. J Bacteriol. 1993;175:2116–2124. doi: 10.1128/jb.175.7.2116-2124.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Struhl K, Magasanik B. Ammonia-sensitive mutant of Klebsiella aerogenes. J Bacteriol. 1976;126:739–742. doi: 10.1128/jb.126.2.739-742.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valle F, Becerril B, Chen E, Seeburg P, Heyneker H, Bolivar F. Complete nucleotide sequence of the glutamate dehydrogenase gene from Escherichia coli K-12. Gene. 1984;27:193–199. doi: 10.1016/0378-1119(84)90140-9. [DOI] [PubMed] [Google Scholar]
- 34.Wohlheuter R M, Schutt H, Holzer H. Regulation of glutamine synthesis in vivo in Escherichia coli. In: Prusiner S, Stadtman E R, editors. The enzymes of glutamine metabolism. New York, N.Y: Academic Press, Inc.; 1973. pp. 45–64. [Google Scholar]
- 35.Wooten J C, McPherson M J. Genes of nitrate and ammonium assimilation. In: Lea P J, Stewart G R, editors. Annual proceedings of the Phytochemical Society of Europe. Vol. 23. Oxford, United Kingdom: Clarendon Press; 1984. pp. 89–114. [Google Scholar]